Abstract
Objective
The aim of this study was to evaluate the surgical impact of benign ovarian mass on ovarian reserve as measured by serum follicle stimulating hormone (FSH), estradiol (E2) and anti-Müllerian hormone (AMH) levels, antral follicle count (AFC) and ovarian volumes. In addition, the differences in ovarian reserve impairment between endometrioma cystectomy and non-endometrioma cystectomy were investigated.
Methods
In this prospective study, 22 patients of reproductive age (range, 18.35 years) with benign ovarian masses were enrolled to undergo laparoscopic cystectomy. Of whom 12 had endometriomas and 10 had non-endometriomas. On early follicular phase (day 3) of the cycle preceding the operation and three months after the laparoscopic cystectomy, serum levels of FSH, E2 and AMH, AFC and ovarian volumes were measured in all patients. Data were analyzed with Mann-Whitney U-test and Wilcoxon rank test using SPSS ver. 12.0 for statistic analysis.
Results
Median level of serum AMH was significantly decreased from 5.48 ng/mL (interquartile range [IQR], 2.80-7.47) before cystectomy to 2.56 ng/mL (IQR, 1.74-4.32) 3 months postoperation (P<0.05). On the other hand, no significant differences in FSH, E2, AFC and ovarian volumes were found between the preoperative and three months postoperative levels. In a subgroup analysis of the pathologic type of the ovarian cyst, postoperative serum AMH levels were significantly decreased in the endometrioma group, but not in the non-endometrioma group.
Conclusion
Serum AMH levels were significantly decreased after laparoscopic cystectomy without any changes of other ovarian reserve tests.
Keywords: Anti-Müllerian hormone, Benign ovarian mass, Endometrioma, Laparoscopic cystectomy
Introduction
Benign ovarian cysts such as endometrioma, dermoid, serous or mucinous cystadenoma are seen frequently in young or reproductive aged women. Several surgical modalities can be performed to manage benign ovarian cysts. Over the last 10 years, laparoscopic techniques such as fenestration and drainage of the cyst and stripping/electrocoagulation of the cyst wall, and minilaparotomy have become increasingly common approaches for the surgical removal of benign ovarian tumors [1]. Recently, laparoscopic ovarian cystectomy is considered as the first-line choice and has gained increasing acceptance among gynecologic surgeons [1,2,3,4]. However, the safety of these techniques in terms of ovarian damage to the operated gonad has recently been questioned. Also, surgical approach for benign ovarian tumor may cause unavoidable risk of damage to residual normal ovarian tissue or ovarian reserve. A great deal of evidence supports that the removal of ovarian cysts is associated with injury to the ovarian reserve [4,5]. During cystectomy, the damage to the ovarian reserve is not only due to the inadvertent removal of healthy ovarian tissue, but also vascular compromise due to electrosurgical coagulation or postsurgical inflammation may also cause damage [5,6]. The technique of the surgery may also affect the change in the ovarian reserve.
Ovarian reserve is defined as the functional potential of the ovary, and reflects the number of the follicles left in the ovary at any given time. Recently, it has been shown that the serum anti-Müllerian hormone (AMH) may be a valuable marker of the ovarian reserve, which is menstrual cycle-independent and is unaffected by the use of oral contraceptive pills or gonadotropin-releasing hormone agonists. AMH has been reported to be a good indicator of ovarian reserve and the ovarian response to stimulation for assisted reproductive technology programs [7]. There are some reports examining the role of AMH for the evaluation of ovarian damage after the ovarian surgery [8,9,10,11,12].
The aim of this study was to evaluate the surgical impact of benign ovarian mass on ovarian reserve as measured by serum follicle stimulating hormone (FSH), estradiol (E2) and AMH levels, antral follicle count (AFC) and ovarian volumes. In addition, the differences in ovarian reserve impairment between endometrioma cystectomy and non-endometrioma cystectomy were also investigated.
