Abstract
Aims
The mechanism by which SR48692 inhibits non-small cell lung cancer (NSCLC) proliferation was investigated.
Materials and Methods
The ability of SR48692 to inhibit the proliferation of NSCLC cell lines NCI-H1299 and A549 were investigated in vitro in the presence or absence of neurotensin (NTS). The ability of NTS to cause epidermal growth factor receptor (EGFR) transactivation was investigated by Western blot using NSCLC cells and various inhibitors. The growth effects and Western blot results were determined in cell lines treated with siRNA for NTSR1.
Key findings
Treatment of A549 or NCI-H1299 cells with siRNA for NTSR1, reduced significantly NTSR1 protein and the ability of SR48692 to inhibit the proliferation of A549 or NCI-H1299 NSCLC cells. Treatment of A549 and NCI-H1299 cells with siRNA for NTSR1 reduced the ability of NTS to cause epidermal growth factor receptor (EGFR) transactivation. SR48692 or gefitinib (EGFR tyrosine kinase inhibitor) inhibited the ability of NTS to cause EGFR and ERK tyrosine phosphorylation. NTS transactivation of the EGFR was inhibited by GM6001 (matrix metalloprotease inhibitor), Tiron (superoxide scavenger) or U73122 (phospholipase C inhibitor) but not H89 (PKA inhibitor). NTS stimulates whereas SR48692 or gefitinib inhibits the clonal growth of NSCLC cells.
Significance
These results suggest that SR48692 may inhibit NSCLC proliferation in an EGFR-dependent mechanism.
Keywords: neurotensin, epidermal growth factor receptor, transactivation, lung cancer, siRNA
Introduction
Neurotensin (NTS) (Carraway and Leeman, 1973) has potent growth effects in normal and neoplastic tissues (Evers, 2006). NTS is medullary thyroid carcinoma (Zeytinoglu et al., 1995) and small cell lung cancer (SCLC) cells (Moody et al., 1985). NTS is secreted from SCLC cells and binds with high affinity (Moody et al. 2003). The action of NTS is mediated by NTSR1 and NTSR2 as well as NTSR3, which has a single transmembrane domain and binds sortolin with high affinity (Betancur et al., 1998). SR48692 is a non-peptide NTSR1 antagonist (Gulley et al., 1993) which inhibits the proliferation of pancreatic, prostate and SCLC cells in vitro and in vivo (Moody et al., 2001; Valerie et al., 2011; Wang et al., 2011).
NTSR1 activation causes phosphatidylinositol (PI) turnover in a phospholipase C dependent manner (Dupouy et al., 2011). The inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) released elevation of cytosolic Ca2+ (Staley et al., 1989) and activates protein kinase (PK)C, respectively (Muller et al., 2011). The activation of ERK and PKD is dependent upon PKC activity (Guha et al., 2002, Kisfalvi et al., 2005). NTS activates Akt and NF-κB pathways leading to increased cellular survival (Hassan et al., 2004; Zhao et al., 2003) and inactivates glycogen synthase kinase leading to increased cyclin D1 expression (Wang et al., 2006). NTS causes tyrosine phosphorylation of focal adhesion kinase (FAK) (Leyton et al., 2002) and Src (Lee et al., 2001). NTS causes epidermal growth factor (EGF)R and ERK tyrosine phosphorylation in prostate cancer cells (Hassan et al., 2004). The results indicate that NTS causes tyrosine phosphorylation of numerous proteins (Servotte et al., 2006; Heakal et al., 2011).
The NTSR1 is present in several types of cancer. Reubi et al., (1999) found a high density of specific (125I-Tyr3)NTS binding sites in Ewing’s sarcoma and medullary thyroid cancers. In non-small cell lung cancer (NSCLC), NTS and NTSR1 immunoreactivity are present in approximately 60% of lung adenocarcinoma biopsy specimens (Alfano et al., 2010). Patients with high NTSR1 had significantly lowers relapse-free survival than those with reduced NTSR1 levels. Similarly, high NTSR1 expression is associated with poor prognosis of patients with ductal breast cancer as well as head and neck squamous carcinomas (Dupouy et al., 2009; Shimizu et al., 2008). Treatment of mice containing NSCLC or colon cancer xenografts with the NTSR1 antagonist SR48692 reduced tumor growth (Moody et al., 2001; Maoret et al., 1999). These results suggest that NTSR1 may regulate the proliferation of numerous cancers.
The mechanism by which SR48692 inhibits NSCLC proliferation was investigated. Addition of siRNA to the NSCLC cells decreased significantly NTSR1 protein, decreased NTS transactivaiton of the EGFR and the ability of SR48692 to inhibit proliferation. The ability of NTS to cause EGFR tyrosine phosphorylation was inhibited by SR48692, gefitinib (EGFR TKI), GM6001 (matrix metalloprotease inhibitor), Tiron (superoxide scavenger) and U73122 (phospholipase C inhibitor). NTS stimulated, but gefitinib or SR48692 inhibited the clonal growth of NCI-H1299 cells. These results indicate that SR48692 inhibits the growth of NSCLC cells in an EGFR dependent mechanism.
Materials and Methods
Cell culture
NSCLC NCI-H1299 or A549 cells, which contain NTSR1 and wild type EGFR, were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium containing 10% heat-inactivated fetal bovine serum (Invitrogen, Grand Island, NY). The cells were split weekly 1/20 with trypsin-ethylenediaminotetraacetic acid (EDTA). The cells were mycoplasma-free and were used when they were in exponential growth phase after incubation at 37°C in 5% CO2/95% air.
Receptor binding
NCI-H1299 cells were plated in 24 well plates. When the cells were confluent, they were rinsed 2 times with PBS and placed in PBS containing 0.1 % bovine serum albumin (BSA) and 100 ug/ml bacitracin. (125I-Tyr3)NTS (0.1 nM) was added in the presence or absence of NTS analogs (NTS, NTS8–13, Ac-NTS8–13 and NT1–8) (Bachem, Torrence CA). After 30 min at 37°C, the cells were washed 3 times in PBS containing 0.1% BSA. The cells containing bound radiolabeled NTS were counted in a LKB gamma counter.
