Abstract
Stress is among the strongest signals promoting neuroplasticity: Stress signals, indicating real or perceived danger, lead to alterations of neuronal function and often structure, designed to adapt to the changed conditions and promote survival. Corticotropin releasing factor (CRF) is expressed and released in several types of neuronal populations that are involved in cognition, emotion and the regulation of autonomic and endocrine function. CRF expressing neurons undergo functional and structural plasticity during stress and, in addition, the peptide acts via specific receptors to promote plasticity of target neurons.
Keywords: CRF, hippocampus, hypothalamus, neuropeptide, synapse, plasticity, stress, memory, epigenetics
1. Introduction and scope
Stress is among the strongest signals promoting brain plasticity. A vast body of work has demonstrated that stress signals, indicating real or perceived danger, lead to alterations of neuronal function, and often structure, designed to adapt to the changed conditions and promote survival (Christoffel et al., 2011; McEwen, 2012; Wosiski-Kuhn and Stranahan, 2012). These crucial changes take place at time scales ranging from seconds to months, and are mediated by a complex set of both central and peripheral mediators that together constitute the stress response. Stress-activated mediators have traditionally included neurotransmitters (norepinephrine, serotonin and others), and steroid hormones (cortisol in humans, corticosterone in rodents). The discovery and isolation of corticotropin releasing factor (CRF) by Wylie Vale and his team uncovered the nature of the enigmatic hypothalamic factor that stimulated ACTH release (Vale et al., 1981). In addition, Wylie and his group demonstrated the presence of the peptide in a number of brain regions outside of the hypothalamus and its neurotransmission functions, adding an important element of complexity to the intricate array of stress mediators (Vale et al., 1983). CRF is a neuropeptide, a class of molecules that often demonstrates distinct temporal and spatial action domains (Swanson et al., 1983; Landgraf and Neumann, 2004; Joëls and Baram, 2009; Maras and Baram, 2012), bridging the typical range of temporal actions of neurotransmitters and the genomic actions of steroid hormones. Whereas much overlap exists, the majority of neurotransmitters act via ionotropic or G-protein coupled receptors to evoke effects within millisecond to seconds. Neuropeptides overlap with this range in their synaptic effects (e.g.,Gallagher et al., 2008), but can also act more slowly, likely via polysynaptic (Hollrigel et al., 1998) or non-synaptic pathways (Landgraf and Neumann, 2004; Valentino and Van Bockstaele, 2008, Refojo 2011). The slower time-scale might result from the more long-distance travel of the peptide via volume transmission (Bittencourt and Sawchenko, 2000; Agnati et al., 2010). The time-scale of the action of peptides, including CRF, also differs from the time-scale of steroid hormones. Whereas rapid nongenomic actions of corticosteroid hormones have been well-described (Roozendaal et al, 2010; Groeneweg et al., 2011), the fundamental actions of corticosteroids involve GR and MR mediated genomic effects that require hours. Peptides also occupy a distinct (though overlapping) spatial niche: Because they are often dispersed via volume transmission, neuropeptides such as CRF can affect simultaneously numerous neurons in a brain region (Roozendaal et al., 2002; Agnati et al., 2010) rather than act at single or a few synapses, yet they do not permeate the whole brain, as do steroid hormones.
Wylie Vale isolated CRF from the hypothalamus and initially focused on the role of this peptide in promoting synthesis and release of ACTH from the pituitary gland (Brown et al., 1982; Gibbs and Vale, 1982; Rivier et al., 1982), In addition to the hypothalamus, well discussed in other manuscripts within this collection, Wylie’s group (Swanson et al., 1983) as well as others, found that CRF is expressed or acts in the amygdala (Roozendaal et al., 2002; Gallagher et al., 2008; Regev et al., 2012), hippocampus (Lee et al., 1993; Chen et al., 2001a; Refojo et al, 2011; Chen et al., 2013), cortex (De Souza et al., 1986; Behan et al., 1995a; Gallopin et al., 2006), , inferior olive (Chang et al., 1996), locus coeruleus (Valentino and Van Bockstaele, 2008) bed nucleus of the stria terminalis (Dabrowska et al., 2011), and other areas affected by, or involved in, stress. Importantly, CRF receptors type-1 (CRFR1) and type-2 (CRFR2) are found on target neurons (Van Pett et al., 2000; Reul and Holsboer, 2002), and within specific subcellular compartments (Reyes et al, 2008; Chen et al, 2012), enabling complex and fine-tuned effects of CRF on target neurons throughout the brain.
Thus, CRF is an important component of the complex set of stress-mediators, allowing the brain to mount the entire spectrum of the stress response, ranging from immediate attention and strategic decisions, which are important for survival in the short-term, to storage of information about a stressful situation, which is advantageous in the long-term.
This manuscript focuses on CRF and neuroplasticity. Neuroplasticity may be defined as a long-lasting change in neuronal structure and/or function in response to a trigger. It is commonly believed that both structural and functional plasticity involves altered expression, transport and function of numerous genes in concert. Long-lasting neuroplasticity often derives from epigenetic mechanisms: enduring changes in chromatin structure that influence the presence and degree of transcription of genes and gene families. Here we discuss the CRF neuron in both hypothalamus and hippocampus. We describe how CRF neurons undergo functional and structural plasticity and, in addition, how the peptide acts via specific receptors to promote plasticity of target neurons.
2. Plasticity of the hypothalamic CRF neuron
Hypothalamic CRF coordinates neuroendocrine, autonomic and behavioral responses to stress (Brunson et al., 2001; Coste et al., 2001; Bale and Vale, 2004; de Kloet et al., 2005; Joëls and Baram, 2009; Lightman, 2008; Valentino and Van Bockstaele, 2008; Zoumakis and Chrousos, 2010; Aguilera, 2011; Bonfiglio et al., 2011), and dysregulation of CRF neurons within the paraventricular nucleus of the hypothalamus (PVN) is found in several stress-related affective disorders (de Kloet et al., 2005; Lloyd and Nemeroff, 2011; Flandreau et al., 2012). CRF expression in the PVN is regulated by a variety of factors including stress (Swanson and Simmons, 1989; Watts, 2005; Lightman, 2008). It is generally found that stress activates both CRF release as well as a rapid increase in transcription of the gene (Yi and Baram, 1994; Rivest et. al., 1995; Ma et. al., 1997; Tanimura and Watts, 1998; Dent et al., 2000; Ginsberg et al., 2003; Watts, 2005; Pace et. al., 2009; Osterlund and Spencer, 2011; Liu et al, 2012). Appropriate initiation and termination of CRF secretion and synthesis are crucial for physiological homeostasis (Coste et al., 2001; Bale et al., 2002; Liu and Aguilera, 2009; Aguilera and Liu, 2012). Therefore, the manner by which stress signals reach and activate CRF cells in PVN, and the nature of the regulation of CRF expression levels and release have been subjects of intensive investigation.
Here we focus on certain aspects of stress-related neuroplasticity of the hypothalamic CRF neuron: We describe how synaptic connectivity, which influences neuronal function, is altered by early-life experiences. This unique structural and functional synaptic plasticity initiates intracellular events within CRF neurons that constitute an additional, enduring functional and molecular neuroplasticity.
2.1. Structural neuroplasticity of the hypothalamic CRF neuron
It has been well established that early-life experience induces persistent neuroplasticity of the neuroendocrine stress system, characterized by reduced stress responses (Levine, 1967; Plotsky and Meaney, 1993; Avishai-Eliner et al., 2001a), increased resilience to depressive-like behavior (Meaney et al., 1991) and improved learning and memory (Liu et al, 2000; Fenoglio et al., 2005). This neuroplasticity can be induced by a brief daily separation of rat pups from the dam (handling) during the first weeks of life, a manipulation resulting in augmented maternal-derived sensory input upon the reunification of dam and pups (Liu et al., 1997; Fenoglio et al., 2006a; Korosi et al., 2010). At the level of the hypothalamic CRF neuron, reduced expression of the gene is found in adult rats experiencing handling-related augmented maternal sensory input (Plotsky and Meaney, 1993; Liu et al., 1997; Fenoglio et al., 2005). Experience-induced reduction of CRF expression is observed rapidly, already by postnatal day 9 (P9), preceding the lowered hormonal stress responses and the epigenetically-induced augmentation of hippocampal GR expression (Avishai-Eliner et al., 2001a; Weaver et. al., 2005). These observations suggest that plasticity of the hypothalamic CRF neuron, resulting in repressed gene expression, is an early and important component of the effects of enhanced maternal sensory input on the stress response.
Therefore, experiments were conducted to uncover the nature of the signals, derived from maternal activities, that reach CRF neurons. Experiments also explored the resulting changes (plasticity) of the hypothalamic CRF neuron. Because the CRF neuron is a component of a neuronal network activated by maternal care (Fenoglio et al., 2006a), we examined if its neuroplasticity consisted of altered excitatory and / or inhibitory synaptic input. These changes, in turn, may provoke repression of the crf gene.