Materials and methods
1. Patients
This prospective study was conducted on 24 patients, who were enrolled to undergo laparoscopic cystectomy between December 2010 and September 2011. Before enrollment, they were diagnosed as having uni-/bilateral benign ovarian cyst(s) by transvaginal ultrasound examination and histologically confirmed after surgery. Inclusion criteria were as follows: 1) 18 to 35 years of age with regular menstrual cycles (21 to 35 days); 2) no clinical signs or ultrasound evidence suspicious of ovarian malignancy; and 3) no evidence of any other endocrine disorders such as diabetes mellitus, thyroid dysfunction, hyperprolactinemia, congenital adrenal hyperplasia, Cushing's syndrome, or adrenal insufficiency. Patients with polycystic ovary syndrome according to the Rotterdam criteria [13], under any kind of hormonal treatment for at least 6 months, and with previous history of adnexal surgery were excluded from the study. Among 24 enrolled patients, one with unilateral endometrioma and counterlateral serous cystadenoma was excluded, and one with parovarian cyst was excluded. Finally, twelve with endometriomas (unilateral 6, bilateral 6) and ten with non-endometriomas (unilateral 9, bilateral 1; mature teratoma 6, mucinous cystadenoma 4) were analyzed.
2. Methods
Preoperatively, a blood sample was drawn to measure FSH, luteinizing hormone (LH), E2, and AMH during the early follicular phase (day 3) of the month in which surgery was scheduled. FSH and LH levels were determined using commercial kits by an immunoradiometric assay (DIAsource SA, Nivelles, Belgium) and E2 was measured by radioimmunoassay (DIAsource SA). Serum AMH was assayed using an enzyme immunoassay (Immunotech SAS, Marseilles, France). Intraassay and interassay coefficients of variation of AMH were 12.3% and 14.2%, with detection limit of 0.14 ng/mL. Transvaginal ultrasound examination was performed in the early follicular phase in order to estimate the ovarian volume and the AFC. The AFC was estimated as the total number of follicles measuring 2 to 10 mm in diameter within the ovary. The ovarian volume was calculated using the prolate ellipsoid fomular: π/6 × anteroposterior diameter × transverse diameter × longitudinal diameter (in cm). Preoperational ovarian volume were obtained by removal of the cyst volume from the total ovarian volume. In order to preclude the interobserver variation, all ultrasound examinations were done by one investigator using the same equipment; 5-MHz endovaginal transducer (SSD 1000, Aloka, Tokyo, Japan). The blood sample and transvaginal ultrasound examinations were then repeated three months after the laparoscopic cystectomy in the same cycle phase.
3. Procedure
All the laparoscopic operations were performed under general anesthesia by same team of experienced pelvic endoscopists. The surgery was done as follows. A three-port laparoscopy technique was used: After an 11-mm trocar was inserted through a subumbilical vertical incision, a 10-mm laparoscope was introduced and the pneumo-peritoneum was achieved by inflating with CO2 (12 mmHg); a lateral 10-mm operating port and a central suprapubic 5-mm operating port were also inserted. After inspection of the peritoneal cavity, the cyst was freed and every effort was made to excise the cyst without spilling its contents, especially when the ultrasound diagnosis had revealed a dermoid cyst. Removal of the cyst was carried out carefully by identifying the cyst wall and removing it from the ovarian cortex by traction with grasping forceps. Bipolar electrocoagulation was applied occasionally for hemostasis on the ovarian parenchyma with caution not to damage ovarian hillus and vascularity. The sutures were used for ovarian reconstruction. The cyst wall was removed from the abdomen by means of an endobag and all specimens obtained from operation were submitted for pathology examination.
4. Statistical analysis
Statistical analysis was performed using the SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). Serum levels of FSH, LH, E2, and AMH were compared between each sampling point (preoperatively and postoperatively at month 3) using Mann-Whitney U-test and Wilcoxon rank test. Correlations were calculated using the Pearson's test. P-value <0.05 was considered statistically significant.
