Abstract
This study investigated the hypothesis that wear particle-induced oxidative stress initiates osteolysis after total hip replacement (THR). Patient radiographs were scored for osteolysis and periprosthetic tissues were immunostained and imaged to quantify polyethylene wear, inflammation, and five osteoinflammatory and oxidative stress-responsive factors. These included high mobility group protein-B1 (HMGB1), cyclooxygenase-2 (COX2), inducible nitric oxide synthase (iNOS), 4-hydroxynonenal (4-HNE), and nitrotyrosine (NT). The results show wear debris correlated with inflammation, 4-HNE, NT and HMGB1, whereas inflammation only correlated with NT and HMGB1. Similar to wear debris and inflammation, osteolysis correlated with HMGB1. Additionally, osteolysis correlated with COX2 and 4-HNE, but not iNOS or NT. Understanding the involvement of oxidative stress in wear-induced osteolysis will help identify diagnostic biomarkers and therapeutic targets to prevent osteolysis after THR.
Keywords: osteolysis, high mobility group protein-B1 (HMGB1), cyclooxygenase-2 (COX2), inducible nitric oxide synthase (iNOS), 4-hydroxynonenal (4-HNE), nitrotyrosine (NT)
Introduction
Total joint replacement (TJR) is the standard of care for advanced degenerative joint disease in the United States, with over 600,000 total hip (THR) and total knee replacements (TKR) being performed each year [1]. It is projected that the number of annual TJR surgeries will exceed 4,000,000 by the year 2030. Although complications after joint replacement are relatively low, approximately 10-20% of all TJR surgeries result in additional surgeries, which require implant replacement or other medical interventions to restore mobility. The foremost complication limiting implant longevity in the United States is aseptic loosening due to polyethylene (PE) wear debris-initiated chronic inflammation and inflammatory-mediated bone resorption [2-6]. Progressive bone loss at the bone-implant interface results in implant loosening, instability, and ultimately revision surgery. Due to advanced age and the loss of surrounding bone, revision surgeries have poorer outcomes. Thus, early diagnosis and treatment of osteolysis to reduce the number of revision surgeries would significantly improve patient quality of life and reduce the economic burden. Currently, there is no specific diagnostic marker for the identification of early osteolysis in THR patients, nor is there a treatment to prevent osteolysis.
The generation of implant wear debris from the articulation of metal on PE components is known to affect the activation and senescence of resident cells including macrophages, fibroblasts, osteoclasts, and osteoblasts [2, 7-13]. Activation of both resident and recruited macrophages following ingestion of biologically-indestructible PE wear particles results in the production and release of pro-inflammatory cytokines, chemokines, [2, 13, 14] reactive oxygen species (ROS) [15], and reactive nitrogen species (RNS) [16-18]. These products do little to remove the debris, but inadvertently affect the activity, proliferation, differentiation and apoptotic responses of osteoclasts and osteoblasts. Additionally, resident macrophages have the potential to differentiate into fully functional osteoclasts in response to wear debris-mediated inflammation [7]. Thus, the chronic inflammatory cascade induced by PE wear debris ultimately leads to enhanced bone resorption and the development of osteolysis.
Bone resorption is controlled by a system comprised of three key proteins, RANK (receptor-activator of nuclear factor kappa beta), its ligand RANKL (receptor-activator of nuclear factor kappa beta ligand) and a decoy receptor OPG (osteoprotegerin). Many inflammatory cytokines (e.g. interleukin-1β, tumor necrosis factor-α, prostaglandin E2 (PGE2)) increase the RANKL/OPG ratio and/or have direct effects on osteoclastogenesis and bone resorption [12, 19]. Like RANKL, these factors induce the production of ROS by NADPH-oxidase (NOX), which are required for the differentiation and activation of osteoclasts [20-26]. Thus, ROS and ROS-induced oxidative stress play a major role in regulating osteoclast function and bone resorption.
Despite the importance of ROS in osteoclastogenesis, a limited number of studies have focused on the involvement of oxidative stress in aseptic loosening. A single study suggested that overproduction or inadequate removal of ROS may be involved in the formation of fibrotic pseudocapsular tissues around revised THR components [27]. Indeed, oxidative stress is known to participate in the development of fibrosis associated with TKA [28]. Both phagocytosis and pro-inflammatory cytokines initiate macrophage generation of ROS by NOX and nitric oxide (NO) by iNOS [15]. More importantly, as systemic oxidative stress is associated with age-related loss of bone mass [29] and ROS is known to drive osteoclastogenesis and bone resorption [20-23, 30], the combination of the two most certainly factor into post-THR osteolysis.
RNS is also likely to be involved in the development of post-THR osteolysis, as it is generated by the simultaneous production of NO and ROS (e.g. superoxide anion) by resident macrophages, osteoclasts, or fibroblasts. Detection of RNS in tissues is determined by measuring NT accumulation. Three studies have suggested that iNOS and RNS production play a role in aseptic loosening [16-18]. Suh, Chang et al. reported a significant correlated increase in iNOS expression and NT accumulation in periprosthetic tissue compared to primary surgical control tissues [18]. The levels of both were higher in non-cemented THR tissues compared to cemented THR tissues, but the increases did not correlate with the extent of wear debris-induced osteolysis for either cemented or non-cemented THR. Similarly, Puskas, Menke et al. found significant differences in the amount of iNOS protein and NT accumulation in tissues from loose osteolytic and loose non-osteolytic THR compared to primary surgical controls, but no significant difference between loose osteolytic and loose non-osteolytic tissue levels [16]. Contrary to these studies, Stea, Visentin et al. reported that iNOS protein increased proportionally with the extent of osteolysis [17]. In their study, tissues were collected not only from metal on PE implants, some of which were cemented, but also ceramic on ceramic THRs. Of the three studies, only Stea, Visentin et al. specifically looked at the amounts of iNOS and NT in regards to the degree of osteolysis.
