Abstract
Increased blood cholesterol is an independent risk factor for atherosclerotic cardiovascular disease. Cholesterol homeostasis in the body is controlled mainly by endogenous synthesis, intestinal absorption, and hepatic excretion. Niemann-Pick C1-Like 1 (NPC1L1) is a polytopic transmembrane protein localized at the apical membrane of enterocytes and the canalicular membrane of hepatocytes. It functions as a sterol transporter to mediate intestinal cholesterol absorption and counterbalances hepatobiliary cholesterol excretion. NPC1L1 is the molecular target of ezetimibe, a potent cholesterol absorption inhibitor that is widely used in treating hypercholesterolemia. Recent findings suggest that NPC1L1 deficiency or ezetimibe treatment also prevents diet-induced hepatic steatosis and obesity in addition to reducing blood cholesterol. Future studies should focus on molecular mechanisms underlying NPC1L1-dependent cholesterol transport and elucidation of how a cholesterol transporter modulates the pathogenesis of metabolic diseases.
Keywords: ezetimibe, fat absorption, hepatic steatosis, obesity
INTRODUCTION
In mammals, cholesterol is not only a structural component of cell membranes essential for maintaining membrane integrity, permeability, and fluidity, it is also a signaling molecule as well as the precursor for the synthesis of steroid hormones and bile acids. Throughout evolution, the conversion of cholesterol to bile acids has been critical for energy acquisition and survival because dietary fat is not efficiently absorbed if it is not solubilized in bile acids (1, 2). Cholesterol conversion to bile acids is also critical for intestinal absorption of cholesterol. Although cholesterol can be synthesized in the body from simple substrates, it is a process requiring substantial energy input. In contrast, large amounts of cholesterol enter the intestinal lumen from dietary intake and biliary secretion. The daily intake of cholesterol in humans on a typical Western diet is ~300–500 mg, and the daily secretion of cholesterol into bile is estimated to be ~800–1200 mg (3). To attenuate the burden of energy-consuming de novo cholesterol biosynthesis, a physiological process—intestinal cholesterol absorption—has evolved to take up readily available cholesterol molecules from the gut lumen. Cholesterol molecules derived from both de novo synthesis and intestinal absorption are carried in the blood in the form of protein-lipid complexes termed lipoproteins and are delivered to cells for utilization via the circulation. Excess cholesterol is disposed of in the feces through biliary and perhaps direct intestinal secretion (4).
Efficient transport of cholesterol in the body depends on many proteins. Defects in these transport-related proteins, and in proteins involved in cholesterol biosynthesis and its regulation, disturb whole-body cholesterol homeostasis, resulting in diseases. Increased circulating levels of total cholesterol and low-density-lipoprotein cholesterol (LDL-C) cause atherosclerotic cardiovascular disease (CVD) (5), the leading cause of death in developed countries. According to a report from the American Heart Association, CVD accounted for 34.3% of all deaths in the United States in 2006, and the estimated direct and indirect costs of CVD for 2010 are US$503.2 billion, a 14% increase compared with the US$431.8 billion cost for 2007 (6).
Until the beginning of this century, statins were the major drugs prescribed to lower blood LDL-C. They lower LDL-C by inhibiting 3-hydroxy-3-methyl-glutaryl coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme in cholesterol synthesis (7). However, even with administration of large doses, reductions in the incidence of coronary heart disease were only ~30% for statins (8). Further reductions in LDL-C were recommended (9). The search for novel LDL-C-lowering drugs resulted in the discovery of ezetimibe (10), a potent intestinal cholesterol absorption inhibitor (11, 12). Compared with administration of a statin alone, coadministration of ezetimibe with a statin has led to greater reductions in blood total cholesterol and LDL-C (13–15).
The potency of low doses of ezetimibe in inhibiting intestinal cholesterol absorption has demonstrated that this physiological pathway is a protein-mediated active process. The search for key protein(s) in this process resulted in the discovery of Niemann-Pick C1-Like 1 (NPC1L1) (16). Recent studies show that NPC1L1 is essential for intestinal cholesterol absorption and is the molecular target of ezetimibe (16–18). Here, we discuss NPC1L1 and review its role in intestinal and hepatic cholesterol transport.
NPC1L1 GENE AND PROTEIN
NPC1L1 was identified using a genomic and bioinformatics approach (16, 19). It is a homolog of Niemann-Pick C1 (NPC1) protein, whose mutations cause Niemann-Pick disease type C1, a genetic disorder characterized by lysosomal accumulation of cholesterol and other lipids (20, 21). The NPC1L1 gene spans ~29 kb of human chromosome 7p13, containing 20 exons with an unusually large exon 2 of 1,526 bp and a small exon 14 of 56 bp (16, 19). The full-length cDNA encodes a 1,359-amino-acid protein. However, the predominant mRNA transcript skips exon 15 and produces a protein of 1,332 amino acids, which is the form typically studied in most NPC1L1 experiments (19, 22, 23). There is also a truncated transcript, which skips exon 7 and terminates within intron 8 (19). The biological significance of alternatively spliced transcripts has yet to be determined.
NPC1L1 has a typical signal peptide, an N-terminal NPC1 domain, and extensive N-glycosylation sites (16, 19). The protein is highly N-glycosylated (24). Amino acid sequences of NPC1L1 share 51% similarity and 42% identity with those of NPC1 (19). Like its homolog, NPC1L1 also shares topological similarity with the resistance-nodulation-division family of prokaryotic permeases (25). These permeases mediate the efflux of lipophilic drugs, detergents, fatty acids, bile acids, metal ions, and dyes from the cytosol of bacteria, implying a role for NPC1L1 in lipid transport (25).
Notably, NPC1L1 has 13 transmembrane domains, 5 of which constitute a sterol-sensing domain (SSD) (16, 19, 26). This SSD is conserved in several polytopic transmembrane proteins involved in cholesterol metabolism and regulation (27), including NPC1, HMG-CoA reductase, SCAP [sterol regulatory element–binding protein (SREBP) cleavage-activating protein], and the Hedgehog signaling protein Patched. The purified SSD-containing membrane region of SCAP binds cholesterol (28). NPC1 appears to require a functional SSD to bind a radiolabeled, photoactivatable cholesterol analog (29). The function of NPC1L1’s SSD remains to be elucidated. In cultured cells, NPC1L1 protein cycles between intracellular compartments and the cell membrane in a cholesterol-dependent manner (22). When cells are enriched with cholesterol, NPC1L1 is localized predominantly in the endocytic recycling compartment (ERC). When cells are deprived of cholesterol, NPC1L1 moves to the plasma membrane. The SSD may regulate NPC1L1’s intracellular itineraries by sensing membrane cholesterol content.
TISSUE DISTRIBUTION AND REGULATION OF NPC1L1 EXPRESSION
NPC1L1 is abundantly expressed in the small intestine across species (16, 30). Expression of NPC1L1 in other tissues varies in rodents and humans/nonhuman primates. In rodents, NPC1L1 is almost exclusively expressed in the small intestine (16, 30), but humans and non-human primates express a significant amount of NPC1L1 in the liver as well (22, 31).
Transcriptional regulation of NPC1L1 remains unclear and inconsistent. Several nuclear receptors, including peroxisome proliferator–activated receptor (PPAR)α, PPARδ, liver X receptor (LXR), and retinoid X receptor (RXR), have been implicated in the regulation of intestinal cholesterol absorption (32–39), but whether they function by directly modulating intestinal NPC1L1 expression remains elusive. A series of animal and cell-based studies suggest that these nuclear receptors may not have direct effects on NPC1L1 transcription (39–43).
Several pieces of evidence suggest that NPC1L1 may be transcriptionally regulated by cellular cholesterol content through SREBP-2, a membrane-bound transcription factor that increases expression of cholesterol synthetic genes when cellular cholesterol content is low (44, 45). For instance, feeding mice a diet containing high amounts of cholesterol and bile acids dramatically reduces intestinal NPC1L1 mRNA levels (46). Mice lacking acyl-CoA:cholesterol acyltransferase 2 (ACAT2) or phospholipid transfer protein (PLTP) accumulate unesterified cholesterol in enterocytes and simultaneously show reduced intestinal NPC1L1 mRNA levels (47, 48). Conversely, cholesterol deprivation raises hepatic and intestinal NPC1L1 mRNA levels in mice and miniature pigs (49, 50). SREBP-2 binds the two sterol regulatory elements in the human NPC1L1 promoter and increases NPC1L1 promoter activity in CaCo-2 cells (51) and in HuH7 human hepatoma cells (52). Furthermore, in CaCo-2 cells another transcription factor, hepatocyte nuclear factor 4α (HNF4α), synergistically enhances the effect of SREBP-2 on stimulating human NPC1L1 promoter activity, although HNF4α alone does not have such an effect (53). A recent study, however, did not reproduce in HuH7 human hepatoma cells the role of HNF4α in stimulating SREBP-2-dependent regulation of NPC1L1. This study instead found that HNF1α, a downstream effector of HNF4α, seems to play a direct role in regulating NPC1L1 transcription (52).
Despite strong evidence supporting regulation of NPC1L1 by cholesterol, discrepancies exist. For example, the addition of cholesterol to a chow diet does not suppress intestinal NPC1L1 expression in mice (41, 54). Ezetimibe treatment, a manipulation that reduces cholesterol content in absorptive enterocytes, does not increase NPC1L1 mRNA in the small intestines of chow-fed mice (41). It is unclear whether these discrepancies are attributable to differences in diet composition, animal species, treatment duration, experimental system, and/or other variables.
NPC1L1 MEDIATES INTESTINAL CHOLESTEROL ABSORPTION
Many excellent reviews have discussed lipid absorption (3, 55–58). Intestinal absorption of unesterified cholesterol begins with its incorporation into mixed micelles containing bile acids, phospholipids, and hydrolytic products of triglycerides. This step is critical for cholesterol to diffuse across the unstirred water layer, the major barrier that lies between the bulk water phase and the intestinal brush border membrane (57). The uptake of cholesterol and other nutrients from mixed micelles occurs at the brush border membrane. Given the hydrophobic nature of cholesterol, it was thought that cholesterol may move across the brush border membrane through a passive diffusion process and then enter enterocytes down a concentration gradient (57). It is now clear that the apically localized NPC1L1 mediates the bulk of sterol movement into the enterocyte, because the genetic inactivation of NPC1L1 in mice results in a substantial reduction in intestinal cholesterol absorption to a degree similar to that observed in mice treated with ezetimibe (16). After entering the enterocyte via a NPC1L1-dependent pathway (Figure 1), unesterified cholesterol may be pumped back to the intestinal lumen by an apically localized, heterodimeric sterol efflux transporter, ABCG5/ABCG8 [ATP-binding cassette (ABC) transporters G5 and G8] (59–62), or may be transported to the basolateral membrane of the enterocyte for ABCA1-mediated biogenesis of high-density lipoprotein (HDL) (47, 63, 64). The majority of cholesterol is esterified in the endoplasmic reticulum by ACAT2 (65) and is then incorporated into the nascent chylomicron, along with triglycerides, some unesterified cholesterol, and apolipoprotein B48. The intracellular assembly of this nascent chylomicron requires microsomal triglyceride transfer protein (MTP), a protein that also facilitates the formation of very low density lipoprotein (VLDL) in the hepatocyte (66, 67). Mature chylomicrons are then secreted into lymph across the basolateral membrane of enterocytes and are transported to the bloodstream via the lymphatic system. In the circulation, lipoprotein lipase hydrolyzes triglycerides in the core of chylomicrons for utilization and storage by peripheral tissues such as fat and muscle, whereas the majority of cholesterol in the chylomicron remnant is delivered to the liver.
