Abstract
Background
Peritoneal dissemination of abdominal malignancy (carcinomatosis) has a clinical course marked by bowel obstruction and death; it traditionally does not respond well to systemic therapy and has been approached with nihilism. To treat carcinomatosis, we utilize cytoreductive surgery (CS) with hyperthermic intraperitoneal chemotherapy (HIPEC).
Methods
A prospective database of patients has been maintained since 1992. Patients with biopsy proven peritoneal surface disease (PSD) were uniformly evaluated for, and treated with, CS and HIPEC. Patient demographics, performance status (ECOG), resection status (R), PSD was classified according to primary site. Univariate and multivariate analysis were performed. The experience was divided into quintiles and compared with outcomes.
Results
Between 1991 and 2013, 1,000 patients underwent 1,097 HIPEC procedures. Average age was 52.9 years and 53.1% were female. Primary tumor sites were: appendix 472(47.2%), colorectal 248(24.8%), mesothelioma 72(7.2%), ovary 69(6.9%), gastric 46(4.6%), others 97(9.7%). Thirty day mortality rate was 3.8% and median hospital stay was 8 days. Median overall survival (OS) was 29.4 months, with a 5 year survival of 32.5%. Factors correlating with improved survival on univariate and multivariate analysis (p≤.0001 for each) were preoperative performance status, primary tumor type, resection status, and experience quintile (p=.04). Over the 5 quintiles, the 1 and 5 year survival, as well as the complete cytoreduction score (R0,R1,R2a) have increased, while transfusions, stoma creations, and complications have all significantly decreased (p<.001 for all).
Conclusions
This largest reported single center experience with CS and HIPEC demonstrates that prognostic factors include primary site, performance status, completeness of resection, and institutional experience. The data shows that outcomes have improved over time with more complete cytoreduction and fewer serious complications transfusions and stomas. This was due to both better patient selection, and increased operative experience. CS with HIPEC represents a substantial improvement in outcomes compared to historical series, and shows that meaningful long term survival is possible for selected carcinomatosis patients. Multi-institutional cooperative trials are needed to further refine the utility of CS and HIPEC.
Keywords: Peritoneal dissemination, disseminated peritoneal adenomucinosis, peritoneal mucinous carcinomatosis, intraperitoneal hyperthermic chemotherapy, mitomycin C, cytoreductive surgery
INTRODUCTION
Disseminated peritoneal surface malignant disease (PSD) or "carcinomatosis" has traditionally been approached with therapeutic nihilism, because patients typically progressed to death due to bowel obstruction in less than a year1. PSD results from intracavitary dissemination of tumor from a variety of primary pathologic lesions. Such findings are all too common for gastrointestinal and ovarian carcinomas, and are also seen with unusual malignancies such as sarcoma, mesothelioma and urachal carcinoma.
Frequently, PSD is confined to the peritoneal cavity without extra-abdominal disease. Thus, a regional approach to selected patients with PSD is reasonable. In the 1980’s, aggressive multimodality treatment of peritoneal surface malignancies were attempted to improve outcomes. Centers explored treatment options such as peritonectomy procedures2, intraperitoneal injection of 32P, immunotherapy3, photodynamic therapy4,5 hyperthermic intraperitoneal chemotherapy (HIPEC), and early postoperative intraperitoneal chemotherapy 6,7,8. Over the past two decades there has been ever increasing interest in such regional therapy for PSD. This has been further stimulated by publication of a prospective randomized trials for PSD from colorectal sources9 as well as successes with ovarian cancer10,11.
The optimal management of patients with PSD remains a matter of debate. Systemic chemotherapy for PSD is limited, in part, due to its restricted ability to enter the peritoneal cavity. The localization of tumor within the peritoneum without distant metastasis makes an aggressive regional approach attractive. Several groups have treated peritoneal surface dissemination of appendiceal tumors with debulking procedures12,13,14. However, these procedures are frequently unable to remove all of the microscopic tumor.
Our approach to selected patients with PSD has been to combine aggressive CS (with the goal or resection of all gross disease) with chemoperfusion to address microscopic residual. Since surgery alone can not address such microscopic residual, we have utilized intraoperative intraperitoneal chemotherapy as an adjuvant. An intraperitoneal chemotherapy perfusion done at the same time as CS has several advantages: first, intracavitary chemotherapy achieves drug levels far higher than can be obtained with even the most aggressive systemic administration, which may overcome relative drug resistance; next, following CS, all peritoneal surfaces are exposed (all adhesions lysed), which allows for better drug distribution (versus post-operative); additionally, the single intra-operative dose eliminates significant compliance/tolerance issues encountered with postoperative administration of several cycles of treatment10,11,15. The rationale for hyperthermia is based on laboratory studies showing synergy with certain drugs, and it has the advantage of avoiding hypothermia frequently encountered with prolonged open procedures.
We have previously reported our prior experience8 as well subsets of patients treated with CS and HIPEC for PSD from appendiceal16,17, colo-rectal18,19, gastric20, small bowel21, and urachal22 carcinomas, as well as sarcomatosis23 and mesothelioma24. Herein, we examine our experience with patients undergoing CS and HIPEC for PSD in order to evaluate our outcomes with the first 1,000 patients.
METHODS
Patients who underwent CS and HIPEC for peritoneal surface disease (PSD) at Wake Forest University School of Medicine Baptist Hospital between 1991 and 2013 were identified from a prospective database. This database has been continuously approved by the institutional review board at Wake Forest University. Clinical data on all patients were recorded in the database and maintained by a dedicated data management unit. All patients were evaluated in the Surgical Oncology Clinics preoperatively. Evaluations included, at a minimum, a complete history, examination, pathologic review, CT or MRI imaging, blood counts, renal and liver functions. To be considered for CS and HIPEC, patients needed to have normal organ function (serum creatinine < 3 mg/dL, alkaline phosphatase and serum aspartate transaminase or alanine transaminase <3 times the upper limit of normal, white blood cell count ≥4,000/mm3, and platelet count ≥100,000 mm3). Evaluation of preoperative CT or MRI imaging focused upon the absence of; extra-abdominal metastasis, parenchymal hepatic metastasis (limited, completely resectable and hepatic and liver surface lesions allowed), bulky small bowel disease, multi-station bowel obstruction, ureteral, or biliary obstruction. Tumors were categorized according to the primary site of origin. Prior to CS and HIPEC, patients had their pathology reviewed by the Wake Forest University Department of Pathology. This was compared to final pathology from specimens garnered at the time of CS to reach a final diagnosis for the database. Patients with bulky pelvic disease, or multiple previous pelvic procedures, were routinely considered for urologic consultation for cystoscopy, with temporary externalized ureteral stent placement at the start of the procedure to facilitate retroperitoneal and pelvic dissection. Morbidity was defined according to the Clavien-Dindo25 classification system. Post-operative mortality was assessed at 30 days after the procedure. The clinical experience was divided into 5 quintiles of 200 patients each. These corresponded to cases done between 12/30/91-5/23/00 for the 1st, 5/25/00-3/28/05 for the 2nd, 4/4/05-6/9/08 for the 3rd, 6/10/08-8/30/10 for the 4th and 9/2/10-6/10/13 for the 5th quintiles.
CYTOREDUCTIVE SURGERY
The goal of CS was removal of all gross disease in all cases. CS consisted of the removal of gross tumor and involved organs, peritoneum, or tissue deemed technically feasible and safe for the patient. Upon opening the abdomen, the quantity and distribution of disease and/or ascites present was noted and quantitated (since 2005) by the peritoneal carcinomatosis index (PCI) 26. This included routine supracolic omentectomy in all cases where not previously performed. Peritoniectomy procedures were performed only as indicated by the presence of visible disease.2,8 Any tumors adherent or invasive to vital structures that could not be removed were cytoreduced using standard techniques or the cavitational ultrasonic surgical aspirator (CUSA; Valleylab, Boulder, Colorado.). The resection status of patients was judged after CS using the following classification: R0, complete removal of all visible tumor and negative cytological findings or microscopic margins; R1, complete removal of all visible tumor and positive post-perfusion cytological findings or microscopic margins; R2a, minimal residual tumor, nodule(s) measuring 0.5 cm or less; R2b, gross residual tumor, nodule greater than 0.5 cm but less than or equal to 2 cm; and R2c, extensive disease remaining, nodules greater than 2 cm27.
INTRAPERITONEAL HYPERTHERMIC CHEMOTHERAPY
Near the completion of CS, patients were cooled to a core temperature of approximately 34°C to 35°C by passive measure (i.e., not warming airway gases or intravenous solutions and cooling the room). Constant patient and perfusate temperature monitoring was performed in all cases. After CS was completed, peritoneal perfusion was facilitated via two 22 French inflow and two 32 French outflow catheters, placed percutaneously into the abdominal cavity. Temperature probes were placed on the inflow and outflow tubing and were continuously monitored. The abdominal skin incision was closed temporarily with a running cutaneous suture to prevent leakage of peritoneal perfusate. A perfusion circuit was established with approximately 3 L of crystalloid (typically Ringer’s lactate or plasmalyte). Flow rates of approximately 1L/minute were maintained using a roller pump managed by a perfusionist. The circuit continued through a single roller pump, through a heat exchanger and then to the patient.
Once a stable perfusion circuit was established and outflow temperature was >38.5°C, the chemotherapy was introduced into the perfusion circuit. A maximum inflow temperature of 43°C was tolerated during perfusion, with a target outflow temperature at the pelvis of 40°C. The abdomen was gently massaged throughout perfusion to improve drug distribution to all peritoneal surfaces. Total planned perfusion time after the initial addition of chemotherapy was typically 120 minutes. Although several chemotherapeutic agents were utilized, most patients received mitomycin c (MMC). The MMC was dosed based on volume of perfusate necessary to establish a stable circuit (typically 3 liters). When MMC was utilized, 30 mg was added to the perfusate at the initiation of the HIPEC, and at 60 minutes an additional 10 mg of MMC was added to keep MMC perfusate concentrations higher than 5µg/mL. In certain patients (elderly individuals, those with extensive previous chemotherapy, poor performance status), reductions in the dose of MMC (to 30 mg total) or perfusion time (to 60–90 minutes) were made to minimize hematotoxicity. Other chemotherapeutic agents were also utilized based on primary tumor site and previous systemic therapy. Since 2004, we have used cisplatin 250mg/M2 with sodium thiosulfate for mesothelioma cases24. Ovarian cases utilized cisplatin or carboplatinum (1,000mg/M2) 28. Sarcoma cases (and GIST prior to the introduction of imatinib) were perfused with MMC ± mitoxantrone. We are also used oxaliplatin (200mg/M2) 29 for select appendiceal and colonic cases.
