Abstract
Objective:
Attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorder frequently co-occur. Several studies show increased risk of substance use disorders in ADHD, yet there is limited information related to how ADHD symptoms, autistic traits, and their combined effects are associated with nicotine, alcohol, and cannabis use and use disorders in the general population.
Method:
Cross-sectional interview and self-report questionnaire data from 3,080 young adult Australian twins (mean age 31.9 years) were used to assess ADHD symptoms, autistic traits, substance use, and substance use disorders. Substance use disorders—based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria—were assessed in the full sample as well as in those who reported substance use. Logistic regression analyses were used for comparing the associations between ADHD symptoms, autistic traits, substance use, and substance misuse after conduct disorder, sex, age, and zygosity were controlled for.
Results:
Greater ADHD symptoms and autistic traits scores were associated with elevated levels of regular smoking; cannabis use; and nicotine, alcohol, and cannabis use disorders, even after conduct disorder was adjusted for. In contrast, for alcohol use, those with high autistic traits scores were less likely to report drinking to intoxication. However, upon initiation, and similar to the findings for nicotine and cannabis, they were at elevated risk for developing alcohol dependence.
Conclusions:
Increased liability to ADHD and elevated autistic traits scores were associated with substance use and misuse, with the exception of alcohol use. Given the social underpinnings of drinking, persons with autistic traits may be less likely to engage in it; however, upon engagement in drinking, their vulnerability to alcohol dependence is elevated.
Little is known about the relationship between autism spectrum disorder (ASD) and substance use and substance use disorders (SUDs) (Sizoo et al., 2010). In general, it is expected that substance use might be rare in individuals with ASD (Santosh and Mijovic, 2006) because of a lack of social skills, resulting in reduced access to substance-using peers (Bauminger and Kasari, 2000; Prendeville et al., 2006) as well as lower-than-average novelty-seeking behavior (Sizoo et al., 2009; Soderstrom et al., 2002)—all key facilitators of substance use. In one study comparing children and adolescents ages 12–18 years with ASD versus psychiatric controls, Santosh and Mijovic (2006) found lower rates of drug and alcohol use and misuse in those with ASD (3% vs. 17%). Another study of 122 adults with ASD with normal intelligence reported that 16% of the participants, no greater than expected in the general population, met lifetime criteria for an SUD (Hofvander et al., 2009).
Although ASDs are relatively uncommon (estimated to be about 1% in adulthood; Brugha et al., 2011), there is accumulating evidence that autistic traits (AT) are more prevalent. Numerous quantitative indices assess AT (see Constantino et al., 2003). One in particular, the Social Responsiveness Scale (SRS; Constantino and Gruber, 2005) has been shown to be a robust index of ASD and a useful index of AT (Bölte et al., 2008; Constantino et al., 2003; Kamio et al., 2013). The SRS provides information related to deficits in reciprocal social interactions (receiving and interpreting social cues, responding appropriately and engaging suitably in reciprocal social interactions) that are of considerable significance in the onset of substance use and the development of SUDs. The SRS also includes items on stereotyped and repetitive behaviors and communication problems that impair social interactions (Constantino and Gruber, 2005). In the context of substance use, such stereotyped and repetitive behaviors might also influence the transition from substance use to SUD. The SRS correlates well (r = .7) with clinical diagnostic assessments of ASD (Constantino et al., 2003) and also has been found to be predictive of interpersonal problems and difficulties related to AT in the general population (Kanne et al., 2009). However, no study to date has explored whether liability to AT differs from clinically diagnosed ASD in its relationship with substance use and SUD.
ASD and AT (Reiersen et al., 2007, 2008) commonly co-occur with attention-deficit/hyperactivity disorder (ADHD) symptoms. Clinic and population-based studies have found elevated AT scores in children and adults meeting diagnostic criteria for ADHD according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; American Psychiatric Association, 1994; Clark et al., 1999; Nydén et al., 2010; Reiersen et al., 2007). Similarly, the presence of clinically significant ADHD symptoms has been identified in children and adults with ASD (Goldstein and Schwebach, 2004).
In contrast to the poorly understood links between ASD, AT, and substance involvement, the relationship between ADHD and substance use and misuse has been studied extensively. Relative to those with ASD, individuals with ADHD are more likely to report substance use and misuse (Lee et al., 2011). Both adolescents and adults with ADHD show elevated rates of comorbid SUD (Molina and Pelham, 2003; Sobanski, 2006; Tamm et al., 2012; Wilens et al., 1997). In this regard, the most commonly studied psychiatric comorbidity is conduct disorder. For instance, Lynskey and Fergusson (1995) found that accounting for conduct disorder eliminated associations between early attention problems and later substance use (Disney et al., 1999; Fergusson et al., 1997; Greenbaum et al., 1991). Likewise, Molina and colleagues (2003) found that ADHD, particularly inattention problems, did not correlate with adolescent substance use in the absence of conduct disorder. In contrast, Elkins et al (2007) found that hyperactive-impulsive symptoms continued to predict initiation of substance use, nicotine dependence, and cannabis use disorders, even after conduct disorder was controlled for.
Despite the high, and presumed independent, comorbidity between ADHD and SUD and that of ADHD and ASD, few studies have explored whether ADHD and ASD, when studied together, show varying rates of co-aggregation with substance use and SUD. To our knowledge, only three studies (Hallerbäck et al., 2012; Santosh and Mijovic, 2006; Sizoo et al., 2010) have explored the combined effects of ADHD and ASD on substance use in small (N = 54–123) treatment/clinical samples. Sizoo et al. (2010) found that, despite similar rates of shared risk influences (e.g., parental SUD, early smoking) in those with either diagnosis, the prevalence of comorbid SUD was higher in ADHD (58%) compared with individuals diagnosed with ASD (30%). The other studies explored the combined effects of ADHD and ASD on nicotine use (Hallerbäck et al., 2012) and involvement with alcohol and other drugs (Santosh and Mijovic, 2006). Overall, results in both studies indicated comorbidity between ADHD and ASD, with lower prevalence of substance use in ASD individuals, independent of ADHD.
Not surprisingly, studies relying on clinically diagnosed ADHD and ASD tend to have smaller sample sizes. One unexplored possibility is to replace reliance on clinical diagnosis in favor of examining liability to ADHD and AT using dimensional conceptualizations of vulnerability. For ADHD, this technique might be as straightforward as examining individuals with varying levels of symptoms (e.g., Fergusson et al., 1997). ASDs, however, have traditionally been conceptualized as diagnostic categories (i.e., case-ness). However, there is strong historical evidence that such diagnoses, even for disorders imbuing a high degree of impairment, such as ASD, represent the dichotomization of a quantitative liability, in this case to traits representing stereotypy and deficits in social interactions and communication (Robinson et al., 2011).
Therefore, examination of a quantitative index of AT might facilitate such analyses. Such dimensional indices of symptomatology also allow the examination of whether rates of substance involvement might be elevated at nonclinical thresholds of ADHD and AT, hence providing better clues regarding potential prevention and intervention.