Western Blot
The ability of NTS to stimulate tyrosine phosphorylation of EGFR was investigated by Western blotting. NCI-H1299 or A549 cells were cultured in 10 cm dishes. When a monolayer of cells formed they were placed in SIT media for 3 hr. Routinely, NSCLC cells were pre-treated with SR48692, gefitinib (Tocris Bioscience, Ellisville, MO), GM6001, Tiron or U73122 (Sigma-Aldrich, St. Louis, MO) for 30 min. Then cells were treated with 100 nM NTS for 2 min, washed twice with PBS and lysed in buffer containing 50 mM Tris.HCl (pH 7.5), 150 mM sodium chloride, 1% Triton X-100, 1% deoxycholate, 1% sodium azide, 1 mM ethyleneglycoltetraacetic acid, 0.4 M EDTA, 1.5 µg/ml aprotinin, 1.5 µg/ml leupeptin, 1 mM phenylmethylsulfonylfluoride and 0.2 mM sodium vanadate (Sigma-Aldrich, St. Louis, MO). The lysate was sonicated for 5 s at 4°C and centrifuged at 10,000 × g for 15 min. Protein concentration was measured using the BCA reagent (Thermo Scientific, Rockford, IL), and 600 µg of protein was incubated with 4 µg of antiphosphotyrosine (PY) monoclonal antibody, 4 µg of goat anti-mouse immunoglobulin IgG and 30 µl of immobilized protein G (Thermo Scientific, Rockford, IL) overnight at 4°C. The immunoprecipitates were washed 3 times with phosphate buffered saline and analyzed by sodium dodecyl sulfate/polyacrylamide gel electrophoresis and Western blotting. Immunoprecipitates were fractionated using 4–20% polyacrylamide gels (Novex, San Diego, CA). Proteins were transferred to nitrocellulose membranes and the membranes were blocked overnight at 4°C using blotto [5% non-fat dried milk in solution containing 50 mM Tris/HCl (pH 8.0), 2 mM CaCl2, 80 mM sodium chloride, 0.05% Tween 20 and 0.02% sodium azide (Sigma-Aldrich, St. Louis, MO)] and incubated for 16 h at 4°C with 1 µg/ml anti-EGFR antibody (Cell Signaling Technologies, Danvers, MA) followed by anti-rabbit immunoglobulin G-horseradish peroxidase conjugate (Upstate Biotechnologies, Lake Placid, NY). The membrane was washed for 10 min with blotto and twice for 10 min with washing solution (50 mM Tris/HCl (pH 8.0), 2 mM CaCl2, 80 mM sodium chloride, 0.05% Tween 20 and 0.02% sodium azide (Sigma-Aldrich, St. Louis, MO)). The blot was incubated with enhanced chemiluminescence detection reagent for 5 min and exposed to Kodak XAR film. The intensity of the bands was determined using a densitometer.
Alternatively, 20 µg of cellular extract was loaded onto a 15 well 4–20% polyacrylamide gels. After transfer to nitrocellulose, the blot was probed with anti PY1068-EGFR, anti-EGFR, anti-PY-ERK, anti-ERK, anti-PY402PYK2, anti-PY416Src, anti-PY654β-catenin or anti-tubulin (Cell Signaling Technologies, Danvers, MA).
siRNA
A549 and NCI-H1299 cells were cultured in 6 well plates. When the cells were confluent, they were washed in 1 ml of siRNA transfection medium followed by addition of 20 pmol NTSR1 siRNA (Santa Cruz Biotechnology, Santa Cruz, CA.) in 100 µl of siRNA transfection medium. After 6 hr at 37°C, 1 ml of RPMI-1640 with 10% fetal bovine serum was added. After 72 hours, the cells were washed in PBS and the protein lysate prepared for Western blot. The Western blots were probed with NTSR1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA).
Reactive oxygen species
NCI-H1299 or A549 cells were placed in 96 well plates (30,000 cells/well) and cultured overnight. The cells were treated with 10 µM dichlorofluoresceindiacetate for 1 h and washed 3 times with serum free SIT medium. Some of the cells were treated with 5 mM Tiron for 30 min and then stimuli such as 0.1 µM NTS or 10 µM H2O2 added. Fluorescence measurements were taken at the various times using an excitation wavelength of 485 nm and emission wavelength of 585 nm.
Proliferation assays
In the MTT assay, A549 or NCI-H1299 cells were placed in 96 well plates (105 cells/well) in SIT media (100 µl). Varying concentrations of SR48692 (Tocris Biosciences, Ellisville MO) were added. After 3 days, 15 µl of MTT (1 mg/ml) was added. The following day 150 µl of DMSO was added and the absorbance determined at 540 nm using an ELISA reader.
In the clonogenic assay, NCI-H1299 cells were treated with 10 nM NTS in the presence or absence of SR48692 and/or gefitinib. The bottom layer contained 0.5% agarose in SIT medium containing 5% FBS in 6 well plates in 2 ml. The top layer consisted of 2 ml of SIT medium in 0.3% agarose (Lonzo, Rockford, ME), 5 × 104 NCI-H1299 cells, SR48692 and/or gefitinib. Triplicate wells were plated and after 2 weeks, 1 ml of 0.1% p-iodonitrotetrazolium violet (Sigma-Aldirch, St. Louis, MO) was added and after 16 hours at 37°C, the plates were screened for colony formation; the number of colonies larger than 50 µm in diameter were counted using an Omnicon image analysis system.
TGFα ELISA
NCI-H1299 cells in 24 well plates were washed in 0.25 ml of SIT media. The cells were incubated with inhibitors such as 10 µM GM6001 for 30 min, followed by addition of 100 nM NTS for 5 min. The supernatant was collected and assayed for TGFα by ELISA (R & D; Minneapolis, MN).
Results
NTSR1 knock-down and effects of SR48692 on NSCLC cells
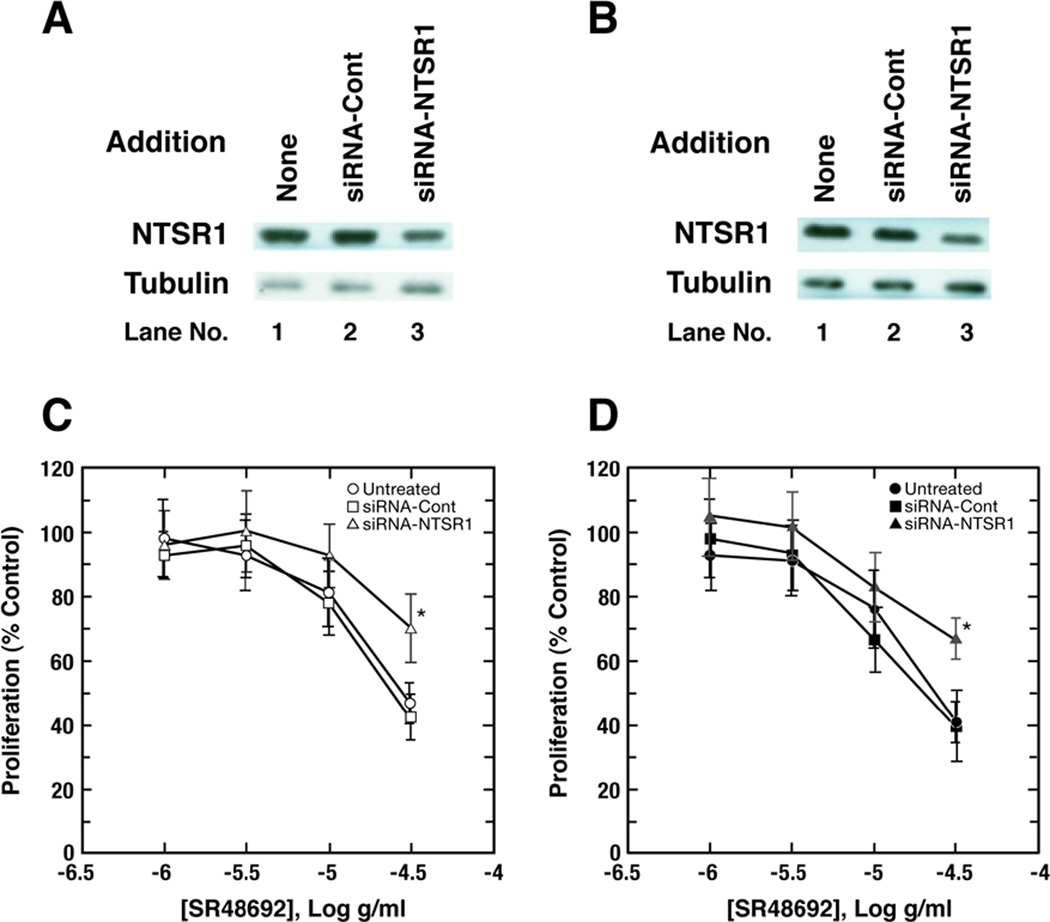
NTSR1 was investigated in NSCLC cells by Western blot and receptor binding. Table I shows that specific 125I-Tyr3-NTS binding to NCI-H1299 cells was inhibited with high affinity by NTS, NTS8–13, Ac-NTS8–13 but not NTS1–8 with IC50 values of 4, 10, 7 and >2000 nM, respectively. Also, SR48692 inhibited specific binding of radiolabelled NTS to NCI-H1299 cells (IC50 value of 205 nM), whereas levocabastine, a NTSR2 agonist, and gefitinib did not (IC50 values of >2000 and >2000 nM, respectively). Table I shows that addition of NTS, NTS8–13 and Ac-NTS8–13 to NCI-H1299 cells significantly increased EGF tyrosine phosphorylation whereas NT1–8, SR48692, gefitinib or levocabastine did not. These results indicate that NTS, NTS8–13 and Ac-NTS8–13 are biologically active. By Western blot, a 55 kDal band was present in extracts derived from A549 cells and NCI-H1299 cells (Fig. 1). Treatment of NCI-H1299 or A549 cells with siRNA for NTSR1 reduced the band intensity by 52 % and 44 % respectively, whereas treatment with control siRNA had little effect (Fig.1A, 1B). These results indicate that NTSR1 is present in NSCLC cells.