CLINICAL FOLLOW-UP
Clinical follow-up occurred at one month and then at least every 6 months thereafter for up to 5 years. After 5 years from the last HIPEC, follow-up was suggested on an annual basis. Blood counts, liver functions and tumor markers (as appropriate), as well as abdominal and pelvic CT or MRI scans with intravenous contrast, were obtained with each follow up visit and when clinically indicated. Patients were typically followed jointly with medical oncologists. Some patients received systemic chemotherapy at the discretion of their medical oncologists. Of the first 1,000 patients on the HIPEC database, 78 were lost to follow-up (7.8%). The longest survivor after HIPEC underwent the procedure 225 months ago.
STATISTICAL ANALYSIS
All data were collected prospectively; descriptive statistics were generated for all measures, including means, ranges, and standard deviations for continuous measures and frequencies and proportions for categorical data. Overall survival (OS) was calculated from the date of CS and HIPEC to the last known date of follow-up or date of death. Estimates of survival were calculated using the Kaplan-Meier (product-limit) method; analysis using Cox proportional hazards was performed on all pertinent clinicopathologic variables to determine each one’s association with survival. Group comparisons of OS were performed using the approximate chi-square statistic for the log-rank test. Additionally, the Cox proportional hazards regression model was used in a stepwise fashion to perform a multivariate analysis of clinic-pathologic factors to determine an overall model of independent predictors of OS. Statistical significance was defined as a P-value ≤ 0.05.
RESULTS
PATIENTS AND CLINICOPATHOLOGIC FEATURES
A total of 1,000 patients underwent 1,097 HIPEC procedures between December 30, 1991 and June 10, 2013. This study was approved by our institutional review board. Patient outcome data stratified by experience quintiles are listed in Table 1. The mean age was 52.9 ± 12.4 years (range 11–87 years of age) with 53.1% being female. The median intensive care unit and hospital stays are currently 1 and 8 days, which has decreased significantly from 2 and 9 days in the first quintile p=.03 and p<.0001 respectively (see Table 1.). As part of the CS 19.0% of patients had an ileostomy (12%) or colostomy (7%) created. However, the frequency of stoma placement has decreased significantly over time. The organs resected as part of the CS are listed in Table 2. Most (68%) of the patients had received systemic chemotherapy prior to HIPEC. The median hospital stay was 9 days with an average of 14.1(±16.3) days. Most (73%) patients were admitted to the ICU with an average stay of 1–2 days, with a decrease in ICU stay found over time.
Table 1.
Clinicopathologic data for 1,000 patients undergoing CS and HIPEC for peritoneal surface disease
| 1st quintile | 2nd | 3rd | 4th | 5th | ||
|---|---|---|---|---|---|---|
| Primary (n) | ||||||
| Appendix (472) | 56 (28%) | 80 (40%) | 116 (58%) | 121 (60.5%) | 99 (49.5%) | |
| Colon (232) | 58 (29%) | 50 (25%) | 40 (20%) | 40 (20%) | 44 (22%) | |
| Gastric (46) | 27 (13.5%) | 12 (6%) | 3 (1.5%) | 2 (1%) | 2 (1%) | |
| Mesothelioma (72) | 12 (6%) | 13 (6.5%) | 12 (6%) | 12 (6%) | 23 (11.5%) | |
| Ovary (69) | 18 (9%) | 25 (12.5%) | 5 (2.5%) | 8 (4%) | 13 (6.5%) | |
| Others (109) | 29 (14.5%) | 20 (10%) | 24 (12%) | 17 (8.5%) | 19 (9.5%) | |
| Complications | P<0.0001 | |||||
| No (N=422) | 92 (46%) | 76 (38%) | 49 (24%) | 90 (45%) | 115 (58%) | |
| Yes (N=578) | 108 (54%) | 124 (62%) | 151 (76%) | 110 (55%) | 85 (42%) | |
| Transfusion | ||||||
| No | 80 (45.2%) | 104 (68.9%) | 129 (68.3%) | 129 (65.2%) | 149 (76.8%) | |
| Yes | 97 (54.8%) | 47 (31.1%) | 60 (31.7%) | 69 (34.8%) | 45 (23.2%) | |
| Stoma | p=0.001 | |||||
| No | 160 (88.4%) | 141 (70.5%) | 160 (80.0%) | 159 (79.5%) | 193 (96.5%) | |
| Colostomy | 15 (8.3%) | 28 (14.0%) | 8 (4.0%) | 14 (7.0%) | 7 (3.5%) | |
| Ileostomy | 6 (3.3%) | 31 (15.5%) | 32 (16.0%) | 27 (13.5%) | 22 (11.0%) | |
| ECOG | P<0.0001 | |||||
| 0 | 53 (26.8%) | 84 (42.0%) | 85 (42.5%) | 115 (59.0%) | 105 (54.7%) | |
| 1 | 99 (50.0%) | 86 (43.0%) | 82 (41.0%) | 57 (29.2%) | 64 (33.3%) | |
| 2 | 36 (18.2%) | 22 (11.0%) | 28 (14.0%) | 19 (9.7%) | 16 (8.3%) | |
| 3 or 4 | 10 (5.1%) | 8 (4.0%) | 5 (2.5%) | 4 (2.1%) | 7 (3.7%) | |
| Resection | P<0.0001 | |||||
| R0/R1 | 71 (35.5%) | 93 (46.5%) | 103 (51.8%) | 93 (46.5%) | 104 (52.8%) | |
| R2a | 39 (19.5%) | 55 (27.5%) | 49 (24.6%) | 73 (36.5%) | 69 (35.0%) | |
| R2b | 31 (15.5%) | 32 (16.0%) | 34 (17.1%) | 28 (14.0%) | 15 (7.6%) | |
| R2c | 59 (29.5%) | 20 (10.0%) | 13 (6.5%) | 6 (3.0%) | 9 (4.6%) | |
| Clavien- Dindo | 1st quintile | 2nd | 3rd | 4th | 5th | P<0.003 |
| 0 | 59 (30.6%) | 72 (36.0%) | 59 (29.5%) | 65 (32.5%) | 39 (29.8%) | |
| I | 11 (5.2%) | 11 (5.5%) | 13 (6.5%) | 31 (15.5%) | 16 (12.2%) | |
| II | 75 (38.9%) | 54 (27.0%) | 53 (26.5%) | 51 (25.5%) | 35 (26.7%) | |
| III | 27 (14.0%) | 34 (17.0%) | 49 (24.5%) | 33 (16.5%) | 23 (17.6%) | |
| IV | 14 (7.2%) | 17 (8.5%) | 21 (10.5%) | 12 (6.0%) | 13 (9.9%) | |
| V | 8 (3.1%) | 12 (6.0%) | 5 (2.5%) | 8 (4.0%) | 5 (3.8%) | |
| Hospital LOS | median 9 | 9 | 10 | 9 | 8 | p=0.03 |
| ICU LOS | 2 | 2 | 1 | 1 | 1 | P<0.0001 |
| Pre HIPEC Chemo | ||||||
| No | 59 (36%) | 122 (61%) | 107 (54%) | 85 (42%) | 48 (32%) | |
| Yes | 105 (64%) | 77 (39%) | 93 (46%) | 115 (58%) | 100 (68%) |
Table 2.
Listing of organs resected as part of the CS in addition to peritoneal resections. Omentectomy (supracolic) was performed routinely if not previously resected.
| Organ Resected | Number | Percent |
|---|---|---|
| Diaphragm | 98 | 9.8% |
| Colon | 500 | 50.0% |
| Rectum | 78 | 7.8% |
| Small bowel | 322 | 32.2% |
| Stomach | 111 | 11.1% |
| Spleen | 416 | 41.6% |
| Uterus | 91 | 17.1%* |
| Ovaries | 170 | 32.0%* |
| Gallbladder | 291 | 29.1% |
| Pancreas | 62 | 6.2% |
| Appendix | 102 | 10.2% |
| Omentum | 717 | 71.7% |
| Kidney | 12 | 1.2% |
| Lung | 4 | 0.4% |
| Liver | 102 | 10.2% |
| Bladder | 25 | 2.5% |
| Adrenal | 3 | .3% |
| Umbilicus | 27 | 2.7% |
percentage of female patients
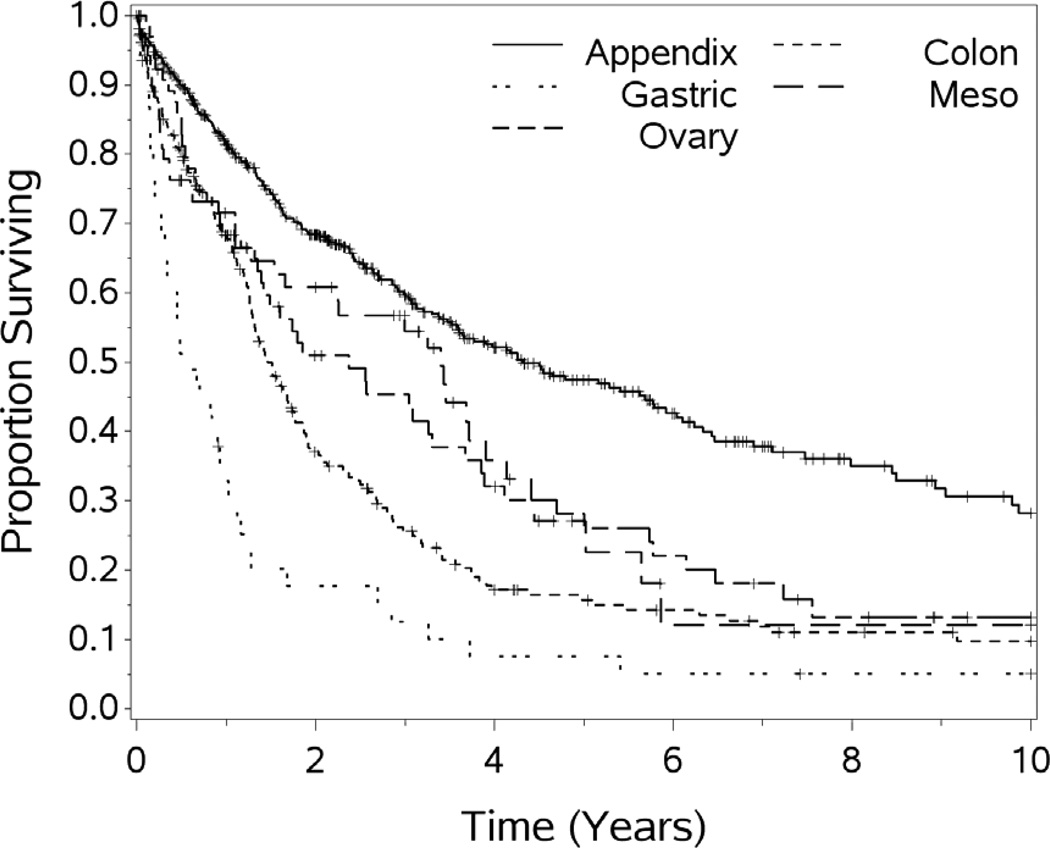
Primary sites of origin for the patients were as follows: adrenal 2 (0.2%), appendix 472 (47.2%), colorectal 248 (24.8%), gall bladder 5 (0.5%), gastric 46 (4.6%), gastrointestinal stromal tumor (GIST) 9 (2%), liver 2 (0.2%), mesothelioma 72 (7.2%), ovary 66 (6.9%), pancreas (cystic neoplasm and IPMT) 6 (.6%), sarcoma 14 (1.4%), small bowel 17 (1.7%), urachal 5 (1.1%), and unknown 19 (1.9%). The median survival (months) was significantly different by site of origin as follows: appendix 63.5, colorectal 16.4, gastric 6.1, mesothelioma 27.1, ovary 28.5, sarcoma 28.1, p=.0001. For other histologic sites of origin the series has too few cases for meaningful analysis. The distribution of the primary sources of PSD has changed over time with increases in appendiceal primary and decreases in gastric and sarcoma cases.
