ABSTRACT
BACKGROUND
In patients hospitalized with community-acquired pneumonia (CAP), indicators of clinical instability at discharge (fever, tachycardia, tachypnea, hypotension, hypoxia, decreased oral intake and altered mental status) are associated with poor outcomes. It is not known whether the order of indicator stabilization is associated with outcomes.
OBJECTIVES
To describe variation in the sequences, including whether and in what order, indicators of clinical instability resolve among patients hospitalized with CAP, and to assess associations between patterns of stabilization and patient-level outcomes.
DESIGN / PARTICIPANTS / MAIN MEASURES
Chart review ascertained whether and when indicators stabilized and other data for 1,326 adult CAP patients in six U.S. academic medical centers. The sequences of indicator stabilization were characterized using sequence analysis and grouped using cluster analysis. Associations between sequence patterns and 30-day mortality, length of stay (LOS), and total costs were modeled using regression analysis.
KEY RESULTS
We found 986 unique sequences of indicator stabilization. Sequence analysis identified eight clusters of sequences (patterns) derived by the order or speed in which instabilities resolved or remained at discharge and inpatient mortality. Two of the clusters (56 % of patients) were characterized by almost complete stabilization prior to discharge alive, but differing in the rank orders of four indicators and time to maximum stabilization. Five other clusters (42 % of patients) were characterized by one to three instabilities at discharge with variable orderings of indicator stabilization. In models with fast and almost complete stabilization as the referent, 30-day mortality was lowest in clusters with slow and almost complete stabilization or tachycardia or fever at discharge [OR = 0.73, 95 % CI = (0.28–1.92)], and highest in those with hypoxia with instabilities in mental status or oral intake at discharge [OR = 3.99, 95 % CI = (1.68–9.50)].
CONCLUSIONS
Sequences of clinical instability resolution exhibit great heterogeneity, yet certain sequence patterns may be associated with differences in days to maximum stabilization, mortality, LOS, and hospital costs.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-013-2626-7) contains supplementary material, which is available to authorized users.
KEY WORDS: community-acquired pneumonia, hospitalization, social sequence analysis, process of care, hospital discharge
INTRODUCTION
From the work of the Community-Acquired Pneumonia (CAP) Patient Outcomes Research Team (PORT), mortality and readmission of CAP patients after discharge have been known to be associated with seven measures of clinical instability at discharge: fever, tachycardia, tachypnea, hypotension, hypoxia, the inability to maintain oral intake, and failure to return to baseline mental status.1–6 Based on this, practice guidelines recommend delaying discharge among patients with continuing clinical instability.2,3,7
While the clinical significance of the number of clinical instabilities at discharge among CAP patients has received attention,4,5 the temporal order in which these instabilities resolve may also be associated with outcomes, but this has not been investigated.8 If variation in the way instabilities resolve matters in terms of important clinical outcomes, then adjustments to the in-hospital management of CAP patients may be warranted. The prognostic importance of the order of clinical events or interventions has been studied in only a few, very simple, clinical contexts,9 but has been associated with important outcomes. For example, preexisting congestive heart failure (CHF) predicts higher mortality among patients who develop atrial fibrillation, but preexisting atrial fibrillation does not predict higher mortality among patients who develop CHF.10 Similarly, sequencing of measles, diphtheria-tetanus-pertussis, and inactivated poliovirus vaccinations predict mortality risk.11,12
The number of CAP indicators presents a larger methodological challenge in characterizing and studying the consequences of the sequence of clinical events. However, methods for sequence analysis that can handle a large number of categorically coded events or states are widely used in genomics (e.g., sequences of amino acids)13,14 and computational linguistics and computer science (e.g., strings of text),15 and are increasingly used in the social sciences.16,17 These methods typically use optimal matching (OM) algorithms18 and statistical cluster analysis19 to find and categorize observed sequences into a smaller number of groups based on degree of similarity, which can then be used as categorical covariates for subsequent analysis. Sequence analysis also often uses graphical depictions of sequences for exploratory analyses.20
This study uses sequence analysis to describe and categorize patterns in which clinical instabilities resolve in hospitalized CAP patients, and analyzes the association of these sequences with clinical outcomes and resource utilization.
METHODS
Study Subjects and Study Design
Data were retrospectively abstracted from the charts of 1,461 patients age 18 years and older hospitalized for CAP on general medical/hospitalist ward services from 2000 to 2003 across five academic medical centers [Brigham and Women’s Hospital (BWH), University of California at San Francisco (UCSF), University of Iowa (UI), University of New Mexico (UNM), University of Wisconsin (UW)], and from 2001 to 2006 at the University of Chicago (UC), all of whom gave informed consent to participate in the Multicenter Hospitalist Study, a larger study examining the effects of academic hospitalists on resource allocation and quality of care.21,22 Pneumonia cases were identified if they had a principal discharge diagnosis of pneumonia (International Classification of Disease, Ninth Edition, Clinical Modification [ICD-9-CM] codes 480.0–480.9, 481, 482.0–482.9, 483.0–483.8, 485, 486, 487.0, 507.0) or a principal discharge diagnosis of acute respiratory failure (ICD-9-CM code 518.81) and a secondary discharge diagnosis of pneumonia.23 The following patients were excluded: HIV positive, history of organ transplant, prior hospitalization within 10 days of presentation, or no chest X-ray within 24 h of presentation. Admission and discharge dates were available for all sites except UNM. The study was approved by all sites’ Institutional Review Boards.
Self-reported health status (excellent–poor), race/ethnicity, and Charlson Comorbidity Index24 were ascertained during a patient interview conducted by trained research assistants shortly after admission. Symptoms on presentation, indicator stabilization times and dates, radiology and laboratory data were obtained by chart review and used to calculate the Pneumonia Severity Index (PSI) scores.25 Insurance status (private, Medicare, Medicaid or no insurance), in-hospital mortality, hospitalization costs, and attending physician were obtained from each hospital’s administrative databases. Attending physicians were identified as hospitalist or not by the site principal investigators. Statistics on death within 30 days of admission were obtained via linkage to the National Death Index.
