Abstract
Although drugs may serve as reinforcers or punishers of operant behavior, the punishing function has received much less experimental attention than the reinforcing function. A sensitive method for studying drug-induced punishment is to assess choice for a punished response over an unpunished response. In these experiments, rats chose between pressing one lever and receiving a sucrose pellet or pressing another lever and receiving a sucrose pellet plus an intravenous injection of histamine. When sucrose was delivered equally frequently for either the punished or the unpunished response, rats selected the unpunished lever consistently, but decreases in the punished response did not differ as a function of intravenous histamine dose (0.1–1 mg/kg/inj). Changing the procedure so that sucrose was delivered on the unpunished lever with p = .5 increased the rats’ responding on the punished lever with saline injections. In addition, the same range of histamine doses produced a much larger range of responses on the punished lever that was dose dependent. Using these procedures to assess the receptors mediating histamine’s effects, the histamine H1-receptor antagonists, pyrilamine and ketotifen, antagonized the punishing effect of histamine, but the histamine H2-receptor antagonist ranitidine did not. However, ranitidine pretreatments reduced histamine-induced heart-rate increases to a greater extent than did the histamine H1-receptor antagonists when administered at the same doses examined under conditions of histamine punishment. Overall, the present findings extend the general hypothesis that activation of histamine H1-receptors mediates the punishing effects of histamine. They also introduce methods for rapidly assessing pharmacological mechanisms underlying drug-induced punishment.
Keywords: drug punishment, choice, histamine, antagonist, cardiovascular effects, lever press, rats
Reinforcing drug effects have been studied extensively under a wide range of experimental conditions, whereas punishing drug effects have been explored far less. Despite the relevance of such drug effects, ranging from understanding of fundamental behavioral processes to issues of drug treatment compliance, the study of punishing drug effects remain limited compared with study of reinforcing drug effects. This state of affairs mirrors the difference in knowledge of reinforcement and punishment processes generally (Lerman & Vorndran, 2002).
Studies of punishing drug effects typically have arranged extensive training of steady-state operant behavior with primates (Goldberg, 1980; Goldberg & Spealman, 1983; Katz & Goldberg, 1986; Negus, 2005; Takada, Barrett, Allen, Cook, & Katz, 1992; Woolverton, 2003). For instance, Goldberg arranged a multiple schedule in which food pellets were presented to squirrel monkeys for pressing a lever on a fixed-ratio (FR) 30 schedule of food reinforcement in two components. Intravenous injections of histamine, an endogenous neurotransmitter involved in pain and inflammation (Hough, 2001), were arranged for the 11th and 22nd response of each ratio in one component. Histamine produced a dose-dependent (0.03 – 0.1 mg/kg/inj) decrease in responding that was selective to responding in that component, that is, responding in the unpunished component remained unchanged. These findings suggest that, as with punishment with electric shock, histamine punishment could come under discriminative-stimulus control and be directly related to the dose (i.e., intensity) of the punishing stimulus (see Azrin & Holz, 1966). Goldberg also showed that presession injections of diphenhydramine, a selective histamine H1-receptor antagonist, blocked the punishing effects of histamine, as indicated by elevations in responding previously suppressed by histamine administration. Taken together, these findings revealed that such methods might be used quite generally to study behavioral and pharmacological determinants of the punishing effects of intravenous drugs.
Podlesnik, Jimenez-Gomez, and Woods (2010) described an operant procedure to assess the punishing effects of intravenous drugs in rats. These procedures were developed to assess drug effects quickly because of limited patency of catheters in rats. In 100 discrete trials per session, rats were trained to respond on two concurrently available levers for the presentation of sucrose pellets on FR 1 reinforcement schedules with 5-s intertrial intervals (ITI). Indwelling intravenous catheters were implanted, and intravenous injections of histamine (1 mg/kg/inj), in addition to sucrose-pellet delivery, were made contingent on responses on one lever (sucrose- plus-injection lever). Generally consistent with the findings of Goldberg (1980), histamine injections selectively punished responding. Specifically, histamine injections decreased responding on the injection lever while responding increased on the lever that produced only sucrose pellets.
Although a selective punishing effect of histamine could be observed rapidly, the procedure was limited with regard to assessing the dose-dependent effects of histamine. When the histamine injection was discontinued, responding on the sucrose and injection lever often remained as suppressed as it had been when it resulted in histamine delivery (see also Katz & Goldberg, 1986). This continued suppression in the absence of histamine indicated that simply substituting various doses of histamine likely would not result in reliable dose-dependent responding on the sucrose-plus-injection lever.
Dose-dependent effects of histamine were established in subsequent conditions when the lever that produced sucrose plus histamine and the lever that produced sucrose alone were reversed for several sessions at each histamine dose (0.1–1 mg/kg/inj). Therefore, in another experiment, Podlesnik et al. (2010) arranged daily lever reversals in an attempt to reveal the dose-dependent punishing effects of histamine more quickly. Instead, daily lever reversals with the 1 mg/kg/inj dose of histamine resulted in mixed effects. In several cases, more responding consistently was allocated to one of the levers regardless of which lever the injection was contingent upon (see also Banks, 1973; Karsh, 1970, 1971, for similar findings with shock punishment).
Given the problems observed with reversals of the histamine-injection lever in Podlesnik et al. (2010), we conducted the present study to develop a general set of methods to assess rapid changes in the aversiveness of intravenous drug injections without the need to reverse the injection lever. In the present study, we assessed the same dose range as in Podlesnik et al. but the manipulated histamine dose was contingent on responding on the same lever throughout the experiment. Furthermore, we examined the effects of changing the probability of histamine injections or sucrose-pellet presentations to enhance the sensitivity of responding to changes in histamine dose. Thus, our general goal was to establish methods sensitive to revealing dose-dependent effects of aversive drugs. Such methods would provide a framework from which to assess pharmacological processes that serve to mediate aversive drug effects.
Experiment 1
The objective of Experiment 1 was to assess procedures that would reveal dose-dependent punishing effects of histamine. Ideally, high levels of responding would occur on the lever that produced an intravenous injection when injections were saline, and progressive declines in responding would occur selectively when injections were increasing doses of histamine. Such dose-dependent declines in responding with increasing dose would be taken to indicate increases in the aversive effects of histamine.
We arranged the same doses that produced dose-dependent effects with injection-lever reversals in Podlesnik et al. (2010). In that study, the 0.1 mg/kg/inj dose produced levels of responding on the sucrose-plus-injection lever that were similar to injections of saline. The 0.32 mg/kg/inj dose produced moderate suppression, and the 1 mg/kg/inj dose produced almost complete suppression of responding on the sucrose-plus-injection lever. In the present experiment, we first arranged contingent injections of the 1 mg/kg/inj histamine dose on one lever followed by the manipulation of the injections among saline and a range of histamine doses across successive sessions.
We examined three different procedures to develop methods that might be more sensitive to the described range of histamine doses (0.1–1 mg/kg/inj). In one group of rats, we arranged the same general procedure used by Podlesnik et al. (2010). A response on either concurrently available lever produced a sucrose pellet; responses on one of the levers also resulted in an intravenous injection. We refer to this group as the equal-reinforcement group. We predicted that responding on the sucrose-plus-injection lever would remain suppressed at all histamine doses due to the association between the sucrose-plus-injection lever and the initial exposure to the 1 mg/kg/inj histamine dose.
The next group was identical to the equal-reinforcement group, except that the probability of an injection when responses were made on the sucrose-plus-injection lever was set at .5 per response. We refer to this group as the reduced-punishment group. The rationale for decreasing the probability of injections was that others have found that the suppressive effects of shock punishment decrease as the probability of shock decreases (Azrin & Holz, 1966; Simon, Gilbert, Mayse, Bizon, & Setlow, 2009). Therefore, changes in histamine dose might result in a broad range of responding on the sucrose-plus-injection lever, which is necessary for detecting differences in the aversiveness of histamine as a function of dose.
The final group was identical to the equal-reinforcement group with the exception that the probability of a sucrose presentation on the sucrose-alone lever was set at .5 per response. We refer to this group as the differential-reinforcement group. The lower probability of sucrose on the sucrose-alone lever was arranged to provide an incentive for rats to respond more on the sucrose-plus-injection lever in the absence of punishment, given that twice the amount of sucrose could be obtained for responding on the sucrose-plus-injection lever. We predict that providing greater incentive to respond on the injection lever will produce maximal responding with saline injections with progressive decreases with increases in histamine dose.
Method
Subjects
Sixteen male Sprague-Dawley rats obtained from Harlan (Indianapolis, IN), weighing approximately 300 g before experimentation, were maintained in a temperature- and humidity-controlled environment on a 12-hr light/dark cycle with lights on at 7:00 a.m. Rats were housed in groups of two to three prior to intravenous catheterization and housed individually thereafter. Food was restricted to keep rats at 85% of free-feeding weights, with approximately 20 g of postsession supplemental feeding. Water was provided freely in the home cage throughout the experiment. All studies were carried out in accordance with the Guide for Care and Use of Laboratory Animals as adopted by the National Institutes of Health. University of Michigan’s Committee on the Use and Care of Animals approved all experimental protocols.
Apparatus
Six Med Associates® (St. Albans, VT, USA) operant-conditioning chambers (Model # ENV-007CT) were used. Each chamber was approximately 30 cm long, 24 cm wide, and 21 cm high, and housed in a sound-attenuating cubicle. The front panel of each chamber was equipped with two retractable response levers 6.8 cm above the grid floor and 1.3 cm from the side walls with a horizontal array of red, yellow, and green LEDs 4 cm above each lever. A 28-V DC houselight was located at the top center of the opposite panel 3 cm from the ceiling. Between the two levers was a pellet receptacle with its bottom edge 2 cm above a grid floor in which 45-mg sucrose pellets (Bio-Serv, Frenchtown, NJ) could be presented. Injections were delivered with no accompanying auditory stimulus, through iv catheters attached to a tether joined by a swivel to a syringe pump (MED Associates, St. Albans, VT) located outside of the sound attenuating cubicle. A chamber ventilation fan masked extraneous noise. Injection duration was based on weight (kg) of each rat divided by drug-delivery pump flow rate (0.072 ml per s), yielding an injection of 4.16 s for a 300-g rat. Swivels were held in place by a counterbalanced arm (MED Associates). Control of experimental events and data recording were conducted with Med Associates interfacing and programming with a time resolution of 10 ms (Version IV).