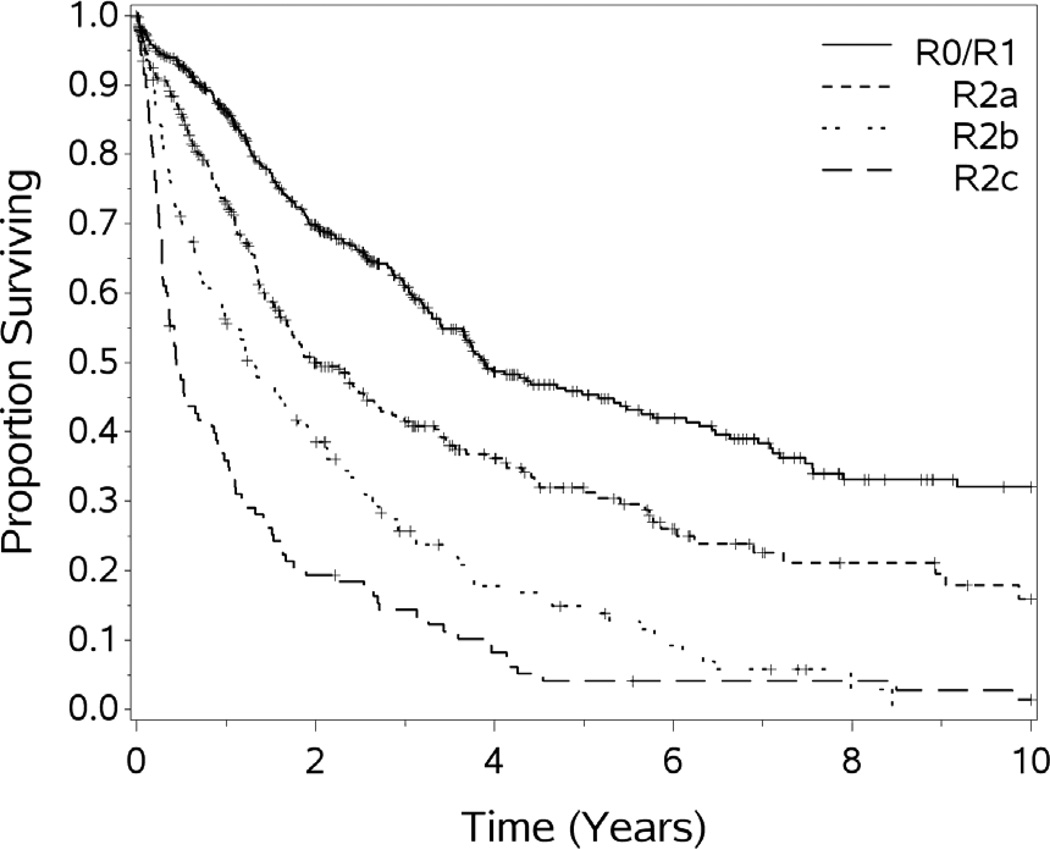
The operative and perfusion data are summarized in Table 1. The mean PCI was 12. The length of the operation (range 183-1,531 minutes) was dependent on the extent and location of disease at exploration, but averaged just under 10 hours. The quantity of residual disease was recorded by the primary surgeon and was scored according to the R status for residual disease30 The R status of all patients undergoing HIPEC is listed in Table 1. The resection status was a significant predictor of survival p<.0001. For the purposes of survival calculations, R0 and R1 were combined due to the difficulties in clearly separating them (as radial margins are typically positive) in the setting of PSD.
MORBIDITY AND MORTALITY
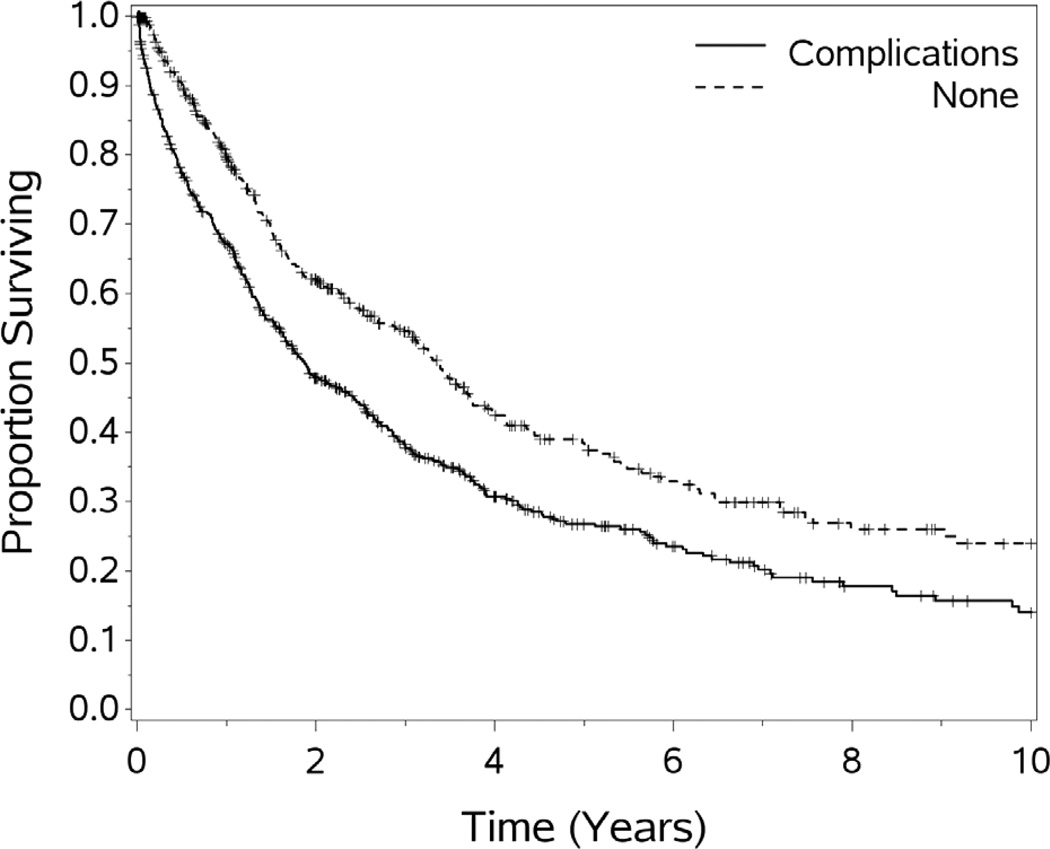
The thirty day postoperative morbidity and mortality were 42% and 3.8%, respectively. Thirty eight patients in this study died within 30 days of HIPEC. Wound infection, hematologic toxicity, sepsis, respiratory failure, anastomotic leak, pneumonia, and enterocutaneous fistula account for the majority of the postoperative complications in this cohort of patients. The mortality rates did not change significantly by quintile and ranges from 2.5% (5th quintile) to 6% (2nd quintile). Patients who experienced a complication had poorer survival than those who did not, p<.001. This difference remained significant on multivariate analysis. Complications were less common in patients undergoing R0/1 resections, when compared to cases with more residual, p=.04.
EXPERIENCE OVER TIME
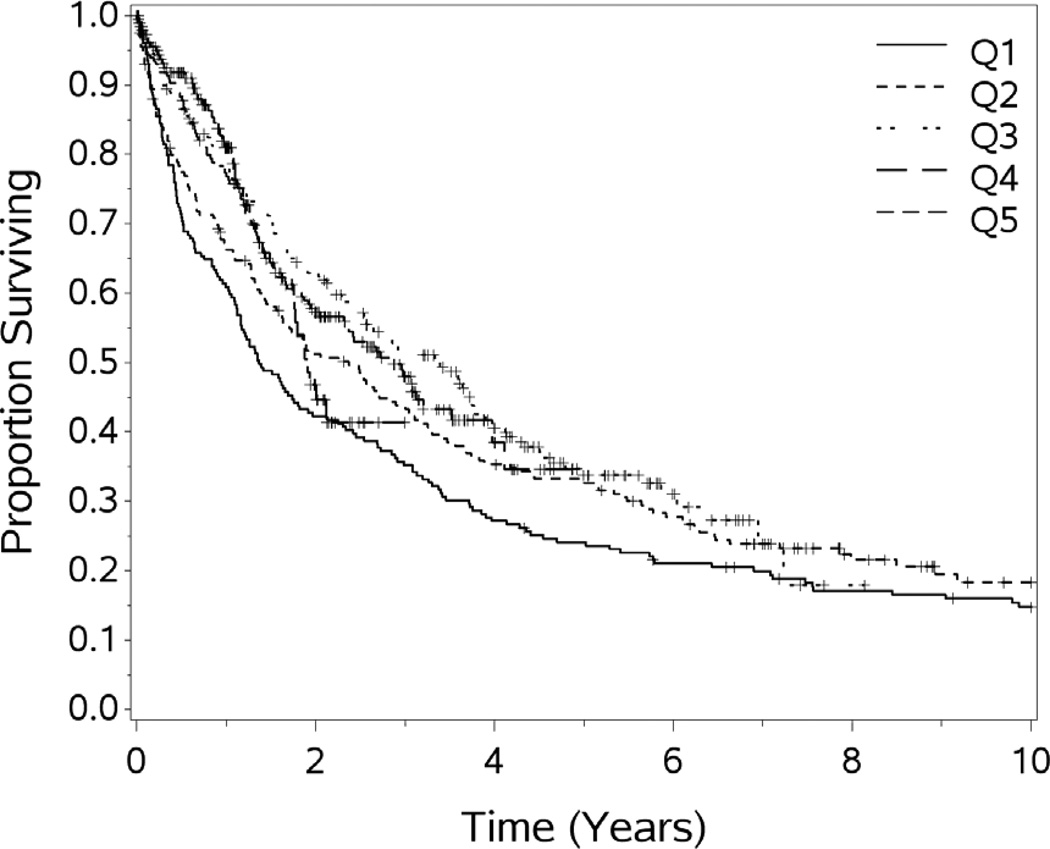
In order to evaluate our experience over time, we divided our patient experience into 5 time periods (quintiles) of 200 patients each. The median survival in months for the 5 quintiles are 16.4, 28.5, 40.7, 34.3+ and 22.9+ respectively, p =.006 (see figure 1). However, the median length of stay was evenly distributed over the quintiles, the medians are 9, 9, 10, 8, 9; the means (±SD) are 14.7 (±17.7), 15.3 (±17.9), 17.0 (±20.3), 12.1 (±13.1), and 13.2 (±13.7) days, respectively, p= 0.048. The middle quintile is significantly higher than the 4th (p=0.005) and the last (p=0.028).
