Abstract
Non-steroidal anti-inflammatory drugs (NSAIDs) are used extensively for analgesic and antipyretic treatments. In addition, NSAIDs reduce the risk and mortality to several cancers. Their mechanisms in anti-tumorigenesis are not fully understood, but both cyclooxygenase (COX)-dependent and -independent pathways play a role. We and others have been interested in elucidating molecular targets of NSAID-induced apoptosis. In this review, we summarize updated literature regarding cellular and molecular targets modulated by NSAIDs. Among those NSAIDs, sulindac sulfide and tolfenamic acid are emphasized in this review because these two drugs have been well investigated for their anti-tumorigenic activity in many different types of cancer.
Keywords: NSAIDs, COX, sulindac sulfide, tolfenamic acid, NAG-1
1. Introduction
Despite advancements in modern medicine, cancer is a major cause of death worldwide, with a death toll of 7.6 million or about 13% of all deaths per year [1]. This along with increasing life expectancies in the developed world produces more chances of acquiring cancer and makes research into the mechanisms of cancer prevention a high priority.
Non-steroidal anti-inflammatory drugs (NSAIDs) are a class of drugs that have been utilized for their analgesic and antipyretic effects for centuries. In more recent history, NSAIDs have also been widely used and studied for their anti-tumorigenic and chemopreventive properties. The classical pathway of action for NSAIDs is blocking the generation of prostaglandins from arachidonic acid (AA) via inhibition of cyclooxygenase-1 and -2 (COX-1 and -2) activities. COX-1 is present and expressed constitutively in most tissues, whereas COX-2 expression is usually transient and can be rapidly induced by cytokines, lipopolysaccharide (LPS), phorbol myristate acetate (PMA), or growth factors [2–4]. The inhibition of both COX-1 and CO-2 by conventional NSAIDs such as aspirin, ibuprofen, and naproxen, could have negative consequences due to inhibition of prostaglandin (PG) synthesis. Particularly, PGE2 has a cytoprotective effects on the gastric mucosa and renal epithelial cells [5]. Therefore, the prolonged use of conventional NSAIDs that inhibit both COX-1 and COX-2 can lead to side effects including gastrointestinal tract bleeding and kidney failure, which may be attributable to COX-1 inhibition. In addition, a recent clinical trial of familial adenomatous polyposis (FAP) patients show a three-fold increase in the risk of cardio-toxicity from treatment with the COX-2 inhibitor celecoxib [6, 7]. These side effects are part of the motivation that drives research into the COX-independent mechanisms of NSAIDs. Among NSAIDs, tolfenamic acid (TA) and sulindac sulfide (SS) have been extensively investigated for their anti-tumorigenic properties because these two drugs exhibit better anti-cancer activity than many other NSAIDs [8–10]. There is still much work to be done elucidating the pathways of COX-independent activity of NSAIDs, and this review primarily focuses on intracellular molecular targets that are affected by NSAIDs.
2. Transcription factors
NSAIDs have been shown to affect a wide variety of transcription factors, including EGR-1, ATF3, ATF4, Sp1, CHOP, NF-κB, β-catenin/TCF, and Smad. These transcription factors have been identified as either pro-tumorigenic or anti-tumorigenic, depending on cell context and types. In this section, we summarize how these transcription factors are altered by NSAIDs as related to tumorigenesis (Fig. 1).
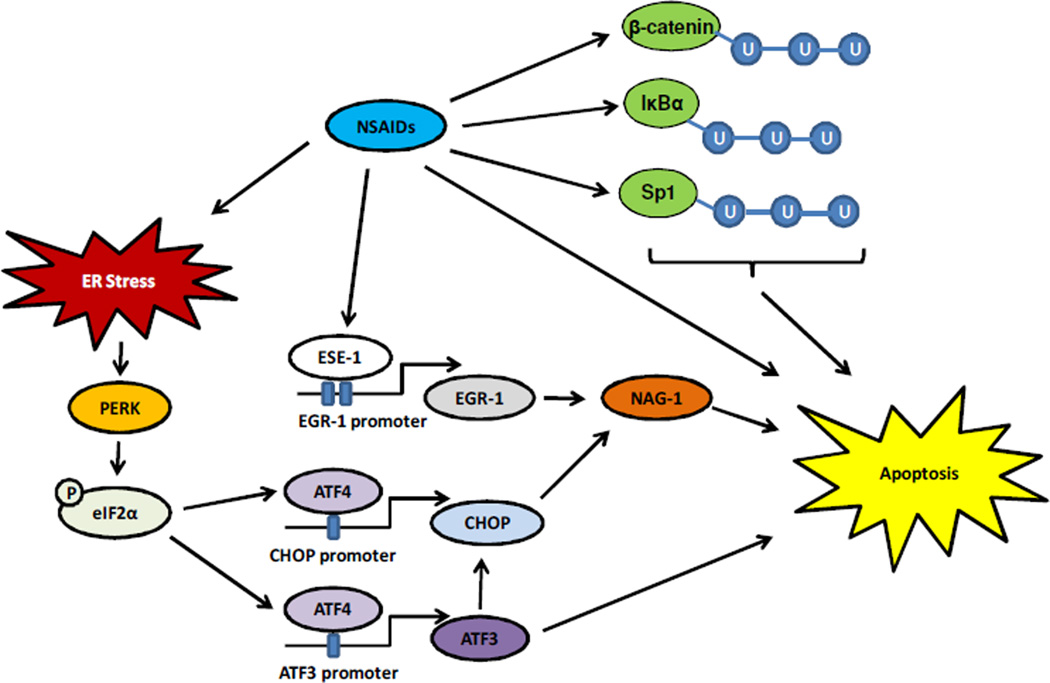
Fig. 1.
NSAIDs’ effects on transcriptional factors. NSAIDs induce apoptosis through a variety of transcription factors and pathways. Pro-tumorigenic proteins such as β-catenin, IκBα, and Sp1 are degraded by NSAIDs through ubiquitination pathways. NSAIDs can also induce ESE-1 nuclear translocation, where it binds to EGR-1’s promoter and up-regulates pro-apoptotic NAG-1 protein expression. ER stress is induced by NSAID treatment as well, which causes the ER membrane protein PERK to phosphorylate eIF2α. The phospho-eIF2α protein activates ATF4, which translocates to the nucleus where it binds to the promoter regions of the transcription factors CHOP or ATF3. This leads to up-regulation of CHOP and NAG-1. NSAIDs also induce apoptosis via unknown mechanisms. All of these pathways funnel into induction of apoptosis.
2.1. EGR-1
The zinc-finger DNA binding protein early growth respose-1 (EGR-1) is involved in many activities including differentiation and tumorigenesis. EGR-1 acts as a tumor suppressor in mouse two-step skin carcinogenesis using EGR-1 knock-out mice, and binds to the p53 promoter region both in vitro and in vivo [11]. Furthermore, EGR-1 plays an important role in up-regulating the expression of the anti-tumorigenic/pro-apoptotic protein NSAID activated gene-1 (NAG-1) and the anti-angiogenic protein thrombospondin-1 (TSP-1) in colorectal and lung cancer cells, respectively, during sulindac sulfide (SS) treatment [12, 13]. NSAIDs up-regulate EGR-1, which binds to the promoter regions of NAG-1 and TSP-1. This up-regulation has been confirmed in vivo, using a Sprague-Dawley rat model for early colorectal tumorigenesis, which uses the colon-specific pro-carcinogen 1,2-dimethylhydrazine dihydrochloride (DMH) [14]. EGR-1 protein expression is induced in rats treated with sulindac or celecoxib, along with less tumor formation compared to control rats [14]. Another conventional NSAID, TA, also induces EGR-1 at the transcription level via enhancement of epithelium-specific ETS transcription factor-1 (ESE-1) nuclear translocation, which leads to activation of apoptosis in colorectal cancer cells [15]. In addition, natural anti-cancer compounds such as resveratrol can induce apoptosis via EGR-1 induction [16]. However, depending on the cell type and conditions, EGR-1 can also play a negative role in tumorigenesis. In androgen-independent prostate cancer, EGR-1 is required for mediating CXCL5/ENA78’s oncogenic activity in cell growth, migration, and epithelial-to-mesenchymal transition (EMT) [17]. Thus, EGR-1 plays a complex role, acting through many pathways including anti- and/or pro-tumorigenic activity. Therefore, although NSAIDs increase EGR-1 expression, further study of EGR-1 function in tumorigenesis is needed.