Prior studies on ADHD, ASD, and SUD also did not include comprehensive measures of substance use and SUD but instead focused on nicotine use (Hallerbäck et al., 2012) or a combined alcohol and other drug use group (Santosh and Mijovic, 2006). In addition, we are not aware of studies that have taken the multistage contingent nature of substance involvement into account when examining the relationship with ADHD and ASD. For instance, given the prominence of peer influences on the initiation and regular use of alcohol, one might posit that individuals with ASD or higher levels of AT, unlike those with ADHD, might be at a lower likelihood of alcohol use. However, on initiation of alcohol use, progression to alcohol dependence may be accelerated in those with ASD or higher AT scores, as well as those with ADHD, via repetitive reinforcing behaviors (Sizoo et al., 2009), the co-aggregation of other psychopathologies (Caamaño et al., 2013), or other processes.
In an effort to extend this modest literature, the current analysis used a large general-population sample of Australian twins ages 27–40 years to investigate the effects of liability to ADHD and AT and their combined influence on alcohol, nicotine, and cannabis use and misuse. The main goals of this study were to examine (a) whether rates and likelihood of alcohol (monthly drinking, intoxication, and DSM-IV alcohol dependence), nicotine (ever smoking, smoking ≥100 cigarettes, and DSM-IV nicotine dependence), and cannabis (ever used, repeated use [≥11 times], and cannabis abuse/dependence) involvement varied as a function of ADHD and AT symptomatology, when studied in conjunction; and (b) whether elevated rates of alcohol dependence, nicotine dependence, and cannabis abuse/dependence were noted in individuals with varying degrees of ADHD and AT symptomatology after their associations with initial stages of experimentation and regular use were accounted for.
Method
Sample
The sample for the current analysis included 3,080 White twins. The full study included 3,824 twins and their siblings who participated in a computer-assisted telephone interview. Of these participants, 3,676 also completed mail-in or online questionnaires. For the present study, we selected twin individuals only (because doing so allowed for a more restricted age range than when including siblings who might have been older), resulting in 3,143 twins who had participated in both the interview and questionnaire phase of assessment. Of these twins, 28 did not respond to questionnaire items related to AT, and 35 twins did not respond to interview questions on ADHD; hence, they were excluded. This resulted in the current study sample size of 3,080.
The twin pairs included in the study were born between 1972 and 1979 and were enrolled in the volunteer Australian Twin Registry. The Australian Twin Registry obtained consent from the twin pairs to forward their contact information to participate in an interview-based study of substance use and mental health. Information about the interested participants was forwarded to the Queensland Institute of Medical Research, which made independent efforts to enroll the twins. The data used for these analyses included information on (a) 925 monozygotic females, (b) 434 monozygotic males, (c) 712 dizygotic females, (d) 326 dizygotic males, and (e) 683 twins from opposite-sex pairs. More women than men participated in the study (which is a typical pattern seen in twin studies), and all participants were between ages 27 and 40 years. For detailed information on participant recruitment procedures and on the sociodemographic characteristics of the full sample, see Lynskey et al. (2012).
Assessment protocol
All participants completed a computer-administered semi-structured interview along with a brief questionnaire, which included questions related to physical health, personality, and other measures. The interview assessment (SSAGA-OZ; Lynskey et al., 2012) was derived from the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA), which has been found to be a reliable and valid measure of standardized DSM-IV diagnostic criteria for a range of psychiatric disorders as well as alcohol, nicotine, cannabis, and other illicit drug abuse and dependence (Bucholz et al., 1994). Responses to the questionnaires were submitted by mailing in a completed paper-and-pen survey to Queensland Institute of Medical Research or by completing the survey online. A majority of participants (93%) who completed the interview also completed the questionnaires (75% of participants completed the online version of the questionnaire) (Lynskey et al., 2012). The key elements from the interview and questionnaire used in the current analysis are described below.
Adult attention-deficit/hyperactivity disorder symptoms
The SSAGA-OZ interview included nine questions assessing inattentive symptoms and nine questions assessing hyperactive-impulsive symptoms derived from the DSM-IV. Respondents were asked to refer to symptoms occurring during childhood and adulthood (i.e., across the lifetime) and to compare themselves with other individuals of the same age. A diagnosis of ADHD (six or more inattentive symptoms or six or more hyperactive-impulsive symptoms) was uncommon (N = 122); hence, for these analyses, ADHD liability was coded as a three-level variable, with (a) individuals endorsing none of the 18 symptoms (59.7%), (b) those endorsing 1 or 2 symptoms (20.8%), and (c) the remainder endorsing 3 or more symptoms.
Autistic traits
The self-report questionnaire included a self-administered brief (11 -item) measure of AT, derived from an early version of the SRS. The full SRS (Constantino et al., 2003) includes 65 questions encompassing the three DSM-IV autistic symptom domains of social impairment, communication impairment, and stereotyped/repetitive behaviors (Constantino and Todd, 2000). In samples including normally developing and clinically diagnosed children, it has been shown, cross-culturally (Bölte et al., 2008; Kamio et al., 2013), to have excellent psychometric properties, which include good internal consistency (Cronbach’s α = .97), good short-term test–retest reliability (r = .88), and satisfactory interrater reliability (between parent and teacher, r = .73) (Constantino et al., 2000). The SRS also correlates highly (r = .70) with the Autism Diagnostic Interview-Revised, which is a standard measure for establishing a diagnosis of ASD (Constantino et al., 2003) and moderately with the Autistic Diagnostic Observation Schedule (Constantino et al., 2007), which is a semi-structured observational assessment to elicit criterion symptoms related to autistic disorder (Lord et al. 1999). Therefore, the SRS is a reliable index of AT with moderate to good convergent and discriminant validity (Bölte et al., 2008).
Eleven representative SRS items, each of which had high loadings on the first unrotated factor from principal components analysis of the full parent-report SRS in a pediatric sample (Reiersen et al., 2008), were used to assess AT in this sample. This brief assessment retains the psychometric features of the full SRS while reducing subject burden, with scores in the top 5th percentile (≥13) in the general population denoting a greater degree of problems with executive functioning related to ASD (Christ et al., 2010). Most of these items related to reciprocal social interaction, but others measured stereotyped/repetitive behaviors or communication impairment. These items were modified for self-report, and respondents rated items on a 4-point Likert scale (1 = false, not at all true; 2 = slightly true; 3 = mainly true; 4 = very true). Despite its abbreviated length, the short scale used here has shown good inter-item reliability (Cronbach’s α = .81; Reiersen et al., 2008) and test–retest reliability (r = .78; Kanne et al., 2009) and is correlated (r = .65; Kanne et al., 2009) with a more commonly used self-report ASD symptom measure, the Adult Autism Spectrum Quotient (Baron-Cohen et al., 2001).
For the current study, the 11 items (each with four response levels; e.g., “I avoid eye contact with most people,” with responses false, not at all true, slightly true, mainly true, very true) were summed to create a score (M = 3.6, SD = 4.0, range: 0–29), and a quantitative four-level index of AT was constructed as follows: those with a score of 0 (21%), those with a score of 1–2 (31.9%), those with a score of 3–5 (24.4%), and the remainder with scores of 6 or more. We did not use the previously defined “high-risk” score of 13 or greater, because only 3.8% (N = 118) of the sample could be categorized as such.
