Abstract
Objective
Under physiological conditions brain natriuretic peptide (BNP) is inversely associated with metabolic risk factors, but under pathological conditions these associations may tend to plateau.
Material end methods
5597 individuals in the Multi-Ethnic Study of Atherosclerosis (MESA), 45–84 years of age, free of overt cardiovascular disease in 2000–02 and then again in 2003–05 participated in this study. Associations between NT-proBNP and BMI, blood lipids, homeostasis model of insulin resistance (HOMA-IR) using linear regression models were adjusted for age, race, sex, BMI, % of energy from saturated fats, intentional exercise, statin use, antihypertensive medication use, diabetes and glomerular filtration rate. The inflection points (IP) at which these associations became nonlinear were determined using linear splines with knots at different levels of NT-proBNP.
Results
Participants with NT-proBNP ≥100 pg/mL (29%) tended to be older, on statins and anti-hypertensive medications vs. those with NT-proBNP <100 pg/mL. The IP point varies among variables and ranged from 50–120 pg/mL. NT-proBNP <IP, associated inversely with BMI, total cholesterol (TC), LDL-C, triglycerides (TG) and HOMA-IR, but positively with HDL-C. A higher proportion of participants with NT-proBNP ≥100 pg/mL had subclinical CVD. All associations with NT-proBNP plateaued when NT-proBNP ≥IP. Baseline level in NT-proBNP was not associated with 3-year change in BMI, TG, HDL-C or fasting glucose.
Conclusions
In a large cardiovascular disease-free cohort, NT-proBNP within the lower (physiological) range was inversely associated with TC, LDL-C, TG and insulin resistance with different inflection points, but at higher (pathological) levels these associations were blunted.
Keywords: NT-proBNP, lipids, inflammatory markers, HOMA, BMI
Introduction
Elevated levels of the amino-terminal-probrain natriuretic peptide (NT-proBNP) and BNP are well-known for increasing the risk of morbidity and mortality from cardiovascular diseases (CVD) [1, 2]. Paradoxically, low levels of NT-proBNP are more frequent in obese, those with elevated triglyceride levels [3, 4] and NT-proBNP is predictive of type 2 diabetes [5]. All of these variables are important risk factors in the development of CVD. Thus, NT-proBNP concentrations appear to have pathological implications at both low and high values.
In the absence of pathological influences, blood levels of NT-proBNP fluctuate in response to physiological variations in blood volume and pressure load in the heart [6] in an age and gender dependent manner [7, 8]. Under this condition, BMI, blood lipids and insulin resistance (IR) have been shown to have an inverse association with NT-proBNP [3]. However, the presence of cardiovascular and inflammatory pathologies can substantially increase NT-proBNP [9, 10] and induce a state of hypo-responsiveness to natriuretic peptides [11]. A state of hypo-responsiveness to natriuretic peptides would make it possible that the inverse association between NT-proBNP and BMI, blood lipids and IR seen under physiologic conditions would be lost when pathologic influences predominate. Whether this supposition is true is currently not known.
Previous reports on the association between NT-proBNP and BMI, blood lipids and fasting glucose have been obtained from cross sectional studies. Unfortunately, longitudinal studies which would lend further support for a cause and effect relationship have not been reported. The Multi-Ethnic Study on Atherosclerosis (MESA) offers the opportunity to assess changes in BMI, blood lipids and fasting glucose as a function of baseline and change in NT-proBNP.
Therefore, we hypothesized that cross-sectionally in asymptomatic adults free of overt cardiovascular disease the inverse association between NT-proBNP and BMI, blood lipids and insulin resistance plateau at the higher levels of NT-proBNP. To study this hypothesis, we used linear spline models to determine the inflection point at which the linear association between NT-proBNP with BMI, blood lipids and insulin resistance is lost. In addition, we hypothesized that baseline NT-proBNP predicts the direction of change in BMI and TC, LDL-C, triglycerides (TG) and fasting glucose and that change in NT-proBNP will be associated with change in BMI, blood lipids and blood glucose.
Methods
Study Subjects
We studied participants in the Multi-Ethnic Study of Atherosclerosis (MESA) recruited in 2000–2002 and during their third visit in 2003–2005. They were initially free of self-reported overt cardiovascular disease and renal failure. Included here were those in whom NT-proBNP were assayed at baseline, n = 5597 of the 6814 total participants in MESA and n = 4694 during the third visit. Details of study recruitment and design have been previously published [12].
Blood measurements
Blood lipids, insulin, and glucose were measured in blood samples following a 12 hour fast and sent to (Collaborative Studies Clinical Laboratory at Fairview University Medical Center, Minneapolis, Minnesota). Serum glucose and insulin were measured by the Vitros analyzer (Johnson & Johnson Clinical Diagnostics, Rochester, New York) and by a radioimmunoassay method using the Linco Human Insulin Specific RIA kit (Linco Research, St. Charles, MO), respectively. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated according to [13]. TG, total cholesterol (TC), and high density lipoprotein cholesterol (HDL-C) concentrations were measured using the cholesterol oxidase method (Roche Diagnostics, Indianapolis, IN) and low density lipoprotein cholesterol (LDL-C) was estimated according to [14]. NT-proBNP was measured at the VA San Diego Health Care System, using an ElecSys 2010 analyzer (Roche Diagnostics, Indianapolis, IN) with intra-assay and interassay coefficients of variation of 1.3% and 4.8%, respectively [15]. Serum creatinine levels were used to estimate glomerular filtration rate (eGFR) according to Chronic Kidney Disease-EPI (CKD Epidemiology Collaboration) equations [16]. IL-6 concentrations were measured by ultrasensitive enzyme-linked immunosorbent assay (Quantikine HS human IL-6 immunoassay; R&D Systems, Minneapolis, MN). NT-proBNP, height, weight, and blood lipids were remeasured at visit 3, but IL-6 and insulin were not measured then.