Results
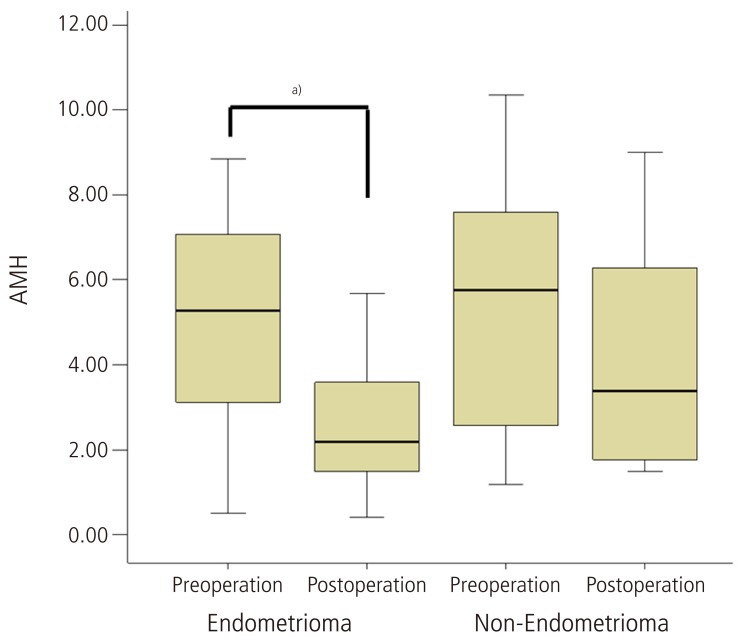
A total of 22 patients were included in this prospective study with a mean age of 26.4 ± 4.6 years. Patients ages (mean ± SD) were 27.0 ± 4.3 years in the endometrioma group, and 25.6 ± 5.3 years in the non-endometrioma group. Hormonal serum levels before the operation and 3 months after surgery are shown in Table 1. Median level of preoperative serum AMH was 5.48 ng/mL (interquartile range [IQR], 2.80-7.47) similar with regular menstruated control women (5.40 ng/mL; IQR, 3.02-7.04) at same age range in our previous study [14]. However, median level of serum AMH was significantly decreased to 2.56 ng/mL (IQR, 1.74-4.32) at 3 months after operation (P < 0.05). On the other hand, no significant differences in FSH, E2, AFC and ovarian volumes were found between the preoperative and three months postoperative levels. Compared with postoperative AFC, postoperative serum AMH levels showed significant positive correlations (r = 0.505, P = 0.01). In a subgroup analysis of the pathologic type of the ovarian cyst, 3 months postoperative serum AMH levels, of which the preoperative median level was 5.27 ng/mL (IQR, 3.12-7.08), were significantly decreased to 2.19 ng/mL (IQR, 1.49-3.60) in the endometrioma group compared with non-endometrioma group (Table 2, Fig. 1). Before cystectomy, serum AMH levels of women with bilateral endometrioma were 4.48 ng/mL (IQR, 3.44-6.52), already lower than those of women with unilateral endometrioma (6.36 ng/mL; IQR, 2.80-7.56), however, it was not statistically significant. Postoperative AMH levels of unilateral and also bilateral endometrioma were significantly decreased compared to preoperative AMH levels by Wilcoxon rank test. However, postoperative AMH levels of unilateral non-endometrioma were not significantly decreased compared to preoperative AMH levels.
Table 1.