2.2. ATF3
ATF3 is a cyclic adenosine monophosphate (cAMP)-dependent transcription factor that is a member of the basic region leucine zipper (bZIP) family [18]. ATF3 is induced by the conventional NSAIDs SS and TA in human colorectal cancer cells [19]. Treatment of colorectal cancer cells (CRC) with TA increases ATF3 protein expression, which is highly associated with apoptosis induction. Furthermore, ATF2 phosphorylation by TA increases ATF3 promoter activity via direct binding of phospho-ATF2 in the ATF3 promoter [19].
Both ATF3 over-expression and SS treatment down-regulate metastasis-associated protein (MTA1) and β-catenin in HCT-116 cells [20]. Thus, ATF3 has been implicated in several anti-tumorigenic pathways and represents one explanation for NSAIDs’ COX-independent activity.
2.3. ATF4
ATF4 is another bZIP transcriptional factor that is usually up-regulated under cellular stress by a translational control mechanism [21]. In addition, hypoxia can induce ATF4 up-regulation by increasing its stability [22]. Various NSAIDs have been shown to trigger endoplasmic reticulum (ER) stress response, resulting in the induction of ATF4, which is involved in NSAID-induced apoptosis in cultured guinea-pig gastric mucosal cells [23]. Moreover, celecoxib activates the Ca2+-dependent PERK-eIF2a-ATF4 pathway, which causes apoptosis through up-regulation of PUMA expression in human gastric carcinoma cell lines [24]. On the other hand, celecoxib-induced ATF4 expression could contribute to the up-regulation of glucose-regulated protein 78 (GRP78), an ER chaperone, and S100P, a member of the S100 family of EF-hand Ca2+-binding proteins, both of which may partially decrease the potential capability of celecoxib against cancer [25, 26]. More recently, we also showed evidence that ATF4 could play a role in apoptosis induced by TA in CRC cells [9]. Collectively, NSAIDs increase ATF4 expression via inducing ER stress, which may play complicated roles in gastric tumor suppression, and possibly drug resistance as well.
2.4 CHOP
The C/EBP-homologous protein (CHOP) is an ER stress response protein and member of the bZIP transcription factor family; CHOP inhibits CCAAT/enhancer-binding protein (C/EBP) and liver-enriched transcriptional activator protein (LAP) DNA-binding activity [27]. CHOP mRNA and protein levels are both elevated by SS treatment in CRC cells [28]. Celecoxib also induces CHOP protein expression in malignant glioma cells, whereas SS does not unless co-treated with carbonyl cyanide 4-(trifluoromethoxy)-phenylhyrazone (FCCP) [29]. FCCP is a protonophore which uncouples oxidative phosphorylation causing the loss of mitochondrial membrane potential; FCCP does not induce CHOP protein by itself in malignant glioma cells [29, 30]. These results suggest that mitochondrial disruption accompanied by ER stress response is needed to induce CHOP expression in malignant glioma cells. Subsequently, recent study from our group suggests that TA affects ER stress, thereby enhancing apoptosis via CHOP activity in human CRC cells [9]. Interestingly, pranoprofen suppresses ER stress-induced CHOP expression, likely through the inhibition of XBP-1 splicing in glial cells [31]. Taken together, these results suggest that there are complicated mechanisms involved in NSAID-induced CHOP expression, and that these mechanisms are dependent on cell context and chemical structure.
2.5. Sp
Specificity proteins (Sp) are zinc-finger transcription factors that are members of the Sp/KLF sub-family [32]. Sp proteins bind to the GC and/or GT boxes of many promoters. There are several Sp proteins, including Sp1, Sp2, Sp3, Sp4, Sp5, Sp6/KLF14, Sp7, and Sp8. This section focuses on Sp1, Sp3, and Sp4, which are phylogenetically more similar to one another than to the other Sp proteins [33, 34].
Sp1 is normally responsible for controlling various housekeeping genes; however, Sp1 has been linked to the regulation of tumorigenesis [35]. Sp proteins contribute to the proliferation of metastatic tumor phenotypes, and thus overexpression of these transcription factors is a negative survival prognostic factor in many human cancers [35].
TA has been shown to decrease Sp1, Sp3, and Sp4 protein expression as well as vascular endothelial growth factor receptor-1 (VEGFR-1) protein expression in pancreatic cancer cells [36]. Interestingly, small interfering RNA (siRNA) for Sp1, Sp3, and Sp4 also lead to decreased VEGFR-1 [36]. Thus, TA decreases this important angiogenic factor via regulation of Sp proteins. These results are supported by nude mouse data that show that TA reduces Sp1, Sp3, and Sp4 protein expression in vivo as well [8]. TA has also been shown to decrease Sp1, Sp3, and Sp4 in rhabdomyosarcoma (RMS) cells and in lung cancer cells both in vitro as well as in mouse models [37, 38]. COX-2-preferential NSAIDs, including celecoxib, also down-regulate Sp1 and Sp4 activity in human CRC cells; however, Sp3 protein expression is unchanged in this case [39]. This action was shown to be through COX-2-independent activation of Sp1 and Sp4 proteasomal degradation [39]. Recently, Dr. Safe’s group has further shown that TA and the novel nitric oxide (NO) chimera containing NSAID ethyl 2-((2,3-bis(nitrooxy)propyl) disulfanyl) benzoate (GT-094) down-regulate Sp1, Sp3, and Sp4 in human CRC cells both in vitro and in a mouse xenograft model, leading to decreased expression of VEGF/VEGFR-1, cyclin D1, hepatocyte growth factor receptor, survivin, Bcl-2, and NF-κB [40, 41]. Aspirin and sodium salicylate have also recently been shown to decrease Sp1, Sp3, and Sp4 in a caspase-dependent manner in human CRC cells in vitro as well as in a mouse model [42]. Taken together, these data show that NSAIDs down-regulate Sp proteins in several different cancers including pancreas, lung, rhabdomyosarcoma, and colon cancers. The consistency of these results makes NSAID action through Sp protein regulation a promising pathway.
2.6. NF-κB
The roles of NF-κB in cancer progression and anti-cancer therapeutics are complex. There is evidence to suggest that NF-κB activation is associated with increased survival of cancer cells and resistance to chemotherapy; thus inhibition of NF-κB activity is regarded as a target for anti-cancer therapy [43, 44]. Aspirin inhibits the NF-κB pathway by interaction with IKKβ [45]. In an in vivo model using male Sprague-Dawley rats given DMH to produce early stages of CRC, SS and celecoxib inhibited DMH-mediated IκBβ down-regulation and DMH-mediated IKKβ up-regulation, followed by interfering nuclear localization of NF-κB [46]. Through in silico 3D crystal structure modeling, this study also suggests that both SS and celecoxib directly bind to NF-κB docking sites [46]. However, NF-κB activation is capable of promoting a pro-apoptotic response under different circumstances [47, 48]. SS and TA induce NF-κB activity and apoptosis in human CRC cells [49, 50]. Thus, further study is needed to elucidate NF-κB’s role in tumorigenesis, although NSAIDs could activate or inhibit NF-κB activity.
2.7. β-catenin/TCF
The β-catenin/TCF signaling pathway plays a key role in regulation of tumorigenesis. In colorectal tumor cells, loss of APC function results in increased levels of β-catenin. The elevated levels of β-catenin promote its interaction with transcription factors of the TCF/Lef family and translocation of the β-catenin/TCF-complex to the nucleus, where it modulates transcription of downstream genes involved in pro-tumorigenesis. Both aspirin and indomethacin suppress β-catenin target genes by modulating TCF activity [51]. Nuclear β-catenin content is also decreased by treatment with several other NSAIDs [52, 53]. However, this effect of NSAIDs on β-catenin suppression is dependent on PPARγ and RXRα expression [54]. Thus, NSAIDs down-regulate β-catenin expression at the transcriptional and/or translational levels, leading to its reduced nuclear translocation. Furthermore, it is speculated that NSAIDs may be useful as anti-metastatic drugs by inhibiting β-catenin activity, leading to inhibition of metastatic protein S100A4 transcription [55].