Based on the hypothesis that oxidative stress mediates wear particle-induced bone resorption and osteolysis, we focused on five specific osteoinflammatory and oxidative stress responsive factors. The first two potential serum diagnostic factors were HMGB1, a cytokine and alarmin released from macrophages, dendritic cells, and osteoblasts, which regulates RANKL-induced osteoclastogenesis [31], and 4-HNE, an oxidized lipid product that accumulates during age-related bone loss [29]. COX2, an ROS-producing enzyme, was included based on the ability of this enzyme to generate 4-HNE and a previous study showing it played a role in aseptic loosening [32]. iNOS and its RNS product NT were included based on their potential role in wear debris-mediated inflammation and osteolysis [17, 18]. The objectives of this study were to 1) quantify the levels of five oxidative stress markers, three that play a direct role in osteoclastogenesis or bone resorption (COX2, HMGB1, iNOS) and two products that are elevated in conjunction with the loss of bone mass (NT and 4-HNE), and 2) correlate the amounts of these markers with the presence of wear debris, inflammation and the degree of THR osteolysis. Looking at the levels of these markers based on the severity of osteolysis provides insight into a potential role for oxidative stress as a mediator of both the onset and progression of THR osteolysis, and that inhibitors of oxidative stress may slow wear debris-associated osteolysis.
Methods
Tissue Collection and Patient Clinical Information
Hip tissue specimens from regions adjacent to the implanted device were obtained from 18 THR patients at the time of revision surgery. All tissue specimens were collected by surgeons at the Rothman Institute, Thomas Jefferson University Hospital, Philadelphia, PA. All identifying information was removed, and the tissues were processed according to the IRB guidelines at Drexel University. Inclusion in this study was based on patient consent to participate and implant type. To standardize the type of bearing surface material, the patient had to have received a conventional, gamma-air sterilized THR polyethylene component. Exclusion criteria included revision for infection, a previous revision surgery, or a cemented implant. The patients were placed into four groups based on the degree of osteolysis (Table 1). Based on radiographic scoring the severe osteolysis (>2 mm) cohort included six patients (implantation time 11.5-19.8 yr; average 16.0 yr). The moderate osteolysis (< 2 mm) cohort included four patients (10.8-25.0 yr; 16.7 yr). The mild osteolysis cohort based on intraoperative observation and not visible radiographic osteolysis included seven patients (5.1-20.2 yr; 16.0 yr). Controls included two patients who received highly cross-linked, gamma-inert sterilized polyethylene components with neither radiographic osteolysis nor intraoperative osteolysis (2.9-5.2 yr, 4.1 yr).
Table 1.
Patient clinical information, wear particle number and inflammation score.
| Patient No. | Revision Reason | Radiographic/Intraoperative Osteolysis | Osteolysis Location | Implantation Time (y) | Age at Revision (y) | Sex | Wear Particle (#/mm2) | Inflam mation (0-3+) |
|---|---|---|---|---|---|---|---|---|
| Severe Osteolysis | ||||||||
| 1 | Osteolysis | Y/Y | Acetabular | 15.7 | 67 | M | 130 | 2 |
| 2 | Acetabular Loosening | Y/Y | Acetabular Zones II, III; Femoral Zones II, VI | 17.6 | 54 | M | 1 | 0.5 |
| 3 | Acetabular Loosening | Y/Y | Acetabular Zones I-III | 19.8 | 45 | M | 2 | 0.75 |
| 4 | Osteolysis (periacetabulum, proximal femur) | Y/Y | Peri-acetabulum & proximal femur | 15.7 | 76 | M | 5 | 1 |
| 5 | Acetabular Loosening, | Y/Y | Acetabular | 16.0 | 70 | M | 11 | 1.5 |
| Moderate Osteolysis | ||||||||
| 1 | Osteolysis | Y/Y | Femoral | 15.0 | 59 | M | 0 | 0.5 |
| 2 | Periprosthetic fracture (femur) | Y/Y | Proximal Femoral | 10.8 | 59 | M | 3 | 0.5 |
| 3 | Loosening | Y/Y | Femoral | 16.0 | 73 | F | 677 | 2.5 |
| 4 | Acetabular loosening, Subsidence | Y/Y | Acetabular Zones I-IV; Femoral Zones I, II, VI & VII | 25.0 | 77 | M | 38 | 1.5 |
| Mild Osteolysis | ||||||||
| 1 | Loosening | N/Y | Retro-acetabular & ischium | 20.2 | 75 | F | 18 | 2.3 |
| 2 | Instability | N/Y | Acetabular | 5.1 | 63 | F | 6 | 0 |
| 3 | Osteolysis | N/Y | Right Hip | 15.8 | 56 | M | 62 | 3 |
| 4 | Acetabular Loosening | N/Y | Acetabular | 18.5 | 69 | F | 3 | 0.5 |
| 5 | Wear and Lysis | N/Y | Acetabular Zone II | 18.0 | 46 | F | 2 | 1 |
| 6 | PE Wear | N/Y | Acetabular Zone III | 15.1 | 63 | M | 0 | 1 |
| 7 | Loosening and Lysis | N/Y | Acetabular | 19.2 | 72 | M | 9 | 2 |
| Group 1 - Control Group | ||||||||
| 1 | Loosening | N/N | N/A | 2.9 | 62 | F | 1 | 0 |
| 2 | Subluxation, impingement | N/N | N/A | 5.1 | 71 | F | 0 | 0 |
Radiograph Scores
To determine the extent and location of osteolysis, serial anteroposterior and lateral radiographs of the affected hip joint were scored by a qualified orthopaedic surgeon. Loosened components were defined as those that demonstrated a complete lucent line on any radiograph, femoral subsidence of >2 mm, or acetabular component migration or tilt, and osteolysis was defined by lucent areas adjacent to the implanted device in either the acetabular or femoral zone. The femur was divided into seven zones and the acetabulum into three zones to evaluate the location of lucent lines of osteolysis [33].