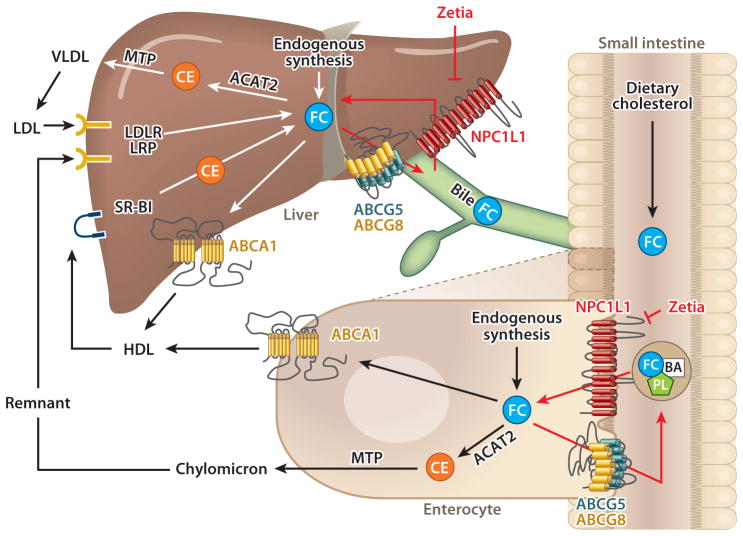
Figure 1.
The role of Niemann-Pick C1-Like 1 (NPC1L1) in cholesterol transport in the small intestine and liver. In the lumen of the small intestine, unesterified free cholesterol (FC) from dietary intake and biliary secretion is solubilized in mixed micelles containing bile acids (BA) and phospholipids (PL). This solubilization is critical for the diffusion of FC across the unstirred water layer to reach intestinal brush border membranes, where FC is taken up into enterocytes by the apically localized NPC1L1 protein. Ezetimibe (Zetia) can inhibit this NPC1L1-dependent cholesterol uptake. The majority of absorbed and endogenously synthesized cholesterol is transported to the endoplasmic reticulum, where it is converted to cholesterol ester (CE) by acyl-CoA:cholesterol acyltransferase 2 (ACAT2) and is then assembled into chylomicrons in a microsomal triglyceride transfer protein (MTP)-dependent manner for secretion into the circulation via the lymphatic system. Unesterified FC may be transported back to the intestinal lumen by the apically localized heterodimeric sterol transporter ABCG5/ABCG8 [ATP-binding cassette (ABC) transporters G5 and G8]. FC may also be transported into the circulation as a constituent of high-density lipoprotein (HDL) via ABCA1 located at the basolateral membrane of enterocytes. In the liver, cholesterol can be synthesized locally or taken up by hepatocytes from circulating lipoproteins such as low-density lipoprotein (LDL), HDL, and chylomicron remnants via LDL receptor (LDLR), HDL receptor scavenger receptor class B type I (SR-BI), and LDLR-related protein (LRP) localized at the basolateral membrane of hepatocytes. A large amount of FC is converted to bile acids for hepatobiliary secretion. A proportion of FC is esterified by ACAT2 and then packaged into nascent very low density lipoprotein (VLDL) particles in an MTP-dependent manner for secretion into the circulation as a constituent of VLDL. Unmetabolized FC can be transported to ABCA1 localized at the sinusoidal membrane of hepatocytes for the biogenesis of HDL or to ABCG5/ABCG8 localized at the canalicular membrane of hepatocytes for direct secretion into bile. In humans and nonhuman primates, NPC1L1 is also localized at the apical membrane of hepatocytes, where it may counterbalance the function of ABCG5/ABCG8 by transporting newly secreted biliary cholesterol back into hepatocytes, thereby preventing excessive loss of endogenous cholesterol.
NPC1L1 DEFICIENCY DISRUPTS ENTEROHEPATIC CIRCULATION OF ENDOGENOUS CHOLESTEROL
Biliary cholesterol accounts for more than two-thirds of the total amount of cholesterol in the gut lumen of humans (68). Approximately 800–1,200 mg of unesterified cholesterol are secreted daily from the liver into the intestinal lumen via the biliary route (3). If one considers that the average fractional cholesterol absorption is ~50% (69), humans reabsorb ~500 mg of bile-derived cholesterol daily, the majority of which is ultimately delivered to the liver. This process is the enterohepatic circulation of endogenous cholesterol, resembling the enterohepatic circulation of bile acids (70), although at a much lower efficiency. NPC1L1 deficiency or ezetimibe treatment disrupts enterohepatic circulation of cholesterol, thereby causing a substantial loss of endogenous cholesterol from the body and a dramatic feedback upregulation of endogenous cholesterol synthesis (16, 46, 71, 72). Importantly, this feedback upregulation of cholesterol synthesis does not appear to fully compensate for the excessive loss of cholesterol because NPC1L1 deficiency or ezetimibe treatment can significantly reduce blood cholesterol in pure vegetarians (73) and in rodents on low-cholesterol diets (11, 74). Thus, disruption of the enterohepatic circulation of endogenous cholesterol plays a key role in the hypocholesterolemic effects of ezetimibe.
HEPATIC NPC1L1 INHIBITS BILIARY CHOLESTEROL EXCRETION
Liver plays a central role in whole-body cholesterol homeostasis (Figure 1). First, it contributes a significant proportion of cholesterol via synthesis in the whole animal (75). Second, it produces and takes up lipoprotein cholesterol, critical for regulating blood cholesterol concentrations. It also converts cholesterol to bile acids and therefore regulates intestinal absorption efficiencies of lipids and vitamins. Liver secretes both unesterified cholesterol and cholesterol-derived bile acids into bile and thus is important for cholesterol disposal. All these pathways in the liver are protein-mediated processes that are metabolically integrated on several levels in response to fluctuations in dietary, systemic, and local cholesterol pools.
As discussed above, in humans and nonhuman primates, NPC1L1 is also expressed in the liver. The protein localizes to the canalicular membrane of hepatocytes (22, 31), where the cholesterol efflux transporter ABCG5/ABCG8 also resides (76, 77). Genetic and animal studies have demonstrated that ABCG5/ABCG8 mediates hepatobiliary secretion of unesterified cholesterol (59–62). What is the physiological role of NPC1L1 in the bile canalicular membrane? To address this question, liver-specific NPC1L1 transgenic mice were generated. Hepatic overexpression of human NPC1L1 dramatically reduces biliary cholesterol concentration and secretion without altering the hepatic expression of ABCG5/ABCG8 (31). Given the critical role of NPC1L1 in intestinal cholesterol uptake (influx), it is tempting to speculate that hepatic NPC1L1 prevents excessive loss of cholesterol by retrieving ABCG5/ABCG8-derived cholesterol from the canalicular bile.
Interestingly, the impaired biliary cholesterol excretion in liver-specific NPC1L1 transgenic mice can be rescued by ezetimibe treatment, indicating that ezetimibe can inhibit NPC1L1 function in both the intestine and the liver. The inhibition of hepatic NPC1L1 by ezetimibe is expected to increase the cholesterol saturation index in bile and therefore has the potential to promote gallstone formation. However, in rodents treated with ezetimibe, the opposite effect was observed, particularly when these animals were on a high-cholesterol-containing diet (71, 78–80), which may be attributable to the tissue distribution pattern of NPC1L1 expression in these species. NPC1L1 is almost exclusively expressed in the rodent intestine, and its inhibition by ezetimibe would result in a substantial reduction in cholesterol transport to the liver, thereby limiting cholesterol for biliary secretion and potential gallstone formation. In a few human subjects treated with ezetimibe, this seems to be the case (79). However, interindividual variations in hepatic and intestinal NPC1L1 expression may exist (31), and in some individuals with higher NPC1L1 expression in the liver relative to intestinal expression, treatment with ezetimibe may predispose to gallstone formation.
ASSOCIATION OF GENETIC VARIATION IN NPC1L1 WITH CHOLESTEROL ABSORPTION AND LOW-DENSITY-LIPOPROTEIN CHOLESTEROL
Fractional intestinal cholesterol absorption varies highly in healthy individuals, ranging from 29% to 80% (69). Increased cholesterol absorption raises cholesterol content in the liver and downregulates hepatic expression and activity of LDL receptors, resulting in reduced LDL clearance by the liver and elevated blood LDL-C levels (81, 82). Although the crosstalk among multiple proteins may ultimately determine how much cholesterol is absorbed, NPC1L1 appears to be a major determinant. Using the nonsynonymous variant technique, Cohen and colleagues (83) showed that variation in human NPC1L1 gene sequences contributes to reduced intestinal cholesterol absorption efficiencies and LDL-C levels. They further showed that these rare variants express lower levels of NPC1L1 protein in cultured cells after transfection (84), suggesting a dose-dependent effect of NPC1L1 protein on intestinal cholesterol absorption and blood LDL-C levels. Other sequence variants are identified in NPC1L1, and these variants are also associated with alterations in sterol absorption, plasma LDL-C levels, or responses to ezetimibe (85–90).
RECONSTITUTION OF NPC1L1-DEPENDENT AND EZETIMIBE-SENSITIVE CHOLESTEROL UPTAKE IN CULTURED CELLS
Animal studies have definitively established the necessity of NPC1L1 in mediating intestinal cholesterol absorption (16, 46, 64, 91, 92). To reconstitute NPC1L1-dependent cholesterol absorption in cultured cells so that the molecular basis of this process can be further explored, stable cell lines overexpressing NPC1L1 have been established and studied. When stably overexpressed in McArdle RH7777 rat hepatoma cells, human NPC1L1 is localized predominantly to the ERC (22). When these cells are depleted of cholesterol by cyclodextrin, the majority of NPC1L1 is quickly translocated to the cell membrane from the ERC and is preferentially concentrated in an apical subdomain on the cell surface. At this stage, the addition of cholesterol to the culture medium results in facilitated cellular uptake of cholesterol, which is coupled with the movement of NPC1L1 protein back to the intracellular compartments (22). Ezetimibe treatment can block this movement of NPC1L1 and cholesterol (22, 93–95). Cholesterol depletion also increases the localization of endogenous NPC1L1 to the cell surface in HepG2 human hepatoma cells (22) and in polarized Madin-Darby canine kidney (MDCK)-II cells (96). Cholesterol depletion–induced increases in localization of endogenous NPC1L1 protein to the cell surface lead to the facilitated and ezetimibe-sensitive cholesterol uptake in MDCK-II cells (96). Overexpression of rat NPC1L1 in CaCo-2 intestinal carcinoma cells also promotes cholesterol uptake in an ezetimibe-sensitive manner (23). Conversely, siRNA-mediated silencing of NPC1L1 in CaCo-2 cells reduces the cellular uptake of free cholesterol (97). Thus, NPC1L1 not only is capable of, but also is essential for, promoting cellular uptake of unesterified cholesterol.
