Abstract
Resolution of severe RSV-induced bronchiolitis is mediated by alternatively activated macrophages (AA-Mϕ) that counteract cyclooxygenase (COX)-2-induced lung pathology. Herein, we report that RSV infection of 5-lipoxygenase (LO)−/− and 15-LO−/− macrophages or mice failed to elicit AA-Mϕ differentiation and concomitantly exhibited increased COX-2 expression. Further, RSV infection of 5-LO−/− mice resulted in enhanced lung pathology. Pharmacologic inhibition of 5-LO or 15-LO also blocked differentiation of RSV-induced AA-Mϕ in vitro and, conversely, treatment of 5-LO−/− macrophages with downstream products, lipoxin A4 (LXA4) and resolvin E1 (RvE1), but not leukotriene B4 (LTB4) or LTD4, partially restored expression of AA-Mϕ markers. Indomethacin blockade of COX activity in RSV-infected macrophages increased 5-LO, and 15-LO, as well as arginase-1 mRNA expression. Treatment of RSV-infected mice with indomethacin also resulted not only in enhanced lung arginase-1 mRNA expression and decreased COX-2, but also, decreased lung pathology in RSV-infected 5-LO−/− mice. Treatment of RSV-infected cotton rats with a COX-2-specific inhibitor resulted in enhanced lung 5-LO mRNA and AA-Mϕ marker expression. Together, these data suggest a novel therapeutic approach for RSV that promotes AA-Mϕ differentiation by activating the 5-LO pathway.
INTRODUCTION
Respiratory Syncytial Virus (RSV) infection generally elicits mild respiratory disease in healthy adults, but is potentially lethal for infants and young children, the elderly, and immunosuppressed individuals.1 In fact, RSV infection is the most common cause of virus-induced death in children under the age of five worldwide.1 Although a highly effective prophylactic monoclonal antibody is available in the USA for at-risk infants2, the cost of such treatment is prohibitive in most countries. Currently, there is no vaccine or therapeutic treatment for severe RSV disease.3
Although airway epithelial cells are the initial targets of RSV infection, RSV also infects lung macrophages. This results in the early release of potent proinflammatory cytokines and chemokines that recruit inflammatory cells that mediate a robust inflammatory response including cyclooxygenase-2 (COX)-2-mediated pathology.4 This proinflammatory profile is typical of classically activated macrophages (CA-Mϕ or M1).5,6 We have previously reported that differentiation of alternatively activated macrophages (AA-Mϕ or M2) occurs later in infection. These cells counteract the earlier inflammatory responses through induction of anti-inflammatory cytokines such as IL-10, IL-4, and IL-13, and mediate repair of lung damage through induction of enzymes such as FIZZ1, Ym1, and arginase-1.7
Arachidonic acid is a substrate for both the COX and lipoxygenase (LO) enzymes. The COX-1 and COX-2 enzymes convert arachidonic acid into prostaglandins and thromboxanes. COX-1 is ubiquitously expressed and functions in homeostatic roles, while COX-2 expression is highly correlated with the inflammatory process.8–10 In contrast to the COX enzymes, the LO pathway converts arachidonic acid to proinflammatory leukotrienes and anti-inflammatory lipoxins through enzymes including 5-, 12-, and 15-LO8,11. This pathway has been implicated in the pathogenesis or resolution of certain inflammatory diseases such as asthma and inflammatory bowel disease, respectively.12,13 Brown and colleagues reported that mice deficient in 5-LO exhibited persistent Lyme disease-induced arthritis.14 Although numerous cell types, including macrophages, are known to express 5-LO, 12-LO, and 15-LO, the level of expression is cell type-dependent.8,15
In this report, we show an unexpected reciprocal relationship between induction of COX-2 and arginase-1 (and other AA-Mϕ markers) in RSV-infected wild-type (WT) vs. 5-LO−/− and 15-LO−/− macrophages. This observation strongly suggested that the balance between COX-2 and LO pathways is critical to the development of pathology versus resolution of lung injury and suggests a novel therapeutic approach for amelioration of RSV-induced disease.
RESULTS
RSV-infected macrophages induce 5-LO and 15-LO mRNA and lipoxygenase enzymatic activity
Previous studies showed that COX-2 is responsible for RSV-induced lung pathology.4 We also reported that pharmacologic inhibition of COX-2 decreased RSV-induced pathology while increasing expression of AA-Mϕ genes.7 Since arachidonic acid is a substrate for both COX-2 and 5-LO, and 5-LO has been shown to resolve inflammation through the release of lipoxins and resolvins8, we sought to evaluate the possibility of a functional relationship between COX-2 and 5-LO with respect to RSV-induced lung pathology.