Histomorphology and Wear Debris Imaging and Analysis
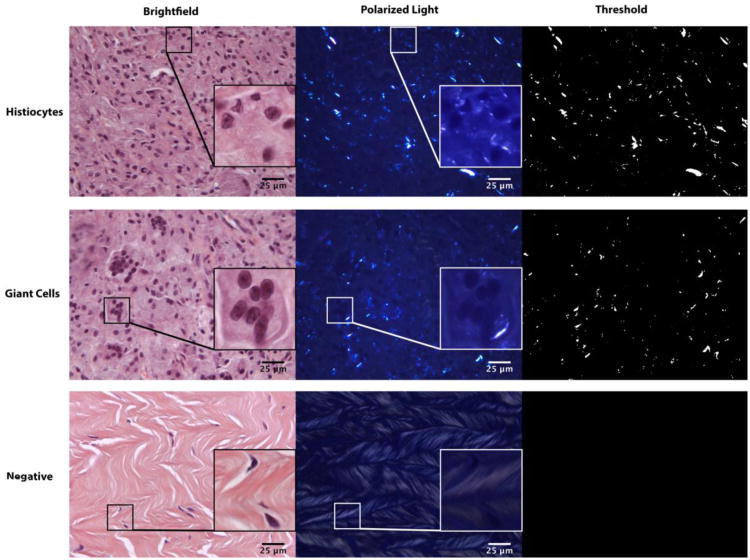
Retrieved tissues were fixed in Universal Tissue Fixative (Sakura Finetek USA, Inc.) and transferred to 70% ethanol 4 days after surgery. Representative regions were selected from each tissue, embedded in paraffin, and 6μm serial sections were mounted onto Fisher Superfrost/Plus slides and used for histological and immunohistochemical staining. Slides were dewaxed, rehydrated, and stained with hematoxylin and eosin (H&E) (ThermoFisher Scientific). Brightfield microscopic images were visually scored for the presence of inflammation (histiocytes and giant cells) [34]. Slides were imaged using an Olympus BX50 microscope (Olympus, Melville, NY), equipped with a stepper motor controlled stage, an elliptically polarized light imaging system, and a PixeLINK camera. A representative 9-image montage was created from each tissue section in brightfield and polarized light.
Polyethylene particle number, size, and shape were determined using a customized macro in NIH ImageJ on the polarized light images based on previous studies [34-36]. In brief, polarized light images were split into three 8-bit channels (red, green, and blue). Signals from the green and blue channels were summated, and the images were converted into masks based on a threshold value relative to the average signal intensity of each image. All images were visually reviewed to ensure that false positive signals from birefringent collagen did not contribute to particle analysis results. The resulting particle number was then converted to number per mm2 area of tissue using a measured conversion factor of 0.29 μm/pixel.
Immunohistochemistry, Imaging and Analysis
Immunohistochemistry was performed to evaluate the presence of osteolysis (HMGB1, Rabbit IgG, Abcam), reactive oxygen species (COX2, Mouse IgG1, Abcam), reactive nitrogen species (iNOS, Mouse IgG1, R&D Systems), and their oxidized products (4-HNE, Mouse IgG1, Percipio Biosciences; and NT, Mouse IgG3, R&D Systems). Optimal conditions for each antibody were determined using tissues retrieved from patients with arthrofibrosis. The antibody concentrations were: 4-HNE 1:50, COX2 1:100, HMGB1 1:200, iNOS 1:50, and NT 1:100. Before incubation at 4°C overnight with primary antibody, the slides were incubated in an antigen retrieval solution (Vector Labs), 0.5% Triton in PBS to enhance permeability, 3% H2O2 in methanol to block endogenous peroxidases, and finally to block non-specific background in 4% BSA, 0.1% Tween 20 in PBS. For antibody visualization, samples were incubated with pan-specific secondary antibody, followed by horseradish peroxidase (Santa Cruz Biotech) and DAB solution (Vector Labs), then counterstained with hematoxylin. Microscopic fields were acquired with a (10×) objective and stitched together to create a 9 image montage of each tissue. To determine the percentage of immune-positive stained area per total tissue area for each antibody, a custom macro was created in ImagePro Plus (Media Cybernetics Inc, Bethesda, MD) and applied to each tissue montage. All image analyses were performed by two observers using the same software macro to determine user variability. The results agreed within 95% of each other.
Statistical Analysis
The normality of the data was determined using the Shapiro-Wilk test (JMP, Cary, NC) and the differences in the amount of wear debris and inflammation for each osteolytic group using Kruskal-Wallis non-parametric test to determine statistical significance (p < 0.05). Correlations for the five immunohistological markers, the amount of wear debris and inflammation based on osteolytic severity and within each osteolytic group were determined using Spearman Rho correlation test for non-parametric data to determine statistical significance (p < 0.05).