Substance use and substance use disorders
DSM-IV symptoms of substance dependence, along with comprehensive information related to substance use and misuse, were obtained using the SSAGA-OZ interview. The Composite International Diagnostic Interview (Robins et al., 1988) assessment was used to obtain information for nicotine dependence. All measures were dichotomous and assessed across the lifetime.
Lifetime tobacco use, regular smoking, and nicotine dependence
Tobacco use was dichotomously defined as ever having tried smoking, even a puff. Based on the Centers for Disease Control and Prevention and National Health Interview Survey recommendations (Centers for Disease Control and Prevention, 2007), regular tobacco smoking was defined as having smoked 100 or more cigarettes during one’s lifetime. The DSM-IV diagnostic criteria were used to assess nicotine dependence. Individuals who endorsed three or more of the seven criteria within a 12-month period were categorized as nicotine dependent.
Lifetime monthly alcohol use, intoxication, and alcohol dependence
As 98.8% of the sample reported using alcohol at least once during the lifetime, monthly alcohol use was defined as drinking at least once a month for 6 months or more. Drinking to intoxication was defined as drinking until drunkenness (i.e., slurred speech, unsteady on one’s feet, or hard to keep one’s balance). Finally, DSM-IV alcohol-dependence diagnostic criteria were used to diagnose alcohol dependence. This diagnosis included the participant’s endorsing three or more of the seven diagnostic criteria within a 12-month period.
Cannabis use, repeated use, and cannabis abuse/dependence
Cannabis use was defined as lifetime use, even once, of cannabis. Repeated use was defined as using cannabis 11 or more times across one’s lifetime. DSM-IV criteria for abuse (one or more of four) and dependence (three or more of six, excluding withdrawal, clustering in a 12-month period) were used to diagnose a lifetime history of cannabis abuse/dependence.
Influential covariate: Childhood conduct disorder
Research suggests that conduct disorder may mediate observed associations between ADHD and substance involvement. To control for this possibility, all analyses included a lifetime diagnosis of DSM-IV conduct disorder (7.8% in this sample). Additional covariates included sex, age, and twin-pair zygosity.
Statistical analyses
Descriptive statistics and cross tabulations were computed in SAS Version 9 (SAS Institute, Inc., Cary, NC), and logistic regressions were conducted in STATA (StataCorp LP, College Station, TX). Sex, age, zygosity (member of identical vs. fraternal twin pair), and conduct disorder were used as covariates in all analyses. The “cluster” option available in STATA was used to adjust the standard errors for nonindependence of data within families.
Both ADHD liability (two dummy-coded variables, representing 1–2 symptoms and ≥3 symptoms) and AT liability (three dummy-coded variables, representing 1–2, 3–5, and ≥6 symptoms) were dummy-coded, with those with no symptoms serving as reference groups. All five dummy-coded variables were jointly entered into the model, along with covariates. Post hoc Wald chi-square tests were used to examine whether the magnitude of association across the dummy-coded variables could be statistically equated to each other.
For the three SUD measures, analyses were conducted in two ways: first, in the entire sample, by coding never users/light users as zero (i.e., not receiving a diagnosis of SUD), and second, by then excluding never/light users from the analysis. The latter analytic strategy allowed us to disentangle the role of liability to ADHD and AT on use from their role in liability to disorder. Therefore, when never/light users were excluded from the data, any observed associations with SUD were not confounded with preexisting associations between ADHD, AT, and substance use.
Results
Sample characteristics
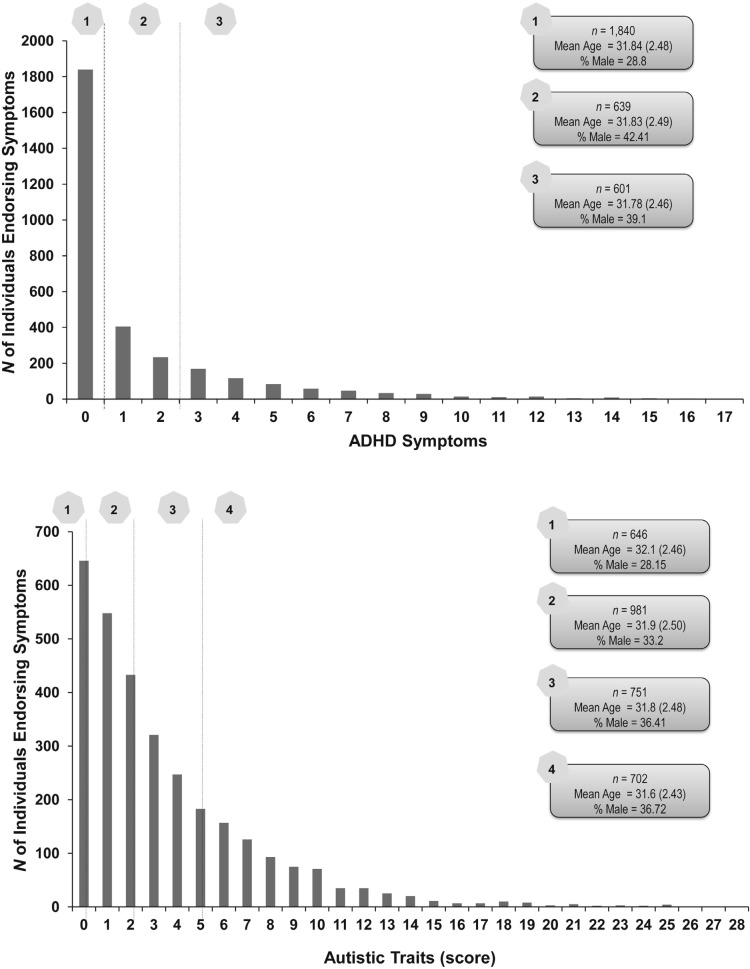
Figure 1 shows the distribution of ADHD symptoms and AT scores. Also shown are mean ages and the proportion of males in each category of scores. Although there were no statistically significant age differences across categories, for both ADHD and AT, those with higher scores were more likely to be male. ADHD symptoms and AT scores were correlated (r = .40, p < .0001). Of the full sample, 502 individuals endorsed no symptoms of ADHD and had AT scores of 0. Correspondingly, 268 individuals were in the highest category for both ADHD (≥3 symptoms) and AT (scores of ≥6). Of those 639 individuals endorsing one to two ADHD symptoms, an approximately equal proportion (27%–30%) had AT scores ranging from 1 to 6 or more. For those with AT scores of 1–2, 18% and 14% reported one to two and three or more ADHD symptoms, respectively. Likewise, for those with AT scores between 3 and 5, 25% and 20% reported one to two and three or more ADHD symptoms, respectively.
Figure 1.