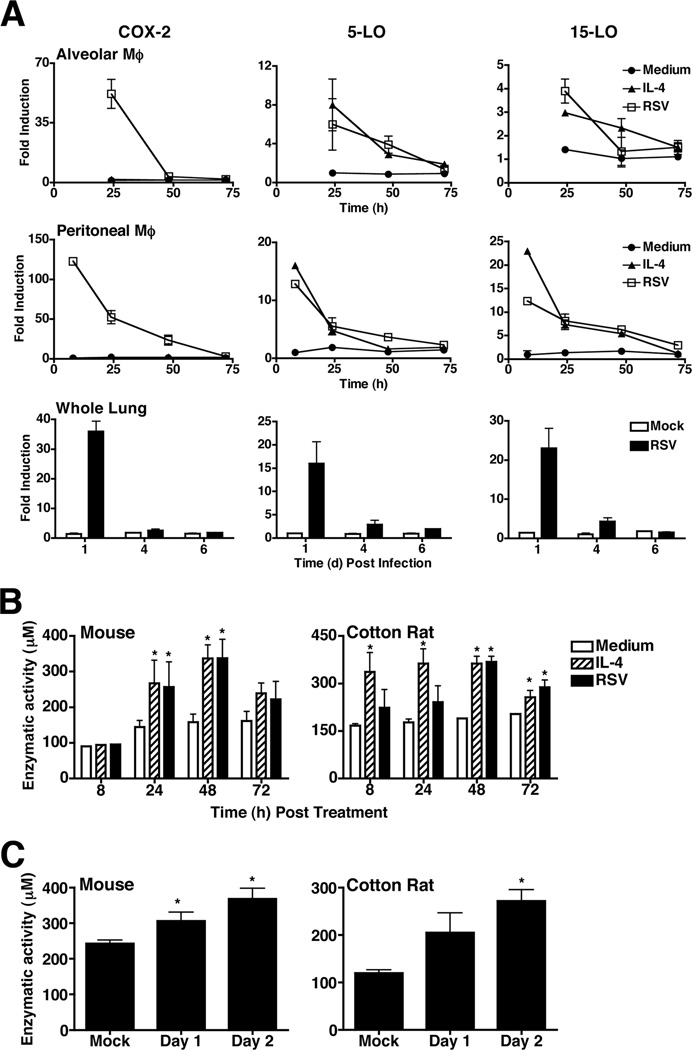
To determine if RSV infection up-regulates expression of 5-LO and/or 15-LO mRNA, purified mouse alveolar and peritoneal exudate macrophages were stimulated in vitro with medium only or rIL-4 (positive control for induction of AA-Mϕ), or infected with RSV (Fig. 1A). While rIL-4 did not induce COX-2, RSV significantly induced COX-2 mRNA early in infection that was reduced to basal levels by 72 h in both alveolar and peritoneal macrophages. Similar to rIL-4, RSV induced expression of both 5-LO and 15-LO mRNA within several hours of infection, and both returned to baseline by 72 h (Fig. 1A). Lung homogenates from RSV-infected mice showed similar results with both 5-LO and 15-LO mRNA being induced early, on day 1 post-infection, and returning to and remaining at basal levels by days 4 and 6 post-infection (Fig. 1A). RSV failed to induce 12-LO mRNA expression either in vitro or in vivo (data not shown).
Figure 1.
RSV infection induces 5-LO and 15-LO mRNA and lipoxygenase activity in vitro. (A) Highly purified WT C57BL/6J alveolar and peritoneal macrophage cultures were treated with medium only, rIL-4 (40 ng/ml), or infected with RSV (MOI = 2). WT C57BL/6J mice were mock infected with PBS or infected with RSV. Lungs were collected on the days indicated. Gene expression was analyzed by qRT-PCR. Data represent means +/− s.e.m from three (macrophages) and 2 (whole lungs) separate experiments. (B) WT C57BL/6J and cotton rat macrophages were treated as in (A). Lipoxygenase enzymatic activity was determined by the FOX assay. Data represent means +/− s.e.m. from 3 separate assays. (C) WT C57BL/6J mice and cotton rats were infected with RSV and lipoxygenase enzymatic activity was determined as in (B). Data represent means +/− s.e.m. from two separate assays with 3–6 animals per treatment group in 2 experiments (mouse) and 1 experiment (cotton rat; representative of 3 separate experiments with similar results).
The cotton rat (Sigmodon hispidus) is the model of choice for RSV infection because the lung responses more closely reflect that of humans.16 LO enzymatic activity peaked between 24 and 48 h in both mouse (Fig. 1B, left panel) and cotton rat macrophages (Fig. 1B, right panel) and in lung homogenates 2 days post-infection in both RSV-infected mice (Fig. 1C, left panel) and cotton rats (Fig. 1C, right panel).
Both the 5-LO and 15-LO enzymes are required for AA-Mϕ induction
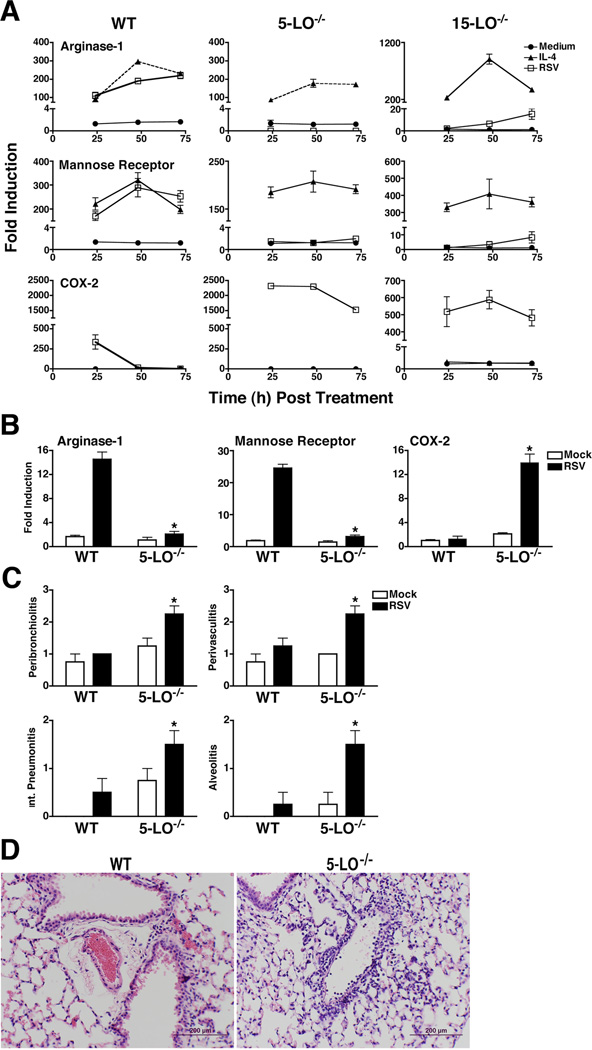
No lipoxygenase activity was observed in RSV-infected 5-LO−/− macrophages (data not shown). Therefore, to assess whether the 5-LO pathway is involved in the differentiation of RSV-induced AA-Mϕ, WT C57BL/6J or 5-LO−/− macrophages (on a C57BL/6 background) were stimulated with medium only or rIL-4, or infected with RSV, followed by analysis of prototype AA-Mϕ markers, arginase-1 and mannose receptor mRNA, by quantitative real-time PCR (qRT-PCR). Both arginase-1 and mannose receptor mRNA levels were increased comparably in rIL-4-treated and RSV-infected WT macrophages, while RSV infection failed to induce arginase-1 mRNA in 5- or 15-LO−/− macrophages (Fig. 2A). Conversely, RSV-infected 5-LO−/− and 15-LO−/− macrophages expressed greatly augmented steady-state levels of COX-2 mRNA (Fig. 2A). Thus, under conditions where AA-Mϕ are not induced by RSV, i.e., in absence of 5-LO or 15-LO, a significant up-regulation of COX-2 mRNA expression was observed. Similar findings were seen in vivo. Figure 2B illustrates that arginase-1 and mannose receptor mRNA expression is greatly increased in the lungs of WT, but not 5-LO−/− mice, whereas COX-2 mRNA is greatly increased in RSV-infected 5-LO−/− mice. That rIL-4 induced arginase-1, mannose receptor, and enhanced COX-2 mRNA expression in the 5/15-LO-deficient mice suggests that these enzymes act upstream of the induction of IL-4. Indeed, levels of IL-4 and IL-13 mRNA, but not levels of IL-4Rα, were decreased in the 5-LO and 15-LO macrophages (data not shown).
