Abstract
Preterm birth occurs in 12.5% of births in the United States and can lead to risk of infant death or to lifelong serious health complications due to prematurity. A greater understanding by which the two main processes, uterine contraction and cervical remodeling are regulated is required to reduce rates of preterm birth. The cervix must undergo extensive remodeling through pregnancy in preparation for parturition, the process of labor and delivery of young. One key aspect of this dynamic process is a change in the composition and abundance of glycosaminoglycans (GAGs) and proteoglycans within the extracellular matrix, which influences the loss of tensile strength or stiffness of the cervix during labor. 23Na NMR spectroscopy has previously been validated as a method to quantiate GAGs in tissues. In the current study the Na+ concentration was measured at several time points through pregnancy in mouse cervices using 23Na NMR spectroscopy. The Na+ concentration increased progressively during pregnancy and peaked one day before birth followed by a rapid decline after birth. The same trend was seen in GAGs as measured by a biochemical assay using independent cervix samples over the course of pregnancy. We suggest that monitoring the Na+ concentration via 23Na NMR spectroscopy can serve as an informative physiological marker in evaluating the stages of cervical remodeling ex vivo and warrants further investigation to determine its utility as a diagnostic tool for the identification of women at risk for impending preterm birth.
Keywords: cervix, cervical remodeling, sodium, 23Na MRS, fixed charge density (FCD), glycosaminoglycan (GAG), hexuronic acid assay
INTRODUCTION
Preterm birth is the primary cause of infant mortality in the first year of life and can lead to life-long health problems for those that do survive (1). Preterm birth defined as birth that occurs prior to 37 weeks of a 40 week gestation occurs at an alarming rate of 12.5% of births in the United States. While the cause of prematurity is unclear in roughly 50% of preterm births, some known factors include infection, previous preterm birth, environmental factors and premature rupture of fetal membranes (2). Regardless of the initiating factor, preterm birth results in the premature acceleration of uterine contractions and/or cervical remodeling. Cervical remodeling, the process by which the cervix goes from a closed rigid structure to one that can open sufficiently during the birth process, requires a complex and carefully orchestrated series of events throughout pregnancy that leads to changes in this connective tissue rich matrix resulting in loss of tensile strength and integrity at birth (3,4). Studies aimed at defining the normal process of cervical remodeling will enhance our ability to develop improved tools for accurate detection of those at risk.
Changes in the composition and abundance of glycosaminoglycans (GAGs) within the extracellular matrix (ECM) accompany the transformation of the cervix through pregnancy, birth and postpartum (3,5–7). GAGs are highly hydrophilic macromolecules and generate an osmotic pressure and hence maintain the tissue hydration and compressive strength. In addition, GAGs influence the functional properties of the major structural protein, collagen, and thereby the tensile strength of the tissue. An increased synthesis of the nonsulfated GAG, hyaluronan (HA), during cervical ripening as a result of increased expression of hyaluronan synthase 2 occurs in women and mice (8). At the end of pregnancy, HA resides predominantly in a large molecular weight form and functions to disperse collagen, increase tissue hydration and tissue viscolelasticity (9). During labor or postpartum low molecular weight HA predominates and may play a role in inflammatory processes required for postpartum tissue repair. Sulfated GAGs such as chondroitin sulfate, dermatan sulfate, heparin sulfate and keratin sulfate are also present in the pregnant cervix (6,7,10,11). Numerous proteoglycans (PGs) with sulfated GAG chains have been described in the pregnant cervix including decorin, biglycan, and versican (12,13). Based on their roles in other biological systems (14,15) they may have similar functions in the cervix, such as modulating collagen fibril packing and tissue viscoelasticity during cervical remodeling. During pregnancy and labor there are changes in the proportion of specific GAGs to the pool of total GAGs with HA contributing to a larger percentage of the GAG pool during ripening. Some studies describing the changes in the total GAG content within the cervix during pregnancy and labor report no change and others report increases in total GAG (5,7,16). These variations may be due to the differences in methodology for the analysis of GAGs or species differences. Premature cervical remodeling resulting from the administration of prostaglandin E2 also leads to changes in cervical GAG composition in women and animal models (12,17,18). Understanding the changes in GAG composition and the specific function of each type of GAG during cervical remodeling will help elucidate the normal physiological process in the cervix. These investigations may enhance our understanding of potential causes of preterm birth as well as lead to the development of new and more accurate clinical tools for the early detection of preterm birth.
At physiological pH, the carboxylate groups and the sulfate groups of GAGs are negatively charged. Since GAGs in the cervix either form high molecular weight macromolecules themselves (mostly HA) or are covalently attached to the protein core of the PG macromolecules, the charges from the anionic groups are restricted in the tissue extracellular matrix (ECM), hence they are referred to as “fixed charges”. Collagen has nearly equal amounts of cationic and anionic groups; it does not contribute much net charge, which leaves GAGs as the major source of the fixed charge density (FCD) (19).
Contrary to the fixed charges, mobile ions such as Na+ and Cl− move freely between ECM and the surrounding fluid. To maintain the overall electroneutrality of the tissue, the mobile ions are distributed such that the negative charge is neutralized. In the connective tissue, the predominant cations are Na+ ions, therefore measuring the change of Na+ ion concentrations enables one to monitor the change of local fixed charge, and the FCD can be further translated to give useful insights about GAGs.