Figure 1.
Overall survival by quintile of experience, difference significant p=.0006.
Selection of patients changed significantly over the experience; with increased rates of appendiceal (p<.0001) and ovarian (p=.0005) primary, and decreases in gastric (p<.0001) and sarcoma (p=.02). Rates over time for mesothelioma and colonic cancer primary have not significantly changed. The rate of colostomy and ileostomy varied significantly over the time quintiles (p=.0003 and p=.0009 respectively). The rate of complications varied significantly over the experience quintiles, with the highest rate during the 3rd quintile, p<.0001. The median hospital and ICU stays decreased over time (p=.03 and p<.0001 respectively).
The mortality rate ranged from 2.6–7.0% over the 5 quintiles without significant differences. The rate of complete resection (as defined by R0,R1 or R2a) increased with each quintile (55.0%, 74.0%, 76.4%, 83%, 88.3% respectively), p<.001. Class IV and V complications decreased over time (45.0%, 26.0%, 23.6%, 17.0%, 11.7% respectively), p <.001. The rates of stoma creation (ileostomy or colostomy) decreased over time (11.8%, 29.5%, 20.0%, 20.6%, 15.1% respectively), p <.001. Further, the 1 and 5 year overall survival has increased over time (with the 5 year median OS, not yet reached for the latest 2 quintiles).
SURVIVAL AND FOLLOW-UP
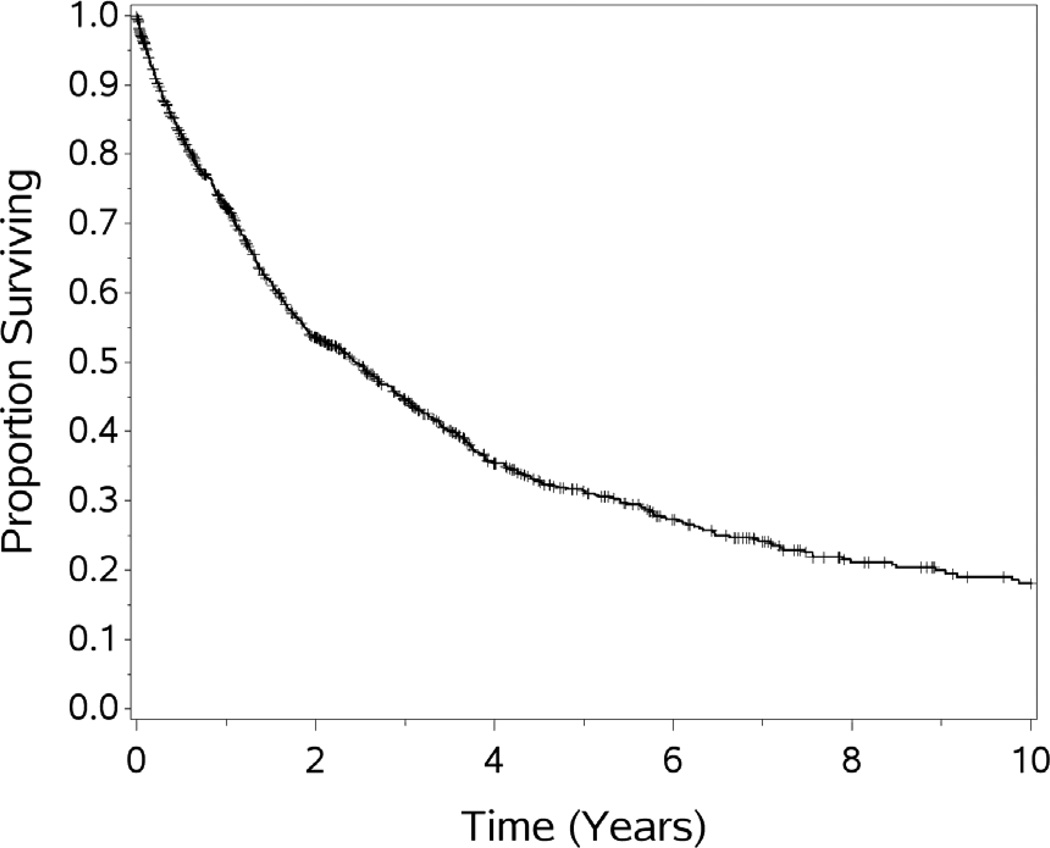
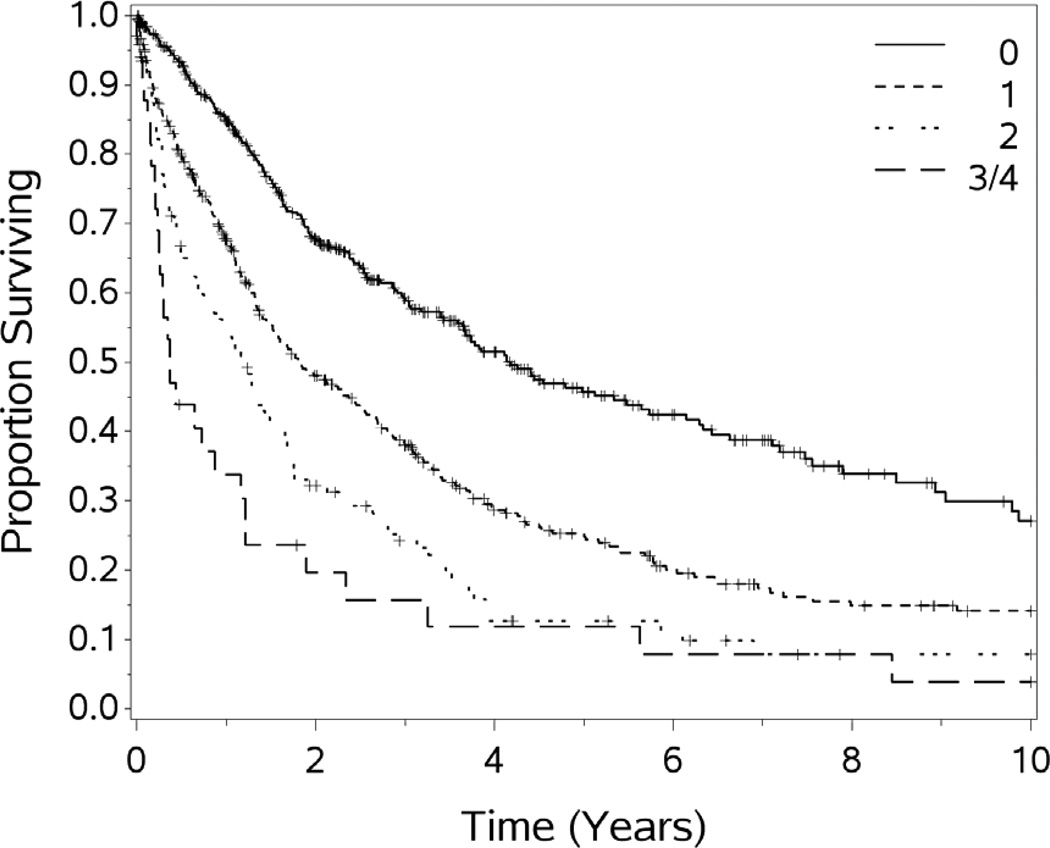
For the cohort of 1,000 patients with a median follow-up of 54.1 months, the median OS was 29.4 months. One, 3-year, 5-year, 10 year and 15 year OS (±standard error) was 72.3 (±1.5%), 44.6(±1.7%), 31.5(±1.8%), 18.1(±1.9%), and 10.7(±2.7%) respectively (see Figures 1–5). Second HIPEC was performed on 89 selected patients for recurrent/persistent disease.30 with 8 subjects undergoing 3 procedures. When plotting the overall survival time after repeat versus initial HIPEC, the results are strikingly similar (see Figure 6.) The survival rates include operative mortality. A univariate analysis of clinic-pathologic factors was performed to identify singularly significant prognostic factors associated with OS after CS and HIPEC for PSD. Multivariate analysis of factors effecting survival was performed via a stepwise regression technique. This analysis allowed for all variables regardless of level of significance in the univariate analysis. The Cox proportional hazards regression model found that 5 clinicopathologic factors were independent predictors of OS: tumor histology, resection status, complications and performance status (see Table 3). The Figures 1–6 depict the Kaplan-Meier actuarial survival curves for these factors.
Figure 5.
Overall survival by resection status, p<.0001.
Figure 6.
Overall survival by postoperative major complication, p<.0001.
Table 3.