Innervation of CRF neurons includes GABAergic and glutamatergic synapses (Boudaba et al., 1997; Miklos and Kovacs, 2002; Ziegler et al., 2005; Karsten and Baram, 2013), that signal via GABAA (Cullinan, 2000) and glutamate receptors (Aubry et al., 1996; Kiss et al., 1996; Di et al., 2003). In addition, functional synaptic plasticity of the CRF neurons in PVN has been extensively studied in the context of stress (e.g., Kuzmiski et al., 2010; see Levy and Tasker, 2012 for review). Using quantitative confocal and electron microscopy coupled with electrophysiology, we examined the effects of early-life experience on excitatory and inhibitory synapses innervating CRF neurons. We found no significant changes in GABAergic synapse number or function. In contrast, there was a significant reduction in both the number and function of excitatory, glutamatergic synapses on the CRF neuron. The majority of the neurochemical, structural and electrophysiological data suggested a presynaptic reduction in release sites (Korosi et al., 2010). Hence, early-life experience promotes structural neuroplasticity of the CRF neuron, consisting of reduced excitatory drive onto this neuron because of reduced glutamatergic synaptic innervation. The hypothalamic CRF neuron is part of the stress network (Hatalski et al., 1998; Chen et al., 2001a; Jankord and Herman, 2008; Dedovic et al., 2009; Bonfiglio et al., 2011); therefore, this neuroplasticity should reduce CRF release and expression in response to stress.
2.2. Functional / molecular neuroplasticity of the hypothalamic CRF neuron following early-life experience, and the potential role of epigenetic mechanisms
Molecular plasticity of the CRF neuron, and specifically alteration of CRF expression, has been described in numerous contexts. Acutely, stress augments transcription of the crf gene (Tanimura and Watts, 1998; Baram and Hatalski, 1998; Dent et al, 2000; Chen et al., 2001b; Ritter et. al., 2003; Fenoglio et al., 2006b; Liu et al., 2012; Cope et al., 2013). However, at longer time frames, stress can either increase (Sterrenburg et al., 2011) or decrease (Pinnock and Herbert, 2001; Ivy et al., 2008; Rice, et al., 2008) CRF levels.
CRF levels are persistently reduced in rodents experiencing augmented early life maternal care (Plotsky and Meaney, 1993; Brunson, et al., 2001; Avishai-Eliner, et al., 2001a). Levels of CRF expression in parvocellular hypothalamic neurons contribute to the fine-tuning of the neuroendocrine response to stress because there is a relationship between the levels of CRF expression and peptide release in response to stressful signals. Therefore, we sought to elucidate how the persistent, life-long reduction in CRF expression, induced by augmented maternal care early in life, is initiated and maintained.
In terms of the initiation of repression of the crf gene, ongoing studies are focusing on the potential causal relationship of reduced excitatory synaptic input to CRF neurons and the reduction of CRF expression. The maintenance of the long-lasting repression of this gene likely involves epigenetic mechanisms, i.e., changes to the conformation of the chromatin around the crf gene (review by Szyf, 2013; Lucassen et al., 2013). Changes in DNA methylation at the promoter region of the CRF gene have been reported in view of the contribution of DNA methylation to transcriptional repression (McGill et al., 2006), an inverse relationship of promoter methylation and crf expression has been sought, and indeed found (Mueller and Bale, 2008; Elliott et al, 2010, Chen et al., 2012a). Surprisingly, in our hands, the study of crf promoter and intron methylation after early-life augmented maternal care failed to find increased methylation as a mechanism for the enduring repression of the crf gene (McClelland, 2011), and these findings are consistent with the emerging complexity of various types of DNA methylation and the relationship of these modifications to gene expression (Lister, et al., 2013). Considering alternative mechanisms to DNA methylation, and focused on the potential role of the transcriptional repressor neuron-restrictive silencing factor (NRSF; Mori et al., 1992; Palm et al., 1998), because of the presence of a functional binding site for this repressor within the crf gene (Seth and Majzoub, 2001). Augmented maternal care was found to increase NRSF levels in the hypothalamus (but not in thalamus, Korosi et al., 2010), and the NRSF bound the NRSE site on the crf gene intron. Thus, NRSF is an attractive molecule to mediate the epigenetic changes underlying the persistent molecular neuroplasticity of the crf gene- and likely of numerous additional stress-related genes.
Whereas there is a general agreement about the molecular neuroplasticity of the CRF neuron following optimal or augmented maternal care early in life (Plotsky and Meaney, 1993; Liu et al., 1997; Korosi and Baram, 2009), this is not the case for the consequences of adverse early-life experience on the CRF neurons and specifically on CRF gene expression. Maternal deprivation has led to an enhanced CRF expression in the PVN (Aisa et al., 2007; Chen et al., 2012a). In contrast, others have found a depletion of steady-state CRF messenger RNA in P9 rat or mouse pups after a week-long chronic stress provoked by limited nesting and bedding material in the cage (Avishai-Eliner et al., 2001b; Rice et al., 2008). These diverse findings suggest that early-life adversity can influence the stress-sensitive CRF neurons in multiple ways, and the distinct parameters involved (e.g., intermittent vs. continuous stress, patterns of maternal behavior [Baram et al., 2012]) as well as the cellular and molecular mechanisms, deserve further study.
3. CRF contributes to stress-induced neuroplasticity of learning and memory
3.1. Role for CRF in stress-induced memory problems
Stress is prevalent and unavoidable. It is biologically important because it enables both rapid and delayed adaptive processes to a changing environment. The adaptive importance of remembering threatening or dangerous events allows learning from them, which promotes survival. Because the hippocampus is a key brain region for learning and memory, many of the effects of stress on these cognitive functions take place within the hippocampus.
The effects of stress on hippocampal structure and function are bi-directional. Acute or short stress, lasting seconds to minutes, enhances hippocampal function by augmenting synaptic plasticity through a variety of mediators and mechanisms (Blank et al., 2002; Joëls and Baram 2009; McEwen and Gianaros, 2011). However, these same mechanisms, when activated intensely or for a prolonged period, may render the hippocampus vulnerable to the detrimental effects of chronic or severe stress (Joëls and Baram, 2009; Ulrich-Lai and Herman, 2009). Thus, the effects of stress on hippocampal functions are complex, depending on whether the stressful stimulus is mild or severe, acute or chronic, and on its perception as controllable and predictable vs. unpredictable and uncontrollable.
Recent studies have uncovered an important role for CRF in the effects of stress on learning and memory, and specifically the effects of stress on hippocampus- dependent learning. For example, blocking the ability of CRF to interact with its receptor attenuated the adverse effects of hours-long stress on memory (Chen et al., 2010), and mice lacking the CRFR1 were resistant to the pervasive effects of chronic early-life stress (Wang et al., 2011a) and chronic social stress (Wang et al., 2011b) on memory.
3.2. Hippocampal CRF: origins and targets
The studies cited above indicate that there is a role for CRF, acting on CRFR1, in the effects of stress on hippocampal function. However, the source of the peptide is not completely resolved. CRF is expressed in both developing and adult rodent hippocampus (Sakanaka et al., 1987; Chen et al., 2001a). The peptide is secreted from the interneuronal axon terminals into the local synaptic space during stress (Chen et al., 2012b). CRF is also expressed and secreted during stress in the amygdala (Roozendaal et al., 2002), locus coeruleus (Valentino and Wehby, 1988; Snyder et al., 2012) and other brain regions. CRF was shown to reach many brain regions when injected into the cerebral ventricles (Bittencourt and Sawchenko, 2000), and to travel from the central amygdala nucleus to the adjacent basolateral nucleus (Roozendaal et al., 2002). The precise mechanism for this transport, and a potential role of the CRF binding protein, remain unclear (Behan et al., 1995b; Seasholtz et al, 2001; Chen et al., 2004). Whereas it conceivable that the source of CRF that influences hippocampal neurons during stress might be from other brain areas, two lines of evidence suggest that the origin of CRF that acts on CRF receptor within hippocampus is local. First, in organotypic cultures of the hippocampus (where other brain regions are not included), the presence of selective blockers of CRFR1 (the receptor most highly expressed in the hippocampal formation) provokes abnormal dendritic growth, suggesting a role for endogenous hippocampal CRF in shaping hippocampal dendritic structure (Chen et al., 2004). Second, electron microscopy studies demonstrate that CRF is stored within releasable vesicle pools at axon terminals of CRF-expressing interneurons (although there are no CRF-containing vesicles in dendrites), consistent with the presence of canonical release machinery for CRF at axon terminals. These data suggest local release of endogenous hippocampal CRF from interneuronal axon terminals (Yan et al., 1998; Chen et al., 2012b). In sum, available evidence supports the notion that CRF is produced in populations of interneurons in the pyramidal cell layers of areas CA1 and CA3 (Sakanaka et al., 1987; Yan et al., 1998; Chen et al., 2001a; Ivy et al., 2010). All of the CRF-expressing cells observed in adult hippocampus seem to express GAD, the GABA synthetic enzyme. Many co-express the calcium binding protein, parvalbumin, typical of hippocampal basket cells within the pyramidal cell layer, but none co-express cholecystokinin, which defines a separate set of interneurons (Chen et al., 2012b). It is still not fully resolved how stress promotes secretion of CRF from axonal terminals of these interneurons, and how the peptide reaches its receptors.
