Abstract
Objective
To analyze how pretreatment body mass index relates to known endometrial cancer prognostic factors and how it impacts the disease-free survival and cause-specific survival of Korean women with endometrial cancer.
Methods
The patients were divided into the non-obese (<25 kg/m2) and obese groups (≥25 kg/m2) according to their pretreatment body mass index. The 25 kg/m2 body mass index cut-off was based on the World Health Organization criteria for Asian people. The two groups were compared in terms of their clinicopathological characteristics and survival outcomes.
Results
A total of 213 consecutive patients with endometrioid adenocarcinoma of the uterus met the eligibility criteria of this study and were included in the analysis. Of these patients, 105 patients had a body mass index less than 25 kg/m2 (non-obese group) and 108 patients had a body mass index equal to or more than 25 kg/m2 (obese group). The two groups did not differ in terms of age, menopause, parity, height, FIGO (International Federation of Obstetrics and Gynecology) stage, tumor grade, tumor size, myometrial invasion, lymphovascular space invasion, cytology, and lymph node metastasis. Body mass index was not a significant factor for disease-free and cause-specific survival in univariate analysis, and after adjusting for all prognostic factors that were significant in univariate analysis, it did not associate significantly with disease-free and cause-specific survival.
Conclusion
In Korean women with endometrioid adenocarcinoma of the uterus, a high pretreatment body mass index did not associate with other prognostic factors and had little impact on the disease-free survival and cause-specific survival of these women.
Keywords: Body mass index, Endometrial neoplasms, Obesity, Prognosis
Introduction
Endometrial cancer is the third most common gynecological cancer and the eleventh most common female cancer in Korea [1]. In 2010, 1,752 new endometrial cancer cases were diagnosed in Korea and 222 patients died of this cancer [1]. However, since its incidence has started increasing rapidly recently, it will soon become the most common gynecological cancer in Korea [2].
Obesity is a well-known risk factor for the development of enImpact cancer [3,4,5,6]. Since the prevalence of obesity is increasing worldwide [7], it is important to understand the influence of obesity on the prognosis of endometrial cancer. However, despite the many studies on the relationship between obesity and endometrial cancer prognosis recently, this relationship remains poorly understood and controversial [8,9,10,11,12,13,14,15,16,17,18,19]. Thus, the present study was performed to analyze how pretreatment body mass index relates to known endometrial cancer prognostic factors and how it impacts the disease-free survival and cause-specific survival of Korean women with endometrial cancer.
Materials and methods
After obtaining approval from the Institutional Review Board, the medical records of all consecutive women who had endometrial adenocarcinoma and underwent surgical management and follow-up at Asan Medical Center (AMC) between 2005 and 2008 were retrieved. Patients were included in this study if they had endometrioid adenocarcinoma of the uterus and underwent surgical staging and/or debulking surgery at AMC. Patients who received fertility-sparing management and who did not undergo hysterectomy were excluded. The following clinicopathological data of the patients were collected from the medical records: their age, body weight, height, body mass index, parity, menopause, tumor stage according to the International Federation of Obstetrics and Gynecology (FIGO), tumor grade, tumor size, depth of myometrial invasion, lymphovascular space invasion, cytology, lymph node metastasis, surgical management and adjuvant therapy, recurrence, and death.
The patients were then divided into the non-obese (<25 kg/m2) and obese (≥25 kg/m2) groups according to their pretreatment body mass index. The 25 kg/m2 body mass index cut-off was based on the World Health Organization criteria for Asian people. The two groups were compared in terms of their clinicopathological characteristics and survival outcomes. The mean values of the two groups were compared by using Student's t-test, while their frequency distributions were compared by using the chi-squared test. Survival curves and rates were obtained by using the Kaplan-Meier method and the differences in the survival rates were compared by using a log-rank test. Multivariate survival analysis was performed by using Cox's proportional hazards model. All prognostic factors that were significant in univariate analysis were included in the multivariate survival analysis. Disease-free survival was calculated in months from the date of surgery until the date of disease recurrence or last follow-up. Cause-specific survival was calculated from the date of surgery until the date of death due to endometrial cancer or last follow-up. P-values less than 0.05 were regarded to indicate statistically significant differences. All statistical analyses were performed by using SPSS ver. 11.0 (SPSS Inc., Chicago, IL, USA).