Distribution of attention-deficit/hyperactivity disorder (ADHD) symptoms (Figure 1a) and autistic traits scores (Figure 1b). ADHD (Figure 1a) categories are defined by endorsement of no symptoms (1), one to two symptoms (2), and three or more symptoms (3). Autistic traits categories (Figure 1b) are defined by endorsement of DSM-IV–related autistic traits on a self-report measure with scores as follows: score of 0 (1), score of 1–2 (2), score of 3–5 (3), and score of 6 or higher (4). Also shown in the figure are mean (standard deviation) age in years and proportion of men in each category.
Association of substance use and substance use disorders across groups
Prevalence of tobacco, alcohol, and cannabis involvement in the full sample and across the categories of ADHD symptoms and AT is summarized in Table 1, and the corresponding odds ratios, adjusted for sex, age, zygosity, and a lifetime history of conduct disorder, are presented in Table 2.
Table 1.
Frequency of tobacco-smoking, alcohol, and cannabis involvement as a function of ADHD symptoms and autistic traits scores in 3,080 Australian twins, ages 27–40 years
| Variable | Full sample (N = 3,080) | ADHD symptoms |
Autistic traits |
|||||
| 0 | 1–2 | ≥3 | 0 | 1–2 | 3–5 | ≥6 | ||
| Smoking | ||||||||
| Ever smoked tobacco | 86.4 | 85.5 | 87.3 | 88.4 | 86.5 | 86.1 | 85.4 | 87.9 |
| Regular tobacco smoking | 41.5 | 36.9 | 44.9 | 51.1 | 32.8 | 38.4 | 44.1 | 51.2 |
| DSM-IV nicotine dependence | 25.0 | 20.2 | 27.1 | 37.1 | 16.0 | 20.2 | 28.5 | 36.2 |
| DSM-IV nicotine dependence (conditional on regular use, n = 1,273) | 60.3 | 54.8 | 60.3 | 72.6 | 48.8 | 52.8 | 64.7 | 70.7 |
| Alcohol | ||||||||
| Monthly drinking | 91.1 | 90.8 | 92.5 | 91.0 | 91.7 | 91.9 | 90.1 | 90.5 |
| Drinking to intoxication | 87.7 | 87.0 | 90.1 | 87.7 | 88.6 | 89.1 | 85.9 | 86.8 |
| DSM-IV alcohol dependence | 24.3 | 18.4 | 29.0 | 37.1 | 18.3 | 21.2 | 26.0 | 32.1 |
| DSM-IV alcohol dependence (conditional on monthly use, n = 2,809) | 26.7 | 20.1 | 31.3 | 40.8 | 19.8 | 23.0 | 28.8 | 35.3 |
| Cannabis | ||||||||
| Ever used cannabis | 68.4 | 65.0 | 73.4 | 73.0 | 64.6 | 68.1 | 68.7 | 71.9 |
| Used cannabis ≥ 11 times | 30.8 | 26.5 | 36.5 | 37.6 | 22.5 | 29.6 | 31.5 | 39.1 |
| DSM-IV cannabis abuse/dependence | 16.2 | 12.3 | 18.9 | 25.0 | 10.0 | 14.1 | 16.9 | 24.2 |
| DSM-IV cannabis abuse/dependence (conditional on repeated use, n = 946) | 52.7 | 46.4 | 52.0 | 66.4 | 44.5 | 47.3 | 53.6 | 61.9 |
Notes: ADHD = attention-deficit/hyperactivity disorder; DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition.
Table 2.
Adjusted odds ratios with their 95% confidence limits reflecting associations between tobacco, alcohol, and cannabis use and misuse and liability to ADHD and autistic traits in 3,080 Australian twins, ages 27–40 years, after sex, age, zygosity (identical vs. fraternal), and a lifetime history of conduct disorder were controlled for
| Variable | ADHD symptoms |
Autistic traits |
|||
| 1–2 | ≥3 | 1–2 | 3–5 | ≥6 | |
| Smoking | |||||
| Ever smoked tobacco | 1.11 [0.83, 1.47] | 1.09 [0.80, 1.48] | 0.94 [0.71, 1.26] | 0.86 [0.63, 1.18] | 1.02 [0.72, 1.44] |
| Regular tobacco smoking | 1.17 [0.97, 1.41] | 1.24*b [1.00, 1.54] | 1.21 [0.98, 1.50] | 1.46**ab [1.16, 1.84] | 1.79**a [1.40, 2.28] |
| DSM-IV nicotine dependence | 1.20 [0.96, 1.49] | 1.52**b [1.22, 1.90] | 1.25 [0.96, 1.62] | 1.84**ab [1.40, 2.43] | 2.30**a [1.74, 3.06] |
| DSM-IV nicotine dependence (conditional on regular use, n = 1,273) | 1.10 [0.82, 1.47] | 1.62**a [1.19, 2.21] | 1.13 [0.80, 1.60] | 1.74**a [1.20, 2.51] | 2.07**a [1.42, 3.00] |
| Alcohol | |||||
| Monthly drinking | 1.23 [0.87, 1.74] | 0.98 [0.68, 1.39] | 0.98 [0.68, 1.42] | 0.75 [0.51, 1.10] | 0.76 [0.50, 1.15] |
| Drinking to intoxication | 1.36 [0.99, 1.84] | 1.02 [0.75, 1.38] | 0.99 [0.72, 1.36] | 0.70* [0.50, 0.97] | 0.71 [0.49, 1.03] |
| DSM-IV alcohol dependence | 1.50**a [1.21, 1.86] | 1.91**a [1.52, 2.39] | 1.06 [0.82, 1.38] | 1.23 [0.94, 1.61] | 1.42*a [1.07, 1.87] |
| DSM-IV alcohol dependence (conditional on monthly use, n = 2,809) | 1.50**a [1.20, 1.86] | 1.96**a [1.55, 2.46] | 1.07 [0.82, 1.39] | 1.28 [0.98, 1.68] | 1.47**a [1.11, 1.95] |
| Cannabis | |||||
| Ever used cannabis | 1.34** [1.09, 1.64] | 1.17 [0.93, 1.47] | 1.10 [0.89, 1.36] | 1.06 [0.84, 1.35] | 1.15 [0.89, 1.49] |
| Used cannabis ≥11 times | 1.31*ab [1.06, 1.61] | 1.09 [0.87, 1.37] | 1.35*a [1.07, 1.70] | 1.36*a [1.06, 1.75] | 1.75**b [1.35, 2.28] |
| DSM-IV cannabis abuse/dependence | 1.24 [0.95, 1.62] | 1.41*ab [1.07, 1.85] | 1.32 [0.96, 1.81] | 1.46*a [1.05, 2.04] | 2.02**b [1.44, 2.85] |
| DSM-IV cannabis abuse/dependence (conditional on repeated use, n = 946) | 1.07 [0.77, 1.49] | 1.68** [1.16, 2.43] | 1.03 [0.68, 1.57] | 1.21 [0.79, 1.85] | 1.50 [0.96, 2.33] |
Notes: ADHD = attention-deficit/hyperactivity disorder; DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition.