Results
Tissue Inflammation and Wear Debris Correlate with the Degree of Osteolysis
Patient tissue samples were grouped based on degree of osteolysis, and wear particle accumulation and the chronic inflammatory response were determined for each patient (Table 1). Severe osteolysis contained an average of 30±25 particles per mm2 of tissue. Moderately osteolytic patient tissues contained an average of 180±166 particles/mm2, however two tissues from one patient contained 1946 and 664 particles/mm2 (without these tissues, the average was 22±12 particles/mm2). Low osteolytic patient tissues contained 14±8 particles/mm2, and non-osteolytic patient tissues contained 0.5±0.5 particles/mm2 of tissue. There was an increase in particle number for the three osteolysis groups compared to control (p<0.001). Both wear debris and chronic inflammation were present in all osteolytic patient tissues, but minimal in control tissues (p<0.0001). The correlation between wear debris and inflammation was significant when all groups were compared (ρ=0.76, p=0.0003), but neither parameter correlated with osteolysis (Table 2). Representative brightfield and polarized light images showing chronic inflammation and polyethylene (PE) wear particles, respectively are shown in figure 1.
Table 2.
Individual patient mean comparisons based on degree of osteolysis.
| Wear Debris | Inflammation | Osteolysis | COX-2 | 4 HNE | iNOS | NT | HMGB1 | |
|---|---|---|---|---|---|---|---|---|
| Wear Debris | 1 |
0.76
0.0003** |
0.25 0.32 |
0.22 0.37 |
0.49
0.04* |
0.14 0.58 |
0.56
0.02* |
0.61
0.01* |
| Inflammation | 1 | 0.24 0.33 |
0.22 0.38 |
0.34 0.17 |
0.21 0.40 |
0.53
0.02* |
0.53
0.02* |
|
| Osteolysis Score | 1 |
0.49
0.04* |
0.54
0.02* |
0.31 0.21 |
0.00 0.99 |
0.47
0.047* |
||
| COX-2 | 1 |
0.77
0.0002** |
0.46 0.05 |
0.45 0.06 |
0.55
0.02* |
|||
| 4 HNE | 1 |
0.48
0.04* |
0.52
0.03* |
0.62
0.006** |
||||
| iNOS | 1 |
0.55
0.02* |
0.61
0.01* |
|||||
| NT | 1 |
0.60
0.01* |
Spearman's Rho score and p value,
correlation is significant at the 0.05 level;
significant at the 0.01 level.
Figure 1. Areas of chronic inflammation and PE particle accumulation.
Brightfield images of representative H&E stained tissues with corresponding polarized light images showing PE particles and threshold images used for particle analysis. The white boxes and insets show areas of histiocyte, giant cell and PE wear particle accumulation. The negative control shows collagen before and after threshold.
Oxidative Stress Markers Increase with Degree of Osteolysis
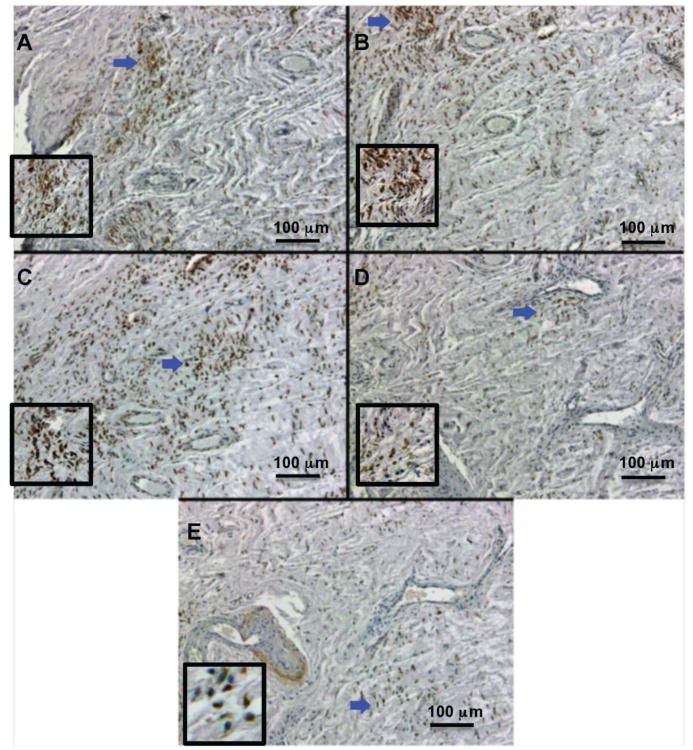
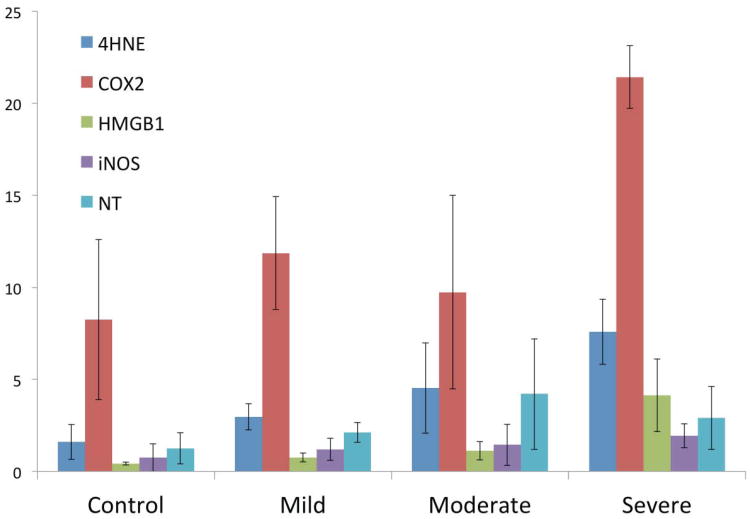
As ingestion of wear debris by macrophages activates NOX production of ROS, we hypothesized this would lead to the increased expression of COX-2 and iNOS; accompanied by additional ROS and RNS generation which may contribute to the severity of osteolysis. To test this hypothesis, five markers of oxidative stress and increased bone turnover were evaluated: an osteoclast activation protein (HMGB1), an ROS enzyme and product (COX2; 4-HNE), and an RNS enzyme and product (iNOS; NT). All five markers were present in revision tissues from patients with varying degress of osteolysis, and to a lesser extent in control tissues from patients without detectable osteolysis. Representative immunohistochemical images are shown in figure 2. The mean percentage of immunopositive area for all five markers is shown in figure 3. Despite the general increase in all five markers, there were no significant correlations between increasing osteolytic severity and group means.