Results
During the study period, 213 consecutive patients with endometrioid adenocarcinoma of the uterus met the eligibility criteria of this study and were included in the analysis. Of these patients, 105 patients had a body mass index less than 25 kg/m2 (non-obese group) and 108 patients had a body mass index equal to or more than 25 kg/m2 (obese group). Table 1 shows the clinicopathological characteristics of the non-obese and obese groups. The two groups did not differ in terms of age, menopause, parity, height, FIGO stage, tumor grade, tumor size, myometrial invasion, lymphovascular space invasion, cytology, and lymph node metastasis. Pelvic lymph node dissection was performed in 93 (88.6%) patients in the non-obese group and 99 (91.7%) patients in the obese group (P = 0.449), while para-aortic lymph node dissection was performed in 37 (35.2%) patients in the non-obese group and 30 (27.8%) patients in the obese group (P = 0.241). After surgery, 29 (27.6%) patients in the non-obese group and 19 (17.6%) patients in the obese group received adjuvant therapy (P = 0.283). The two groups did not differ in terms of the type of adjuvant therapy (Table 1).
Table 1.
Patient characteristics (n=213)
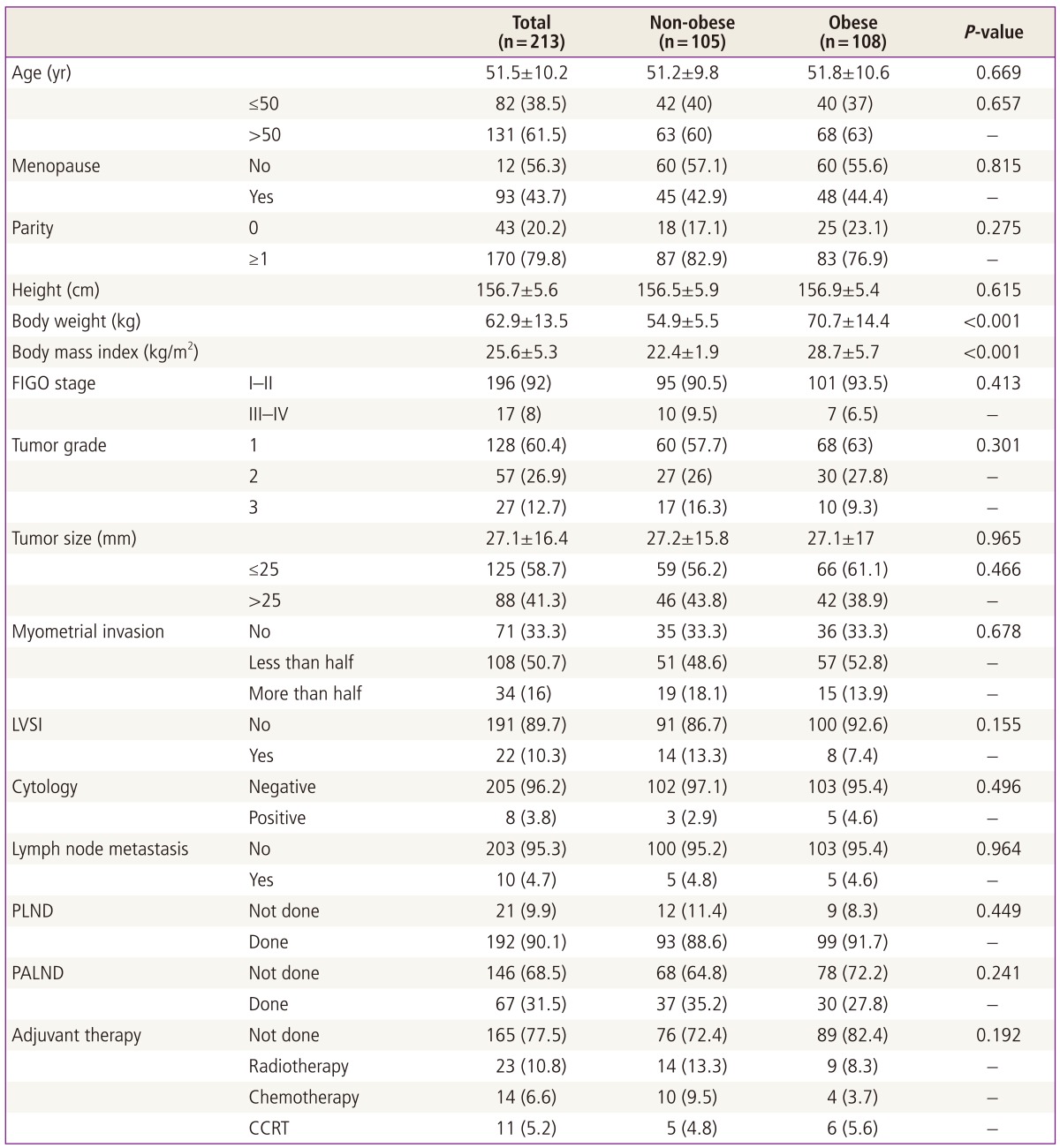
Data are shown as mean±SD or n (%).