Surgery
All rats were surgically implanted with chronic indwelling catheters. Rats were anesthetized with ketamine (100 mg/kg i.m.) and xylazine (10 mg/kg i.m.) before a longitudinal incision was made to expose the femoral vein into which a catheter made of Micro-Renathane tubing (Braintree Scientific, Inc., Braintree, MA) was inserted. This tubing was connected to a metal cannula attached to a mesh backplate that was secured subcutaneously. The metal cannula exited the skin and connected to a plastic cap. Catheters were flushed daily with saline before and after each session to maintain patency.
Procedure
Initial training was identical to that used by Podlesnik et al. (2010). While housed in groups of three, we provided rats with approximately 50 g of sucrose pellets in the home cages to familiarize the rats with the pellets. On a following day, we placed the rats in the operant chamber with the houselight on. Pellets were delivered response independently, according to a random-time (RT) 60-s schedule. The houselight was turned off for 0.25-s during all pellet presentation throughout all conditions. During the next two sessions, we trained the rats to respond on the levers using a modified autoshaping procedure (see Brown & Jenkins, 1968; Peterson, Ackil, Frommer, & Hearst, 1972) with an FR 1 reinforcement schedule. All trials began by randomly extending one lever into the chamber and illuminating the LEDs over that lever. If the rat pressed the lever within 8 s, the lever retracted, LEDs turned off, and a pellet was presented. If the rat did not press the lever within 8 s from trial onset, the lever then retracted, LEDs turned off, and a pellet was presented. Intertrial intervals (ITIs) were 60 s in duration (time between end of sucrose-pellet presentation and lever extension). All training sessions were conducted for 1 hr.
In the final two training sessions, we presented the rats with a choice between two levers. A single response on either lever produced one sucrose pellet (i.e., concurrent FR 1 FR 1 schedule of reinforcement). Trials began with both levers extended into the chamber. A response to either lever produced a food pellet, turned off the houselight for 0.25 s, turned off the LEDs, and retracted the levers. If no response occurred within 60 s, both levers were retracted and LEDs turned off. The next trial was initiated 5 s after the levers were retracted on the previous trial (i.e., 5-s ITI). Sessions continued for 1 hr or until the rat completed 100 trials (including two forced trials, described next).
Two forced trials preceded all training and regular sessions. These trials presented the outcomes that were available throughout the session (i.e., sucrose pellet or sucrose pellet plus injection). The only difference from the stimuli and timing described for a standard trial is that only one lever at a time was extended into the chamber, and only one stimulus light was illuminated.
Following the first choice-training session, during which all animals responded, rats were surgically catheterized, given 1 week to recover, and then given one final choice-training session to determine allocation of performance between the two levers prior to introducing histamine. If more responses were made on one lever than the other, that lever was selected as the one on which intravenous injections were subsequently contingent. Following this, each session involved a choice between a lever that produced sucrose pellets (sucrose-alone lever) and a lever that produced sucrose pellets and an intravenous injection (sucrose-plus-injection lever). Rats were weighed and catheters were flushed with saline prior to all sessions. For all rats, histamine injections (1 mg/kg/inj iv), in addition to sucrose pellets, were made contingent on responding on one lever for three consecutive sessions. Thereafter, the content of the injections were manipulated across daily sessions among saline and doses of histamine (0.1–1 mg/kg/inj), as shown in Table 1.
Table 1.
Response-dependent injections of histamine (mg/kg/inj) and saline presented across successive sessions in Experiment 1
| Session | Condition |
|---|---|
| 1 | 1.00 histamine |
| 2 | 1.00 histamine |
| 3 | 1.00 histamine |
| 4 | saline |
| 5 | 1.00 histamine |
| 6 | saline |
| 7 | 0.32 histamine |
| 8 | saline |
| 9 | 0.10 histamine |
| 10 | 1.00 histamine |
The rats (n = 5) in the equal-reinforcement group experienced the same general procedure as arranged in Podlesnik et al. (2010). Sucrose pellets were presented for a press on either lever with p = 1 and injections were arranged with p = 1 on one lever.
For the rats (n = 5) in the reduced-punishment group, injections were presented following a response with p = 1 for the first session after choice training and with p = .5 for the remainder of the experiment. Specifically, presence versus absence of injections was selected from a list with two out of every four presses delivering an injection. During forced trials, a response on the sucrose-plus-injection lever always produced an injection.
For the rats (n = 6) in the differential-reinforcement group, after the final choice-training session both histamine injections and sucrose were presented contingent on a response to the sucrose-plus-injection lever with p = 1. On the sucrose-alone lever, sucrose pellets were presented following a response with p = .5 (i.e., two out of four responses). During the forced trials, a pellet was always presented for a response on the sucrose-alone lever.
Drugs
Histamine hydrochloride (Sigma Chemical Co., St. Louis, MO) was dissolved in 0.9% saline solution and expressed as weight of the salt.
Dependent Measures
We recorded the number of responses on sucrose-plus-injection and sucrose-alone levers out of the 98 choice trials per session for all conditions. From these data, we calculated the percentage of responses on the sucrose-plus-injection lever relative to responses on both the sucrose-plus-injection and sucrose-alone levers. We also calculated the number of trials completed within every session by summing the number of responses across both levers. Finally, we calculated the percentage of responses on the sucrose-plus-injection and sucrose-alone levers relative to the mean number of responses on those levers during saline control conditions.
Results
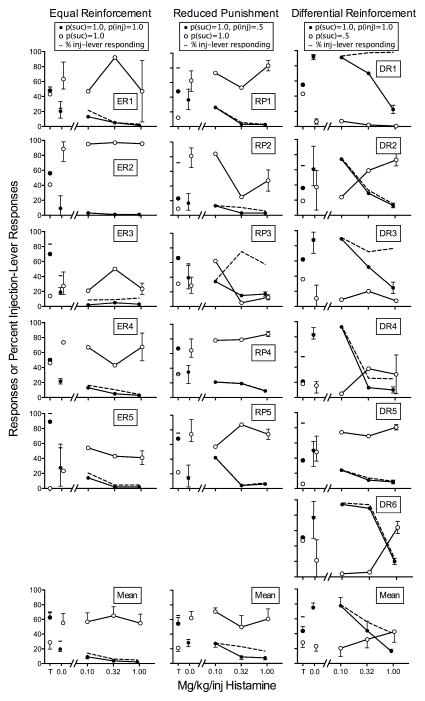
Figure 1 shows responding on the sucrose-plus-injection and sucrose-alone levers during the final choice-training session (T) and as a function of the daily manipulations of saline (0.0) and histamine dose. Note that injections were introduced contingent on the lever with more responses during the final choice-training session, during which no contingent injections were arranged. Data are shown for the equal-reinforcement (ER; left column), reduced-punishment (RP; center column), and differential-reinforcement (DR; right column) rats. All rats responded during the final choice-training session; ER5 responded exclusively on one lever.
Fig. 1.
Responses on the sucrose- (suc) plus-injection (inj) (filled circles) and sucrose-alone (open circles) levers during the final choice-training session (T), and as a function of saline (0.0) and histamine dose for the equal-reinforcement (left column), reduced-punishment (middle column), and differential-reinforcement (right column) rats in Experiment 1. Dashes and dashed lines indicate percent sucrose-plus-injection-lever responding. Error bars are ranges for individual subjects and SEM for means.
In the left column of Figure 1, responding on the sucrose-plus-injection lever decreased from a mean of 69% of all responses during the final choice-training session (T) to a mean of 30% during saline injections (0.0) for the equal-reinforcement rats (see dashes). This decrease with the saline control injections likely was a function of the conditioned punishment from histamine injections administered during the preceding sessions. Histamine injections of 0.1, 0.32, and 1 mg/kg/inj decreased responding on the sucrose-plus-injection lever to 14%, 6%, and 5% of responses on both levers, respectively, averaged across rats. This dose-dependent decrease in responding on the sucrose-plus-injection lever occurred in four of five rats (exception was ER3). Responding on the sucrose-alone lever was not systematically influenced by histamine dose.
In the center column of Figure 1, responding on the sucrose-plus-injection lever decreased from a mean of 66% of all responses during the final choice-training session (T) to a mean of 33% during saline injections (0.0) for the reduced-punishment rats (see dashes). As with the equal-reinforcement rats, decreases with saline control injections likely resulted from the conditioned punishment on prior histamine injections. Contingent histamine injections of 0.1, 0.32, and 1 mg/kg/inj decreased responding on the sucrose-plus-injection lever to 28%, 23%, and 17% of all responses, respectively, averaged across rats. The exception from this pattern was RP3 who responded more on the sucrose-plus-injection lever than the sucrose-only lever at higher histamine doses. For all rats, however, the dose-dependent decrease in responding on the sucrose-plus-injection lever was observed. As with the equal-reinforcement rats, responding on the sucrose-alone lever was not systematically influenced by histamine dose.
In contrast to the other groups of rats, responding on the sucrose-plus-injection lever increased from a mean of 63% of all responses during the final choice-training session (T) to a mean of 76% during saline injections (0.0—see dashes in the right column of Fig. 1) in the differential-reinforcement rats. This finding suggests that the conditioned punishment from prior histamine injections most likely was overcome by the greater probability of reinforcement for sucrose-plus-injection responding. The dashed lines in Figure 1 show that the effects of histamine dose on percentage of responses on the sucrose-plus-injection lever differed across rats. The effects ranged from almost exclusive responding on the sucrose-plus-injection lever (DR1, DR3), reversals in responding with histamine dose (DR2, DR4, DR6), and consistently more responding on the sucrose-alone lever (DR5). Nevertheless, 0.1, 0.32, and 1 mg/kg/inj histamine decreased responding on the sucrose-plus-injection lever to 79%, 56%, and 41% of all responses, respectively, across rats. The clear exception to the decreasing pattern of relative responding was DR1. Overall, histamine dose dependently decreased responding on the sucrose-plus-injection lever, but did not systematically affect responding on the sucrose-alone lever.