Covariates
All study covariates in the cross-sectional analyses were obtained from the MESA baseline clinical examination and assessed using standard protocols, as previously reported [17]. Common carotid intima media thickness (cIMT) was assessed at the common carotid artery as described previously [18]. Coronary artery calcium (CAC) was determined with electron beam or helical computed tomography (CT) as described in [19]. Left ventricular dimensions were determined using cardiac MR images according to [20]. Presence of plaque was defined by a >25% diameter narrowing in the common carotid artery [21]. Antihypertensive medication use was based on names of reported medication use/pill bottle examination. Left ventricular hypertrophy (LVH) was defined as a left ventricular mass in mg corrected by height in cm above the 95th percentiles by gender; 52 and 49 g/cm for males and females, respectively. Metabolic syndrome was defined according to the National Cholesterol Education Program III guidelines [22] and presence of diabetes was based on fasting glucose ≥126 mg/dL or the use of insulin or oral hypoglycemic medications. NT-proBNP, BMI and blood lipids levels at the participant’s third visit at 3.2 years were used in longitudinal analyses.
Statistical analysis
Continuous variables with normal distributions are presented as mean ± SD (or median) and categorical variables as frequencies and percentages. Because we observed a linear association between NT-proBNP with BMI, blood lipids, fasting glucose and insulin resistance, but only across low values of NT-proBNP (see Supplementary Table 1), we used Chi square and t-tests to compare proportion and mean values of categorical and continuous variables between 2 categories of NT-proBNP: < vs ≥100 pg/mL. The value 100 pg/mL was chosen for this comparison as an approximation to the point at which most slopes changed.
We used linear regression to assess the levels of BMI, lipoprotein cholesterol concentrations, TG, fasting glucose, inflammatory markers and HOMA-IR as dependent variables across deciles of baseline values of NT-proBNP as the independent variable, adjusting gender, age, race, body mass index, % of calories from saturated fats, total intentional exercise (MET-min/week), statin use, antihypertensive medication use, presence of diabetes and estimated glomerular filtration rate. Next, we assessed more precisely the NT-proBNP value at which the slopes the dependent variables had a substantial change in slope i.e. the inflection point (IP) was determined using linear splines adjusted for age, race and gender as explained below. Linear splines were performed by fitting the data to the following regression equation: dependent variables = a + b<ip(NT-proBNP − IP) + b>IP (NT-proBNP − IP)INT-proBNP > IP, where a = intercept, IP = inflection point, b<ip = linear coefficient at values below the IP and b>ip = change in the linear coefficient at values above the IP and INT-proBNP = 1 if NT-proBNP ≥ IP and 0 otherwise (covariates and error term are suppressed). Knots for the linear splines were chosen at NT-proBNP values every 5 pg/mL intervals between 20 – 300 pg/mL. The NT-proBNP value at which the linear spline offered the best fit assessed with the highest R2 was chosen as the IP for each of the dependent variables.
Linear regression analysis was used to examine the associations between baseline NT-proBNP values with change (visit 3 years later – baseline) in BMI, blood lipids and fasting blood glucose after adjusting for the variables listed above and for baseline NT-proBNP. Significance was set at p < 0.05. Statistical analysis was performed using SAS v 9.3 by SAS Institute Inc., Cary, NC.
Results
Demographic characteristics of the MESA sample at baseline
Table 1 shows the demographic characteristics of participants as a whole group and also divided by category of NT-proBNP < or ≥100 pg/mL. About two thirds of the population had NT-proBNP values <100 pg/mL. Compared to the lower NT-proBNP category, the proportion of females was 45% higher than that of the males and whites were over-represented, while blacks and Chinese were underrepresented within the higher NT-proBNP category. Participants in the higher NT-proBNP category were on average 9 years older, had lower BMI, diastolic blood pressure, as well as lower LDL-C, fasting glucose and HOMA-IR, but higher HDL-C. Estimated GFR was lower in participants at the highest category of NT-proBNP, but the values were well above the threshold for kidney failure. However, despite the tendency for BMI values, blood lipids and insulin resistance in the higher category of NT-proBNP that were healthier from the cardiovascular perspective, the proportion of participants with subclinical CVD, metabolic syndrome, on antihypertensive medications and statins were greater in the higher category of NT-proBNP. There was no significant difference in the proportion of diabetes, while the proportion of current smokers was lower within the higher NT-proBNP category. TC and percent calories from saturated fats were similar in both groups, but reported intentional exercise was higher in participants within the lower category of NT-proBNP.
Table 1.
Demographic characteristics of subjects by levels of NT-proBNP < or ≥ 100 pg/mL, MESA baseline.
| NT-proBNP pg/mL | |||||
|---|---|---|---|---|---|
| Variable | Total (n=5597) | < 100 (n=3988) | ≥100 (n=1609) | ||
| NT-proBNP, geometric mean (range) |
50.7 (4.9 – 11699) | 29.3 (4.9 – 99.98) | 196.8 (100.1 – 11699) | p value | |
| Gender | <0.0001 | ||||
| Females, % | 51.5 | 46 | 67 | ||
| Race | <0.0001 | ||||
| White, % | 39.6 | 36 | 50 | ||
| Chinese, % | 13.3 | 15 | 10 | ||
| Black, % | 24.3 | 26 | 19 | ||
| Hispanic, % | 22.9 | 23 | 21 | ||
| Diabetes, % | 12.5 | 12 | 13 | 0.26 | |
| Statin use, % | 14.8 | 14 | 17 | 0.007 | |
| HTN meds, % | 36.7 | 31 | 50 | <0.0001 | |
| Metabolic syndrome, % | 36.8 | 35.9 | 39.0 | 0.03 | |
| LVH, % | 5.4 | 3.8 | 9.7 | <0.0001 | |
| cIMT > 1.3 mm, % | 25.3 | 20.5 | 37.4 | <0.0001 | |
| Presence of plaque, % | 41.3 | 36.1 | 54.1 | <0.0001 | |
| Age, years | 62.9 (10.3) | 60.4 (0.16) | 69.0 (0.24) | <0.0001 | |
| BMI, kg/m2 | 28.2 (5.5) | 28.4 (0.09) | 27.7 (0.14) | <0.0001 | |
| Total cholesterol, mg/dL | 194.3 (36) | 194.6 (0.6) | 193.5 (0.9) | 0.6 | |
| LDL-C, mg/dL | 117 (31.5) | 118.4 (0.5) | 113.5 (0.8) | <0.0001 | |
| HDL-C, mg/dL | 50.8 (15) | 49.5 (0.2) | 54.9 (0.4) | <0.0001 | |
| Triglycerides, mg/dL | 133.7 (90.3) | 136.6 (1.5) | 126.5 (1.8) | <0.0001 | |
| Glucose, mg/dL | 97.5 (30.5) | 97.6 (0.5) | 95.6 (0.7) | <0.0001 | |
| HOMA-IR | 2.7 (7.0) | 2.8 (0.12) | 2.4 (0.15) | 0.04 | |
| IL-6, pg/mL | 1.56 (1.23) | 1.44 (0.02) | 1.82 (0.04) | <0.0001 | |
| SBP, mmHg | 132 (18.4) | 122.9 (0.31) | 134.7 (0.64) | <0.0001 | |
| DBP, mmHg | 71.8 (18.4) | 72.3 (0.16) | 70.2 (0.28) | <0.0001 | |
| CAC, Agatston units | 150.7 (424.4) | 114 (5.3) | 241.9 (14.4) | <0.0001 | |
| eGFR, ml/min | 77.9 (16.4) | 83.2 (0.3) | 75.0 (0.5) | <0.0001 | |
| % calories from sat. fats | 10.1 (3.2) | 10.1 (0.05) | 10.1 (0.09) | 0.08 | |
| Exercise MET-min /week | 1548.3 (2276.9) | 1619.5 (39) | 1400.5 (50) | <0.0001 | |
% indicates percentage of individuals within each category of baseline NT-proBNP. Statin use = participants on statins, HTN meds = participants on anti-hypertensive medications, LVH = left ventricular hypertrophy, BMI = body mass index, SBP = systolic blood pressure, DBP = diastolic blood pressure, cIMT = common carotid intima media thickness, CAC = coronary artery calcium, exercise = Total intentional exercise in MET-min/week. Values represent unadjusted means (SE). For total population column values are mean (SD). p value indicates difference between categories of NT-proBNP < or ≥100 pg/mL.