Trained abstractors at each site used chart review to obtain data on the date and time (hh:mm) of resolution of each of the following seven instabilities: temperature > 37.8 °C (T); heart rate > 100 beats/min (HR); respiratory rate > 24 breaths/min (RR); systolic blood pressure < 90 mm Hg (BP); blood oxygen saturation < 90 % (O2); failure to return to baseline ability to maintain oral intake (OI); and failure to return to baseline mental status (MS).3 These clinical variables were charted according to the usual standard of care at each participating site. Following PORT criteria, an indicator was deemed stabilized after it had remained stable for 24 h.6,26 Days to maximal stability was defined as the number of days from admission to the date that the last indicator was stabilized.
Sequence Characterization and Clustering
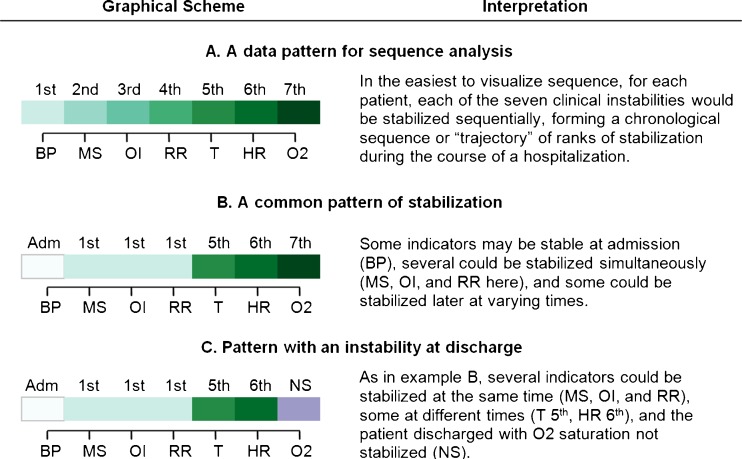
We developed a sequence coding scheme based on whether indicators were stabilized, and in what order, with ties treated as a unique event. (Supplementary Table S1, online, contains further details.) Figure 1 shows sample patterns of indicator stabilization for three patients. Each indicator is assigned a rank ordering of 1 to 7, based on the order of stabilization during the hospital stay, 0 if stable at admission, and 8 or 9 for indicators not stabilized (NS) at the time of discharge alive, and dead (Died), respectively. Patients who had no indicators stabilized (n = 30), any missing stabilization data (n = 99), or a length of stay (LOS) greater than 30 days (n = 6) were excluded from subsequent analyses, yielding 1,326 patients for this study. We conducted a series of comparisons between those excluded and included, which revealed no significant differences in age group, sex, LOS, or PSI. This gave us confidence that exclusion of these subjects would have little to no effect on our findings.
Figure 1.
Basic sequential patterns of stabilization of discharge criteria. This figure shows the graphical scheme used in subsequent figures, with different shades of color assigned to the orders of stabilization, panel A. The rank order of stabilization is shown in a left to right sequence corresponding to the order in which each indicator is stabilized. The order displayed here and in subsequent graphics (BP = blood pressure; MS = return to baseline mental status; OI = ability to feed by oral intake; RR = respiratory rate; T = temperature; HR = heart rate; O2 = Blood oxygen saturation) corresponds to the overall average resolution pattern in our data, so this “baseline” order is adopted for convenience. Several indicators can have the same rank, since multiple indicators can be recorded as stabilized at the same observation point. For example, in panel B, three indicators were stabilized at observation point 1, so they were all ranked “1st.” Varying shades of green = stabilized nth or tied for nth. In addition to the instability resolution coding, we added three additional codes to our data and graphical displays: White = Indicator stable at admission; Purple = Indicator not stabilized before discharge; Orange = Patient died in-hospital before indicator stabilized.
To examine coherence in the large number of sequences of stabilization of the seven indicators, we used an optimal matching (OM) algorithm27 to generate scores that characterize the dissimilarity between sequences and Ward’s clustering method28 to group sequences into clusters based on these scores. As with the application of these methods to some types of DNA sequencing,14,18 the essence of this approach is to define clusters that minimize the number and type of changes (e.g., substitutions, insertions, deletions, etc.) in ranks within each cluster that would be needed to make the sequences the same within that cluster. We used Stata 11 (StataCorp LP, College Station, TX) with the SQ-ADOS sequence analysis routine to perform the regressions and OM,27 and TraMineR version 1.8-3 with WeightedCluster version 0.9-8 in R to compute cluster quality statistics.29
Since we sought to identify clusters that were clinically meaningful and similar enough to permit analysis, and because there is no literature on indicator stabilization sequences in CAP, we performed sensitivity analyses examining two to 20 clusters of sequences [Average Silhouette Width (ASW), a summary measure of cluster quality, ranged from 0.11 to 0.26].19 Clusters were largely distinguished by which instabilities had stabilized by discharge, the order and speed of stabilization, and in-hospital mortality. We used the eight-cluster (ASW = 0.14) solution as an optimal combination of cluster quality (two clusters was best, ASW = 0.26), discrimination between types of sequences and patients, and cluster size (all > 30 patients). We gave clusters descriptive labels based on clinical characteristics. Clusters were used as nominal variables in regression models for clinical outcomes.
Analysis
We compared the sample characteristics across sites using ANOVA for continuous variables and Chi-square analysis for categorical variables. To assess variability in stabilization patterns, we plotted the distributions of rank at which each indicator stabilized (e.g., 1st, 2nd, 3rd,…) for the entire sample. In addition to rank order for each of the seven indicators, several sequence-specific statistics were computed for each patient and aggregated by cluster: the number of indicators stable at admission, the number of instabilities at discharge, and the number of days needed to achieve maximum stabilization. In addition, we calculated the average rank of stabilization of each indicator within each cluster (e.g., an indicator could stabilize, on average, 2.4th within one cluster but 4.3th within another). Logistic and generalized linear regression (GLM) models with log link and robust standard errors were used to examine the relationships between 30-day mortality, log transformed LOS, and log transformed total cost as dependent variables, and PSI score, the number of indicators stable at admission, and categorical indicator variables for each cluster as independent variables, controlling for site and patient characteristics.
RESULTS
Characteristics of the study subjects by site are shown in Table 1. Mean age and percent female varied across sites, from 58.4 to 69.8 years, and 45.8 % to 62.5 %, respectively. The average number of days to maximum stabilization was 3.1 (SD 3.4). The average patient was discharged with 6.3 (SD 0.9) indicators stabilized; 42.9 % of patients were discharged with one or more instabilities. Average LOS was 5.0 (SD 4.3) days and the overall 30-day mortality rate was 8.2 %. The average cost of hospitalization was $9,347 (SD $12,408).