Table I.
NTS ligands
| Addition | IC50, nM | % EGFR transactivation |
|---|---|---|
| NTS | 4 ± 1 | 367 ± 31** |
| NTS8–13 | 10 ± 2 | 282 ± 24** |
| Ac-NTS8–13 | 7 ± 1 | 330 ± 28** |
| NT1–8 | >2000 | 105 ± 7 |
| SR48692 | 205 ± 31 | 95 ± 8 |
| Gefitinib | >2000 | 98 ± 6 |
| Levocabastine | >2000 | 102 ± 5 |
The ability of ligands to half-maximally (IC50) inhibit specific 125I-Tyr3-NTS binding to NCI-H1299 cells was determined at 37°C. The % EGFR tyrosine phosphorylation was determined 2 min after the addition of 100 nM of the indicated compound to NCI-H1299 cells. The mean value ± S.D. of 4 determinations is shown;
p < 0.01, ** by ANOVA.
The NTS structure is:
Pyr-Leu-Tyr-Glu-Asn-Lys-Pro-Arg-Arg-Pro-Tyr-Ile-Leu
Fig. 1.
NTSR1 siRNA. By Western blot, siRNA-NTSR1 but not siRNA-control (Cont) decreased NTSR1 but not tubulin in (A) A549 and (B) NCI-H1299 cells. This experiment is representative of 3 others. Using the MTT assay the ability of varying concentrations of SR48692 to inhibit the proliferation of (C) A549 and (D) NCI-H1299 cells was investigated. The mean value ± S.D. of 8 determinations is indicated; p < 0.05, *; for siRNA-NTSR1 relative to untreated or siRNA-Cont. treated cells by ANOVA. This experiment is representative of 2 others.
Previously NTSR1 knock-down reduced A549 proliferation in vitro and in vivo (Alfano et al., 2010). Here the growth effects of SR48692 were investigated. Fig. 1C shows that using untreated A549 cells, A549 cells treated with NTSR1 siRNA and cells treated with control siRNA, the IC50 values for SR48692 growth reduction were 28 µg/ml, >40 µg/ml and 25 µg/ml respectively. Similarly using untreated NCI-H1299, cells treated with NTSR1 siRNA and cells treated with control siRNA, the IC50 values for SR48692 to inhibit proliferations were 26 µg/ml, >40 µg/ml and 21 µg/ml respectively (Fig. 1D). The results indicate that NTSR1 knock-down significantly impairs the growth inhibitory potency of SR48692 in NSCLC cells.
NTSR1 knock-down and EGFR transactivation
The mechanism by which SR48692 inhibited the growth of NSCLC cells was investigated. By Western blot, the EGFR is present on A549 and NCI-H1299 cells. Fig 2A shows that NTS (100 nM) addition to NCI-H1299 cells, increased significantly phosphorylation of the EGFR to 311%. Addition of NTS plus 0.5 ug/ml SR48692 significantly increased EGFR tyrosine phosphorylation to 193% (Fig. 2E). Similarly addition of 100 nM NTS to A549 cells increased significantly EGFR tyrosine phosphorylation to 248% (Fig 2C). Addition of NTS plus 0.5 µg/ml SR48692 to A549 cells significantly increased EGFR tyrosine phosphorylation to 132% (Fig. 2G). The results indicate that SR48692 significantly inhibits the EGFR transactivation caused by NTS addition to untreated NCI-H1299 or A549 cells.
Fig. 2.
EGFR transactivation. The ability of 100 nM NTS and 0.5 µg/ml SR48692 to alter EGFR tyrosine phosphorylation of Tyr1068 was investigated in untreated NCI-H1299 cells (A) and siRNA-NTSR1 treated NCI-H1299 (B) cells. As controls, total EGFR and tubulin were not affected by siRNA-NTSR1. The mean value ± S.D. of 3 determinations is indicated using NCI-H1299 untreated cells (E) and cells treated with siRNA-NTSR1 (F); p < 0.01, **; p < 0.05, * relative to Lane 1, p < 0.05, a relative to lane 2. The ability of 100 nM NTS and 0.5 µg/ml SR48692 to alter EGFR tyrosine phosphorylation of Tyr1068 was investigated in untreated NCI-A549 cells (C) and siRNA-NTSR1 treated A549 (D) cells was investigated. As controls, total EGFR and tubulin are provided. The mean value ± S.D. of 3 determinations is indicated using A549 untreated cells (G) and cells treated with siRNA-NTSR1 (H); p < 0.01, **; p < 0.05, * relative to Lane 1, p < 0.05, a relative to lane 2 by ANOVA.
The effects of NTSR1 knock-down on EGFR transactivation were investigated. NTS (100 nM) addition to NCI-H1299 cells treated with siRNA for NTSR1 increased EGFR tyrosine phosphorylation significantly to 211% (Fig.2B). Addition of NTS plus 0.5 µg/ml SR48692 significantly increased EGFR transactivation to 179% (Fig. 2F). The results indicate that SR48692 did not significantly alter the ability of NTS to increase EGFR transactivation using NCI-H1299 cells treated with siRNA-NTSR1. As controls, siRNA for NTSR1 had little effect on total EGFR or tubulin. Addition of 100 nM NTS to A549 cells treated with siRNA to NTSR1 increased EGFR tyrosine phosphorylation to 155% (Fig. 2D). Addition of NTS and 0.5 µg/ml SR48692 to NCI-H1299 cells treated with siRNA NTSR1 increased EGFR transactivation significantly to 135% (Fig.2H). SR48692 did not significantly alter the ability of NTS to cause EGFR transactivation in A549 cells treated with siRNA to NTSR1. The results indicate that treatment of NCI-H1299 cells of A549 cells with siRNA-NTSR1 reduces the EGFR transactivation stimulation caused by addition of NTS and impairs the inhibition caused by addition of SR48692.