We defined a selective punishing effect as a decrease in punished responding, with a complementary increase in nonpunished responding. That is, we expected to observe complementary increases in responding on the sucrose-alone lever as sucrose-plus-injection lever responding decreased with increasing histamine dose. We observed this pattern clearly in some cases (e.g., ER2, RP4, DR2 in Fig. 1), but we more often did not observe clear increases in sucrose-alone responding with decreases in sucrose-plus-injection responding. Specifically, rats often did not complete trials under most conditions. Did histamine injections function to generally suppress all behavior?
To assess whether histamine suppressed behavior, Table 2 shows the number of trials completed out of 98 possible trials per session. Overall, only one rat (RP5) across the three groups completed all 98 trials in all conditions. In fact, many rats did not complete all trials during choice training (T), which most likely was a result of it being the rats’ first exposure to moving about the chamber with an implanted catheter. This interpretation is supported by the finding that rats that completed 60 or fewer trials in the choice-training session, completed 98 trials during later saline control sessions. During histamine injections, the number of trials completed decreased with increasing histamine dose in all groups. These decreases in total number of trials completed with increases in histamine dose suggest that histamine injections might have had general suppressive effects in all groups. Nevertheless, there was overlap in the range of trials completed among all groups, suggesting no clear systematic differences with regard to the number of trials completed as a function of group.
Table 2.
Mean number of trials completed by individual rats across all conditions in Experiments (Expt) 1 and 2.
| Expt | Group | Condition | Rat number
|
|||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| 1 | ER | T | 91 | 97 | 84 | 96 | 89 | -- |
| saline | 84(71–97) | 98(98–98) | 46(36–62) | 95(90–98) | 51(27–81) | -- | ||
| 0.10 hist | 60 | 98 | 23 | 80 | 68 | -- | ||
| 0.32 hist | 97 | 98 | 55 | 48 | 45 | -- | ||
| 1.00 hist | 7 | 96 | 33 | 90 | 51 | -- | ||
| RP | T | 60 | 32 | 97 | 98 | 90 | -- | |
| saline | 98(98–98) | 98(95–98) | 68(42–83) | 98(98–98) | 88(72–96) | -- | ||
| 0.10 hist | 98 | 97 | 96 | 98 | 98 | -- | ||
| 0.32 hist | 56 | 28 | 20 | 98 | 91 | -- | ||
| 1.00 hist | 93 | 37 | 30 | 98 | 85 | -- | ||
| DR | T | 98 | 55 | 98 | 39 | 43 | 98 | |
| saline | 98(98–98) | 98(98–98) | 98(98–98) | 98(98–98) | 98(98–98) | 98(98–98) | ||
| 0.10 hist | 98 | 98 | 98 | 98 | 98 | 98 | ||
| 0.32 hist | 72 | 88 | 72 | 51 | 80 | 95 | ||
| 1.00 hist | 28 | 91 | 23 | 62 | 90 | 80 | ||
| 2 | Pyrilamine (DR) | T | 98 | 77 | 96 | 98 | 98 | -- |
| sal + sal | 98(98–98) | 98(95–98) | 98(97–98) | 98(98–98) | 98(98–98) | -- | ||
| hist + sal | 92(92–92) | 87(80–93) | 38(16–60) | 90(87–93) | 93(91–95) | -- | ||
| hist + 0.10 | 87 | 79 | 98 | 76 | 86 | -- | ||
| hist + 0.32 | 89 | 81 | 79 | 78 | 98 | -- | ||
| hist + 1.00 | 97 | 66 | 73 | 78 | 97 | -- | ||
| hist + 3.20 | 94 | 87 | 93 | 87 | 92 | -- | ||
| Ketotifen (DR) | T | 97 | 86 | 21 | 87 | 36 | -- | |
| sal + sal | 96(90–98) | 82(50–98) | 48(18–98) | 91(64–98) | 79(20–98) | -- | ||
| hist + sal | 15(9–21) | 24(10–38) | 11(10–12) | 37(18–56) | 23(2–43) | -- | ||
| hist + 0.01 | 37 | 80 | 39 | 40 | 43 | -- | ||
| hist + 0.10 | 55 | 60 | 38 | 82 | 68 | -- | ||
| hist + 1.00 | 49 | 11 | 18 | 25 | 17 | -- | ||
| Ranitidine (DR) | T | 98 | 96 | 98 | 83 | 71 | -- | |
| sal + sal | 98(98–98) | 98(98–98) | 98(98–98) | 97(97–98) | 98(98–98) | -- | ||
| hist + sal | 90(85–96) | 38(29–45) | 27(19–41) | 37(13–82) | 50(42–61) | -- | ||
| hist + 3.2 | 86 | 42 | 35 | 6 | 63 | -- | ||
| hist + 5.6 | 96 | 42 | 27 | 36 | 70 | -- | ||
| Pyrilamine (ER) | T | 94 | 98 | 98 | 98 | 91 | -- | |
| sal + sal | 98(98–98) | 97(94–98) | 98(98–98) | 98(98–98) | 98(98–98) | -- | ||
| hist + sal | 78(66–89) | 72(62–81) | 51(26–75) | 92(91–92) | 77(69–85) | -- | ||
| hist + 0.10 | 90 | 92 | 60 | 98 | 81 | -- | ||
| hist + 0.32 | 94 | 94 | 98 | 98 | 96 | -- | ||
| hist + 1.00 | 98 | 89 | 74 | 98 | 77 | -- | ||
| hist + 3.20 | 94 | 95 | 92 | 98 | 95 | -- | ||
| Ketotifen (ER) | T | 94 | 95 | 66 | 97 | 53 | -- | |
| sal + sal | 98(98–98) | 98(98–98) | 96(88–98) | 98(98–98) | 87(70–98) | -- | ||
| hist + sal | 72(46–98) | 74(66–82) | 74(60–87) | 68(62–73) | 57(29–84) | -- | ||
| hist + 0.01 | 98 | 67 | 61 | 28 | 85 | -- | ||
| hist + 0.10 | 75 | 73 | 93 | 68 | 83 | -- | ||
| hist + 1.00 | 46 | 82 | 60 | 73 | 29 | -- | ||
Note. Ranges are indicated in parentheses.
Decreases in number of trials completed might suggest a general suppression of behavior; however, a general suppression should be accompanied by decreases in responding on both the sucrose-plus-injection lever and the sucrose-alone lever. Inconsistent with general suppression, Figure 1 revealed a dose-dependent decrease in responding on the sucrose-plus-injection lever, but no systematic dose-related change in responding on the sucrose-alone lever. To better isolate the effects of histamine injections on responding, we examined responding on both levers individually during histamine injections as a function of the percentage of responding on that lever when saline was administered.
Figure 2 shows the means of rats’ responding on both levers across doses of histamine relative to responding during saline injections for all three groups. Appendix 1 shows individual-rat data. Overall responding on the sucrose-plus-injection lever decreased more for the equal-reinforcement rats than for the other groups. Mean percent responding for the equal-reinforcement rats was 43%, 18%, and 11% across the three doses. Overall levels of mean percent of saline responding across the three histamine doses between the reduced-punishment rats (119%, 30%, and 27%) and the differential-reinforcement rats (101%, 56%, and 22%) did not differ substantially. Conversely, we observed no systematic effect of histamine dose on responding to the sucrose-alone lever relative to saline controls in any group. An increasing trend across the three histamine doses appears for the differential-reinforcement rats (77%, 132%, 156%), but the trend was observed only in two of six rats (DR2, DR6), as can be seen in Appendix 1.
Fig. 2.
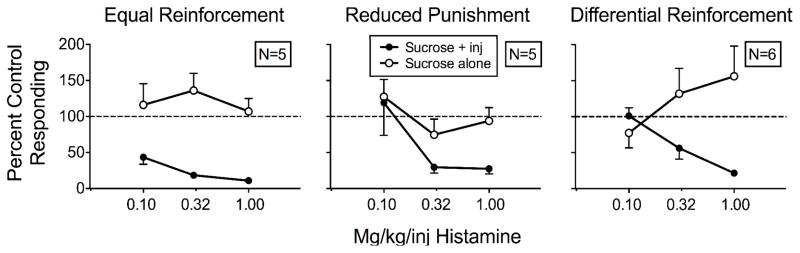
Mean percent control responding (contingent saline injections) for the sucrose-plus-injection (inj) lever and sucrose-alone lever as a function of histamine dose for the equal-reinforcement (left), reduced-punishment (middle), and differential-reinforcement (right) rats in Experiment 1.
Appendix 1.