Figure 2.
The 5-LO pathway is required for RSV-induced AA-Mϕ differentiation and lack of 5-LO results in increased pathology in response to RSV infection in vivo. (A) WT C57BL/6J, 5-LO−/−, and 15-LO−/− peritoneal macrophages were treated as in Figure 1 and mRNA expression measured. Data are means +/− s.e.m. from 2 separate experiments. (B) WT and 5-LO−/− mice were mock- or RSV-infected. Mice were killed 6 days post-infection, and arginase-1 and COX-2 mRNA measured in lungs by qRT-PCR. (C) WT and 5-LO−/− mice were treated as in panel (B). Lung pathology was scored as described in “Methods.” Results are compiled from 2 independent experiments. (D) Lung sections derived from WT and 5LO−/− mice that were RSV-infected. Lungs were harvested 6 days post infection. Images were taken at 400X magnification and the bars shown are 200 µM.
Consistent with these findings, analysis of lung sections from RSV-infected 5-LO−/− mice revealed significantly greater pathology than seen in RSV-infected WT mice (Fig. 2C and D) for each histological parameter scored (i.e., peribronchiolitis, perivasculitis, interstitial pneumonitis, and alveolitis). Thus, 5/15-LO enzymes contribute to both the differentiation of AA-Mϕ and to amelioration of lung pathology induced by RSV infection.
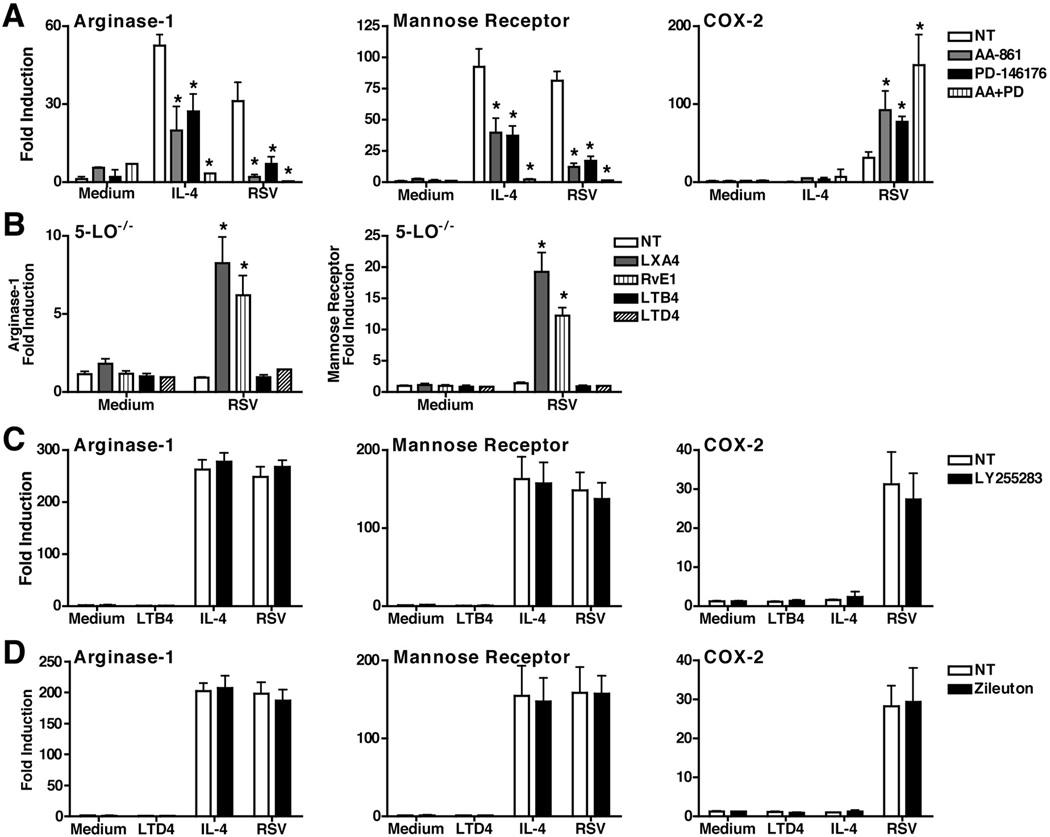
To confirm the observation that the 5-LO and/or 15-LO pathways reciprocally regulate AA-Mϕ differentiation and COX-2 expression, WT macrophages were treated with 5-LO pathway inhibitor AA-861 or 15-LO pathway inhibitor, PD-146176. Blocking either 5-LO and/or 15-LO inhibited the induction of both arginase-1 and mannose receptor mRNA in both rIL-4-treated and RSV-infected WT macrophages, while enhancing the induction of COX-2 mRNA (Fig. 3A). To challenge the hypothesis further, 5-LO−/− macrophages were treated exogenously with either lipoxin A4 (LXA4) leukotriene B4 (LTB4), or leukotriene D4 (LTD4), end products of the 5-LO pathway. LXA4 has been shown to exert anti-inflammatory actions whereas LTB4 and LTD4 have been shown to exert pro-inflammatory actions.8 Macrophages were also treated with resolvin E1 (RvE1), an endogenous lipid mediator known to be involved in the resolution of acute inflammation that is also an end product of the LO pathway, but is derived from the 5-LO substrate, eicosapentaenoic acid (EPA), rather than arachidonic acid.8,17 Figure 3B shows that LXA4 and RvE1, but neither LTB4 nor LTD4, increased expression of arginase-1 and mannose receptor mRNA significantly in 5-LO−/− macrophages infected with RSV.