2.8. Smad
Smad transcription factors serve as vital mediators in TGF-β signaling; these transcription factors normally form heteromeric complexes and then translocate into the nucleus where they activate target gene expression together with co-activators and/or suppressors [56]. Smad signaling has tumor-suppressive effects in the early stages of tumorigenesis; however, paradoxically, Smad signaling contributes to metastasis in advanced stage tumors. There are a large number of proteins targeted by the Smad pathway, over half of which may be involved in cancer cell invasion and migration [57]. Therefore, disruption of Smad signaling in late-stage tumorigenesis could be a potential cancer therapeutic strategy. The NSAID, 5-aminosalicylic acid (5-ASA) has been reported to suppress TGF-β-driven Smad2/3 phosphorylation and subsequently inhibit translocation of Smad2/3 into nucleus in CRC cells [58]. Moreover, the intake of aspirin seems to modulate Smad gene variation in colorectal tumors [59]. Our recent finding also shows that TA inhibits TGF-β-induced Smad phosphorylation via the ERK MAP kinase pathway in various cancer cells [60]. More importantly, TA had the best inhibitory activity of Smad phosphorylation compared with other tested NSAIDs. These data suggest that the interference of Smad signaling could be a novel mechanism of NSAID anti-tumor activity.
3. Cell signaling proteins
Cell signaling proteins are a diverse group of proteins that perform a wide variety of functions. Some are adhesion modules responsible for cell-cell binding while others are responsible for anchoring the plasma membrane to the actin cytoskeleton. Cell surface proteins can act as receptors for paracrine and autocrine cell signaling and are responsible for signal transduction to cytoplasmic signaling proteins and even translocation into the nucleus to affect transcription. These proteins are highly studied in cancer research due to their potential prognostic value as cancer markers and the promising chemotherapy potential of cancer cell-specific drug delivery (Fig. 2).
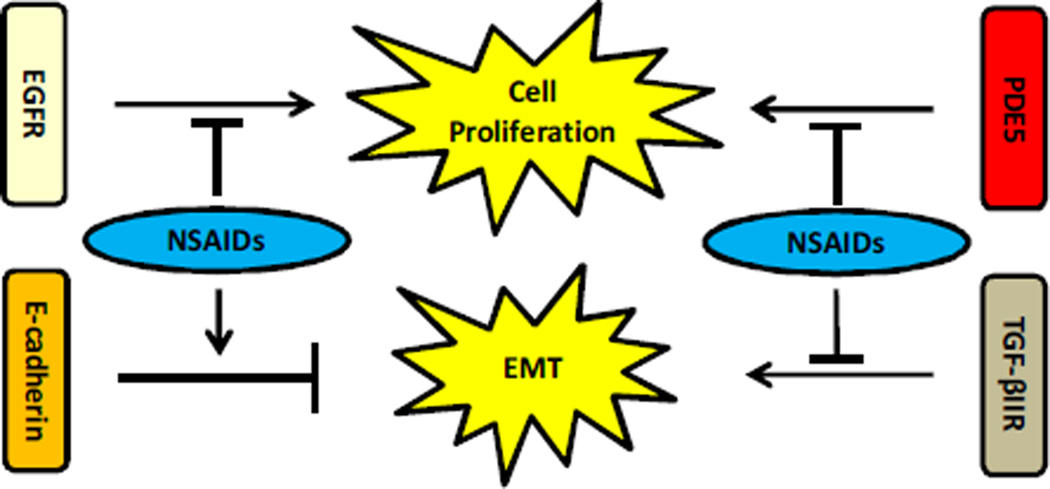
Fig. 2.
NSAIDs’ effects on cell signaling proteins. EGFR signaling and PDE5’s effect on cAMP and cGMP drive increased cell proliferation. NSAIDs inhibit cell proliferation through down-regulation of EGFR protein expression and inhibition of PDE5 activity. E-cadherin is an adhesion molecule that is important for maintaining tight cell junctions and is lost or reduced during EMT. TGF-β signaling also drives EMT. NSAIDs block EMT through reduction of Smad activity or serum levels of TGF-β1 ligand, and through protection of E-cadherin protein expression.
3.1 EGFR
Epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase that is mutated and/or over-active in a high percentage of cancers. EGFR signaling can lead to cell proliferation through the Erk1/2 pathway and cell survival, invasion, and angiogenesis through the AKT pathway. Sulindac metabolites, SS and sulindac sulfone, down-regulate EGFR signaling by inhibiting activation and/or expression of EGFR in CRC cell lines [61]. The dual NSAID licofelone, which inhibits both COX and 5-lipoxygenase (5-LOX), has also been shown to decrease CRC membrane fluidity leading to the inhibition of EGFR signaling, which contributes to apoptosis [62]. Thus, NSAIDs could be another attractive agent to target EGFR by co-treatment with other EGFR target drugs.
3.2. E-cadherin
E-cadherin is a calcium-dependent type-1 transmembrane protein. It is important for tight cell-cell junctions and anchors to the cytoskeleton. E-cadherin can play an important role in tumor progression through its sequestration of β-catenin to the plasma membrane, which prevents β-catenin from acting in the Wnt signaling pathway and from performing its job as a cell adhesion molecule. Loss of E-cadherin function and/or expression is an important step in epithelial-mechenchymal transition (EMT) leading to invasion and migration. In an animal model, sulindac treatment protected APCMin/+ mice from E-cadherin loss and accumulation of nuclear β-catenin in colon tumors [63]. On the contrary, the non-selective NSAID indomethacin reduced protein levels of E-cadherin and collagen IV, while increasing the activity of matrix metalloprotease-9 (MMP-9) leading to enhanced motility in vitro in lung cancer cell lines [64]. Furthermore, low doses of celecoxib treatment resulted in loss of E-cadherin, followed by induction of EMT [65]. Thus, further study may need to clarify the role of E-cadherin in NSAID-induced anti-tumorigenesis.
3.3. Phosphodiesterase-5 (PDE5)
Accumulating evidence suggests that modulating the intracellular secondary messengers, cAMP and cGMP may be a promising approach in the field of anti-cancer therapy. Inhibiting PDE5 leads to increased intracellular cGMP levels and activated cGMP-dependent protein kinase (PKG). PDE5 was found to be overexpressed in colorectal cancer cells as well as in colon cancer tissues compared to adjacent normal tissues [66]. Indeed, Dr. Piazza’s group has reported that SS and other NSAIDs inhibit PDE5 activity, and this leads to induction of apoptosis in cancer cells [67]. Therefore, inhibition of PDE5 may contribute to the anti-cancer properties of NSAIDs. Since PDE5 inhibitors have been previously reported to have chemo-sensitizing effects [68], NSAIDs like SS may be useful chemo-sensitizers along with other cancer drugs.
4. Cytoskeletal proteins
Cytoskeletal proteins are critical for maintaining normal cellular form and function. They represent the highways for vesicle trafficking, which is a key element of autocrine and paracrine cellular signaling. Cytoskeletal protein rearrangement is also a critical part of cell migration in cancer. All of these aspects are areas that would need to be disregulated to allow a pre-cancerous cell to go through EMT and metastasize to new sites.
4.1. Nesprin-2
Nesprin-2 is a 796 kDa protein containing an N-terminal actin-binding domain consisting of two calponin homology domains, a central coiled-coil rod domain, and a C-terminal transmembrane KASH domain responsible for targeting to the nuclear envelop and interaction with inner nuclear membrane SUN proteins [69–71]. Also, the Nesprin-2/SUN complex is important for the proper nuclear positioning that is necessary for fibroblast migration, suggesting that Nesprin-2 may have an important role in cell motility [72]. Recently, a link between Nesprin-2 and cancer has been suggested because it was observed in tumor tissues more frequently and at higher expression levels than normal tissues [73]. SS and other NSAIDs down-regulate Nesprin-2 expression in human colorectal cancer cells and its down-regulation is highly associated with NSAIDs’ effect on anti-tumorigenesis as assessed by cell impedance experiments [73]. Therefore, Nesprin-2 is likely a molecular target of NSAIDs in a group of cell assembly proteins.