The median levels of ovarian reserve markers before and 3 months after laparoscopic ovarian cystectomy (n=22)
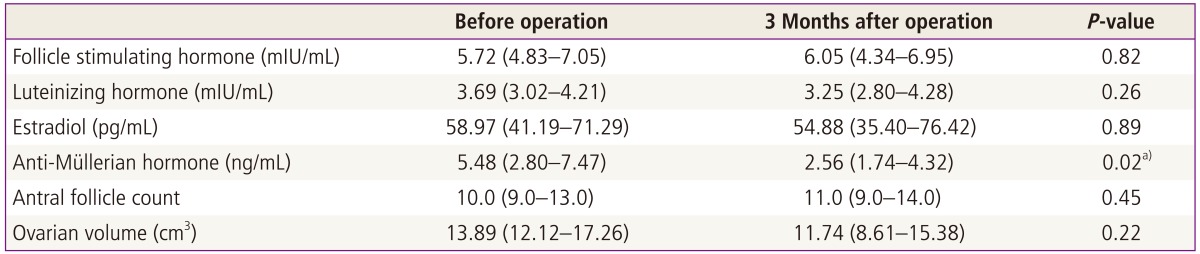
Values are median and interquartile range.
a)P<0.05 by Mann-Whitney test.
Table 2.
Comparison of ovarian reserve markers before and 3 months after surgery according to pathologic type of the ovarian cysts
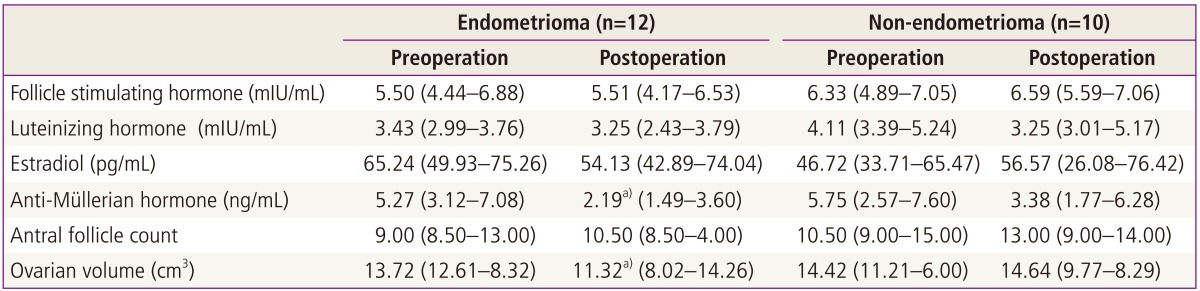
Values are median and interquartile range.
a)P<0.05 by Mann-Whitney test.
Fig. 1.
The box plot represents the serum anti-Müllerian hormone (AMH) levels before and 3 months after laparoscopic cystectomy according to the pathologic type. Serum AMH levels were significantly decreased after laparoscopic cystectomy for endometriomas. Data are given as Box-and-whisker plots. Lines inside boxes indicate median, and the upper and lower limits of the boxes and whiskers indicate interquartile and total ranges. a)P<0.05 by Mann-Whitney test.
Discussion
The treatment of benign ovarian cysts should be both curative and conservative [15]. Laparoscopic ovarian cystectomy usually is considered as the approach of choice for treating benign ovarian cysts. Compared with laparotomy, laparoscopy is associated with a faster recovery, better and closer visualization, shorter hospital stays, and less possible adhesion formation [16]. In the present study, a total of 22 patients underwent surgery by laparoscopic ovarian cystectomy. Benign ovarian cysts such as ovarian endometrioma are common in young women, so the technique used for surgery is crucial for sparing ovarian tissue and residual ovarian function after operation is important [17]. The present study was attempted to investigate the influence of ovarian cystectomy on the ovarian reserve by assessing the levels of serum FSH, E2 and AMH, AFC and ovarian volumes. Also, the differences in ovarian reserve impairment between endometrioma cystectomy and non-endometrioma cystectomy were investigated. Considering the folliculogenesis initiated before 3 months ago, markers of ovarian reserve were measured before the operation and 3 months after surgery in this study. And also, there were inconsistent results about the serial changes in the serum AMH after cystectomy. Celik et al. [8] measured the serum AMH until 6 month after cystectomy which was gradually declined. Chang et al. [9] reported the serum AMH was declined after cystectomy, then, gradually increased after one month, and that there was 65% recovery of the preoperative level after 3 months.