Figure 3.
Pharmacological blockade of the LO pathway inhibits AA-Mϕ differentiation. (A) WT C57BL/6J peritoneal macrophages were pre-treated with AA-861 (5-LO inhibitor; 1 µg/ml), PD-146176 (15-LO inhibitor; 1 µg/ml), or both AA-861 and PD-146176 for 2 h. Macrophages were then treated with medium alone, rIL-4, or infected with RSV for 48 h. Gene expression was measured by qRT-PCR. (B) 5-LO−/− peritoneal macrophages were pre-treated with LXA4 (1 µg/ml) overnight, RvE1 (1 µg/ml), LTB4 (1 µg/ml), or LTD4 (1 µg/ml) for 1 h. Macrophages were treated with medium alone or infected with RSV for 48 h. Gene expression was measured by qRT- PCR. (C and D) WT C57BL/6J peritoneal macrophages were pre-treated with LY25583 (100 µg/ml) or zileuton (1 µM) for 1 h. Macrophages were then treated with medium alone, LTB4 (1 µg/ml) or LTD4 (1 µg/ml), rIL-4 (40 ng/ml) or infected with RSV for 48 h. Gene expression was measured by qRT-PCR. All data represent means +/− s.e.m. from 2 experiments.
Although it has been previously reported that infection with RSV can lead to the production of leukotrienes18,19, neither study showed that these molecules contributed to the cytokine induction or inflammatory responses associated with RSV. To assess further the possible involvement of leukotrienes during RSV infection in macrophages and their role in AA-Mϕ development, WT macrophages were treated with either an antagonist of the leukotriene receptors, BLT1 and BLT2 (LY255283), or an inhibitor of the cysteinyl leukotriene pathway (zileuton), and then infected with RSV. Neither inhibitor affected induction of RSV-induced arginase-1, mannose receptor, or COX-2 mRNA (Fig. 3C and 3D, respectively), suggesting that in contrast to lipoxins and resolvins, leukotrienes do not mediated the macrophage inflammatory response induced by RSV. Together, these observations strengthen the hypothesis that the 5-LO pathway reciprocally regulates expression of COX-2 and AA-Mϕ gene expression and favors the production of the anti-inflammatory lipoxins or resolvins during RSV infection.
Inhibition of COX enzymes in vitro or in vivo enhances 5-LO leading to AA-Mϕ induction
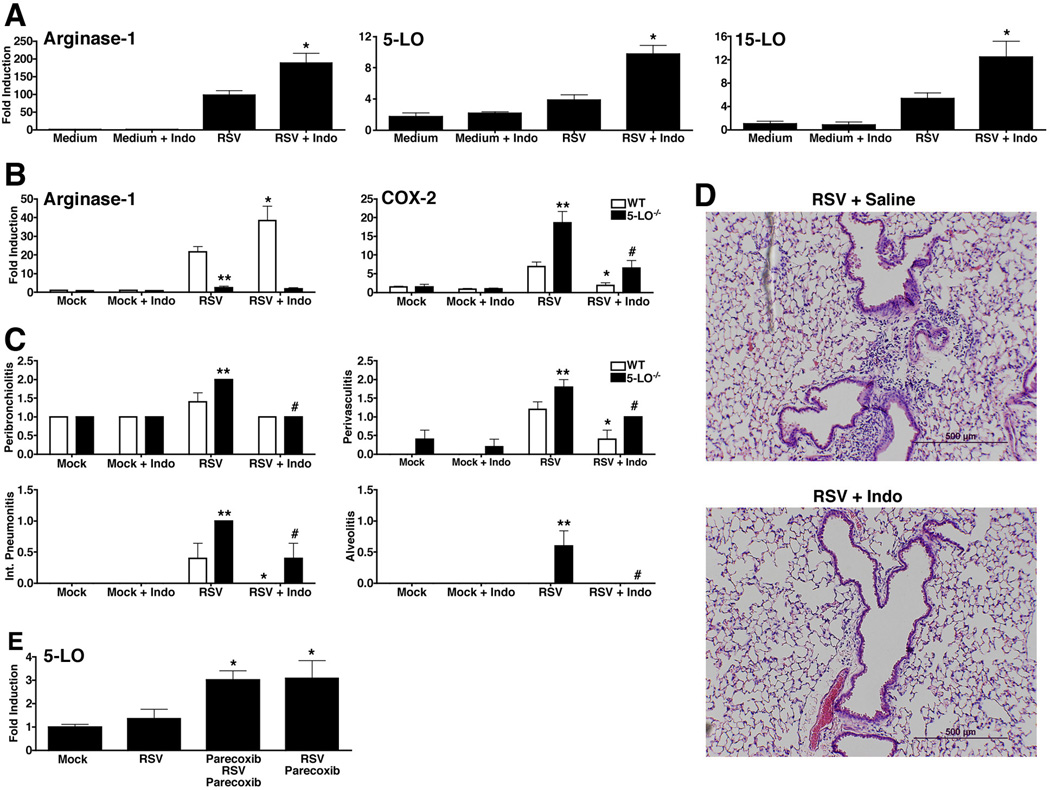
Richardson et al. previously showed that RSV selectively up-regulates COX-2 mRNA and protein in lung macrophages and that the pan-COX inhibitor, indomethacin, blocked RSV-induced pathology.4 To extend these findings, murine macrophages were infected with RSV in the absence or presence of indomethacin followed by analysis of arginase-1 mRNA. RSV-induced arginase-1 mRNA was increased when indomethacin was included in the macrophage cultures (Fig. 4A). In parallel, both 5-LO and 15-LO mRNA were also significantly increased during RSV infection in indomethacin-treated macrophages (Fig. 4A).