5. TGF-β signaling
The mixtures of cytokines that are produced in the tumor microenvironment have important roles in cancer. TGF-β is a multifunctional regulatory polypeptide that is part of a large family of cytokines that control many aspects of cellular function, including cellular proliferation, differentiation, migration, apoptosis, adhesion, angiogenesis, immune surveillance, and survival. TGF-β signaling can drive either tumor suppression or tumor promotion. The TGF-β superfamily protein NAG-1 has been extensively studied in NSAID-induced anti-tumorigenesis.
5.1. NAG-1
NAG-1, also known as GDF15, MIC-1, and PDF, is a divergent member of the TGF-β superfamily. NAG-1 is one of the few TGF-β superfamily proteins whose receptors remain unknown. NAG-1 is highly induced by most conventional NSAIDs [74] and its induction by NSAIDs is mediated in a COX-independent manner [75]. NAG-1 induction by NSAIDs not only plays a role in colorectal anti-tumorigenesis, but also in others including head and neck cancer [76], prostate cancer [77], ovarian cancer [78], and thyroid cancer [79]. Mechanistically, it has been reported that NSAIDs up-regulate EGR-1 in vitro and in vivo, and HER-1 then binds to the promoter region of NAG-1 [14, 80]. SS also induces NAG-1 expression, and its induction can be blocked by dominate negative PERK and by dominant negative CHOP [28], indicating that NAG-1 is downstream of PERK and CHOP (Fig. 1). Since NAG-1 transgenic mice exhibit anti-cancer activity and anti-inflammation activity [81, 82] in the colon and lung, NAG-1 must be a key target protein to control anti-inflammation and anti-cancer activity, mediated by NSAIDs.
6. Cell cycle regulators
Several studies have shown that NSAIDs can modulate cell cycle progression. Expression and activity of cyclins and CDKs are modulated by several NSAIDs in a COX-independent manner. Two important cell cycle regulators, cyclin D1 and p21 are discussed.
6.1. Cyclin D1
Cyclin D1 serves as a key regulator in cell cycle progression by activating cyclin dependent kinase (CDK) 4/6 and is frequently over-expressed in tumor cells. There is a substantial body of experimental evidence showing that cyclin D1 could be a potential cancer therapeutic target [83]. NSAIDs, as potential anti-cancer agents, have been well documented to down-regulate cyclin D1 accompanied by G1 cell cycle arrest; however, the underlying mechanisms could be diverse. Various NSAIDs can down-regulate cyclin D1 expression by suppressing NF-κB activation in the tumor necrosis factor (TNF)-induced KMB-5 cell model [84]. Also, aspirin cause cyclin D1 degradation in SW480 cells through the p38 MAP kinase pathway, which, in turn, results in NF-κB pathway activation and subsequently apoptosis [85]. On the other hand, the suppression of Wnt/β-catenin signaling by NSAIDs could also contribute to cyclin D1 down-regulation in colorectal cancer cells [86–90]. Although TA-mediated cyclin D1 decreases have been proposed to result from degradation of Sp transcription factor protein [91], we recently showed that TA can inhibit cyclin D1 translation via PERK/eIF2α pathway activation in colorectal cancer cells [9]. Apparently, NSAIDs alter cyclin D1 expression by various transcriptional and post-transcriptional mechanisms.
6.2. p21
SS increases p21 mRNA expression in the immortalized human breast epithelial cell line MCF-10F [92] and increases p21 protein in ovarian cancer cells [78]. Celecoxib also increases p21 protein expression in the human CRC cell lines HCT-15, Caco-2, and HT-29 [93]. COX-2-preferential NSAIDs NS398 and nimesulide also increase p21 expression at the transcriptional level [94]. Collectively, a decrease in p21 expression may be one of the main oncogenic events in the development of cancer, and thus p21 induction by NSAIDs could be the molecular link between NSAIDs and chemopreventive activity in several cancers.
7. Conclusion
NSAIDs interact with many pathways in cancer cells. There are many promising cyclooxygenase-independent mechanisms utilized by NSAIDs that provide potential avenues for developing new and better drugs that minimize or eliminate the undesirable side effects of cyclooxygenase inhibition such as gastric bleeding and cardiovascular risks. NSAIDs up-regulate a number of transcription factors such as the tumor suppressors EGR-1, ATF3, and CHOP and down-regulate oncogenic transcription factors Sp1 and β-catenin (Fig. 1). The oncogenic cell membrane protein EGFR is down-regulated, and the activity of the metastatic protein complex Smad2/3 is decreased by NSAID treatment in CRC cell lines, while the cell adhesion protein E-cadherin is protected in APCMin/+ mice from loss of expression by NSAID treatment (Fig. 2). Some NSAIDs also affect cytoskeletal reorganization and loss of actin stress fibers. The secreted tumor suppressor protein NAG-1 is induced by NSAIDs both in vitro and in vivo via p53, EGR-1, CHOP, and other pathways. Thus, NSAIDs and improved derivatives of NSAIDs have great potential as both chemotherapeutic and chemopreventive agents in cancer. Molecular targets of two NSAIDs, SS and TA, are summarized in Table 1, and more targets could be identified in the future.
Table 1.
Molecular targets of sulindac sulfide and tolfenamic acid in different cancer types.
| NSAIDs | Up- regulated targets |
Down- regulated targets |
Cancer types [References] |
|---|---|---|---|
| Sulindac sulfide | EGR-1 | Colon [80], Lung [13], brain [95] | |
| ATF3 | Colon [20] | ||
| NF-κB | Colon [46], Breast [96] | ||
| NF-κB | Colon [50] | ||
| β-catenin | Colon [53], lung and breast [97] | ||
| EGFR | Colon [61] | ||
| E-cadherin | Colon [63] | ||
| PDE5 | Colon [67] | ||
| NAG-1 | Colon [74], ovarian [78], Gastric [98], Prostate [99] | ||
| Nesprin-2 | Colon [73] | ||
| p21 | Breast [92], ovarian [78], Head/Neck [100], Pancreas [101] | ||
| CHOP | Colon [28] | ||
| Cyclin D1 | Breast [92], pancreas [101], colon [102] | ||
| Tolfenamic acid | EGR-1 | Colon [15] | |
| ATF3 | Colon [19] | ||
| ATF4 | Colon [9] | ||
| CHOP | Colon [9] | ||
| Sp1 | Pancreas [36], Rhabdomyosarcoma [37], lung [38], esophageal [91]. Ovarian [103], colon [40] | ||
| NF-κB | Colon [49] | ||
| Smad2/3 | Lung [60] | ||
| NAG-1 | Colon [15], Oral [76], thyroid [79] | ||
| p21 | Breast [104] | ||
| Cyclin D1 | Colon [9] |
Acknowledgements
We apologize to all colleagues whose important work we could not cite due to space restrictions. The authors thank Misty Bailey for her critical review. This work was supported by grants from the American Cancer Society (CNE-111611), National Institutes of Health (R01CA108975), and the University of Tennessee, Center of Excellence in Livestock Diseases and Human Health.