Although various test and markers of ovarian reserve have been reported, the clinical value of testing for basal FSH and inhibin-B value is limited. Ultrasonographic markers, such as AFC and ovarian volume, can be used as indicators of ovarian reserve, but it is difficult to assess the exact number of antral follicles and ovarian volume of the cystic ovary before cystectomy [18,19]. In this study, AFC was similar even after cystectomy. In other studies, AFC were found to be significantly increased after cystectomy unexpectedly [8,20]. We speculated that it could be a consequence that the preoperative AFC may be undercounted beyond the ovarian tumor or it may be due to a reactive response of ovarian parenchyma after surgery [8]. Recently, AMH produced by granulosa cells of the preantral follicles and antral follicles was suggested to be a good indicator of ovarian reserve and ovarian response to gonadotropin stimulation. Serum AMH levels appears to correspond well with AFC and ovarian response to hyperstimulation in in vitro fertilization [10]. It was suggested that AMH was a superior marker for predicting ovarian response over either age, FSH, or inhibin B [7,21]. Our study demonstrated that serum AMH levels were significantly decreased after 3 months postoperatively. Also, postoperative serum AMH level has significant positive correlations with postoperative AFC. On the other hand, there were no significant differences in FSH and E2 between the preoperative and three months postoperative levels. Considering serum FSH levels were not elevated until the perimenopausal transition even though the ovarian reserve was reduced after mid-thirties, serum FSH could be less sensitive to detect the change of ovarian reserve after meticulous cystectomy. Celik et al. [8] also reported that serum levels of FSH and E2 at 6 months after operation were not different compared with preoperative levels. We agree with their opinion that basal FSH, E2, and inhibin B have low sensitivity in detecting the early decrease in the ovarian reserve [22].
Several possible mechanisms underlying the reduction of ovarian reserve related ovarian cystectomy can be hypothesized [5]. First, the damage may precede surgery, i.e., the cyst itself may cause negative effects on the surrounding tissue [23]. Based on the histological analysis, it has been reported that the ovarian tissue surrounding the endometrioma cyst wall is morphologically altered and possibly not functional, thus suggesting that a functional disruption may already be present before surgery [24]. Histological alterations involving endometriomas did not appear to be present when the ovarian cortex surrounding mature teratoma and cystadenomas were studied [5,25]. Second, a considerable amount of ovarian tissue containing follicles is unintentionally removed during cystectomy, which leads to the decrease in ovarian reserve [23,25]. The removal of any benign cyst inadvertently leads to the removal of ovarian tissue, but the effect is more significant in endometriomas [17]. Muzii et al. [25] proposed that this difference was due to the presence of a pseudocapsule in an endometrioma versus a real capsule in a non-endometriotic cyst, which had a separate tissue plane making the dissection from tissue easy [17]. We also found that according to the pathologic type of the cyst, patients with ovarian endometriomas had significantly decreased serum AMH level at 3 months postoperativly compared with non-endometrioma cyst. Third, it could be directly damaged from electrosurgical coagulation for hemostasis. Furthermore, the reduction could be a consequence of damage to ovarian vasculature or an inflammation-mediated injury resulting in the loss of healthy ovarian follicles [9].
There are some reports examining the role of AMH for the evaluation of ovarian damage after the ovarian surgery [8,9,10,11,12]. Most of them were retrospective study or investigated only with endometrioma, or measured the serum AMH only. In this prospective study, we measured the serum AMH, FSH, E2, AFC, and ovarian volume in women with the endometrioma and non-endometrioma. Serum AMH levels were significantly decreased after laparoscopic cystectomy without the changes of other ovarian reserve markers. Serum AMH could be a delicate marker to provide surgical impact on ovarian reserve. Postoperative serum AMH levels were significantly decreased especially in endometrioma group, but not in non-endometrioma group. However, we could not analyze in the aspect of endometriosis stage or cyst size because of limited number. Further studies are required with larger sample size and histological evaluation to clarify the relation between the change of serum AMH and actual loss of ovarian reserve.
