Abstract
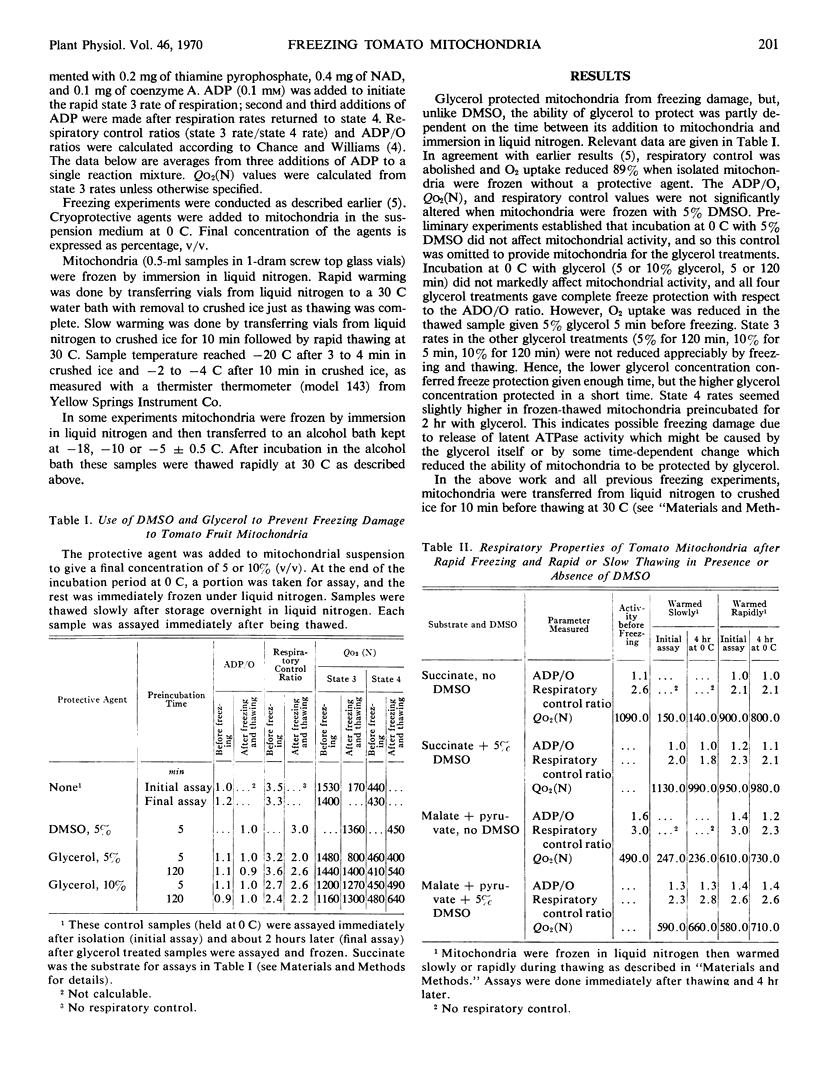
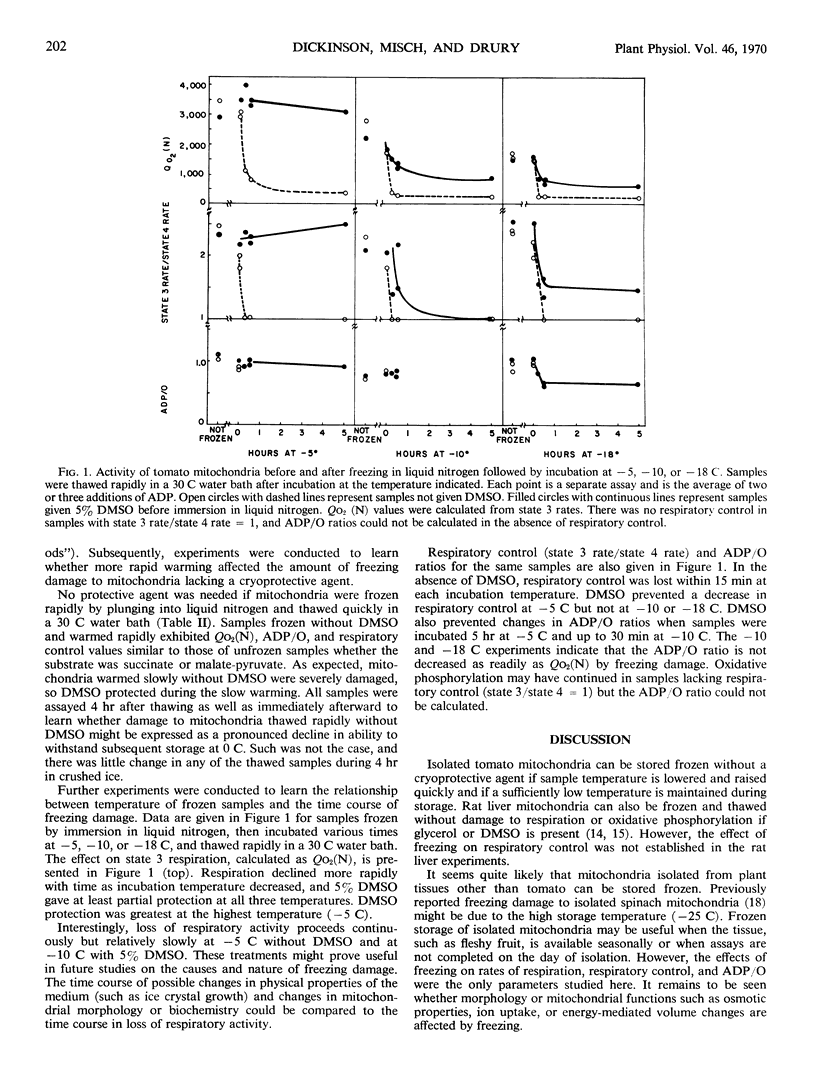
Isolated tomato (Lycopersicon esculentum var. Kc 146) fruit mitochondria could be stored successfully in the frozen state without a cryoprotective agent if the mitochondria were frozen quickly by immersion in liquid nitrogen and later thawed quickly at 30 C. Criteria of freezing damage were rate of respiration, adenosine diphosphate to oxygen ratio, and respiratory control ratio. Marked reduction in respiration and loss of respiratory control occurred when mitochondria were transferred from liquid nitrogen to −5, −10, or −18 C for 15 minutes prior to thawing at 30 C. Dimethylsulfoxide (5%) prevented freezing damage when mitochondria were incubated at −5 C but did not prevent freezing damage at −10 or −18 C. Isolated tomato mitochondria show promise as a model system for studying the nature of freezing damage and the mode of action of cryo-protective agents.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bickis I. J., Kazaks K., Finn J. J., Henderson I. W. Permeation kinetics of glycerol and dimethyl sulfoxide in Novikoff hepatoma ascites cells. Cryobiology. 1967 Jul-Aug;4(1):1–10. doi: 10.1016/s0011-2240(67)80180-9. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Dickinson D. B., Misch M. J., Drury R. E. Dimethyl sulfoxide protects tightly coupled mitochondria from freezing damage. Science. 1967 Jun 30;156(3783):1738–1739. doi: 10.1126/science.156.3783.1738. [DOI] [PubMed] [Google Scholar]
- Drury R. E., McCollum J. P., Garrison S. A. Properties of succinate oxidation in tomato fruit mitochondria. Plant Physiol. 1968 Feb;43(2):248–254. doi: 10.1104/pp.43.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein W. N., Stowell R. E. Studies on the mechanism of freezing damage to mouse liver using a mitochondrial enzyme assay. I. Temporal localization of the injury phase during slow freezing. Cryobiology. 1968 May-Jun;4(6):283–289. doi: 10.1016/s0011-2240(68)80125-7. [DOI] [PubMed] [Google Scholar]
- GREIFF D., MYERS M. Effect of dimethyl sulphoxide on the cryo-tolerance of mitochondria. Nature. 1961 Jun 24;190:1202–1204. doi: 10.1038/1901202b0. [DOI] [PubMed] [Google Scholar]