Recently, 23Na NMR spectroscopy and imaging has been used as a tool to noninvasively evaluate the integrity of connective tissue such as cartilage, (20–22) and intervertebral disc (23). In the early onset of osteoarthritis, a loss of PG is observed, leading to a decline in the fixed negative charge density of the tissue. Hence 23Na NMR spectroscopy has been shown to accurately measure the decline in fixed charge density associated with osteoarthritis (21). In the current study, 23Na NMR spectroscopy is used to evaluate the changes in Na+ concentration in the mouse cervix during pregnancy, labor and postpartum as a means of evaluating the GAG changes during each stage of cervical remodeling. The objective of this ex vivo study is to evaluate the utility of 23Na NMR spectroscopy for the assessment of the degree of cervical remodeling.
MATERIALS AND METHODS
Mice
Animals were housed under a 12L:12D photoperiod (lights-on, 0600–1800 h) at 22°C. Mice used in the present studies were of mixed strain (C57BL/6×129SvEv). The C57BL/6×129SvEv mice were generated and maintained as a breeder colony at the University of Texas Southwestern Medical Center (Dallas, TX). In general, mice in these studies were 3 to 6 months old and nulliparous. Female mice were housed overnight with males and checked at midday for vaginal plugs in order to obtain accurately timed pregnant mice. The day of plug formation was counted as day zero, and birth occurred in the early morning hours of day 19. All studies were conducted in accordance with the standards of humane animal care as described in the NIH Guide for the Care and Use of Laboratory Animals. The research protocols were approved by the institutional animal care and research advisory committee.
Sample preparation
Cervices at gestation days 10, 12, 14, 16, 17, 18, 24 hour postpartum (24h pp) and 48 hour postpartum (48h pp) were collected. For each time point, 5 samples were tested (N=5). At the time of collection, samples were weighed, flash frozen in liquid nitrogen and transported to New York University on dry ice. Before the NMR experiments, the cervix samples were rehydrated in phosphate buffered saline (PBS, Sigma-Aldrich, St Louis, MO) for at least 45 minutes. After rehydration, each sample was taken out of the buffer, blot dried, and placed into an NMR tube. To preserve the sample and avoid susceptibility effects, the void space around the sample was filled with fluorinated oil (Sigma-Aldrich, St Louis, MO).
Determination of the water content
The water content was determined by the difference between tissue wet weight (taken at the time of tissue collection) and dry weight (after overnight lyophilization).
Hexuronic acid assay
Hexuronic acid (glucuronic acid or iduronic acid) is a component of the repeating unit of all glycosaminoglycans with the exception of keratin sulfate and this assay provides a useful estimate of the total GAG concentration (minus keratin) in tissue (24,25). Cervices were lyophilized overnight, dry weight was determined and tissues digested in ammonium acetate (100 mM) with phenol red (0.0005%), pH 7.0 containing 250 ug/ml proteinase K (Roche, Indianapolis, IN) for 4 h at 60°C. Proteinase K was inactivated by boiling at 100°C for 10 min. Undigested tissues were pelleted by centrifugation at 12000×g for 15 min and supernatant stored at −80°C.
An aliquot of supernatant equal to 0.5 mg dry weight of digested cervix was assayed as described (25) with slight modifications. Total GAGs were precipitated from each aliquot by addition of 800 ul of cold 100% ethanol and incubation at −20°C for 16 h. GAGs were pelleted by centrifugation (12000×g for 15 min) followed by a second precipitation with 1 ml of 80% ethanol at −20°C for 2 h. After centrifugation, pellets were air dried for 30 min and resuspended in 100 ul ultra pure water. 750 ul of sulfuric acid tetraborate was added to the sample, heated to 100°C for 5 min, cooled, and mixed with 10 ul of hydroxyphenol reagent (0.15% 2-phenylphenol in 0.5% NaOH). The samples (250 ul) in triplicates were then transferred to a 96-well flat bottom clear plate and absorbance read using the Tecan Safire2 plate reader (Durham, NC) at 532 nm with 750 nm background subtraction. Data were converted to hexuronic acid concentrations by comparison to a standard curve of D-glucuronic acid. The results were normalized to the molecular weight of D-glucuronic acid (194.14 g/mol). All reagents are acquired from Sigma- Aldrich (St. Louis, MO) if it is not indicated otherwise.
NMR method
All experiments were carried out on an 11.7 Tesla Bruker Avance spectrometer with a broad-band direct observe (BBO) probe tuned to sodium frequency (132.31 MHz).
The sodium NMR experiments on NaCl standard solution and the cervical tissue were performed back-to-back. Before each experiment, the probe was tuned and matched. The 900 pulse was calibrated for each sample at powers ranging from 31.2 kHz to 33.7 kHz. The pre-scan delay was 6 μs. The spectra were recorded with a spectral width of 5 kHz and 4096 data point. 128 transients were acquired for each spectrum with a repetition delay of 1s. Although the repetition delay could be shortened significantly, this was not needed since adequate signal-to-noise ratios were obtained rapidly. The Na NMR signals were integrated and calibrated by comparing with the signal of a 25 mM NaCl standard solution in water to obtain the absolute Na+ content.