Abbreviations
- AA
arachidonic acid
- NSAID
non-steroidal anti-inflammatory drug
- FAP
familial adenomatous polyposis
- TA
tolfenamic acid
- SS
sulindac sulfide
- EGR-1
early growth response-1
- NAG-1
NSAID-activated gene-1
- EMT
epithelial-mesenchymal transition
- bZIP
basic leucine zipper domain
- EGFR
epidermal growth factor receptor
- PDE5
phosphodiesterase type 5
- TGF-β
transforming growth factor-beta
- CRC
colorectal cancer
- CHOP
C/EBP homologous transcription factor
- SP
specific protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Ineterest Statement
None
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 3.Herschman HR. Prostaglandin synthase 2. Biochim Biophys Acta. 1996;1299:125. doi: 10.1016/0005-2760(95)00194-8. [DOI] [PubMed] [Google Scholar]
- 4.Tanabe T, Tohnai N. Cyclooxygenase isozymes and their gene structures and expression. Prostag Oth Lipid M. 2002;68:95–114. doi: 10.1016/s0090-6980(02)00024-2. [DOI] [PubMed] [Google Scholar]
- 5.Hoshino T, Tsutsumi S, Tomisato W, Hwang H-J, Tsuchiya T, Mizushima T. Prostaglandin E2 Protects Gastric Mucosal Cells from Apoptosis via EP2 and EP4 Receptor Activation. J Biol Chem. 2003;278:12752–12758. doi: 10.1074/jbc.M212097200. [DOI] [PubMed] [Google Scholar]
- 6.Khan M, Fraser A. Cox-2 inhibitors and the risk of cardiovascular thrombotic events. Ir Med J. 2012;105:119–121. [PubMed] [Google Scholar]
- 7.Bjarnason I. Gastrointestinal safety of NSAIDs and over-the-counter analgesics. Int J Clin Pract Suppl. 2013;178:37–42. doi: 10.1111/ijcp.12048. [DOI] [PubMed] [Google Scholar]
- 8.Abdelrahim M, Baker CH, Abbruzzese JL, Safe S. Tolfenamic acid and pancreatic cancer growth, angiogenesis, and Sp protein degradation. J Natl Cancer Inst. 2006;98:855–868. doi: 10.1093/jnci/djj232. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Lee SH, Min KW, McEntee MF, Jeong J, Li Q, Baek SJ. The involvement of endoplasmic reticulum stress in the suppression of colorectal tumorigenesis by tolfenamic acid. Cancer Prev Res (Phila) 2013;6:1337–1347. doi: 10.1158/1940-6207.CAPR-13-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, Booker SV, Robinson CR, Offerhaus GJ. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 11.Krones-Herzig A, Mittal S, Yule K, Liang H, English C, Urcis R, Soni T, Adamson ED, Mercola D. Early growth response 1 acts as a tumor suppressor in vivo and in vitro via regulation of p53. Cancer Res. 2005;65:5133–5143. doi: 10.1158/0008-5472.CAN-04-3742. [DOI] [PubMed] [Google Scholar]
- 12.Baek SJ, Kim J-S, Moore SM, Lee S-H, Martinez J, Eling TE. Cyclooxygenase inhibitors induce the expression of the tumor suppressor gene EGR-1, which results in the up-regulation of NAG-1, an antitumorigenic protein. Mol Pharmacol. 2005;67:356–364. doi: 10.1124/mol.104.005108. [DOI] [PubMed] [Google Scholar]
- 13.Moon Y, Bottone FG, Jr, McEntee MF, Eling TE. Suppression of tumor cell invasion by cyclooxygenase inhibitors is mediated by thrombospondin-1 via the early growth response gene Egr-1. Mol Cancer Ther. 2005;4:1551–1558. doi: 10.1158/1535-7163.MCT-05-0213. [DOI] [PubMed] [Google Scholar]
- 14.Vaish V, Piplani H, Rana C, Vaiphei K, Sanyal SN. NSAIDs may regulate EGR-1-mediated induction of reactive oxygen species and non-steroidal anti-inflammatory drug-induced gene (NAG)-1 to initiate intrinsic pathway of apoptosis for the chemoprevention of colorectal cancer. Mol Cell Biochem. 2013;378:47–64. doi: 10.1007/s11010-013-1593-y. [DOI] [PubMed] [Google Scholar]
- 15.Lee SH, Bahn JH, Choi CK, Whitlock NC, English AE, Safe S, Baek SJ. ESE-1/EGR-1 pathway plays a role in tolfenamic acid-induced apoptosis in colorectal cancer cells. Mol Cancer Ther. 2008;7:3739–3750. doi: 10.1158/1535-7163.MCT-08-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitlock NC, Bahn JH, Lee S-H, Eling TE, Baek SJ. Resveratrol-induced apoptosis is mediated by early growth response-1, Krüppel-like factor 4, and activating transcription factor 3. Cancer Prev Res (Phila) 2011;4:116–127. doi: 10.1158/1940-6207.CAPR-10-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuo PL, Chen YH, Chen TC, Shen KH, Hsu YL. CXCL5/ENA78 increased cell migration and epithelial-to-mesenchymal transition of hormone-independent prostate cancer by early growth response-1/snail signaling pathway. J Cell Physiol. 2011;226:1224–1231. doi: 10.1002/jcp.22445. [DOI] [PubMed] [Google Scholar]
- 18.Schjerning Olsen A-M, Fosbøl EL, Lindhardsen J, Andersson C, Folke F, Nielsen MB, Køber L, Hansen PR, Torp-Pedersen C, Gislason GH. Cause-Specific Cardiovascular Risk Associated with Nonsteroidal Anti-Inflammatory Drugs among Myocardial Infarction Patients - A Nationwide Study. PLoS ONE. 2013;8:e54309. doi: 10.1371/journal.pone.0054309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SH, Bahn JH, Whitlock NC, Baek SJ. Activating transcription factor 2 (ATF2) controls tolfenamic acid-induced ATF3 expression via MAP kinase pathways. Oncogene. 2010;29:5182–5192. doi: 10.1038/onc.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bottone FG, Moon Y, Kim JS, Alston-Mills B, Ishibashi M, Eling TE. The anti-invasive activity of cyclooxygenase inhibitors is regulated by the transcription factor ATF3 (activating transcription factor 3) Mol Cancer Ther. 2005;4:693–703. doi: 10.1158/1535-7163.MCT-04-0337. [DOI] [PubMed] [Google Scholar]
- 21.Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci U S A. 2004;101:11269–11274. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koditz J, Nesper J, Wottawa M, Stiehl DP, Camenisch G, Franke C, Myllyharju J, Wenger RH, Katschinski DM. Oxygen-dependent ATF-4 stability is mediated by the PHD3 oxygen sensor. Blood. 2007;110:3610–3617. doi: 10.1182/blood-2007-06-094441. [DOI] [PubMed] [Google Scholar]
- 23.Tsutsumi S, Gotoh T, Tomisato W, Mima S, Hoshino T, Hwang HJ, Takenaka H, Tsuchiya T, Mori M, Mizushima T. Endoplasmic reticulum stress response is involved in nonsteroidal anti-inflammatory drug-induced apoptosis. Cell Death Differ. 2004;11:1009–1016. doi: 10.1038/sj.cdd.4401436. [DOI] [PubMed] [Google Scholar]
- 24.Ishihara T, Hoshino T, Namba T, Tanaka K, Mizushima T. Involvement of up-regulation of PUMA in non-steroidal anti-inflammatory drug-induced apoptosis. Biochem Biophys Res Commun. 2007;356:711–717. doi: 10.1016/j.bbrc.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 25.Tsutsumi S, Namba T, Tanaka KI, Arai Y, Ishihara T, Aburaya M, Mima S, Hoshino T, Mizushima T. Celecoxib upregulates endoplasmic reticulum chaperones that inhibit celecoxib-induced apoptosis in human gastric cells. Oncogene. 2006;25:1018–1029. doi: 10.1038/sj.onc.1209139. [DOI] [PubMed] [Google Scholar]
- 26.Namba T, Homan T, Nishimura T, Mima S, Hoshino T, Mizushima T. Up-regulation of S100P expression by non-steroidal anti-inflammatory drugs and its role in anti-tumorigenic effects. J Biol Chem. 2009;284:4158–4167. doi: 10.1074/jbc.M806051200. [DOI] [PubMed] [Google Scholar]
- 27.Ron D, Habener JF. CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Gene Dev. 1992;6:439–453. doi: 10.1101/gad.6.3.439. [DOI] [PubMed] [Google Scholar]