Cross-sectional associations of metabolic parameters with NT-proBNP at baseline
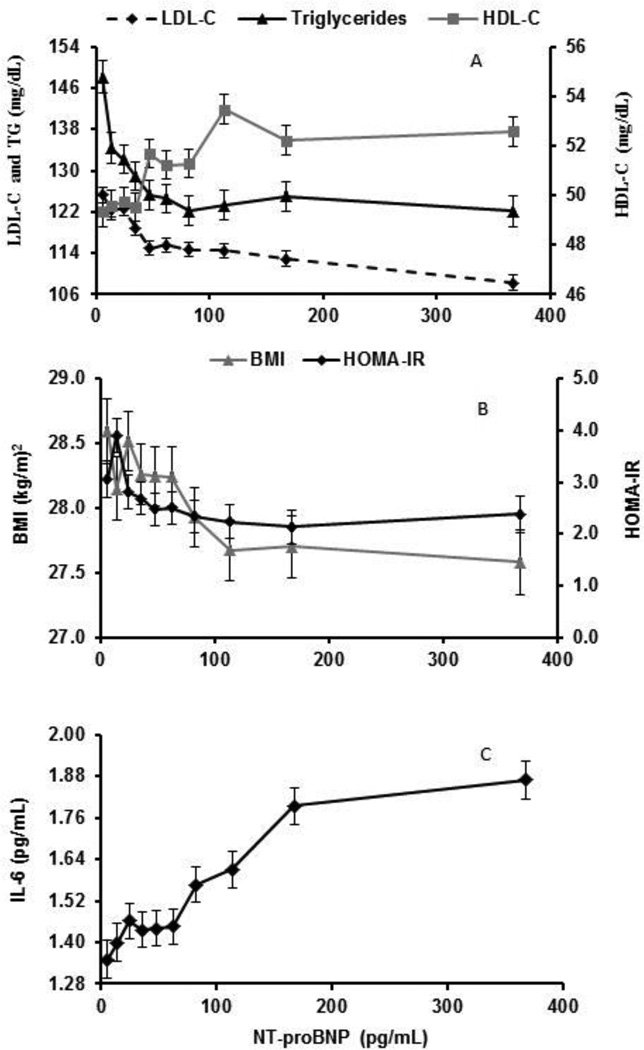
Supplemental Table 1 shows that BMI, TC, LDL-C, TG, and HOMA-IR each have an inverse association and HDL-C had a positive association with NT-proBNP within NT-proBNP <100 pg/mL, but not at values ≥100, p < 0.003, for difference between slopes. BMI, blood lipids, inflammatory markers and HOMA-IR were plotted as a function of deciles of NT-proBNP (Figures 1A–C) and corresponding linear regression analyses performed (Table 2). BMI, TC, LDL-C, TG, fasting glucose and HOMA-IR each showed an inverse association and HDL-C had a positive association with NT-proBNP within NT-proBNP <IP, which plateaus for NT-proBNP ≥IP. The IP for BMI, TC, LDL-C, HDL-C, triglycerides, fasting glucose and HOMA-IR were at NT-proBNP values of 95, 50, 90, 120, 60, 65 and 65 pg/mL, for the respective variables, as marked on Figures 1A and 1B. The inflammatory marker IL-6 was positively associated with NT-proBNP throughout its whole range of values, figure 1C.
Figure 1. Adjusted means of blood lipids (A), BMI and HOMA-IR (B) and IL-6 (C) by deciles of NT-proBNP at MESA baseline.
Data are presented as mean values ± SE at each decile of NT-proBNP adjusted for age, race, sex, BMI (except when BMI was the dependent variable), % of total calories from saturated fats, total intentional exercise (MET-min/week), statin use, antihypertensive medications, presence of diabetes and glomerular filtration rate. (A) LDL-C, HDL-C and triglycerides (B) BMI and HOMA-IR and (C) IL-6.
Table 2.