Table 1.
Characteristics of the Sample of Community-Acquired Pneumonia Patients at Six University Hospital Sites
| All | UC | BWH | UCSF | UI | UNM | UW | P* | |
|---|---|---|---|---|---|---|---|---|
| (n = 1,326) | (n = 647) | (n = 27) | (n = 306) | (n = 70) | (n = 156) | (n = 120) | ||
| Age, mean (SD) | 64.5 (19.8) | 64.0 (20.2) | 62.1 (18.3) | 69.8 (19.1) | 62.2 (18.8) | 58.4 (18.2) | 64.4 (18.8) | < 0.001 |
| Female, % | 56.7 | 62.4 | 51.9 | 51.5 | 55.7 | 52.6 | 45.8 | 0.002 |
| Black, % | 42.0 | 78.1 | 14.8 | 12.1 | 2.9 | 1.3 | 5.8 | < 0.001 |
| Insurance | ||||||||
| Private, % | 11.1 | 17.3 | 9.5 | 2.0 | 2.9 | 14.3 | 4.2 | < 0.001 |
| Medicare, % | 61.2 | 55.9 | 52.4 | 73.5 | 68.1 | 42.9 | 67.5 | < 0.001 |
| Medicaid, % | 15.4 | 20.9 | 14.3 | 12.1 | 5.8 | 10.4 | 3.3 | < 0.001 |
| None, % | 12.3 | 5.9 | 23.8 | 12.4 | 23.2 | 32.5 | 25.0 | < 0.001 |
| Health: fair or poor, % | 49.1 | 47.8 | 57.7 | 51.7 | 50.0 | 50.7 | 44.7 | 0.72 |
| Charlson index, mean (SD) | 1.2 (1.3) | 1.2 (1.2) | 1.2 (1.1) | 1.2 (1.3) | 1.5 (1.5) | 0.6 (1.1) | 1.2 (1.3) | < 0.001 |
| Weekend admission, % | 25.2 | 23.8 | 48.2 | 29.7 | 21.4 | — | 18.3 | 0.005 |
| Days to maximum stabilization, mean (SD) | 3.1 (3.4) | 2.9 (3.4) | 3.7 (3.9) | 3.0 (3.3) | 4.3 (3.6) | — | 3.5 (3.3) | 0.004 |
| # Factors stabilized before discharge, mean (SD) | 6.3 (0.9) | 6.4 (0.9) | 5.9 (1.0) | 6.3 (1.0) | 6.4 (0.9) | 6.3 (1.0) | 6.2 (0.9) | 0.05 |
| Discharged with ≥ 1 instability, % | 42.9 | 39.7 | 55.6 | 41.5 | 41.4 | 46.8 | 56.7 | 0.01 |
| Length of Stay, mean (SD) | 5.0 (4.3) | 4.5 (4.0) | 7.3 (7.1) | 5.2 (4.8) | 5.8 (4.2) | 5.2 (4.4) | 5.4 (3.6) | 0.000 |
| In-hospital mortality, % | 3.4 | 3.4 | 7.4 | 4.9 | 1.4 | 2.0 | 1.7 | 0.29 |
| 30-day mortality, % | 8.2 | 7.0 | 11.1 | 11.8 | 4.3 | — | 7.5 | 0.08 |
| Total hospitalization cost, $ mean (SD) | 9,347 (12,408) | 9,126 (11,270) | 14,098 (21,049) | 11,318 (16,633) | 6,290 (5,199) | 7,820 (9,756) | 8,173 (7,218) | 0.002 |
UC University of Chicago, BWH Brigham and Women’s Hospital, UCSF University of California at San Francisco, UI University of Iowa, UNM University of New Mexico, UW University of Wisconsin
*P values indicate tests of differences across sites by ANOVA or Chi-square tests
—Data not available
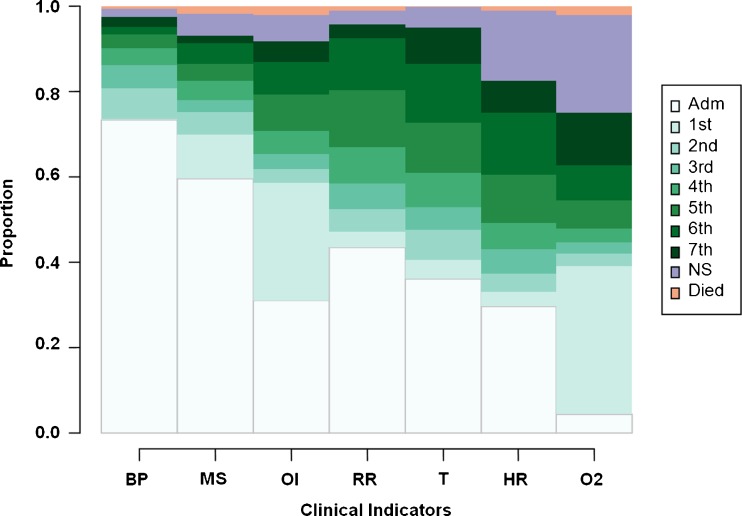
Figure 2 depicts the proportional distribution of the rank order of stabilizations for each of the seven indicators, remaining instabilities, in-hospital deaths, and indicators already stable at admission for the full sample of patients. (Supplementary Figure S1, online, displays this by site.)
Figure 2.
Proportional distributions of the rank order of indicator stabilizations ( N = 1,326). This figure shows the proportional distributions of the rank orders of indicator stabilization as stacked bars, with different shades of color assigned to each order of stabilization. For example, the white bars show that the BP and MS instabilities were most often (73 % and 59 %, respectively) already recorded as stable at admission. The palest green bars show that the OI and O2 instabilities were most often (28 % and 35 %, respectively) resolved first or tied for first in rank order of resolution of all seven instabilities. The darkest green portion of the O2 saturation instability shows that O2 was resolved last (or 7th) for about 13 % of patients, and the purple portion shows that it remained unstable on discharge for about 23 % of patients. White = indicator stable at admission; Varying shades of green = stabilized nth or tied for nth; Purple = Indicator not stabilized before discharge; Orange = Patient died in-hospital before indicator stabilized. BP = blood pressure; MS = return to baseline mental status; OI = ability to feed by oral intake; RR = respiratory rate; T = temperature; HR = heart rate; O2 = Blood oxygen saturation.