Effect of SR48692 or gefitinib dose on EGFR and ERK tyrosine phosphorylation
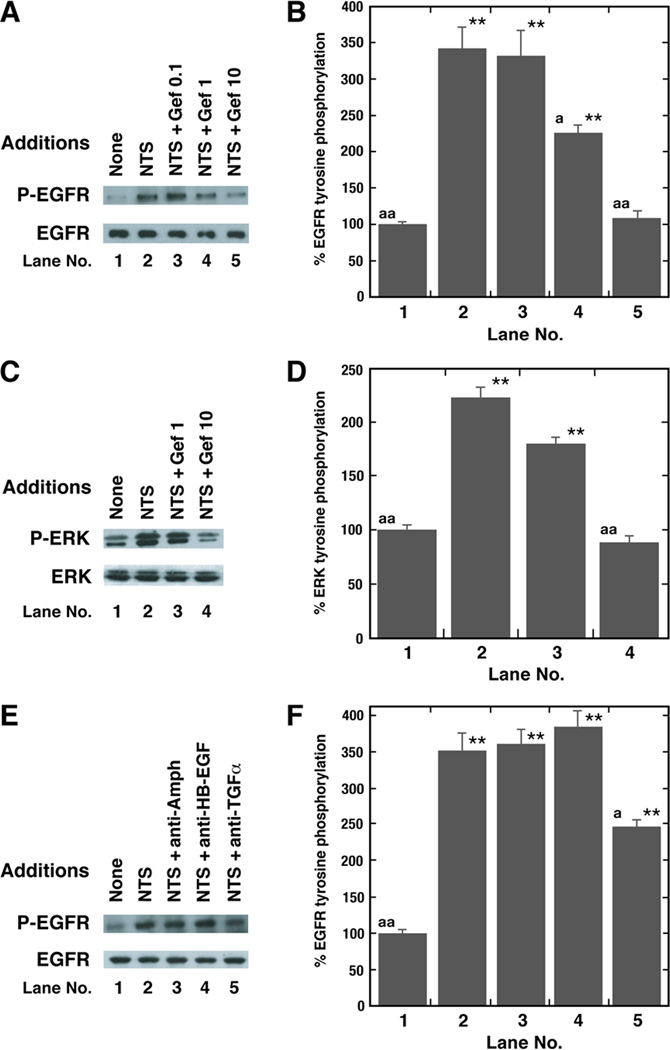
The dose-response curve of SR48692 to inhibit EGFR or ERK tyrosine phosphorylation was investigated in untreated NCI-H1299 cells. Figure 3A shows that 1 µg/ml SR48692 moderately and 10 µg/ml SR48692 strongly inhibited the increase in EGFR tyrosine phosphorylation caused by the addition of 100 nM NTS to NCI-H1299 cells. In contrast, SR48692 had no effect on basal P-EGFR. Fig. 3B shows that NTS addition to NCI-H1299 cells significantly increased EGFR tyrosine phosphorylation by 392% and this increase was significantly inhibited by 1 µg/ml SR48692 (p < 0.05) and 10 µg/ml SR48692 (p < 0.01). SR48692 (10 µg/ml) by itself had little effect on total EGFR or ERK. Addition of 100 nM NTS to NCI-H1299 cells significantly increased ERK tyrosine phosphorylation by 186% (Fig. 3C). Similarly, 1 µg/ml SR48692 (p < 0.05) and 10 µg/ml SR48692 (p < 0.01) significantly inhibited the ability of NTS to increase ERK tyrosine in NCI-H1299 cells (Fig. 3D). These results suggest that the NTSR1 regulates EGFR and ERK tyrosine phosphorylation in lung cancer cells.
Fig. 3.
Inhibition of EGFR and ERK tyrosine phosphorylation by SR48692. (A) The ability of NTS to increase EGFR or ERK tyrosine phosphorylation was investigated as a function of SR48692 (SR) concentration using NCI-H1299 cells. The P-EGFR and total EGFR is shown. (B) The mean value ± S.D. of 3 experiments is indicated; p < 0.01, ** relative to control; p < 0.05, a, p < 0.01, aa relative to NTS by ANOVA. (C) The ability of NTS addition to NCI-H1299 cells to cause ERK tyrosine phosphorylation was investigated using 1 or 10 µg/ml SR48692. (D) The mean values ± S.D. of 3 experiments is indicated; p < 0.01, ** relative to control; p < 0.05, a; p < 0.01, aa relative to NTS by ANOVA.
The ability of gefitinib to inhibit EGFR and ERK tyrosine phosphorylation was investigated. Figure 4A shows that using NCI-H1299 cells 1 or 10 µg/ml, but not 0.1 µg/ml gefitinib, impaired the ability of NTS to cause EGFR transactivation. In contrast, gefitinib had no effect on total EGFR. Figure 4B shows that NTS increased EGFR tyrosine phosphorylation by 346% and the increase caused by NTS was reduced significantly by 1 µg/ml gefitinib (p < 0.05) and 10 µg/ml gefitinib ((p < 0.01), whereas 0.1 µg/ml gefitinib had little effect. Figure 4C shows that addition of 100 nM NTS to NCI-H1299 cells increased ERK tyrosine phosphorylation which was inhibited by 10 µg/ml gefitinib. NTS increased ERK tyrosine phosphorylation by 226% and the increase was significantly inhibited by 10 µg/ml gefitinib (p < 0.01), but not 1 µg/ml gefitinib (Fig. 4D). The MEK inhibitor PD98059 inhibited the ability of NTS to increase ERK, but not EGFR tyrosine phosphorylation (data not shown). These results indicate that the NTS tyrosine phosphorylation of ERK is downstream from that of the EGFR.
Fig. 4.
Inhibition of EGFR and ERK tyrosine phosphorylation by gefitinib. (A) The ability of NTS to caused EGFR tyrosine phosphorylation was investigated as a function of gefitinib concentration using NCI-H1299 cells. The P-EGFR and total EGFR is shown. (B) The mean value ± S.D. of 4 determinations is indicated; p < 0.01, ** relative to control; p < 0.05a; p < 0.01, aa relative to NTS by ANOVA. (C) ERK tyrosine phosphorylation was determined after addition of 100 nM NTS to NCI-H1299 cells in the absence or presence of 1 or 10 µg/ml gefitinib. The P-ERK and total ERK are shown. (D) The mean value ± S.D. of 3 experiments is indicated; p < 0.01 relative to control, **; p < 0.01 relative to 100 nM NTS; p < 0.05, a; p < 0.01, aa by ANOVA. (E) The increase in EGFR transactivation caused by addition of 100 nM NTS to NCI-H1299 cells was inhibited by 5 µg/ml anti-TGFα but not anti-HB-EGF or anti-amphiregulin (Amph). The P-EGFR and total EGFR are shown. (F) The mean value ± S.D. of 4 experiments is indicated; p < 0.05, *; p , 0.01, ** relative to control; p < 0.05, a; p < 0.01, aa relative to 100 nM NTS by ANOVA.
TGFα and EGFR transactivation
The ligand which causes EGFR transactivation was investigated. Figure 4E shows that NTS addition to NCI-H1299 cells increased EGFR tyrosine phosphorylation which was altered by anti-transforming growth factor (TGFα) not altered by anti-amphiregulin (Amph) or anti-heparin binding-EGF (HB-EGF). NTS increased EGFR tyrosine phosphorylation by 351% which was significantly inhibited by anti-transforming growth factor (TGF)-α (p < 0.05) but not anti-amphiregulin or anti-HB-EGF (Fig. 4F). NTS significantly increased by 550% the secretion rate of TGFα from NCI-H1299 cells (Table II). In contrast, GM6001, a matrix metalloprotease (MMP) inhibitor reversed the TGFα secretion caused by NTS addition to NCI-H1299 cells. These results suggest that prepro-TGFα is metabolized by MMP in lung cancer cells to biologically active TGFα.
Table II.