Unadjusted linear regression coefficients between NT-proBNP and lipid and metabolic variables at levels above and below NT-proBNP inflection point. MESA baseline.
| Dependent Variables | NT-proBNP IP (pg/mL) |
R2 | b<IP | p value at <IP |
b>IP | p value at >IP |
|---|---|---|---|---|---|---|
| BMI, kg/m2 | 95 | 0.007 | −0.01 (0.002) | <.0001 | −0.0003 (0.0003) | 0.4 |
| Total Cholesterol, mg/dL | 50 | 0.03 | −0.21 (0.03) | <.0001 | −0.001 (0.002) | 0.6 |
| LDL-C, mg/dL | 90 | 0.01 | −0.11 (0.02) | <.0001 | 0.0008 (0.002) | 0.7 |
| HDL-C, mg/dL | 120 | 0.17 | 0.04 (0.005) | <.0001 | −0.00002 (0.0008) | 1.0 |
| Triglycerides, mg/dL | 60 | 0.01 | −0.39 (0.07) | <.0001 | 0.0005 (0.005) | 0.9 |
| Glucose, mg/dL | 65 | 0.05 | −0.14 (0.02) | <.0001 | 0.002 (0.002) | 0.4 |
| HOMA-IR | 65 | 0.01 | −0.02 (0.005) | <.0001 | 0.0003 (0.0004) | 0.5 |
BMI = body mass index. IP = inflection point. The unadjusted b values are slopes for each dependent variable regressed on baseline NT-proBNP < and > the IP value. Each row represents a single linear spline model, that is, the regression equation is dependent variables = a + b<IP*(NT-proBNP − IP) + b>IP*(NT-proBNP − IP) * INT-proBNP >IP. Where a = intercept. The IP was chosen as the NT-proBNP value between 20 – 300 pg/mL with the highest R2.
Longitudinal associations of NT-proBNP with BMI and blood lipid variables
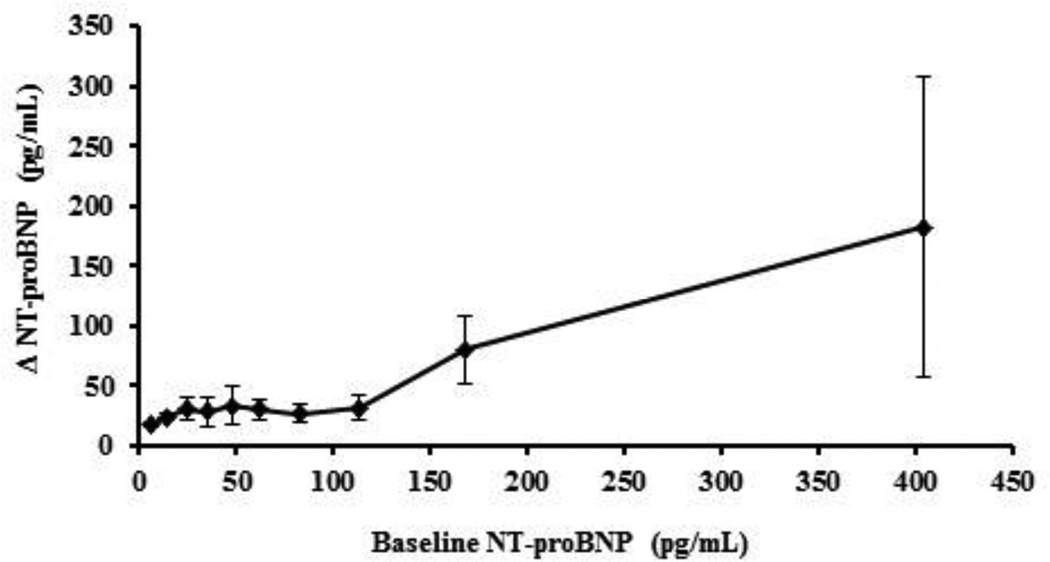
Figure 2 shows the three-year unadjusted change in NT-proBNP as a function of baseline NT-proBNP. The mean changes in NT-proBNP when baseline NT-proBNP was < vs. ≥100 pg/mL were 32.0 (95% CI: 17.7, 48.3) pg/mL vs. 85.5 (95% CI: (59.3, 111.7) pg/mL, respectively, p<0.0001. Table 3 shows the regression coefficients for the associations between baseline NT-proBNP and change in BMI, blood lipids and fasting glucose adjusted for the variables mentioned above, within categories of baseline NT-proBNP < or ≥ 100 pg/mL. The adjusted change in BMI, HDL-C, TG and fasting glucose were not associated with baseline NT-proBNP within either of the baseline NT-proBNP categories. Contrary to the cross-sectional analyses, there was a positive association between change in NT-proBNP and the adjusted change in TC and LDL-C, but only within values of NT-proBNP <100 pg/mL. In those individuals with NT-proBNP baseline values ≥100 pg/mL the adjusted change in TC and LDL-C plateaued with no further increases. The change in BMI, blood lipids and glucose regressed on change in NT-proBNP showed an inverse association with BMI, blood lipids and glucose, but only when baseline NT-proBNP values were <100 pg/mL.
Figure 2. Three-year change in NT-proBNP by deciles of baseline NT-proBNP.
Data are presented as mean ± 95% CI. ΔNT-proBNP, where change is computed as visit 3 – baseline values.
Table 3.
Adjusted regression coefficients for the change in BMI and blood lipids predicted from baseline NT-proBNP or from change in NT-proBNP, by categories of baseline NT-proBNP < or ≥ 100 pg/mL.
| Baseline NT-proBNP | Δ in NT-proBNP | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline NT-proBNP | < 100 pg/mL | ≥ 100 pg/mL | < 100 pg/mL | ≥ 100 pg/mL | ||||
| Beta coefficient | p value | Beta coefficient | p value | Beta coefficient | p value | Beta coefficient | p value | |
| Δ BMI | −0.002 (0.001) | 0.13 | −0.0002 (0.0002) | 0.37 | −0.0006 (0.0003) | 0.02 | −0.0001 (0.00007) | 0.09 |
| Δ Total cholesterol | 0.09 (0.02) | 0.0001 | −0.003 (0.005) | 0.5 | −0.02 (0.0052) | <.0001 | 0.001 (0.001) | 0.4 |
| Δ LDL-C | 0.09 (0.02) | 0.0001 | −0.003 (0.004) | 0.5 | −0.02 (0.005) | 0.0009 | 0.002 (0.001) | 0.1 |
| Δ HDL-C | −0.003 (0.006) | 0.62 | −0.0002 (0.001) | 0.9 | −0.003 (0.001) | 0.04 | 0.00002 (0.0004) | 1 |
| Δ Triglycerides | 0.05 (0.07) | 0.5 | −0.003 (0.008) | 0.7 | −0.02 (0.02) | 0.1 | −0.003 (0.002) | 0.2 |
| Δ Glucose | 0.02 (0.02) | 0.2 | − 0.005 (0.003) | 0.07 | −0.01 (0.004) | 0.002 | −0.001 (0.0008) | 0.08 |
Δ = change between visit 3 - baseline. Adjusted for age, race, sex, ΔBMI (except when BMI was the dependent variable), % of total calories from saturated fats, total intentional exercise, statin use, presence of diabetes, glomerular filtration rate, days of follow-up and baseline NT-proBNP when ΔNT-proBNP was the independent variable.