Figure 4.
Blocking COX activity enhances AA-Mϕ and 5-LO mRNA. (A) WT C57BL/6J macrophages were treated with medium alone or infected with RSV (MOI = 2) in the absence or presence of indomethacin (10 µM/ml) for 48 h. Gene expression for mRNA was measured by quantitative real-time PCR. Data represents means +/− s.e.m. from 2 experiments (* p < 0.05). (B-D) WT and 5-LO−/− mice were mock- or RSV-infected. Starting at day 2 post-infection, mice were either treated with saline or indomethacin (0.3 mg/kg) for 5 consecutive days. Mice were killed 6 days post-infection, and (B) arginase-1 and COX-2 mRNA measured in lungs by qRT-PCR. (C) Lung pathology was scored as described in “Methods.” (B-C: * is WT vs. WT comparison, p < 0.05; # is 5-LO−/− vs. 5-LO−/− comparison, p < 0.05; ** WT vs. 5-LO−/− comparison, p < 0.05). (D) Lung sections derived from 5-LO−/− mice that were RSV-infected and treated with either saline or indomethacin. Lungs were harvested 6 days post infection. Images were taken at 100X magnification and the bars shown are 500 µM. (E) Cotton rats were treated daily with vehicle (saline) or parecoxib (50 mg/kg) in 200 µl volume i.p. starting two days prior to infection for prophylactic group and starting on the day of infection (day 0) for therapeutic group. Animals were infected i.n. with RSV A/Long (1×106 pfu/100µl/animal) i.n. on day 0. Treatment on day 0 was done ~4 h post-infection. Animals were sacrificed on day 6 p.i. and 5-LO mRNA expression was analyzed by qRT-PCR. Uninfected and non-treated animals were used as controls.
When RSV-infected WT or 5-LO−/− mice were treated with indomethacin and their lungs harvested on day 6 post-infection, again, indomethacin treatment increased arginase-1 mRNA in the WT lungs, but failed to upregulate arginase 1 in the 5-LO−/− mice (Fig. 4B, left panel). Conversely, indomethacin treatment significantly decreased the RSV-induced COX-2 mRNA expression in both the WT and the 5-LO−/− mice (Fig. 4B, right panel). This decreased COX-2 correlated with a significant decrease in RSV-induced lung pathology that was more striking in the 5-LO−/− lungs (Figs. 4C and 4D).
Parecoxib, a COX-2-specific inhibitor, was shown previously to block COX-2 while up-regulating expression of arginase-1 and mannose receptor gene expression in lungs of RSV-infected cotton rats.7 To test the hypothesis that COX-2 and 5-LO induction are reciprocally regulated in response to RSV infection, cotton rats were pretreated with saline or parecoxib, infected with RSV, then treated with saline or parecoxib after infection or paracoxib was administered after RSV infection only. Both groups of parecoxib-treated, RSV-infected cotton rats exhibited significantly increased expression of 5-LO mRNA (Fig. 4E). Collectively, these data indicate that increased COX-2 inhibits both 5-LO induction and AA-Mϕ differentiation, underlying increased pathology in RSV-infected mice and cotton rats.
DISCUSSION
RSV, the most serious lower respiratory tract infection in infants and young children1, results in up to 125,000 hospitalizations20 and ~500 deaths yearly in the USA.21 RSV-specific immunity has been implicated in both protection and the immunopathological mechanism(s) that lead to severe lower respiratory tract disease and long-term changes in the immunological environment of the lung, i.e., RSV infection in early infancy has been correlated with development of allergic and asthmatic symptoms later in life.22 The idea that the immune response plays an adverse role in RSV-induced disease is based largely on the outcome of a failed clinical trial in the 1960’s in which vaccination of infants with formalin-inactivated RSV (FI-RSV) resulted in enhanced lower respiratory tract involvement upon subsequent RSV infection, including 2 deaths. Graham et al.23 first reported that FI-RSV-induced enhanced disease was associated with a “Th2 type” response. BALB/c mice immunized with FI-RSV then challenged with RSV exhibited a pattern of cytokine mRNA expression characterized by an increased ratio of IL-4/IFN-γ and eosinophilia, whereas unvaccinated animals showed a “Th1 type” response to RSV infection, with undetectable levels of IL-4.23 This led to the long-held idea that the Th2 response to RSV is pathologic.