Univariate and multivariate analysis of prognostic significance of clinicopathologic variables, based on stepwise regression analysis.
| Variable | p-value | Hazard Ratio |
|---|---|---|
| Univariate | ||
| Race | 0.31 | |
| Peritoneal Carcinomatosis Index (PCI) | <.0001 | 1.28 for each 5 unit increase |
| Resection Status | <0.0001 | 1.6–5.7 |
| Sex | 0.039 | .85 |
| Complications | <.0001 | 1.5 |
| Length of operation | 0.041 | 1.03(each additional hour) |
| Previous CS & HIPEC | <.0001 | .45 |
| Age | 0.028 | 1.04 (each 5 year increase) |
| Temperature of perfusate | 0.73 | |
| Length of perfusion with chemotherapy | 0.21 | |
| Primary tumor histology (site) | <0.0001 | .24–2.7 (depending on primary) |
| Performance status (ECOG) | <0.0001 | 2.8 for 2, 4.3 for 3 or 4 |
| Experience Quintile | .006 | 1.5 (quintile 1 vs. 5) |
| Multivariate | ||
| Resection status | <0.0001 | |
| Performance status (ECOG) | <0.0001 | |
| Primary tumor histology (site) | <0.0001 | |
| Complications | <0.0001 | |
| Experience Quintile | .043 |
DISCUSSION
CS and HIPEC represent a substantial operative undertaking for both patient and surgeon. Average operative times are approximately 10 hours, with ICU and hospital stays which consume substantial resources. Morbidity and mortality have improved over time, but remain significant; straight forward preoperative discussions with the patient and family are, therefore, necessary. However, properly selected patients have a real chance at long term survival rarely, if ever, realized without such aggressive efforts. Clearly, long term survival is possible for patients with PSD. Over our experience, we have modified our approach to PSD in terms of patient selection, and to a lesser extent, operative techniques.
Systemic chemotherapy for PSD has been the traditional approach, but is hampered by limited entry into the peritoneum. Any systemic chemotherapy for intraperitoneal disease must overcome the plasma-peritoneal partition in order to reach molecular targets. Pharmacokinetic studies have confirmed the presence of this peritoneal-plasma partition, by demonstrating that drugs delivered into the peritoneal cavity have a clearance that is inversely proportional to the square root of its molecular weight.31,32,33 The delivery of intraperitoneal chemotherapy can be viewed as a tool to overcome this drug resistance, as well the toxicity attendant to systemic administration. Because of this partition, drugs without lipophilic properties and high molecular weights have optimal characteristics for intraperitoneal application. The pharmacokinetic advantage of intraperitoneal perfusion is substantial, and can be quantified by the area under the curve ratios of peritoneal fluid to plasma that favor retention of drug in the peritoneum.34,35,36,37,38,39,40
In addition to the pharmacokinetic advantage that intraperitoneal chemotherapy infusion (after maximal tumor debulking) offers, the addition of hyperthermia effects cell membranes, cytoskeletons, synthesis of macromolecules and DNA repair mechanisms.41,42 Our institution, and others, have primarily used mitomycin c (MMC). The synergy between MMC and hyperthermia occurs independent of the cell cycle, thus allowing for significant tumoricidal activity with relatively brief exposures.43,44,45 Additionally, the hyperthermia ameliorates the hypothermia frequently encountered during long open operative procedures.
There is a paucity of data regarding the utility of systemic therapy for PSD in general, and for appendiceal tumors46, specifically the more common low grade tumors. Therefore, the foundation of treatment for PSD of appendiceal malignancies remains aggressive CS followed by hyperthermic peritoneal perfusion. Removal of bulk disease is imperative, however, as even the most ambitious perfusion strategies penetrate a maximum 5 millimeters into peritoneal surfaces. Aggressive CS allows hyperthermic chemoperfusion to address the microscopic or small volume residual. Consequently, the foundation of treatment of PSD for appendiceal disease remains aggressive CS followed by HIPEC.
Pathologic characteristics clearly impact the clinical outcomes of patients with PSD. For appendiceal tumors, patients with low grade mucinous carcinoma peritonei (which is also described as disseminated peritoneal adenomucinosis or pseudomyxoma peritonei (PMP)) experience better clinical outcomes than those with higher grade non-mucinous appendiceal malignancies.47,48,49,50,51,52,53,54 We (and others) have previously shown that the survival rate in patients with high-grade lesions was significantly lower than for the low grade PSD 8,16,17. This is not an unexpected finding based on the biological and molecular differences between low and high-grade non-mucinous appendiceal tumors.16,17,49 We have recently described the genomics of appendiceal tumors and have found them to be dramatically different from colorectal epithelial neoplasms.49 Such studies are important in determining the biologic underpinnings of PMP, and to seek actionable targets for personalized therapies.
Appendiceal cancer with PMP has been considered the classic indication for HIPEC, as PMP rarely metastasizes beyond the peritoneal space and pelvis. Five year survival after HIPEC for PMP range between 66 and 97%, and our experience is consistent with those results. 50,51,52,53 Tumor histology is a major driver of prognosis for patients with PMP. The outcome with the low grade disease with PSD is significantly better than that of intermediate or high grade in the original description of the histologic subtypes of PMP.55 We believe that the behavior of PMP/appendiceal carcinoma is best described simply as low and high grade rather than a more cumbersome three tier classification.16,17 This clearly demonstrates the differences in tumor biology among these histologic subgroups of PMP, and is readily reproducible when reviewed by pathologists.
This study confirms our previous reports that patients with ECOG performance scores of 2 to 3 had significantly poorer overall survival than those with scores of 0 or 1.8,18 This also highlights the importance of evaluating candidates for the procedure while they are medically fit to undergo such a large-scale intervention. Preoperative performance scores and quality of life indices clearly predict outcomes56 Therefore, we select patients for HIPEC with ECOG scores of 2 or better. Despite recent improvements in systemic therapy for colorectal cancer, treating patients with second line therapy while their performance status declines may deprive candidates of the opportunity to be salvaged with CS and HIPEC. We suggest that if systemic chemotherapy will be utilized preoperatively, that it be limited to 3–6 cycles to avoid substantial decrements in performance status attendant to prolonged systemic chemotherapy. 56
Patients undergoing complete CS prior to HIPEC had superior outcomes compared to those who underwent incomplete CS regardless of site of the primary lesion (see Figure 5). This finding confirms data from our institution, and others, that demonstrate a significant survival advantage for patients undergoing R0/R1 resection compared to those with R2 resections.8,18,57,58 In a review of 506 patients, Glehen et. al. analyzed the survival of patients with peritoneal surface malignancies from colorectal primary tumors undergoing incomplete CS followed by HIPEC, and found that this treatment paradigm resulted in limited long-term survival. Patients who are unable to undergo significant CS (R2a or better) at laparotomy may be spared the potential toxicity of HIPEC. Our rate of complete cytoreduction increased significantly over time, which likely results more from better patient selection than from improvements in surgical techniques.
Surgical resection remains the primary mode of therapy for colon and rectal cancer. Treatment options for patients with unresectable metastatic disease have improved significantly in the past few years. Patients with Stage IV colorectal cancer treated with newer combinations of cytotoxic chemotherapy,59 and/or biological agents,60 have resulted in an unprecedented median survival of approximately 20 months, though at considerable cost. However, such therapeutic combinations are not an optimal treatment strategy for all categories of Stage IV disease. Patients with PSD from colorectal cancer treated with modern systemic therapy have poorer survival than those with metastases to other sites, with 5 year survival of 6.0 vs 4.1% with modern chemotherapy 61. Patients undergoing CS and HIPEC had a 5 year survival of 17%, with those undergoing R0/1 resections being more than four times that.19 This finding is consistent with other high volume centers.62,63,64 Further, it must be kept in mind that most of the patients undergoing HIPEC for colorectal cancer have been treated with systemic chemotherapy prior to HIPEC. Therefore they are well into the 12.7 month median survival found with systemic chemotherapy alone, and present a treatment lead time against any benefit of HIPEC versus systemic therapy61.
This experience is supported by the randomized trial from the Netherlands that compared palliative surgery with chemotherapy to CS and HIPEC with the same systemic chemotherapy.9 That randomized trial found a doubling of survival for patients treated with CS and HIPEC.9 Therefore, we concur with the consensus statement from XXXX in that systemic therapy alone is no longer appropriate for patients with limited peritoneal dissemination from a primary or recurrent colon cancer.65 The surgical management of PSD of colorectal origin with CS and HIPEC has been clearly defined and continues to improve. This aggressive strategy has resulted in long term survival rates which are unprecedented in the literature. Despite the cost of significant morbidity, properly selected patients have a real opportunity for survival for in a situation which was previously approached with purely palliative intent.
We have avoided addressing PSD from hepatic, biliary, pancreatic sources principally due to difficulty in obtaining control of the primary lesion, and a paucity of agents with significant activity. Similarly we currently consider patients with gastric cancer after a response to systemic chemotherapy and only if R0/1 resection can be anticipated. PSD from sarcoma (sarcomatosis) is now a rare indication for HIPEC. Although we had some success with the procedure and have long term survivors, we have no confidence in the activity of the chemotherapy in this setting, and no longer offer the procedure to patients with disseminated gastrointestinal stromal tumors or liposarcoma23.
Primary peritoneal mesothelioma is a much less common entity than the pleural malignancy.57 Although the molecular characteristics of peritoneal disease differ only slightly from the pleural disease, the clinical courses are disparate.66,67 Peritoneal disease typically presents with ascites, abdominal pain and eventually bowel obstruction. The disease tends to remain within the abdominal cavity until late in the course and distant metastasis is distinctly uncommon, thus making this an excellent candidate for CS and HIPEC. We and others have previously reported our experience with this modality24,68,69,70,71,72,73,74 which represents a great improvement over even the best systemic therapy.66,24,68,69,70 In our initial study, we utilized MMC as the agent, 24 but have changed to cisplatin after the reports from the surgery branch of the National Cancer Institute.24,68,69 The experience with CS and HIPEC for peritoneal mesothelioma has lead to a proposed staging system, which we support74. We believe that mesothelioma represents one of the strongest cases for combining HIPEC with CS.