Percent control responding for individual rats in Experiments 1 and 2
| Experiment | Group | Condition | Rat number
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||||||||
|
| ||||||||||||||
| Suc + inj | Suc alone | Suc + inj | Suc alone | Suc + inj | Suc alone | Suc + inj | Suc alone | Suc + inj | Suc alone | Suc + inj | Suc alone | |||
| 1 | ER | 0.10 hist | 64 | 74 | 32 | 107 | 11 | 77 | 60 | 91 | 51 | 231 | -- | -- |
| 0.32 hist | 25 | 145 | 11 | 109 | 26 | 183 | 23 | 58 | 7 | 184 | -- | -- | ||
| 1.00 hist | 7 | 74 | 11 | 108 | 16 | 86 | 14 | 92 | 7 | 176 | -- | -- | ||
| RP | 0.10 hist | 72 | 116 | 78 | 104 | 86 | 219 | 61 | 121 | 300 | 77 | -- | -- | |
| 0.32 hist | 8 | 85 | 18 | 31 | 38 | 18 | 55 | 123 | 29 | 118 | -- | -- | ||
| 1.00 hist | 7 | 132 | 18 | 59 | 43 | 44 | 26 | 134 | 43 | 100 | -- | -- | ||
| DR | 0.10 hist | 99 | 111 | 122 | 64 | 102 | 84 | 113 | 32 | 48 | 154 | 123 | 19 | |
| 0.32 hist | 76 | 32 | 48 | 158 | 60 | 188 | 16 | 243 | 22 | 144 | 116 | 28 | ||
| 1.00 hist | 25 | 8 | 21 | 196 | 28 | 70 | 12 | 195 | 17 | 168 | 26 | 300 | ||
| 2 | Pyr (PD) | hist + 0.10 | 106 | 92 | 133 | 86 | 47 | 1050 | 467 | 50 | 49 | 108 | -- | -- |
| hist + 0.32 | 222 | 66 | 181 | 82 | 93 | 638 | 947 | 8 | 78 | 115 | -- | -- | ||
| hist + 1.00 | 311 | 55 | 362 | 37 | 187 | 213 | 907 | 12 | 216 | 64 | -- | -- | ||
| hist + 3.20 | 289 | 57 | 210 | 86 | 97 | 800 | 387 | 70 | 363 | 4 | -- | -- | ||
| Keto (KD) | hist + 0.01 | 492 | 59 | 257 | 393 | 433 | 0 | 123 | 81 | 195 | 133 | -- | -- | |
| hist + 0.10 | 738 | 82 | 457 | 89 | 289 | 600 | 289 | 104 | 229 | 1333 | -- | -- | ||
| hist + 1.00 | 508 | 188 | 86 | 15 | 111 | 400 | 85 | 37 | 57 | 333 | -- | -- | ||
| Ran (RD) | hist + 3.2 | 177 | 87 | 148 | 39 | 126 | 92 | 229 | 75 | 9 | 120 | -- | -- | |
| hist + 5.6 | 55 | 63 | 115 | 11 | 151 | 109 | 371 | 55 | 122 | 0 | -- | -- | ||
| Pyr (PE) | hist + 0.10 | 160 | 115 | 67 | 133 | 29 | 133 | 0 | 109 | 0 | 107 | -- | -- | |
| hist + 0.32 | 160 | 120 | 44 | 137 | 0 | 225 | 250 | 104 | 0 | 126 | -- | -- | ||
| hist + 1.00 | 160 | 125 | 267 | 115 | 386 | 108 | 400 | 101 | 400 | 96 | -- | -- | ||
| hist + 3.20 | 80 | 123 | 44 | 139 | 57 | 202 | 150 | 106 | 0 | 125 | -- | -- | ||
| Keto (KE) | hist + 0.01 | 80 | 140 | 109 | 89 | 240 | 77 | 80 | 38 | 0 | 157 | -- | -- | |
| hist + 0.10 | 220 | 96 | 309 | 82 | 280 | 121 | 240 | 90 | 80 | 150 | -- | -- | ||
| hist + 1.00 | 140 | 58 | 73 | 114 | 160 | 79 | 100 | 109 | 120 | 48 | -- | -- | ||
These findings suggest histamine injections had at least two effects. First, histamine injections likely had some suppressive effects on all behavior evidenced by decreases in trials completed and the absence of complementary increases in responding on the sucrose-alone lever with decreases in responding to the sucrose-plus-injection lever. Second, histamine injections differentially punished responding on the sucrose-plus-injection lever as revealed by the more reliable decreases on the sucrose-plus-injection lever and no systematic change on the sucrose-alone lever. Moreover, the procedures used with the differential-reinforcement group produce more graded effects in responding with changes in histamine dose than the procedures used with the other groups. Only for the differential-reinforcement rats was percent of saline responding during the intermediate 0.32 mg/kg/inj histamine dose reliably between response levels obtained with the other doses.
Discussion
Histamine dose dependently decreased responding on the sucrose-plus-injection lever in all groups tested in the present experiment, supporting findings from Podlesnik et al. (2010) with the same range of histamine doses (0.1–1 mg/kg/inj). Podlesnik et al. obtained dose-dependent effects, however, only by maintaining a dosing condition for several sessions and reversing lever assignments. In the current experiment, dose-dependent punishing effects of histamine occurred with manipulations of histamine dose across daily experimental sessions. Thus, the present methods allow the assessment of dose-dependent effects of aversive drugs more rapidly than the procedures developed by Podlesnik et al.
Moreover, the present experiment showed that the punishing effects of histamine are dependent on the arranged reinforcement and punishment contingencies, shown previously only with monkeys (Negus, 2005). When sucrose was presented with p = .5 on the sucrose-alone lever (i.e., differential-reinforcement group), relatively high levels of responding occurred on the sucrose-plus-injection lever across saline controls and all histamine doses. This is in contrast to the relatively low levels of responding on the sucrose-plus-injection lever observed across saline and all histamine doses in the equal-reinforcement and reduced-punishment groups. Thus, providing a greater incentive to respond on the sucrose-plus-injection lever resulted in greater levels of responding allocated to the lever arranging punishment, consistent with the findings of Negus (see also Simon et al., 2009).
When assessing the effects of histamine dose relative to saline controls, histamine differentially decreased responding on the sucrose-plus-injection lever, but did not systematically affect responding on the sucrose-alone lever. These findings suggest that histamine injections differentially decreased responding to the punished lever rather than solely decreasing all behavior occurring in the experimental context, consistent with the findings of Goldberg (1980). We did not, however, observe a selective punishing effect of histamine as responding did not increase on the sucrose-alone lever as responding decreased on the sucrose-plus-injection lever. Instead, the number of trials completed decreased as histamine dose increased in all three groups. Therefore, contingent histamine injections differentially punished responding on the sucrose-plus-injection lever, but with some general suppressive effects on all behavior that did not appear to differ among the groups.
Finally, we observed that decreases in responding on the sucrose-plus-injection lever were most systematically influenced by histamine dose for the differential-reinforcement rats. These effects were most clearly demonstrated by responding on the sucrose-plus-injection lever relative to saline control. In all rats, responding to the middle, 0.32 mg/kg/inj histamine dose fell between levels of responding with the higher and lower doses. These more systematic effects of histamine dose on the sucrose-plus-injection lever likely come from the greater range of absolute levels of responding on that lever compared to the other groups. Specifically, a greater range of absolute levels of responding allows better separation of the effects of different histamine doses from all other sources of variability in responding. These large, dose-dependent effects of histamine dose suggest that the procedure developed with the differential-reinforcement rats could provide a sensitive tool for testing a wide range of behavioral and pharmacological manipulations that might influence the punishing effects of drugs. One question relevant to behavioral pharmacologists is whether we can reveal the receptor types mediating aversive drug effects with these procedures. In the next experiment, we used the procedures developed in the present experiment to study some potential receptor types mediating the punishing effects of histamine in rats.
Experiment 2
Experiment 1 revealed a range of punishing effects of histamine that were dependent on the reinforcement and punishment contingencies. Responding on the sucrose-plus-injection lever decreased with increases in histamine dose relative to saline controls in all three groups. However, arranging a higher probability of sucrose for responding on the punished lever resulted in overall higher levels of responding on that lever and more systematic effects of histamine dose when compared to the other groups. Thus, arranging greater incentive to respond on the punished alternative with the differential-reinforcement procedure enhanced the likelihood of detecting changes in behavior as a function of histamine dose.
The systematic decreases in responding on the sucrose-plus-injection lever with increasing histamine dose suggest that dose mediated the magnitude of histamine’s effects via some population of endogenous receptors. By logical extension, these methods also could be used to assess how the effects of histamine are mediated pharmacologically. Systemic injections of selective histamine-receptor antagonists that decrease the punishing effects of histamine should produce a pattern of behavior analogous to decreasing histamine dose. Specifically, as with decreasing histamine dose, pharmacologically antagonizing histamine’s effects also should result in increases in responding to the sucrose-plus-injection lever.
Goldberg (1980) found that histamine H1, but not H2 receptors mediated the punishing effects of histamine in squirrel monkeys. Using the equal-reinforcement and differential-reinforcement procedures developed in Experiment 1, we assessed whether histamine H1 and/or H2 receptors also mediated the punishing effects of histamine in rats. We investigated the effects of presession injections of the selective histamine H1-receptor antagonists, pyrilamine and ketotifen, and the selective histamine H2-receptor antagonist, ranitidine (see Hough, 2001; Martin & Romer, 1978). Revealing antagonism of histamine’s punishing effects using the present methods would suggest the usefulness of these methods generally for assessing the receptor systems mediating punishing drug effects.
In addition to showing that histamine H1 receptors mediate the punishing effects of histamine in squirrel monkeys (see also Katz & Goldberg, 1986), Goldberg (1980) showed that H2 receptors primarily mediate the cardiovascular effects of intravenous histamine (i.e., histamine-mediated increases in heart rate and decreases in blood pressure). These findings indicate that the punishing effects of histamine are not a function of cardiovascular changes produced by histamine. Podlesnik et al. (2010) made similar conclusions based on the finding that cardiovascular changes were observed at doses lower than those producing punishing effects. However, the dose of histamine that produces punishing effects of histamine can be altered by the procedure used, as observed in Experiment 1. Therefore, assessing the receptors mediating the punishing and cardiovascular effects provides a more thorough test for revealing whether these effects of histamine are independent in rats.
Method
Behavioral Study
Subjects and apparatus
Twenty-five male Harlan rats were obtained, housed, and catheterized identically as those described for Experiment 1. Each group consisted of five rats. The groups were pyrilamine (equal reinforcement and differential reinforcement), ketotifen (equal reinforcement and differential reinforcement) and ranitidine (differential reinforcement only). Because we observed that ranitidine was not effective with the differential-reinforcement procedure, which was more sensitive to histamine dose in Experiment 1, ranitidine was not assessed with the equal-reinforcement procedure.
Procedure
Initial training was identical to that described for Experiment 1. Following intravenous catheterization, rats were provided with a choice-training session. Thereafter, histamine injections were contingent on responses on the lever that maintained more responses during the final choice-training session. The effects of histamine receptor antagonists were assessed under two procedures examined in Experiment 1: equal-reinforcement and differential-reinforcement. For rats in the equal-reinforcement groups, responses on the sucrose-alone lever produced sucrose with p = 1 and responses on the sucrose-plus-injection lever produced sucrose and an injection with p = 1. For the rats in the differential-reinforcement groups, responses on the sucrose-alone lever produced sucrose with p = .5 and responses on the sucrose-plus-injection lever produced sucrose and an injection with p = 1. Either 1 mg/kg/inj histamine or saline was presented contingent on responses on the sucrose-plus-injection lever.
Five min before the beginning of each experimental session, intravenous pretreatments of saline or a selective histamine-receptor antagonist were administered immediately prior to receiving a saline flush. Table 3 shows the presession (antagonists) and within-session (histamine or saline) injections for all groups.
Table 3.