FCD calculation
The Fixed charge density (FCD) was calculated using the ideal Donnan equilibrium.
| [1] |
In which [Na+]b is the PBS bath concentration and [Na+]t is the intratissue Na+ concentration. The Na+ concentration in PBS bath is 154 mM. The intratissue Na+ concentration was calculated using the Na+ concentration acquired from NMR experiments normalized to the water content assuming the density of water is 1.0 g/mL (0.9970 g/mL at room temperature). The intratissue FCD was then converted to reflect the charges per total tissue weight, since this would be the number of interest for in vivo situations.
RESULTS
Measurement of GAG concentration
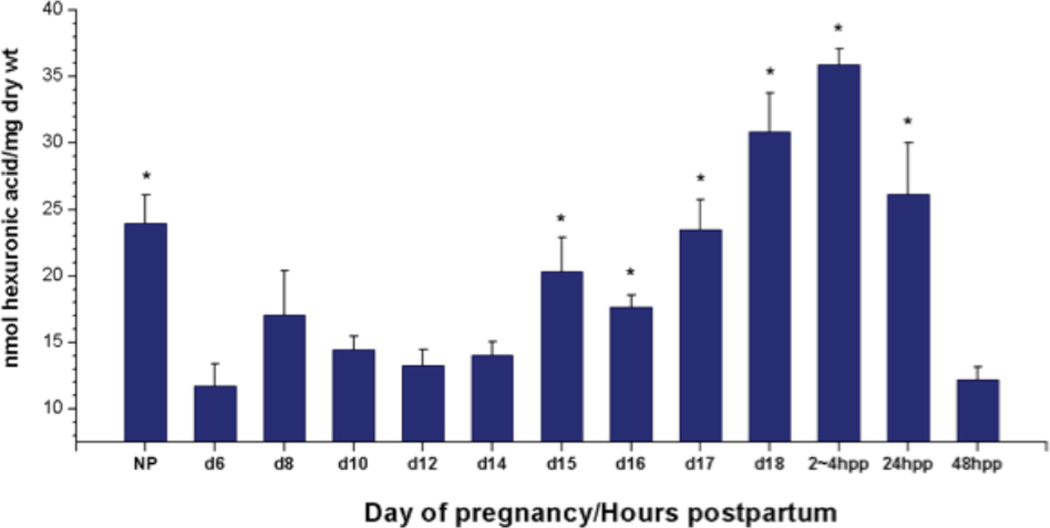
GAG concentration in the mouse cervix through pregnancy and postpartum was evaluated to confirm an increase in total GAGs at the end of pregnancy as previously described in human (5,7). The hexuronic acid assay was performed as described and data is presented as nmol uronic acid/mg dry weight of tissue. Similar to studies in human and animal models, cervical total GAGs steadily increased with progression of pregnancy in the mouse. Compared to early pregnancy (gestation day 6) there was a significant increase in hexuronic acid by gestation day 15 which reached a peak 2 to 4 hours after birth and began to decline by 24 hours postpartum (Fig. 1).
Figure 1.
Hexuronic acid levels in proestrus nonpregnant (NP), pregnant (d6-18) and cervix collected 2–4, 24, and 48 hours postpartum (pp2–4, 24 and 48 h). Each group represents the mean ± S.E.M. of 5 samples. (*) P < 0.05 when compared to d6. Data was analyzed by One Way ANOVA. Differences among treatment means were determined using Student-Newman-Keuls’ test.
Changes in Na+ concentration
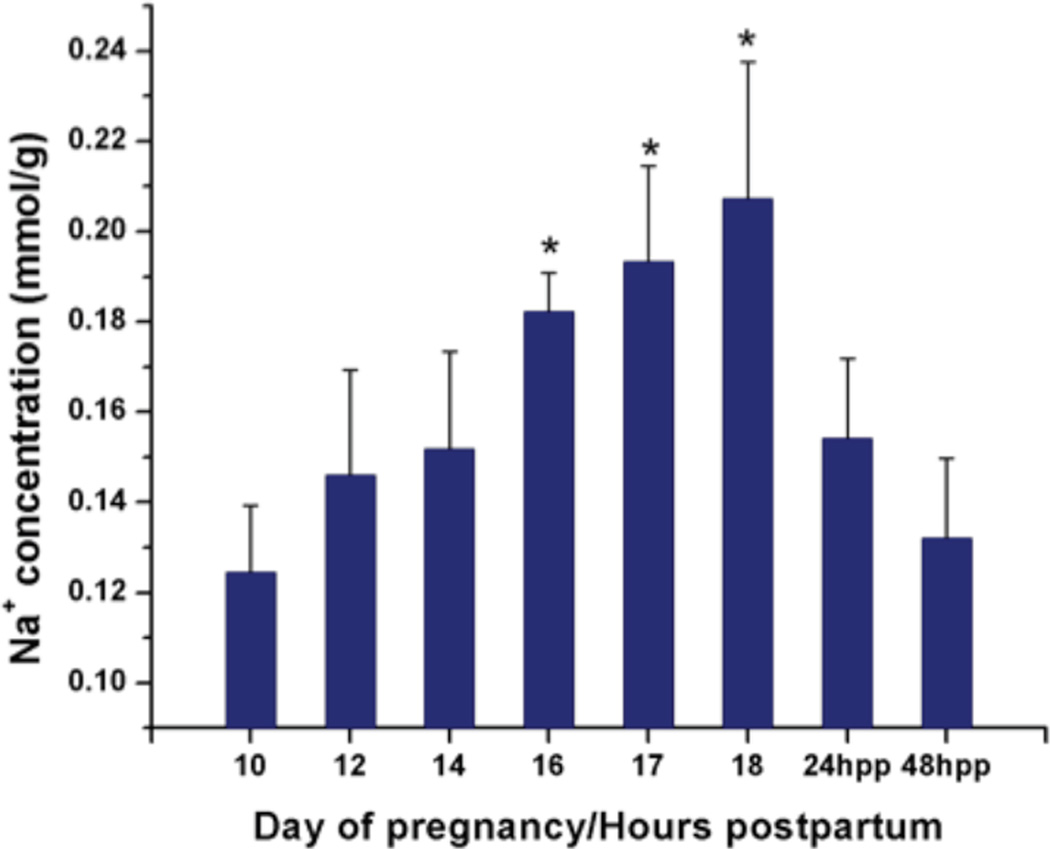