3.3. Stress-induced, CRF-mediated neuroplasticity of hippocampal cells
The evidence above suggests that during stress, hippocampal neurons are impacted by several stress-mediators: glucocorticoids reach the hippocampus from the adrenal, and act via both glucocorticoid and mineralocorticoid receptors (McEwen, 1999; Kim and Diamond, 2002; Roozendal et al., 2003; Joëls and Baram, 2009). During stress, hippocampal synapses are impacted by neurotransmitters such as serotonin and norepinephrine, and, as shown above, by hippocampal CRF. Hence, it is likely that hippocampal CRF contributes to an orchestrated set of cellular and molecular events initiated by stress within hippocampal neurons, which promote plasticity.
The duration of stress is especially important in determining its effects on learning and memory (Zoladz and Diamond, 2008; Joëls and Baram, 2009; Joëls et al., 2011; McEwen and Gianaros, 2011): Stress (and CRF application) lasting for minutes alters the structural and functional properties of neurons in a manner that is distinct from observations after stress lasting for hours, although both of these time-frames often designate “acute” stress. Chronic stress, lasting days and weeks, exerts still more distinctive changes in hippocampal function and structure. How does CRF contribute to these distinct, time-dependent effects of stress on memory?
During minutes-long stress, CRF release potentiates synaptic plasticity (Wang et al., 1998, 2000). It also leads to priming of long-term potentiation (Blank et al, 2002, 2003,). LTP is a cellular process that is generally considered to represent learning and memory (Larson and Lynch, 1986; Bliss and Collingridge, 1993). The ‘positive’ effect of CRF on hippocampal function during stress is supported by the fact that several hippocampus-dependent learning tasks are improved upon administration of CRF into the brain (Hung et al., 1992; Ma et al, 1999; Radulovic et al, 1999; Blank et al., 2002, 2003; Row and Dohanich, 2008). The mechanisms by which CRF influences synaptic function and memory at the seconds-to-minutes time window are not fully known. It is clear that CRFCRFR1 signaling is involved, because synaptic potentiation is attenuated in hippocampal slices from mice lacking CRFR1 (Schierloh et al, 2007), and, in addition, these mice have memory problems (Contarino et al, 1999). There is evidence for CRF-mediated increase of presynaptic glutamate release (Hollrigel et al., 1998), as well as enhanced postsynaptic excitability (Aldenhoff et al., 1983), potentially related to CRF-induced suppression of after-hyperpolarization (Aldenhoff et al., 1983). In addition, activation of several molecular cascades by crf receptor activation has been demonstrated, including specific kinases (Refojo et al., 2005; Punn et al., 2006) beta-arrestins (Holmes et al., 2006; Oakley et al., 2007) and Rho GTPases (Swinny and Valentino, 2006; Chen et al., 2013).
A role for CRF in the effects of longer stress on hippocampus is suggested by several lines of evidence. First, genetic manipulation of CRF levels in hippocampus accelerated cognitive problems and structural decline in models of Alzheimer disorder (Dong et al., 2012). Indeed, overexpression of CRF in forebrain led to learning and memory defects (Wang et al., 2011a). CRF has also been implicated in dendritic and spine changes in hippocampus after a shorter stress, lasting several hours (Pawlak et al., 2005; Chen et al., 2013), and these effects involve both inter-cellular molecules such as nectin 3 (Wang et al., 2013) and intracellular mechanisms for destabilizing dendritic spines (Chen et al., 2013). Finally, exposing hippocampal explants to CRF chronically promotes loss of dendritic arborization (Chen et al., 2004). These data, suggest that CRF contributes to the established effects of hours-lasting and chronic stress on synaptic plasticity that underlies hippocampus-mediated learning and memory.
4. The hippocampal CRF neuron: target and mediator of cognitive effects of early life stress
Whereas stress during adult life leads to significant effects on neuronal function and on learning and memory, this type of neuroplasticity generally does not persist. In contrast, early-life stress may contribute to severe and enduring cognitive impairments (Nelson et al., 2007). These problems may emerge during adulthood, and seem to progress with age (Kaplan et al., 2001; Wilson et al., 2007). Is there a role for CRF in these long-lasting effects of early-life stress?
Early-life stress augments the expression levels of hippocampal CRF chronically: We generated chronic stress during postnatal days P2-P9 employing a naturalistic rodent model of limited resources by limiting the nesting and bedding material in the cages. This led to frequent sorties of the dam from the nest area, and to fragmented maternal care, with little effect on total care. Remarkably, the abnormal patterns and rhythms of maternal care generated chronic stress in the pups, as evident from adrenal hypertrophy and elevated plasma corticosterone (Avishai-Eliner et al., 2001b; Brunson et al., 2005). As adults, graduates of this early-life stress had apparent normal stress-hormone levels, but their memory was impaired on several tests (Brunson et al., 2005; Ivy et al., 2010).. These memory defects were associated with major deficits of LTP. The LTP defects were not global, but centered on the commissural / associational synapses in stratum radiatum of CA1 and CA3 (Brunson et al., 2005). Interestingly, the basis of the LTP problems was, at least in part, a loss of these synapses: the structure of the corresponding neurons was quite abnormal, including impoverished dendritic arborization and hence reduced numbers of dendritic spines and excitatory synapses (Brunson et al, 2005; Ivy et al., 2010; Wang et al., 2011a).
There is evidence that CRF contributes to the abnormal synaptic plasticity and synaptic loss generated by early-life stress: As mentioned, expression levels of hippocampal CRF were chronically augmented in this model (Fenoglio et al., 2006b; Ivy et al., 2010). In addition, selective blocking of central CRFR1 receptors during the week that followed the early-life stress period sufficed to abolish deficits in hippocampus-dependent memory, LTP and dendritic structure in selective regions of hippocampal CA1 (Ivy et al., 2010). These mechanistic studies support the role of CRF in these effects. Further support for a role of CRF in the effects of early-life stress on hippocampal structure and function is apparent from the fact that mice with a conditional forebrain CRFR1 knockout were resistant to the effects of chronic early-life stress (Wang et al., 2011a). Interestingly, the consequences of chronic early-life stress were reproduced by simply over-expressing CRF postnatally in forebrain neurons (Wang et al., 2011a). Together, these studies demonstrate that functional, structural and molecular changes evoked by early-life stress are at least partly dependent on CRF over-activity via forebrain CRFR1 signaling.
It is intriguing to consider potential adaptive aspects of these neuroanatomical and functional changes. A cumulative stress hypothesis suggests that adults experiencing chronic early life stress might be more vulnerable to future stress (McEwen 2012; Nederhof and Schmidt, 2012). In contrast, a match-mismatch theory might suggest that the loss of memory processing capacity of adults subjected to chronic stress early in life might prepare them to an adult life fraught with stress. Reduced memory of stressful occurrences could be protective in this context (Wang et al, 2011a).
An important question with clinical significance involves the time frame of the actions of early life stress and the increased levels of CRF, and their potential reversibility. Two alternative scenarios are possible:
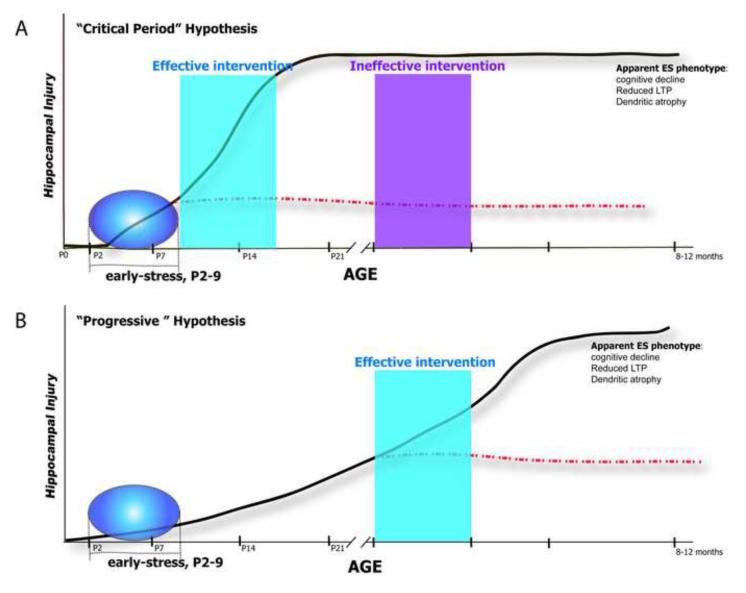
The “Critical Period’ hypothesis posits that the effects of early-life stress on hippocampal structure and function require excessive CRF-CRFR1 signaling during a critical period of brain programming within the first three weeks of life (Bale et al., 2010). If this hypothesis is true, then the first few postnatal weeks are the only period when intervention can prevent the detrimental effects of early-stress on hippocampus; after this time-period, the consequences of early-stress are programmed for life (Fig1A).