Odds ratios with identical superscripts could be statistically equated to each other at p > .05.
Indicates significant at p < .05;
indicates significant at p < .008, which refers to the Bonferroni correction for two phenotypes (ADHD vs. autistic traits) and three substances (alcohol, nicotine, and cannabis), that is, .05 / 6 = .0083.
Lifetime tobacco use, regular smoking, and nicotine dependence
There were no differences in the rate of ever smoking across the range of ADHD or AT scores. However, as ADHD as well as AT scores increased, so did rates of regular tobacco smoking and nicotine dependence. Those with ADHD symptoms or AT scores of 3 or more were more likely to report regular tobacco smoking and to meet criteria for nicotine dependence. The association with nicotine dependence persisted even after we controlled for regular smoking. Those with AT scores of 6 or more appeared to be at a somewhat greater likelihood of meeting criteria for nicotine dependence than those with three or more ADHD symptoms. However, these differences between the highest categories of ADHD and AT vulnerability no longer persisted when nicotine dependence was examined conditional on regular smoking.
Lifetime monthly alcohol use, intoxication, and alcohol dependence
Monthly drinking was not associated with ADHD symptoms or AT scores. Drinking to intoxication was associated with AT but not ADHD liability; however, higher AT scores appeared to be negatively associated with the likelihood of drinking to intoxication. In contrast, both high ADHD symptoms (i.e., ≥3 symptoms) and AT scores (i.e., scores of ≥6) were similarly and positively associated with alcohol dependence.
Cannabis use, repeated use, and cannabis abuse/dependence
Relative to those without any ADHD symptoms, there was limited evidence for an association between cannabis use and fewer symptoms of ADHD (i.e., 1–2 symptoms). These individuals also were more likely to report using cannabis 11 or more times, as were those with an AT score of even 1 (relative to those with AT scores of 0). Cannabis dependence, on the other hand, was associated with higher AT and ADHD (symptoms/scores of ≥3). However, when those exposed to repeated cannabis use were examined, higher ADHD, but not AT, scores were associated.
Discussion
In a sample of adult Australian twins from the general population, we found that increased liability to ADHD and AT is associated, to varying degrees, with a lifetime history of regular substance use and SUDs. However, the nature and degree of association across the groups varied for the individual substances, alluding to possibly distinct comorbidities.
Tobacco
In the current study, increased ADHD symptoms were associated with regular tobacco smoking and nicotine dependence. Tobacco, particularly cigarette smoking, is well recognized for its association with ADHD (Glass and Flory, 2010). Studies have shown a higher prevalence of cigarette smoking in adults with current ADHD symptoms as well as in those with a lifetime diagnosis of ADHD in the absence of current symptoms (Pomerleau et al., 1995, 2003). Those with ADHD are not only more likely to start smoking at an early age (Rohde et al., 2003) but also to progress more rapidly to regular smoking and nicotine dependence (Lambert and Hartsough, 1998). In a review of the literature, Glass and Flory (2010) posit multiple psychosocial pathways that might connect ADHD to tobacco smoking. These include self-medication of inattentive symptoms via the stimulant effects of nicotine as well as cognitive (e.g., coping strategies), individual (e.g., educational attainment), and social (e.g., peer delinquency) factors. In addition, there are numerous studies that document an increased risk of ADHD in offspring prenatally exposed to tobacco (Cornelius and Day, 2000; Knopik, 2009). These studies have found that the evidence for a direct causal influence of tobacco exposure on ADHD is limited; instead, overlapping genetic and environmental factors connect prenatal cigarette smoking, offspring smoking, and offspring ADHD.
Higher AT scores also were associated with a heightened vulnerability to regular smoking and nicotine dependence. This finding is in contrast to that of other studies (Bejerot and Nylander, 2003; Hallerbäck et al., 2012). Bejerot and Nylander (2003) showed significantly lower levels of smoking (smoking at least one cigarette daily for the past 6 months) in Swedish adults with ASD compared with adults with schizophrenia and the general population. Similarly, in the study by Hallerbäck et al. (2012), low nicotine use was seen in adult patients with Asperger syndrome. There are numerous possible reasons for these cross-study differences. First, our study used lifetime measures of tobacco smoking, including regular smoking and nicotine dependence. Second, in addition to being an index of ASD-related problems, our measure of AT likely identifies individuals with other non-ASD behavioral problems. In fact, in a recent study of 2,368 ASD probands and their 1,913 siblings, raw SRS scores were highly associated with ASD symptoms and reduced social development but also with internalizing and externalizing problem scales of the Child Behavior Check List (Hus et al., 2013). The association between SRS scores and externalizing problems was pronounced even in those with low scores on the social development scale, indicating a potentially independent, but also bi-directional (i.e. higher SRS scores contribute to ASD risk which, in turn, increases likelihood of externalizing problems; Constantino and Frazier, 2013), relationship between AT and externalizing problems, which include substance use and misuse.
Alcohol
Perhaps the most intriguing observation from the present study is the relationship between ADHD, AT, and alcohol involvement. Consistent with the literature, rates of monthly drinking and drinking to intoxication were not associated with increasing ADHD symptomatology; however, individuals with higher ADHD symptoms were considerably more likely to meet criteria for a lifetime history of alcohol dependence (Disney et al., 1999; Flory et al., 2003; Knopik et al., 2009; Kuperman et al., 2001). However, the presence of AT appeared to significantly reverse the direction of association with alcohol (but not nicotine and cannabis) use measures such that individuals with higher AT scores were less likely to report drinking to intoxication. Santosh and Mijovic (2006) reported a similar result in adolescents with ASD (with normal intelligence), in the absence of ADHD, being associated with a lower frequency of alcohol (and other drug) use.
One possible explanation for this finding is that individuals with higher AT scores experience demonstrable interpersonal difficulties, which may distance them from the social context of normative alcohol consumption. Research has shown that alcohol use and facilitation of use by older siblings and peers are among the most robust and proximal risk influences on the onset of alcohol use and drinking to intoxication (Chartier et al., 2011). Peers play a significant role in influencing alcohol consumption, especially in young adults (Andrews et al., 2002). Consistent with a deviance proneness model (Sher, 1991), studies show that adolescents with ADHD are more likely to associate with peers who engage in risky behaviors and that this agglomeration of deviant behaviors, including conduct disorder, contribute to a heightened vulnerability to alcohol and substance use (Marshal et al., 2003; Wilens, 2007; Wilens and Biederman, 2006). On the other hand, individuals with AT, particularly those with clinical ASD, have poor peer interactions, lack social skills, and may be socially isolated (Bauminger and Kasari, 2000; Prendeville et al., 2006). As a result, they might have reduced exposure to peers and social settings that encourage recreational drinking and alcohol intoxication (Santosh and Mijovic, 2006).