Injections of saline or antagonists pyrilamine (pyr), ketotifen (keto), and ranitidine (ran) in mg/kg administered before sessions (presession) and response-dependent injections during sessions (during) across successive sessions in Experiment 2.
| Session | Pyrilamine (pyr)
|
Ketotifen (keto)
|
Ranitidine (ran)
|
|||
|---|---|---|---|---|---|---|
| Presession | During | Presession | During | Presession | During | |
| 1 | saline | histamine | saline | histamine | saline | histamine |
| 2 | saline | histamine | saline | histamine | saline | histamine |
| 3 | saline | histamine | saline | histamine | saline | histamine |
| 4 | saline | saline | saline | saline | saline | saline |
| 5 | saline | histamine | saline | histamine | saline | histamine |
| 6 | saline | saline | saline | saline | saline | saline |
| 7 | 1.00 pyr | histamine | 1.00 keto | histamine | 3.20 ran | histamine |
| 8 | saline | histamine | saline | saline | saline | saline |
| 9 | saline | saline | 0.10 keto | histamine | 3.20 ran | histamine |
| 10 | 3.20 pyr | histamine | saline | saline | saline | histamine |
| 11 | saline | saline | saline | histamine | 5.60 ran | histamine |
| 12 | 0.32 pyr | histamine | saline | saline | saline | saline |
| 13 | saline | saline | 0.01 keto | histamine | 5.60 ran | histamine |
| 14 | 0.10 pyr | histamine | -- | -- | -- | -- |
| 15 | saline | saline | -- | -- | -- | -- |
Cardiovascular Study
Subjects and apparatus
Rats were obtained and housed identically as those described in Experiment 1. Implantable telemetry devices (PA-C40) and receivers (RPC-1) were used for recording heart rate and blood pressure (Data Sciences International, St. Paul, MN). Data recording was conducted with DataQuestART (Data Sciences International, St. Paul, MN) with a 10-s resolution.
Surgery
Four rats were used to study all drugs and drug interactions. They were anesthetized with ketamine (100 mg/kg i.m.) and xylazine (10 mg/kg i.m.). The femoral vein and artery were exposed with a longitudinal incision. A catheter of Micro-Renathane tubing (Braintree Scientific, Inc., Braintree, MA) was inserted into the vein and the pressure sensor tip of the telemetry device (Data Sciences International, St. Paul, MN) was inserted into the artery. The telemetry device was placed in a subcutaneous pocket by the hind leg. The distal end of the intravenous catheter was run subcutaneously and exited through the back, where it was secured. The distal end of the catheter was covered with a metal obturator when not in use. Rats were given 1 week to recover from surgery, during which time catheters were flushed daily with saline.
Procedure
Rats were placed in a covered Plexiglas® cage (28 cm long × 19 cm wide × 20 cm high) with corncob bedding. The distal end of the catheter, which exited through the rat’s back, was connected to a tether that exited from the top of the cage. The end of the tether was connected to a 1-ml syringe used to inject drugs intravenously. For experimental sessions, telemetry devices were activated and cages were placed on top of the receivers; recording began when the cage was placed on the receiver. Approximately 30 min after data recording began, an injection of saline or antagonist was delivered. After an additional 20 min, saline or 1 mg/kg dose of histamine was injected. Injections were 1 ml/kg injected across 5 s and were immediately followed by a saline flush (0.5 ml).
Drugs
Histamine hydrochloride, pyrilamine maleate, ketotifen fumarate, and ranitidine hydrochloride (Sigma Chemical Co., St. Louis, MO) were dissolved in 0.9% saline solution and are expressed as weights of the salt.
Results
Behavioral Study
Differential-reinforcement groups
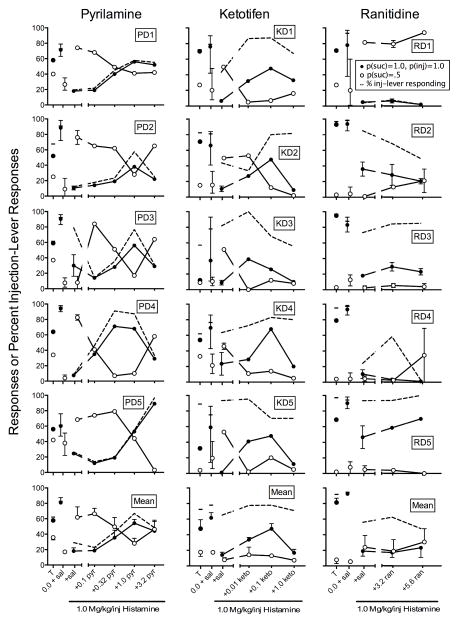
Figure 3 shows responding on the sucrose-plus-injection and sucrose-alone levers for the groups receiving the differential-reinforcement procedure during the final choice-training session (T), with intravenous saline injections plus saline pretreatment (0 + sal), and intravenous injections of 1 mg/kg/inj histamine with pretreatment of saline or histamine antagonist. All rats responded on both levers during the final choice-training session. Contingent injections were introduced to the lever with more responses during the final choice-training session. Rats received pretreatments of the histamine H1-receptor antagonists, pyrilamine or ketotifen (left and center columns) and the histamine H2-receptor antagonist, ranitidine (right column).
Fig. 3.
The y-axis shows responses on the sucrose (suc) plus injection (inj) (filled circles) and sucrose-alone (open circles) levers during the final choice-training session (T), and as a function of saline (sal) and pretreatment dose for the Pyrilamine (left column), Ketotifen (middle column), and Ranitidine (right column) rats with the differential-reinforcement procedure in Experiment 2. Dashes and dashed lines indicate percent sucrose-plus-injection-lever responding. Error bars are ranges for individual subjects and SEM for means.
In the left column of Figure 3, saline injections with saline pretreatment increased the Pyrilamine rats’ responding on the sucrose-plus-injection lever relative to all responses to a mean of 83% from 62% during the final choice-training session (T; see dashes). These findings are consistent with the differential-reinforcement rats in Experiment 1. Responding in the presence of saline injections with histamine pretreatment provided a reference from which to compare any changes in responding with contingent histamine injections in the presence of pyrilamine pretreatments (see also Goldberg, 1980). When contingent 1 mg/kg/inj histamine was administered after saline pretreatment, responding on the sucrose-plus-injection lever decreased relative to responding on the sucrose-alone lever in four out of five rats (M = 29%; PD3 was the exception). Increasing pyrilamine dose produced an overall bitonic function in all rats except PD5. That is, the percentage of responding on the sucrose-plus-injection lever remained unchanged with the 0.1 mg/kg pyrilamine dose (M = 23%), increased across the 0.32 (M = 43%) and 1 mg/kg doses (M = 67%), and decreased at the 3.2 mg/kg pyrilamine dose (M = 48%).
In the center column of Figure 3, relative responding increased in four out of five of the Ketotifen rats from the final choice training (M = 72%) to saline injections with saline pretreatment (M = 80%); the exception was KD2 (see dashes). With histamine injections and saline pretreatment, responding on both levers decreased, but relative responding between levers did not change systematically across rats (M = 65%). Compared to contingent histamine injections and saline pretreatment, pretreatments with ketotifen did not systematically change responding on the sucrose-plus-injection lever relative to saline-alone responding at any dose. Mean relative responding to the sucrose-plus-injection lever was 78% at the 0.01 mg/kg dose, 78% at the 0.1 mg/kg dose, and 71% at the 1 mg/kg dose. However, absolute responses increased only on the sucrose-plus-injection lever at the 0.01 and 0.1 mg/kg doses of ketotifen. These effects are discussed in detail below (see Figure 4).
Fig. 4.
Mean percent control responding (contingent histamine injections with saline pretreatment) for the sucrose-plus-injection (inj) lever and sucrose-alone lever as a function of antagonist dose for the Pyrilamine (left), Ketotifen (middle), and Ranitidine (right) rats with the differential-reinforcement procedure in Experiment 2. Error bars are SEM.
In the right column of Figure 3, relative responding increased in four out of five of the Ranitidine rats from the final choice training (M = 92%) to saline injections with saline pretreatment (M = 94%); the exception was RD3 (see dashes). With histamine injections and saline pretreatment, absolute numbers of responses decreased for all rats on the sucrose-plus-injection lever, but did not change on the sucrose-alone lever. This resulted in relative responding on the sucrose-plus-injection lever decreasing in four out of five rats (M = 56%); RD5 was the exception. Nevertheless, relative responding to the sucrose-plus-injection lever remained positive in four out of five rats; RD4 was the exception. We observed no systematic effect of ranitidine on relative responding at the 3.2 mg/kg dose (M = 62%) or the 5.6 mg/kg dose (M = 48%).
Table 2 shows the mean number of trials completed out of 98 possible trials per session across all dosing conditions for the three differential-reinforcement groups. In all three groups, trials completed during choice training (T) tended to be similar to or lower than the control conditions of contingent saline injections plus saline pretreatment, consistent with findings from the differential-reinforcement rats in Experiment 1.
Contingent saline injections with saline pretreatments resulted in a mean of 98 trials completed for the Pyrilamine group, 79 trials completed for the Ketotifen group, and 98 trials completed for the Ranitidine group. Of primary importance is that, relative to these control conditions of contingent saline injections plus saline pretreatment, introducing histamine injections produced relatively little change in trials completed for the Pyrilamine rats (decrease of 18 trials) compared to the large decreases for the Ketotifen (decrease of 57 trials) and Ranitidine rats (decrease of 49 trials). These differences among the groups largely continued throughout the administration of the antagonists. Trials completed across doses of pyrilamine did not change systematically from the numbers observed during the contingent saline plus saline pretreatment control conditions (see Table 2). Conversely, ketotifen pretreatments increased the number of trials completed from a mean of 22 trials completed with saline pretreatments to a mean of 48 trials at the 0.01 mg/kg dose, a mean of 61 trials at the 0.1 mg/kg dose, but a return to lower levels with a mean of 24 trials completed at the 1 mg/kg dose. Finally, trials completed with ranitidine pretreatments did not change systematically from the low levels observed with saline pretreatments (M = 48 trials), with the 3.2 mg/kg dose (M = 46 trials), or 5.6 mg/kg dose (M = 54 trials). These findings suggest that contingent histamine injections suppressed all responding for the Ketotifen and Ranitidine rats, but not for the Pyrilamine rats. Moreover, the H1 histamine-receptor antagonist, ketotifen, reduced histamine’s suppressive effects but H2 histamine-receptor antagonist, ranitidine, did not.