In conclusion, our results suggest that the laparoscopic cystectomy for benign ovarian masses, especially endometrioma, causes a postoperative decrease of serum AMH levels. We believe serum AMH could be reliable and easily measurable test to evaluate the surgical impact on ovarian reserve.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Medeiros LR, Rosa DD, Bozzetti MC, Fachel JM, Furness S, Garry R, et al. Laparoscopy versus laparotomy for benign ovarian tumour. Cochrane Database Syst Rev. 2009;(2):CD004751. doi: 10.1002/14651858.CD004751.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Canis M, Rabischong B, Houlle C, Botchorishvili R, Jardon K, Safi A, et al. Laparoscopic management of adnexal masses: a gold standard? Curr Opin Obstet Gynecol. 2002;14:423–428. doi: 10.1097/00001703-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Chapron C, Fauconnier A, Goffinet F, Breart G, Dubuisson JB. Laparoscopic surgery is not inherently dangerous for patients presenting with benign gynaecologic pathology. Results of a meta-analysis. Hum Reprod. 2002;17:1334–1342. doi: 10.1093/humrep/17.5.1334. [DOI] [PubMed] [Google Scholar]
- 4.Candiani M, Barbieri M, Bottani B, Bertulessi C, Vignali M, Agnoli B, et al. Ovarian recovery after laparoscopic enucleation of ovarian cysts: insights from echographic short-term postsurgical follow-up. J Minim Invasive Gynecol. 2005;12:409–414. doi: 10.1016/j.jmig.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Li CZ, Liu B, Wen ZQ, Sun Q. The impact of electrocoagulation on ovarian reserve after laparoscopic excision of ovarian cysts: a prospective clinical study of 191 patients. Fertil Steril. 2009;92:1428–1435. doi: 10.1016/j.fertnstert.2008.08.071. [DOI] [PubMed] [Google Scholar]
- 6.Hachisuga T, Kawarabayashi T. Histopathological analysis of laparoscopically treated ovarian endometriotic cysts with special reference to loss of follicles. Hum Reprod. 2002;17:432–435. doi: 10.1093/humrep/17.2.432. [DOI] [PubMed] [Google Scholar]
- 7.La Marca A, Broekmans FJ, Volpe A, Fauser BC, Macklon NS ESHRE Special Interest Group for Reproductive Endocrinology: AMH Round Table. Anti-Müllerian hormone (AMH): what do we still need to know? Hum Reprod. 2009;24:2264–2275. doi: 10.1093/humrep/dep210. [DOI] [PubMed] [Google Scholar]
- 8.Celik HG, Dogan E, Okyay E, Ulukus C, Saatli B, Uysal S, et al. Effect of laparoscopic excision of endometriomas on ovarian reserve: serial changes in the serum antiMüllerian hormone levels. Fertil Steril. 2012;97:1472–1478. doi: 10.1016/j.fertnstert.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 9.Chang HJ, Han SH, Lee JR, Jee BC, Lee BI, Suh CS, et al. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-Müllerian hormone levels. Fertil Steril. 2010;94:343–349. doi: 10.1016/j.fertnstert.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 10.Ercan CM, Sakinci M, Duru NK, Alanbay I, Karasahin KE, Baser I. AntiMüllerian hormone levels after laparoscopic endometrioma stripping surgery. Gynecol Endocrinol. 2010;26:468–472. doi: 10.3109/09513591003632134. [DOI] [PubMed] [Google Scholar]
- 11.Lee DY, Kim NY, Kim MJ, Yoon BK, Choi D. Effects of laparoscopic surgery on serum anti-Müllerian hormone levels in reproductive-aged women with endometrioma. Gynecol Endocrinol. 2011;27:733–736. doi: 10.3109/09513590.2010.538098. [DOI] [PubMed] [Google Scholar]
- 12.Raffi F, Metwally M, Amer S. The impact of excision of ovarian endometrioma on ovarian reserve: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2012;97:3146–3154. doi: 10.1210/jc.2012-1558. [DOI] [PubMed] [Google Scholar]