The amount of Na+ was normalized to the weight obtained after collection to determine the tissue Na+ concentration. Fig. 2 shows the tissue Na+ concentration as a function of gestation day and the hours postpartum. We observed that the Na+ concentration increased progressively starting at gestation day 10, prior to the softening phase (0.12±0.01mmol/g wet weight of tissue, mean±SD), and continuing through the course of cervical remodeling. A maximum is reached immediately prior to labor during cervical ripening (gestation day 18, 0.21±0.03mmol/g wet tissue, mean±SD). Compared to gestation day 10, Na+ concentration was significantly increased by day 16 consistent with the rise in hexuronic acid measured at this time point. The concentration decreased significantly and rapidly at the postpartum phase (24 and 48 hours postpartum), (0.15±0.02mmol/g and 0.13±0.02mmol/g, respectively) consistent with the decline in hexuronic acid measured at these time points. The temporal pattern of Na+ correlates with the GAG measurements in the hexuronic acid assay.
Figure 2.
Na+ concentration (mmol/g tissue wet weight) as a function of gestation day/hour post partum (hpp). Each group represents the mean ± S.D. of 5 samples. (*) P < 0.05 when compared to d10. Data was analyzed by One-Way ANOVA.
Change in water content
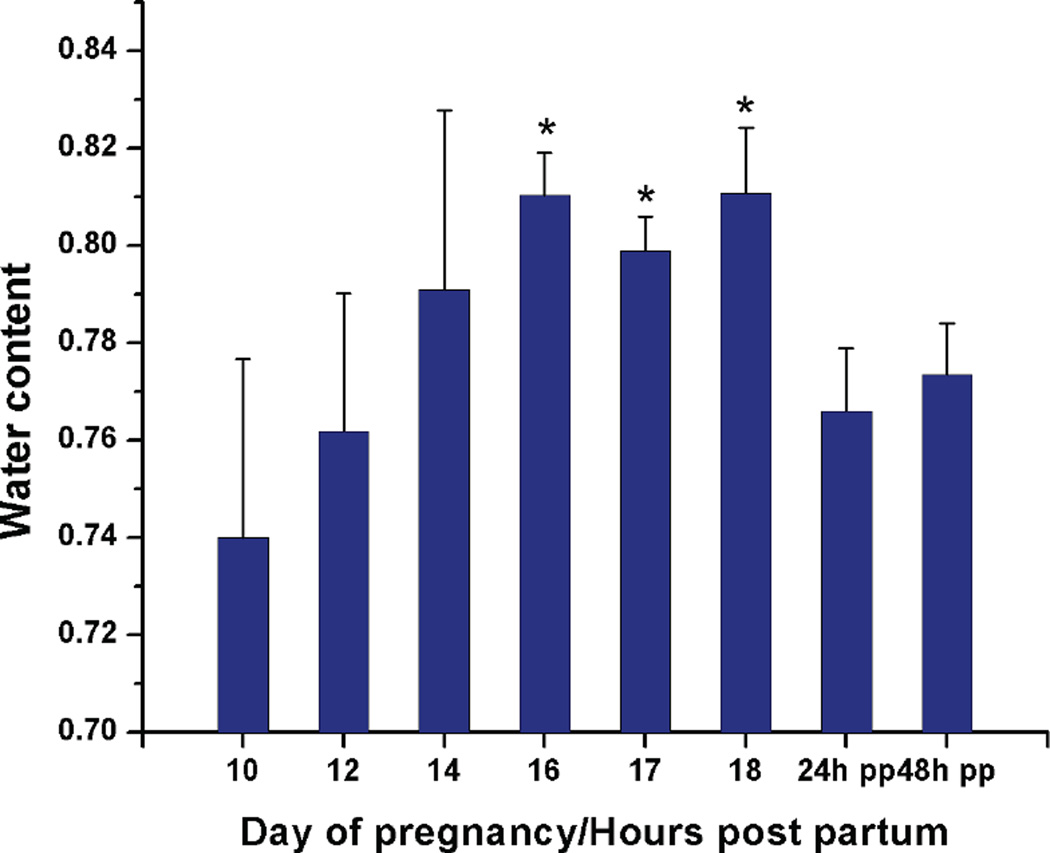
The water content as a function of gestation day was plotted (mean±SD) in Fig.3. Compared to earlier pregnancy (gestation day 10), cervical water content increased significantly by gestation day 16 (81.0±0.9%, mean±SD) and peaked at the end of pregnancy (gestation day 18, 81.1±1.3%). The water content declined as the postpartum cervix started to recover (24 and 48 hours postpartum, 76.6±1.3% and 77.3±1.1%, respectively).