TGFα release from NSCLC cells.
| Addition | TGFα (pg/ml) |
|---|---|
| None | 8 ± 2 |
| NTS (100 nM) | 44 ± 7** |
| NTS + GM6001 (10 µM) | 9 ± 3 |
NCI-H1299 cells were treated with 100 nM NTS for 5 min and the supernatant removed and assayed for immunoreactive TGFα. The mean value ± S.D. of 3 determinations is indicated;
, p < 0.01 by ANOVA.
Mechanisms of EGFR transactivation
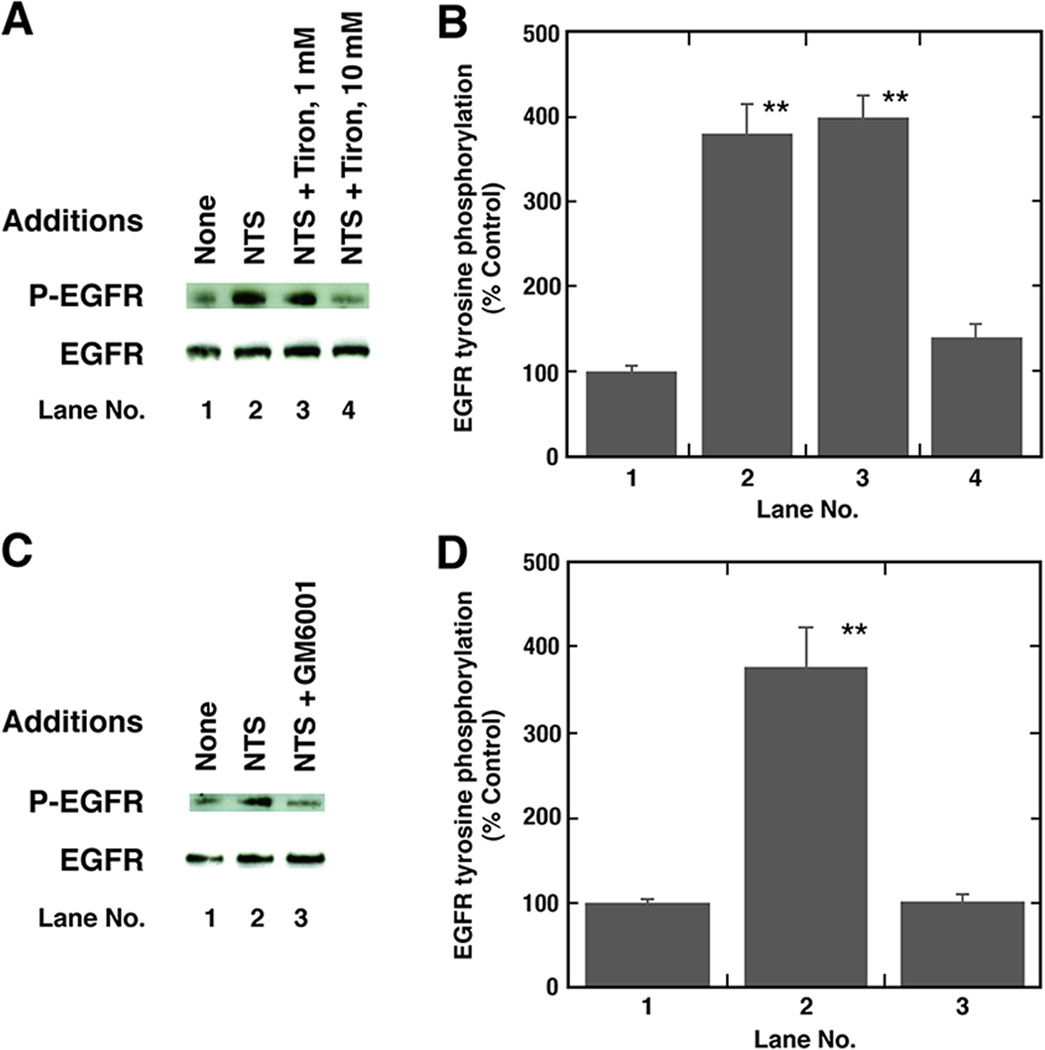
To further investigate the cellular signaling involved in EGFR transactivation, various inhibitors were used. Figure 5A shows that Tiron, a superoxide scavenger, in a dose-dependent manner inhibited the ability of NTS to increase EGFR tyrosine phosphorylation caused by the addition of NTS to NCI-H1299 cells. Fig. 5B shows that NTS addition to NCI-H1299 cells increased EGFR tyrosine phoshorylation by 381% and this increase was significantly inhibited by 10 mM but not 1 mM Tiron. NTS and H2O2 significantly increased reactive oxygen species (ROS) in NCI-H1299 cells by 27% and 66%, respectively (Table III). The increase in ROS caused by addition of NTS to NCI-H1299 cells was significantly inhibited by the addition of Tiron to NCI-H1299 cells. These results indicate that NTSR1 regulation of EGFR transactivation is dependent upon ROS.
Fig. 5.
Inhibition of EGFR transactivation by tiron and GM6001. (A) The increase in EGFR tyrosine phosphorylation caused by NTS was investigated as a function of Tiron (Tir) addition to NCI-H1299 cells. (B) The mean value ± S.D. of 4 experiments is indicated; p < 0.05, *; p < 0.01, **. (C) Ten µM GM6001 inhibited EGFR tyrosine phoshorylation caused by addition of 100 nM NTS to NCI-H1299 cells. (D) The mean value ± S.D. of 3 experiments is indicated; p < 0.05, *; p < 0.01, ** by ANOVA.
Table III.
Reactive oxygen species.
| Addition | Relative fluorescence |
|---|---|
| None | 100 ± 3 |
| NTS (100 nM) | 127 ±8** |
| NTS + Tiron (5 mM) | 99 ± 4 |
| H2O2 | 166 ± 16** |
NCI-H1299 cells were treated with 100 nM NTS for 15 min and the relative fluorescence determined. The mean value ± S.D. of 8 determinations is indicated;
, p < 0.01 by ANOVA.
Also, GM6001, which inhibits MMP, impaired the ability of NTS to cause EGFR tyrosine phosphorylation (Fig 5C). Figure 5D shows that NTS increased EGFR tyrosine phosphorylation by 378% which was inhibited significantly by GM6001. These data suggest that NTS causes EGFR transactivation in lung cancer cells in a MMP-dependent manner.
Figure 6A shows that 2 min after the addition of NTS to NCI-H1299 cells, EGFR, PYK-2, Src and β-catenin tyrosine phosphorylation increased significantly. As a control, NTS had no effect on tubulin. Fig. 6B shows that NTS significantly increased EGFR, PYK-2, Src and β-catenin tyrosine phosphorylation by 366%, 266%, 292% and 183%, respectively. Addition of the PLC inhibitor U73122, but not the PKA inhibitor H89, significantly inhibited the ability of NTS to cause tyrosine phosphorylation of the EGFR, PYK-2, Src and β-catenin. Addition of BAPTA, which reduces cytosolic Ca2+, impaired the ability of NTS to cause tyrosine phosphorylation of PYK-2 but not the phosphorylation of the EGFR, Src or β-catenin. The results show that NTS addition to NSCLC cells increases EGFR, PYK-2, Src and β-catenin tyrosine phosphorylation in a PLC but not PKA dependent manner. Further NTS causes PYK-2 tyrosine phosphorylation in a Ca-2+ dependent manner.
Fig. 6.