FIGO, International Federation of Obstetrics and Gynecology; LVSI, lymphovascular space invasion; PLND, pelvic lymph node dissection; PALND, para-aortic lymph node dissection; CCRT, concurrent chemoradiation therapy.
The median follow-up time for the 213 patients was 64 months (range, 2-94 months). The two groups did not differin terms of the median follow-up time (65 vs. 67 months, P = 0.449). Twenty-five patients had recurrent disease and 14 of these patients died of the disease. For the whole cohort, the 5 year disease-free survival and cause-specific survival rates were 89% and 93%, respectively.
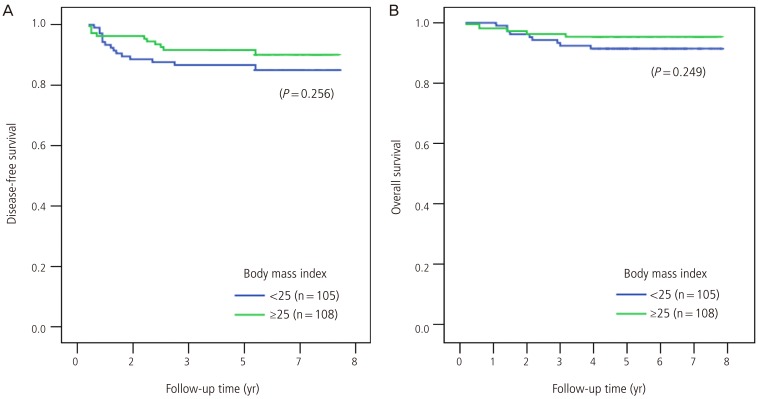
In univariate analysis, FIGO stage (I-II vs. III-IV), tumor grade, tumor size, myometrial invasion, lymphovascular space invasion, cytology, lymph node metastasis, and the need for adjuvant therapy associated significantly with disease-free survival (Table 2). In the multivariate analysis that included the significant prognostic factors in the univariate analysis, only tumor grade associated significantly with disease-free survival: compared to grade 1, grade 2 disease was associated with a poorer disease-free survival (odds ratio, 2.7; 95% confidence intervals, 0.9-8.0; P = 0.075). This was also true for grade 3 disease (odds ratio, 6.7; 95% confidence intervals, 2.0-22.7; P = 0.002). Body mass index was not a significant factor for disease-free survival in univariate analysis (Fig. 1). After adjusting for all prognostic factors that were significant in univariate analysis, body mass index did not associate significantly with disease-free survival.
Table 2.