ENDOCYTOSIS AND NPC1L1-DEPENDENT CHOLESTEROL UPTAKE
Findings from cell biology studies support that NPC1L1-dependent cholesterol uptake may be a cholesterol-regulated endocytic process, and in this case, NPC1L1 functions as a receptor rather than as a transporter for unesterified cholesterol in the plasma membrane (22, 94). This is similar to the function of SCAP, which is a receptor for unesterified cholesterol in the endoplasmic reticulum (28). NPC1L1 protein is seen in the plasma membrane and the intracellular vesicular compartments in cultured cells and in the small intestine and colocalizes with the distribution of unesterified cholesterol in the cell (16, 17, 22–24, 31, 97–99). NPC1L1 can facilitate cellular cholesterol uptake only when it is localized at the cell surface, and during the process of cholesterol uptake, NPC1L1 disappears from the cell membrane and reappears in the intracellular compartments (22, 94, 96). Additionally, NPC1L1-mediated cholesterol uptake can be blocked by potassium depletion (100), a condition that arrests the formation of coated pits and LDL receptor–mediated endocytosis in fibroblasts (101). Mice lacking caveolin-1, a structural component of caveolae, show normal intestinal cholesterol absorption (102). This observation is important because it excludes another important endocytic pathway, caveolin-mediated endocytosis, as the cellular basis of NPC1L1-dependent cholesterol uptake. In contrast, a recent cell biology study using McArdle hepatoma cells shows that NPC1L1 interacts in a cholesterol-dependent manner with the μ2 subunit of the adaptor protein complex 2 (AP2) and the heavy chain of clathrin, two critical proteins involved in clathrin-mediated endocytosis (94). This finding strongly supports the notion that NPC1L1-dependent cholesterol uptake is a clathrin-mediated endocytic process (Figure 2).
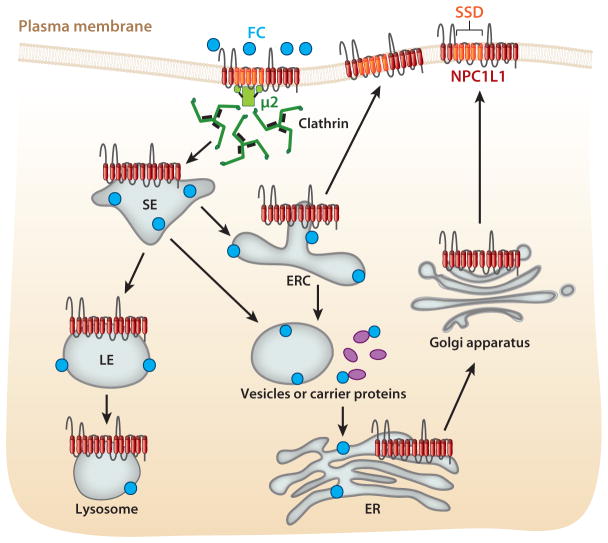
Figure 2.
The proposed cellular basis for Niemann-Pick C1-Like 1 (NPC1L1)-dependent cholesterol uptake. The polytopic transmembrane NPC1L1 protein is synthesized in the rough endoplasmic reticulum (ER) and then traffics to the plasma membrane via the Golgi apparatus. When cellular cholesterol is low, the protein preferentially localizes at the apical membrane of enterocytes and the canalicular membrane of hepatocytes, the two membrane domains that are exposed to high concentrations of unesterified free cholesterol (FC) in mixed micelles. On the basis of current knowledge about NPC1L1, we propose the following mechanism for NPC1L1-dependent cholesterol uptake. Through direct binding of FC to the extracellular N-terminal domain of NPC1L1, NPC1L1 may recruit FC to the membrane microdomain, where the sterol-sensing domain (SSD) of NPC1L1 is localized. When FC is enriched at this microdomain to a certain extent, it can be sensed by NPC1L1’s SSD, resulting in conformational changes of the protein, which exposes NPC1L1’s YXXØ motif and causes the direct binding of this motif to the μ2 subunit of adaptor protein complex 2 in the clathrin-mediated endocytic pathway and subsequent internalization of NPC1L1-containing and FC-rich membrane microdomains. The intracellular sorting of NPC1L1 in these internalized membrane microdomains may follow typical vesicular trafficking itineraries, moving from sorting endosome (SE) to late endosome (LE) to lysosome for degradation. A large proportion of NPC1L1 may be trafficked to the endocytic recycling compartment (ERC) and then recycled back to the cell surface for another round of sterol-dependent endocytosis. During this intracellular sorting, FC may be dissociated from SE or ERC and may be carried via vesicles or proteins to the ER for esterification and assembly into chylomicrons in the intestine and very low density lipoprotein (VLDL) particles in the liver.
Is there a signal in the NPC1L1 protein that has the potential to direct this protein to the clathrin-mediated endocytic pathway? A cytosolic tyrosine-based sorting signal, YXXØ (tetrapeptide), in many plasma membrane proteins can mediate the clathrin-dependent endocytosis of these proteins by interacting with the μ subunit of AP2 (103). In this YXXØ motif, the tyrosine (Y) residue is functionally indispensable. The Ø represents a residue with a bulky hydrophobic side chain. The X residues are highly variable. NPC1L1 has two potential YXXØ motifs, Y721QRL and Y836APF (104), one of which (Y721QRL) is located within the SSD region. The two motifs are conserved in NPC1L1 proteins from Drosophila to mammals. One of these motifs may regulate NPC1L1-dependent cholesterol uptake through interaction with proteins in the clathrin-mediated endocytic pathway in a cholesterol-dependent manner.
CHOLESTEROL-BINDING SITES IN NPC1L1
If NPC1L1 is a plasma membrane receptor for unesterified cholesterol, where does cholesterol bind? Surprisingly, a crystallization study exposed a sterol-binding pocket in the cysteine-rich (18-cysteine) N-terminal domain of NPC1 instead of in the SSD region (105). The N terminus of NPC1L1 also contains 18 cysteines (106). Given the high similarity in this region between the two proteins, it is reasonable to speculate that the N terminus of NPC1L1 binds sterols (104), although a definitive answer awaits the outcome of structure studies of NPC1L1 protein.
If cholesterol indeed binds to the N terminus of NPC1L1, what is the functional significance of this binding? Is this binding sufficient to trigger internalization of the cholesterol-NPC1L1 complex? How does the process of cholesterol-NPC1L1 binding efficiently transport the bulk of cholesterol into a cell? One possibility is that the N terminus of NPC1L1 helps move extracellular cholesterol to the membrane-localized SSD region, which may create a raft-like plasma membrane microdomain. The SSD somehow senses cholesterol content in this microdomain through protein conformational changes, which may cause subsequent interactions of NPC1L1’s YXXØ motif with proteins in the clathrin-mediated endocytic pathway and the internalization of the entire cholesterol-rich microdomain.
In the small intestine, internalized unesterified cholesterol has to be sorted to the endoplasmic reticulum for esterification by ACAT2 before it can be packaged into chylomicrons for secretion into lymph. It is currently unknown how NPC1L1-derived cholesterol is sorted to the endoplasmic reticulum. Is it sorted out directly from the early endosome or from the ERC? Are cytosolic carrier proteins needed for the transport of cholesterol to its metabolic sites? To molecularly define NPC1L1-dependent cholesterol uptake, these questions must be addressed. Nevertheless, after dissociation from cholesterol, the NPC1L1 protein can be recycled back to the cell surface (22, 94, 95). A recent study shows that this recycling is a vesicular process requiring microfilaments and the microfilament-associated triple complex (myosin Vb, Rab11a, and Rab11-FIP2) (107).
NPC1L1 IS THE MOLECULAR TARGET OF EZETIMIBE
After the discovery and development of statins to inhibit endogenous cholesterol biosynthesis, the hunt for hypocholesterolemic agents targeting other major cholesterol metabolic pathways became the focus of drug companies to more aggressively lower blood cholesterol levels. This search led to the discovery and development of a potent intestinal cholesterol absorption inhibitor, ezetimibe (commercially known as Zetia), by Schering-Plough Research Institute and Merck & Co. Ezetimibe was approved by the U.S. Food and Drug Administration to treat hypercholesterolemia in humans before its molecular target and mechanism of action were confirmed. Later, Altmann and colleagues (16) at Schering-Plough Research Institute identified NPC1L1 as the molecular target of ezetimibe. They found that NPC1L1 knockout mice and ezetimibe-treated mice show similarly reduced intestinal cholesterol absorption, demonstrating that NPC1L1 is in the ezetimibe-sensitive pathway (16). Although several other proteins, including scavenger receptor class B type I (SR-BI) (98, 99, 108), the annexin 2–caveolin-1 complex (109), and aminopeptidase N (110), have been reported to bind ezetimibe in biochemical assays, the genetic inactivation of SR-BI and caveolin-1 in mice causes no reduction in overall intestinal cholesterol absorption (111, 112). In the case of SR-BI knockout mice, cluster determinant (CD)36 was thought to compensate for SR-BI’s function. However, mice lacking both SR-BI and CD36 do not have a defect in overall intestinal cholesterol absorption (113). Ezetimibe still inhibits intestinal cholesterol absorption in SR-BI knockout mice (114). Although the role of aminopeptidase N (CD13) in intestinal cholesterol absorption has not been examined in animal models, the majority of animal and human genetic studies provide strong evidence that NPC1L1 is the target of ezetimibe. For example, genetic inactivation of NPC1L1 in mice reduces intestinal cholesterol absorption to the level seen in ezetimibe-treated mice (16); NPC1L1 is enriched at the apical surface of absorptive enterocytes (16, 46, 97); the in vivo responsiveness to ezetimibe correlates well with different NPC1L1 binding affinities of species (115); sequence variations in the human NPC1L1 gene are associated with varying sterol absorption efficiencies (83, 90), LDL-C levels (83), and responsiveness of LDL-C levels to ezetimibe therapy (86–88); and overexpression of NPC1L1 in cultured cells facilitates cellular cholesterol uptake, which can be blocked by ezetimibe treatment (22, 23, 94). Importantly, ezetimibe binds to a single site on intestinal brush border membrane vesicles and to cells overexpressing NPC1L1, and this binding is absent in intestinal brush border membrane vesicles isolated from NPC1L1-null mice (17). More definitively, the ezetimibe-NPC1L1 complex has been purified elegantly, showing that NPC1L1 accounts for essentially all the ezetimibe binding to protein (18). The ezetimibe-binding site on NPC1L1 has been mapped to the second extracellular loop of the protein (18).