It is estimated that only a handful of patients who are potential candidates for CS and HIPEC actually receive it, which is underscored by the relatively small number of patients accrued to the trials and studies for PSD at large “perfusion centers.” It is clear that expanding the number of centers should be done by surgical oncologists who have more than a passing knowledge of systemic chemotherapy and are comfortable with the rigors of aggressive operative procedures in the abdomen.30 This has led to consensus statements by a group of surgeons with an interest in CS and HIPEC, which outlines an evaluation strategy for PSD from colorectal carcinoma.62,65
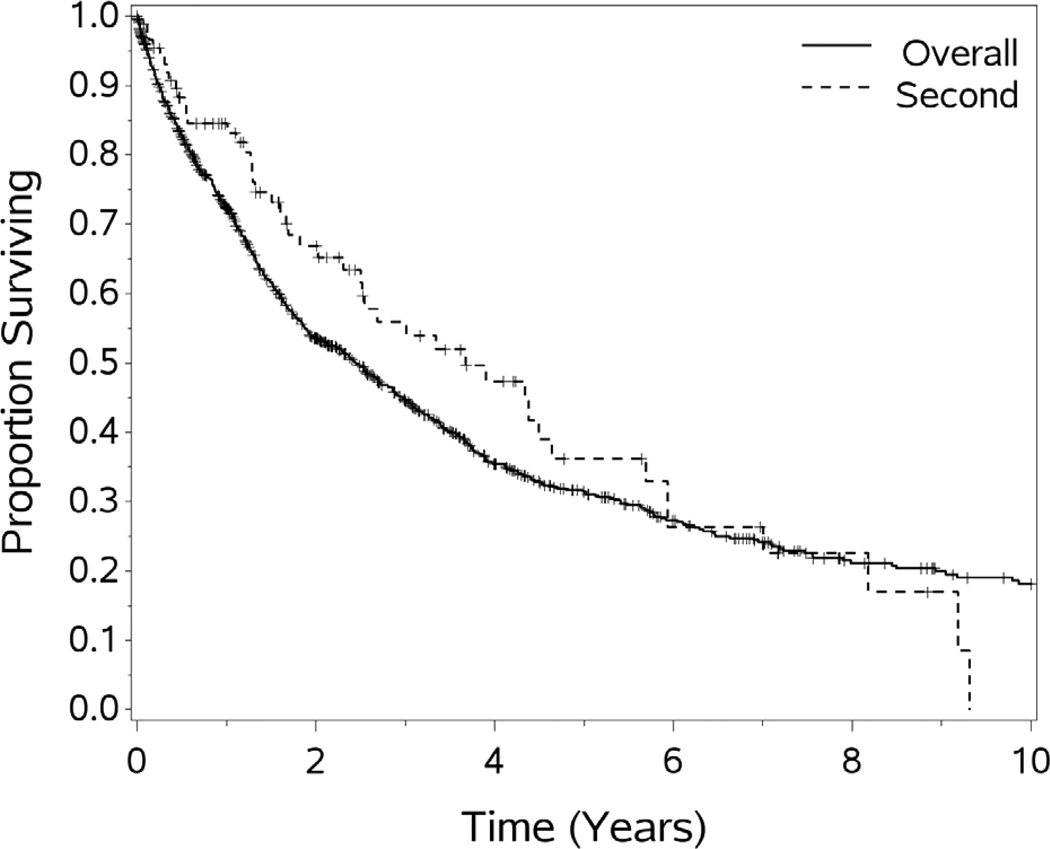
While reported results from “perfusion centers” represent a substantial improvement in duration and likely quality of life,75,76,77,78 the majority of patients undergoing these procedures will experience tumor recurrence. Evaluating patients for a second CS and HIPEC will become an ever more common problem as patients with PMP survive long enough to require multiple procedures.30,79,80 We, and others, believe that in selected patients, a second CS and chemoperfusion may be of value (see Figure 7.). In evaluating patients for second cytoreduction, the same criteria which are used to select patients for the first remain important. Specifically, the patients must remain medically fit enough to tolerate a major operative procedure, be free of extra-abdominal metastasis, and have disease that seems amenable to complete cytoreduction. Additionally, the time to recurrence after initial cytoreduction and the completeness of the initial cytoreduction should be considered in deciding to proceed with another procedure. Patients with bulk residual disease after an initial cytoreduction for PSD should not be considered candidates for second cytoreductive procedures.79,30,80 In this study, 89 patients underwent a second (or third) HIPEC. While such cases had good outcomes, with survival similar to the experience with an initial procedure, when chosen appropriately, iterative procedures can “reset the clock” to the time of the initial HIPEC. We do recognize that this survival advantage clearly represents a selection bias in choosing patients for repeat procedures.
Figure 7.
Overall survival for second vs. initial HIPEC procedures
Several issues surround the future of CS and HIPEC for PSD. Chief among them is how to make such therapy standardized and available to large numbers of patients. At present there are approximately 100 active centers in the United States, but only approximately a dozen with experience of greater than 100 cases. These operative procedures require aggressive cytoreduction and are lengthy, challenging, potentially morbid, and utilize a great deal of hospital, blood bank, and surgical house officer resources. Resource utilization (and safety) of chemotherapy in the operating room remainss daunting for many centers. Additionally, great care needs to be taken in selecting patients to undergo this procedure. Further, the financial cost of these procedures can be significant. Even considering the potential feasibility of laparoscopic approaches to selected patients with PSD, the cost of these procedures will remain significant. However, when viewed in the context of the skyrocketing costs for multi-agent chemotherapy59,60 with increasing use of biologic agents costing in excess of $100,00081,82, we maintain that HIPEC should be cost effective for appropriately selected patients.
Our experience has evolved and improved over the two decades of this study. This implies a learning curve, which we estimate to be in the range of 50–200 cases. While we would like to think our surgical techniques have improved significantly over time, we believe that it is primarily better patient selection which accounts for the improved outcomes. Further, even with our experience, for most primary site tumors the optimal time, dose, temperature and chemotherapeutic agent for perfusion are not based upon class I data. Therefore, further investigation into these variables remains important.
Fundamental questions regarding HIPEC for PSD need to be addressed. Foremost among these is whether the addition of HIPEC after CS is of value. It seems obvious that the value of HIPEC should depend on the tumor being treated. The only completed randomized trial for CS and HIPEC evaluated patients with PSD from colorectal primary and appendiceal lesions9. That trial compared CS and HIPEC with standard systemic chemotherapy to standard systemic therapy (fluorouracil and leucovorin) and found the CS and HIPEC doubled the survival.9 However, to date, no study has compared CS with or without HIPEC.9,62 Clearly, it would be desirable to evaluate the value of HIPEC versus CS alone, in a multicenter prospective randomized trial, and such a trial in France is now accruing patients. However, such a randomized controlled trial has proven difficult to complete and efforts have previously failed as many patients presenting themselves for evaluation refuse to consider such a randomization.62,83 Efforts to bring CS and HIPEC to multicenter trials have not been embraced by the cooperative oncology groups to date. A recent study offered via the American College of Surgeons Oncology Group accrued a single patient (coincidentally from our site) prior to closure due to lack of accrual. However, such difficulties in performing randomized trials do not mean they should not be pursued.
The advancement of centers of excellence as well as the initiation of cooperative group trials will help to define the improved approaches for peritoneal spread for PSD. The future of CS and HIPEC for PSD lies in a multi-center and randomized trials that not only investigate response and survival, but also standardization of techniques, quality of life, and integration with ever improving systemic therapy. Our experience clearly shows that long term survival is possible after a diagnosis of PSD, and that approaching such patients with therapeutic nihilism is no longer appropriate.
Figure 2.
Overall survival for 1,000 patients treated with CS and HIPEC.
Figure 3.
Overall survival by primary tumor site, differences significant p<.0001.
Figure 4.
Overall survival by preoperative performance status, differences significant p<.0001.
Acknowledgements
The authors wish to acknowledge the substantial contributions of fellows, house officers, perfusionists, nurses, enterostomal therapists, psychosocial oncologists, pathologists, urologists and radiologists who contributed to the care of these patients. We would also like to thank Joan L. Feder for her editorial support and Kathleen Cummins for her ongoing support of the database.
Supported, in part, by the Smith family fund and the Comprehensive Cancer Center of Wake Forest University. Biostatistics shared resource supported by NCI CCSG P30CA012197.
Abbreviations
- PSD
peritoneal surface (malignant) disease
- HIPEC
Hyperthermic intraperitoneal chemotherapy
- CS
cytoreductive surgery
- MMC
mitomycin C
- GIST
gastrointestinal stromal tumor
- ECOG
Eastern cooperative oncology group
- OS
overall survival
- PMP
pseudomyxoma peritonei
- CT
computerized tomography
- R
Resection score
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the Southern Surgical Association 125th Annual Meeting, Hot Springs, VA, December 2013.