Figure 2. Immunohistochemistry for osteolysis and oxidative stress markers.
Representative images of positive immunohistochemical staining within accumulated macrophages. A: 4-HNE accumulation, ROS product; B: COX2 expression, ROS enzyme; C: HMGB1 expression, osteolysis marker; D: iNOS expression, RNS enzyme; E: NT accumulation, RNS product. Blue arrows indicate inset location.
Figure 3. Positive area percentage means for each marker, grouped by osteolytic severity.
The mean correlations for each marker and the severity of osteolysis in decreasing order were 4HNE (p = 0.13), COX2 (p = 0.13) and HMGB1 (p = 0.27), iNOS (p = 0.62) and NT accumulation (p = 0.92). Error bars represent standard error of the mean.
Correlations between Wear Debris, Inflammation, Osteolysis, and Oxidative Stress Markers
In contrast to the group mean comparisons, when individual patient mean values were ranked using Spearman's Rho rank correlation coefficient, associations for all five oxidative stress markers, inflammation, wear debris, and osteolysis were observed (Table 2). We observed a strong correlation between wear debris and inflammation, but neither correlated with the degree of osteolysis. The amount of wear debris did however correlate with the presence of 4-HNE (ρ=0.49, p=0.04), NT (ρ=0.56, p=0.02), and HMGB1 (ρ=0.61, p=0.01), whereas inflammation correlated with only NT (ρ=0.53, p=0.02) and HMGB1 (ρ=0.53, p=0.02). Similar to wear debris and inflammation, the degree of osteolysis showed a correlation with HMGB1 (ρ=0.47, p=0.047). In addition, osteolysis correlated with the amounts of COX2 (ρ=0.49, p=0.04) and 4-HNE (ρ=0.54, p=0.02), but not iNOS or NT.
Correlations between Oxidative Enzyme Expression and their Products
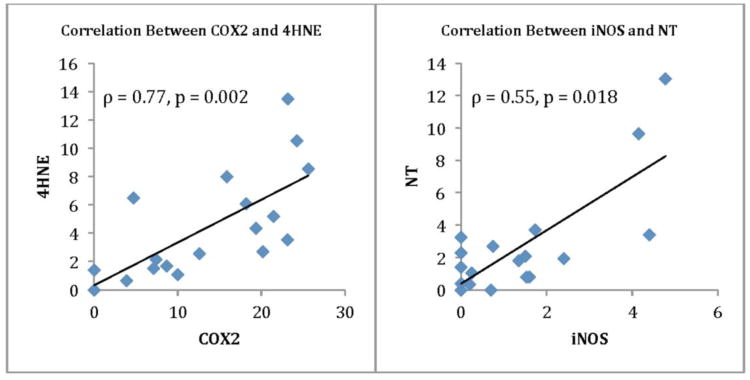
To determine whether COX2 and iNOS were activated, the amount of accumulated 4-HNE and NT were compared to the expression levels of these enzymes (Fig. 4). There was a strong correlation between COX2 expression and 4-HNE accumulation (ρ=0.77, p= 0.0002), and a correlation for the amounts of NT and the expression of iNOS (ρ=0.55, p=0.02) (Table 2).
Figure 4.
Spearman Rho correlations are provided for the ROS enzyme COX-2 and its oxidized product 4HNE, and the RNS enzyme iNOS and its oxidized product NT.
Correlations between Oxidative Stress Markers
In addition to correlations between the oxidative enzymes and their products, we looked for correlations amongst all five markers. HMGB1 was the only marker that correlated with increased amounts of the other 4 markers, iNOS (ρ=0.61, p=0.01), NT (ρ=0.60, p=0.01), 4-HNE (ρ=0.62, p=0.006), and COX2 (ρ=0.55, p=0.02) (Table 2). The only other observed correlations were for 4-HNE with iNOS expression (ρ=0.48, p= 0.04) and NT accumulation (ρ=0.52, p=0.03).
These comparisons highlight that all five markers can be detected and that the amounts increase, but the proportional increases or correlations for the five markers differ resulting in only three of the markers showing an association with osteolysis.
Discussion
Numerous studies have focused on pro-inflammatory mediators as potential biomarkers of osteolysis, but these studies have met with limited success in establishing specific osteolytic markers [10, 13, 14, 37]. Two promising serum markers that have been correlated with the degree of osteolysis are OPG, which may be up-regulated by cells within the periprosthetic tissue to prevent bone loss, and tartrate-resistant acid phosphatase 5b (TRAP 5b), a marker of osteoclast activity [37, 38]. In the current study, we looked for additional prospective serum markers based on the potential involvement of oxidative stress in the development of post-THR osteolysis in response to PE wear debris-induced inflammation. We looked for the presence of five markers of oxidative stress, which included a ROS and a RNS generating enzyme, ROS and RNS products and HMGB1, an oxidative stress responsive and pro-osteoclastic inflammatory factor [31].