- GREIFF D., MYERS M., PRIVITERA C. A. The effects of glycerol, freezing and storage at low temperatures, and drying by vacuum sublimation on oxidative phosphorvlation by mitochondrial suspensions. Biochim Biophys Acta. 1961 Jun 24;50:233–242. doi: 10.1016/0006-3002(61)90321-3. [DOI] [PubMed] [Google Scholar]
- Geronimo J., Beevers H. Effects of Aging and Temperature on Respiratory Metabolism of Green Leaves. Plant Physiol. 1964 Sep;39(5):786–793. doi: 10.1104/pp.39.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant N. H., Alburn H. E. Acceleration of enzyme reactions in ice. Nature. 1966 Oct 8;212(5058):194–194. doi: 10.1038/212194a0. [DOI] [PubMed] [Google Scholar]
- Grant N. H., Alburn H. E. Fast Reactions of Ascorbic Acid and Hydrogen Peroxide in Ice, a Presumptive Early Environment. Science. 1965 Dec 17;150(3703):1589–1590. doi: 10.1126/science.150.3703.1589. [DOI] [PubMed] [Google Scholar]
- Grant N. H., Alburn H. E. Reactions in frozen systems. VI. Ice as a possible model for biological structured-water systems. Arch Biochem Biophys. 1967 Feb;118(2):292–296. doi: 10.1016/0003-9861(67)90351-7. [DOI] [PubMed] [Google Scholar]
- Heber U. W., Santarius K. A. Loss of Adenosine Triphosphate Synthesis Caused by Freezing and Its Relationship to Frost Hardiness Problems. Plant Physiol. 1964 Sep;39(5):712–719. doi: 10.1104/pp.39.5.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U. Freezing injury and uncoupling of phosphorylation from electron transport in chloroplasts. Plant Physiol. 1967 Oct;42(10):1343–1350. doi: 10.1104/pp.42.10.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U. Freezing injury in relation to loss of enzyme activities and protection against freezing. Cryobiology. 1968 Nov-Dec;5(3):188–201. doi: 10.1016/s0011-2240(68)80163-4. [DOI] [PubMed] [Google Scholar]
- LOVELOCK J. E., BISHOP M. W. Prevention of freezing damage to living cells by dimethyl sulphoxide. Nature. 1959 May 16;183(4672):1394–1395. doi: 10.1038/1831394a0. [DOI] [PubMed] [Google Scholar]
- LOVELOCK J. E. The denaturation of lipid-protein complexes as a cause of damage by freezing. Proc R Soc Lond B Biol Sci. 1957 Dec 17;147(929):427–433. doi: 10.1098/rspb.1957.0062. [DOI] [PubMed] [Google Scholar]