RSV infection leads to an early “cytokine storm” and production of prostaglandins.4,24 Prostaglandins have an important role in the pathophysiology following RSV infection. There is a strong up-regulation of COX-2 in bronchiolar epithelial cells and macrophages after RSV.24 COX-2 has been reported to mediate lung pathology in both RSV and influenza infections.4,25 Later during infection, RSV-infected macrophages differentiate into the AA-Mϕ phenotype that repairs the damage from inflammation.7 This is seen in both BALB/c and C57BL/6 mice with peak induction at 48 h post-infection in vitro in both strains.7 Chen et al. showed that infection with influenza in mice can also lead to the development of AA-Mϕ that were proposed to facilitate secondary bacterial infections such as Streptococcus pneumoniae.26 A recent study by von Moltke et al. showed macrophage-induced inflammation occurred through inflammasome-dependent production of eicosanoids, such as prostaglandins and leukotrienes, that occurred within minutes of treating cells with either anthrax lethal toxin or flagella and had pathological effects.27 Tam et al. recently showed a lipidomic profile during influenza infection in both mouse and human that supports the lipoxygenase pathway for resolution of infection-induced inflammation.28 While prostaglandins and leukotrienes are well-known as inflammatory mediators, lipoxins and resolvins require the LO pathway for their synthesis and stimulate the resolution of inflammation in multiple diseases.8 The data presented herein extend these observations by showing that RSV-infected 5-LO−/− mice fail to develop AA-Mϕ and exhibit increased lung pathology due to elevated COX-2, suggesting the essentiality of the LO pathway for RSV-induced AA-Mϕ and resolution of lung injury. Blocking COX-2 leads to increased 5-LO and 15-LO mRNA, as well as enhanced arginase-1 and mannose receptor mRNA.7 That RSV induces both COX-2 and lipoxygenases, and both compete for the same substrate, arachidonic acid, supports the concept of an “eicosanoid storm,” as recently proposed by Vance and colleagues27, that contributes to both the pathology by COX-2, as well as the amelioration of the initial inflammatory damage by AA-Mϕ. However, if the anti-inflammatory responses are not well-controlled, then this pathway may result in a predisposition to subsequent airway hyperreactivity. In fact, asthma often develops in children following severe RSV and other respiratory viral infections such as rhinovirus and influenza early in life.29 Overall, our data i) indicate that targeting COX-2/5-LO pathways therapeutically may provide benefit in the context of severe RSV infection, and ii) provide a novel mechanism for the control and regulation of AA-Mϕ that may have broader implications in the context of other intracellular pathogens.
MATERIALS AND METHODS
Virus and Reagents for in vitro and in vivo studies
RSV Long strain (group A) was obtained from American Type Culture Collection (Manassas, VA), and propagated as described.4 Parecoxib was obtained from Exim-Pharm International (Mumbai, India). Indomethacin was purchased from Sigma-Aldrich (St. Louis, MO). AA-861 and PD-1746, 5-LO and 15-LO specific inhibitors, respectively, were purchased from Biomol International Inc. (Farmingdale, NY).30,31 LTD4, LY25583, and zileuton were purchased from Cayman Chemicals.32,33
Animals and macrophage cell cultures
Six to 8-week old, WT C57BL/6J and Alox5−/− (5-LO−/−) mice were purchased (Jackson Laboratory, Bar Harbor, ME). Alox15−/− (15-LO−/−) mice were bred at Cincinnati Children’s Hospital. Four to six-week old, inbred cotton rats (Sigmodon hispidus) were bred at Sigmovir Biosystems, Inc. (Rockville, MD). All animal experiments were conducted with institutional approval.
Highly purified (>97%) murine or cotton rat thioglycollate-elicited peritoneal macrophages, and murine BAL macrophages (>98%) were enriched as previously described (Shirey et al., 2010). Peritoneal macrophages were plated in 6-well (4×106 cells/well) or 12-well (2×106 cells/well), and BAL macrophages were plated in 24-well (2.5×105 cells per well) tissue culture plates. Macrophages were stimulated with medium alone, or murine or cotton rat rIL-4 (40 ng/ml; R&D Systems, Inc.), or infected with RSV (multiplicity of infection = 2) and incubated at 37° C for the indicated times.
For in vivo studies, WT C57BL/6J and 5-LO−/− mice and cotton rats were inoculated with sterile PBS or infected with RSV (1×106 pfu/animal) i.n. Lungs were harvested 1, 4, or 6 days later for RNA and histopathology.
Quantitative real-time reverse transcription PCR (qRT-PCR)
Total RNA isolation and real-time PCR were performed as previously described.7,34 Levels of mRNA for specific genes are reported as relative gene expression normalized to medium-treated samples. Cotton rat primers for 5-LO: forward 5’-TCAAGCAGCACAGGCGTAAAG-3’; reverse 5’-GTCCACGATGAAAATGTTCCCTTC.
Ferrous Oxidation-xylenol Orange (FOX) Assay for Lipoxygenase Activity
Lipoxygenase enzymatic activity was measured using the FOX assay.35,36 Treated macrophages were washed with cold 1X PBS twice, then lysed with 50 µl Cell Lysis Buffer (0.1% Triton X-100 containing 5 µg pepstatin, 5 µg aprotinin and 5 µg antipain) by gentle shaking for 30 min at room temperature. The lysates were diluted 1:1 by adding 50 µl 50 mM Tris-HCl pH 7.4 and heated at 55° C for 10 min and cooled for 5 minutes at 4°C. Cell lysis was incubated at 4° C for 5 min and 50 µl of cell lysate was transferred to a second 1.5 ml microcentrifuge tube where 50 µl of arachidonic acid was added to a final concentration 70 µM. The mixture was incubated at 37°C for 10 min with a ferrous II sulfate and methanol solution to stop the reaction. Aliquot 100 µl cell lysis solution to 96 well plate triplicate in each condition with standard curve, read at 630 nm.