“Progressive” Hypothesis: Early-life stress sets in motion progressive molecular events that result in cognitive defects during adulthood, and perhaps an acceleration of aging-related cognitive decline (Ivy et al., 2010). In this scenario, blocking CRF signaling later in life might still prevent the negative outcomes resulting from early-life stress (Fig1B).
Figure 1. The long-term consequences of early-life stress may result from two alternative processes.
(A) Chronic early-life stress (ES) may exert its long-term effects on hippocampal structure and function by interfering with hippocampal maturation during a vulnerable / critical developmental period; this possibility predicts that whereas interventions within the critical window will be effective (blue box), interventions initiated later in life will be ineffective (purple box). (B) Alternatively, ES may set in motion molecular cascades that progress over time to lead to functional / structural deficits apparent during middle age. This possibility predicts that therapeutic interventions initiated during young adulthood (blue box) will ameliorate the cognitive deficits.
Existing data support both hypotheses: The resistance of mice with a conditional knockout of the CRFR1 gene to chronic early-life stress supports the progressive hypothesis, because these receptors, repressed via a CaMKinase II mechanism, are still expressed during the first 10 days of life, the period of chronic stress. In addition, in wild-type mice and rats, the progressive emergence of cognitive problems and the progression of the hippocampal cell injury also support the idea that chronic early-life stress induces progressive functional and structural changes in the hippocampus. This is likely to be at least in part via increased expression levels of CRF.
In support of the critical period scenario, whereas blocking CRFR1 immediately following the early-stress period (Ivy et al., 2010) reversed the effect of such stress, administration of CRFR1 blocker several months later had only a partial effect (unpublished observations). This result is expected if reversal of the consequences of chronic early-life stress was not possible beyond a ‘critical period’ (Figure 1).
5. Summary
Since the isolation and characterization of CRF by Wylie Vale, numerous studies--by his group and many others-- have demonstrated the importance of this peptide and its significance for myriad physiological as well as pathological conditions. Remarkably, the effects of CRF take place both peripherally, through its neuroendocrine functions, as well as within specific brain regions, as highlighted here.
CRF is a pivotal modulator of the stress response, and the effects of the peptide may be influenced by the amount / levels of peptide released as well as the duration of CRF action. Indeed, knock-out studies suggest that the two CRF receptors, R1 and R2 might contribute to the initiation of stress-related actions of the peptide and their termination, respectively (Bale et al., 2002; Bale and Vale, 2004; Coste et al., 2001). Further complexity derives from the fact that different targets of CRF seem to have distinct sensitivities to the peptide. Thus, low level, short CRF exposure promotes synapse function and plasticity, whereas high levels and long-duration exposure to the peptide results in elimination of synapses, as shown using live multi-photon imaging (Chen et al., 2013). This complexity endows CRF with both positive as well as detrimental effects. CRF promotes survival under stressful conditions (Denver et al., 2013), yet its excess can induce or contribute to a number of maladaptive consequences. Here we reviewed the role of CRF in neuroplasticity. Neuroplasticity underlies our ability to learn and to change, and is often helpful in the context of stress. However, excessive exposure to stress, e.g. chronic stress, severe stress or stress during critical periods in life can lead to maladaptive plasticity, accompanied by cognitive impairments. Together with other important components of the stress system (e.g. glucocorticoids; Timmermans et al., 2013), CRF plays a central role in these neuroplastic changes.
Whereas the majority of evidence for the role of CRF in neuroplasticity has arisen in rodent models, these studies carry important implications to human health and disease. CRF is chemically identical in rat and human, and a large number of human studies support a role for CRF in stress responses and coping (Bradley et al., 2008). Changes in CRF expression are found in aging (Behan et al., 1995a), depression (Raadsheer et al., 1995; Arborelius et al., 1999; Merali et al., 2006) and epilepsy (Wang et al., 2001), supporting a role for the peptide in neurological disorders. Together with the mechanistic animal studies discussed here, these findings suggest that a better understanding of the role of CRF in neuroplasticity should provide new avenues for developing intervention and perhaps prevention of stress-related cognitive and emotional disorders.
Highlights.
Early-life experience contributes to plasticity of the neuroendocrine stress system.
The CRF neuron in PVN is a key site of such plasticity via altered excitatory synapses.
The hippocampal CRF neuron releases the peptide locally during stress.
CRF contributes to stress-induced structural and functional hippocampal plasticity
Chronic early-life stress persistently increases CRF expression in hippocampus.
Acknowledgements
The excellent editorial assistance of Barbara Cartwright is appreciated. The authors’ research has been supported by NIH grants NS28912; NS45260; MH73136 and P50 MH096889.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agnati LF, Guidolin D, Guescini M, Genedani S, Fuxe K. Understanding wiring and volume transmission. Brain Res Rev. 2010;64:137–59. doi: 10.1016/j.brainresrev.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Aguilera G, Liu Y. The molecular physiology of CRH neurons. Front. Neuroendocrinol. 2012;33:67–84. doi: 10.1016/j.yfrne.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera G. HPA axis responsiveness to stress: implications for healthy aging. Exp. Gerontol. 2011;46:90–95. doi: 10.1016/j.exger.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aisa B, Tordera R, Lasheras B, Del Río J, Ramírez MJ. Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology. 2007;32:256–66. doi: 10.1016/j.psyneuen.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Aldenhoff JB, Gruol DL, Rivier J, Vale W, Siggins GR. Corticotropin releasing factor decreases postburst hyperpolarizations and excites hippocampal neurons. Science. 1983;221:875–877. doi: 10.1126/science.6603658. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Owens M.J,, Plotsky, P.M., Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J. Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Aubry JM, Bartanusz V, Pagliusi S, Schulz P, Kiss JZ. Expression of ionotropic glutamate receptor subunit mRNAs by paraventricular corticotropin-releasing factor (CRF) neurons. Neurosci. Lett. 1996;205:95–98. doi: 10.1016/0304-3940(96)12380-6. [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, Eghbal-Ahmadi M, Tabachnik E, Brunson KL, Baram TZ. Down-regulation of hypothalamic corticotropin-releasing hormone messenger ribonucleic acid (mRNA) precedes early-life experience-induced changes in hippocampal glucocorticoid receptor mRNA. Endocrinology. 2001a;142:89–97. doi: 10.1210/endo.142.1.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avishai-Eliner S, Gilles EE, Eghbal-Ahmadi Y, Bar-El Y, Baram TZ. Altered regulation of gene and protein expression of hypothalamic-pituitary-adrenal axis components in an immature rat model of chronic stress. J. Neuroendocrinol. 2001b;13:799–807. doi: 10.1046/j.1365-2826.2001.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, Nemeroff CB, Reyes TM, Simerly RB, Susser ES, Nestler EJ. Early life programming and neurodevelopmental disorders. Biol. Psychiatry. 2010;68:314–319. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu. Rev. Pharmacol. Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bale TL, Adan R, Contarino A, Koob GF, Vale WW, Lee KF. Mice deficient for both corticotropin-releasing factor receptor 1 (CRFR1) and CRFR2 have an impaired stress response and display sexually dichotomous anxiety-like behavior. J. Neurosci. 2002;22:193. doi: 10.1523/JNEUROSCI.22-01-00193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Davis EP, Obenaus A, Sandman CA, Small SL, Solodkin A, Stern H. Fragmentation and Unpredictability of Early-Life Experience in Mental Disorders. Am. J. Psychiatry. 2012;169:907–915. doi: 10.1176/appi.ajp.2012.11091347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Hatalski CG. Neuropeptide-mediated excitability: A key triggering mechanism for seizure generation in the developing brain. Trends Neurosci. 1998;21:471–476. doi: 10.1016/s0166-2236(98)01275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan DP, Heinrichs SC, Troncoso JC, Liu XJ, Kawas CH, Ling N, De Souza EB. Displacement of corticotropin releasing factor from its binding protein as a possible treatment for Alzheimer’s disease. Nature. 1995a;378:284–287. doi: 10.1038/378284a0. [DOI] [PubMed] [Google Scholar]