All five markers were present in increased amounts in patient tissues revised for osteolysis as compared to non-osteolytic revisions, and the amounts increased based on the severity of osteolysis. Their presence was localized to regions with wear debris and chronic inflammation, and correlations were observed for wear debris with NT, 4-HNE, and HMGB1 and inflammation with NT and HMGB1. The lack of correlation for wear debris or inflammation with either COX2 or iNOS, despite the strong correlations between the enzymes and their products, suggests other cells such as fibroblasts may be expressing these enzymes and producing 4-HNE and NT in response to wear debris [39]. However, the formation of both ROS and RNS products confirms COX2 and iNOS were activated, and based on the correlation of COX2 and 4-HNE with osteolysis, that oxidative stress is involved in the development of aseptic osteolysis. In addition, the correlation between osteolysis, as well as the other 4 factors, with the oxidative stress responsive and pro-osteoclast factor HMGB1 further supports a relationship between oxidative stress and aseptic osteolysis.
There are several limitations in the current study. First, the lack of statistical difference for group means was due to the large standard errors, owing to individual patient variation in both wear debris generation and the inflammatory response. For this reason, we looked for relationships based on individual patient means for each parameter. Second, the sample sizes for each group were small. However, when all 18 individual patient means were analyzed we observed correlations for wear debris, inflammation, the oxidative stress markers, and osteolysis. Third, the availability of revision tissue for the control group was very limited but represented a group that had undergone THR. Others have shown that primary hip tissues from patients with osteoarthritis or non-osteolytic controls express inflammatory cytokines and iNOS, albeit at lower levels [16, 18]. Thus, analysis of serum to detect these osteolytic markers should not be a concern as the levels will be limited for non-osteolytic patients and patients that have recently undergone THR. Furthermore, establishing a baseline level for each patient after THR will provide the necessary comparison to detect osteolysis.
In a prior study, the production of ROS in THR tissues from patients revised for aseptic loosening was proposed to be involved in the formation of the fibrous pseudocapsule based on the presence of oxidized glutathione and malondialdehyde [27]. XXX and others have also shown that oxidative stress participates in the development of fibrosis associated with TKA [28], and that ROS are required for normal bone remodeling [20-25, 30, 40, 41]. Thus, based on previous studies and the current study, we propose that ROS may play a dual role in the osteolytic process, initiation of osteoclast mediated bone resorption and fibrotic tissue formation.
Three previous studies looked at the role of RNS in THR revision tissues, and found conflicting results [16-18]. Stea and colleagues reported that the amount of iNOS was directly proportional to the extent of osteolysis, and highly expressed in severely osteolytic tissues from non-cemented THRs [17]. However, the other two groups found increased iNOS expression and NT accumulation were associated but not directly correlated with the development of osteolysis [16, 18]. We also observed a proportional increase in iNOS and NT with increasing severity of osteolysis in non-cemented THRs, but did not observe a direct correlation of either factor with osteolysis. The increase in iNOS and NT may represent a negative feedback to limit osteoclastogenesis, similar to the observed increase in OPG [38], as increased NO inhibits osteoclast activity [42].
The increased presence of COX2 in revision tissues is in agreement with a previous study, implicating COX2 and its product PGE2 as possible mediators of early prosthesis failure [32]. Similarly, we observed a correlation between osteolysis and the expression of COX2, and in our study the accumulation of 4-HNE. 4-HNE is a biomarker of pathophysiological processes that are associated with age-related loss of bone mass, but has not been evaluated in revision tissues [29].
Finally, HMGB1 was evaluated, as it is an oxidative stress responsive protein required for RANKL-induced osteoclastogenesis [31]. The increase in HMGB1 was the only factor that correlated with increased amounts of the other four markers, inflammation, wear debris, and osteolysis. The increase in HMGB1 was directly proportional to the degree of osteolytic severity, suggesting that serum levels of this protein may be a sensitive marker for the onset and progression of osteolysis.
One of the most significant outcomes of this study was finding a connection between oxidative stress and the development of osteolysis in THR patients. In support of this statement, we observed increased expression of two oxidative stress responsive enzymes, COX2 (ROS) and iNOS (RNS), and a corresponding accumulation of their products. We also observed an increase in HMGB1, an oxidative stress-responsive osteoclast differentiation factor, in all patients with osteolysis. Furthermore, the correlation between osteolysis and three major oxidative factors, COX2, 4-HNE and HMGB1, provides insight into the involvement of oxidative stress in the progression of osteolysis. In summary, monitoring changes in the serum levels of these proteins at various time points after THR in conjunction with OPG and TRAP 5b provides a highly sensitive means of detecting osteolysis [37, 38]. Moreover, demonstrating the involvement of the oxidative stress and HMGB1 pathways provides potential targets for the development of therapeutic interventions.