- 28.Yang H, Park SH, Choi HJ, Moon Y. The integrated stress response-associated signals modulates intestinal tumor cell growth by NSAID-activated gene 1 (NAG-1/MIC-1/PTGF-beta) Carcinogenesis. 2010;31:703–711. doi: 10.1093/carcin/bgq008. [DOI] [PubMed] [Google Scholar]
- 29.White M, Johnson G, Zhang W, Hobrath J, Piazza G, Grimaldi M. Sulindac sulfide inhibits sarcoendoplasmic reticulum Ca2+ ATPase, induces endoplasmic reticulum stress response, and exerts toxicity in glioma cells: Relevant similarities to and important differences from celecoxib. J Neurosci Res. 2013;91:393–406. doi: 10.1002/jnr.23169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haak LL, Grimaldi M, Smaili SS, Russell JT. Mitochondria regulate Ca2+ wave initiation and inositol trisphosphate signal transduction in oligodendrocyte progenitors. J Neurochem. 2002;80:405–415. doi: 10.1046/j.0022-3042.2001.00727.x. [DOI] [PubMed] [Google Scholar]
- 31.Hosoi T, Sasaki M, Baba S, Ozawa K. Effect of pranoprofen on endoplasmic reticulum stress in the primary cultured glial cells. Neurochem Int. 2009;54:1–6. doi: 10.1016/j.neuint.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 32.Suske G. The Sp-family of transcription factors. Gene. 1999;238:291–300. doi: 10.1016/s0378-1119(99)00357-1. [DOI] [PubMed] [Google Scholar]
- 33.Turner J, Crossley M. Mammalian Kruppel-like transcription factors: more than just a pretty finger. Trends Biochem Sci. 1999;24:236–240. doi: 10.1016/s0968-0004(99)01406-1. [DOI] [PubMed] [Google Scholar]
- 34.Scohy S, Gabant P, Van Reeth T, Hertveldt V, Dreze PL, Van Vooren P, Riviere M, Szpirer J, Szpirer C. Identification of KLF13 and KLF14 (SP6), novel members of the SP/XKLF transcription factor family. Genomics. 2000;70:93–101. doi: 10.1006/geno.2000.6362. [DOI] [PubMed] [Google Scholar]
- 35.Safe S, Abdelrahim M. Sp transcription factor family and its role in cancer. Eur J Cancer. 2005;41:2438–2448. doi: 10.1016/j.ejca.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Abdelrahim M, Baker CH, Abbruzzese JL, Sheikh-Hamad D, Liu S, Cho SD, Yoon K, Safe S. Regulation of vascular endothelial growth factor receptor-1 expression by specificity proteins 1, 3, and 4 in pancreatic cancer cells. Cancer Res. 2007;67:3286–3294. doi: 10.1158/0008-5472.CAN-06-3831. [DOI] [PubMed] [Google Scholar]
- 37.Chadalapaka G, Jutooru I, Sreevalsan S, Pathi S, Kim K, Chen C, Crose L, Linardic C, Safe S. Inhibition of rhabdomyosarcoma cell and tumor growth by targeting specificity protein (Sp) transcription factors. Int J Cancer. 2013;132:795–806. doi: 10.1002/ijc.27730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colon J, Basha MR, Madero-Visbal R, Konduri S, Baker CH, Herrera LJ, Safe S, Sheikh-Hamad D, Abudayyeh A, Alvarado B, Abdelrahim M. Tolfenamic acid decreases c-Met expression through Sp proteins degradation and inhibits lung cancer cells growth and tumor formation in orthotopic mice. Invest New Drugs. 2011;29:41–51. doi: 10.1007/s10637-009-9331-8. [DOI] [PubMed] [Google Scholar]
- 39.Abdelrahim M, Safe S. Cyclooxygenase-2 inhibitors decrease vascular endothelial growth factor expression in colon cancer cells by enhanced degradation of Sp1 and Sp4 proteins. Mol Pharmacol. 2005;68:317–329. doi: 10.1124/mol.105.011825. [DOI] [PubMed] [Google Scholar]
- 40.Pathi S, Li X, Safe S. Tolfenamic acid inhibits colon cancer cell and tumor growth and induces degradation of spn cancer cells by enhanced degradation of Sp1 and Sp4 proteins, Molecular pharmacologyecificity protein (Sp) transcription factors. Mol Carcinog. 2014 doi: 10.1002/mc.22010. In Press. [DOI] [PubMed] [Google Scholar]
- 41.Pathi SS, Jutooru I, Chadalapaka G, Sreevalsan S, Anand S, Thatcher GR, Safe S. GT-094, a NO-NSAID, inhibits colon cancer cell growth by activation of a reactive oxygen species-microRNA-27a: ZBTB10-specificity protein pathway. Mol Cancer Res. 2011;9:195–202. doi: 10.1158/1541-7786.MCR-10-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pathi S, Jutooru I, Chadalapaka G, Nair V, Lee S-O, Safe S. Aspirin Inhibits Colon Cancer Cell and Tumor Growth and Downregulates Specificity Protein (Sp) Transcription Factors. PLoS ONE. 2012;7:e48208. doi: 10.1371/journal.pone.0048208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verma IM. Nuclear factor (NF)-kappaB proteins: therapeutic targets. Ann Rheum Dis. 2004;63(Suppl 2):ii57–ii61. doi: 10.1136/ard.2004.028266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim HJ, Hawke N, Baldwin AS. NF-kappa B and IKK as therapeutic targets in cancer. Cell Death Differ. 2006;13:738–747. doi: 10.1038/sj.cdd.4401877. [DOI] [PubMed] [Google Scholar]
- 45.Kopp E, Ghosh S. Inhibition of NF-kB by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 46.Vaish V, Rana C, Piplani H, Vaiphei K, Sanyal SN. Sulindac and Celecoxib Regulate Cell Cycle Progression by p53/p21 Up Regulation to Induce Apoptosis During Initial Stages of Experimental Colorectal Cancer. Cell Biochem Biophys. 2013:1–19. doi: 10.1007/s12013-013-9711-8. [DOI] [PubMed] [Google Scholar]
- 47.Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8:33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perkins ND, Gilmore TD. Good cop, bad cop: the different faces of NF-kappaB. Cell Death Differ. 2006;13:759–772. doi: 10.1038/sj.cdd.4401838. [DOI] [PubMed] [Google Scholar]
- 49.Jeong JB, Yang X, Clark R, Choi J, Baek SJ, Lee SH. A mechanistic study of the proapoptotic effect of tolfenamic acid: involvement of NF-kappaB activation. Carcinogenesis. 2013;34:2350–2360. doi: 10.1093/carcin/bgt224. [DOI] [PubMed] [Google Scholar]
- 50.Mladenova D, Pangon L, Currey N, Ng I, Musgrove EA, Grey ST, Kohonen-Corish MR. Sulindac activates NF-kappaB signaling in colon cancer cells. Cell Commun Signal. 2013;11:73. doi: 10.1186/1478-811X-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dihlmann S, Siermann A, von Knebel Doeberitz M. The nonsteroidal anti-inflammatory drugs aspirin and indomethacin attenuate beta-catenin/TCF-4 signaling. Oncogene. 2001;20:645–653. doi: 10.1038/sj.onc.1204123. [DOI] [PubMed] [Google Scholar]
- 52.Gardner SH, Hawcroft G, Hull MA. Effect of nonsteroidal anti-inflammatory drugs on beta-catenin protein levels and catenin-related transcription in human colorectal cancer cells. Br J Cancer. 2004;91:153–163. doi: 10.1038/sj.bjc.6601901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McEntee MF, Chiu CH, Whelan J. Relationship of beta-catenin and Bcl-2 expression to sulindac-induced regression of intestinal tumors in Min mice. Carcinogenesis. 1999;20:635–640. doi: 10.1093/carcin/20.4.635. [DOI] [PubMed] [Google Scholar]