- Behan DP, De Souza EB, Lowry PJ, Potter E, Sawchenko P, Vale WW. Corticotropin releasing factor (CRF) binding protein: a novel regulator of CRF and related peptides. Front. Neuroendocrinol. 1995b;16:362–82. doi: 10.1006/frne.1995.1013. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Sawchenko PE. Do centrally administered neuropeptides access cognate receptors? An analysis in the central corticotropin-releasing factor system. J. Neurosci. 2000;20:1142–1156. doi: 10.1523/JNEUROSCI.20-03-01142.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Grammatopoulos DK, Randeva HS, Hillhouse EW, Spiess J. Corticotropin-releasing factor receptors couple to multiple G-proteins to activate diverse intracellular signaling pathways in mouse hippocampus: role in neuronal excitability and associative learning. J. Neurosci. 2003;23:700–707. doi: 10.1523/JNEUROSCI.23-02-00700.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Eckart K, Spiess J. Priming of long-term potentiation in mouse hippocampus by corticotropin-releasing factor and acute stress: implications for hippocampus-dependent learning. J. Neurosci. 2002;22:3788–3794. doi: 10.1523/JNEUROSCI.22-09-03788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bonfiglio JJ, Inda C, Refojo D, Holsboer F, Arzt E, Silberstein S. The corticotropin-releasing hormone network and the hypothalamic-pituitary-adrenal axis: molecular and cellular mechanisms involved. Neuroendocrinology. 2011;94:12–20. doi: 10.1159/000328226. [DOI] [PubMed] [Google Scholar]
- Boudaba C, Schrader LA, Tasker JG. Physiological evidence for local excitatory synaptic circuits in the rat hypothalamus. J. Neurophysiol. 1997;77:3396–3400. doi: 10.1152/jn.1997.77.6.3396. [DOI] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ, Stowe ZN, Heim CM, Nemeroff CB, Schwartz A, Cubells JF, Ressler KJ. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch. Gen. Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MR, Fisher LA, Spiess J, Rivier J, Rivier C, Vale W. Comparison of the biologic actions of corticotropin-releasing factor and sauvagine. Regul. Pept. 1982;4:107–14. doi: 10.1016/0167-0115(82)90101-x. [DOI] [PubMed] [Google Scholar]
- Brunson KL, Kramár E, Lin B, Chen Y, Colgin LL, Yanagihara TL, Lynch G, Baram TZ. Mechanisms of late-onset cognitive decline after early life stress. J. Neurosci. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Avishai-Eliner S, Hatalski CG, Baram TZ. Neurobiology of the stress response early in life: evolution of a concept and the role of corticotropin releasing hormone. Mol. Psychiatry. 2001;6:647–656. doi: 10.1038/sj.mp.4000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D, Yi SJ, Baram TZ. Developmental profile of corticotropin releasing hormone messenger RNA in the rat inferior olive. Int. J. Dev. Neurosci. 1996;14:69–76. doi: 10.1016/0736-5748(95)00072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Evans AN, Liu Y, Honda M, Saavedra JM, Aguilera G. Maternal deprivation in rats is associated with corticotrophin-releasing hormone (CRH) promoter hypomethylation and enhances CRH transcriptional responses to stress in adulthood. J. Neuroendocrinol. 2012a;24:1055–1064. doi: 10.1111/j.1365-2826.2012.02306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Kramár EA, Chen LY, Babayan AH, Andres AL, Gall CM, Lynch G, Baram TZ. Impairment of synaptic plasticity by the stress mediator CRH involves selective destruction of thin dendritic spines via RhoA signaling. Mol. Psychiatry. 2013;18:485–496. doi: 10.1038/mp.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Andres AL, Frotscher M, Baram TZ. Tuning synaptic transmission in the hippocampus by stress: the CRH system. Front. Cell. Neurosci. 2012b;6:13. doi: 10.3389/fncel.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Rex CS, Rice CJ, Dubé CM, Gall CM, Lynch G, Baram TZ. Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. Proc. Natl. Acad. Sci. U.S.A. 2010;107:13123–13128. doi: 10.1073/pnas.1003825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bender RA, Brunson KL, Pomper JK, Grigoriadis DE, Wurst W, Baram TZ. Modulation of dendritic differentiation by corticotropin-releasing factor in the developing hippocampus. Proc. Natl. Acad. Sci. U.S.A. 2004;101:15782–15787. doi: 10.1073/pnas.0403975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Brunson KL, Adelmann G, Bender RA, Frotscher M, Baram TZ. Hippocampal corticotropin releasing hormone: pre- and postsynaptic location and release by stress. Neuroscience. 2004;126:533–540. doi: 10.1016/j.neuroscience.2004.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bender RA, Frotscher M, Baram TZ. Novel and transient populations of corticotropin-releasing hormone-expressing neurons in developing hippocampus suggest unique functional roles: a quantitative spatiotemporal analysis. J. Neurosci. 2001a;21:7171–7181. doi: 10.1523/JNEUROSCI.21-18-07171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hatalski CG, Brunson KL, Baram TZ. Rapid phosphorylation of the CRE binding protein precedes stress-induced activation of the corticotropin releasing hormone gene in medial parvocellular hypothalamic neurons of the immature rat. Mol. Brain Res. 2001b;96:39–49. doi: 10.1016/s0169-328x(01)00265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Russo SJ. Structural and synaptic plasticity in stress-related disorders. Rev. Neurosci. 2011;22:535–49. doi: 10.1515/RNS.2011.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contarino A, Dellu F, Koob GF, Smith GW, Lee KF, Vale W, Gold LH. Reduced anxiety-like and cognitive performance in mice lacking the corticotropin-releasing factor receptor 1. Brain Res. 1999;835:1–9. doi: 10.1016/s0006-8993(98)01158-5. [DOI] [PubMed] [Google Scholar]
- Cope JL, Regev L, Chen Y, Korosi A, Rice CJ, Ji S, Rogge GA, Wood MA, Baram TZ. Differential contribution of CBP:CREB binding to corticotropin-releasing hormone expression in the infant and adult hypothalamus. Stress. 2013 doi: 10.3109/10253890.2013.806907. Available on line, doi:10.3109/10253890.2013.806907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste SC, Murray SE, Stenzel-Poore MP. Animal models of CRH excess and CRH receptor deficiency display altered adaptations to stress. Peptides. 2001;22:733–741. doi: 10.1016/s0196-9781(01)00386-2. [DOI] [PubMed] [Google Scholar]
- Cullinan WE. GABA(A) receptor subunit expression within hypophysiotropic CRH neurons: a dual hybridization histochemical study. J. Comp. Neurol. 2000;419:344–351. doi: 10.1002/(sici)1096-9861(20000410)419:3<344::aid-cne6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Dabrowska J, Hazra R, Ahern TH, Guo JD, McDonald AJ, Mascagni F, Muller JF, Young LJ, Rainnie DG. Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: Implications for balancing stress and affect. Psychoneuroendocrinology. 2011;36:1312–26. doi: 10.1016/j.psyneuen.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedovic K, Duchesne A, Andrews J, Engert V, Pruessner JC. The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. Neuroimage. 2009;47:864–871. doi: 10.1016/j.neuroimage.2009.05.074. [DOI] [PubMed] [Google Scholar]
- Dent GW, Smith MA, Levine S. Rapid induction of corticotropin-releasing hormone gene transcription in the paraventricular nucleus of the developing rat. Endocrinology. 2000;141:1593–1598. doi: 10.1210/endo.141.5.7455. [DOI] [PubMed] [Google Scholar]
- Denver RJ. Neuroendocrinology of amphibian metamorphosis. Curr. Top Dev. Biol. 2013;103:195–227. doi: 10.1016/B978-0-12-385979-2.00007-1. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- De Souza EB, Whitehouse PJ, Kuhar MJ, Price DL, Vale WW. Reciprocal changes in corticotropin-releasing factor (CRF)-like immunoreactivity and CRF receptors in cerebral cortex of Alzheimer’s disease. Nature. 1986;319:593–595. doi: 10.1038/319593a0. [DOI] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J. Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Murphy KM, Meng L, Montalvo-Ortiz J, Zeng Z, Kolber BJ, Zhang S, Muglia LJ, Csernansky JG. Corticotrophin releasing factor accelerates neuropathology and cognitive decline in a mouse model of Alzheimer’s disease. J Alzheimers Dis. 2012;28:579–592. doi: 10.3233/JAD-2011-111328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott E, Ezra-Nevo G, Regev L, Neufeld-Cohen A, Chen A. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat. Neurosci. 2010;13:1351–1353. doi: 10.1038/nn.2642. [DOI] [PubMed] [Google Scholar]