NPC1L1 IS ESSENTIAL FOR INTESTINAL ABSORPTION OF PLANT STEROLS
Each day, a healthy individual on a typical Western diet consumes ~250 mg of plant sterols (116). The two major plant sterols are sitosterol and campesterol. Although the average efficiency of intestinal cholesterol absorption is ~50% (69), only ~5% of plant sterols are absorbed in healthy humans (116). In a rare autosomal recessive genetic disorder known as sitosterolemia (117), plant sterols and shellfish sterols accumulate in blood and tissues. Blood cholesterol is often elevated in sitosterolemic patients, resulting in premature coronary heart disease and xanthomas (cholesterol deposits in skin and tendons) (118). The disease is caused by mutations in the heterodimeric sterol transporter ABCG5/ABCG8 (59, 60). Interestingly, ezetimibe treatment can dramatically reduce plant sterol accumulation in sitosterolemic patients (119, 120) and in mice (72). NPC1L1 knockout mice also absorb fewer plant sterols (46, 91). Genetic deletion of NPC1L1 in mice lacking ABCG5/ABCG8 completely normalizes sitosterolemia but fails to rescue biliary cholesterol secretion (91). These findings demonstrate that a transporter is needed for intestinal absorption of plant sterols and that NPC1L1 is such a transporter.
IS NPC1L1 THE FIRST GENETIC DEFENSE AGAINST PLANT STEROLS?
As mentioned above, the efficiency of intestinal plant sterol absorption is only ~5%, whereas the average fractional absorption of cholesterol is ~50%. What is the genetic defense against plant sterols? Because mutations in ABCG5/ABCG8 cause sitosterolemia, it was believed that the ABCG5/ABCG8 heterodimer plays a critical role in discriminating plant sterols from cholesterol by preferentially transporting plant sterols out of cells (3, 121). However, animal and biochemical studies have shown that ABCG5/ABCG8 can also efficiently transport cholesterol and is essential for hepatobiliary cholesterol secretion (62, 122–124). The rank order of fractional intestinal absorption for various sterols (cholesterol > cholestanol > campesterol > sitosterol) is maintained in mice lacking ABCG5/ABCG8 (61, 122). It is therefore unlikely that this heterodimeric sterol transporter has higher affinity for plant sterols than for cholesterol.
The in vitro sterol uptake studies suggest that NPC1L1 may play a part in discriminating plant sterols from cholesterol. In McArdle hepatoma cells overexpressing NPC1L1, facilitated uptake is evident for cholesterol, but not for β-sitosterol (93, 94). In CaCo-2 cells overexpressing NPC1L1, β-sitosterol uptake is facilitated, but its efficiency is ~60% lower compared with cholesterol uptake (23). Although NPC1L1 is essential for plant sterols to enter the body in mice (91), these cell-based uptake assays suggest that NPC1L1 may be a low-affinity transporter for phytosterols and thus represents the first genetic defense against intestinal absorption of diet-derived plant sterols.
NPC1L1 AND ATHEROSCLEROSIS
The strong positive correlation between blood LDL-C levels and atherosclerotic CVD (5, 6) implies that a significant reduction in LDL-C induced by NPC1L1 deficiency or ezetimibe treatment would decrease atherogenesis. Indeed, ezetimibe treatment or genetic inactivation of NPC1L1 significantly reduces atherosclerosis in mice deficient in apolipoprotein E (11, 92). However, the outcome of the clinical trial ENHANCE (Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression) in 720 subjects with heterozygous familial hypercholesterolemia was disappointing, with no differences observed in carotid intima-media thickness between the group on ezetimibe/simvastatin and the group on simvastatin alone after two years of treatment (125). Improvements in study design—such as including heart attack and stroke as endpoints, increasing participant numbers, and extending treatment duration—may be needed. An ongoing clinical trial, IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial), involves 18,000 subjects (126). Although the outcome of this long-term large clinical trial may be more conclusive, all subjects involved in the IMPROVE-IT trial have normal blood LDL-C, and total cardiovascular events may be limited, which has the potential to confound the outcome.
NPC1L1 DEFICIENCY PREVENTS FATTY LIVER DISEASE
Hepatic steatosis, hepatic steatosis with inflammation, fibrosis, and cirrhosis are seen in alcoholic fatty liver disease and virus-associated hepatitis. In patients without hepatic viral infection and excessive alcohol use, these liver abnormalities are collectively referred to as nonalcoholic fatty liver disease (NAFLD) (127–130). The prevalence of NAFLD is estimated to range from 3% to 24% in the general population (131) and to reach ~10% in children (132) in developed countries. In one study, the frequency of hepatic steatosis determined by proton magnetic resonance spectroscopy was even higher: 45% in Hispanics, 33% in whites, and 24% in blacks (133). A detailed lipidomic analysis of liver biopsy samples showed that all subjects with NAFLD accumulated triglyceride and unesterified cholesterol in the liver (134). It has long been known that increased dietary cholesterol promotes hepatic accumulation of not only cholesterol but also triglycerides in animals (135). Thus, reducing intestinal cholesterol absorption may decrease triglyceride accumulation in liver. In agreement with this, inactivation of NPC1L1 prevents the development of fatty liver in mice fed a cholesterol-rich and bile acid–containing Paigen diet (30). Ezetimibe treatment also improves hepatic steatosis in wild-type mice fed a high-fat, high-cholesterol diet (136) and in Zucker fatty (fa/fa) rats (137), the latter of which develop metabolic syndrome and hepatic steatosis as a result of leptin receptor deficiency (138). In a rat model of NAFLD induced by a methionine choline–deficient diet, ezetimibe administration reduces hepatic triglyceride content (139). Interestingly, ezetimibe treatment or NPC1L1 disruption in mice can even prevent hepatic steatosis induced by a high-fat diet that contains only a trace amount of cholesterol (140, 140a), implying a potential role for the enterohepatic circulation of endogenous cholesterol in promoting fatty liver disease.
Researchers recently examined the beneficial effect of ezetimibe on fatty liver disease in humans. In obese subjects on a weight loss diet, ezetimibe treatment significantly reduced hepatic fat content as determined by magnetic resonance techniques (141). In ten Japanese patients with nonalcoholic steatohepatitis and dyslipidemia, ezetimibe treatment for six months substantially improved serum profiles of liver enzymes and lipids as well as histological scores for fatty liver (142). In another study, although ezetimibe treatment did not improve fatty liver, it significantly reduced serum levels of alanine aminotransferase in nonobese subjects with NAFLD (143).
Hepatic steatosis is often associated with insulin resistance, a pathological condition characterized by increased circulating insulin levels. Under this condition, insulin cannot suppress hepatic gluconeogenesis, but it can promote hepatic lipogenesis (144). Ezetimibe treatment improves insulin resistance in rodents with NAFLD (74, 137, 139, 145) and in human subjects with hypercholesterolemia (146). Genetic ablation of NPC1L1 also improves glucose tolerance and increases insulin sensitivity in mice on a high-fat diet (74). Although the improved insulin resistance under these conditions may result from the reduced fat accumulation in the liver, there are situations in which animals have severe fatty degeneration in the liver, yet show no alterations in insulin sensitivity (147). Thus, ezetimibe treatment or NPC1L1 disruption may prevent hepatic steatosis primarily by improving insulin sensitivity.
Several other mechanisms have been proposed to explain how ezetimibe treatment or NPC1L1 deficiency protects against hepatic steatosis, including reduced generation of reactive oxygen species (ROS), elevated C-Jun N-terminal kinase (JNK) activation, and decreased endoplasmic reticulum stress (145). However, all these alterations may be a result of reduced hepatic accumulation of triglyceride and cholesterol, rather than the cause of decreased steatosis.
Figure 3 summarizes mechanisms by which ezetimibe treatment or NPC1L1 ablation may prevent fatty liver disease. By inhibiting intestinal cholesterol absorption, ezetimibe treatment or NPC1L1 disruption may reduce cholesterol-dependent LXR activation (148) and subsequent LXR activation–induced hepatic lipogenesis (149). Intestinal cholesterol absorption may be important for maintaining basal LXR activity in the body. Consistent with this notion, NPC1L1 knockout mice are less sensitive to a synthetic LXR agonist relative to wild-type mice in terms of activation of lipogenic gene expression and fat accumulation in the liver (64). Additionally, the steatoprotective effect of ezetimibe or NPC1L1 deficiency may be associated with improved insulin sensitivity and decreased circulating insulin concentrations. In support of this, NPC1L1 knockout compared with wild-type mice have a dramatic reduction in plasma insulin concentrations and are completely protected against hepatic steatosis induced by long-term high-fat-diet feeding (140a). In this study, however, we were unable to determine the cause-effect relationship between reduced hepatosteatosis and low blood insulin levels because protection from hepatic steatosis per se may improve insulin sensitivity, thereby reducing circulating insulin concentration. Alternatively, protection of NPC1L1-null mice from diet-induced obesity may result in improved insulin sensitivity (74) and low blood insulin levels, thus preventing insulin-induced hepatic steatosis (144).
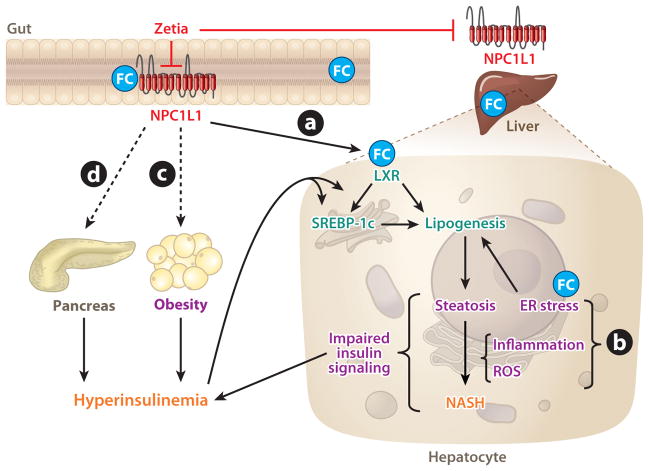
Figure 3.
Proposed mechanisms for Niemann-Pick C1-Like 1 (NPC1L1) deficiency or ezetimibe (Zetia) treatment to prevent nonalcoholic fatty liver disease (NAFLD). (a) NPC1L1 inhibition blocks intestinal absorption and promotes biliary excretion of free cholesterol (FC). Therefore, NPC1L1 may prevent hepatic steatosis by reducing hepatic cholesterol content and cholesterol-dependent activation of liver X receptor (LXR), a nuclear receptor that promotes hepatic lipogenesis. (b) Reduced lipid accumulation in the liver as a result of NPC1L1 inhibition may improve liver insulin signaling by reducing hepatic content of lipotoxic lipids, endoplasmic reticulum (ER) stress, and/or the production of proinflammatory cytokines and reactive oxygen species (ROS). Such events thereby inhibit simple steatosis progression to nonalcoholic steatohepatitis (NASH) and blunt diet-induced insulin resistance and the development of hyperinsulinemia, a condition that promotes hepatic lipogenesis via a mechanism dependent on a membrane-bound transcription factor, sterol regulatory element–binding protein (SREBP)-1c. (c) For unknown reasons, NPC1L1 inhibition prevents diet-induced obesity, which may further protect the liver from steatosis by improving whole-body insulin sensitivity. (d) NPC1L1 inhibition dramatically reduces blood insulin concentrations. It is unclear if this reduction is attributable solely to improved insulin sensitivity. NPC1L1 inhibition may somehow directly impair insulin secretion by pancreatic β cells, thereby preventing the liver from undergoing insulin-driven lipogenesis.