In contrast, there was a positive (i.e., risk) association between higher AT scores and alcohol dependence, with odds ratios being comparable to those with greater ADHD symptoms. This finding underscores the importance of disentangling early stages of alcohol use (e.g., intoxication) that are more sensitive to the social context from later, maladaptive stages, which may be differentially influenced. For instance, although social isolation may prohibit social drinking, it is well recognized as a risk factor for alcohol dependence (Chou et al., 2011; Santosh and Mijovic, 2006). Social support, which may be lacking in individuals with higher AT scores, may protect against pathological drinking, directly, or indirectly via buffering from other stressful life events (Cohen and Wills, 1985) and comorbidities with other psychopathologies, such as depression. Self-medication of depressed mood as well as other internalizing behaviors also might encourage the development of alcohol dependence—Kanne et al. (2009), for example, found higher rates of anxiety, depressive symptoms, and low self-esteem and correspondingly higher levels of social stress in those with high AT scores. Another possibility is that repetitive preoccupations that are part of the repertoire of ASD-like features may accelerate transitions from alcohol use, once it is initiated, to alcoholism (Lewis and Bodfish, 1998).
Cannabis
Similar to literature on the links between tobacco smoking, drinking, and ADHD, studies highlight increased rates of cannabis use and, potentially, cannabis use disorder in individuals with ADHD (Elkins et al., 2007; Flory et al., 2003). In contrast, we expected a lower likelihood of cannabis use in individuals with elevated AT scores, because individuals at risk for ASD are known to demonstrate behaviors associated with low risk taking and high harm avoidance, which might lead to lower experimentation with drugs such as cannabis (Santosh and Mijovic, 2006; Soderstrom et al., 2002). Based on the prior literature, such as the study by Sizoo et al. (2010) reporting higher rates of cannabis use disorder (17% vs. 7%) in those with ADHD relative to ASD, we also expected associations with ADHD to be stronger than those with AT.
Although we did identify the association between repeated cannabis use (as well as cannabis use disorder conditional on repeated use) and greater ADHD symptoms, in contrast to prior studies, those with AT scores of 3 or more also were more (not less) likely to report repeated use of cannabis and to meet criteria for cannabis use disorders. Once again, this result might indicate that lower levels of AT, as indexed by the SRS, correlate robustly with childhood externalizing problems (Hus et al., 2013) such as the onset of use. The elevated levels of cannabis use and misuse associated with high AT scores also could be a result of repetitive preoccupations associated with AT traits, which might be an underlying factor facilitating the transition from repeated cannabis use to cannabis abuse/dependence.
Role of conduct disorder
Several studies have noted that the relationship between ADHD and substance use as well as SUD is mediated by co-morbid conduct disorder and, in some instances, that ADHD symptoms—particularly inattention—may be unrelated to subsequent risk for substance use and misuse in the absence of conduct disorder (Disney et al., 1999; Elkins et al., 2007; Fergusson et al., 1997; Lynskey and Fergusson, 1995; Molina and Pelham, 2003). In the present study, although controlling for conduct disorder may have attenuated some of the associations between substance involvement and ADHD, some significant relationships continued to emerge. Therefore, it is possible that the comorbidity of other psychiatric disorders, over and above conduct disorder, may be increasing the risk for SUD in our sample.
Limitations and conclusions
Some limitations are noteworthy. First, formal diagnostic criteria were not used to define ADHD and ASD. Thus, findings in this study may differ from studies that use clinical diagnosis and may reflect a milder risk related to substance use and SUD. Even though a proportion of those with three or more ADHD symptoms and those with AT scores of 6 or greater are clinically impaired, an overwhelming majority of those in those highest categories are likely to manifest subthreshold levels of symptomatology. Second, because this is a cross-sectional study, we cannot be certain of the direction of causation of effects. Although it is presumed that ADHD and AT contribute to the onset and progression of substance involvement, there also is evidence that cannabis and other drug misuse may, in turn, lead to increased ADHD symptoms (Fergusson and Boden, 2008). This study cannot disentangle such causal effects. Finally, the sample consisted of educated, White, young adult Australian twins; consequently, results from other studies may differ.
Despite these limitations, our study is among the first to examine the liability to ADHD and AT and their combined effects on substance use and SUD in an epidemiological sample. Our use of subthreshold, traitlike definitions of both liabilities may have revealed novel findings for substance involvement. The authors of future studies may wish to further disentangle the components of ADHD (i.e., inattention vs. hyperactivity-impulsivity) and the subscales of the SRS and relate them to substance involvement.
Acknowledgments
The authors thank Dixie Statham, Richard Parker, Soad Hancock, Judith Moir, Sally Rodda, Pieta-Maree Shertock, Heather Park, Jill Wood, Pam Barton, Fran Husband, and Adele Somerville, who worked on this project, and the twins and their siblings for participating. Arpana Agrawal has received peer-reviewed grant funds from ABMRF/Foundation for Alcohol Research. John N. Constantino receives royalties from Western Psychological Services for commercial distribution of the Social Responsiveness Scale.
Footnotes
This research was supported by National Institute on Drug Abuse Grants T32DA007313 (to Duneesha De Alwis), DA18267 (to Michael T. Lynskey), DA23668 (to Arpana Agrawal), DA32573 (to Arpana Agrawal), and HD042541 (to John N. Constantino). Data collection also was made possible through access to the Australian Twin Registry, which is supported by an Enabling Grant (ID 628911) from the Australian National Health and Medical Research Council. Angela M. Reiersen received grant support from National Institute of Mental Health Grant MH-080287 and from the McDonnell Center for Systems Neuroscience and the McDonnell Center for Cellular and Molecular Neurobiology. The content of this article is the responsibility of the authors and does not represent the official views of the funding bodies
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Andrews JA, Tildesley E, Hops H, Li F. The influence of peers on young adult substance use. Health Psychology. 2002;21:349–357. doi: 10.1037//0278-6133.21.4.349. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): Evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Bauminger N, Kasari C. Loneliness and friendship in high-functioning children with autism. Child Development. 2000;71:447–456. doi: 10.1111/1467-8624.00156. [DOI] [PubMed] [Google Scholar]
- Bejerot S, Nylander L. Low prevalence of smoking in patients with autism spectrum disorders. Psychiatry Research. 2003;119:177–182. doi: 10.1016/s0165-1781(03)00123-9. [DOI] [PubMed] [Google Scholar]