Discussion
Our results demonstrate that the associations between NT-proBNP with BMI, blood lipids and insulin resistance do not follow a linear pattern throughout the whole range of NT-proBNP values. In fact, the initial linear association is followed by a tendency towards a plateau at higher NT-proBNP concentrations. The biphasic nature of these associations suggests an upper threshold which varies between 50 and 120 pg/mL on the association between NT-proBNP and BMI, blood lipids and insulin resistance. Substantially elevated levels of NT-proBNP, potentially due to the presence of subclinical CVD, is no longer able to influence BMI, blood lipids and insulin resistance. Unexpectedly, NT-proBNP at baseline was unable to predict change in BMI, blood lipids and glucose over a three year period suggesting either that the effects of natriuretic peptides are of short duration (e.g. lasting only weeks or months) or that longer follow-up periods are required to predict the long-term effect of NT-proBNP on BMI, lipid and glucose metabolism.
Factors affecting blood levels of NT-proBNP
An increase in myocardial wall strain and hypoxia, in addition to an increase in IL-6 can induce a dose dependent increase in the expression and release of BNP [9, 23]. Therefore, any physiological or pathological increase in cardiac wall strain [24, 25], cardiac hypoxia [26] or chronic inflammatory conditions [27] can also explain an increase in NT-proBNP. Although at MESA baseline individuals with clinical cardiovascular disease and renal failure were excluded, the presence of a number of participants with subclinical atherosclerosis, LVH and higher values of IL-6 and potentially myocardial wall strain [28] explained the large variation in the levels of NT-proBNP.
Cross sectional associations of NT-proBNP with blood lipids and HOMA-IR at levels < or ≥ 100 pg/mL
Although an NT-proBNP value of approximately 100 pg/mL should not be interpreted as a definite cutoff point to differentiate between physiological and pathological levels, we showed that below a value that ranged across variables from 50 to 120 pg/mL there was a linear association between NT-proBNP and BMI, blood lipids and insulin resistance, which disappeared at values above. In addition, it has been shown that the risk of CVD and all-cause mortality increases when levels of NT-proBNP were >100 pg/mL [29, 30]. These facts, in addition to our own findings provide supporting evidence to propose the presence of an upper threshold above which the physiological effects of natriuretic peptides have little influence on metabolic variables. The differences in BMI, blood lipids and insulin resistance in individuals with NT-proBNP < or ≥ 100 pg/mL could be explained by differences in the percent of energy intake from saturated fats, which influences TC [31], but this is unlikely because this variable was not different between the two categories of NT-proBNP. Physical activity, which is inversely related to BMI and TG and positively associated with HDL-C [32], was higher in the category with NT-proBNP <100 pg/mL and therefore could not explain the higher BMI, TG and lower HDL-C observed with lower values of NT-proBNP. Diabetes, which influences blood lipid levels, was not different between the two categories of NT-proBNP. The slightly higher percentage of individuals using statins in the group with the highest values of NT-proBNP could potentially contribute to the lower blood lipid values with higher levels of NT-proBNP. However, the associations between NT-proBNP with blood lipids were maintained after adjusting for the use of statins. These facts further support the hypothesis that NT-proBNP independently reflects the actions of BNP on weight regulation, blood lipids and insulin resistance.
An increase in BMI can affect BNP levels due to an increase in receptor mediated clearance [33, 34] and a decrease in the synthesis of BNP as observed in an animal model of obese mice [35]. In addition, low levels of BNP can also affect BMI through its effects on mitochondrial genesis [36]. Therefore, obesity can be both a cause and a consequence of low BNP levels. The inverse associations of NT-proBNP at values below the IP with blood lipids and insulin resistance likely reflect the combined actions at physiological levels of BNP on adipose cell lipolysis and mitochondrial function [36, 37]. The actions of BNP in promoting mitochondrial biogenesis, increasing oxygen consumption, and fat oxidation [36] explain how lower levels of BNP might lead to the variations in BMI, blood lipids and insulin resistance observed in this study. We propose that low levels of BNP leads to mitochondrial dysfunction, increasing the synthesis of lipids and the levels of ApoB-100 [38, 39]. Furthermore, the decreased oxidation of fat increases available free fatty acids that are then transferred to lipoproteins, producing triglyceride rich lipoproteins [40]. These physiological and biochemical pathways provide potential biological explanations for the associations between low levels of NT-proBNP with higher BMI, blood lipids and insulin resistance. Although the changes in BMI, blood lipids and insulin resistance are not of considerable magnitude low BNP should be an additional factor to be considered in individuals with dyslipidemia and impaired glucose tolerance.
The lack of consistency in the IP’s among the different variables should not deny the presence of a threshold above which natriuretic peptides are no longer able to influence BMI, blood lipids and glucose metabolism. The large difference, more than 10 times, in the slope for the associations between NT-proBNP with BMI, blood lipids and insulin resistance and the lack of linear associations above the IP are strong supporting arguments in favor of an IP. However, determining the reasons for the variations in IP among the different variables may require further studies aimed at evaluating the acute and long-term dose-response relationship between natriuretic peptides and glucose and lipid metabolism.
The lack of association at high levels of NT-proBNP with BMI, blood lipids and insulin resistance could reflect the combination of saturation and down regulation of natriuretic peptide receptors-A at high BNP concentrations [41, 42]. Another plausible explanation is that the cross-reactivity between NT-proBNP and proBNP in the assay for NT-proBNP can over estimate NT-proBNP influencing the linear association with the different metabolic variables in this study [43]. By measuring NT-proBNP it is assumed that it is reflecting BNP synthesis, but the fact that patients with heart failure produce a proBNP molecule that is O-glycated at position 71 interfering with the cleavage by furin or corin [44], suggests the possibility that the presence of high concentrations of NT-proBNP may be reflecting an increase in proBNP while masking a decrease in the active BNP.