There were 986 unique stabilization patterns among the 1,326 patients studied. Table 2 shows the 21 patterns with five or more occurrences in the sample, accounting for 197 (14.9 %) of all patients. The most common pattern (n = 36) is that of six indicators already stable at admission, with O2 stabilized later at observation point 1. A similar sequence [BP-MS-OI-RR-T-HR-O2] corresponds to the overall average sequence of resolution, calculated using the average of ranks for all patients. However, patients exhibited great heterogeneity in resolution, with only 35.9 % of patients sharing the same sequence with at least one other patient.
Table 2.
Most Common Instability Resolution Patterns Among 1,326 Community-Acquired Pneumonia Patients
| Most common instability resolution patterns | |||||||
|---|---|---|---|---|---|---|---|
| Pattern | Order of stabilization | n | % | ||||
| At admission | 1st | 2nd | 3rd | 4th | |||
| 1 | [BP - MS - OI - RR - T - HR] | [O2] | 36 | 2.71 | |||
| 2 | [BP - MS - RR - T - HR] | [OI - O2] | 19 | 1.43 | |||
| 3 | [BP - MS - OI - RR] | [O2] | [T] | [HR] | 12 | 0.90 | |
| 4 | [BP - MS - RR - T - HR] | [O2] | [OI] | 12 | 0.90 | ||
| 5 | [BP - MS - OI - RR - HR] | [O2] | [T] | 11 | 0.83 | ||
| 6 | [BP - MS - RR] | [OI - O2] | [HR] | [T] | 11 | 0.83 | |
| 7 | [BP - MS - OI - RR - T] | [O2] | [HR] | 10 | 0.75 | ||
| 8 | [BP - MS - OI - RR] | [O2] | [HR] | [T] | 9 | 0.68 | |
| 9 | [BP - MS - OI - RR - T] | [O2] [hr→] | 8 | 0.60 | |||
| 10 | [BP - MS - RR - T - HR] | [OI] | [O2] | 8 | 0.60 | ||
| 11 | [BP - MS - RR - HR] | [OI - O2] | [T] | 7 | 0.53 | ||
| 12 | [BP - MS - RR - T] | [OI - O2] | [HR] | 6 | 0.45 | ||
| 13 | [BP - MS - RR - T] | [OI - O2] [hr→] | 6 | 0.45 | |||
| 14 | [BP - MS - RR] | [OI - O2] | [T] | [HR] | 6 | 0.45 | |
| 15 | [BP - MS - RR] | [OI - O2] | [T] [hr→] | 6 | 0.45 | ||
| 16 | [BP - MS - OI - RR - T] | [HR] | [O2] | 5 | 0.38 | ||
| 17 | [BP - MS - OI] | [O2] | [RR] | [HR] | [T] | 5 | 0.38 |
| 18 | [BP - MS - RR - T] | [OI] | [HR] [o2→] | 5 | 0.38 | ||
| 19 | [BP – MS – RR – HR] | [OI] | [T] [o2→] | 5 | 0.38 | ||
| 20 | [BP - MS - T - HR] | [OI] | [RR] [o2→] | 5 | 0.38 | ||
| 21 | [BP - MS - T] | [OI] | [HR] | [RR] [o2→] | 5 | 0.38 | |
| TOTALS | 197 | 14.86 | |||||
All indicator stabilization sequences with five or more occurrences among the 1,326 patients in the sample are shown. These 21 of 986 unique patterns accounted for 197 patients (14.86 % of sample). The “Stable at Admission” column in the table shows the indicators that were already stable at admission. The stabilized “1st” column shows the indicators that were stabilized at the same, first, observation point during the hospitalization before discharge, and, if any, the indicators that remained unstable before discharge (lowercase and →). The following three columns show the indicators stabilized second, third, or fourth before discharge, along with indicators, if any, that remained unstable. The last two columns show how many (n, %) patients in the sample had each respective instability resolution pattern. For example, for pattern 15, six patients presented with BP, MS, and RR already stable, OI and O2 indicators were later observed to stabilize together, T was next observed to stabilize by itself, and these patients were discharged before HR could be stabilized.
BP blood pressure; MS return to baseline mental status; OI ability to feed by oral intake; RR respiratory rate; T temperature; HR heart rate; O2 Blood oxygen saturation
Results of the cluster analysis allow us to further examine patterns among the sequences. (Sequence index plots for all eight clusters are in Supplementary Figure S2, online.) Two clusters (Stabilized Fast, Stabilized Slow) accounted for 56 % of patients, and were characterized by complete or almost complete stabilization prior to discharge alive, differing in the rank orders of RR, T, HR, and O2 and time to maximum stabilization (Table 3). Patients in both of these clusters were close to the overall sample average distributions of age, gender, PSI score, and Charlson Index. Five of the clusters, capturing 42 % of patients (Tachycardic, Febrile, Hypoxic, Tachycardic/Hypoxic, and Mental/Oral/Hypoxic), were characterized by one to three instabilities at discharge, with varying rank orderings of indicators that stabilized. The Tachycardic/Hypoxic cluster patients were characterized by tachycardia and hypoxia rarely controlled or controlled late in the hospitalization. The Mental/Oral/Hypoxic cluster was characterized by failures to return to baseline mental status, anorexia, hypoxia, and the highest 30-day mortality of those surviving to discharge (26.3 %). The final cluster (Inpatient Mortality) captured most of the patients who died in hospital with low rates of instability resolution except T and BP. This cluster was older and sicker than patients in the other clusters, and had the highest LOS and total cost. All but one of the patients in the Inpatient Mortality cluster died before discharge, capturing 69 % (n = 31) of all in-hospital deaths (n = 45), while the Hypoxic cluster had 16 % (n = 7) of in-hospital deaths.
Table 3.