Figure 3.
Water content in tissue wet weight as a function of gestation day (10–18) /hours post partum(24hpp and 48hpp). Each group represents the mean ± S.D. of 5 samples. (*) P < 0.05 when compared to gestation day 10. Data was analyzed by One-Way ANOVA.
FCD calculation
The concentration of mobile ions can be translated to FCD using the ideal Donnan theory as described in the Methods section. This model was originally developed to describe the partitioning of ions and water across a semi-permeable membrane and later proved to be analogous to tissue immersed in buffer/extracellular fluid (26–28). In vivo, the tissue is always under Donnan equilibrium with the surrounding fluid, while in vitro, the Donnan equilibrium can be achieved by equilibrating the tissue in a bath solution.
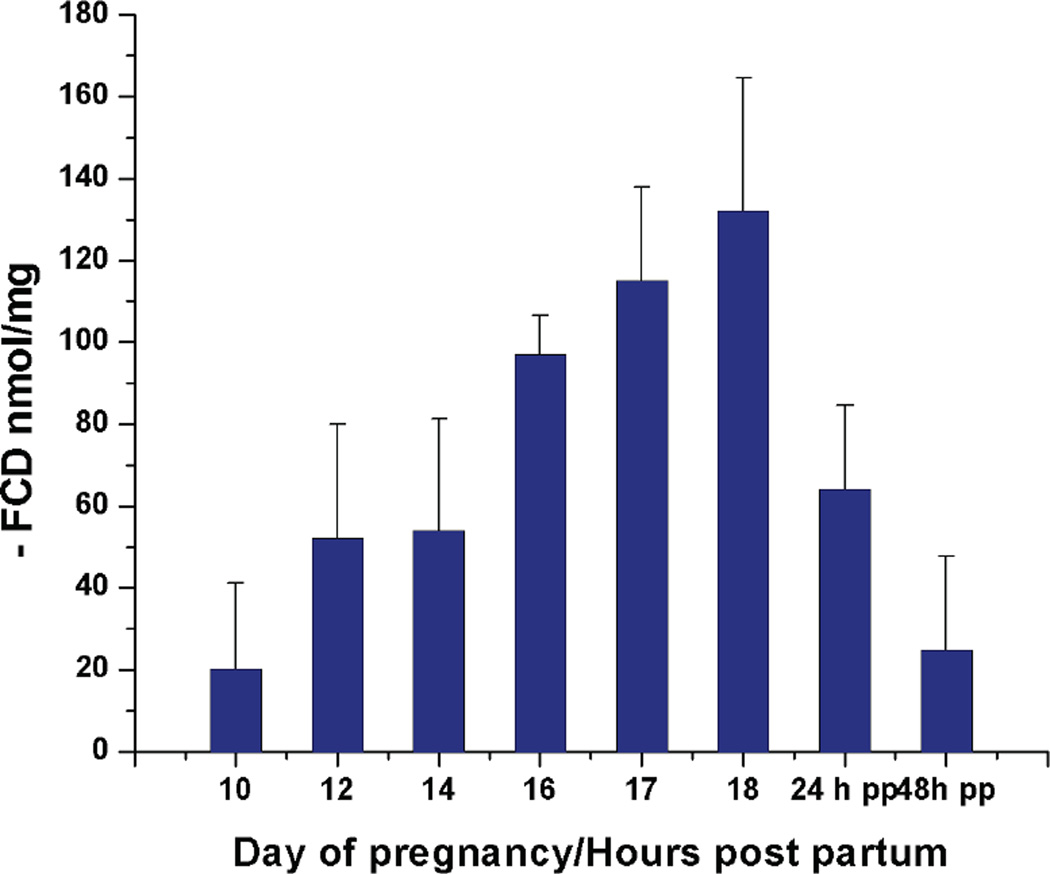
The calculated FCD is negative by definition based on the derivation of Eq. [1]. In Fig.4, the negative FCD in the tissue was plotted as a function of gestation day or hours postpartum.
Figure 4.
Calculated fixed charge density using Na+ concentration and water content. (−)FCD is plotted as a function of gestation day/hours post partum. Error analysis is applied in the calculation.
DISCUSSION
The data suggest that the Na+ concentration increases progressively during the course of pregnancy and declines dramatically postpartum. This trend may reflect the increase of charged species within the tissue and may provide an indirect reporter of the changes of total GAG concentrations, as well as a biomarker for normal pregnancy progression. The weight of the collected mouse cervices is in the range of 5mg to 30mg, depending on the stage of pregnancy. The sample sizes are small with respect to the RF coil; hence there should be no positioning issues. Any magnetic field inhomogeneities would only affect the linewidths, not the signal integrals used in this study. With the method used, however, no distinction between different pools can be made, and the total Na+ concentration is measured. The variation between two independent NMR measurements of the same sample is within 5% (results not shown). Hence the larger variations of the measured concentrations are likely due to individual differences between mice.