Physiological and pathological influences of NT-proBNP on BMI and metabolic variables
We propose that associations of metabolic factors with NT-proBNP result from the combined contributions of physiological and pathological pathways. In this model, the levels of BMI, blood lipids and insulin resistance depend on the relative weighting (w vs. 1−w) of the physiological pathway (expressed as a mathematical function phys(NT-proBNP)) and the pathological pathway (expressed mathematically as path(NT-proBNP)). Algebraically, the relative influence of these pathways on a metabolic variable Y can be written (suppressing the error term):
Y = w * phys(NT-proBNP) + (1−w) * path(NT-proBNP).
The function phys(NT-proBNP) reflects normal physiology of BNP. In addition, NT-proBNP is assumed to be influenced by cardiac pathology and inflammation, indicated by a function path1(cardiac dysfunction or inflammation), so that there is another component of the model,
NT-proBNP = normal level of NT-proBNP + path1(cardiac dysfunction or inflammation)
Although the weight w and the functions phys(NT-proBNP), path(NT-proBNP), and path1(cardiac dysfunction or inflammation) are not known, the point at which Y(NT-proBNP) becomes primarily dependent on the pathological pathways is suggested by the IP in the empirical fit of a linear spline with a single join point. Therefore, as cardiovascular disease or inflammation progresses, the model suggests that NT-proBNP rises and the influence of (1−w) path(NT-proBNP) on the dependent variable increases. Empirically, at levels of NT-proBNP above the IP the metabolic variables studied here tend to plateau with increasing NT-proBNP.
Longitudinal associations between baseline NT-proBNP and change in BMI, blood glucose and blood lipids
The larger 3-year change in NT-proBNP in individuals with baseline NT-proBNP ≥100 pg/mL could be due to the fact that participants in the higher category of baseline NT-proBNP were 10 years older and a higher proportion of them were hypertensive, had LVH, higher levels of cIMT, CAC and higher IL-6 values. Thus, the larger 3-year rise in NT-proBNP in that group may reflect the effects of the progression of subclinical cardiovascular disease or the presence of chronic inflammation.
The lack of an association between baseline NT-proBNP and change in BMI, HDL-C, TG and glucose suggests that the effect of NT-proBNP on BMI, blood lipids and glucose are of short duration and inadequate to predict long-term variations in these variables. However, recent reports indicating that NT-proBNP as well as atrial natriuretic peptide are inversely associated with incident diabetes [5, 45, 46], raises doubt about the previous statement. An alternate explanation could be that the difference in follow-up time, which in those studies was between 12 and 16 years, suggests that a follow-up time of only 3 years is not of sufficient duration to assess the long-term effects of NT-proBNP on BMI, blood lipids and glucose metabolism. The inverse association between change in NT-proBNP and change in BMI, blood lipids and blood glucose confirms our cross sectional data and provide further support of the influence of natriuretic peptides on glucose and lipid metabolism.
Strength and limitations
A major strength of this study is the large cohort free of overt CVD and with different lipid components, insulin sensitivity, and some inflammatory parameters available for the cross sectional data analysis. One of the limitations is that for the longitudinal analysis we did not have measurements of inflammatory markers and HOMA-IR. Furthermore, only NT-proBNP was measured rather than specifically measuring BNP and proBNP. Further studies that simultaneously evaluate the blood levels of proBNP, BNP and NT-proBNP, their biological actions, and their effects on receptor number at high blood levels of NT-proBNP are necessary next steps. In addition, the effects of atrial natriuretic peptide (ANP) in regulating blood volume are slightly different in magnitude and timing to those of BNP. Therefore, the metabolic effects of ANP may also be different than the ones observed for BNP and this would be an important aspect to evaluate in future studies as well
Conclusions
This study provides cross-sectional evidence in a large cohort group consistent with the concept that the association between NT-proBNP and BMI, blood lipids and insulin resistance follows a biphasic response. The level at which the linear association between NT-proBNP and BMI, blood lipids and insulin resistance plateaus varies among these variables and ranges between 50 and 120 pg/mL. Individuals with subclinical CVD and/or elevated IL-6 tend to have substantially higher levels of NT-proBNP and at those levels the physiological effects of natriuretic peptides on BMI, blood lipids and insulin resistance substantially decrease and tend to plateau. In addition, change in NT-proBNP depends on baseline values with larger change occurring in those with initially greater levels of NT-proBNP. The lack of ability for baseline NT-proBNP to predict change in BMI, blood lipids and glucose may be due to the short term effect of natriuretic peptides or on the contrary, the follow-up time might be too short to detect long-term effects of natriuretic peptides.
Supplementary Material
Acknowledgments
This research was supported by contracts N01-HC-95159 through N01-HC-95165, N01-HC-95169 grants R01-HL 66075, N01-HC-95168, N01-HC 9808, and N01-HC 95168 from the National Heart, Lung, and Blood Institute. The authors also thank the investigators and staff of the Multi-Ethnic Study of Atherosclerosis (MESA) for their valuable contributions. A full list of MESA investigators and institutions can be found at http://www.mesa-nhlbi.org/.
Abbreviations
- BMI
body mass index
- CAC
coronary artery calcium
- cIMT
common carotid intima media thickness
- CT
computed tomography
- CVD
cardiovascular disease
- eGFR
estimate glomerular filtration rate
- HDL-C
high density lipoprotein cholesterol
- HOMA-IR
homeostasis model of insulin resistance
- IL-6
interleukin-6
- IP
inflection point
- IR
insulin resistance
- LDL-C
low density lipoprotein cholesterol
- MESA
Multi-Ethnic Study of Atherosclerosis
- NT-proBNP
N-terminal-pro-brain natriuretic peptide
- TC
total cholesterol
- TG
triglycerides
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
OS wrote the major portions of the paper and performed the statistical analysis. DD and DJ were the main supervisors of the paper, contributed to the analysis and editing. AM, HB and LD contributed with their expertise in NT-proBNP. All other authors contributed equally to critical review of the paper.
Disclosures
Otto A. Sanchez, no disclosures. Daniel Duprez MD, PhD consultant to Genentech, Novartis. Hossein Bahrami no disclosures. Lori B Daniels consultant to Singlulex, Critical Diagnostics and Alere, Inc. Aaron R. Folsom, no disclosures. Joao A. Lima, consultant to Toshiba Medical Systems, Bracco. Alan Maisel, consultant to Alere, BG Medicine, Brahms, Critical Diagnostics, EFG diagnostics, Novartis, Abbott. Carmen A. Peralta, no disclosures. David R Jacobs, no disclosures.