Reconstitution Assay
5-LO−/− macrophages were treated with either LXA4 (1 µg/ml; all reagents kindly provided by Christopher L. Karp) overnight or LTB4 (1 µg/ml), LTD4 (1 µg/ml) or Resolvin E1 (1 µg/ml) for 1 h under a nitrogen blanket. Macrophages were then treated with medium alone or infected with RSV (MOI = 2) for 48 h before total RNA was isolated and gene expression analyzed by quantitative real-time PCR.
Inhibitor Assays
For inhibition of 5-LO or 15-LO, WT C57BL/6J thioglycollate-elicited macrophages were treated with either AA-861 (1 µg/ml) and/or PD-146176 (1 µg/ml), 5-LO and 15-LO inhibitors, respectively, for 2 h. For inhibition of LTB4 or LTD4, WT C57BL/6J thioglycollate-elicited macrophages were treated with either LY25583 (100 µg/ml) or zileuton (1 µM) for 1 h. Macrophages then treated with medium alone, rIL-4 (40 ng/ml), or infected with RVS (MOI = 2) for 48 h. Total RNA was isolated and gene expression for arginase-1 and COX-2 were analyzed by real time PCR.
To assess the effect of inhibiting COX induction, WT C57BL/6J thioglycollate-elicited macrophages were treated with medium alone or infected with RVS (MOI = 2) in the presence or absence of indomethacin (10 µM/ml) for 48 h. Total RNA was isolated and arginase-1 gene expression was analyzed by real time PCR.
For the in vivo therapeutic treatment of mice with indomethacin, WT C57BL/6J and 5-LO−/− mice were inoculated with sterile PBS or infected with RSV (1×106 pfu/animal) i.n. Starting at day 2 post-infection mice were either injected with saline or indomethacin (0.3 mg/kg in 100 ul) i.p. for 5 consecutive days. Lungs were harvested on day 6 for RNA and histopathology.
For the prophylactic and therapeutic treatment of RSV infections with the COX-2 specific inhibitor, parecoxib, cotton rats were treated daily with vehicle (saline) or parecoxib (50 mg/kg) in 200 µl volume i.p. starting two days prior to infection for prophylactic group and starting on the day of infection (day 0) for therapeutic group. Animals were infected with RSV A/Long (106 pfu/100 µl/animal) i.n. on day 0. Treatment on day 0 was done ~4 h post-infection. Animals were sacrificed on day 6 p.i. and 5-LO mRNA expression was analyzed by quantitative real-time PCR. Uninfected and non-treated animals were used as a control.
Histopathology
Fixed sections (10 µm) of paraffin-embedded lungs were stained with hematoxylin and eosin (H&E). Four inflammatory parameters were scored independently from 0 – 4 for each section as previously describedPrince LI 99: peribronchiolitis (inflammatory cells, primarily lymphocytes, surrounding a bronchiole), perivasculitis (inflammatory cells, primarily lymphocytes, surrounding a blood vessel), alveolitis (inflammatory cells within alveolar spaces), and interstitial pneumonitis (increased thickness of alveolar walls associated with inflammatory cells). Slides were randomized, read blindly, and scored for each.
Statistics
Statistical differences between two groups were determined using an unpaired, two-tailed Student’s t test with significance set at p < 0.05. For comparisons between three or more groups, analysis was carried out by one-way ANOVA, followed by Tukey’s multiple comparison test with significance determined at p < 0.05.
ACKNOWLEDGEMENTS
This study was supported by NIH grants AI-057575 (J.C.B.) and AI-18797 (S.N.V.) and Cystic Fibrosis Foundation RDP Center Grant (C.L.K.).
Footnotes
Disclosure: The authors have no conflict of interest to declare.
The authors declare no competing financial interests.
Author contributions: K.A.S. and S.N.V. carried out the study design, with advice from J.C.B. and C.L.K. K.A.S., W.L., J.C.B., and L.M.P. performed experiments. C.L.K. and S.D. provided crucial reagents for study. K.A.S. and S.N.V. prepared the manuscript, with input and approval of all other co-authors.
References
- 1.Welliver RC. Review of epidemiology and clinical risk factors for severe respiratory syncytial virus (RSV) infection. J Pediatr. 2003;143:S112–S117. doi: 10.1067/s0022-3476(03)00508-0. [DOI] [PubMed] [Google Scholar]
- 2.IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–537. [PubMed] [Google Scholar]
- 3.Blanco JCG, Boukhvalova MS, Shirey K, Prince GA, Vogel SN. New insights for development of a safe and protective RSV vaccine. Human. Vaccine. 2010;6:1–11. doi: 10.4161/hv.6.6.11562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson JY, et al. Respiratory syncytial virus (RSV) infection induces cyclooxygenase 2: a potential target for RSV therapy. J. Immunol. 2005;174:4356–4364. doi: 10.4049/jimmunol.174.7.4356. [DOI] [PubMed] [Google Scholar]
- 5.Gordon S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 6.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shirey K, et al. Control of RSV-induced lung injury by alternatively activated macrophages is IL-4Rα-. TLR4-, and IFN-β-dependent. Mucosal Immunol. 2010;3:291–300. doi: 10.1038/mi.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serhan CN, Haeggstrom JZ. Lipid mediators in acute inflammation and resolution: eicosanoids PAF, resolvins, and protectins. In: CN Serhan CN, Ward PA, Gilroy DW, editors. Fundamentals of Inflammation. New York, NY: Cambridge University Press; 2010. pp. 153–174. [Google Scholar]
- 9.Smith WL. Prostanoid biosynthesis and mechanisms of action. Am. J. Physiol. 1992;263:F18. doi: 10.1152/ajprenal.1992.263.2.F181. [DOI] [PubMed] [Google Scholar]
- 10.Dubois RN, et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063. [PubMed] [Google Scholar]