- Fenoglio KA, Chen Y, Baram TZ. Neuroplasticity of the hypothalamic-pituitary-adrenal axis early in life requires recurrent recruitment of stress-regulating brain regions. J. Neurosci. 2006a;26:2434–2442. doi: 10.1523/JNEUROSCI.4080-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio KA, Brunson KL, Baram TZ. Hippocampal neuroplasticity induced by early-life stress: functional and molecular aspects. Front. Neuroendocrinol. 2006b;27:180–192. doi: 10.1016/j.yfrne.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio KA, Brunson KL, Avishai-Eliner S, Stone BA, Kapadia BJ, Baram TZ. Enduring, handling-evoked enhancement of hippocampal memory function and glucocorticoid receptor expression involves activation of the corticotropin-releasing factor type 1 receptor. Endocrinology. 2005;146:4090–4096. doi: 10.1210/en.2004-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flandreau EI, Ressler KJ, Owens MJ, Nemeroff CB. Chronic overexpression of corticotropin-releasing factor from the central amygdala produces HPA axis hyperactivity and behavioral anxiety associated with gene-expression changes in the hippocampus and paraventricular nucleus of the hypothalamus. Psychoneuroendocrinology. 2012;37:27–38. doi: 10.1016/j.psyneuen.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher JP, Orozco-Cabal LF, Liu J, Shinnick-Gallagher P. Synaptic physiology of central CRH system. Eur. J. Pharmacol. 2008;583:215–25. doi: 10.1016/j.ejphar.2007.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallopin T, Geoffroy H, Rossier J, Lambolez B. Cortical sources of CRF, NKB, and CCK and their effects on pyramidal cells in the neocortex. Cereb. Cortex. 2006;16:1440–1452. doi: 10.1093/cercor/bhj081. [DOI] [PubMed] [Google Scholar]
- Gibbs DM, Vale W. Presence of corticotropin releasing factor-like immunoreactivity in hypophysial portal blood. Endocrinology. 1982;111:1418–20. doi: 10.1210/endo-111-4-1418. [DOI] [PubMed] [Google Scholar]
- Ginsberg AB, Campeau S, Day HE, Spencer RL. Acute glucocorticoid pretreatment suppresses stress-induced hypothalamic-pituitary-adrenal axis hormone secretion and expression of corticotropin-releasing hormone hnRNA but does not affect c-fos mRNA or Fos protein expression in the paraventricular nucleus of the hypothalamus. J. Neuroendocrinol. 2003;15:1075–1083. doi: 10.1046/j.1365-2826.2003.01100.x. [DOI] [PubMed] [Google Scholar]
- Groeneweg FL, Karst H, de Kloet ER, Joëls M. Rapid non-genomic effects of corticosteroids and their role in the central stress response. J. Endocrinol. 2011;209:153–167. doi: 10.1530/JOE-10-0472. [DOI] [PubMed] [Google Scholar]
- Hatalski CG, Guirguis C, Baram TZ. Corticotropin releasing factor mRNA expression in the hypothalamic paraventricular nucleus and the central nucleus of the amygdala is modulated by repeated acute stress in the immature rat. J. Neuroendocrinol. 1998;10:663–669. doi: 10.1046/j.1365-2826.1998.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollrigel GS, Chen K, Baram TZ, Soltesz I. The pro-convulsant actions of corticotropin-releasing hormone in the hippocampus of infant rats. Neuroscience. 1998;84:71–79. doi: 10.1016/s0306-4522(97)00499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes KD, Babwah AV, Dale LB, Poulter MO, Ferguson SS. Differential regulation of corticotropin releasing factor 1alpha receptor endocytosis and trafficking by beta-arrestins and Rab GTPases. J. Neurochem. 2006;96:934–949. doi: 10.1111/j.1471-4159.2005.03603.x. [DOI] [PubMed] [Google Scholar]
- Hung HC, Chou CK, Chiu TH, Lee EH. CRF increases protein phosphorylation and enhances retention performance in rats. Neuroreport. 1992;3:181–184. doi: 10.1097/00001756-199202000-00015. [DOI] [PubMed] [Google Scholar]
- Ivy AS, Rex CS, Chen Y, Dubé C, Maras PM, Grigoriadis DE, Gall CM, Lynch G, Baram TZ. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J. Neurosci. 2010;30:13005–13015. doi: 10.1523/JNEUROSCI.1784-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy AS, Brunson KL, Sandman C, Baram TZ. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: A clinically relevant model for early-life stress. Neuroscience. 2008;154:1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankord R, Herman JP. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann. N.Y. Acad. Sci. 2008;1148:64–73. doi: 10.1196/annals.1410.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M, Baram TZ. The neuro-symphony of stress. Nat. Rev. Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M, Fernandez G, Roozendaal B. Stress and emotional memory: a matter of timing. Trends Cogn. Sci. 2011;15:280–288. doi: 10.1016/j.tics.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Kaplan GA, Turrell G, Lynch JW, Everson SA, Helkala EL, Salonen JT. Childhood socioeconomic position and cognitive function in adulthood. Int. J. Epidemiol. 2001;30:256–263. doi: 10.1093/ije/30.2.256. [DOI] [PubMed] [Google Scholar]
- Karsten CA, Baram TZ. How Does a Neuron “know” to Modulate Its Epigenetic Machinery in Response to Early-Life Environment/Experience? Front. Psychiatry. 2013;4:89. doi: 10.3389/fpsyt.2013.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat. Rev. Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kiss J, Gorc TJ, Kuhn R, Knopfel T, Csaky A, Halasz B. Distribution of metabotropic glutamate receptor 1a in the rat hypothalamus: an immunocytochemical study using monoclonal and polyclonal antibody. Acta. Biol. Hung. 1996;47:221–237. [PubMed] [Google Scholar]
- Korosi A, Shanabrough M, McClelland S, Liu ZW, Borok E, Gao XB, Horvath TL, Baram TZ. Early-life experience reduces excitation to stress-responsive hypothalamic neurons and reprograms the expression of corticotropin-releasing hormone. J. Neurosci. 2010;30:703–713. doi: 10.1523/JNEUROSCI.4214-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korosi A, Baram TZ. The pathways from mother’s love to baby’s future. Front. Behav. Neurosci. 2009;3:27. doi: 10.3389/neuro.08.027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmiski JB, Marty V, Baimoukhametova DV, Bains JS. Stress-induced priming of glutamate synapses unmasks associative short-term plasticity. Nat. Neurosci. 2010;13:1257–1264. doi: 10.1038/nn.2629. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Larson J, Lynch G. Induction of synaptic potentiation in hippocampus by patterned stimulation involves two events. Science. 1986;232:985–988. doi: 10.1126/science.3704635. [DOI] [PubMed] [Google Scholar]
- Lee EH, Lee CP, Wang HI, Lin WR. Hippocampal CRF, NE, and NMDA system interactions in memory processing in the rat. Synapse. 1993;14:144–153. doi: 10.1002/syn.890140207. [DOI] [PubMed] [Google Scholar]
- Levine S. Maternal and environmental influences on the adrenocortical response to stress in weanling rats. Science. 1967;156:258–260. doi: 10.1126/science.156.3772.258. [DOI] [PubMed] [Google Scholar]
- Levy BH, Tasker JG. Synaptic regulation of the hypothalamic-pituitary-adrenal axis and its modulation by glucocorticoids and stress. Front. Cell. Neurosci. 2012;6:24. doi: 10.3389/fncel.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightman SL. The neuroendocrinology of stress: a never ending story. J. Neuroendocrinol. 2008;20:880–884. doi: 10.1111/j.1365-2826.2008.01711.x. [DOI] [PubMed] [Google Scholar]
- Lister R, Mukamel EA, Nery JR, Urich M, Puddifoot CA, Johnson ND, Lucero J, Huang Y, Dwork AJ, Schultz MD, Yu M, Tonti-Filippini J, Heyn H, Hu S, Wu JC, Rao A, Esteller M, He C, Haghighi FG, Sejnowski TJ, Behrens MM, Ecker J,R. Global epigenomic reconfiguration during mammalian brain development. Science. 2013;341:1237905. doi: 10.1126/science.1237905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Poon V, Sanchez-Watts G, Watts AG, Takemori H, Aguilera G. Salt-inducible kinase is involved in the regulation of corticotropin-releasing hormone transcription in hypothalamic neurons in rats. Endocrinology. 2012;153:223–233. doi: 10.1210/en.2011-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Aguilera G. Cyclic AMP inducible early repressor mediates the termination of corticotropin releasing hormone transcription in hypothalamic neurons. Cell. Mol. Neurobiol. 2009;29:1275–1281. doi: 10.1007/s10571-009-9423-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat. Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lloyd RB, Nemeroff CB. The role of corticotropin-releasing hormone in the pathophysiology of depression: therapeutic implications. Curr. Top. Med. Chem. 2011;11:609–617. doi: 10.2174/1568026611109060609. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Naninck EF, van Goudoever JB, Fitzsimons C, Joels M, Korosi A. Perinatal programming of adult hippocampal structure and function; emerging roles of stress, nutrition and epigenetics. Trends Neurosci. 2013 doi: 10.1016/j.tins.2013.08.002. doi: 10.1016/j.tins.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Ma XM, Levy A, Lightman SL. Rapid changes in heteronuclear RNA for corticotrophin-releasing hormone and arginine vasopressin in response to acute stress. J. Endocrinol. 1997;152:81–89. doi: 10.1677/joe.0.1520081. [DOI] [PubMed] [Google Scholar]