- Bölte S, Poustka F, Constantino JN. Assessing autistic traits: Cross-cultural validation of the social responsiveness scale (SRS) Autism Research. 2008;1:354–363. doi: 10.1002/aur.49. [DOI] [PubMed] [Google Scholar]
- Brugha TS, McManus S, Bankart J, Scott F, Purdon S, Smith J, Meltzer H. Epidemiology of autism spectrum disorders in adults in the community in England. Archives of General Psychiatry. 2011;68:459–465. doi: 10.1001/archgenpsychiatry.2011.38. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: A report on the reliability of the SSAGA. Journal of Studies on Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Caamaño M, Boada L, Merchán-Naranjo J, Moreno C, Llorente C, Moreno D, Parellada M. Psychopathology in children and adolescents with ASD without mental retardation. Journal of Autism and Developmental Disorders. 2013;43:2442–2449. doi: 10.1007/s10803-013-1792-0. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Cigarette smoking among adults—United States, 2006. Morbidity and Mortality Weekly Report. 2007, November 9;56(44):1157–1161. [PubMed] [Google Scholar]
- Chartier KG, Hesselbrock MN, Hesselbrock VM. Alcohol problems in young adults transitioning from adolescence to adulthood: The association with race and gender. Addictive Behaviors. 2011;36:167–174. doi: 10.1016/j.addbeh.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou KL, Liang K, Sareen J. The association between social isolation and DSM-IV mood, anxiety, and substance use disorders: Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry. 2011;72:1468–1476. doi: 10.4088/JCP.10m06019gry. [DOI] [PubMed] [Google Scholar]
- Christ SE, Kanne SM, Reiersen AM. Executive function in individuals with subthreshold autism traits. Neuropsychology. 2010;24:590–598. doi: 10.1037/a0019176. [DOI] [PubMed] [Google Scholar]
- Clark T, Feehan C, Tinline C, Vostanis P. Autistic symptoms in children with attention deficit-hyperactivity disorder. European Child & Adolescent Psychiatry. 1999;8:50–55. doi: 10.1007/s007870050083. [DOI] [PubMed] [Google Scholar]
- Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychological Bulletin. 1985;98:310–357. [PubMed] [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, Reich W. Validation of a brief quantitative measure of autistic traits: Comparison of the social responsiveness scale with the autism diagnostic interview-revised. Journal of Autism and Developmental Disorders. 2003;33:427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Frazier TW. Commentary: The observed association between autistic severity measured by the social responsiveness scale (SRS) and general psychopathology—a response to Hus et al. (2013) Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2013;54:695–697. doi: 10.1111/jcpp.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. Social Responsiveness Scale (SRS) Manual. Los Angeles CA: Western Psychological Services; 2005. [Google Scholar]
- Constantino JN, Lavesser PD, Zhang Y, Abbacchi AM, Gray T, Todd RD. Rapid quantitative assessment of autistic social impairment by classroom teachers. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:1668–1676. doi: 10.1097/chi.0b013e318157cb23. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Przybeck T, Friesen D, Todd RD. Reciprocal social behavior in children with and without pervasive developmental disorders. Journal of Developmental and Behavioral Pediatrics. 2000;21:2–11. doi: 10.1097/00004703-200002000-00002. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Genetic structure of reciprocal social behavior. American Journal of Psychiatry. 2000;157:2043–2045. doi: 10.1176/appi.ajp.157.12.2043. [DOI] [PubMed] [Google Scholar]
- Cornelius MD, Day NL. The effects of tobacco use during and after pregnancy on exposed children. Alcohol Research & Health. 2000;24:242–249. [PMC free article] [PubMed] [Google Scholar]
- Disney ER, Elkins IJ, McGue M, Iacono WG. Effects of ADHD, conduct disorder, and gender on substance use and abuse in adolescence. American Journal of Psychiatry. 1999;156:1515–1521. doi: 10.1176/ajp.156.10.1515. [DOI] [PubMed] [Google Scholar]
- Elkins IJ, McGue M, Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Archives of General Psychiatry. 2007;64:1145–1152. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM. Cannabis use and adult ADHD symptoms. Drug and Alcohol Dependence. 2008;95:90–96. doi: 10.1016/j.drugalcdep.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Lynskey MT, Horwood LJ. Attentional difficulties in middle childhood and psychosocial outcomes in young adulthood. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1997;38:633–644. doi: 10.1111/j.1469-7610.1997.tb01690.x. [DOI] [PubMed] [Google Scholar]
- Flory K, Milich R, Lynam DR, Leukefeld C, Clayton R. Relation between childhood disruptive behavior disorders and substance use and dependence symptoms in young adulthood: Individuals with symptoms of attention-deficit/hyperactivity disorder are uniquely at risk. Psychology of Addictive Behaviors. 2003;17:151–158. doi: 10.1037/0893-164x.17.2.151. [DOI] [PubMed] [Google Scholar]
- Glass K, Flory K. Why does ADHD confer risk for cigarette smoking? A review of psychosocial mechanisms. Clinical Child and Family Psychology Review. 2010;13:291–313. doi: 10.1007/s10567-010-0070-3. [DOI] [PubMed] [Google Scholar]
- Goldstein S, Schwebach AJ. The comorbidity of pervasive developmental disorder and attention deficit hyperactivity disorder: Results of a retrospective chart review. Journal of Autism and Developmental Disorders. 2004;34:329–339. doi: 10.1023/b:jadd.0000029554.46570.68. [DOI] [PubMed] [Google Scholar]
- Greenbaum PE, Prange ME, Friedman RM, Silver SE. Substance abuse prevalence and comorbidity with other psychiatric disorders among adolescents with severe emotional disturbances. Journal of the American Academy of Child and Adolescent Psychiatry. 1991;30:575–583. doi: 10.1097/00004583-199107000-00008. [DOI] [PubMed] [Google Scholar]
- Hallerbäck MU, Lugnegård T, Gillberg C. ADHD and nicotine use in schizophrenia and Asperger syndrome: A controlled study. Journal of Attention Disorders. 2012 doi: 10.1177/1087054712439099. Advance on-line publication. Retrieved from http://jad.sagepub.com/content/early/2012/04/11/1087054712439099. [DOI] [PubMed] [Google Scholar]
- Hofvander B, Delorme R, Chaste P, Nydén A, Wentz E, Ståhlberg O, Leboyer M. Psychiatric and psychosocial problems in adults with normal-intelligence autism spectrum disorders. BMC Psychiatry. 2009;9:35. doi: 10.1186/1471-244X-9-35. Retrieved from http://www.biomedcentral.com/1471-244X/9/35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hus V, Bishop S, Gotham K, Huerta M, Lord C. Factors influencing scores on the social responsiveness scale. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2013;54:216–224. doi: 10.1111/j.1469-7610.2012.02589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamio Y, Inada N, Moriwaki A, Kuroda M, Koyama T, Tsujii H, Constantino JN. Quantitative autistic traits ascertained in a national survey of 22 529 Japanese schoolchildren. Acta Psychiatrica Scandinavica. 2013;128:45–53. doi: 10.1111/acps.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanne SM, Christ SE, Reiersen AM. Psychiatric symptoms and psychosocial difficulties in young adults with autistic traits. Journal of Autism and Developmental Disorders. 2009;39:827–833. doi: 10.1007/s10803-008-0688-x. [DOI] [PubMed] [Google Scholar]