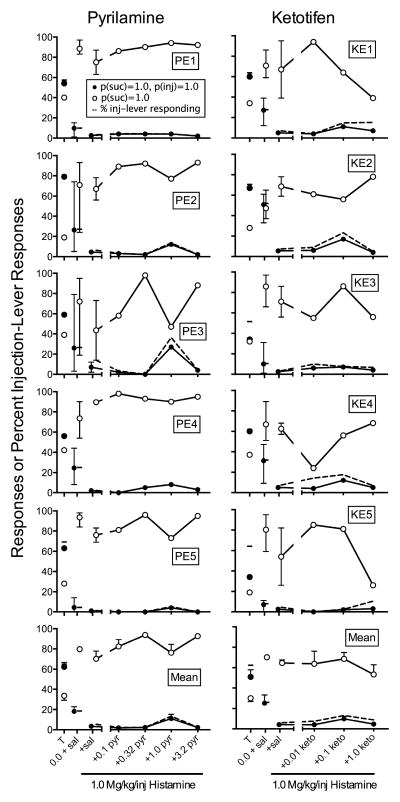
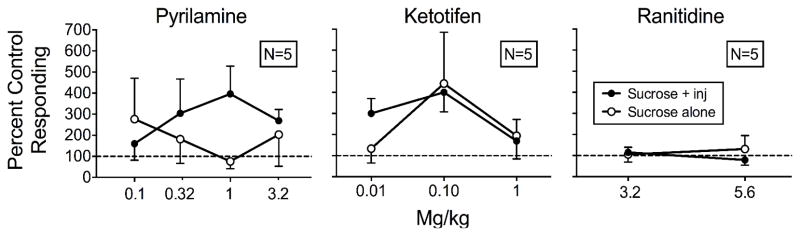
To assess the effects of the histamine antagonists relative to the appropriate control, Figure 4 shows the means of rats’ responding on both levers with contingent histamine injections plus antagonist pretreatments relative to responding during saline pretreatment and contingent histamine injections for all three groups. Appendix 1 shows individual-rat data. Antagonism of histamine’s punishing effects would produce increases in responding on the sucrose-plus-injection lever. Overall, responding on the sucrose-plus-injection lever increased with pyrilamine and ketotifen, but not with ranitidine.
For the Pyrilamine rats in the left panel of Figure 4, responding on the sucrose-plus-injection lever did not differ reliably from control levels with saline pretreatment and histamine injections at the 0.1 mg/kg pyrilamine dose (M = 160%). Responding increased at the 0.32 mg/kg (M = 304%), 1 mg/kg dose (M = 397%), and 3.2 mg/kg dose (M = 269%). Conversely, responding on the sucrose-alone lever followed an opposite pattern from sucrose-plus-injection responding. Note that mean values are inflated due to suppressed sucrose-alone responding at the saline control condition for PR3. From levels observed at the 0.1 mg/kg dose (M = 277%), responding decreased at the 0.32 mg/kg dose (M = 182%), the 1 mg/kg dose (M = 76%), but increased again at the 3.2 mg/kg dose (M = 203%).
For the Ketotifen rats in the center panel of Figure 4, sucrose-plus-injection responding consistently was greater than control levels with saline pretreatment and histamine injections at the 0.01 mg/kg (M = 300%) and 0.1 mg/kg (M = 400%) doses, but decreased toward saline levels at the 1 mg/kg dose (M = 169%). Responding on the sucrose-alone lever did not differ reliably from saline levels. Mean percent saline responding was consistently above control levels with 133% at the 0.01 mg/kg dose, 400% at the 0.1 mg/kg dose, and 195% at the 1 mg/kg dose. However, examining the individual-rat data in Appendix 1 shows no reliable effects of ketotifen dose.
For the Ranitidine rats in the right panel of Figure 4, sucrose-plus-injection responding did not change reliably from control levels with saline pretreatment and histamine injections at the 3.2 mg/kg (M = 138%) or 5.6 mg/kg (M = 163%) doses. Consistent with the individual-rat data in Appendix 1, mean percent responding on the sucrose-alone lever also did not differ reliably from saline controls at the 3.2 mg/kg (M = 83%) or 5.6 mg/kg (M = 43%) doses.
Equal-reinforcement groups
Figure 5 shows responding on the sucrose-plus-injection and sucrose-alone levers for the groups receiving the equal-reinforcement procedure during the final choice-training session (T), with intravenous saline injections plus saline pretreatment (0.0 + sal), and intravenous injections of 1 mg/kg/inj histamine with pretreatment of saline or histamine antagonist. All rats responded on both levers during the final choice-training session and contingent injections were introduced on the lever with more responses during the final choice-training session. Rats received pretreatments only of the histamine H1-receptor antagonists, pyrilamine or ketotifen, shown in the left and right columns, respectively.
Fig. 5.
The y-axis shows responses on the sucrose (suc) plus injection (inj) (filled circles) and sucrose-alone (open circles) levers during the final choice-training session (T), and as a function of saline (sal) and pretreatment dose for the Pyrilamine (left column) and Ketotifen (right column) rats with the equal-reinforcement procedure in Experiment 2. Dashes and dashed lines indicate percent sucrose-plus-injection-lever responding. Error bars are ranges for individual subjects and SEM for means.
Overall, we observed very similar patterns of responding across levers for both groups. Less responding was allocated to the sucrose-plus-injection lever than the sucrose-alone lever during conditions with contingent saline injections and saline pretreatments for both the Pyrilamine rats (M = 19%) and Ketotifen rats (M = 26%; exception was KE2; see dashes). These findings are consistent with the pattern of responding by the equal-reinforcement rats in Experiment 1 and are probably due to the history of exposure to contingent histamine injections. With contingent histamine injections plus saline pretreatment, responding consistently was lower on the sucrose-plus-injection lever relative to the sucrose-alone lever for the Pyrilamine rats (M = 5%) and Ketotifen rats (M = 6%), replicating findings from Experiment 1. This general pattern of greater responding on the sucrose-plus-injection lever relative to the sucrose-alone lever continued across all pretreatment doses of pyrilamine and ketotifen. We observed small increases in some rats’ responding to the sucrose-plus-injection lever relative to the sucrose-alone lever with the 1 mg/kg pyrilamine dose (M = 13%) and the 0.1 mg/kg ketotifen dose (M = 13%). However, these increases were not apparent at all for some rats (e.g., PE1, KE5).
Table 2 shows the mean number of trials completed out of 98 possible trials per session across all dosing conditions for the two equal-reinforcement groups. For both groups, trials completed during choice training (T) tended to be similar to or lower than the control conditions of contingent saline injections plus saline pretreatment. Contingent saline injections with saline pretreatments resulted in a mean of 98 trials completed for the Pyrilamine group and 95 trials completed for the Ketotifen group. Introducing contingent histamine injections decreased trials completed for both the Pyrilamine rats (M = 74 trials) and Ketotifen rats (M = 69 trials). As shown in Table 2, pyrilamine increased trials completed to a mean of 96 trials at the 0.32 mg/kg dose but no such systematic increase was observed across doses of ketotifen.
Figure 6 shows the means of individual rats’ responding on both levers with contingent histamine injections plus antagonist pretreatments relative to responding during saline pretreatment and contingent histamine injections for both groups. Appendix 1 shows individual-rat data. Relative to controls, responding on the sucrose-plus-injection lever increased only at the 1 mg/kg pyrilamine dose (322%) for the Pyrilamine rats and at the 0.1 mg/kg ketotifen dose (226%) for the Ketotifen rats. We observed no systematic changes in responding to the sucrose-alone lever for either group at any dose.
Fig. 6.
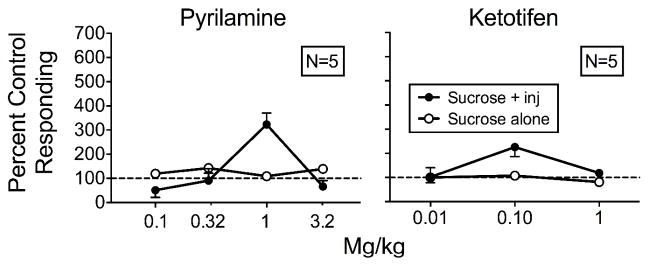
Mean percent control responding (contingent histamine injections with saline pretreatment) for the sucrose-plus-injection (inj) lever and sucrose-alone lever as a function of antagonist dose for the Pyrilamine (left) and Ketotifen (right) rats with the equal-reinforcement procedure in Experiment 2. Error bars are SEM.
Cardiovascular Study
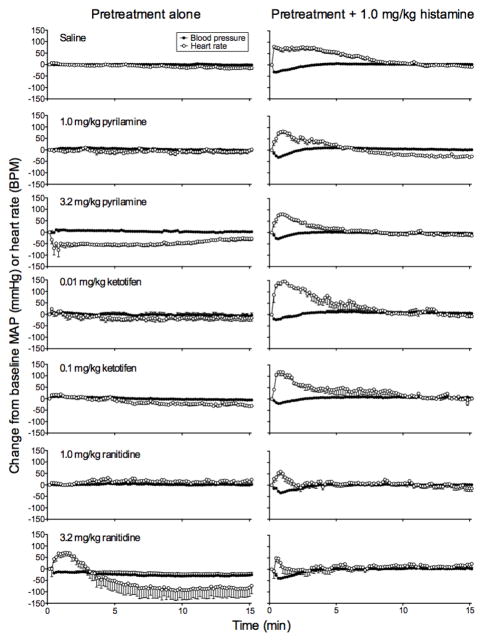
Figure 7 shows effects of pretreatments alone and their combination with 1 mg/kg/inj histamine on blood pressure and heart rate relative to resting control levels averaged across the four rats. Saline alone had no systematic effect (top left). Histamine alone produced a maximal decrease in blood pressure to 32 mmHg from control, which returned to control levels at 3 min. Histamine alone also produced a maximal increase in heart rate of 81 beats per min over controls, which returned to control levels at 11.3 min (i.e., tachychardia; top right). The effects of pyrilamine, ketotifen, and ranitidine are shown in the left side and the effects of these pretreatments on the cardiovascular effects of histamine are shown in the right side. No pretreatment affected histamine-induced changes in blood pressure. When injected alone, the 1 mg/kg dose of pyrilamine had no effect on heart rate, but the 3.2 mg/kg dose maximally decreased heart rate by 78 beats per min, and heart rate remained decreased for the entire 15 min of testing. Both the 1 and 3.2 mg/kg doses of pyrilamine reduced the duration of histamine-induced tachychardia to 6.5 min and 8.2 min, respectively. Note that the 1 mg/kg pyrilamine pretreatment further decreased heart rate, which stabilized at 9 beats per min. Both the 0.01 mg/kg and 1 mg/kg ketotifen doses had no effect alone, but enhanced the tachycardia produced by histamine to 146 and 119 beats per min, respectively. The 1 mg/kg dose of ranitidine had no effects alone, but the 3.2 mg/kg dose first increased heart rate to 71 beats per min above control and then, at 3 min, decreased heart rate to 95 beats per min below control, which continued through the rest of testing. Both ranitidine doses decreased heart rate and shortened the tachycardia effect produced by histamine: 1 mg/kg ranitidine decreased the histamine effect to 60 beats per min and 3.5 min in duration, and 3.2 mg/kg ranitidine decreased the histamine effect to 49 beats per min and 1.5 min in duration. Because larger doses of pyrilamine and ranitidine exerted strong effects on their own, these findings suggest complexity of combined drug effects in the cardiovascular system.