NTS causes phosphorylation of EGFR, Src, PYK-2 and β-catenin. (A) NCI-H1299 cells were treated with BAPTA, H89 or U73122 for 30 min and 100 nM NTS added for 2 min. Protein tyrosine phosphorylation was determined for EGFR, PYK-2, Src and β-catenin. Also, total tubulin is indicated. (B) The mean value ± S.D. of 4 determinations is indicated; p < 0.05, *; p < 0.01, ** relative to control by ANOVA.
Gefitinib and SR48692 inhibit NSCLC proliferation
The ability of SR48692 and gefitinib to inhibit the growth of NCI-H1299 cells was investigated. SR48692 and gefitinib reduced significantly NCI-H1299 proliferation in the clonogenic assay (Table IV). SR48692 and gefitinib moderately inhibited NCI-H1299 colony formation alone, however, when combined SR48692 and gefitinib strongly reduced colony number. NTS (10 nM) significantly increased colony number. The increase in colony number caused by NTS was inhibited by SR48692 and/or gefitinib.
Table IV.
Clonal growth of NCI-H1299 cells
| Addition | Colony number |
|---|---|
| None | 21 ± 3 |
| Gef, 1 µg/ml | 13 ± 2* |
| SR48692 3 µg/ml | 10 ± 5* |
| SR48692 + Gef | 5 ± 1** |
| NTS, 10 nM | 37 ± 5** |
| NTS + Gef | 25 ± 4 |
| NTS + SR48692 | 22 ± 2 |
| NTS + Gef + SR | 15 ± 2* |
The ability of gefitinib (Gef), SR48692 (SR) and NTS to alter NCI-H1299 colony number was determined. The mean colony number ± S.D. of 3 determinations each repeated in triplicate is indicated;
p < 0.05; *;
p < 0.01; ** relative to no addition by ANOVA.
Discussion
NSCLC patients are treated traditionally with combination chemotherapy, however, the 5 year patient survival rate is only 16%. EGFR tyrosine kinase inhibitors have been approved for treating NSCLC patients who fail chemotherapy (Lynch et al., 2004; Paez et al., 2004). EGFR mutations of the ATP binding site are associated with patient response to EGFR tyrosine kinase inhibitors (Helfrich et al., 2006). Approximately 88% of the NSCLC patients, however, have wild type EGFR with reduced sensitivity to gefitinib, suggesting the need for new therapeutic approaches.
NTS is synthesized in, secreted from and binds with high affinity to SCLC cells (Moody et al., 1985). Because NTS stimulated and SR48692 inhibited the growth of SCLC cells in vitro and in vivo, NTSR1 may regulate the proliferation of SCLC cells (Moody et al., 2001). In this communication, NTS binds with high affinity to NSCLC cells and specific NTS binding is inhibited with high affinity by NTS, NTS8–13, Ac-NTS8–13 and SR48692, but not levocabastine. By Western blot a major 55 KDal band of NTSR1 immunoreactivity was present in extracts derived from NCI-H1299 and A549 cells. Treatment of NCI-H1299 and A549 cells with siRNA-NTSR1 but not siRNA control reduced the NTSR1 by approximately 50% but had little effect on total EGFR or tubulin. Further siRNA-NTSR1, but not siRNA-control, reduced the growth inhibitory potency of SR48692 on NCI-H1299 and A549 cells. Routinely, 0.5 µg/ml SR48692 patially inhibited the EGFR transactivation caused by the addition of 100 nM NTS to lung cancer cells, whereas 10 µg/ml SR48692 completely inhibited the increase in EGFR and ERK tyrosine phosphorylation of the EGFR and ERK caused by the addition of NTS to lung cancer cells for 2 min. The ability of SR48692 to inhibit NSCLC growth may be a function of NTSR1 density.
Recent crystal structure studies indicate that NTS8–13 binds to the NTSR1 perpendicular to the membrane plane with the C-terminus oriented toward the receptor core (White et al., 2012). The activated NTSR1 interacts with a guanine nucleotide binding subunit (Gq) leading to PI turnover (Moody et al., 2003). PI metabolites cause elevation of cytosolic Ca2+ within seconds after addition of NTS to NSCLC cells and PKC activation (Jensen and Moody, 2006). The metabolism of PI can be impaired by the phospholipase C inhibitor U73122, but not the PKA inhibitor H89. In our studies, U73122 but not H89, inhibited the ability of NTS to cause tyrosine phosphorylation of the EGFR, Src, PYK-2 and β-catenin demonstrating their activation was dependent on NTS stimulated PLC activation. BAPTA, which reduces cytosolic Ca2+, impaired the ability of NTS to cause PYK-2 but not EGFR, Src or β-catenin tyrosine phosphorylation in lung cancer cells demonstrating that PYK-2 activation is different from EGFR, Src or β-catenin. In prostate cancer cells, NTS increased tyrosine phosphorylation of c-Src, Stat5b and the EGFR (Amorino et al., 2007). Using PC-3 cells, NTS slowly increased phosphorylation of Y845 of the EGFR or Y419 of Src after 60 min. In our studies, 2 min after addition of NTS to NSCLC cells, phosphorylation of the EGFR, ERK, and Src rapidly increased demonstrating that NTS-mediated EGFR activation can vary in different cells.
Src interacts with FAK and/or PYK-2 causing their tyrosine phosphorylation. NTS addition to NSCLC cells causes increased FAK phosphorylation which is inhibited by SR48692 (Leyton et al., 2002). FAK is a 125 kDal non-receptor tyrosine kinase which is structurally related to the 116 kDal PYK-2, which is activated by an increase in cytoplasmic Ca2+. FAK and PYK-2 have a central catalytic domain flanked by an N-terminal which has SH2- and SH3-binding sites and a C-terminal which contains two proline-rich domains (Hall et al., 2011). PYK-2 is expressed at high levels in 62% of the NSCLC tumors and higher expression of PYK-2 was associated with NSCLC metastasis form the lung to the lymph node (Zhang et al., 2008). PYK-2 is present in several NSCLC cell lines including NCI-H1299 and A549. The role of FAK or PYK-2 in the proliferation of NSCLC cells remains to be determined.
When β-catenin is phosphorylated it dissociates from E-cadherin, redistributes to the nucleus and the forms a transcriptionally active β-catenin/TCF complex. The NTSR1 gene is a target of Wnt/APC associated with the β-catenin/Tcf transcriptional complex (Souaze et al., 2006). Thus increased tyrosine phosphorylation of β-catenin may lead to increased expression of NTSR1 resulting in cancer proliferation.
The MMP inhibitor GM6001 reduced the phosphorylation of the EGFR caused by NTS addition to lung cancer cells. GM6001 may inhibit lung cancer MMP activity leading to reduced secretion of TGFα from lung cancer cells. NTS addition to NCI-H1299 cells increased TGFα in conditioned media by 550% and the increased secretion was reversed by GM6001. Furthermore, antibodies to TGFα, but not amphiregulin or heparin binding growth factor impaired the ability of NTS to cause EGFR transactivation. These results suggest that NTSR1 regulates matrix metalloprotease to convert prepro-TGFα into biologically active TGFα which binds with high affinity to the EGFR causing phosphorylation of Tyr1068. Endothelin addition to ovarian cancer cells caused Y654 phosphorylation of β-catenin and dissociation from E-cadherin complexes. The activated β-catenin localized to the nucleus altering Tcf activity. Activation of the Tcf transcriptional complex increases expression of MMP genes, resulting in increased invasion behavior of tumor cells (Cianfrocca et al., 2012).