The water content, as observed in the study, also increased as pregnancy advanced. This coincided with the observed increase of Na+ concentration which may be a reflection of the increased osmotic pressure induced by the charged species. An increased expression of aquaporin (AQP) water channels during pregnancy (29) which was reported in a previous study may, however, also account for the increase in water. The water content measurement was needed for calculating the intratissue Na+ concentration. The cervical tissue is composed of 10- 15% smooth muscle cells and 85–90% fibrous connective tissue (30–32). While Na+ exists both in the extracellular matrix (ECM) and in the cells, the cellular portion was not taken into account in the current study. Typical Na+ concentrations in smooth muscle cells are roughly 30mmol/kg (33), while the total Na+ concentration measured in this study was around 200mmol/kg. The uneven distribution of Na+ in the cells and in the connective tissue introduces a source of variation in calculating the FCD where the Na+ content is normalized to the total water content which includes both the intracellular and the extracellular fraction. Another possible source of error regards the change of the cellular portion during the course of pregnancy. Apoptosis, the process of programmed cell death, was reported to be associated with the change of cervical softening (34). This process could change the cellular volume fraction of the cervical tissue and hence introduce an error in the calculation. The error would, however be relatively minor and would not change the trend of the observation. Assuming that the cell fraction would change from 15 to 10%, the ensuing error in the FCD calculation would amount to approximately 5%, which is well within the reported error bars.
The change of FCD through cervical remodeling and postpartum recovery mimics the temporal increase in total GAGs measured by the biochemical assay. While the temporal increase was similar, the FCD calculated from the Na+ concentration is higher than the result from the biochemical assay. One possibility is that the increased water content resulting from rehydration may account for an additional overestimation of the FCD. This effect, however, is only problematic in these ex vivo studies, and would not occur in vivo. A second point for consideration is that the biochemical assay quantifies uronic acid residues and thus is independent of whether the GAGs are sulfated or not. Na+ concentration, however, is sensitive to the total negative charge arising from both the sulfate groups on the GAG disaccharide unit as well as, the carboxylate residues (one per repeat unit). Since hyaluronan is not sulfated, a sulfated proteoglycan could have twice or more the number of charges than hyaluronan. The higher concentration in FCD as compared to the one determined from the hexuronic acid assay may suggest that increased sulfation of GAG in cervical tissue is a regulatory step along with increased GAG synthesis. Indeed, both the types of GAG and the degree of sulfation regulate many biological and pathological processes that include collagen fibril organization and tensile properties (35), growth factor signaling (36), neuronal function (37), and lung development (38). Altered GAG sulfation patterns are further described in hepatic carcinomas (39). Identification of changes in GAG type, amount and degree of sulfation in the cervix through pregnancy and birth will be an important goal of future studies.
Compared to an earlier pregnancy day (day 10), the Na+ concentration increased by approximately a factor of 1.7, and approximately 2.1 in the assay measurement. The water content, however, only increased by 5%. The existing proton MRI studies reported no or very low correlation between T2 weighted signal intensity and the gestational age (40, 41). Given the significant change in Na+ concentration, the results presented in this work suggest that change in Na+ concentration by itself can serve as an informative physiological marker for cervical remodeling. Further steps will be focused on studying the translation of these spectroscopic results into in vivo settings for the purpose of developing a diagnostic tool. In an in vivo MRI setting, issues such as resolution, sensitivity, and inhomogeneities would have to be addressed.
CONCLUSION
We present here the observation of the progressive increase in Na+ concentration during the course of cervical remodeling using 23Na NMR spectroscopy. The significant changes seen over this time course render Na+ a potential biomarker of cervical remodeling ex vivo. A hexuronic acid assay showed similar trends for GAG concentration as a function of gestation day. The noninvasive 23Na NMR method could open the door to evaluating the integrity of the cervix during pregnancy in vivo, and may have potential as a tool for the identification of women at risk for preterm birth.
Acknowledgements
This work was supported in part by the National Institutes of Health Grants P01 HD11149 (To M.M.) and 1R21AR054002-01A1 (to A.J.).
Abbreviations used
- GAG
glycosaminoglycan
- ECM
extracellular matrix
- HA
hyaluronan
- PG
proteoglycan
- PBS
phosphate buffered saline