- Knopik VS. Maternal smoking during pregnancy and child outcomes: Real or spurious effect? Developmental Neuropsychology. 2009;34:1–36. doi: 10.1080/87565640802564366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Bucholz KK, Madden PA, Waldron M. Genetic and environmental influences on externalizing behavior and alcohol problems in adolescence: A female twin study. Pharmacology, Biochemistry, and Behavior. 2009;93:313–321. doi: 10.1016/j.pbb.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperman S, Schlosser SS, Kramer JR, Bucholz K, Hesselbrock V, Reich T, Reich W. Developmental sequence from disruptive behavior diagnosis to adolescent alcohol dependence. American Journal of Psychiatry. 2001;158:2022–2026. doi: 10.1176/appi.ajp.158.12.2022. [DOI] [PubMed] [Google Scholar]
- Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. Journal of Learning Disabilities. 1998;31:533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- Lee SS, Humphreys KL, Flory K, Liu R, Glass K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: A meta-analytic review. Clinical Psychology Review. 2011;31:328–341. doi: 10.1016/j.cpr.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MH, Bodfish JW. Repetitive behavior disorders in autism. Mental Retardation and Developmental Disabilities Research Reviews. 1998;4:80–89. [Google Scholar]
- Lord C, Rutter M, Dilavore PC, Risi S. Autism Diagnostic Observation Schedule-WPS (WPS Edition) Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- Lynskey MT, Agrawal A, Henders A, Nelson EC, Madden PAF, Martin NG. An Australian twin study of cannabis and other illicit drug use and misuse, and other psychopathology. Twin Research and Human Genetics. 2012;15:631–641. doi: 10.1017/thg.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynskey MT, Fergusson DM. Childhood conduct problems, attention deficit behaviors, and adolescent alcohol, tobacco, and illicit drug use. Journal of Abnormal Child Psychology. 1995;23:281–302. doi: 10.1007/BF01447558. [DOI] [PubMed] [Google Scholar]
- Marshal MP, Molina BS, Pelham WE., Jr Childhood ADHD and adolescent substance use: An examination of deviant peer group affiliation as a risk factor. Psychology of Addictive Behaviors. 2003;17:293–302. doi: 10.1037/0893-164X.17.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BS, Pelham WE., Jr Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. Journal of Abnormal Psychology. 2003;112:497–507. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- Nydén A, Niklasson L, Stahlberg O, Anckarsater H, Wentz E, Rastam M, Gillberg C. Adults with autism spectrum disorders and ADHD neuropsychological aspects. Research in Developmental Disabilities. 2010;31:1659–1668. doi: 10.1016/j.ridd.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Downey KK, Snedecor SM, Mehringer AM, Marks JL, Pomerleau OF. Smoking patterns and abstinence effects in smokers with no ADHD, childhood ADHD, and adult ADHD symptomatology. Addictive Behaviors. 2003;28:1149–1157. doi: 10.1016/s0306-4603(02)00223-x. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Downey KK, Stelson FW, Pomerleau CS. Cigarette smoking in adult patients diagnosed with attention deficit hyperactivity disorder. Journal of Substance Abuse. 1995;7:373–378. doi: 10.1016/0899-3289(95)90030-6. [DOI] [PubMed] [Google Scholar]
- Prendeville JA, Prelock PA, Unwin G. Peer play interventions to support the social competence of children with autism spectrum disorders. Seminars in Speech and Language. 2006;27:32–46. doi: 10.1055/s-2006-932437. [DOI] [PubMed] [Google Scholar]
- Reiersen AM, Constantino JN, Grimmer M, Martin NG, Todd RD. Evidence for shared genetic influences on self-reported ADHD and autistic symptoms in young adult Australian twins. Twin Research and Human Genetics. 2008;11:579–585. doi: 10.1375/twin.11.6.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiersen AM, Constantino JN, Volk HE, Todd RD. Autistic traits in a population-based ADHD twin sample. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2007;48:464–472. doi: 10.1111/j.1469-7610.2006.01720.x. [DOI] [PubMed] [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, Towle LH. The Composite International Diagnostic Interview: An Epidemiologic Instrument Suitable for Use in Conjunction with Different Diagnostic Systems and in Different Cultures. Archives of General Psychiatry. 1988;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- Robinson EB, Koenen KC, McCormick MC, Munir K, Hallett V, Happé F, Ronald A. Evidence that autistic traits show the same etiology in the general population and at the quantitative extremes (5%, 2.5%, and 1%) Archives of General Psychiatry. 2011;68:1113–1121. doi: 10.1001/archgenpsychiatry.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde P, Lewinsohn PM, Brown RA, Gau JM, Kahler CW. Psychiatric disorders, familial factors and cigarette smoking: I. Associations with smoking initiation. Nicotine & Tobacco Research. 2003;5:85–98. doi: 10.1080/1462220031000070507. [DOI] [PubMed] [Google Scholar]
- Santosh PJ, Mijovic A. Does pervasive developmental disorder protect children and adolescents against drug and alcohol use? European Child & Adolescent Psychiatry. 2006;15:183–188. doi: 10.1007/s00787-005-0517-0. [DOI] [PubMed] [Google Scholar]
- Sher KJ. Children of alcoholics: A critical appraisal of theory and research. Chicago, IL: University of Chicago Press; 1991. [Google Scholar]
- Sizoo B, van den Brink W, Gorissen van Eenige M, van der Gaag RJ. Personality characteristics of adults with autism spectrum disorders or attention deficit hyperactivity disorder with and without substance use disorders. Journal of Nervous and Mental Disease. 2009;197:450–454. doi: 10.1097/NMD.0b013e3181a61dd0. [DOI] [PubMed] [Google Scholar]
- Sizoo B, van den Brink W, Koeter M, Gorissen van Eenige M, van Wijngaarden-Cremers P, van der Gaag RJ. Treatment seeking adults with autism or ADHD and co-morbid substance use disorder: Prevalence, risk factors and functional disability. Drug and Alcohol Dependence. 2010;107:44–50. doi: 10.1016/j.drugalcdep.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Sobanski E. Psychiatric comorbidity in adults with attention-deficit/hyperactivity disorder (ADHD) European Archives of Psychiatry and Clinical Neuroscience, 256, Supplement. 2006;1:i26–i31. doi: 10.1007/s00406-006-1004-4. [DOI] [PubMed] [Google Scholar]
- Soderstrom H, Rastam M, Gillberg C. Temperament and character in adults with Asperger syndrome. Autism. 2002;6:287–297. doi: 10.1177/1362361302006003006. [DOI] [PubMed] [Google Scholar]
- Tamm L, Adinoff B, Nakonezny PA, Winhusen T, Riggs P. Attention-deficit/hyperactivity disorder subtypes in adolescents with co-morbid substance-use disorder. American Journal of Drug and Alcohol Abuse. 2012;38:93–100. doi: 10.3109/00952990.2011.600395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE. The nature of the relationship between attention-deficit/hyperactivity disorder and substance use. Journal of Clinical Psychiatry, 68, Supplement. 2007;11:4–8. [PubMed] [Google Scholar]
- Wilens TE, Biederman J. Alcohol, drugs, and attention-deficit/hyperactivity disorder: A model for the study of addictions in youth. Journal of Psychopharmacology. 2006;20:580–588. doi: 10.1177/0269881105058776. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Mick E, Faraone SV, Spencer T. Attention deficit hyperactivity disorder (ADHD) is associated with early onset substance use disorders. Journal of Nervous and Mental Disease. 1997;185:475–482. doi: 10.1097/00005053-199708000-00001. [DOI] [PubMed] [Google Scholar]