The activated NTSR1 mediates EGFR transactivation that is dependent on generation of reactive oxygen species. The transactivation of the EGFR caused by NTS addition to NSCLC cells is reversed by tiron. Furthermore, we found an increase in reactive oxygen species caused by NTS addition to NSCLC cells that was reversed by tiron. Previously it was shown that the endothelin 1 receptor regulates oxidation of cysteine amino acid residues in the catalytic Src homology 2-containing tyrosine phosphatase, which reduces phosphatase enzymatic activity (Cheng-Hsien et al., 2006). It remains to be determined if NTS addition to lung cancer cells alters phosphatase activity.
Using the clonogenic assay, 3 µg/ml SR48692 significantly reduced NCI-H1299 colony number. Furthermore the growth inhibitory effects of SR48692 were reversed by NTS. Gefitinib (1 µg/ml) moderately reduced, whereas gefitinib plus SR48692 strongly reduced NCI-H1299 colony number. The concentration of gefitinib used here is similar to the plasma level found in patients (0.5 µg/ml) 4 hours after injection of 250 mg of gefitinib (Faivre et al., 2011). SR48692 does not inhibit all NSCLC proliferation because SR48692 blocks the EGFR transactivation caused by NTS, but has no effect on EGFR transactivation caused by other endogenous growth factors such as bombesin (BB)-like peptides or pituitary adenylate cyclase activating polypeptide (PACAP) (Moody et al, 2010, 2012). In contrast, gefitinib blocks the RTK downstream from the GPCR.
Summary.
SR48692, a NTSR1 antagonist, decreases the proliferation of NSCLC cells in an EGFR-dependent manner. Knock-down of the NTSR1 decreases the growth inhibitory and EGFR transactivation effects of SR48692 on NSCLC cells. Using untreated NSCLC cells, the ability of NTS to increase EGFR transactivation is inhibited by gefitinib or SR48692. The proliferation of NSCLC cells is inhibited by gefitinib or SR48692 in vitro, however their combination has enhanced activity. It remains to be determined if the potency of gefitinib in NSCLC cells with wild type EGFR may be increased in vivo, using GPCR antagonists such as SR48692.
Acknowledgments
The authors thank Dr. David Wink for helpful discussions. This research is partially supported by the intramural research program of the NIDDK and NCI, of NIH.
Footnotes
Conflicts of Interest statement:
The authors declare that there is no conflict of interest.
References
- 1.Alfano M, Souaze F, Dupouy S, Camilleri-Broet S, Younes M, Ahmed-Zaid SM, et al. Neurotensin receptor 1 determines the outcome of non-small cell lung cancer. Clin Cancer Res. 2010;16:4401–4410. doi: 10.1158/1078-0432.CCR-10-0659. [DOI] [PubMed] [Google Scholar]
- 2.Amorino GP, Deeble PD, Parsons SJ. Neurotensin stimulates mitogenesis of prostate cancer cells through a novel c-Src/Stat5b pathway. Oncogene. 2007;26:745–756. doi: 10.1038/sj.onc.1209814. [DOI] [PubMed] [Google Scholar]
- 3.Betancur C, Azzi M, Rostene W. Nonpeptide antagonists of neuropeptide receptors: Tools for research and therapy. TIPS. 1998;18:372–386. doi: 10.1016/s0165-6147(97)01109-7. [DOI] [PubMed] [Google Scholar]
- 4.Carraway R, Leeman SE. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalamus. J Biol Chem. 1978;248:6854–6861. [PubMed] [Google Scholar]
- 5.Cheng-Hsien C, Yung-Ho H, Yuh-Mou S, Chun-Cheng H, Horng-Mo L, Huei-Mei H, et al. Src homology 2-containing phosphotyrosine phosphatase regulates endothelin 1-induced epidermal growth factor receptor transactivation in rat renal tubular cell NRK-52E. Pflugers Arc. 2006:452, 16–24. doi: 10.1007/s00424-005-0006-9. [DOI] [PubMed] [Google Scholar]
- 6.Cianfrocca R, Tocci P, Spinella F, DiCastro V, Bagnato A, Rosano L. The endothelin A receptor and epidermal growth factor receptor signaling converge on β-catenin to promote ovarian cancer metastasis. Life Sci. 2012;91:550–556. doi: 10.1016/j.lfs.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 7.Dupouy S, Mourra N, Doan WK, Gompel A, Alifano M, Forgez P. The potential use of the neurotensin high affinity receptor 1 as a biomarker for cancer progression and as a component of personalized medicine in selective cancers. Biochimie. 2011;93:1369–1378. doi: 10.1016/j.biochi.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 8.Dupouy S, Viardot-Foucault V, Alifano M, Souaze F Pul-Dureau G, Chaouat M, et al. The neurotensin receptor-1 pathway contributes to human ductal breast cancer progression. PLoS ONE. 2009;4:e4223. doi: 10.1371/journal.pone.0004223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evers BM. Neurotensin and growth of normal and neoplastic tissues. Peptides. 2006;27:2424–2433. doi: 10.1016/j.peptides.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 10.Faivre L, Gomo C, Mir O, Taieb F, Schoemann-Thomas SR, Vidal M, et al. A simple HPLC-UV method for the simultaneous quantification of gefitinib and erlotinib in human plasma. J. Chromatography B. 2011;879:2345–2350. doi: 10.1016/j.jchromb.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 11.Guha S, Rey O, Rozengurt E. Neurotensin induces protein kinase C-dependent protein kinase D activation and DNA synthesis in human pancreatic carcinoma cell line PANC-1. Cancer Res. 2002;62:1632–1640. [PubMed] [Google Scholar]
- 12.Gulley D, Canton M, Boigegrain R, Jeanjean G, Molimard JD, Poncelet M, et al. Biochemical and pharmacological profile of a potent and selective nonpeptide antagonist of the neurotensin receptor. Proc Natl Acad Sci USA. 1993;90:65–69. doi: 10.1073/pnas.90.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall JE, Fu W, Schaller MD. Focal adhesion kinase: Exploring FAK structure to gain insight into function. Int. Rev Cell Mol Biol. 2011;288:185–225. doi: 10.1016/B978-0-12-386041-5.00005-4. [DOI] [PubMed] [Google Scholar]
- 14.Hassan S, Dobner PR, Carraway RE. Involvement of MAP kinase, PI-3 kinase and EGF-receptor in the stimulatory effect of neurotensin on DNA synthesis in PC3 cells. Regul Pept. 2004;120:155–166. doi: 10.1016/j.regpep.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Heakal Y, Woll MP, Fox T, Seaton K, Levenson R, Kester M. Neurotensin receptor-1 inducible palmitoylation is required for efficient receptor-mediated mitogenic-signaling within structured membrane microdomains. Cell Biology & Therapy. 2011;12:427–435. doi: 10.4161/cbt.12.5.15984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helfrich BA, Raben D, Varella-Garcia M, Gustafson D, Chan DC, Bemis L, et al. Antitumor activity of the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor gefitinib (ZD1839, Iressa) in non-small cell lung cancer cell lines correlates with gene copy number and EGFR mutations but not EGFR protein levels. Clin Cancer Res. 2006;12:7117–7125. doi: 10.1158/1078-0432.CCR-06-0760. [DOI] [PubMed] [Google Scholar]