- LOVELOCK J. E. The mechanism of the protective action of glycerol against haemolysis by freezing and thawing. Biochim Biophys Acta. 1953 May;11(1):28–36. doi: 10.1016/0006-3002(53)90005-5. [DOI] [PubMed] [Google Scholar]
- Lusena C. V., Dass C. M. Structural changes associated with the release of enzymes from rat-liver mitochondria by freezing. Can J Biochem. 1966 Jun;44(6):775–781. doi: 10.1139/o66-095. [DOI] [PubMed] [Google Scholar]
- Lusena C. V., Depocas F. Heterogeneity and differential fragility of rat liver mitochondria. Can J Biochem. 1966 May;44(5):497–508. doi: 10.1139/o66-060. [DOI] [PubMed] [Google Scholar]
- Lusena C. V. Release of enzymes from rat liver mitochondria by freezing. Can J Biochem. 1965 Nov;43(11):1787–1798. doi: 10.1139/o65-199. [DOI] [PubMed] [Google Scholar]
- Mazur P., Farrant J., Leibo S. P., Chu E. H. Survival of hamster tissue culture cells after freezing and thawing. Interactions between protective solutes and cooling and warming rates. Cryobiology. 1969 Jul-Aug;6(1):1–9. doi: 10.1016/s0011-2240(69)80002-7. [DOI] [PubMed] [Google Scholar]
- Meryman H. T. Modified model for the mechanism of freezing injury in erythrocytes. Nature. 1968 Apr 27;218(5139):333–336. doi: 10.1038/218333a0. [DOI] [PubMed] [Google Scholar]
- Mohr W. P., Stein M. Effect of different freeze-thaw regimes on ice formation and ultrastructural changes in tomato fruit parenchyma tissue. Cryobiology. 1969 Jul-Aug;6(1):15–31. doi: 10.1016/s0011-2240(69)80004-0. [DOI] [PubMed] [Google Scholar]
- SHERMAN J. K. Questionable protection by intracellular glycerol during freezing and thawing. J Cell Comp Physiol. 1963 Feb;61:67–83. doi: 10.1002/jcp.1030610108. [DOI] [PubMed] [Google Scholar]
- TRUMP B. F., YOUNG D. E., ARNOLD E. A., STOWELL R. E. EFFECTS OF FREEZING AND THAWING ON THE STRUCTURE, CHEMICAL CONSTITUTION, AND FUNCTION OF CYTOPLASMIC STRUCTURES. Fed Proc. 1965 Mar-Apr;24:S144–S168. [PubMed] [Google Scholar]
- van den Berg L., Soliman F. S. Composition and ph changes during freezing of solutions containing calcium and magnesium phosphate. Cryobiology. 1969 Jul-Aug;6(1):10–14. doi: 10.1016/s0011-2240(69)80003-9. [DOI] [PubMed] [Google Scholar]