Univariate analysis of factors that associate with disease-free survival and cause-specific survival (n=213)
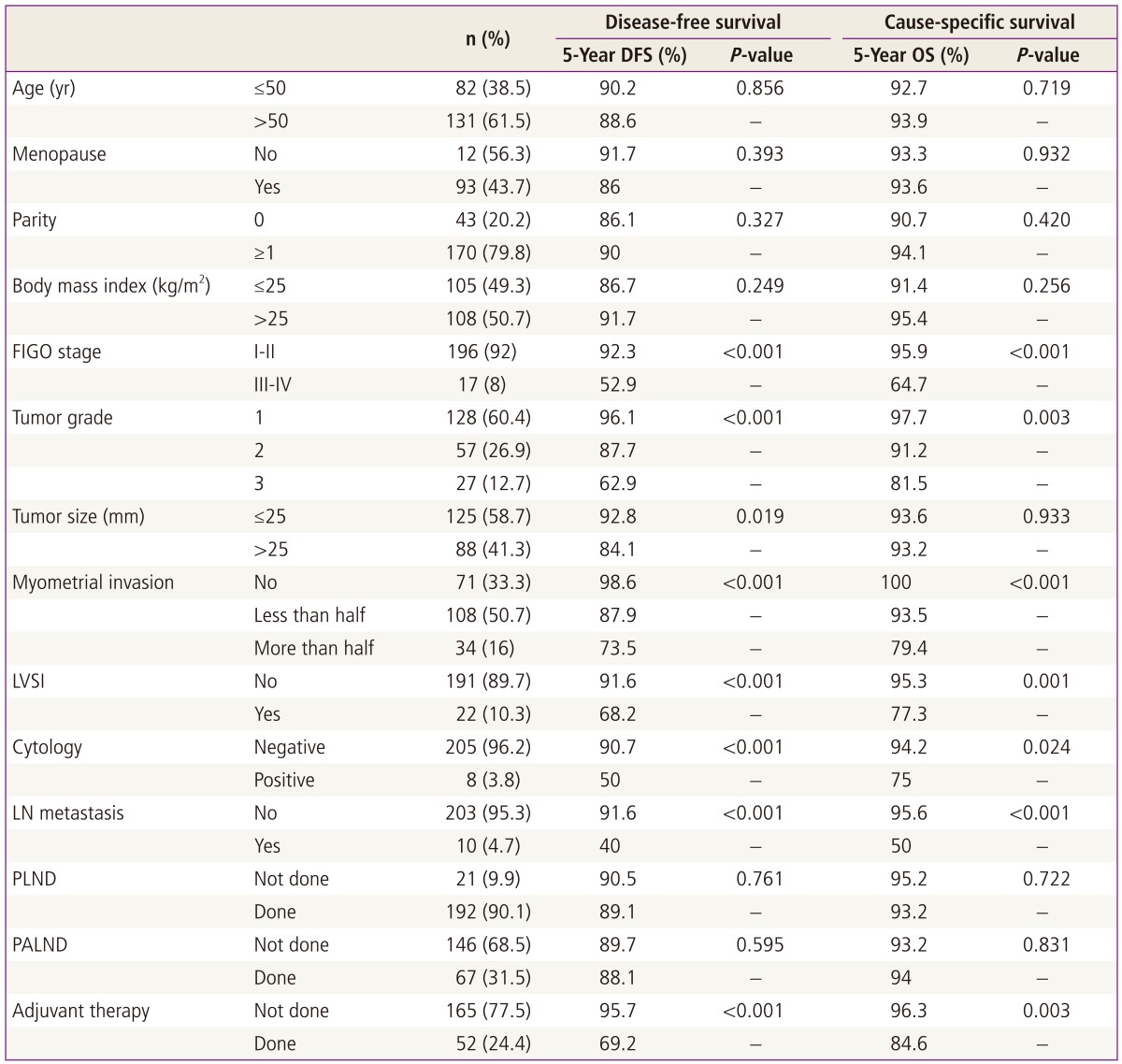
DFS, disease-free survival; OS, overall survival; FIGO, International Federation of Obstetrics and Gynecology; LVSI, lymphovascular space invasion; LN, lymph node; PLND, pelvic lymph node dissection; PALND, para-aortic lymph node dissection.
Fig. 1.
Effect of pretreatment body mass index on disease-free survival (A) and cause-specific survival (B).