Supplementary Material
Acknowledgments
This work was supported by a grant from the NIAMS (NIH R01 AR47904). We also thank Madhuri Penmatsa, Christine Ho, and Eual Phillips for their help in performing the image analyses for this paper, and Dr. XXXXXX for performing the statistical analysis of the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bozic KJ, Kurtz SM, Lau E, Ong K, Chiu V, Vail TP, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468:45–51. doi: 10.1007/s11999-009-0945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Purdue PE, Koulouvaris P, Potter HG, Nestor BJ, Sculco TP. The cellular and molecular biology of periprosthetic osteolysis. Clin Orthop Relat Res. 2007;454:251–261. doi: 10.1097/01.blo.0000238813.95035.1b. [DOI] [PubMed] [Google Scholar]
- 3.Goodman SB. Wear particles, periprosthetic osteolysis and the immune system. Biomaterials. 2007;28:5044–5048. doi: 10.1016/j.biomaterials.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konttinen YT, Zhao D, Beklen A, Ma G, Takagi M, Kivela-Rajamaki M, et al. The microenvironment around total hip replacement prostheses. Clin Orthop Relat Res. 2005:28–38. doi: 10.1097/01.blo.0000150451.50452.da. [DOI] [PubMed] [Google Scholar]
- 5.Revell PA. The combined role of wear particles, macrophages and lymphocytes in the loosening of total joint prostheses. J R Soc Interface. 2008;5:1263–1278. doi: 10.1098/rsif.2008.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldring SR, Clark CR, Wright TM. The problem in total joint arthroplasty: aseptic loosening. J Bone Joint Surg Am. 1993;75:799–801. doi: 10.2106/00004623-199306000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Sabokbar A, Fujikawa Y, Neale S, Murrary D, Athanasou N. Human arthroplasty derived macrophages differentiate into osteoclastic bone resorbing cells. Annals of Rheumatism Disease. 1997;56:414–420. doi: 10.1136/ard.56.7.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atkins GJ, Welldon KJ, Holding CA, Haynes DR, Howie DW, Findlay DM. The induction of a catabolic phenotype in human primary osteoblasts and osteocytes by polyethylene particles. Biomaterials. 2009;30:3672–3681. doi: 10.1016/j.biomaterials.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 9.Goodman SB, Ma T, Chiu R, Ramachandran R, Smith RL. Effects of orthopaedic wear particles on osteoprogenitor cells. Biomaterials. 2006;27:6096–6101. doi: 10.1016/j.biomaterials.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 10.Granchi D, Amato I, Battistelli L, Ciapette G, Pagani S, Avnet S, et al. Molecular basis of osteoclastogenesis induced by osteoblasts exposed to wear particles. Biomaterials. 2005;26:9. doi: 10.1016/j.biomaterials.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 11.Sakai H, Jingushi S, Shuto T, Urabe K, Ikenoue T, Okazaki K, et al. Fibroblasts from the inner granulation tissue of the pseudocapsule in hips at revision arthroplasty induce osteoclast differentiation, as do stromal cells. Annals of the Rheumatic Diseases. 2002;61:103–109. doi: 10.1136/ard.61.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabokbar A, Itonaga I, Sun SG, Kudo O, Athanasou NA. Arthroplasty membrane-derived fibroblasts directly induce osteoclast formation and osteolysis in aseptic loosening. Journal of Orthopaedic Research. 2005;23:511–519. doi: 10.1016/j.orthres.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Koulouvaris P, Ly K, Ivashkiv LB, Bostrom MP, Nestor BJ, Sculco TP, et al. Expression profiling reveals alternative macrophage activation and impaired osteogenesis in periprosthetic osteolysis. J Orthop Res. 2008;26:106–116. doi: 10.1002/jor.20486. [DOI] [PubMed] [Google Scholar]
- 14.Shanbhag AS, Kaufman AM, Hayata K, Rubash HE. Assessing osteolysis with use of high-throughput protein chips. J Bone Joint Surg Am. 2007;89:1081–1089. doi: 10.2106/JBJS.F.00330. [DOI] [PubMed] [Google Scholar]
- 15.Wang ML, Hauschka PV, Tuan RS, Steinbeck MJ. Exposure to particles stimulates superoxide production by human THP-1 macrophages and avian HD-11EM osteoclasts activated by tumor necrosis factor-alpha and PMA. J Arthroplasty. 2002;17:335–346. doi: 10.1054/arth.2002.30416. [DOI] [PubMed] [Google Scholar]
- 16.Puskas BL, Menke NE, Huie P, Song Y, Ecklund K, Trindade MC, et al. Expression of nitric oxide, peroxynitrite, and apoptosis in loose total hip replacements. J Biomed Mater Res A. 2003;66:541–549. doi: 10.1002/jbm.a.10010. [DOI] [PubMed] [Google Scholar]
- 17.Stea S, Visentin M, Donati ME, Granchi D, Ciapetti G, Sudanese A, et al. Nitric oxide synthase in tissues around failed hip prostheses. Biomaterials. 2002;23:4833–4838. doi: 10.1016/s0142-9612(02)00236-3. [DOI] [PubMed] [Google Scholar]
- 18.Suh KT, Chang JW, Jung JS. The role of inducible nitric oxide synthase in aseptic loosening after total hip arthroplasty. J Bone Joint Surg Br. 2002;84:753–757. doi: 10.1302/0301-620x.84b5.12314. [DOI] [PubMed] [Google Scholar]
- 19.Gehrke T, Sers C, Morawietz L, Fernahl G, Neidel J, Frommelt L, et al. Receptor activator of nuclear factor kappaB ligand is expressed in resident and inflammatory cells in aseptic and septic prosthesis loosening. Scandinavian Journal of Rheumatology. 2003;32:287–294. doi: 10.1080/03009740310003929. [DOI] [PubMed] [Google Scholar]
- 20.Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS, et al. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood. 2005;106:852–859. doi: 10.1182/blood-2004-09-3662. [DOI] [PubMed] [Google Scholar]