- 54.Lu D, Cottam HB, Corr M, Carson DA. Repression of β-catenin function in malignant cells by nonsteroidal antiinflammatory drugs. Proc Natl Acad Sci U S A. 2005;102:18567–18571. doi: 10.1073/pnas.0509316102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stein U, Arlt F, Smith J, Sack U, Herrmann P, Walther W, Lemm M, Fichtner I, Shoemaker RH, Schlag PM. Intervening in beta-catenin signaling by sulindac inhibits S100A4-dependent colon cancer metastasis. Neoplasia. 2011;13:131–144. doi: 10.1593/neo.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 57.Adorno M, Cordenonsi M, Montagner M, Dupont S, Wong C, Hann B, Solari A, Bobisse S, Rondina MB, Guzzardo V, Parenti AR, Rosato A, Bicciato S, Balmain A, Piccolo S. A Mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell. 2009;137:87–98. doi: 10.1016/j.cell.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 58.Koelink PJ, Hawinkels LJ, Wiercinska E, Sier CF, ten Dijke P, Lamers CB, Hommes DW, Verspaget HW. 5-Aminosalicylic acid inhibits TGF-beta1 signalling in colorectal cancer cells. Cancer Lett. 2010;287:82–90. doi: 10.1016/j.canlet.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 59.Slattery ML, Herrick JS, Lundgreen A, Wolff RK. Genetic variation in the TGF-beta signaling pathway and colon and rectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2011;20:57–69. doi: 10.1158/1055-9965.EPI-10-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang X, Min KW, Liggett J, Baek SJ. Disruption of the transforming growth factor-beta pathway by tolfenamic acid via the ERK MAP kinase pathway. Carcinogenesis. 2013;34:2900–2907. doi: 10.1093/carcin/bgt250. [DOI] [PubMed] [Google Scholar]
- 61.Pangburn HA, Kraus H, Ahnen DJ, Rice PL. Sulindac metabolites inhibit epidermal growth factor receptor activation and expression. J Carcinog. 2005;4:16. doi: 10.1186/1477-3163-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tavolari S, Munarini A, Storci G, Laufer S, Chieco P, Guarnieri T. The decrease of cell membrane fluidity by the non-steroidal anti-inflammatory drug Licofelone inhibits epidermal growth factor receptor signalling and triggers apoptosis in HCA-7 colon cancer cells. Cancer Lett. 2012;321:187–194. doi: 10.1016/j.canlet.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 63.Greenspan EJ, Nichols FC, Rosenberg DW. Molecular alterations associated with sulindac-resistant colon tumors in ApcMin/+ mice. Cancer Prev Res. 2010;3:1187–1197. doi: 10.1158/1940-6207.CAPR-09-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kato T, Fujino H, Oyama S, Kawashima T, Murayama T. Indomethacin induces cellular morphological change and migration via epithelial-mesenchymal transition in A549 human lung cancer cells: a novel cyclooxygenase-inhibition-independent effect. Biochem Pharmacol. 2011;82:1781–1791. doi: 10.1016/j.bcp.2011.07.096. [DOI] [PubMed] [Google Scholar]
- 65.Wang ZL, Fan ZQ, Jiang HD, Qu JM. Selective Cox-2 inhibitor celecoxib induces epithelial-mesenchymal transition in human lung cancer cells via activating MEK-ERK signaling. Carcinogenesis. 2013;34:638–646. doi: 10.1093/carcin/bgs367. [DOI] [PubMed] [Google Scholar]
- 66.Tinsley HN, Gary BD, Keeton AB, Lu W, Li Y, Piazza GA. Inhibition of PDE5 by Sulindac Sulfide Selectively Induces Apoptosis and Attenuates Oncogenic Wnt/β-Catenin–Mediated Transcription in Human Breast Tumor Cells. Cancer Prev Res. 2011;4:1275–1284. doi: 10.1158/1940-6207.CAPR-11-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li N, Xi Y, Tinsley HN, Gurpinar E, Gary BD, Zhu B, Li Y, Chen X, Keeton AB, Abadi AH, Moyer MP, Grizzle WE, Chang W-C, Clapper ML, Piazza GA. Sulindac Selectively Inhibits Colon Tumor Cell Growth by Activating the cGMP/PKG Pathway to Suppress Wnt/β-Catenin Signaling. Mol Cancer Ther. 2013;12:1848–1859. doi: 10.1158/1535-7163.MCT-13-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Black KL, Yin D, Ong JM, Hu J, Konda BM, Wang X, Ko MK, Bayan J-A, Sacapano MR, Espinoza A, Irvin DK, Shu Y. PDE5 inhibitors enhance tumor permeability and efficacy of chemotherapy in a rat brain tumor model. Brain Res. 2008;1230:290–302. doi: 10.1016/j.brainres.2008.06.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhen YY, Libotte T, Munck M, Noegel AA, Korenbaum E. NUANCE, a giant protein connecting the nucleus and actin cytoskeleton. J Cell Sci. 2002;115:3207–3222. doi: 10.1242/jcs.115.15.3207. [DOI] [PubMed] [Google Scholar]
- 70.Padmakumar VC, Abraham S, Braune S, Noegel AA, Tunggal B, Karakesisoglou I, Korenbaum E. Enaptin, a giant actin-binding protein, is an element of the nuclear membrane and the actin cytoskeleton. Exp Cell Res. 2004;295:330–339. doi: 10.1016/j.yexcr.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 71.Padmakumar V, Libotte T, Lu W, Zaim H, Abraham S, Noegel AA, Gotzmann J, Foisner R, Karakesisoglou I. The inner nuclear membrane protein Sun1 mediates the anchorage of Nesprin-2 to the nuclear envelope. J Cell Sci. 2005;118:3419–3430. doi: 10.1242/jcs.02471. [DOI] [PubMed] [Google Scholar]
- 72.Luxton GG, Gomes ER, Folker ES, Vintinner E, Gundersen GG. Linear arrays of nuclear envelope proteins harness retrograde actin flow for nuclear movement. Science. 2010;329:956–959. doi: 10.1126/science.1189072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liggett JL, Choi CK, Donnell RL, Kihm KD, Kim JS, Min KW, Noegel AA, Baek SJ. Nonsteroidal anti-inflammatory drug sulindac sulfide suppresses structural protein Nesprin-2 expression in colorectal cancer cells. Biochim Biophys Acta. 2013;1840:322–331. doi: 10.1016/j.bbagen.2013.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baek SJ, Kim KS, Nixon JB, Wilson LC, Eling TE. Cyclooxygenase inhibitors regulate the expression of a TGF-beta superfamily member that has proapoptotic and antitumorigenic activities. Mol Pharmacol. 2001;59:901–908. [PubMed] [Google Scholar]
- 75.Baek SJ, Wilson LC, Lee CH, Eling TE. Dual function of nonsteroidal anti-inflammatory drugs (NSAIDs): inhibition of cyclooxygenase and induction of NSAID-activated gene. J Pharmacol Exp Ther. 2002;301:1126–1131. doi: 10.1124/jpet.301.3.1126. [DOI] [PubMed] [Google Scholar]
- 76.Kang SU, Shin YS, Hwang HS, Baek SJ, Lee SH, Kim CH. Tolfenamic acid induces apoptosis and growth inhibition in head and neck cancer: involvement of NAG-1 expression. PLoS ONE. 2012;7:e34988. doi: 10.1371/journal.pone.0034988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wynne S, Djakiew D. NSAID inhibition of prostate cancer cell migration is mediated by Nag-1 Induction via the p38 MAPK-p75(NTR) pathway. Mol Cancer Res. 2010;8:1656–1664. doi: 10.1158/1541-7786.MCR-10-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim JS, Baek SJ, Sali T, Eling TE. The conventional nonsteroidal anti-inflammatory drug sulindac sulfide arrests ovarian cancer cell growth via the expression of NAG-1/MIC-1/GDF-15. Mol Cancer Ther. 2005;4:487–493. doi: 10.1158/1535-7163.MCT-04-0201. [DOI] [PubMed] [Google Scholar]
- 79.Chang JW, Kang SU, Choi JW, Shin YS, Baek SJ, Lee SH, Kim CH. Tolfenamic Acid Induces Apoptosis and Growth Inhibition in Anaplastic Thyroid Cancer: Involvement of NAG-1 Expression and Intracellular ROS Generation. Free Radic Biol Med. 2014;67:115–130. doi: 10.1016/j.freeradbiomed.2013.10.818. [DOI] [PubMed] [Google Scholar]
- 80.Baek SJ, Kim JS, Moore SM, Lee SH, Martinez J, Eling TE. Cyclooxygenase inhibitors induce the expression of the tumor suppressor gene EGR-1, which results in the up-regulation of NAG-1, an antitumorigenic protein. Mol Pharmacol. 2005;67:356–364. doi: 10.1124/mol.104.005108. [DOI] [PubMed] [Google Scholar]