In univariate analysis, FIGO stage, tumor grade, myometrial invasion, lymphovascular space invasion, cytology, lymph node metastasis, and the need for adjuvant therapy associated significantly with cause-specific survival. In multivariate analysis that included these prognostic factors, none of these factors associated significantly with cause-specific survival. Body mass index was not a significant factor for cause-specific survival in univariate analysis (Fig. 1), and after adjusting for all prognostic factors that were significant in univariate analysis, it did not associate significantly with cause-specific survival.
Discussion
The present study showed that pretreatment obesity (≥25 kg/m2) did not associate with other prognostic factors, including FIGO stage, tumor grade, tumor size, myometrial invasion, lymphovascular space invasion, cytology, lymph node metastasis, and the need for adjuvant therapy in Korean women with endometrioid adenocarcinoma of the uterus. Obesity also had little impact on the disease-free survival and cause-specific survival of these women.
Several studies have shown that obesity is an important risk factor for the development of endometrial adenocarcinoma [3,4,5,6]. This may reflect the fact that endometrial cancer is usually a disease of postmenopausal women and that obesity is associated with increased production of estrogen in adipose tissue, particularly in postmenopausal women [20]. One study has shown that a high body mass index (≥25 kg/m2) is a significant risk factor for endometrial cancer in Korean women as well [12]. However, the association between obesity and prognostic factors of endometrial cancer is controversial. Some studies suggest that a high body mass index associates with more favorable tumor characteristics [9,10,11,12,13,14,21,22], but this was not supported by other studies. Indeed, in our series, a high pretreatment body mass index did not associate significantly with other prognostic factors. The impact of obesity on the prognosis of endometrial cancer is also controversial. While several studies suggest that a high body mass index associates with a worse prognosis of endometrial cancer [15,16,17,18,19], other studies did not observe this association [9,10,11,12,13,14]. Indeed, in our series, the non-obese and obese patients did not differ in terms of disease-free survival and cause-specific survival in either univariate analysis or after multivariate adjustment.
Our study is limited because of the retrospective nature of its study design. However, the follow-up time was sufficiently long and the number of subjects was sufficiently large to analyze the association between obesity and prognostic factors and to compare the survival outcomes of obese and non-obese patients. Since only patients with the endometrioid histological type of adenocarcinoma of the uterus were included in the study, the clarity of conclusion seems to be high.
In conclusion, in Korean women with endometrioid adenocarcinoma of the uterus, a high pretreatment body mass index did not associate with other prognostic factors and had little impact on the disease-free survival and cause-specific survival of these women.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013;45:1–14. doi: 10.4143/crt.2013.45.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jung KW, Park S, Won YJ, Kong HJ, Lee JY, Park EC, et al. Prediction of cancer incidence and mortality in Korea, 2011. Cancer Res Treat. 2011;43:12–18. doi: 10.4143/crt.2011.43.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindemann K, Vatten LJ, Ellstrom-Engh M, Eskild A. Body mass, diabetes and smoking, and endometrial cancer risk: a follow-up study. Br J Cancer. 2008;98:1582–1585. doi: 10.1038/sj.bjc.6604313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjorge T, Engeland A, Tretli S, Weiderpass E. Body size in relation to cancer of the uterine corpus in 1 million Norwegian women. Int J Cancer. 2007;120:378–383. doi: 10.1002/ijc.22260. [DOI] [PubMed] [Google Scholar]
- 5.Furberg AS, Thune I. Metabolic abnormalities (hypertension, hyperglycemia and overweight), lifestyle (high energy intake and physical inactivity) and endometrial cancer risk in a Norwegian cohort. Int J Cancer. 2003;104:669–676. doi: 10.1002/ijc.10974. [DOI] [PubMed] [Google Scholar]