NPC1L1 DEFICIENCY PREVENTS DIET-INDUCED OBESITY
A recent study showed that genetic inactivation of NPC1L1 or ezetimibe treatment protects C57BL/6 mice from weight gain induced by a high-fat diet (74). Such an effect was not seen in NPC1L1 knockout mice on a 129/OlaHsd and C57BL/6 mixed background after they were fed a diet containing 0.15% cholesterol (92) or in ezetimibe-treated wild-type C57BL/6 mice on a diet containing 0.12% cholesterol (136). Although the discrepancy between these studies may be attributable to differences in genetic background, gene targeting strategies, experimental duration, or fat source, we found that C57BL/6 mice deficient in NPC1L1 are protected against diet-induced obesity only on a low-cholesterol (~0.02%)-containing diet, but not on a high-cholesterol (~0.2%)-containing diet (150). Large clinical trials have not reported changes in weight gain in human subjects treated with ezetimibe (151). However, in a small clinical study conducted on 38 Japanese hypercholesterolemic subjects, ezetimibe treatment for approximately five weeks did significantly reduce body weight gain, body mass index, and waist circumference (146). Because this ethnic group generally consumes a diet low in cholesterol, the weight-reducing effect of ezetimibe may be seen only in people consuming a low-cholesterol diet.
How does an intestinal cholesterol transporter modulate diet-induced obesity? NPC1L1 deficiency or ezetimibe treatment slightly but significantly reduces intestinal absorption of total fat and two saturated fatty acids, stearate and palmitate, in mice on a high-fat diet, despite having no effects on food intake (74). Although this modest reduction in fat absorption may account for a proportion of reduced weight gain over a long period, we found that NPC1L1-deficient mice have similar calorie intakes and fat absorption on several low-fat diets and a high-fat diet of different composition, but they still gain significantly less weight relative to wild-type mice (150). Thus, energy expenditure must be increased in NPC1L1-deficient mice.
FUTURE DIRECTIONS
The molecular mechanisms for NPC1L1-dependent cholesterol uptake remain elusive. Dissection of molecular components in this process may hold promise for the development of novel hypocholesterolemic agents. It is also unclear how NPC1L1 modulates the pathogenesis of fatty liver disease, insulin sensitivity, and diet-induced obesity, which should be an important topic of research. Given the upsurge in prevalence of these metabolic diseases, this research has tremendous translational potential for improving public health.
SUMMARY POINTS.
NPC1L1 mediates the uptake of unesterified cholesterol from the intestinal lumen into absorptive enterocytes and is essential for intestinal cholesterol absorption.
In humans and nonhuman primates, NPC1L1 may have evolved to prevent excessive loss of cholesterol from bile, likely by transporting cholesterol in the bile canaliculus back into hepatocytes.
NPC1L1 may be a weak transporter for plant sterols but is essential for intestinal absorption of these sterols, thus serving as the first genetic defense against phytosterols.
Both intestinal NPC1L1 and hepatic NPC1L1 are targets of the cholesterol-lowering drug ezetimibe.
NPC1L1-dependent cholesterol uptake may be a clathrin-mediated endocytic process.
Animal studies show that genetic inactivation of NPC1L1 or ezetimibe treatment attenuates atherosclerosis, NAFLD, insulin resistance, and, under certain circumstances, diet-induced obesity.
Acknowledgments
This work is supported in part by a Scientist Development Grant from the American Heart Association (to L.Y.) and by award number R01DK085176 from the National Institute of Diabetes and Digestive and Kidney Diseases (to L.Y.). J.L.B. is supported by a Ruth L. Kirschstein National Research Service Award (NRSA) (#1F32DK084582-01) provided by the National Institute of Diabetes and Digestive and Kidney Diseases.
Glossary
- Intestinal cholesterol absorption
the movement of cholesterol from intestinal lumen into the intestinal or thoracic duct lymph across intestinal mucosa
- CVD
cardiovascular disease
- LDL-C
low-density-lipoprotein cholesterol
- HMG-CoA
3-hydroxy-3-methyl-glutaryl coenzyme A
- NPC1L1
Niemann-Pick C1-Like 1
- SSD
sterol-sensing domain
- SREBP
sterol regulatory element–binding protein
- ERC
endocytic recycling compartment
- LXR
liver X receptor
- ACAT2
acyl-CoA:cholesterol acyltransferase 2
- Intestinal uptake of cholesterol
the movement of cholesterol from mixed micelles at the surface of intestinal mucosa into absorptive enterocytes
- ABC transporter
ATP-binding cassette transporter
- HDL
high-density lipoprotein
- MTP
microsomal triglyceride transfer protein
- Enterohepatic circulation of cholesterol
the cycling of biliary cholesterol from the liver, where it is produced and secreted into bile, to the small intestine, where it is reabsorbed and transported back to the liver via circulation
- Hepatobiliary secretion of cholesterol
the transportation of unesterified cholesterol from hepatocytes into the bile canaliculus, a process mediated primarily by the ABCG5/ABCG8 heterodimer
- Bile canaliculus
the tiny tubular space formed by part of the lateral membrane of hepatocytes; collects newly secreted bile that is eventually delivered to the duodenal lumen
- Endocytosis
uptake of material into a cell by invagination of the plasma membrane and its internalization in a membrane-bounded vesicle
- Clathrin-mediated endocytosis
the endocytic process mediated by small-membrane vesicles coated with clathrin and its associated proteins
- SR-BI
scavenger receptor class B type I
- NAFLD
nonalcoholic fatty liver disease
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institute of Health.
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Dawson AM, Isselbacher KJ. Studies on lipid metabolism in the small intestine with observations on the role of bile salts. J Clin Investig. 1960;39:730–40. doi: 10.1172/JCI104090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hofmann AF, Borgstroem B. The intraluminal phase of fat digestion in man: the lipid content of the micellar and oil phases of intestinal content obtained during fat digestion and absorption. J Clin Investig. 1964;43:247–57. doi: 10.1172/JCI104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang DQ. Regulation of intestinal cholesterol absorption. Annu Rev Physiol. 2007;69:221–48. doi: 10.1146/annurev.physiol.69.031905.160725. [DOI] [PubMed] [Google Scholar]
- 4.van Der Velde AE, Vrins CL, Van Den Oever K, Kunne C, Oude Elferink RP, et al. Direct intestinal cholesterol secretion contributes significantly to total fecal neutral sterol excretion in mice. Gastroenterology. 2007;133:967–75. doi: 10.1053/j.gastro.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 5.Kreisberg RA, Oberman A. Lipids and atherosclerosis: lessons learned from randomized controlled trials of lipid lowering and other relevant studies. J Clin Endocrinol Metab. 2002;87:423–37. doi: 10.1210/jcem.87.2.8057. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, et al. Heart disease and stroke statistics—2010 update. A report from the American Heart Association. Circulation. 2010;121:e46–215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 7.Endo A. The discovery and development of HMG-CoA reductase inhibitors. J Lipid Res. 1992;33:1569–82. [PubMed] [Google Scholar]
- 8.Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 9.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–39. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 10.Rosenblum SB, Huynh T, Afonso A, Davis HR, Jr, Yumibe N, et al. Discovery of 1-(4-fluorophenyl)-(3R)-[3-(4-fluorophenyl)-(3S)-hydroxypropyl]-(4S)-(4-hydroxyphenyl)-2-azetidinone (SCH 58235): a designed, potent, orally active inhibitor of cholesterol absorption. J Med Chem. 1998;41:973–80. doi: 10.1021/jm970701f. [DOI] [PubMed] [Google Scholar]
- 11.Davis HR, Jr, Compton DS, Hoos L, Tetzloff G. Ezetimibe, a potent cholesterol absorption inhibitor, inhibits the development of atherosclerosis in ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2001;21:2032–38. doi: 10.1161/hq1201.100260. [DOI] [PubMed] [Google Scholar]
- 12.van Heek M, Farley C, Compton DS, Hoos L, Alton KB, et al. Comparison of the activity and disposition of the novel cholesterol absorption inhibitor, SCH58235, and its glucuronide, SCH60663. Br J Pharmacol. 2000;129:1748–54. doi: 10.1038/sj.bjp.0703235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis HR, Jr, Pula KK, Alton KB, Burrier RE, Watkins RW. The synergistic hypocholesterolemic activity of the potent cholesterol absorption inhibitor, ezetimibe, in combination with 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors in dogs. Metabolism. 2001;50:1234–41. doi: 10.1053/meta.2001.26737. [DOI] [PubMed] [Google Scholar]
- 14.Gagne C, Bays HE, Weiss SR, Mata P, Quinto K, et al. Efficacy and safety of ezetimibe added to ongoing statin therapy for treatment of patients with primary hypercholesterolemia. Am J Cardiol. 2002;90:1084–91. doi: 10.1016/s0002-9149(02)02774-1. [DOI] [PubMed] [Google Scholar]
- 15.Davidson MH, McGarry T, Bettis R, Melani L, Lipka LJ, et al. Ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia. J Am Coll Cardiol. 2002;40:2125–34. doi: 10.1016/s0735-1097(02)02610-4. [DOI] [PubMed] [Google Scholar]
- 16.Altmann SW, Davis HR, Jr, Zhu LJ, Yao X, Hoos LM, et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–4. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Calvo M, Lisnock J, Bull HG, Hawes BE, Burnett DA, et al. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1) Proc Natl Acad Sci USA. 2005;102:8132–37. doi: 10.1073/pnas.0500269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinglass AB, Kohler M, Schulte U, Liu J, Nketiah EO, et al. Extracellular loop C of NPC1L1 is important for binding to ezetimibe. Proc Natl Acad Sci USA. 2008;105:11140–45. doi: 10.1073/pnas.0800936105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies JP, Levy B, Ioannou YA. Evidence for a Niemann-Pick C (NPC) gene family: identification and characterization of NPC1L1. Genomics. 2000;65:137–45. doi: 10.1006/geno.2000.6151. [DOI] [PubMed] [Google Scholar]
- 20.Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, et al. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277:228–31. doi: 10.1126/science.277.5323.228. [DOI] [PubMed] [Google Scholar]