References
- 1.Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88:358–363. doi: 10.1002/(sici)1097-0142(20000115)88:2<358::aid-cncr16>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 2.Sugarbaker PH. Peritonectomy procedures. Annals of Surgery. 1995;221:29–42. doi: 10.1097/00000658-199501000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torisu M, Katano M, Kimura Y, et al. New approach to management of malignant ascitis with a streptococcal preparation OK 432: improvement of host immunity and prolongation of survival. Surgery. 1983;93:357–353. [PubMed] [Google Scholar]
- 4.Hendren SK, Hahn SM, Spitz FR, et al. Phase II trial of debulking surgery and photodynamic therapy for disseminated intraperitoneal tumors. Annals of Surgical Oncoogyl. 2001;8:65–71. doi: 10.1007/s10434-001-0065-x. [DOI] [PubMed] [Google Scholar]
- 5.Sindelar WF, DeLaney TF, Tochner Z, et al. Technique of photodynamic therapy for disseminated intraperitoneal malignant neoplasms. Phase I study. Archives of Surgery. 1991;126:318–324. doi: 10.1001/archsurg.1991.01410270062011. [DOI] [PubMed] [Google Scholar]
- 6.Sugarbaker PH, Jablonski KA. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Annals of Surgery. 1995;221:124–132. doi: 10.1097/00000658-199502000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beaujard AC, Glehen O, Caillot JL, et al. Intraperitoneal chemohyperthermia with mitomycin C for digestive tract cancer patients with peritoneal carcinomatosis. Cancer. 2000;88:2512–2519. doi: 10.1002/1097-0142(20000601)88:11<2512::aid-cncr12>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 8.Levine EA, Stewart JH, Russell G, et al. Cytoreductive Surgery and Intraperitoneal Hyperthermic Chemotherapy for Peritoneal Surface Malignancy: Experience with 501 Procedures. Journal of the American College of Surgeons. 2007;204:943–955. doi: 10.1016/j.jamcollsurg.2006.12.048. [DOI] [PubMed] [Google Scholar]
- 9.Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clinical Oncology. 2003;21(20):3737–3743. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal Cisplatin and Paclitaxel in Ovarian Cancer. New England Journal of Medicine. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 11.Markman M, Walker JL. Intraperitoneal Chemotherapy in Ovarian Cancer: A review, with a focus on practical aspects of treatment. J. Clinical Oncology. 2006;24:988–994. doi: 10.1200/JCO.2005.05.2456. [DOI] [PubMed] [Google Scholar]
- 12.Miner TJ, Shia J, Jaques DP, et al. Long-term survival following treatment of pseudomyxoma peritonei: an analysis of surgical therapy. Annals of Surgery. 2005;241:300–308. doi: 10.1097/01.sla.0000152015.76731.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gough DB, Donohue JH, Schutt AJ, et al. Pseudomyxoma peritonei. Long-term patient survival with an aggressive regional approach. Annals of Surgery. 1994;219:112–119. doi: 10.1097/00000658-199402000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinson FL, Ambrose NS. Pseudomyxoma peritonei. British J Surgery. 1998;85:1332–1339. doi: 10.1046/j.1365-2168.1998.00882.x. [DOI] [PubMed] [Google Scholar]
- 15.Cannistra SA. Intraperitoneal chemotherapy comes of age. New Engleand Journal of Medicine. 2006;354:77–79. doi: 10.1056/NEJMe058308. [DOI] [PubMed] [Google Scholar]
- 16.Stewart JH, Shen P, Russell GB, et al. Appendiceal Neoplasms with Peritoneal Dissemination: Outcomes after Cytoreductive Surgery and Intraperitoneal Hyperthermic Chemotherapy. Annals of Surgical Oncology. 2006;13:1–11. doi: 10.1007/s10434-006-9708-2. [DOI] [PubMed] [Google Scholar]
- 17.Bradley RF, Stewart JH, Russell GB, et al. Pseudomyxoma peritonei of appendiceal orgin: a clinicopathologic analysis of 101 uniformly treated patients at a single institution, with literature review. American Journal of Surgical Pathology. 2006;30:551–559. doi: 10.1097/01.pas.0000202039.74837.7d. [DOI] [PubMed] [Google Scholar]
- 18.Shen P, Levine EA, Hall J, et al. Factors predicting survival after intraperitoneal hyperthermic chemotherapy with mitomycin C after cytoreductive surgery for patients with peritoneal carcinomatosis. Archives of Surgery. 2003;138:26–33. doi: 10.1001/archsurg.138.1.26. [DOI] [PubMed] [Google Scholar]
- 19.Shen P, Hawksworth J, Lovato J, et al. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy with mitomycin C for peritoneal carcinomatosis from nonappendiceal colorectal carcinoma. Annals of Surgical Oncology. 2004;11(2):178–186. doi: 10.1245/aso.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Hall JJ, Loggie BW, Shen P, et al. Cytoreductive Surgery with Intraperitoneal Hyperthermic Chemotherapy for Advanced Gastric Cancer. Journal of Gastrointestinal Surgery. 2004;8:454–463. doi: 10.1016/j.gassur.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Sun Y, Shen P, Stewart JH, et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Carcinomatosis from Small Bowel Adenocarcinoma. The American Surgeon. 2013;79:644–648. [PMC free article] [PubMed] [Google Scholar]
- 22.Krane LS, Kader AK, Levine EA. Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Patients with Peritoneal Carcinomatosis Secondary to Urachal Adenocarcinoma. Journal of Surgical Oncology. 2012;105:258–260. doi: 10.1002/jso.22081. [DOI] [PubMed] [Google Scholar]
- 23.Randle RW, Swett K, Shen P, et al. Cytoreductive Surgery (CRS) with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Peritoneal Sarcomatosis. American Surgeon. 2013;79:620–624. [PMC free article] [PubMed] [Google Scholar]
- 24.Blackham AU, Shen P, Stewart JH, et al. Cytoreductive Surgery with Intraperitoneal Hyperthermic Chemotherapy for Malignant Peritoneal Mesothelioma: Mitomycin vs. Cisplatin. Annals of Surgical Oncology. 2010;17:2720–2727. doi: 10.1245/s10434-010-1080-6. [DOI] [PubMed] [Google Scholar]
- 25.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harmon RL, Sugarbaker PH. Prognostic indicators in peritoneal carcinomatosis from gastrointestinal cancer. Int. Seminars Surgical Oncology. 2005;2:3. doi: 10.1186/1477-7800-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.AJCC Cancer Staging Manual. 7th Ed. New York: Springer-Verlag; [Google Scholar]
- 28.Lentz S, Miller BE, Kucera GL, Levine EA. Intraperitoneal Hyperthermic Chemotherapy Using Carboplatin: A Phase I Analysis in Ovarian Carcinoma. Gynecologic Oncology. 2007;106:207–210. doi: 10.1016/j.ygyno.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 29.Stewart JH, Shen P, Russell GB, et al. A Phase I Trial of Oxaliplatin for Intraperitoneal Hyperthermic Chemoperfusion for the Treatment of Peritoneal Surface Dissemination From Colorectal and Appendiceal Cancer. Annals of Surgical Oncology. 2008;15:2137–2145. doi: 10.1245/s10434-008-9967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levine EA. Problems of success and problems of failure: recurrent disease after cytoreductive surgery and intraperitoneal chemoperfusion. Annals of Surgical Oncology. 2004;11:351–353. doi: 10.1245/ASO.2004.10.928. [DOI] [PubMed] [Google Scholar]
- 31.Dedrick RL, Flessner MF. Pharmacokinetic problems in peritoneal drug administration: tissue penetration and surface exposure. J National Cancer Institute. 1997;89:480–487. doi: 10.1093/jnci/89.7.480. [DOI] [PubMed] [Google Scholar]
- 32.Dedrick RL. Interspecies scaling of regional drug delivery. J Pharm Sci. 1986;75:1047–1052. doi: 10.1002/jps.2600751106. [DOI] [PubMed] [Google Scholar]
- 33.Flessner MF, Dedrick RL, Schultz JS. A distributed model of peritoneal-plasma transport: analysis of experimental data in the rat. Am J Physiology. 1985;248:F413–F424. doi: 10.1152/ajprenal.1985.248.3.F413. [DOI] [PubMed] [Google Scholar]
- 34.Kuzuya T, Yamauchi M, Ito A, et al. Pharmacokinetic characteristics of 5-fluorouracil and mitomycin C in intraperitoneal chemotherapy. J Pharm Pharmacol. 1994;46:685–689. doi: 10.1111/j.2042-7158.1994.tb03883.x. [DOI] [PubMed] [Google Scholar]
- 35.Speyer JL, Collins JM, Dedrick RL, et al. Phase I and pharmacological studies of 5-fluorouracil administered intraperitoneally. Cancer Research. 1980;40:567–572. [PubMed] [Google Scholar]
- 36.Israel VK, Jiang C, Muggia FM, et al. Intraperitoneal 5-fluoro-2’-deoxyuridine (FUDR) and (S)-leucovorin for disease predominantly confined to the peritoneal cavity: a pharmacokinetic and toxicity study. Cancer Chemother Pharmacol. 1995;37:32–38. doi: 10.1007/BF00685626. [DOI] [PubMed] [Google Scholar]
- 37.Ozols RF, Young RC, Speyer JL, et al. Phase I and pharmacological studies of adriamycin administered intraperitoneally to patients with ovarian cancer. Cancer Research. 1982;42:4265–4269. [PubMed] [Google Scholar]
- 38.Bartlett DL, Buell JF, Libutti SK, et al. A phase I trial of continuous hyperthermic peritoneal perfusion with tumor necrosis factor and cisplatin in the treatment of peritoneal carcinomatosis. Cancer. 1998;83:1251–1261. [PubMed] [Google Scholar]
- 39.Markman M, Kelsen D. Efficacy of cisplatin-based intraperitoneal chemotherapy as treatment of malignant peritoneal mesothelioma. J Cancer Res Clinical Oncology. 1992;118:547–550. doi: 10.1007/BF01225271. [DOI] [PubMed] [Google Scholar]
- 40.Markman M, Brady MF, Spirtos NM, et al. Phase II trial of intraperitoneal paclitaxel in carcinoma of the ovary, tube, and peritoneum: a Gynecologic Oncology Group Study. J Clinical Oncology. 1998;16:2620–2624. doi: 10.1200/JCO.1998.16.8.2620. [DOI] [PubMed] [Google Scholar]
- 41.Dikomey E, Franzke J. Effect of heat on induction and repair of DNA strand breaks in X-irradiated CHO cells. Int J Radiationt Biology. 1992;61:221–233. doi: 10.1080/09553009214550851. [DOI] [PubMed] [Google Scholar]
- 42.Roti JL, Kampinga HH, Malyapa RS, et al. Nuclear matrix as a target for hyperthermic killing of cancer cells. Cell Stress Chaperones. 1998;3:245–255. doi: 10.1379/1466-1268(1998)003<0245:nmaatf>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barlogie B, Corry PM, Drewinko B. In vitro thermochemotherapy of human colon cancer cells with cis-dichlorodiammineplatinum(II) and mitomycin C. Cancer Research. 1980;40:1165–1168. [PubMed] [Google Scholar]
- 44.Jacquet P, Averbach A, Stuart OA, et al. Hyperthermic Intraperitoneal doxorubicin:pharmacokinetics, metabolism and tissue distribution in a rat model. Cancer Chemotherapy Pharmacology. 1998;41:147–154. doi: 10.1007/s002800050721. [DOI] [PubMed] [Google Scholar]
- 45.Los G, Smals OAG, van Vugt MJH, et al. A rationale for carboplatin treatment and abdominal hyperthermia in cancers restricted to the peritoneal cavity. Cancer Research. 1992;52:1252–1258. [PubMed] [Google Scholar]