- 17.Jensen RT, Moody TW. Bombesin-related peptides and neurotensin: Effects on cancer growth/proliferation and cellular signaling in cancer, in. In: Kastin AJ, editor. Handbook of Biologically Active Peptides. Amsterdam: Elsevier; 2006. pp. 429–434. [Google Scholar]
- 18.Kisfalvi K, Guha S, Rozengurt E. Neurotensin and EGF induce synergistic stimulation of DNA synthesis by increasing the duration of ERK signaling in ductal pancreatic cancer cells. J Cell Physiol. 2005;202:880–890. doi: 10.1002/jcp.20187. [DOI] [PubMed] [Google Scholar]
- 19.Lee LF, Guan J, Qiu Y, Kung HJ. Nonpeptide-induced androgen independence in prostate cancer cells: Roles of nonreceptor tyrosine kinases Etk/Bmx, Src and focal adhesion kinase. Mol. Cell Biol. 2001;21:8385–8397. doi: 10.1128/MCB.21.24.8385-8397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leyton J, Garcia-Marin L, Jensen RT, Moody TW. Neurotensin causes tyrosine phosphorylation of focal adhesion kinase in lung cancer cells. Eur J Pharmacol. 2002;442(3):179–186. doi: 10.1016/s0014-2999(02)01539-x. [DOI] [PubMed] [Google Scholar]
- 21.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 22.Maoret JJ, Anini Y, Rouyer-Fessard C, Gully D, Laburthe M. Neurotensin and a non-peptide neurotensin receptor antagonist control human colon cancer cell growth in cell culture and in cells xenografted into nude mice. Int J Cancer. 1999;80:448–454. doi: 10.1002/(sici)1097-0215(19990129)80:3<448::aid-ijc19>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 23.Moody TW, Berna MJ, Mantey S, Sancho V, Ridnour L, Wink DA, et al. Neuromedin B receptors regulate EGF receptor tyrosine phosphorylation in lung cancer cells. Eur J Pharm. 2010;637:38–45. doi: 10.1016/j.ejphar.2010.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moody TW, Carney DN, Korman LY, Gazdar AF, Minna JD. Neurotensin is produced by and secreted from classic small cell lung cancer cells. Life Sci. 1985;36:1727–1732. doi: 10.1016/0024-3205(85)90555-7. [DOI] [PubMed] [Google Scholar]
- 25.Moody TW, Chiles J, Casibang M, Moody E, Chan D, Davis TP. SR48692 is a neurotensin receptor antagonist which inhibits the growth of small cell lung cancer cells. Peptides. 2001;22:109–115. doi: 10.1016/s0196-9781(00)00362-4. [DOI] [PubMed] [Google Scholar]
- 26.Moody TW, Chan D, Fahrenkrug J, Jensen RT. Neuropeptides as autocrine growth factors in cancer cells. Current Pharmaceutical Design. 2003;9:495–509. doi: 10.2174/1381612033391621. [DOI] [PubMed] [Google Scholar]
- 27.Moody TW, Osefo N, Nuche-Berenguer B, Ridnour L, Wink D, Jensen RT. Pituitary adenylate cyclase activating polypeptide causes tyrosine phosphorylation of the EGF receptor in lung cancer cells. J. Pharmacol. Exp. Ther. 2012;341:873–881. doi: 10.1124/jpet.111.190033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muller KM, Tveteraas IH, Aasrum M, Odegard J, Dawood M, Dajani O, et al. Role of protein kinase C and epidermal growth factor receptor signaling in growth stimulation by neurotensin in colon carcinoma cells. BMC Cancer. 2011;11:421–432. doi: 10.1186/1471-2407-11-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paez JG, Janne PA, Lee JC, Tracey S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 30.Reubi JC, Waser B, Schaer JC, Laissue JA. Neurotensin receptors in human neoplasem: High incidence in Ewing’s Sarcoma. Int J Cancer. 1999;82:213–218. doi: 10.1002/(sici)1097-0215(19990719)82:2<213::aid-ijc11>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 31.Servotte S, Camby O, Debeir FC, Deroanne C, Lambert CA, Lapiere CM, et al. The in vitro influences of neurotensin of the motility characteristics of human U373 cells. Neuropathol Appl Neurobiol. 2006;32:575–584. doi: 10.1111/j.1365-2990.2006.00760.x. [DOI] [PubMed] [Google Scholar]
- 32.Shimizu S, Tsukada J, Sugimoto T, Kikkawa K, Sasaki H, Chazono T, et al. Identification of a novel therapeutic target for head and neck squamous cell carcinomas: A role for the neurotensin-neurotensin receptor 1 oncogenic signaling pathway. Int J Cancer. 2008;123:1816–1823. doi: 10.1002/ijc.23710. [DOI] [PubMed] [Google Scholar]
- 33.Souaze R, Viardot-Foucault V, Roullet N, Tor-Miou–Leong M, Gompel A, Bruyneel E, et al. Neurotensin receptor 1 gene activation activation by the Tcf/β-catenen pathway is an early event in human colonic adenomas. Carcinogenesis. 2006;27:708–716. doi: 10.1093/carcin/bgi269. [DOI] [PubMed] [Google Scholar]
- 34.Staley J, Fiskum G, Davis TP, Moody TW. Neurotensin elevates cytosolic calcium in small cell lung cancer cells. Peptides. 1989;10:1217–1221. doi: 10.1016/0196-9781(89)90015-6. [DOI] [PubMed] [Google Scholar]
- 35.Valerie NC, Casarez EV, Dasilva JO, Dunlap-Brown ME, Parsons SJ, Amorino GP, et al. Inhibition of neurotensin receptor1 selectively sensitizes prostate cancer to ionizing radiation. Cancer Res. 2011;71:6817–6826. doi: 10.1158/0008-5472.CAN-11-1646. [DOI] [PubMed] [Google Scholar]
- 36.Wang JI, Li NN, Li HN, Cui L, Wang P. Pancreatic cancer bears overexpression of neurotensin and neurotensin receptor subtype-1 and SR48692 counteracts neurotensin induced cell proliferation in human pancreatic ductal carcinoma cell line PANC-1. Neuropeptides. 2011;45:151–156. doi: 10.1016/j.npep.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Wang Q, Zhou Y, Evers BM. Neurotensin phosphorylates GSK-3alpha/beta through the activation of PKC in human colon cancer cells. Neoplasia. 2006;8:239–246. doi: 10.1593/neo.06259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Jackson LN, Johnson SM, Wang Q, Evers BM. Suppression of neurotensin receptor type 1 expression and function by histone deacetylase inhibitors in human colorectal cancers. Cancer Res. 2010;9(8):2389–2398. doi: 10.1158/1535-7163.MCT-09-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White JF, Noinaj N, Shibata Y, Love J, Kloss B, Xu F, et al. Structure of the agonist-bound neurotensin receptor. Nature. 2012:490, 508–513. doi: 10.1038/nature11558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeytinoglu FN, Gabel RF, Tashjian AH, Hammer RA, Leeman SE. Characterization of neurotensin production in medullary thyroid cells. Proc Natl Acad Sci USA. 1995;77:3741–3745. doi: 10.1073/pnas.77.6.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang S, Qiu X, Gu Y, Wang Y. Up-regulation of proline-rich tyrosine kinase 2 in non-small cell lung cancer. Lung Cancer. 2008;62:295–301. doi: 10.1016/j.lungcan.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 42.Zhao D, Kuhnt-Moore S, Zeng H, Wu JS, Moyer MP, Pothoulakis C. Neurotensin stimulates IL-8 expression in human colonic epithelial cells through Rho GTPase-mediated NF-kappa B pathways. Am J Physiol Cell Physiol. 2003;284:C1397–C1404. doi: 10.1152/ajpcell.00328.2002. [DOI] [PubMed] [Google Scholar]