- 21.Loftus SK, Morris JA, Carstea ED, Gu JZ, Cummings C, et al. Murine model of Niemann-Pick C disease: mutation in a cholesterol homeostasis gene. Science. 1997;277:232–35. doi: 10.1126/science.277.5323.232. [DOI] [PubMed] [Google Scholar]
- 22.Yu L, Bharadwaj S, Brown JM, Ma Y, Du W, et al. Cholesterol-regulated translocation of NPC1L1 to the cell surface facilitates free cholesterol uptake. J Biol Chem. 2006;281:6616–24. doi: 10.1074/jbc.M511123200. [DOI] [PubMed] [Google Scholar]
- 23.Yamanashi Y, Takada T, Suzuki H. Niemann-Pick C1-like 1 overexpression facilitates ezetimibe-sensitive cholesterol and β-sitosterol uptake in CaCo-2 cells. J Pharmacol Exp Ther. 2007;320:559–64. doi: 10.1124/jpet.106.114181. [DOI] [PubMed] [Google Scholar]
- 24.Iyer SP, Yao X, Crona JH, Hoos LM, Tetzloff G, et al. Characterization of the putative native and recombinant rat sterol transporter Niemann-Pick C1 Like 1 (NPC1L1) protein. Biochim Biophys Acta. 2005;1722:282–92. doi: 10.1016/j.bbagen.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 25.Davies JP, Chen FW, Ioannou YA. Transmembrane molecular pump activity of Niemann-Pick C1 protein. Science. 2000;290:2295–98. doi: 10.1126/science.290.5500.2295. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Chu BB, Ge L, Li BL, Yan Y, Song BL. Membrane topology of human NPC1L1, a key protein in enterohepatic cholesterol absorption. J Lipid Res. 2009;50:1653–62. doi: 10.1194/jlr.M800669-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuwabara PE, Labouesse M. The sterol-sensing domain: multiple families, a unique role? Trends Genet. 2002;18:193–201. doi: 10.1016/s0168-9525(02)02640-9. [DOI] [PubMed] [Google Scholar]
- 28.Radhakrishnan A, Sun LP, Kwon HJ, Brown MS, Goldstein JL. Direct binding of cholesterol to the purified membrane region of SCAP: mechanism for a sterol-sensing domain. Mol Cell. 2004;15:259–68. doi: 10.1016/j.molcel.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 29.Ohgami N, Ko DC, Thomas M, Scott MP, Chang CC, Chang TY. Binding between the Niemann-Pick C1 protein and a photoactivatable cholesterol analog requires a functional sterol-sensing domain. Proc Natl Acad Sci USA. 2004;101:12473–78. doi: 10.1073/pnas.0405255101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davies JP, Scott C, Oishi K, Liapis A, Ioannou YA. Inactivation of NPC1L1 causes multiple lipid transport defects and protects against diet-induced hypercholesterolemia. J Biol Chem. 2005;280:12710–20. doi: 10.1074/jbc.M409110200. [DOI] [PubMed] [Google Scholar]
- 31.Temel RE, Tang W, Ma Y, Rudel LL, Willingham MC, et al. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J Clin Investig. 2007;117:1968–78. doi: 10.1172/JCI30060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knight BL, Patel DD, Humphreys SM, Wiggins D, Gibbons GF. Inhibition of cholesterol absorption associated with a PPAR-α dependent increase in ABC binding cassette transporter A1 in mice. J Lipid Res. 2003;44:2049–58. doi: 10.1194/jlr.M300042-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Repa JJ, Turley SD, Lobaccaro JA, Medina J, Li L, et al. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science. 2000;289:1524–29. doi: 10.1126/science.289.5484.1524. [DOI] [PubMed] [Google Scholar]
- 34.McNamara DJ, Davidson NO, Samuel P, Ahrens EH., Jr Cholesterol absorption in man: effect of administration of clofibrate and/or cholestyramine. J Lipid Res. 1980;21:1058–64. [PubMed] [Google Scholar]
- 35.Umeda Y, Kako Y, Mizutani K, Iikura Y, Kawamura M, et al. Inhibitory action of gemfibrozil on cholesterol absorption in rat intestine. J Lipid Res. 2001;42:1214–19. [PubMed] [Google Scholar]
- 36.Vanhanen HT, Miettinen TA. Cholesterol absorption and synthesis during pravastatin, gemfibrozil and their combination. Atherosclerosis. 1995;115:135–46. doi: 10.1016/0021-9150(94)05474-w. [DOI] [PubMed] [Google Scholar]
- 37.Yu L, York J, von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Stimulation of cholesterol excretion by the liver X receptor agonist requires ATP-binding cassette transporters G5 and G8. J Biol Chem. 2003;278:15565–70. doi: 10.1074/jbc.M301311200. [DOI] [PubMed] [Google Scholar]
- 38.Oliver WR, Jr, Shenk JL, Snaith MR, Russell CS, Plunket KD, et al. A selective peroxisome proliferator-activated receptor δ agonist promotes reverse cholesterol transport. Proc Natl Acad Sci USA. 2001;98:5306–11. doi: 10.1073/pnas.091021198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Der Veen JN, Kruit JK, Havinga R, Baller JF, Chimini G, et al. Reduced cholesterol absorption upon PPARδ activation coincides with decreased intestinal expression of NPC1L1. J Lipid Res. 2005;46:526–34. doi: 10.1194/jlr.M400400-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Mathur SN, Watt KR, Field FJ. Regulation of intestinal NPC1L1 expression by dietary fish oil and docosahexaenoic acid. J Lipid Res. 2007;48:395–404. doi: 10.1194/jlr.M600325-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Valasek MA, Clarke SL, Repa JJ. Fenofibrate reduces intestinal cholesterol absorption via PPARα-dependent modulation of NPC1L1 expression in mouse. J Lipid Res. 2007;48:2725–35. doi: 10.1194/jlr.M700345-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Duval C, Touche V, Tailleux A, Fruchart JC, Fievet C, et al. Niemann-Pick C1 like 1 gene expression is down-regulated by LXR activators in the intestine. Biochem Biophys Res Commun. 2006;340:1259–63. doi: 10.1016/j.bbrc.2005.12.137. [DOI] [PubMed] [Google Scholar]
- 43.Kruit JK, Plosch T, Havinga R, Boverhof R, Groot PH, et al. Increased fecal neutral sterol loss upon liver X receptor activation is independent of biliary sterol secretion in mice. Gastroenterology. 2005;128:147–56. doi: 10.1053/j.gastro.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 44.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–40. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 45.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Investig. 2002;109:1125–31. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis HR, Jr, Zhu LJ, Hoos LM, Tetzloff G, Maguire M, et al. Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J Biol Chem. 2004;279:33586–92. doi: 10.1074/jbc.M405817200. [DOI] [PubMed] [Google Scholar]
- 47.Temel RE, Lee RG, Kelley KL, Davis MA, Shah R, et al. Intestinal cholesterol absorption is substantially reduced in mice deficient in both ATP-binding cassette transporter A1 (ABCA1) and acyl-CoA:cholesterol O-acyltransferase 2 (ACAT2) J Lipid Res. 2005;46:2423–31. doi: 10.1194/jlr.M500232-JLR200. [DOI] [PubMed] [Google Scholar]
- 48.Liu R, Iqbal J, Yeang C, Wang DQ, Hussain MM, Jiang XC. Phospholipid transfer protein-deficient mice absorb less cholesterol. Arterioscler Thromb Vasc Biol. 2007;27:2014–21. doi: 10.1161/ATVBAHA.107.149914. [DOI] [PubMed] [Google Scholar]
- 49.Tang W, Ma Y, Yu L. Plasma cholesterol is hyperresponsive to statin in ABCG5/ABCG8 transgenic mice. Hepatology. 2006;44:1259–66. doi: 10.1002/hep.21380. [DOI] [PubMed] [Google Scholar]
- 50.Telford DE, Sutherland BG, Edwards JY, Andrews JD, Barrett PH, Huff MW. The molecular mechanisms underlying the reduction of LDL apoB-100 by ezetimibe plus simvastatin. J Lipid Res. 2007;48:699–708. doi: 10.1194/jlr.M600439-JLR200. [DOI] [PubMed] [Google Scholar]
- 51.Alrefai WA, Annaba F, Sarwar Z, Dwivedi A, Saksena S, et al. Modulation of human Niemann-Pick C1-like 1 gene expression by sterol: role of sterol regulatory element binding protein 2. Am J Physiol Gastrointest Liver Physiol. 2007;292:369–76. doi: 10.1152/ajpgi.00306.2006. [DOI] [PubMed] [Google Scholar]
- 52.Pramfalk C, Jiang ZY, Cai Q, Hu H, Zhang SD, et al. HNF1α and SREBP2 are important regulators of NPC1L1 in human liver. J Lipid Res. 2010;51:1354–62. doi: 10.1194/jlr.M900274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iwayanagi Y, Takada T, Suzuki H. HNF4α is a crucial modulator of the cholesterol-dependent regulation of NPC1L1. Pharm Res. 2008;25:1134–41. doi: 10.1007/s11095-007-9496-9. [DOI] [PubMed] [Google Scholar]
- 54.Plosch T, Kruit JK, Bloks VW, Huijkman NC, Havinga R, et al. Reduction of cholesterol absorption by dietary plant sterols and stanols in mice is independent of the Abcg5/8 transporter. J Nutr. 2006;136:2135–40. doi: 10.1093/jn/136.8.2135. [DOI] [PubMed] [Google Scholar]
- 55.Tso P, Fujimoto K. The absorption and transport of lipids by the small intestine. Brain Res Bull. 1991;27:477–82. doi: 10.1016/0361-9230(91)90145-a. [DOI] [PubMed] [Google Scholar]
- 56.Wilson MD, Rudel LL. Review of cholesterol absorption with emphasis on dietary and biliary cholesterol. J Lipid Res. 1994;35:943–55. [PubMed] [Google Scholar]
- 57.Turley SD, Dietschy JM. Sterol absorption by the small intestine. Curr Opin Lipidol. 2003;14:233–40. doi: 10.1097/00041433-200306000-00002. [DOI] [PubMed] [Google Scholar]
- 58.Grundy SM. Absorption and metabolism of dietary cholesterol. Annu Rev Nutr. 1983;3:71–96. doi: 10.1146/annurev.nu.03.070183.000443. [DOI] [PubMed] [Google Scholar]
- 59.Berge KE, Tian H, Graf GA, Yu L, Grishin NV, et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–75. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- 60.Lee MH, Lu K, Hazard S, Yu H, Shulenin S, et al. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat Genet. 2001;27:79–83. doi: 10.1038/83799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu L, Hammer RE, Li-Hawkins J, Von Bergmann K, Lutjohann D, et al. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc Natl Acad Sci USA. 2002;99:16237–42. doi: 10.1073/pnas.252582399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu L, Li-Hawkins J, Hammer RE, Berge KE, Horton JD, et al. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J Clin Investig. 2002;110:671–80. doi: 10.1172/JCI16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brunham LR, Kruit JK, Iqbal J, Fievet C, Timmins JM, et al. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J Clin Investig. 2006;116:1052–62. doi: 10.1172/JCI27352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang W, Ma Y, Jia L, Ioannou YA, Davies JP, Yu L. Niemann-Pick C1-Like 1 is required for an LXR agonist to raise plasma HDL cholesterol in mice. Arterioscler Thromb Vasc Biol. 2008;28:448–54. doi: 10.1161/ATVBAHA.107.160465. [DOI] [PubMed] [Google Scholar]