- 11.Karp CL, Cooper AM. An oily, sustained counter-regulatory response to TB. J Clin Invest. 2005;115:1473–1476. doi: 10.1172/JCI25353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wenzel SE. Arachidonic acid metabolites: mediators of inflammation in asthma. Pharmacotherapy. 1997;17:3S–12S. [PubMed] [Google Scholar]
- 13.Rask-Madsen J, Bukhave K, Laursen LS, Lauritsen K. 5-Lipoxygenase inhibitors for the treatment of inflammatory bowel disease. Agents Action. Spec. 1992;36:C37–C46. [PubMed] [Google Scholar]
- 14.Blaho VA, Zhang Y, Hugh-Hanks JM, Brown CR. 5-Lipoxygenase-deficient mice infected with Borrelia burgdorferi develop persistent arthritis. J Immunol. 2011;186:3076–3084. doi: 10.4049/jimmunol.1003473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhn H, O’Donnell VB. Inflammation and immune regulation by 12/15-lipoxygenases. Progress in Lipid Res. 2006;45:334–356. doi: 10.1016/j.plipres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Prince GA, Prieels JP, Slaoui M, Porter DD. Pulmonary lesions in primary respiratory syncytial virus infection, reinfection, and vaccine-enhanced disease in the cotton rat (Sigmodon hispidus) Lab. Invest. 1999;79:1385–1392. [PubMed] [Google Scholar]
- 17.Ohira T, et al. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J Biol Chem. 2010;285:3451–3461. doi: 10.1074/jbc.M109.044131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behera AK, Kumar M, Matsuse H, Lockey RF, Mohapatra SS. Respiratory syncytial virus induces the expression of 5-lipoxygenase and endothelin- in bronchial epithelial cells. Biochem Biophys Res Commun. 1998;251:704–709. doi: 10.1006/bbrc.1998.9537. [DOI] [PubMed] [Google Scholar]
- 19.Weede-Beer K, Hu C, Rodriguez MM, Piedimonte G. Leukotrienes mediate inflammation in lungs of young rats infected with respiratory syncytial virus. Am J Physiol Lung Cell Mol Physiol. 1988;282:L1143–L1150. doi: 10.1152/ajplung.00323.2001. [DOI] [PubMed] [Google Scholar]
- 20.Shay DK, et al. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 21.Shay DK, Holman RC, Roosevelt GE, Clarke MJ, Anderson LJ. Bronchiolitis-associated mortality and estimates of respiratory syncytial virus-associated deaths among US children, 1979–1997. J Infect. Dis. 2001;183:16–22. doi: 10.1086/317655. [DOI] [PubMed] [Google Scholar]
- 22.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B, Bjorksten B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95:500–505. [PubMed] [Google Scholar]
- 23.Graham BS, et al. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J Immunol. 1993;151:2032–2040. [PubMed] [Google Scholar]
- 24.Radi ZA, Meyerholz DK, Ackermann MR. Pulmonary cyclooxygenase-1 (COX-1) and COX-2 cellular expression and distribution after respiratory syncytial virus and parainfluenza virus infection. Viral Immunol. 2010;23:43–48. doi: 10.1089/vim.2009.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carey MA, et al. Contrasting effects of cyclooxygenase-1 (COX-1) and COX-2 deficiency on the host response to influenza A viral infection. J Immunol. 2005;175:6878–6884. doi: 10.4049/jimmunol.175.10.6878. [DOI] [PubMed] [Google Scholar]
- 26.Chen WH, et al. Potential role for alternatively activated macrophages in the secondary bacterial infection during recovery from influenza. Immunol Lett. 2012;141:227–234. doi: 10.1016/j.imlet.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Moltke J, et al. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature. 2012;490:107–111. doi: 10.1038/nature11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tam VC, et al. Lipidomic profiling of influenza infection identifies mediators that induce and resolve inflammation. Cell. 2013;154:213–227. doi: 10.1016/j.cell.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dulek DE, Peebles RS., Jr Viruses and asthma. Biochim Biophys Acta. 2011;1810:1080–1090. doi: 10.1016/j.bbagen.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du L, et al. Binding investigation of human 5-lipoxygenase with its inhibitors by SPR technology correlating with molecular docking simulation. J Biochem. 2006;139:715–23. doi: 10.1093/jb/mvj084. [DOI] [PubMed] [Google Scholar]
- 31.Sordillo LM, et al. Enhanced 15-HPETE production during oxidant stress induces apoptosis on endothelial cells. Prostaglandins Other Lipid Mediat. 2005;76:19–34. doi: 10.1016/j.prostaglandins.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Yokomizo T, Kato K, Hagiya H, Izumi T, Shimizu T. Hydroxyeicosanoids bind to and activate the low affinity leukotriene B4 receptor, BLT2. J Biol Chem. 2001;276:12454–12459. doi: 10.1074/jbc.M011361200. [DOI] [PubMed] [Google Scholar]
- 33.Carter GW, et al. 5-lipoxygenase inhibitory activity of zileuton. J Pharmacol Exp Ther. 1991;256:929–937. [PubMed] [Google Scholar]
- 34.Shirey K, Cole LE, Keegan AD, Vogel SN. Francisella tularensis LVS induces macrophage alternative activation as a survival mechanism. J Immunol. 2008;181:4159–4167. doi: 10.4049/jimmunol.181.6.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waslidge NB, Hayes DJ. A colorimetric method for the determination of lipoxygenase activity suitable for use in a high throughput assay format. Anal Biochem. 1995;231:354–358. doi: 10.1006/abio.1995.0063. [DOI] [PubMed] [Google Scholar]
- 36.Cho YS, Kim HS, Kim CH, Cheon HG. Application of the ferrous oxidation-xylenol orange assay for screening of 5-lipoxygenaes inhibitors. Anal Biochem. 2006;351:62–68. doi: 10.1016/j.ab.2005.12.025. [DOI] [PubMed] [Google Scholar]