- Ma YL, Chen KY, Wei CL, Lee EH. Corticotropin-releasing factor enhances brain-derived neurotrophic factor gene expression to facilitate memory retention in rats. Chin. J. Physiol. 1999;42:73–81. [PubMed] [Google Scholar]
- Maras PM, Baram TZ. Sculpting the hippocampus from within: stress, spines, and CRH. Trends Neurosci. 2012;35:315–24. doi: 10.1016/j.tins.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland S, Korosi A, Cope J, Ivy A, Baram TZ. Emerging roles of epigenetic mechanisms in the enduring effects of early-life stress and experience on learning and memory. Neurobiol. Learn Mem. 2011;96:79–88. doi: 10.1016/j.nlm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Brain on stress: how the social environment gets under the skin. Proc. Natl. Acad. Sci. U.S.A. 2012;109:17180–17185. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu. Rev. Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu. Rev. Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McGill BE, Bundle SF, Yaylaoglu MB, Carson JP, Thaller C, Zoghbi HY. Enhanced anxiety and stress-induced corticosterone release are associated with increased Crh expression in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. U.S.A. 2006;103:18267–18272. doi: 10.1073/pnas.0608702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Viau V, Bhatnagar S, Betito K, Iny LJ, O’Donnell D, Mitchell JB. Cellular mechanisms underlying the development and expression of individual differences in the hypothalamic-pituitary-adrenal stress response. J. Steroid Biochem. Mol. Biol. 1991;39:265–274. doi: 10.1016/0960-0760(91)90072-d. [DOI] [PubMed] [Google Scholar]
- Merali Z, Kent P, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO, Bédard T, Anisman H. Corticotropin-releasing hormone, arginine vasopressin, gastrin-releasing peptide, and neuromedin B alterations in stress-relevant brain regions of suicides and control subjects. Biol. Psychiatry. 2006;59:594–602. doi: 10.1016/j.biopsych.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Miklos IH, Kovacs KJ. GABAergic innervation of corticotropin-releasing hormone (CRH)-secreting parvocellular neurons and its plasticity as demonstrated by quantitative immunoelectron microscopy. Neuroscience. 2002;113:581–592. doi: 10.1016/s0306-4522(02)00147-1. [DOI] [PubMed] [Google Scholar]
- Mori N, Schoenherr C, Vandenbergh DJ, Anderson DJ. A common silencer element in the SCG10 and type II Na channel genes binds a factor present in nonneuronal cells but not in neuronal cells. Neuron. 1992;9:45–54. doi: 10.1016/0896-6273(92)90219-4. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J. Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nederhof E, Schmidt MV. Mismatch or cumulative stress: toward an integrated hypothesis of programming effects. Physiol. Behav. 2012;106:691–700. doi: 10.1016/j.physbeh.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Nelson CA, 3rd, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: the Bucharest Early Intervention Project. Science. 2007;318:1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Olivares-Reyes JA, Hudson CC, Flores-Vega F, Dautzenberg FM, Hauger RL. Carboxyl-terminal and intracellular loop sites for CRF1 receptor phosphorylation and beta-arrestin-2 recruitment: a mechanism regulating stress and anxiety responses. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R209–222. doi: 10.1152/ajpregu.00099.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund C, Spencer RL. Inhibitory effects of corticosterone in the hypothalamic paraventricular nucleus (PVN) on stress-induced adrenocorticotrophic hormone secretion and gene expression in the PVN and anterior pituitary. J. Neuroendocrinol. 2011;23:1231–1240. doi: 10.1111/j.1365-2826.2011.02217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Gaylord RI, Jarvis E, Girotti M, Spencer RL. Differential glucocorticoid effects on stress-induced gene expression in the paraventricular nucleus of the hypothalamus and ACTH secretion in the rat. Stress. 2009;12:400–411. doi: 10.1080/10253890802530730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm K, Belluardo N, Metsis M, Timmusk T. Neuronal expression of zinc finger transcription factor REST/NRSF/XBR gene. J. Neurosci. 1998;18:1280–1296. doi: 10.1523/JNEUROSCI.18-04-01280.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak R, Rao BS, Melchor JP, Chattarji S, McEwen B, Strickland S. Tissue plasminogen activator and plasminogen mediate stress-induced decline of neuronal and cognitive functions in the mouse hippocampus. Proc. Natl. Acad. Sci. U.S.A. 2005;102:18201–18206. doi: 10.1073/pnas.0509232102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnock SB, Herbert J. Corticosterone differentially modulates expression of corticotropin releasing factor and arginine vasopressin mRNA in the hypothalamic paraventricular nucleus following either acute or repeated restraint stress. Eur. J. Neurosci. 2001;13:576–584. doi: 10.1046/j.0953-816x.2000.01406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res. Mol. Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Punn A, Levine MA, Grammatopoulos DK. Identification of signaling molecules mediating corticotropin-releasing hormone-R1alpha-mitogen-activated protein kinase (MAPK) interactions: the critical role of phosphatidylinositol 3-kinase in regulating ERK1/2 but not p38 MAPK activation. Mol. Endocrinol. 2006;20:3179–3195. doi: 10.1210/me.2006-0255. [DOI] [PubMed] [Google Scholar]
- Raadsheer FC, van Heerikhuize JJ, Lucassen PJ, Hoogendijk WJ, Tilders FJ, Swaab DF. Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer’s disease and depression. Am. J. Psychiatry. 1995;152:1372–1376. doi: 10.1176/ajp.152.9.1372. [DOI] [PubMed] [Google Scholar]
- Radulovic J, Rühmann A, Liepold T, Spiess J. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2. J. Neurosci. 1999;19:5016–5025. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BA, Valentino RJ, Van Bockstaele EJ. Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology. 2008;149:122–130. doi: 10.1210/en.2007-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refojo D, Echenique C, Müller MB, Reul JM, Deussing JM, Wurst W, Sillaber I, Paez-Pereda M, Holsboer F, Arzt E. Corticotropin-releasing hormone activates ERK1/2 MAPK in specific brain areas. Proc. Natl. Acad. Sci. U.S.A. 2005;102:6183–6188. doi: 10.1073/pnas.0502070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refojo D, Schweizer M, Kuehne C, Ehrenberg S, Thoeringer C, Vogl AM, Dedic N, Schumacher M, von Wolff G, Avrabos C, Touma C, Engblom D, Schütz G, Nave KA, Eder M, Wotjak CT, Sillaber I, Holsboer F, Wurst W, Deussing JM. Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science. 2011;333:1903–1907. doi: 10.1126/science.1202107. [DOI] [PubMed] [Google Scholar]
- Regev L, Tsoory M, Gil S, Chen A. Site-specific genetic manipulation of amygdala corticotropin-releasing factor reveals its imperative role in mediating behavioral response to challenge. Biol. Psychiatry. 2012;71:317–326. doi: 10.1016/j.biopsych.2011.05.036. [DOI] [PubMed] [Google Scholar]
- Reul JM, Holsboer F. Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr. Opin. Pharmacol. 2002;2:23–33. doi: 10.1016/s1471-4892(01)00117-5. [DOI] [PubMed] [Google Scholar]
- Rice CJ, Sandman CA, Lenjavi MR, Baram TZ. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149:4892–4900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter S, Watts AG, Dinh TT, Sanchez-Watts G, Pedrow C. Immunotoxin lesion of hypothalamically projecting norepinephrine and epinephrine neurons differentially affects circadian and stressor-stimulated corticosterone secretion. Endocrinology. 2003;144:1357–1367. doi: 10.1210/en.2002-221076. [DOI] [PubMed] [Google Scholar]