- FCD
fixed charge density
REFERENCES
- 1.Committee on understanding premature birth and assuring healthy outcomes board on health sciences policy. Preterm birth: Causes, consequences, and prevention. Washington, D.C: National Academies Press; 2006. [Google Scholar]
- 2.Cunningham GL, Kenneth J, Bloom Steven L, Hauth John C, Gilstrap Larry C, Wenstrom Katharine D, editors. Williams obstetrics. ed 22nd Edition. McGraw-Hill Professional; 2005. [Google Scholar]
- 3.Leppert PC. Anatomy and physiology of cervical ripening. Clinical Obstetrics and Gynecology. 1995;38:267–279. doi: 10.1097/00003081-199506000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Word RA, Li XH, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: Mechanisms and current concepts. Seminars in Reproductive Medicine. 2007;25:69–79. doi: 10.1055/s-2006-956777. [DOI] [PubMed] [Google Scholar]
- 5.Myers K, Socrate S, Tzeranis D, House M. Changes in the biochemical constituents and morphologic appearance of the human cervical stroma during pregnancy. Elsevier Ireland Ltd; 2009. pp. S82–S89. [DOI] [PubMed] [Google Scholar]
- 6.Danforth DN, Veis A, Breen M, Weinstei Hg, Buckingh Jc, Manalo P. Effect of pregnancy and labor on human cervix - changes in collagen, glycoproteins, and glycosaminoglycans. Am J Obstet Gynecol. 1974;120:641–651. doi: 10.1016/0002-9378(74)90608-5. [DOI] [PubMed] [Google Scholar]
- 7.Osmers R, Rath W, Pflanz MA, Kuhn W, Stuhlsatz HW, Szeverenyi M. Glycosaminoglycans in cervical connective-tissue during pregnancy and parturition. Obstetrics and Gynecology. 1993;81:88–92. [PubMed] [Google Scholar]
- 8.Straach KJ, Shelton JM, Richardson JA, Hascall VC, Mahendroo MS. Regulation of hyaluronan expression during cervical ripening. Glycobiology. 2005;15:55–65. doi: 10.1093/glycob/cwh137. [DOI] [PubMed] [Google Scholar]
- 9.Ruscheinsky M, De la Motte C, Mahendroo M. Hyaluronan and its binding proteins during cervical ripening and parturition: Dynamic changes in size, distribution and temporal sequence. Matrix Biology. 2008;27:487–497. doi: 10.1016/j.matbio.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obara M, Hirano H, Ogawa M, Tsubaki H, Yoshida Y, Miyauchi S, Tanaka T. Does chondroitin sulfate defend the human uterine cervix against ripening in threatened premature labor? Am J Obstet Gynecol. 2000;182:334–339. doi: 10.1016/s0002-9378(00)70220-1. [DOI] [PubMed] [Google Scholar]
- 11.Von Maillot K, Stuhlsatz HW, Mohanaradhakrishnan V, Greiling H. Changes in the glycosamino glycans distribution pattern in the human uterine cervix during pregnancy and labor. Am J Obstet Gynecol. 1979;135:503–506. doi: 10.1016/0002-9378(79)90440-x. [DOI] [PubMed] [Google Scholar]
- 12.Ji H, Dailey TL, Long V, Chien EK. Prostaglandin e2-regulated cervical ripening: Analysis of proteoglycan expression in the rat cervix. Am J Obstet Gynecol. 2008;198:536.e531–537.e531. doi: 10.1016/j.ajog.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 13.Westergren-Thorsson G, Norman M, Bjornsson S, Endresen U, Stjernholm Y, Ekman G, Malmstrom A. Differential expressions of mrna for proteoglycans, collagens and transforming growth factor-beta in the human cervix during pregnancy and involution. Biochimica Et Biophysica Acta-Molecular Basis of Disease. 1998;1406:203–213. doi: 10.1016/s0925-4439(98)00005-2. [DOI] [PubMed] [Google Scholar]
- 14.Ameye L, Young MF. Mice deficient in small leucine-rich proteoglycans: Novel in vivo models for osteoporosis, osteoarthritis, ehlers-danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology. 2002;12:107R–116R. doi: 10.1093/glycob/cwf065. [DOI] [PubMed] [Google Scholar]
- 15.Handley CJ, Samiric T, Ilic MZ. Structure, metabolism, and tissue roles of chondroitin sulfate proteoglycans. Adv Pharmacol. 2006;53:219–232. doi: 10.1016/S1054-3589(05)53010-2. [DOI] [PubMed] [Google Scholar]
- 16.Golichowski AM, King SR, Mascaro K. Pregnancy-related changes in rat cervical glycosaminoglycans. Biochemical Journal. 1980;192:1–8. doi: 10.1042/bj1920001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rath W, Osmers R, Adelmanngrill BC, Stuhlsatz HW, Szevereny M, Kuhn W. Biochemical-changes in human cervical connective-tissue after intracervical application of prostaglandin-e(2) Prostaglandins. 1993;45:375–384. doi: 10.1016/0090-6980(93)90114-m. [DOI] [PubMed] [Google Scholar]
- 18.Cabrol D, Dubois P, Sedbon E, Dallot E, Legagneux J, Amichot G, Cedard L, Sureau C. Prostaglandin-e2-induced changes in the distribution of glycosaminoglycans in the isolated rat uterine cervix. European Journal of Obstetrics Gynecology and Reproductive Biology. 1987;26:359–365. doi: 10.1016/0028-2243(87)90135-3. [DOI] [PubMed] [Google Scholar]
- 19.Li ST, Katz EP. Electrostatic model for collagen fibrils - interaction of reconstituted collagen with Ca++, Na+, and Cl. Biopolymers. 1976;15:1439–1460. doi: 10.1002/bip.1976.360150802. [DOI] [PubMed] [Google Scholar]