Fig. 7.
Cardiovascular effects as a function of time (min) in Experiment 2. Open symbols indicate changes from baseline heart rate as beats per min (BPM), and closed symbols indicate changes from baseline blood pressure as mean arterial pressure (MAP) every 10 s following histamine, saline, and the antagonists prior to saline (left side) or prior to histamine (right side). Pretreatment type is indicated in the left side. Error bars are SEM.
Discussion
The histamine H1-receptor antagonists pyrilamine and ketotifen partially blocked the punishing effects of histamine, as indicated by intermediate doses of both drugs producing increases in responding to the sucrose-plus-injection lever in the differential-reinforcement group (Fig. 2). Under similar conditions, ranitidine, a selective H2-receptor antagonist, did not block the punishing effects of histamine. Nevertheless, ranitidine was active over the same dose range in reducing the cardiovascular effects of histamine. These findings suggest that the punishing effects of histamine primarily are mediated by H1 receptors in rats, a finding generally consistent with those of Goldberg (1980) in squirrel monkeys. In view of the present findings and the fact that histamine does not readily cross the blood-brain barrier (Snyder, Axelrod, & Bauer, 1964), the punishing effects of histamine likely result from peripheral histamine H1 receptors. Nevertheless, the locus of the stimulus for the punishing effect is unknown.
In the absence of any antagonist pretreatments, we observed different patterns of responding during contingent histamine injections between the Pyrilamine and Ketotifen rats in the differential-reinforcement procedure. Histamine injections differentially decreased responding on the sucrose-plus-injection lever for the Pyrilamine rats, but suppressed responding on both levers for the Ketotifen rats. With pyrilamine pretreatments, responding on the sucrose-alone lever increased when responding decreased on the sucrose-plus-injection lever. Conversely, responding generally predominated on the sucrose-plus-injection lever at all doses of ketotifen. Although pyrilamine and ketotifen similarly increased responding that was decreased by contingent histamine injections, these different baseline patterns of responding complicate the comparison of the behavioral effects of these antagonists to some extent. It is unclear the extent to which the differences in patterns of responding might have influenced the relative potency between pyrilamine and ketotifen. This concern over relative potency is supported, at least in part, by findings showing that baseline rates of operant behavior influence the behavioral effects of a range of drugs, that is, the rate-dependency hypothesis of drug effects first described by Dews (1955, 1977; see McMillan & Katz, 2002, for a review). However, it is important to note that little is understood about the rate-dependent effects of histamine H1-receptor antagonists (see White & Rumbold, 1988).
We originally suggested that effectively antagonizing histamine’s effects should resemble the effects of decreasing histamine dose, which increased responding on the sucrose-plus-injection lever. How closely do the H1-receptor antagonists resemble reductions in histamine dose? Until reaching the largest doses of antagonists tested (i.e., 3.2 mg/kg pyrilamine, 1 mg/kg ketotifen), increasing the antagonist dose also increased sucrose plus histamine responding to levels approaching contingent injections of saline. These finding suggest similar effects of decreasing histamine dose and increasing antagonist dose. However, responding in the presence of the antagonists rarely reached the same levels of responding as with contingent injections of saline (see Figs. 3 and 5), which would indicate a complete antagonism of histamine’s effects. A likely explanation for this incomplete antagonism of histamine’s effects is that, as histamine’s effects are antagonized, more responding is allocated to the sucrose-plus-histamine lever. As a result, more histamine is injected, thereby surmounting the antagonism of histamine by the antagonist pretreatments.
More generally, the similar effects of decreasing histamine dose and increasing the doses of the antagonists speak to behavioral mechanisms of drug action, which can be described as drug effects that produce changes in behavior that are similar in form to the behavioral effects of environmental manipulations (see Witkin & Katz, 1990, for a discussion). Behavioral mechanisms of drug action were demonstrated in the present study when the effects of pyrilamine and ketotifen as pretreatments are compared to the effects of manipulating the magnitude of the punisher through changes in histamine dose. The pyrilamine and ketotifen pretreatments generally produced similar effects as reducing histamine dose under the equal-reinforcement and differential-reinforcement. Specifically, with the equal-reinforcement rats, we observed only small and inconsistent increases in responding on the sucrose-plus-injection lever when the dose of pyrilamine and ketotifen increased or the histamine dose decreased. Conversely, with the differential-reinforcement rats, we observed much larger effects with both manipulations. Therefore, these comparisons provide independent evidence of the concept of behavioral mechanisms of drug action. Furthermore, these findings show that the behavioral mechanisms of drug action depended on the reinforcement and punishment contingencies; that is, reducing histamine dose and increasing pyrilamine and ketotifen dose only had large effects when arranged in the context of the differential-reinforcement procedure.
The H1- and H2-receptor antagonists both impacted the cardiovascular effects of histamine at doses examined in the behavioral study, indicating roles of both histamine H1 and H2 receptors. However, ranitidine, the H2-receptor antagonist, more fully antagonized those effects than did pyrilamine or ketotifen. These findings generally are consistent with those of Goldberg (1980) with squirrel monkeys. He showed that an H2-receptor antagonist (cimetidine) decreased the cardiovascular effects of histamine, while an H1-receptor antagonist (diphenhydramine) enhanced the cardiovascular effects of histamine. Those findings are opposed to the slight antagonistic effects of pyrilamine found in the present study, but are consistent with the enhancing effect of ketotifen on heart rate. Nonetheless, findings from both of these studies support the notion that H1 (and not H2) histamine receptors mediate histamine’s punishing effects. In addition, the present findings that different receptors mediate cardiovascular and punishing effects also support the claim by Podlesnik et al. (2010) that cardiovascular effects per se likely do not mediate the punishing effects of histamine.
One limitation of the present experiment was the absence of data on the effects of the antagonists in the absence of response-dependent intravenous histamine injections (i.e., with intravenous saline injections). Those data would be useful in showing directly whether the biphasic patterns of responding following pretreatments with pyrilamine and ketotifen with the differential-reinforcement procedure (see Fig. 3) were due to general suppressive effects of the antagonists alone or an interaction between the antagonists and histamine’s effects. The cardiovascular effects resulting from pyrilamine and ranitidine alone suggest that histamine-receptor antagonists could be producing generally suppressive effects on behavior, especially at larger doses (see White & Rumbold, 1988, for a review).
General Discussion
Podlesnik et al. (2010) developed a choice procedure to assess the punishing effects of intravenous drugs in rats. The goal of the present study was to assess more rapidly dose effects of a punishing drug, histamine (Experiment 1), and then to determine the receptors mediating these effects (Experiment 2). In Experiment 1, three procedures were assessed, each arranging a choice between a sucrose pellet and a sucrose pellet plus an intravenous injection of histamine (0.1–1 mg/kg/inj). Responding was dose-dependently punished with each procedure, but the range of effects varied depending on the procedure. Equal probabilities of sucrose presentation on both levers produced the narrowest range of responding on the sucrose-plus-injection lever that did not change systematically with changes in histamine dose. Conversely, providing an incentive to responding on the sucrose-plus-injection lever, by lowering the probability of a sucrose presentation (p = .5) on the sucrose-alone lever, resulted in systematic changes in responding to the sucrose-plus-injection lever across the range of histamine doses. These findings show that the conditions of reinforcement influence the effectiveness of drug punishment, as has been shown with other punishers. Specifically, the present study supports other studies of choice showing that more responding is allocated to punished alternatives when arranging relatively high rates of reinforcement for engaging in punished responses compared to any unpunished alternatives (see Azrin & Holz, 1966; Negus, 2005; Simon et al., 2009). We compared these two general procedures arranging equal or differential reinforcement again in Experiment 2 to examine the effects of drug pretreatments on the punishing effects of histamine.
Two selective H1 histamine receptor antagonists, pyrilamine and ketotifen, antagonized the punishing effects of histamine, whereas the H2 receptor antagonist, ranitidine, did not. Conversely, ranitidine dampened the cardiovascular effects of histamine to a greater extent than did pyrilamine or ketotifen. These findings generally are consistent with those of Goldberg (1980) suggesting that H1 receptors mediate the punishing effects of histamine in rats and squirrel monkeys to a greater extent than do H2 receptors. Overall, these studies suggest choice procedures like those used in the present study can be used to identify punishing drug effects rapidly across a range of doses and the receptor systems mediating those effects.
The capability to identify these aspects of punishing drug effects has implications for preclinical drug development. Despite having therapeutic effects, individuals prescribed drugs producing aversive effects potentially could discontinue use. If therapeutic and aversive effects of a drug are mediated by different receptors, a selective antagonist could be combined with the drug of interest to block the aversive component, leaving intact the therapeutic drug effect.