Characteristics of Clusters Derived from 1,326 Patient Sequences of Instability Stabilization
| Characteristic | Stabilized fast | Stabilized Slow | Tachy-cardic | Febrile | Hypoxic | Tachy-cardic/Hypoxic | Mental/Oral/Hypoxic | Inpatient mortality | P* |
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| N (% of 1,326) | 434 (32.7) | 307 (23.2) | 120 (9.1) | 45 (3.4) | 196 (14.8) | 84 (6.3) | 108 (8.1) | 32 (2.4) | |
| Rank orders of stabilization† | |||||||||
| Average Order of Stabilization‡ | [MS-RR-BP-OI-O2-HR-T] | [BP-MS-OI-T-RR-HR-O2] | [BP-MS-OI-RR-O2-T-HR] | [MS-BP-OI- O2-RR-HR-T] | [BP-MS-OI-T-HR-RR-O2] | [BP-MS-OI-T-RR-HR-O2] | [BP-T-RR-HR-O2-MS-OI] | [T-BP-HR-RR-O2-MS-OI] | |
| Blood pressure (BP) | 1.05 (2.48) | 1.28 (1.90) | 0.51 (1.31) | 1.13 (2.27) | 1.07 (1.80) | 0.80 (1.60) | 1.22 (1.75) | 2.94 (3.34) | < 0.001 |
| Mental status (MS) | 0.98 (2.01) | 1.30 (2.03) | 1.20 (2.02) | 0.56 (1.08) | 1.38 (2.09) | 0.89 (1.55) | 5.93 (2.93) | 6.68 (3.63) | < 0.001 |
| Oral intake (OI) | 2.23 (2.64) | 2.04 (2.31) | 1.94 (2.03) | 1.84 (2.42) | 2.11 (2.10) | 1.48 (1.86) | 6.42 (2.73) | 7.93 (2.86) | < 0.001 |
| Respiratory rate (RR) | 1.05 (2.18) | 4.06 (2.14) | 2.48 (2.56) | 2.31 (2.52) | 3.16 (2.45) | 4.30 (3.40) | 2.36 (2.29) | 5.19 (3.54) | < 0.001 |
| Temperature (T) | 2.89 (3.11) | 3.44 (2.40) | 2.94 (2.56) | 7.93 (0.45) | 2.60 (2.31) | 3.29 (2.99) | 2.10 (2.19) | 1.78 (2.04) | < 0.001 |
| Heart rate (HR) | 2.42 (3.01) | 4.34 (2.06) | 8.00 (0.00) | 5.47 (2.90) | 3.03 (2.42) | 6.71 (2.42) | 2.64 (2.76) | 4.88 (3.76) | < 0.001 |
| Oxygen saturation (O2) | 2.26 (2.40) | 4.59 (2.48) | 2.71 (2.20) | 2.29 (1.93) | 8.04 (0.19) | 7.01 (2.27) | 5.08 (2.79) | 6.19 (3.68) | < 0.001 |
| Stabilization | |||||||||
| Hospitalist, n (%) | 134 (30.9) | 92 (30.0) | 39 (32.5) | 16 (35.6) | 65 (33.2) | 31 (36.9) | 34 (31.5) | 15 (46.9) | 0.61 |
| # factors stabilized at presentation | 3.81 (1.30) | 2.08 (1.34) | 2.76 (1.32) | 2.38 (1.53) | 2.43 (1.48) | 2.54 (1.28) | 1.94 (1.31) | 1.56 (0.91) | < 0.001 |
| Fully stabilized, n (%) | 411 (94.7) | 304 (99.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | < 0.001 |
| # instabilities at discharge | 0.08 (0.39) | 0.01 (0.10) | 1.01 (0.09) | 1.62 (0.68) | 1.03 (0.19) | 2.13 (0.65) | 1.89 (0.90) | 3.19 (1.23) | < 0.001 |
| Days to maximum stabilization§ | 2.14 (2.04) | 4.45 (4.19) | 2.56 (3.35) | 1.60 (1.84) | 2.94 (3.32) | 2.39 (2.61) | 4.55 (4.40) | 4.41 (3.61) | < 0.001 |
| Demographics/Health status | |||||||||
| Age, years | 66.5 (19.7) | 61.6 (19.6) | 57.2 (20.2) | 58.0 (18.2) | 66.5 (17.8) | 58.0 (19.9) | 73.5 (17.5) | 79.4 (16.6) | < 0.001 |
| Female, n (%) | 255 (58.8) | 172 (56.2) | 78 (65.0) | 24 (53.3) | 108 (55.1) | 42 (50.0) | 56 (51.9) | 16 (50.0) | 0.36 |
| PSI score | 75.7 (43.6) | 79.2 (46.2) | 64.6 (42.3) | 61.1 (45.3) | 87.7 (46.2) | 72.8 (52.8) | 117.2 (47.6) | 137.9 (53.5) | 0.001 |
| Charlson Index | 1.19 (1.30) | 1.28 (1.36) | 0.97 (0.99) | 1.00 (1.04) | 1.05 (1.19) | 1.15 (1.15) | 1.10 (1.23) | 1.34 (1.60) | 0.25 |
| Hospital stay | |||||||||
| Length of stay, days | 4.07 (3.40) | 5.92 (4.58) | 3.90 (3.38) | 2.42 (1.44) | 5.54 (4.84) | 4.01 (3.48) | 6.42 (5.08) | 9.50 (6.25) | < 0.001 |
| 30-day mortality§, n (%) | 15 (3.85) | 11 (4.09) | 0 (0.00) | 0 (0.00) | 14 (8.59) | 2 (2.74) | 25 (26.3) | 29 (100.0) | < 0.001 |
| Inpatient mortality, n (%) | 3 (0.70) | 3 (1.00) | 0 (0.00) | 0 (0.00) | 7 (3.57) | 1 (1.19) | 0 (0.00) | 31 (96.9) | < 0.001 |
| Total cost, $ | 6,210 (6,626) | 11,789 (14,319) | 6,967 (9588) | 3,700 (3,031) | 11,313 (14,907) | 7,908 (8,506) | 12,913 (14,408) | 24,860 (26,011) | < 0.001 |
BP blood pressure, MS return to baseline mental status, OI ability to feed by oral intake; RR respiratory rate, T temperature, HR heart rate, O2 blood oxygen saturation
*P values indicate tests of differences across cluster by ANOVA or Chi-square tests
†Rank order of stabilizations ranged from 1 to 7; 0 indicates stable on presentation; 8 indicates instability at discharge; 9 indicates not stabilized prior to inpatient death. Lower means indicate lower overall rank order of resolution for those indicators, which results from early stabilization. Indicators with means 5.0–7.0 have influence from late stabilization and/or persisting instabilities, and means > 7.0 indicate substantial influence from instabilities and inpatient mortality. Lower SDs indicate more homogeneous ranks among patients
‡These are the average orders of stabilization for patients in each cluster, based on the average ranks of stabilization of each indicator within each cluster
§Excludes University of New Mexico due to partial data unavailability
In regression-based models of associations between sequence clusters and outcomes, greater PSI score and inclusion in the Mental/Oral/Hypoxic cluster (with Stabilized Fast as reference cluster) were both associated with increased odds of 30-day mortality (all p < 0.05) (see Table 4). The Febrile cluster was associated with shorter LOS, while the PSI score and the Stabilized Slow, Hypoxic, Mental/Oral/Hypoxic and Inpatient Mortality clusters were all associated with longer LOS (all p < 0.05). PSI score, Stabilized Slow, Hypoxic, Mental/Oral/Hypoxic, and Inpatient Mortality clusters were all associated with higher total costs, while only the Febrile cluster was associated with lower costs (all p < 0.05). (Supplementary Figure S3, online, plots mean 30-day mortality, mean LOS, and mean total hospital costs by time to maximum stabilization for each cluster.)