- 20.Lesperance LM, Gray ML, Burstein D. Determination of fixed charge-density in cartilage using nuclear-magnetic-resonance. Journal of Orthopaedic Research. 1992;10:1–13. doi: 10.1002/jor.1100100102. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro EM, Borthakur A, Gougoutas A, Reddy R. Na-23 mri accurately measures fixed charge density in articular cartilage. Magn Reson Med. 2002;47:284–291. doi: 10.1002/mrm.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reddy R, Insko EK, Noyszewski EA, Dandora R, Kneeland JB, Leigh JS. Sodium mri of human articular cartilage in vivo. Magn Reson Med. 1998;39:697–701. doi: 10.1002/mrm.1910390505. [DOI] [PubMed] [Google Scholar]
- 23.Insko EK, Clayton DB, Elliott MA. In vivo sodium mr imaging of the intervertebral disk at 4 t. Acad Radiol. 2002;9:800–804. doi: 10.1016/s1076-6332(03)80350-1. [DOI] [PubMed] [Google Scholar]
- 24.Blumenkr N, Asboehan G. New method for quantitative-determination of uronic acids. Anal Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- 25.Grande-Allen KJ, Mako WJ, Calabro A, Shi YL, Ratliff NB, Vesely I. Loss of chondroitin 6-sulfate and hyaluronan from failed porcine bioprosthetic valves. J Biomed Mater Res Part A. 2003;65A:251–259. doi: 10.1002/jbm.a.10475. [DOI] [PubMed] [Google Scholar]
- 26.Maroudas A, Muir H, Wingham J. Correlation of fixed negative charge with glycosaminoglycan content of human articular cartilage. Biochimica Et Biophysica Acta. 1969;177:492–500. doi: 10.1016/0304-4165(69)90311-0. [DOI] [PubMed] [Google Scholar]
- 27.Maroudas A, Thomas H. A simple physicochemical micromethod for determining fixed anionic groups in connective tissue. Biochimica Et Biophysica Acta. 1970;215:214–216. [PubMed] [Google Scholar]
- 28.Schneiderman R, Keret D, Maroudas A. Effects of mechanical and osmotic-pressure on the rate of glycosaminoglycan synthesis in the human adult femoral-head cartilage - an invitro study. J Orthop Res. 1986;4:393–408. doi: 10.1002/jor.1100040402. [DOI] [PubMed] [Google Scholar]
- 29.Anderson J, Brown N, Mahendroo MS, Reese J. Utilization of different aquaporin water channels in the mouse cervix during pregnancy and parturition and in models of preterm and delayed cervical ripening. Endocrinology. 2006;147:130–140. doi: 10.1210/en.2005-0896. [DOI] [PubMed] [Google Scholar]
- 30.Leppert PC. Anatomy and physiology of cervical ripening. Clinical Obstetrics and Gynecology. 1995;38:267–279. doi: 10.1097/00003081-199506000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Ludmir JM, Sehdev HMM. Anatomy and physiology of the uterine cervix. Clinical Obstetrics & Gynecology. 2000;43:433–439. doi: 10.1097/00003081-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Schlembach D, MacKay L, Shi LL, Maner WL, Garfield RE, Maul H. Cervical ripening and insufficiency: From biochemical and molecular studies to in vivo clinical examination. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2009;144:S70–S76. doi: 10.1016/j.ejogrb.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 33.Djurhuus MS, Klitgaard NAH, Tveskov C, Madsen K, Guldager B, Jelnes R, Petersen PH, Beck-Nielsen H. Methodological aspects of measuring human skeletal muscle electrolyte content and ouabain binding capacity. Anal Biochem. 1998;260:218–222. doi: 10.1006/abio.1998.2625. [DOI] [PubMed] [Google Scholar]
- 34.Leppert PC, Yu SY. Apoptosis in the cervix of pregnant rats in association with cervical softening. Gynecol Obstetlnvest. 1994;37:150–154. doi: 10.1159/000292546. [DOI] [PubMed] [Google Scholar]
- 35.Kuc IM, Scott PG. Increased diameters of collagen fibrils precipitated in vitro in the presence of decorin from various connective tissues. Connective Tissue Research. 1997;36:287–296. doi: 10.3109/03008209709160228. [DOI] [PubMed] [Google Scholar]
- 36.Cortes M, Baria AT, Schwartz NB. Sulfation of chondroitin sulfate proteoglycans is necessary for proper indian hedgehog signaling in the developing growth plate. Development. 2009;136:1697–1706. doi: 10.1242/dev.030742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H, Katagiri Y, McCann TE, Unsworth E, Goldsmith P, Yu ZX, Tan F, Santiago L, Mills EM, Wang Y, Symes AJ, Geller HM. Chondroitin-4-sulfation negatively regulates axonal guidance and growth. J Cell Sci. 2008;121:3083–3091. doi: 10.1242/jcs.032649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faggian J, Fosang AJ, Zieba M, Wallace MJ, Hooper SB. Changes in versican and chondroitin sulfate proteoglycans during structural development of the lung. Am J Physiol-Regul Integr Comp Physiol. 2007;293:R784–R792. doi: 10.1152/ajpregu.00801.2006. [DOI] [PubMed] [Google Scholar]
- 39.Lv HZ, Yu GL, Sun LL, Zhang Z, Zhao X, Chai WG. Elevate level of glycosaminoglycans and altered sulfation pattern of chondroitin sulfate are associated with differentiation status and histological type of human primary hepatic carcinoma. Oncology. 2007;72:347–356. doi: 10.1159/000113145. [DOI] [PubMed] [Google Scholar]
- 40.Pates JA, Zaretsky MV, Alexander JM, Babcock EE, McIntire DD, Twickler DM. Determining cervical ripeness and labor outcome - the efficacy of magnetic resonance t-2 relaxation times. Obstetrics and Gynecology. 2007;109:326–330. doi: 10.1097/01.AOG.0000252711.30867.a1. [DOI] [PubMed] [Google Scholar]
- 41.Chan YL, Lam WWM, Lau TK, Wong SP, Li CY, Metreweli C. Cervical assessment by magnetic resonance imaging - its relationship to gestational age and interval to delivery. Br J Radiol. 1998;71:155–159. doi: 10.1259/bjr.71.842.9579179. [DOI] [PubMed] [Google Scholar]