- Rivest S, Laflamme N, Nappi RE. Immune challenge and immobilization stress induce transcription of the gene encoding the CRF receptor in selective nuclei of the rat hypothalamus. J. Neurosci. 1995;15:2680–2695. doi: 10.1523/JNEUROSCI.15-04-02680.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C, Rivier J, Vale W. Inhibition of adrenocorticotropic hormone secretion in the rat by immunoneutralization of corticotropin-releasing factor. Science. 1982;218:377–379. doi: 10.1126/science.6289439. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Hernandez A, Cabrera SM, Hagewoud R, Malvaez M, Stefanko DP, Haettig J, Wood MA. Membrane-associated glucocorticoid activity is necessary for modulation of long-term memory via chromatin modification. J. Neurosci. 2010;30:5037–5046. doi: 10.1523/JNEUROSCI.5717-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Griffith QK, Buranday J, De Quervain DJ, McGaugh JL. The hippocampus mediates glucocorticoid-induced impairment of spatial memory retrieval: dependence on the basolateral amygdala. Proc. Natl. Acad. Sci. U.S.A. 2003;100:1328–1333. doi: 10.1073/pnas.0337480100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ. Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proc. Natl. Acad. Sci. U.S.A. 2002;99:13908–13913. doi: 10.1073/pnas.212504599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Row BW, Dohanich GP. Post-training administration of corticotropin-releasing hormone (CRH) enhances retention of a spatial memory through a noradrenergic mechanism in male rats. Neurobiol. Learn. Mem. 2008;89:370–378. doi: 10.1016/j.nlm.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K. Corticotropin releasing factor-like immunoreactivity in the rat brain as revealed by a modified cobalt-glucose oxidase-diaminobenzidine method. J. Comp. Neurol. 1987;260:256–298. doi: 10.1002/cne.902600209. [DOI] [PubMed] [Google Scholar]
- Schierloh A, Deussing J, Wurst W, Zieglgänsberger W, Rammes G. Corticotropin-releasing factor (CRF) receptor type 1-dependent modulation of synaptic plasticity. Neurosci. Lett. 2007;416:82–86. doi: 10.1016/j.neulet.2007.01.047. [DOI] [PubMed] [Google Scholar]
- Seasholtz AF, Burrows HL, Karolyi IJ, Camper SA. Mouse models of altered CRH-binding protein expression. Peptides. 2001;22:743–751. doi: 10.1016/s0196-9781(01)00387-4. [DOI] [PubMed] [Google Scholar]
- Seth KA, Majzoub JA. Repressor element silencing transcription factor/neuron-restrictive silencing factor (REST/NRSF) can act as an enhancer as well as a repressor of corticotropin-releasing hormone gene transcription. J. Biol. Chem. 2001;276:13917–13923. doi: 10.1074/jbc.M007745200. [DOI] [PubMed] [Google Scholar]
- Snyder K, Wang WW, Han R, McFadden K, Valentino RJ. Corticotropin-releasing factor in the norepinephrine nucleus, locus coeruleus, facilitates behavioral flexibility. Neuropsychopharmacology. 2012;37:520–530. doi: 10.1038/npp.2011.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterrenburg L, Gaszner B, Boerrigter J, Santbergen L, Bramini M, Elliott E, Chen A, Peeters BW, Roubos EW, Kozicz T. Chronic stress induces sex-specific alterations in methylation and expression of corticotropin-releasing factor gene in the rat. PLoS One. 2011;6:e28128. doi: 10.1371/journal.pone.0028128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Simmons DM. Differential steroid hormone and neural influences on peptide mRNA levels in CRH cells of the paraventricular nucleus: a hybridization histochemical study in the rat. J. Comp. Neurol. 1989;285:413–435. doi: 10.1002/cne.902850402. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Swinny JD, Valentino RJ. Corticotropin-releasing factor promotes growth of brain norepinephrine neuronal processes through Rho GTPase regulators of the actin cytoskeleton in rat. Eur. J. Neurosci. 2006;24:2481–2490. doi: 10.1111/j.1460-9568.2006.05129.x. [DOI] [PubMed] [Google Scholar]
- Szyf M, Bick J. DNA methylation: a mechanism for embedding early life experiences in the genome. Child Dev. 2013;84:49–57. doi: 10.1111/j.1467-8624.2012.01793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura SM, Watts AG. Corticosterone can facilitate as well as inhibit corticotropin-releasing hormone gene expression in the rat hypothalamic paraventricular nucleus. Endocrinology. 1998;139:3830–3836. doi: 10.1210/endo.139.9.6192. [DOI] [PubMed] [Google Scholar]
- Timmermans W, Xiong H, Hoogenraad CC, Krugers HJ. Stress and excitatory synapses: From health to disease. Neuroscience. 2013;248:626–636. doi: 10.1016/j.neuroscience.2013.05.043. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale W, Rivier C, Brown MR, Spiess J, Koob G, Swanson L, Bilezikjian L, Bloom F, Rivier J. Chemical and biological characterization of corticotropin releasing factor. Recent Prog. Horm. Res. 1983;39:245–70. doi: 10.1016/b978-0-12-571139-5.50010-0. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur. J. Pharmacol. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Wehby RG. Corticotropin-releasing factor: evidence for a neurotransmitter role in the locus coeruleus during hemodynamic stress. Neuroendocrinology. 1988;48:674–677. doi: 10.1159/000125081. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J. Comp. Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Wang HL, Tsai LY, Lee EH. Corticotropin-releasing factor produces a protein synthesis – dependent long-lasting potentiation in dentate gyrus neurons. J. Neurophysiol. 2000;83:343–349. doi: 10.1152/jn.2000.83.1.343. [DOI] [PubMed] [Google Scholar]
- Wang HL, Wayner MJ, Chai CY, Lee EH. Corticotrophin-releasing factor produces a long-lasting enhancement of synaptic efficacy in the hippocampus. Eur. J. Neurosci. 1998;10:3428–3437. doi: 10.1046/j.1460-9568.1998.00352.x. [DOI] [PubMed] [Google Scholar]
- Wang XD, Su YA, Wagner KV, Avrabos C, Scharf SH, Hartmann J, Wolf M, Liebl C, Kühne C, Wurst W, Holsboer F, Eder M, Deussing JM, Müller MB, Schmidt MV. Nectin-3 links CRHR1 signaling to stress-induced memory deficits and spine loss. Nat. Neurosci. 2013;16:706–713. doi: 10.1038/nn.3395. [DOI] [PubMed] [Google Scholar]
- Wang XD, Rammes G, Kraev I, Wolf M, Liebl C, Scharf SH, Rice CJ, Wurst W, Holsboer F, Deussing JM, Baram TZ, Stewart MG, Müller MB, Schmidt MV. Forebrain CRF1 modulates early-life stress-programmed cognitive deficits. J. Neurosci. 2011a;31:13625–13634. doi: 10.1523/JNEUROSCI.2259-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XD, Chen Y, Wolf M, Wagner KV, Liebl C, Scharf SH, Harbich D, Mayer B, Wurst W, Holsboer F, Deussing JM, Baram TZ, Müller MB, Schmidt MV. Forebrain CRHR1 deficiency attenuates chronic stress-induced cognitive deficits and dendritic remodeling. Neurobiol. Dis. 2011b;42:300–310. doi: 10.1016/j.nbd.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Dow KE, Fraser DD. Elevated corticotropin releasing hormone/corticotropin releasing hormone-R1 expression in postmortem brain obtained from children with generalized epilepsy. Ann. Neurol. 2001;50:404–409. doi: 10.1002/ana.1138. [DOI] [PubMed] [Google Scholar]
- Watts AG. Glucocorticoid regulation of peptide genes in neuroendocrine CRH neurons: a complexity beyond negative feedback. Front. Neuroendo. 2005;26:109–130. doi: 10.1016/j.yfrne.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, Szyf M. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J. Neurosci. 2005;25:11045–11054. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Boyle PA, Arnold SE, Tang Y, Bennett DA. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68:2085–2092. doi: 10.1212/01.wnl.0000264930.97061.82. [DOI] [PubMed] [Google Scholar]
- Wosiski-Kuhn M, Stranahan AM. Opposing effects of positive and negative stress on hippocampal plasticity over the lifespan. Ageing Res. Rev. 2012;11:399–403. doi: 10.1016/j.arr.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Yan XX, Toth Z, Schultz L, Ribak CE, Baram TZ. Corticotropin-releasing hormone (CRH)-containing neurons in the immature rat hippocampal formation: light and electron microscopic features and colocalization with glutamate decarboxylase and parvalbumin. Hippocampus. 1998;8:231–243. doi: 10.1002/(SICI)1098-1063(1998)8:3<231::AID-HIPO6>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi SJ, Baram TZ. Corticotropin-releasing hormone mediates the response to cold stress in the neonatal rat without compensatory enhancement of the peptide’s gene expression. Endocrinology. 1994;135:2364–2368. doi: 10.1210/endo.135.6.7988418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DR, Cullinan WE, Herman JP. Organization and regulation of paraventricular nucleus glutamate signaling systems: N-methyl-D- aspartate receptors. J. Comp. Neurol. 2005;484:43–56. doi: 10.1002/cne.20445. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Diamond DM. Linear and non-linear dose-response functions reveal a hormetic relationship between stress and learning. Dose Response. 2008;7:132–148. doi: 10.2203/dose-response.08-015.Zoladz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoumakis E, Chrousos GP. Corticotropin-releasing hormone receptor antagonists: an update. Endocr. Dev. 2010;17:36–43. doi: 10.1159/000262526. [DOI] [PubMed] [Google Scholar]