These procedures also offer a framework from which to assess behavioral processes mediating the effects of punishing stimuli. For instance, despite identical training conditions, it is clear that differential punishing effects of histamine are observed in some rats but not others (e.g., differential-reinforcement group, Experiment 1) and in some groups but not in others (e.g., pyrilamine vs. ketotifen in differential-reinforcement groups, Experiment 2). These differences potentially are a result of differentially encountering the punishing effects of histamine and the differential payoff of sucrose. Perhaps the contingency between a particular response and histamine delivery, encountered first, results in selective punishing effects. Conversely, first encountering the differential reinforcement with sucrose between levers could result in overall greater levels of responding on the sucrose-plus-injection lever, regardless of current histamine or saline dosing conditions. Support for this hypothesis is that, when only histamine injections are introduced in the absence of changing the probability of sucrose on the sucrose-alone lever (e.g., Podlesnik et al., 2010; equal-reinforcement group, Experiment 1) histamine injections more reliably decreased responding only on the sucrose-plus-injection lever. Further studies are needed to reveal how encountering histamine injections versus changes in probability of sucrose presentation at different times in training might impact the selective punishing effects of histamine.
Findings from the present study suggest the punishing effects of drugs and their antagonists can be assessed rapidly in rats. This experimental framework could provide a starting point to explore rapidly and more fully the conditions in which other drugs increase responding suppressed by punishment. For instance, a different class of drugs, benzodiazepines (e.g., diazepam, chlordiazepoxide), also produce dose-dependent increases in reinforced operant responding that has been suppressed by punishment (see McMillan & Katz, 2002, for a review). Unlike pharmacological antagonists, benzodiazepines increase punished responding in general, not just responding punished by drugs (e.g., Katz & Goldberg, 1986; van Haaren & Anderson, 1997). Increases in punished responding with benzodiazepines in animal models are indicative of anxiolytic-like effects in humans (Cook & Davidson, 1973; Kleven & Koek, 1999; Rowlett, Lelas, Tornatzky, & Licata, 2006) and have been used to identify novel drugs with potential anxiolytic-like effects (e.g., Evenden, Duncan, & Ko, 2006). Given that the methods developed here can rapidly identify drugs producing increases in responding punished by drugs in rats, the present framework should be of interest to those developing behavioral and pharmacological treatments for anxiety disorders or relapse from drug abuse.
In summary, the present study revealed that reinforcement and punishment contingencies determine the punishing effects of intravenous histamine. The same contingencies also determine the effectiveness by which selective antagonists block the aversive effects of histamine. These findings provide independent evidence in support of behavioral mechanisms of drug action because reducing histamine dose and increasing the doses of the H1-receptor antagonists produced similar behavioral effects. Furthermore, the pharmacological antagonism of the punishing effects of histamine by H1- but not H2-receptor antagonists supports findings in squirrel monkeys (e.g., Goldberg, 1980) that H1 receptors mediate the aversive effects of histamine. We also suggest that histamine-induced changes in cardiovascular effects likely do not mediate the aversive effects of histamine because H1-receptor antagonists did not suppress the cardiovascular effects of histamine as effectively as an H2-receptor antagonist. Overall, these methods could be used to identify rapidly the receptors mediating the aversive effects of drugs as a tool for preclinical drug development.
Acknowledgments
The authors would like to thank Jim Woods for his many contributions to the project. We also thank Adam Kynaston, Jessica Priebe, Davina Barron, Yong Gong Shi, A.J. Flores, Chris Sbonek, Ivan Collado, and Matt Zedro for their technical assistance and Gail Winger for her helpful comments on a previous version of this manuscript. CAP was supported by the National Institutes of Health under Ruth L. Kirschstein National Service Research Service Award T32 DA007268. CJ was supported by the National Institutes of Health under Ruth L. Kirschstein National Service Research Service Award T32 DA007267. The University of Michigan Substance Abuse Research Center (UMSARC) Innovative Approaches to Investigate Aspects of Drug Use and Abuse Grant (U026035) to CAP funded this study.
References
- Azrin NH, Holz WC. Punishment. In: Honig WK, editor. Operant behavior: Areas of research and application. New York: Appleton-Century-Crofts; 1966. pp. 380–447. [Google Scholar]
- Banks RK. Impairment of reversal learning in the rat: A mediational explanation. Journal of Comparative and Physiological Psychology. 1973;82:322–327. doi: 10.1037/h0033926. [DOI] [PubMed] [Google Scholar]
- Brown PL, Jenkins HM. Autoshaping of the pigeon’s key peck. Journal of the Experimental Analysis of Behavior. 1968;11:1–8. doi: 10.1901/jeab.1968.11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook L, Davidson AB. Effects of behaviorally active drugs in a conflict-punishment procedure in rats. In: Garattini S, Mussini E, Randall LO, editors. The benzodiazepines. New York: Raven Press; 1973. pp. 604–673. [Google Scholar]
- Dews PB. Studies on behavior I: Differential sensitivity to pentobarbital of pecking performance in pigeons depending on the schedule of reward. Journal of Pharmacology and Experimental Therapeutics. 1955;138:393–401. [PubMed] [Google Scholar]
- Dews PB. Rate-dependency hypothesis. Science. 1977;198:1182–1183. doi: 10.1126/science.563103. [DOI] [PubMed] [Google Scholar]
- Evenden J, Duncan B, Ko T. A comparison of the effects of psychotomimetics and anxiolytics on punished and unpunished responding maintained by fixed interval schedules of food reinforcement in the rat. Behavioural Pharmacology. 2006;17:87–99. doi: 10.1097/01.fbp.0000189812.77049.e5. [DOI] [PubMed] [Google Scholar]
- Goldberg SR. Histamine as a punisher in squirrel monkeys: Effects of pentobarbital, chlordiazepoxide and H1- and H2-receptor antagonists on behavior and cardiovascular responses. Journal of Pharmacology and Experimental Therapeutics. 1980;214:726–736. [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD. Suppression of behavior by intravenous injections of nicotine or by electric shocks in squirrel monkeys: Effects of chlordiazepoxide and mecamylamine. Journal of Pharmacology and Experimental Therapeutics. 1983;224:334–340. [PubMed] [Google Scholar]
- Hough LB. Genomics meets histamine receptors: New subtypes, new receptors. Molecular Pharmacology. 2001;59:415–419. [PubMed] [Google Scholar]
- Karsh EB. Fixation produced by conflict. Science. 1970;168:873–875. doi: 10.1126/science.168.3933.873. [DOI] [PubMed] [Google Scholar]
- Karsh EB. Effects of conflict on choice behavior of rats. Journal of Comparative and Physiological Psychology. 1971;76:505–514. doi: 10.1037/h0031411. [DOI] [PubMed] [Google Scholar]
- Katz JL, Goldberg SR. Effects of H1-receptor antagonists on responding punished by histamine injection or electric shock presentation in squirrel monkeys. Psychopharmacology. 1986;90:461–467. doi: 10.1007/BF00174061. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Koek W. Effects of benzodiazepine agonists on punished responding in pigeons and their relationship with clinical doses in humans. Psychopharmacology. 1999;141:206–212. doi: 10.1007/s002130050826. [DOI] [PubMed] [Google Scholar]
- Lerman DC, Vorndran CM. On the status of knowledge for using punishment: Implications for treating behavior disorders. Journal of Applied Behavior Analysis. 2002;35:431–464. doi: 10.1901/jaba.2002.35-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin U, Romer D. The pharmacological properties of a new orally active antiphalactic compound: a benzocyclohepthathiophene. Arzneimittelforshung. 1978;28:770–782. [PubMed] [Google Scholar]
- McMillan DE, Katz JL. Continuing implication of the early evidence against the drive-reduction hypothesis of the behavioral effects of drugs. Psychopharmacology. 2002;163:251–264. doi: 10.1007/s00213-002-1185-0. [DOI] [PubMed] [Google Scholar]
- Negus SS. Effects of punishment on choice between cocaine and food in rhesus monkeys. Psychopharmacology. 2005;181:244–252. doi: 10.1007/s00213-005-2266-7. [DOI] [PubMed] [Google Scholar]
- Peterson GB, Ackil JE, Frommer GP, Hearst ES. Conditioned approach and contact behavior toward signals for food or brain-stimulation reinforcement. Science. 1972;177:1009–1011. doi: 10.1126/science.177.4053.1009. [DOI] [PubMed] [Google Scholar]
- Podlesnik CA, Jimenez-Gomez C, Woods JH. A choice procedure to assess the aversive effects of drugs in rodents. Journal of the Experimental Analysis of Behavior. 2010;93:203–223. doi: 10.1901/jeab.2010.93-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlett JK, Lelas S, Tornatzky W, Licata SC. Anti-conflict effects of benzodiazepines in rhesus monkeys: Relationship with therapeutic doses in humans and role of GABAA receptors. Psychopharmacology. 2006;184:201–211. doi: 10.1007/s00213-005-0228-8. [DOI] [PubMed] [Google Scholar]
- Simon NW, Gilbert RJ, Mayse JD, Bizon JL, Setlow B. Balancing risk and reward: A rat model of risky decision making. Neuropsychopharmacology. 2009;34:2208–2217. doi: 10.1038/npp.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder SH, Axelrod J, Bauer H. The fate of C14-histamine in animal tissues. Journal of Pharmacology and Experimental Therapeutics. 1964;143:373–379. [Google Scholar]
- Takada K, Barrett JE, Allen MS, Cook JM, Katz JL. Punishment of schedule-controlled behavior with β-carboline injections: Antagonism and comparisons with other compounds. Journal of Pharmacology and Experimental Therapeutics. 1992;261:138–145. [PubMed] [Google Scholar]
- van Haaren F, Anderson KG. Effects of chlordiazepoxide, buspirone and cocaine on behavior suppressed by timeout presentation. Behavioral Pharmacology. 1997;8:174–182. [PubMed] [Google Scholar]
- White JM, Rumbold GR. Behavioural effects of histamine and its antagonists: A review. Psychopharmacology. 1988;95:1–14. doi: 10.1007/BF00212757. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Katz JL. Analysis of behavioral effects of drugs. Drug Development Research. 1990;20:389–409. [Google Scholar]
- Woolverton WL. A novel choice method for studying drugs as punishers. Pharmacology, Biochemistry, and Behavior. 2003;76:125–131. doi: 10.1016/s0091-3057(03)00219-3. [DOI] [PubMed] [Google Scholar]