- 65.Anderson RA, Joyce C, Davis M, Reagan JW, Clark M, et al. Identification of a form of acyl-CoA:cholesterol acyltransferase specific to liver and intestine in nonhuman primates. J Biol Chem. 1998;273:26747–54. doi: 10.1074/jbc.273.41.26747. [DOI] [PubMed] [Google Scholar]
- 66.Ingram MF, Shelness GS. Folding of the amino-terminal domain of apolipoprotein B initiates microsomal triglyceride transfer protein-dependent lipid transfer to nascent very low density lipoprotein. J Biol Chem. 1997;272:10279–86. doi: 10.1074/jbc.272.15.10279. [DOI] [PubMed] [Google Scholar]
- 67.Raabe M, Veniant MM, Sullivan MA, Zlot CH, Bjorkegren J, et al. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J Clin Investig. 1999;103:1287–98. doi: 10.1172/JCI6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grundy SM, Metzger AL. A physiological method for estimation of hepatic secretion of biliary lipids in man. Gastroenterology. 1972;62:1200–17. [PubMed] [Google Scholar]
- 69.Bosner MS, Lange LG, Stenson WF, Ostlund RE., Jr Percent cholesterol absorption in normal women and men quantified with dual stable isotopic tracers and negative ion mass spectrometry. J Lipid Res. 1999;40:302–8. [PubMed] [Google Scholar]
- 70.Small DM, Dowling RH, Redinger RN. The enterohepatic circulation of bile salts. Arch Intern Med. 1972;130:552–73. [PubMed] [Google Scholar]
- 71.Repa JJ, Dietschy JM, Turley SD. Inhibition of cholesterol absorption by SCH 58053 in the mouse is not mediated via changes in the expression of mRNA for ABCA1, ABCG5, or ABCG8 in the enterocyte. J Lipid Res. 2002;43:1864–74. doi: 10.1194/jlr.m200144-jlr200. [DOI] [PubMed] [Google Scholar]
- 72.Yu L, von Bergmann K, Lutjohann D, Hobbs HH, Cohen JC. Ezetimibe normalizes metabolic defects in mice lacking ABCG5 and ABCG8. J Lipid Res. 2005;46:1739–44. doi: 10.1194/jlr.M500124-JLR200. [DOI] [PubMed] [Google Scholar]
- 73.Clarenbach JJ, Reber M, Lutjohann D, von Bergmann K, Sudhop T. The lipid-lowering effect of ezetimibe in pure vegetarians. J Lipid Res. 2006;47:2820–24. doi: 10.1194/jlr.P600009-JLR200. [DOI] [PubMed] [Google Scholar]
- 74.Labonte ED, Camarota LM, Rojas JC, Jandacek RJ, Gilham DE, et al. Reduced absorption of saturated fatty acids and resistance to diet-induced obesity and diabetes by ezetimibe-treated and Npc1l1−/− mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:776–83. doi: 10.1152/ajpgi.90275.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dietschy JM, Turley SD, Spady DK. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J Lipid Res. 1993;34:1637–59. [PubMed] [Google Scholar]
- 76.Graf GA, Yu L, Li WP, Gerard R, Tuma PL, et al. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J Biol Chem. 2003;278:48275–82. doi: 10.1074/jbc.M310223200. [DOI] [PubMed] [Google Scholar]
- 77.Klett EL, Lee MH, Adams DB, Chavin KD, Patel SB. Localization of ABCG5 and ABCG8 proteins in human liver, gall bladder and intestine. BMC Gastroenterol. 2004;4:21. doi: 10.1186/1471-230X-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Valasek MA, Repa JJ, Quan G, Dietschy JM, Turley SD. Inhibiting intestinal NPC1L1 activity prevents diet-induced increase in biliary cholesterol in Golden Syrian hamsters. Am J Physiol Gastrointest Liver Physiol. 2008;295:813–22. doi: 10.1152/ajpgi.90372.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang HH, Portincasa P, Mendez-Sanchez N, Uribe M, Wang DQ. Effect of ezetimibe on the prevention and dissolution of cholesterol gallstones. Gastroenterology. 2008;134:2101–10. doi: 10.1053/j.gastro.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zuniga S, Molina H, Azocar L, Amigo L, Nervi F, et al. Ezetimibe prevents cholesterol gallstone formation in mice. Liver Int. 2008;28:935–47. doi: 10.1111/j.1478-3231.2008.01808.x. [DOI] [PubMed] [Google Scholar]
- 81.Kovanen PT, Brown MS, Basu SK, Bilheimer DW, Goldstein JL. Saturation and suppression of hepatic lipoprotein receptors: a mechanism for the hypercholesterolemia of cholesterol-fed rabbits. Proc Natl Acad Sci USA. 1981;78:1396–400. doi: 10.1073/pnas.78.3.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spady DK, Dietschy JM. Interaction of dietary cholesterol and triglycerides in the regulation of hepatic low density lipoprotein transport in the hamster. J Clin Investig. 1988;81:300–9. doi: 10.1172/JCI113321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cohen JC, Pertsemlidis A, Fahmi S, Esmail S, Vega GL, et al. Multiple rare variants in NPC1L1 associated with reduced sterol absorption and plasma low-density lipoprotein levels. Proc Natl Acad Sci USA. 2006;103:1810–15. doi: 10.1073/pnas.0508483103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fahmi S, Yang C, Esmail S, Hobbs HH, Cohen JC. Functional characterization of genetic variants in NPC1L1 supports the sequencing extremes strategy to identify complex trait genes. Hum Mol Genet. 2008;17:2101–7. doi: 10.1093/hmg/ddn108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Polisecki E, Peter I, Simon JS, Hegele RA, Robertson M, et al. Genetic variation at the NPC1L1 gene locus, plasma lipoproteins, and heart disease risk in the elderly. J Lipid Res. 2010;51:1201–7. doi: 10.1194/jlr.P001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hegele RA, Guy J, Ban MR, Wang J. NPC1L1 haplotype is associated with interindividual variation in plasma low-density lipoprotein response to ezetimibe. Lipids Health Dis. 2005;4:16. doi: 10.1186/1476-511X-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Simon JS, Karnoub MC, Devlin DJ, Arreaza MG, Qiu P, et al. Sequence variation in NPC1L1 and association with improved LDL-cholesterol lowering in response to ezetimibe treatment. Genomics. 2005;86:648–56. doi: 10.1016/j.ygeno.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 88.Wang J, Williams C, Hegele R. Compound heterozygosity for two nonsynonymous polymorphisms in NPC1L1 in a nonresponder to ezetimibe. Clin Genet. 2005;67:175–77. doi: 10.1111/j.1399-0004.2004.00388.x. [DOI] [PubMed] [Google Scholar]
- 89.Chan DC, Watts GF, Wang J, Hegele RA, van Bockxmeer FM, Barrett PH. Variation in Niemann-Pick C1-like 1 gene as a determinant of apolipoprotein B-100 kinetics and response to statin therapy in centrally obese men. Clin Endocrinol. 2008;69:45–51. doi: 10.1111/j.1365-2265.2007.03144.x. [DOI] [PubMed] [Google Scholar]
- 90.Martin B, Solanas-Barca M, Garcia-Otin AL, Pampin S, Cofan M, et al. An NPC1L1 gene promoter variant is associated with autosomal dominant hypercholesterolemia. Nutr Metab Cardiovasc Dis. 2010;20:236–42. doi: 10.1016/j.numecd.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 91.Tang W, Ma Y, Jia L, Ioannou YA, Davies JP, Yu L. Genetic inactivation of NPC1L1 protects against sitosterolemia in mice lacking ABCG5/ABCG8. J Lipid Res. 2009;50:293–300. doi: 10.1194/jlr.M800439-JLR200. [DOI] [PubMed] [Google Scholar]
- 92.Davis HR, Jr, Hoos LM, Tetzloff G, Maguire M, Zhu LJ, et al. Deficiency of Niemann-Pick C1 Like 1 prevents atherosclerosis in ApoE−/− mice. Arterioscler Thromb Vasc Biol. 2007;27:841–49. doi: 10.1161/01.ATV.0000257627.40486.46. [DOI] [PubMed] [Google Scholar]
- 93.Brown JM, Rudel LL, Yu L. NPC1L1 (Niemann-Pick C1-like 1) mediates sterol-specific unidirectional transport of nonesterified cholesterol in McArdle-RH7777 hepatoma cells. Biochem J. 2007;406:273–83. doi: 10.1042/BJ20070168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ge L, Wang J, Qi W, Miao HH, Cao J, et al. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab. 2008;7:508–19. doi: 10.1016/j.cmet.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 95.Petersen NH, Faergeman NJ, Yu L, Wustner D. Kinetic imaging of NPC1L1 and sterol trafficking between plasma membrane and recycling endosomes in hepatoma cells. J Lipid Res. 2008;49:2023–37. doi: 10.1194/jlr.M800145-JLR200. [DOI] [PubMed] [Google Scholar]
- 96.Weinglass AB, Kohler MG, Nketiah EO, Liu J, Schmalhofer W, et al. Madin-Darby canine kidney II cells: a pharmacologically validated system for NPC1L1-mediated cholesterol uptake. Mol Pharmacol. 2008;73:1072–84. doi: 10.1124/mol.107.043844. [DOI] [PubMed] [Google Scholar]
- 97.Sane AT, Sinnett D, Delvin E, Bendayan M, Marcil V, et al. Localization and role of NPC1L1 in cholesterol absorption in human intestine. J Lipid Res. 2006;47:2112–20. doi: 10.1194/jlr.M600174-JLR200. [DOI] [PubMed] [Google Scholar]
- 98.Labonte ED, Howles PN, Granholm NA, Rojas JC, Davies JP, et al. Class B type I scavenger receptor is responsible for the high affinity cholesterol binding activity of intestinal brush border membrane vesicles. Biochim Biophys Acta. 2007;1771:1132–39. doi: 10.1016/j.bbalip.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Knopfel M, Davies JP, Duong PT, Kvaerno L, Carreira EM, et al. Multiple plasma membrane receptors but not NPC1L1 mediate high-affinity, ezetimibe-sensitive cholesterol uptake into the intestinal brush border membrane. Biochim Biophys Acta. 2007;1771:1140–47. doi: 10.1016/j.bbalip.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 100.Brown JM, Rudel LL, Yu L. Niemann-Pick C1-like 1 (NPC1L1) mediates sterol-specific unidirectional transport of unesterified cholesterol in McArdle-RH7777 hepatoma cells. Biochem J. 2007;406:273–83. doi: 10.1042/BJ20070168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Larkin JM, Brown MS, Goldstein JL, Anderson RG. Depletion of intracellular potassium arrests coated pit formation and receptor-mediated endocytosis in fibroblasts. Cell. 1983;33:273–85. doi: 10.1016/0092-8674(83)90356-2. [DOI] [PubMed] [Google Scholar]
- 102.Valasek MA, Weng J, Shaul PW, Anderson RG, Repa JJ. Caveolin-1 is not required for murine intestinal cholesterol transport. J Biol Chem. 2005;280:28103–9. doi: 10.1074/jbc.M504609200. [DOI] [PubMed] [Google Scholar]
- 103.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 104.Betters JL, Yu L. Transporters as drug targets: discovery and development of NPC1L1 inhibitors. Clin Pharmacol Ther. 2010;87:117–21. doi: 10.1038/clpt.2009.209. [DOI] [PubMed] [Google Scholar]