- 6.Fader AN, Arriba LN, Frasure HE, von Gruenigen VE. Endometrial cancer and obesity: epidemiology, biomarkers, prevention and survivorship. Gynecol Oncol. 2009;114:121–127. doi: 10.1016/j.ygyno.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Obesity: preventing and managing the global epidemic. Geneva: World Health Organization; 2000. [PubMed] [Google Scholar]
- 8.Arem H, Irwin ML. Obesity and endometrial cancer survival: a systematic review. Int J Obes (Lond) 2013;37:634–639. doi: 10.1038/ijo.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson B, Connor JP, Andrews JI, Davis CS, Buller RE, Sorosky JI, et al. Obesity and prognosis in endometrial cancer. Am J Obstet Gynecol. 1996;174:1171–1178. doi: 10.1016/s0002-9378(96)70659-2. [DOI] [PubMed] [Google Scholar]
- 10.Everett E, Tamimi H, Greer B, Swisher E, Paley P, Mandel L, et al. The effect of body mass index on clinical/pathologic features, surgical morbidity, and outcome in patients with endometrial cancer. Gynecol Oncol. 2003;90:150–157. doi: 10.1016/s0090-8258(03)00232-4. [DOI] [PubMed] [Google Scholar]
- 11.Gates EJ, Hirschfield L, Matthews RP, Yap OW. Body mass index as a prognostic factor in endometrioid adenocarcinoma of the endometrium. J Natl Med Assoc. 2006;98:1814–1822. [PMC free article] [PubMed] [Google Scholar]
- 12.Jeong NH, Lee JM, Lee JK, Kim JW, Cho CH, Kim SM, et al. Role of body mass index as a risk and prognostic factor of endometrioid uterine cancer in Korean women. Gynecol Oncol. 2010;118:24–28. doi: 10.1016/j.ygyno.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Munstedt K, Wagner M, Kullmer U, Hackethal A, Franke FE. Influence of body mass index on prognosis in gynecological malignancies. Cancer Causes Control. 2008;19:909–916. [Google Scholar]
- 14.Temkin SM, Pezzullo JC, Hellmann M, Lee YC, Abulafia O. Is body mass index an independent risk factor of survival among patients with endometrial cancer? Am J Clin Oncol. 2007;30:8–14. doi: 10.1097/01.coc.0000236047.42283.b8. [DOI] [PubMed] [Google Scholar]
- 15.Arem H, Chlebowski R, Stefanick ML, Anderson G, Wactawski-Wende J, Sims S, et al. Body mass index, physical activity, and survival after endometrial cancer diagnosis: results from the Women's Health Initiative. Gynecol Oncol. 2013;128:181–186. doi: 10.1016/j.ygyno.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chia VM, Newcomb PA, Trentham-Dietz A, Hampton JM. Obesity, diabetes, and other factors in relation to survival after endometrial cancer diagnosis. Int J Gynecol Cancer. 2007;17:441–446. doi: 10.1111/j.1525-1438.2007.00790.x. [DOI] [PubMed] [Google Scholar]
- 17.Mauland KK, Trovik J, Wik E, Raeder MB, Njolstad TS, Stefansson IM, et al. High BMI is significantly associated with positive progesterone receptor status and clinico-pathological markers for non-aggressive disease in endometrial cancer. Br J Cancer. 2011;104:921–926. doi: 10.1038/bjc.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Modesitt SC, Tian C, Kryscio R, Thigpen JT, Randall ME, Gallion HH, et al. Impact of body mass index on treatment outcomes in endometrial cancer patients receiving doxorubicin and cisplatin: a Gynecologic Oncology Group study. Gynecol Oncol. 2007;105:59–65. doi: 10.1016/j.ygyno.2006.10.045. [DOI] [PubMed] [Google Scholar]
- 19.Arem H, Park Y, Pelser C, Ballard-Barbash R, Irwin ML, Hollenbeck A, et al. Prediagnosis body mass index, physical activity, and mortality in endometrial cancer patients. J Natl Cancer Inst. 2013;105:342–349. doi: 10.1093/jnci/djs530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. 2002;11:1531–1543. [PubMed] [Google Scholar]
- 21.Kodama J, Seki N, Ojima Y, Nakamura K, Hongo A, Hiramatsu Y. Correlation of presenting symptoms and patient characteristics with endometrial cancer prognosis in Japanese women. Int J Gynaecol Obstet. 2005;91:151–156. doi: 10.1016/j.ijgo.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Studzĩnski Z, Zajewski W. Factors affecting the survival of 121 patients treated for endometrial carcinoma at a Polish hospital. Arch Gynecol Obstet. 2003;267:145–147. doi: 10.1007/s00404-001-0288-x. [DOI] [PubMed] [Google Scholar]