- 13.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Jang TG, Lee A, Kim YJ, Lee KY, Rhee JH, Park JC. Age-related normal serum concentrations of anti-Müllerian hormone and comparison of ovarian reserve tests in a cohort of healthy Korean women. Fertil Steril. 2012;98:S85. [Google Scholar]
- 15.Mohamed ML, Nouh AA, El-Behery MM, Mansour SA. Effect on ovarian reserve of laparoscopic bipolar electrocoagulation versus laparotomic hemostatic sutures during unilateral ovarian cystectomy. Int J Gynaecol Obstet. 2011;114:69–72. doi: 10.1016/j.ijgo.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Alborzi S, Foroughinia L, Kumar PV, Asadi N, Alborzi S. A comparison of histopathologic findings of ovarian tissue inadvertently excised with endometrioma and other kinds of benign ovarian cyst in patients undergoing laparoscopy versus laparotomy. Fertil Steril. 2009;92:2004–2007. doi: 10.1016/j.fertnstert.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Dogan E, Ulukus EC, Okyay E, Ertugrul C, Saygili U, Koyuncuoglu M. Retrospective analysis of follicle loss after laparoscopic excision of endometrioma compared with benign nonendometriotic ovarian cysts. Int J Gynaecol Obstet. 2011;114:124–127. doi: 10.1016/j.ijgo.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Kitajima M, Khan KN, Hiraki K, Inoue T, Fujishita A, Masuzaki H. Changes in serum anti-Müllerian hormone levels may predict damage to residual normal ovarian tissue after laparoscopic surgery for women with ovarian endometrioma. Fertil Steril. 2011;95:2589–2591. doi: 10.1016/j.fertnstert.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 19.Maheshwari A, Fowler P, Bhattacharya S. Assessment of ovarian reserve: should we perform tests of ovarian reserve routinely? Hum Reprod. 2006;21:2729–2735. doi: 10.1093/humrep/del188. [DOI] [PubMed] [Google Scholar]
- 20.Tsolakidis D, Pados G, Vavilis D, Athanatos D, Tsalikis T, Giannakou A, et al. The impact on ovarian reserve after laparoscopic ovarian cystectomy versus three-stage management in patients with endometriomas: a prospective randomized study. Fertil Steril. 2010;94:71–77. doi: 10.1016/j.fertnstert.2009.01.138. [DOI] [PubMed] [Google Scholar]
- 21.Hwu YM, Wu FS, Li SH, Sun FJ, Lin MH, Lee RK. The impact of endometrioma and laparoscopic cystectomy on serum anti-Müllerian hormone levels. Reprod Biol Endocrinol. 2011;9:80. doi: 10.1186/1477-7827-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park JC, Bae JG, Kim JI, Rhee JH. Assessment of ovarian volume and hormonal changes after ovarian cystectomy in the different ovarian tumor. Korean J Reprod Med. 2008;35:155–162. [Google Scholar]
- 23.Hirokawa W, Iwase A, Goto M, Takikawa S, Nagatomo Y, Nakahara T, et al. The post-operative decline in serum anti-Müllerian hormone correlates with the bilaterality and severity of endometriosis. Hum Reprod. 2011;26:904–910. doi: 10.1093/humrep/der006. [DOI] [PubMed] [Google Scholar]
- 24.Maneschi F, Marasa L, Incandela S, Mazzarese M, Zupi E. Ovarian cortex surrounding benign neoplasms: a histologic study. Am J Obstet Gynecol. 1993;169(2 Pt 1):388–393. doi: 10.1016/0002-9378(93)90093-x. [DOI] [PubMed] [Google Scholar]
- 25.Muzii L, Bianchi A, Croce C, Manci N, Panici PB. Laparoscopic excision of ovarian cysts: is the stripping technique a tissue-sparing procedure? Fertil Steril. 2002;77:609–614. doi: 10.1016/s0015-0282(01)03203-4. [DOI] [PubMed] [Google Scholar]