Contributor Information
Daniel A. Duprez, Division of Cardiology, University of Minnesota.
Hossein Bahrami, Stanford University.
Lori B. Daniels, School of Medicine, University of California, San Diego.
Aaron R. Folsom, School of Public Health, Division of Epidemiology & Community Health, University of Minnesota.
Joao A. Lima, Division of Cardiology, Johns Hopkins Bayview Medical Center.
Alan Maisel, School of Medicine, University of California, San Diego.
Carmen A. Peralta, School of Medicine, University of California San Francisco.
David R. Jacobs, Jr, School of Public Health, Division of Epidemiology & Community Health, University of Minnesota and University of Oslo.
References
- 1.Di Angelantonio E, Chowdhury R, Sarwar N, et al. B-type natriuretic peptides and cardiovascular risk: Systematic review and meta-analysis of 40 prospective studies. Circulation. 2009;120(22):2177–2187. doi: 10.1161/CIRCULATIONAHA.109.884866. [DOI] [PubMed] [Google Scholar]
- 2.Melander O, Newton-Cheh C, Almgren P, et al. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA. 2009;302(1):49–57. doi: 10.1001/jama.2009.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olsen MH, Hansen TW, Christensen MK, et al. N-terminal pro brain natriuretic peptide is inversely related to metabolic cardiovascular risk factors and the metabolic syndrome. Hypertension. 2005;46(4):660–666. doi: 10.1161/01.HYP.0000179575.13739.72. [DOI] [PubMed] [Google Scholar]
- 4.Asferg CL, Nielsen SJ, Andersen UB, et al. Relative atrial natriuretic peptide deficiency and inadequate renin and angiotensin ii suppression in obese hypertensive men. Hypertension. 2013;62(1):147–153. doi: 10.1161/HYPERTENSIONAHA.111.00791. [DOI] [PubMed] [Google Scholar]
- 5.Lazo M, Young JH, Brancati FL, et al. N-terminal pro-brain natriuretic peptide and risk of diabetes. Diabetes. 2013 doi: 10.2337/db13-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houben AJ, van der Zander K, de Leeuw PW. Vascular and renal actions of brain natriuretic peptide in man: Physiology and pharmacology. Fundamental and Clinical Pharmacology. 2005;19(4):411–419. doi: 10.1111/j.1472-8206.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- 7.Fradley MG, Larson MG, Cheng S, et al. Reference limits for n-terminal-pro-b-type natriuretic peptide in healthy individuals (from the framingham heart study) American Journal of Cardiology. 2011;108(9):1341–1345. doi: 10.1016/j.amjcard.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of b-type natriuretic peptide in the emergency diagnosis of heart failure. New England Journal of Medicine. 2002;347(3):161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 9.Xia WJ, Huang YY, Chen YL, et al. Acute myocardial ischemia directly modulates the expression of brain natriuretic peptide at the transcriptional and translational levels via inflammatory cytokines. European Journal of Pharmacology. 2011;670(1):7–12. doi: 10.1016/j.ejphar.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Abdullah SM, Khera A, Das SR, et al. Relation of coronary atherosclerosis determined by electron beam computed tomography and plasma levels of n-terminal pro-brain natriuretic peptide in a multiethnic population-based sample (the dallas heart study) American Journal of Cardiology. 2005;96(9):1284–1289. doi: 10.1016/j.amjcard.2005.06.073. [DOI] [PubMed] [Google Scholar]
- 11.Bryan PM, Xu X, Dickey DM, et al. Renal hyporesponsiveness to atrial natriuretic peptide in congestive heart failure results from reduced atrial natriuretic peptide receptor concentrations. Am J Physiol Renal Physiol. 2007;292(5):F1636–F1644. doi: 10.1152/ajprenal.00418.2006. [DOI] [PubMed] [Google Scholar]
- 12.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: Objectives and design. American Journal of Epidemiology. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 13.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 14.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical Chemistry. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 15.Karl J, Borgya A, Gallusser A, et al. Development of a novel, n-terminal-probnp (nt-probnp) assay with a low detection limit. Scandinavian Journal of Clinical and Laboratory Investigation. Supplement. 1999;230:177–181. [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of Internal Medicine. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peralta CA, Jacobs DR, Jr, Katz R, et al. Association of pulse pressure, arterial elasticity, and endothelial function with kidney function decline among adults with estimated gfr >60 ml/min/1.73 m(2): The multi-ethnic study of atherosclerosis (mesa) American Journal of Kidney Diseases. 2012;59(1):41–49. doi: 10.1053/j.ajkd.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polak JF, Szklo M, Kronmal RA, et al. The value of carotid artery plaque and intima-media thickness for incident cardiovascular disease: The multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2013;2(2):e000087. doi: 10.1161/JAHA.113.000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. Journal of the American College of Cardiology. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 20.Natori S, Lai S, Finn JP, et al. Cardiovascular function in multi-ethnic study of atherosclerosis: Normal values by age, sex, and ethnicity. AJR. American Journal of Roentgenology. 2006;186(6 Suppl 2):S357–S365. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 21.Polak JF, Tracy R, Harrington A, et al. Carotid artery plaque and progression of coronary artery calcium: The multi-ethnic study of atherosclerosis. Journal of the American Society of Echocardiography. 2013;26(5):548–555. doi: 10.1016/j.echo.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Third report of the national cholesterol education program (ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 23.Liang FQ, Gardner DG. Mechanical strain activates brain natriuretic peptide gene transcription through p38 mapk. Hypertension. 1999;34(2):357-. [Google Scholar]