Table 4.
Associations Between Clusters and Mortality, Length of Stay, and Hospitalization Costs
| Characteristic | 30-day mortality (N = 942)* | Length of stay (N = 1,204)† | Total cost (N = 1,201)† | |||
|---|---|---|---|---|---|---|
| OR | OR CI | β | 95 % CI | β | 95 % CI | |
| PSI score | 1.02 | (1.01–1.03) | 0.03 | (0.02–0.04) | 93 | (73–114) |
| # indicators stabilized on presentation | 0.99 | (0.78–1.25) | −0.07 | (−0.24–0.09) | −238 | (−636–160) |
| Clusters | ||||||
| Stabilized, fast | 1.00 | 0.00 | 0 | |||
| Stabilized, slow | 0.73 | (0.28–1.92) | 1.18 | (0.55–0.81) | 3,502 | (1,985–5,018) |
| Tachycardic | NA | −0.44 | (−1.13–0.29) | 1 | (−1,696–1,698) | |
| Febrile | NA | −1.85 | (−2.45– −1.30) | −3,434 | (−4,517– −2,351) | |
| Hypoxic | 1.61 | (0.66–3.94) | 0.77 | (0.04–1.51) | 2,942 | (1,218–4,666) |
| Tachycardic/Hypoxic | 0.60 | (0.21–2.98) | −0.66 | (−1.53–0.20) | −237 | (−2,036–1,562) |
| Mental/Oral/Hypoxic | 3.99 | (1.68–9.50) | 1.06 | (0.07–2.04) | 3,099 | (804–5,394) |
| Inpatient mortality | NA | 2.88 | (1.24–4.51) | 5,819 | (2,924–8,714) | |
| R 2= | 0.21 | NA | NA | |||
Parameters and CIs in bold are significant. Adjusted for gender, age, race, site, Charlson Index Score, insurance type, hospitalist status
NA not applicable
*Logistic regression
†Generalized linear model regression (GLM) on log length of stay, and log total cost
DISCUSSION
This is the first study to use tools of sequence analysis to characterize variations in temporal patterns in the resolution of CAP instabilities, and the first to detect associations between those variations and mortality, LOS, and total hospitalization costs. This study makes three main contributions to the literature.
The first contribution is to show the vast heterogeneity in the sequences in which, whether, and the speed that instabilities resolved in a diverse sample of 1,326 patients across six geographically dispersed sites. The top five most common patterns totaled less than 6 % of all patients, and yet we were able to extract an “average” order of stabilization [BP-MS-OI-RR-T-HR-O2] that may be relevant to a “natural history” of disease regression in hospitalized CAP patients, although our study was not designed to test this further.
Our second contribution is to show that sequence analysis techniques can be used, despite sequence heterogeneity, to identify a smaller number of sequence clusters, or types. Our clusters were jointly characterized by a combination of differences in the number of instabilities that did not stabilize by discharge, the specific instabilities that were or were not stabilized, and the sequence or speed with which instabilities stabilized. Moreover, these clusters have meaningful clinical interpretations. More generally, our analysis suggests potential to apply sequence analysis in other areas of clinical medicine in which the temporal trajectory or a portion thereof might be viewed as the analytical unit of analysis. For example, given the great interest in preventing unnecessary hospital readmissions,30,31 sequence analysis of ambulatory and hospital use over time may generate important and novel hypotheses that could be tested in predictive models, such as whether particular patterns of ambulatory and emergency department visits predict hospitalization.
A final contribution of this study is that particular patterns of clinical instability resolution are associated with health and resource utilization outcomes. Several of the patterns that include unresolved instabilities on discharge do not appear to be deleterious to some patients, a finding that deserves additional study among different populations. Those in the Febrile, Tachycardic, and Tachycardic/Hypoxic clusters had 30-day mortality rates similar to those in the two Stable clusters, and therefore may be candidates for early discharge with resulting shorter LOS and lower hospitalization costs. On the other hand, hospital courses in which hypoxia alone, or where hypoxia is found in combination with oral intake insufficiency and altered mental status, were associated with higher levels of mortality, longer LOS, and higher levels of resource utilization. Some of these findings, such as the relatively favorable outcomes of patients discharged while still febrile, have a physiologic logic that may help explain them and justify further testing and possible inclusion in future practice guidelines. Our finding that patients in the Febrile cluster were discharged faster (LOS = 2.4 days) than those in other clusters could reflect physician clinical judgments that fever is less worrisome than other instabilities, evidenced by the 0.0 % mortality rates in that cluster. The lower mortality rates in the Tachycardic and Tachycardic/Hypoxic clusters compared to those in the Stabilized Fast cluster (0.0 %, 2.7 %, 3.9 %, respectively) might similarly reflect tachycardia as an adaptive physiologic response. These results suggest that a shorter LOS is not necessarily associated with worse outcomes in CAP, which is not inconsistent with an older Medicare claims study reporting equivocal findings32 or a later reanalysis of PORT data that demonstrated that reductions in LOS are possible without adverse effects on outcomes.33 In contrast, hypoxia with failure to return to baseline mental status and oral intake deficits (i.e., those in the Mental/Oral/Hypoxic cluster) is associated with an almost four-fold increase in the likelihood of mortality relative to those in the Stabilized Fast cluster. Future work might be clinical trials in which patients are randomized to different care management arms that prioritize resolution of specific clinical instabilities to find an optimal balance between clinical stabilization, LOS, and costs.34 Other studies might exploit our growing ability to use the electronic medical record in real time to automatically generate notifications of higher-risk sequencing of CAP clinical instability resolution.