- 81.Baek SJ, Okazaki R, Lee SH, Martinez J, Kim JS, Yamaguchi K, Mishina Y, Martin DW, Shoieb A, McEntee MF, Eling TE. Nonsteroidal anti-inflammatory drug-activated gene-1 over expression in transgenic mice suppresses intestinal neoplasia. Gastroenterology. 2006;131:1553–1560. doi: 10.1053/j.gastro.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 82.Cekanova M, Lee SH, Donnell RL, Sukhthankar M, Eling TE, Fischer SM, Baek SJ. Nonsteroidal anti-inflammatory drug-activated gene-1 expression inhibits urethane-induced pulmonary tumorigenesis in transgenic mice. Cancer Prev Res (Phila) 2009;2:450–458. doi: 10.1158/1940-6207.CAPR-09-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 2011;11:558–572. doi: 10.1038/nrc3090. [DOI] [PubMed] [Google Scholar]
- 84.Takada Y, Bhardwaj A, Potdar P, Aggarwal BB. Nonsteroidal anti-inflammatory agents differ in their ability to suppress NF-kappaB activation, inhibition of expression of cyclooxygenase-2 and cyclin D1, and abrogation of tumor cell proliferation. Oncogene. 2004;23:9247–9258. doi: 10.1038/sj.onc.1208169. [DOI] [PubMed] [Google Scholar]
- 85.Thoms HC, Dunlop MG, Stark LA. p38-mediated inactivation of cyclin D1/cyclin-dependent kinase 4 stimulates nucleolar translocation of RelA and apoptosis in colorectal cancer cells. Cancer Res. 2007;67:1660–1669. doi: 10.1158/0008-5472.CAN-06-1038. [DOI] [PubMed] [Google Scholar]
- 86.Tinsley HN, Gary BD, Thaiparambil J, Li N, Lu W, Li Y, Maxuitenko YY, Keeton AB, Piazza GA. Colon Tumor Cell Growth–Inhibitory Activity of Sulindac Sulfide and Other Nonsteroidal Anti-Inflammatory Drugs Is Associated with Phosphodiesterase 5 Inhibition. Cancer Prev Res. 2010;3:1303–1313. doi: 10.1158/1940-6207.CAPR-10-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hawcroft G, D'Amico M, Albanese C, Markham AF, Pestell RG, Hull MA. Indomethacin induces differential expression of beta-catenin, gamma-catenin and T-cell factor target genes in human colorectal cancer cells. Carcinogenesis. 2002;23:107–114. doi: 10.1093/carcin/23.1.107. [DOI] [PubMed] [Google Scholar]
- 88.Boon EM, Keller JJ, Wormhoudt TA, Giardiello FM, Offerhaus GJ, van der Neut R, Pals ST. Sulindac targets nuclear beta-catenin accumulation and Wnt signalling in adenomas of patients with familial adenomatous polyposis and in human colorectal cancer cell lines. Br J Cancer. 2004;90:224–229. doi: 10.1038/sj.bjc.6601505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cho M, Gwak J, Park S, Won J, Kim DE, Yea SS, Cha IJ, Kim TK, Shin JG, Oh S. Diclofenac attenuates Wnt/beta-catenin signaling in colon cancer cells by activation of NF-kappaB. FEBS lett. 2005;579:4213–4218. doi: 10.1016/j.febslet.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 90.Greenspan EJ, Madigan JP, Boardman LA, Rosenberg DW. Ibuprofen inhibits activation of nuclear {beta}-catenin in human colon adenomas and induces the phosphorylation of GSK-3{beta} Cancer Prev Res. 2011;4:161–171. doi: 10.1158/1940-6207.CAPR-10-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Papineni S, Chintharlapalli S, Abdelrahim M, Lee SO, Burghardt R, Abudayyeh A, Baker C, Herrera L, Safe S. Tolfenamic acid inhibits esophageal cancer through repression of specificity proteins and c-Met. Carcinogenesis. 2009;30:1193–1201. doi: 10.1093/carcin/bgp092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Han EK, Arber N, Yamamoto H, Lim JT, Delohery T, Pamukcu R, Piazza GA, Xing WQ, Weinstein IB. Effects of sulindac and its metabolites on growth and apoptosis in human mammary epithelial and breast carcinoma cell lines. Breast Cancer Res Treat. 1998;48:195–203. doi: 10.1023/a:1005924730450. [DOI] [PubMed] [Google Scholar]
- 93.Grosch S, Tegeder I, Niederberger E, Brautigam L, Geisslinger G. COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. FASEB J. 2001;15:2742–2744. doi: 10.1096/fj.01-0299fje. [DOI] [PubMed] [Google Scholar]
- 94.Han S, Roman J. COX-2 inhibitors suppress lung cancer cell growth by inducing p21 via COX-2 independent signals. Lung Cancer. 2006;51:283–296. doi: 10.1016/j.lungcan.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 95.Kambe A, Yoshioka H, Kamitani H, Watanabe T, Baek SJ, Eling TE. The cyclooxygenase inhibitor sulindac sulfide inhibits EP4 expression and suppresses the growth of glioblastoma cells. Cancer Prev Res (Phila Pa) 2009;2:1088–1099. doi: 10.1158/1940-6207.CAPR-09-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li X, Gao L, Cui Q, Gary BD, Dyess DL, Taylor W, Shevde LA, Samant RS, Dean-Colomb W, Piazza GA, Xi Y. Sulindac inhibits tumor cell invasion by suppressing NF-[kappa]B-mediated transcription of microRNAs. Oncogene. 2012;31:4979–4986. doi: 10.1038/onc.2011.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Han A, Song Z, Tong C, Hu D, Bi X, Augenlicht LH, Yang W. Sulindac suppresses beta-catenin expression in human cancer cells. Eur J Pharmacol. 2008;583:26–31. doi: 10.1016/j.ejphar.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jang TJ, Kang HJ, Kim JR, Yang CH. Non-steroidal anti-inflammatory drug activated gene (NAG-1) expression is closely related to death receptor-4 and-5 induction, which may explain sulindac sulfide induced gastric cancer cell apoptosis. Carcinogenesis. 2004;25:1853–1858. doi: 10.1093/carcin/bgh199. [DOI] [PubMed] [Google Scholar]
- 99.Shim M, Eling TE. Protein kinase C-dependent regulation of NAG-1/placental bone morphogenic protein/MIC-1 expression in LNCaP prostate carcinoma cells. J Biol Chem. 2005;280:18636–18642. doi: 10.1074/jbc.M414613200. [DOI] [PubMed] [Google Scholar]
- 100.Bock JM, Menon SG, Goswami PC, Sinclair LL, Bedford NS, Jackson RE, Trask DK. Differential activity of sulindac metabolites against squamous cell carcinoma of the head and neck is mediated by p21waf1/cip1 induction and cell cycle inhibition. Cancer Biol Ther. 2007;6:30–39. doi: 10.4161/cbt.6.1.3470. [DOI] [PubMed] [Google Scholar]
- 101.Yip-Schneider MT, Sweeney CJ, Jung SH, Crowell PL, Marshall MS. Cell cycle effects of nonsteroidal anti-inflammatory drugs and enhanced growth inhibition in combination with gemcitabine in pancreatic carcinoma cells. J Pharmacol Exp Ther. 2001;298:976–985. [PubMed] [Google Scholar]
- 102.Qiao L, Shiff SJ, Rigas B. Sulindac sulfide alters the expression of cyclin proteins in HT-29 colon adenocarcinoma cells. Int J Cancer. 1998;76:99–104. doi: 10.1002/(sici)1097-0215(19980330)76:1<99::aid-ijc16>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 103.Basha R, Ingersoll SB, Sankpal UT, Ahmad S, Baker CH, Edwards JR, Holloway RW, Kaja S, Abdelrahim M. Tolfenamic acid inhibits ovarian cancer cell growth and decreases the expression of c-Met and survivin through suppressing specificity protein transcription factors. Gynecol Oncology. 2011;122:163–170. doi: 10.1016/j.ygyno.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 104.Kim HJ, Cho SD, Kim J, Kim SJ, Choi C, Kim JS, Nam JS, Han Kwon K, Kang KS, Jung JY. Apoptotic effect of tolfenamic acid on MDA-MB-231 breast cancer cells and xenograft tumors. J Clin Biochem Nutr. 2013;53:21–26. doi: 10.3164/jcbn.12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]