- 46.Shapiro JF, Chase JL, Wolff RA, et al. Modern Systemic Chemotherapy in Surgically Unresectable Neoplasms of Appendiceal Origin. Cancer. 2010;116:316–322. doi: 10.1002/cncr.24715. [DOI] [PubMed] [Google Scholar]
- 47.van Ruth S, Acherman YI, van de Vijver MJ, et al. Pseudomyxoma peritonei: a review of 62 cases. Eur J Surgical Oncology. 2003;29:682–688. doi: 10.1016/s0748-7983(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 48.Glehen O, Mohamed F, Gilly FN. Peritoneal carcinomatosis from digestive tract cancer: new management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncology. 2004;5:219–228. doi: 10.1016/S1470-2045(04)01425-1. [DOI] [PubMed] [Google Scholar]
- 49.Levine EA, Blazer DG, Kim MK, et al. Gene Expression Profiling of Peritoneal Metastases from Appendiceal and Colon Cancer Demonstrates Unique Biologic Signatures and Predicts Patient Outcomes. Journal of the American College of Surgeons. 2012;214:599–607. doi: 10.1016/j.jamcollsurg.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sugarbaker PH, Chang D. Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Annals Surgical Oncology. 1999;6:727–731. doi: 10.1007/s10434-999-0727-7. [DOI] [PubMed] [Google Scholar]
- 51.Witkamp AJ, de Bree E, Kaag MM, et al. Extensive surgical cytoreduction and intraoperative hyperthermic intraperitoneal chemotherapy in patients with pseudomyxoma peritonei. British J Surgery. 2001;88:458–463. doi: 10.1046/j.1365-2168.2001.01701.x. [DOI] [PubMed] [Google Scholar]
- 52.Chua TC, Moran BJ, Sugarbaker PH, et al. Early and Long-term Outcome Data on 2298 Patients with Pseudomyxoma Peritonei of Appendiceal Origin Treated by a Strategy of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Journal of Clinical Oncology. 2012;30:2449–2456. doi: 10.1200/JCO.2011.39.7166. [DOI] [PubMed] [Google Scholar]
- 53.Deraco M, Baratti D, Inglese MG, et al. Peritonectomy and intraperitoneal hyperthermic perfusion (IPHP): a strategy that has confirmed its efficacy in patients with pseudomyxoma peritonei. Annals Surgical Oncology. 2004;11:393–398. doi: 10.1245/ASO.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 54.Shetty S, Natarajan B, Thomas P, et al. Proposed Classification of Pseudomyxoma Peritonei: Influence of Signet Ring Cells on Survival. American Surgeon. 2013;79:1171–1176. [PubMed] [Google Scholar]
- 55.Ronnett BM, Zahn CM, Kurman RJ, et al. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to “pseudomyxoma peritonei.” Am J Surgical Pathology. 1995;19:1390–1408. doi: 10.1097/00000478-199512000-00006. [DOI] [PubMed] [Google Scholar]
- 56.Ihemelandu CU, McQuellon R, Shen P, et al. Predicting Postoperative Morbidity following Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy (CS + HIPEC) with preoperative FACT - C (Functional Assessment of Cancer Therapy) and Patient Rated Performance Status. Annals of Surgical Oncology. 2013;20:3519–3526. doi: 10.1245/s10434-013-3049-8. [DOI] [PubMed] [Google Scholar]
- 57.Culliford AT, Brooks AD, Sharma S, et al. Surgical debulking and intraperitoneal chemotherapy for established peritoneal metastases from colon and appendix cancer. Annals Surgical Oncology. 2001;8:787–795. doi: 10.1007/s10434-001-0787-9. [DOI] [PubMed] [Google Scholar]
- 58.Marcus EA, Weber TK, Rodriguez-Bigas MA, et al. Prognostic factors affecting survival in patients with colorectal carcinomatosis. Cancer Investigation. 1999;17:249–252. doi: 10.3109/07357909909040593. [DOI] [PubMed] [Google Scholar]
- 59.Rothenberg ML, Oza AM, Bigelow RH, et al. Superiority of oxaliplatin and fluorouracil-leukovorin as first-line treatment of metastatic colorectal cancer. Journal of Clinical Oncology. 2003;21:2059–2069. doi: 10.1200/JCO.2003.11.126. [DOI] [PubMed] [Google Scholar]
- 60.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil and leucovorin for metastatic colorectal cancer. New England Journal of Medicine. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 61.Franko J, Shi Q, Goldman CD, et al. Treatment of Colorectal Peritoneal Carcinomatosis with Systemic Chemotherapy: A Pooled Analysis of North Central Cancer Treatment Group Phase III Trials N9741 and N9841. Journal of Clinical Oncology. 2012;30:263–267. doi: 10.1200/JCO.2011.37.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yan T, Black D, Savady R, Sugarbaker PHA. Systematic Review on the efficacy of Cytoreductive Surgery Combined with Perioperative Intraperitoneal Chemotherpy for Peritoneal Carcinomatosis from Colorectal Carcinoma. Journal of Clincal Oncology. 2006;24:4011–4019. doi: 10.1200/JCO.2006.07.1142. [DOI] [PubMed] [Google Scholar]
- 63.Glehen O, Kwiakowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multiinstitutional study. J Clin Oncol. 2004;22(16):3284–3292. doi: 10.1200/JCO.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 64.Franko J, Ibrahim Z, Gusani et. al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemoperfusion Versus Systemic Chemotherapy Alone for Colorectal Peritoneal Carcinomatosis. Cancer. 2010;116:3756–3762. doi: 10.1002/cncr.25116. [DOI] [PubMed] [Google Scholar]
- 65.Turaga K, Levine EA, Barone R, et al. Consensus Guidelines from The American Society of Peritoneal Surface Malignancies on Standardizing the Delivery of Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Colorectal Cancer Patients in the United States. Annals of Surgical Oncology. 2013 doi: 10.1245/s10434-013-3061-z. [DOI] [PubMed] [Google Scholar]
- 66.Robinson WS, Lake RA. Advances in Malignant Mesothelioma. New England Journal of Medicine. 2005;353:1591–1603. doi: 10.1056/NEJMra050152. [DOI] [PubMed] [Google Scholar]
- 67.Trupiano JK, Geisinger KM, Willingham MD, et al. Diffuse Malignant Mesothelioma of the Peritoneum and Pleura, analysis of markers. Modern Pathology. 2004;17:476–481. doi: 10.1038/modpathol.3800067. [DOI] [PubMed] [Google Scholar]
- 68.Feldman AL, Libutti SK, Pingpank JF, et al. Analysis of Factors Associated with Outcome in Patients with Malignant Peritoneal Mesothelioma Undergoing Surgical Debulking and Intraperitoneal Chemotherapy. Journal of Clinical Oncology. 2003;21:4560–4567. doi: 10.1200/JCO.2003.04.150. [DOI] [PubMed] [Google Scholar]
- 69.Park BJ, Alexander R, Lubutti SK, et al. Treatment of Primary Peritoneal Mesothelioma by Continuous Hyperthermic Peritoneal Perfusion. Annals of Surgical Oncology. 1999;6:582–590. doi: 10.1007/s10434-999-0582-6. [DOI] [PubMed] [Google Scholar]
- 70.Cerruto CA, Brun EA, Chang D, Sugarbaker PH. Prognostic Significance of Histomorphologic Parameters in Diffuse Malignant Peritoneal Mesothelioma. Archives Pathology Laboratory Medicine. 2006;130:1654–1661. doi: 10.5858/2006-130-1654-PSOHPI. [DOI] [PubMed] [Google Scholar]
- 71.Cao C, Yan TD, Deraco M, et al. Importance of gender in diffuse malignant peritoneal mesothelioma. Annals of Oncology. 2012;23:1494–1498. doi: 10.1093/annonc/mdr477. [DOI] [PubMed] [Google Scholar]
- 72.Chua TC, Yan TD, Deraco M, et al. Multi-institutional experience of diffuse intra-abdominal multicystic peritoneal mesothelioma. British Journal of Surgery. 2011 Jan;98(1):60–64. doi: 10.1002/bjs.7263. [DOI] [PubMed] [Google Scholar]
- 73.Yan TD, Deraco M, Barratti D, et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Malignant Peritoneal Mesothelioma: Multi-institutional Experience. Journal of Clinical Oncology. 2009;27:6237–6242. doi: 10.1200/JCO.2009.23.9640. [DOI] [PubMed] [Google Scholar]
- 74.Yan TD, Deraco M, Elias D, et al. A Novel Tumor Node Metastasis (TNM) Staging System of Diffuse Malignant Peritoneal Mesothelioma Based on Outcome Analysis of a Multi-institutional Database. Cancer. 2011;117:1855–1863. doi: 10.1002/cncr.25640. [DOI] [PubMed] [Google Scholar]
- 75.McQuellon RP, Loggie BW, Fleming RA, et al. Quality of life after intraperitoneal hyperthermic chemotherapy (HIPEC) for peritoneal carcinomatosis. European J Surgical Oncology. 2001;27:65–73. doi: 10.1053/ejso.2000.1033. [DOI] [PubMed] [Google Scholar]
- 76.McQuellon RP, Loggie BW, Lehman AB, et al. Long-term survivorship and quality of life after cytoreductive surgery plus intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis. Annals Surgical Oncology. 2003;10:155–162. doi: 10.1245/aso.2003.03.067. [DOI] [PubMed] [Google Scholar]
- 77.Duckworth KE, McQuellon RP, Russell GB, et al. Patient Reported Outcomes and Survivorship Following Cytoreductive Surgery Plus Hyperthermic Intraperitoneal Chemotherapy (CS+HIPEC) Journal of Surgical Oncology. 2012;106:376–380. doi: 10.1002/jso.23089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alexander HR, Mavroukakis SM, Libutti SK, et al. Impact of Tumor Resection and Intraperitoneal Chemotherapy on Health Related Quality of Life in Patients with Peritoneal Surface Malignancies; Proceedings of the 57th Annual Society of Surgical Oncology Cancer Symposium; 2004. [Google Scholar]
- 79.Portilla AG, Sugarbaker PH, Chang D. Second-look surgery after cytoreduction and intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal cancer: analysis of prognostic features. World J Surgery. 1999;23:23–29. doi: 10.1007/s002689900560. [DOI] [PubMed] [Google Scholar]
- 80.Votanopoulos K, Ihemelandu C, Shen P, et al. Outcomes of Repeat Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for the Treatment of Peritoneal Surface Malignancy; It is all about Selection and Tumor Biology. Journal of the American College of Surgeons. 2012;215:412–417. doi: 10.1016/j.jamcollsurg.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hoverman R, Cartwright TH, Patt DA, et al. Pathways, Outcomes and Costs in Colon Cancer: Retrospective Evaluations in Two Distinct Databases. J. Oncology Practice. 2011;7:52s–59s. doi: 10.1200/JOP.2011.000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lawrence D, Maschio M, Leahy KJ, et al. Economic analysis of bevacizumab, cetuximab, panitumumab, with fluoropyrimidine-based chemotherapy in the firstline treatment of KRAS wild-type metastatic colorectal cancer(mCRC) J. Medical Economics. 2013;16:1387–1398. doi: 10.3111/13696998.2013.852097. [DOI] [PubMed] [Google Scholar]
- 83.Elias D, Delperro JR, Sideris L, et al. Treatment of peritoneal carcinomatosis from colorectal cancer: Impact of complete cytoreductive surgery and difficulties in conducting randomized trials. Annals of Surgical Oncology. 2004;11:518–521. doi: 10.1245/ASO.2004.09.008. [DOI] [PubMed] [Google Scholar]