A potential limitation of our sample is the exclusion of patients with a principal diagnosis of sepsis and a secondary pneumonia diagnosis, which has become more common in recent years.35 However, at the time these data were collected, sepsis was a less common principal diagnosis among CAP patients, and while their inclusion might have changed our mortality rates somewhat,36 the proportion of CAP patients coded in this way is still small.37 Another potential limitation is that we were not able to account for the possibility that some indicators, once resolved, may subsequently destabilize before discharge, but we have not found literature about how often this may occur. Moreover, this so-called full-information approach to capture all parallel cycling of instability/stability for each indicator would necessitate the use of an even more complex type of sequence analysis, which has limitations of its own. Our definition of stability follows that used in the Pneumonia PORT study, which specified a 24-hour period of stability,6,26 while the 2001 American Thoracic Society (ATS) guidelines suggest that 8 h may be adequate, especially in the context of the decision to switch from intravenous to oral antibiotics.7 Finally, cluster analysis methods have a limitation in that sequences near cluster boundaries may be similar to those in other clusters.
In sum, we used sequence analysis to describe sequences and characterize patterns of resolution of CAP instabilities in hospitalized patients and determined whether there are clinically important implications associated with these patterns. Some patterns do not appear to be deleterious to some patients in terms of 30-day mortality, and safe discharge with fever, tachycardia, or the combination of tachycardia and hypoxia may be possible for some patients with resulting shorter LOS and lower hospitalization costs. In our study, hospitalizations in which hypoxia or mental status instabilities are found in combination with other instabilities typically led to higher levels of mortality, longer LOS, and higher levels of resource utilization.
Electronic Supplementary Material
(PDF 171 KB)
Acknowledgements
The Multicenter Hospitalist Study was supported by grant RO1 HS10597 AHRQ from the Agency for Healthcare Research and Quality. Dr. Hougham and Dr. Ruhnke are supported by a research and training grant (KM1 CA156717) from the National Cancer Institute. Dr. Arora is supported by National Institute on Aging K23AG033763. Dr. Meltzer is supported by a Mid-career K24 Career Development Award from the National Institute on Aging (K24 AG031326-01). The content of this publication is solely the responsibility of the authors and does not necessarily represent the views of the National Cancer Institute or the National Institutes of Health. The authors acknowledge statistical programming assistance from Prof. Hyo Jung Tak. Prof. Andrew Abbott, Prof. John A. Goldsmith, Dr. Elbert S. Huang, and Dr. Michael David read an earlier version of the manuscript and made helpful suggestions.
Prior Presentations
An earlier version of this work was presented as a poster at the Society of Hospital Medicine’s Annual Meeting in San Diego, CA, April 2012.
Conflict of Interest
The authors declare that they do not have any conflicts of interest.
REFERENCES
- 1.Bartlett JG, Dowell SF, Mandell LA, File TM, Jr, Musher DM, Fine MJ. Practice guidelines for the management of community-acquired pneumonia in adults. Infectious Diseases Society of America. Clin Infect Dis. 2000;31(2):347–82. doi: 10.1086/313954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mandell LA, Bartlett JG, Dowell SF, File TM, Jr, Musher DM, Whitney C. Update of practice guidelines for the management of community-acquired pneumonia in immunocompetent adults. Clin Infect Dis. 2003;37(11):1405–33. doi: 10.1086/380488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fine MJ, Medsger AR, Stone RA, Marrie TJ, Coley CM, Singer DE, et al. The hospital discharge decision for patients with community-acquired pneumonia. Results from the Pneumonia Patient Outcomes Research Team cohort study. Arch Intern Med. 1997;157(1):47–56. doi: 10.1001/archinte.1997.00440220051007. [DOI] [PubMed] [Google Scholar]
- 5.Halm EA, Fine MJ, Kapoor WN, Singer DE, Marrie TJ, Siu AL. Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia. Arch Intern Med. 2002;162(11):1278–84. doi: 10.1001/archinte.162.11.1278. [DOI] [PubMed] [Google Scholar]
- 6.Halm EA, Fine MJ, Marrie TJ, Coley CM, Kapoor WN, Obrosky DS, et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA. 1998;279(18):1452–7. doi: 10.1001/jama.279.18.1452. [DOI] [PubMed] [Google Scholar]
- 7.Niederman MS, Mandell LA, Anzueto A, Bass JB, Broughton WA, Campbell GD, et al. Guidelines for the management of adults with community-acquired pneumonia—Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163(7):1730–54. doi: 10.1164/ajrccm.163.7.at1010. [DOI] [PubMed] [Google Scholar]
- 8.Menendez R, Torres A, Rodriguez de Castro F, Zalacain R, Aspa J, Martin Villasclaras JJ, et al. Reaching stability in community-acquired pneumonia: the effects of the severity of disease, treatment, and the characteristics of patients. Clin Infect Dis. 2004;39(12):1783–90. doi: 10.1086/426028. [DOI] [PubMed] [Google Scholar]
- 9.Combi C, Shahar Y. Temporal reasoning and temporal data maintenance in medicine: issues and challenges. Comput Biol Med. 1997;27(5):353–68. doi: 10.1016/S0010-4825(96)00010-8. [DOI] [PubMed] [Google Scholar]
- 10.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107(23):2920–5. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 11.Aaby P, Ibrahim SA, Libman MD, Jensen H. The sequence of vaccinations and increased female mortality after high-titre measles vaccine: trials from rural Sudan and Kinshasa. Vaccine. 2006;24(15):2764–71. doi: 10.1016/j.vaccine.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Aaby P, Jensen H, Whittle H. High-titre measles vaccine and female mortality. Lancet. 2003;362(9397):1765. doi: 10.1016/S0140-6736(03)14867-2. [DOI] [PubMed] [Google Scholar]