- 105.Kwon HJ, Abi-Mosleh L, Wang ML, Deisenhofer J, Goldstein JL, et al. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 2009;137:1213–24. doi: 10.1016/j.cell.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Betters JL, Yu L. NPC1L1 and cholesterol transport. FEBS Lett. 2010;584:2740–47. doi: 10.1016/j.febslet.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chu BB, Ge L, Xie C, Zhao Y, Miao HH, et al. Requirement of myosin Vb. Rab11a Rab11-FIP2 complex in cholesterol-regulated translocation of NPC1L1 to the cell surface. J Biol Chem. 2009;284:22481–90. doi: 10.1074/jbc.M109.034355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Altmann SW, Davis HR, Jr, Yao X, Laverty M, Compton DS, et al. The identification of intestinal scavenger receptor class B, type I (SR-BI) by expression cloning and its role in cholesterol absorption. Biochim Biophys Acta. 2002;1580:77–93. doi: 10.1016/s1388-1981(01)00190-1. [DOI] [PubMed] [Google Scholar]
- 109.Smart EJ, De Rose RA, Farber SA. Annexin 2-caveolin 1 complex is a target of ezetimibe and regulates intestinal cholesterol transport. Proc Natl Acad Sci USA. 2004;101:3450–45. doi: 10.1073/pnas.0400441101. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 110.Kramer W, Girbig F, Corsiero D, Pfenninger A, Frick W, et al. Aminopeptidase N (CD13) is a molecular target of the cholesterol absorption inhibitor ezetimibe in the enterocyte brush border membrane. J Biol Chem. 2005;280:1306–20. doi: 10.1074/jbc.M406309200. [DOI] [PubMed] [Google Scholar]
- 111.Mardones P, Quinones V, Amigo L, Moreno M, Miquel JF, et al. Hepatic cholesterol and bile acid metabolism and intestinal cholesterol absorption in scavenger receptor class B type I-deficient mice. J Lipid Res. 2001;42:170–80. [PubMed] [Google Scholar]
- 112.Valasek MA, Weng J, Shaul PW, Anderson RG, Repa JJ. Caveolin-1 is not required for murine intestinal cholesterol transport. J Biol Chem. 2005;280:28103–9. doi: 10.1074/jbc.M504609200. [DOI] [PubMed] [Google Scholar]
- 113.Nguyen DV, Drover VA, Knopfel M, Dhanasekaran P, Hauser H, Phillips MC. Influence of class B scavenger receptors on cholesterol flux across the brush border membrane and intestinal absorption. J Lipid Res. 2009;50:2235–44. doi: 10.1194/jlr.M900036-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Braun A, Yesilaltay A, Acton S, Broschat KO, Krul ES, et al. Inhibition of intestinal absorption of cholesterol by ezetimibe or bile acids by SC-435 alters lipoprotein metabolism and extends the lifespan of SR-BI/apoE double knockout mice. Atherosclerosis. 2008;198:77–84. doi: 10.1016/j.atherosclerosis.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hawes BE, O’Neill KA, Yao X, Crona JH, Davis HR, Jr, et al. In vivo responsiveness to ezetimibe correlates with Niemann-Pick C1 like-1 (NPC1L1) binding affinity: comparison of multiple species NPC1L1 orthologs. Mol Pharmacol. 2007;71:19–29. doi: 10.1124/mol.106.027896. [DOI] [PubMed] [Google Scholar]
- 116.Salen G, Ahrens EH, Jr, Grundy SM. Metabolism of β-sitosterol in man. J Clin Investig. 1970;49:952–67. doi: 10.1172/JCI106315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bhattacharyya AK, Connor WE. β-Sitosterolemia and xanthomatosis. A newly described lipid storage disease in two sisters. J Clin Investig. 1974;53:1033–43. doi: 10.1172/JCI107640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Salen G, Shefer S, Nguyen L, Ness GC, Tint GS, Shore V. Sitosterolemia. J Lipid Res. 1992;33:945–55. [PubMed] [Google Scholar]
- 119.Salen G, von Bergmann K, Lutjohann D, Kwiterovich P, Kane J, et al. Ezetimibe effectively reduces plasma plant sterols in patients with sitosterolemia. Circulation. 2004;109:966–71. doi: 10.1161/01.CIR.0000116766.31036.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Salen G, Starc T, Sisk CM, Patel SB. Intestinal cholesterol absorption inhibitor ezetimibe added to cholestyramine for sitosterolemia and xanthomatosis. Gastroenterology. 2006;130:1853–57. doi: 10.1053/j.gastro.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 121.Turley SD. The role of Niemann-Pick C1–Like 1 (NPC1L1) in intestinal sterol absorption. J Clin Lipidol. 2008;2:S20–28. doi: 10.1016/j.jacl.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yu L, von Bergmann K, Lutjohann D, Hobbs HH, Cohen JC. Selective sterol accumulation in ABCG5/ABCG8-deficient mice. J Lipid Res. 2004;45:301–7. doi: 10.1194/jlr.M300377-JLR200. [DOI] [PubMed] [Google Scholar]
- 123.Wang J, Sun F, Zhang DW, Ma Y, Xu F, et al. Sterol transfer by ABCG5 and ABCG8: in vitro assay and reconstitution. J Biol Chem. 2006;281:27894–904. doi: 10.1074/jbc.M605603200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang J, Zhang DW, Lei Y, Xu F, Cohen JC, et al. Purification and reconstitution of sterol transfer by native mouse ABCG5 and ABCG8. Biochemistry. 2008;47:5194–204. doi: 10.1021/bi800292v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kastelein JJ, Akdim F, Stroes ES, Zwinderman AH, Bots ML, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358:1431–43. doi: 10.1056/NEJMoa0800742. [DOI] [PubMed] [Google Scholar]
- 126.Davis HR, Veltri EP. Zetia: inhibition of Niemann-Pick C1 Like 1 (NPC1L1) to reduce intestinal cholesterol absorption and treat hyperlipidemia. J Atheroscler Thromb. 2007;14:99–108. doi: 10.5551/jat.14.99. [DOI] [PubMed] [Google Scholar]
- 127.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–19. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 128.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Investig. 2004;114:147–52. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cheung O, Sanyal AJ. Recent advances in nonalcoholic fatty liver disease. Curr Opin Gastroenterol. 2009;25:230–37. doi: 10.1097/mog.0b013e3283294a18. [DOI] [PubMed] [Google Scholar]
- 130.Green RM. NASH: hepatic metabolism and not simply the metabolic syndrome. Hepatology. 2003;38:14–17. doi: 10.1053/jhep.2003.50325. [DOI] [PubMed] [Google Scholar]
- 131.Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40(Suppl 1):5–10. doi: 10.1097/01.mcg.0000168638.84840.ff. [DOI] [PubMed] [Google Scholar]
- 132.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–93. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 133.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 134.Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081–90. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 135.Patil VS, Magar NG. Effect of dietary fat and cholesterol on the polyunsaturated fatty acids of cholesterol esters, phospholipids and triglycerides in the liver of the rat. Biochem J. 1960;74:441–44. doi: 10.1042/bj0740441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zheng S, Hoos L, Cook J, Tetzloff G, Davis H, Jr, et al. Ezetimibe improves high fat and cholesterol diet-induced nonalcoholic fatty liver disease in mice. Eur J Pharmacol. 2008;584:118–24. doi: 10.1016/j.ejphar.2008.01.045. [DOI] [PubMed] [Google Scholar]
- 137.Deushi M, Nomura M, Kawakami A, Haraguchi M, Ito M, et al. Ezetimibe improves liver steatosis and insulin resistance in obese rat model of metabolic syndrome. FEBS Lett. 2007;581:5664–70. doi: 10.1016/j.febslet.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 138.Phillips MS, Liu Q, Hammond HA, Dugan V, Hey PJ, et al. Leptin receptor missense mutation in the fatty Zucker rat. Nat Genet. 1996;13:18–19. doi: 10.1038/ng0596-18. [DOI] [PubMed] [Google Scholar]
- 139.Assy N, Grozovski M, Bersudsky I, Szvalb S, Hussein O. Effect of insulin-sensitizing agents in combination with ezetimibe, and valsartan in rats with nonalcoholic fatty liver disease. World J Gastroenterol. 2006;12:4369–76. doi: 10.3748/wjg.v12.i27.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nozaki Y, Fujita K, Yoneda M, Wada K, Shinohara Y, et al. Long-term combination therapy of ezetimibe and acarbose for nonalcoholic fatty liver disease. J Hepatol. 2009;51:548–56. doi: 10.1016/j.jhep.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 140a.Jia L, Ma Y, Rong S, Betters JL, Xie P, et al. Niemann-Pick C1-Like 1 deletion in mice prevents high-fat diet-induced fatty liver by reducing lipogenesis. J Lipid Res. 2010;51:3135–44. doi: 10.1194/jlr.M006353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chan DC, Watts GF, Gan SK, Ooi EM, Barrett PH. Effect of ezetimibe on hepatic fat, inflammatory markers and apolipoprotein B-100 kinetics in insulin-resistant obese subjects on a weight loss diet. Diabetes Care. 2010;33:1134–39. doi: 10.2337/dc09-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yoneda M, Fujita K, Nozaki Y, Endo H, Takahashi H, et al. Efficacy of ezetimibe for the treatment of nonalcoholic steatohepatitis: an open-label, pilot study. Hepatol Res. 2010;40:566–73. doi: 10.1111/j.1872-034X.2010.00644.x. [DOI] [PubMed] [Google Scholar]
- 143.Enjoji M, Machida K, Kohjima M, Kato M, Kotoh K, et al. NPC1L1 inhibitor ezetimibe is a reliable therapeutic agent for nonobese patients with nonalcoholic fatty liver disease. Lipids Health Dis. 2010;9:29. doi: 10.1186/1476-511X-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 145.Nomura M, Ishii H, Kawakami A, Yoshida M. Inhibition of hepatic Neiman-Pick C1-like 1 improves hepatic insulin resistance. Am J Physiol Endocrinol Metab. 2010;297:1030–38. doi: 10.1152/ajpendo.00343.2009. [DOI] [PubMed] [Google Scholar]
- 146.Yagi S, Akaike M, Aihara K, Iwase T, Ishikawa K, et al. Ezetimibe ameliorates metabolic disorders and microalbuminuria in patients with hypercholesterolemia. J Atheroscler Thromb. 2010;17:173–80. doi: 10.5551/jat.2378. [DOI] [PubMed] [Google Scholar]
- 147.Monetti M, Levin MC, Watt MJ, Sajan MP, Marmor S, et al. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 2007;6:69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 148.Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signaling pathway mediated by the nuclear receptor LXRα. Nature. 1996;383:728–31. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 149.Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, et al. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–38. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jia L, Ma Y, Liu G, Yu L. Dietary cholesterol reverses resistance to diet-induced weight gain in mice lacking Niemann-Pick C1-Like 1. J Lipid Res. 2010;51:3024–33. doi: 10.1194/jlr.M008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bays HE, Ose L, Fraser N, Tribble DL, Quinto K, et al. A multicenter, randomized, double-blind, placebo-controlled, factorial design study to evaluate the lipid-altering efficacy and safety profile of the ezetimibe/simvastatin tablet compared with ezetimibe and simvastatin monotherapy in patients with primary hypercholesterolemia. Clin Ther. 2004;26:1758–73. doi: 10.1016/j.clinthera.2004.11.016. [DOI] [PubMed] [Google Scholar]