- 21.Wauquier F, Leotoing L, Coxam V, Guicheux J, Wittrant Y. Oxidative stress in bone remodelling and disease. Trends Mol Med. 2009;15:468–477. doi: 10.1016/j.molmed.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Steinbeck MJ, Appel WH, Jr, Verhoeven AJ, Karnovsky MJ. NADPH-oxidase expression and in situ production of superoxide by osteoclasts actively resorbing bone. Journal of Cell Biology. 1994;126:765–772. doi: 10.1083/jcb.126.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinbeck MJ, Kim JK, Trudeau MJ, Hauschka PV, Karnovsky MJ. Involvement of hydrogen peroxide in the differentiation of clonal HD-11EM cells into osteoclast-like cells. Journal of Cellular Physiology. 1998;176:574–587. doi: 10.1002/(SICI)1097-4652(199809)176:3<574::AID-JCP14>3.0.CO;2-#. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. Journal of Clinical Investigation. 1990;85:632–639. doi: 10.1172/JCI114485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ha H, Kwak HB, Lee SW, Jin HM, Kim HM, Kim HH, et al. Reactive oxygen species mediate RANK signaling in osteoclasts. Experimental Cell Research. 2004;301:119–127. doi: 10.1016/j.yexcr.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 26.Suda N, Morita I, Kuroda T, Murota S. Participation of oxidative stress in the process of osteoclast differentiation. Biochimica et Biophysica Acta. 1993;1157:318–323. doi: 10.1016/0304-4165(93)90116-p. [DOI] [PubMed] [Google Scholar]
- 27.Kinov P, Leithner A, Radl R, Bodo K, Khoschsorur GA, Schauenstein K, et al. Role of free radicals in aseptic loosening of hip arthroplasty. J Orthop Res. 2006;24:55–62. doi: 10.1002/jor.20013. [DOI] [PubMed] [Google Scholar]
- 28.Freeman TA, Parvizi J, Della Valle CJ, Steinbeck MJ. Reactive oxygen and nitrogen species induce protein and DNA modifications driving arthrofibrosis following total knee arthroplasty. Fibrogenesis Tissue Repair. 2009;2:5. doi: 10.1186/1755-1536-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almeida M, Ambrogini E, Han L, Manolagas SC, Jilka RL. Increased lipid oxidation causes oxidative stress, increased peroxisome proliferator-activated receptor-gamma expression, and diminished pro-osteogenic Wnt signaling in the skeleton. J Biol Chem. 2009;284:27438–27448. doi: 10.1074/jbc.M109.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darden AG, Ries WL, Wolf WC, Rodriguiz RM, Key LL., Jr Osteoclastic superoxide production and bone resorption: stimulation and inhibition by modulators of NADPH oxidase. Journal of Bone & Mineral Research. 1996;11:671–675. doi: 10.1002/jbmr.5650110515. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Z, Han JY, Xi CX, Xie JX, Feng X, Wang CY, et al. HMGB1 regulates RANKL-induced osteoclastogenesis in a manner dependent on RAGE. J Bone Miner Res. 2008;23:1084–1096. doi: 10.1359/JBMR.080234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hukkanen M, Corbett SA, Batten J, Konttinen YT, McCarthy ID, Maclouf J, et al. Aseptic loosening of total hip replacement. Macrophage expression of inducible nitric oxide synthase and cyclo-oxygenase-2, together with peroxynitrite formation, as a possible mechanism for early prosthesis failure. J Bone Joint Surg Br. 1997;79:467–474. doi: 10.1302/0301-620x.79b3.7469. [DOI] [PubMed] [Google Scholar]
- 33.Gruen TA, McNeice GM, Amstutz HC. “Modes of failure” of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979:17–27. [PubMed] [Google Scholar]
- 34.Baxter RM, Ianuzzi A, Freeman TA, Kurtz SM, Steinbeck MJ. Distinct immunohistomorphologic changes in periprosthetic hip tissues from historical and highly crosslinked UHMWPE implant retrievals. J Biomed Mater Res A. 2010;95:68–78. doi: 10.1002/jbm.a.32813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baxter RM, Freeman TA, Kurtz SM, Steinbeck MJ. Do tissues from THA revision of highly crosslinked UHMWPE liners contain wear debris and associated inflammation? Clin Orthop Relat Res. 2011;469:2308–2317. doi: 10.1007/s11999-010-1713-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baxter RM, MacDonald DW, Kurtz SM, Steinbeck MJ. Characteristics of highly cross-linked polyethylene wear debris in vivo. J Biomed Mater Res B Appl Biomater. 2013;101:467–475. doi: 10.1002/jbm.b.32902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landgraeber S, Loer F, Heep H, Classen T, Grabellus F, Totsch M, et al. Tartrate-resistant acid phosphatase 5b and C-terminal telopeptides of type I collagen as markers for diagnosis of aseptic loosening after total hip replacement. Arch Orthop Trauma Surg. 2010;130:441–445. doi: 10.1007/s00402-009-0905-x. [DOI] [PubMed] [Google Scholar]
- 38.Granchi D, Pellacani A, Spina M, Cenni E, Savarino LM, Baldini N, et al. Serum levels of osteoprotegerin and receptor activator of nuclear factor-kappaB ligand as markers of periprosthetic osteolysis. Journal of Bone & Joint Surgery - American Volume. 2006;88:1501–1509. doi: 10.2106/JBJS.E.01038. [DOI] [PubMed] [Google Scholar]
- 39.Koreny T, Tunyogi-Csapó M, Gál I, Vermes C, Jacobs J, Glant T. The Role of Fibroblasts and Fibroblast-derived Factors in Periprosthetic Osteolysis. Arthritis and Rheumatism. 2006;54:3221–3232. doi: 10.1002/art.22134. [DOI] [PubMed] [Google Scholar]
- 40.Key LL, Jr, Ries WL, Taylor RG, Hays BD, Pitzer BL. Oxygen derived free radicals in osteoclasts: the specificity and location of the nitroblue tetrazolium reaction. Bone. 1990;11:115–119. doi: 10.1016/8756-3282(90)90058-7. [DOI] [PubMed] [Google Scholar]
- 41.Suda N. Kokubyo Gakkai Zasshi - the Journal of the Stomatological Society. Vol. 58. Japan: 1991. Role of free radicals in bone resorption; pp. 603–612. [DOI] [PubMed] [Google Scholar]
- 42.Brandi ML, Hukkanen M, Umeda T, Moradi-Bidhendi N, Bianchi S, Gross SS, et al. Bidirectional regulation of osteoclast function by nitric oxide synthase isoforms. Proc Natl Acad Sci U S A. 1995;92:2954–2958. doi: 10.1073/pnas.92.7.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.