- 13.Durbin R. Biological Sequence Analysis: Probabalistic Models of Proteins and Nucleic Acids. Cambridge, UK; New York: Cambridge University Press; 1998. [Google Scholar]
- 14.Needleman SB, Wunsch CD. A general method applicable to search for similarities in amino acid sequence of two proteins. J Mol Biol. 1970;48(3):443. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- 15.Jurafsky D, Martin JH. Speech and Language Processing: An Introduction to Natural Language Processing, Computational Linguistics, and Speech Recognition. 2. Upper Saddle River, N.J.: Pearson Prentice Hall; 2009. [Google Scholar]
- 16.Abbott A. Sequence-analysis—new methods for old ideas. Annu Rev Sociol. 1995;21:93–113. doi: 10.1146/annurev.so.21.080195.000521. [DOI] [Google Scholar]
- 17.Anyadike-Danes M, McVicar D. My brilliant career: characterizing the early labor market trajectories of British women from Generation X. Sociol Method Res. 2010;38(3):482–512. doi: 10.1177/0049124110362968. [DOI] [Google Scholar]
- 18.Gusfield D. Algorithms on Strings, Trees and Sequences: Computer Science and Computational Biology. Cambridge: University Press; 1997. [Google Scholar]
- 19.Tan P-N, Steinbach M, Kumar V. Cluster analysis: basic concepts and algorithms. Introduction to data mining. 1. Boston: Pearson Addison Wesley; 2006. pp. 487–568. [Google Scholar]
- 20.Gabadinho A, Ritschard G, Muller NS, Studer M. Analyzing and visualizing state sequences in R with TraMineR. J Stat Softw. 2011;40(4):1–37. [Google Scholar]
- 21.Auerbach AD, Katz R, Pantilat SZ, Bernacki R, Schnipper J, Kaboli P, et al. Factors associated with discussion of care plans and code status at the time of hospital admission: results from the Multicenter Hospitalist Study. J Hosp Med. 2008;3(6):437–45. doi: 10.1002/jhm.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meltzer D, Manning WG, Morrison J, Shah MN, Jin L, Guth T, et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–74. doi: 10.7326/0003-4819-137-11-200212030-00007. [DOI] [PubMed] [Google Scholar]
- 23.Meehan TP, Fine MJ, Krumholz HM, Scinto JD, Galusha DH, Mockalis JT, et al. Quality of care, process, and outcomes in elderly patients with pneumonia. JAMA. 1997;278(23):2080–4. doi: 10.1001/jama.1997.03550230056037. [DOI] [PubMed] [Google Scholar]
- 24.Chaudhry S, Jin L, Meltzer D. Use of a self-report–generated Charlson comorbidity index for predicting mortality. Med Care. 2005;43(6):607–15. doi: 10.1097/01.mlr.0000163658.65008.ec. [DOI] [PubMed] [Google Scholar]
- 25.Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336(4):243–50. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 26.Fine MJ, Stone RA, Singer DE, Coley CM, Marrie TJ, Lave JR, et al. Processes and outcomes of care for patients with community-acquired pneumonia—results from the Pneumonia Patient Outcomes Research Team (PORT) cohort study. Arch Intern Med. 1999;159(9):970–80. doi: 10.1001/archinte.159.9.970. [DOI] [PubMed] [Google Scholar]
- 27.Brzinsky-Fay C, Kohler U, Luniak M. Sequence analysis with Stata. Stata J. 2006;6(4):435–60. [Google Scholar]
- 28.Ward JH. Hierarchical grouping to optimize an objective function. J Am Stat Assoc. 1963;58(301):236. doi: 10.1080/01621459.1963.10500845. [DOI] [Google Scholar]
- 29.Studer M. Le manuel de la librairie WeightedCluster: Un guide pratique pour la création de typologies de trajectoires en sciences sociales avec R. In: Studer M, editor. Étude des Inégalités de Genre en Début de Carrière Académique à L'aide de Méthodes Innovatrices d'Analyse de Données Séquentielles. Geneva: Thèse SES 777, Faculté des sciences économiques et sociales, Université de Genève; 2012. [Google Scholar]
- 30.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418–28. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 31.Mor V, Besdine RW. Policy options to improve discharge planning and reduce rehospitalization. JAMA. 2011;305(3):302–3. doi: 10.1001/jama.2010.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metersky ML, Tate JP, Fine MJ, Petrillo MK, Meehan TP. Temporal trends in outcomes of older patients with pneumonia. Arch Intern Med. 2000;160(22):3385–91. doi: 10.1001/archinte.160.22.3385. [DOI] [PubMed] [Google Scholar]
- 33.McCormick D, Fine MJ, Coley CM, Marrie TJ, Lave JR, Obrosky DS, et al. Variation in length of hospital stay in patients with community-acquired pneumonia: are shorter stays associated with worse medical outcomes? Am J Med. 1999;107(1):5–12. doi: 10.1016/S0002-9343(99)00158-8. [DOI] [PubMed] [Google Scholar]
- 34.De Saint-Hubert M, Schoevaerdts D, Cornette P, D'Hoore W, Boland B, Swine C. Predicting functional adverse outcomes in hospitalized older patients: a systematic review of screening tools. J Nutr Health Aging. 2010;14(5):394–9. doi: 10.1007/s12603-010-0086-x. [DOI] [PubMed] [Google Scholar]
- 35.Lindenauer PK, Lagu T, Shieh MS, Pekow PS, Rothberg MB. Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003–2009. JAMA. 2012;307(13):1405–13. doi: 10.1001/jama.2012.384. [DOI] [PubMed] [Google Scholar]
- 36.Mortensen EM, Coley CM, Singer DE, Marrie TJ, Obrosky DS, Kapoor WN, et al. Causes of death for patients with community-acquired pneumonia—results from the pneumonia patient outcomes research team cohort study. Arch Int Med. 2002;162(9):1059–64. doi: 10.1001/archinte.162.9.1059. [DOI] [PubMed] [Google Scholar]
- 37.Ruhnke GW, Perraillon MC, Cutler DM. Mortality reduction among pneumonia patients still substantial despite the impact of coding changes. Am J Med. 2013;126(3):266–9. doi: 10.1016/j.amjmed.2012.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 171 KB)