- 24.Maeder MT, Staub D, Surnier Y, et al. Determinants of absolute and relative exercise-induced changes in b-type natriuretic peptides. International Journal of Cardiology. 2011;147(3):409–415. doi: 10.1016/j.ijcard.2009.09.546. [DOI] [PubMed] [Google Scholar]
- 25.Lin S, Yokoyama H, Rac VE, et al. Novel biomarkers in diagnosing cardiac ischemia in the emergency department: A systematic review. Resuscitation. 2012;83(6):684–691. doi: 10.1016/j.resuscitation.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Goetze JP, Gore A, Moller CH, et al. Acute myocardial hypoxia increases bnp gene expression. FASEB Journal. 2004;18(15):1928–1930. doi: 10.1096/fj.03-1336fje. [DOI] [PubMed] [Google Scholar]
- 27.van Diepen S, Roe MT, Lopes RD, et al. Baseline nt-probnp and biomarkers of inflammation and necrosis in patients with st-segment elevation myocardial infarction: Insights from the apex-ami trial. Journal of Thrombosis and Thrombolysis. 2012;34(1):106–113. doi: 10.1007/s11239-012-0691-0. [DOI] [PubMed] [Google Scholar]
- 28.Fernandes VR, Polak JF, Edvardsen T, et al. Subclinical atherosclerosis and incipient regional myocardial dysfunction in asymptomatic individuals: The multi-ethnic study of atherosclerosis (mesa) Journal of the American College of Cardiology. 2006;47(12):2420–2428. doi: 10.1016/j.jacc.2005.12.075. [DOI] [PubMed] [Google Scholar]
- 29.Marz W, Tiran B, Seelhorst U, et al. N-terminal pro-b-type natriuretic peptide predicts total and cardiovascular mortality in individuals with or without stable coronary artery disease: The ludwigshafen risk and cardiovascular health study. Clinical Chemistry. 2007;53(6):1075–1083. doi: 10.1373/clinchem.2006.075929. [DOI] [PubMed] [Google Scholar]
- 30.Paget V, Legedz L, Gaudebout N, et al. N-terminal pro-brain natriuretic peptide: A powerful predictor of mortality in hypertension. Hypertension. 2011;57(4):702–709. doi: 10.1161/HYPERTENSIONAHA.110.163550. [DOI] [PubMed] [Google Scholar]
- 31.Grande F, Anderson JT, Chlouverakis C, et al. Effect of dietary cholesterol on man's serum lipids. Journal of Nutrition. 1965;87(1):52–62. doi: 10.1093/jn/87.1.52. [DOI] [PubMed] [Google Scholar]
- 32.Leon AS, Sanchez OA. Response of blood lipids to exercise training alone or combined with dietary intervention. [review] [86 refs] Medicine & Science in Sports & Exercise. 2001;33(6 Suppl):S502–S515. doi: 10.1097/00005768-200106001-00021. discussion S28-9. [DOI] [PubMed] [Google Scholar]
- 33.Dessi-Fulgheri P, Sarzani R, Tamburrini P, et al. Plasma atrial natriuretic peptide and natriuretic peptide receptor gene expression in adipose tissue of normotensive and hypertensive obese patients. Journal of Hypertension. 1997;15(12 Pt 2):1695–1699. doi: 10.1097/00004872-199715120-00074. [DOI] [PubMed] [Google Scholar]
- 34.Sarzani R, Paci VM, Zingaretti CM, et al. Fasting inhibits natriuretic peptides clearance receptor expression in rat adipose tissue. Journal of Hypertension. 1995;13(11):1241–1246. doi: 10.1097/00004872-199511000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Bartels ED, Nielsen JM, Bisgaard LS, et al. Decreased expression of natriuretic peptides associated with lipid accumulation in cardiac ventricle of obese mice. Endocrinology. 2010;151(11):5218–5225. doi: 10.1210/en.2010-0355. [DOI] [PubMed] [Google Scholar]
- 36.Miyashita K, Itoh H, Tsujimoto H, et al. Natriuretic peptides/cgmp/cgmp-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes. 2009;58(12):2880–2892. doi: 10.2337/db09-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sengenes C, Berlan M, De Glisezinski I, et al. Natriuretic peptides: A new lipolytic pathway in human adipocytes. FASEB Journal. 2000;14(10):1345–1351. [PubMed] [Google Scholar]
- 38.Mailloux R, Lemire J, Appanna V. Aluminum-induced mitochondrial dysfunction leads to lipid accumulation in human hepatocytes: A link to obesity. Cellular Physiology and Biochemistry. 2007;20(5):627–638. doi: 10.1159/000107546. [DOI] [PubMed] [Google Scholar]
- 39.Young SG. Recent progress in understanding apolipoprotein b. Circulation. 1990;82(5):1574–1594. doi: 10.1161/01.cir.82.5.1574. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Peng DQ. New insights into the mechanism of low high-density lipoprotein cholesterol in obesity. Lipids Health Dis. 2011;10:176. doi: 10.1186/1476-511X-10-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura M, Arakawa N, Yoshida H, et al. Vasodilatory effects of b-type natriuretic peptide are impaired in patients with chronic heart failure. American Heart Journal. 1998;135(3):414–420. doi: 10.1016/s0002-8703(98)70316-3. [DOI] [PubMed] [Google Scholar]
- 42.Roubert P, Lonchampt MO, Chabrier PE, et al. Down-regulation of atrial natriuretic factor receptors and correlation with cgmp stimulation in rat cultured vascular smooth muscle cells. Biochemical and Biophysical Research Communications. 1987;148(1):61–67. doi: 10.1016/0006-291x(87)91076-x. [DOI] [PubMed] [Google Scholar]
- 43.Luckenbill KN, Christenson RH, Jaffe AS, et al. Cross-reactivity of bnp, nt-probnp, and probnp in commercial bnp and nt-probnp assays: Preliminary observations from the ifcc committee for standardization of markers of cardiac damage. Clinical Chemistry. 2008;54(3):619–621. doi: 10.1373/clinchem.2007.097998. [DOI] [PubMed] [Google Scholar]
- 44.Clerico A, Vittorini S, Passino C. Circulating forms of the b-type natriuretic peptide prohormone: Pathophysiologic and clinical considerations. Advances in Clinical Chemistry, Vol 58. 2012;58:31–44. doi: 10.1016/b978-0-12-394383-5.00008-4. [DOI] [PubMed] [Google Scholar]
- 45.Magnusson M, Jujic A, Hedblad B, et al. Low plasma level of atrial natriuretic peptide predicts development of diabetes: The prospective malmo diet and cancer study. Journal of Clinical Endocrinology and Metabolism. 2012;97(2):638–645. doi: 10.1210/jc.2011-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfister R, Sharp S, Luben R, et al. Mendelian randomization study of b-type natriuretic peptide and type 2 diabetes: Evidence of causal association from population studies. PLoS Med. 2011;8(10):e1001112. doi: 10.1371/journal.pmed.1001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.