ABSTRACT
BACKGROUND
Because pregnancy complications, including gestational diabetes mellitus (GDM) and hypertensive disorders in pregnancy, are risk factors for diabetes and cardiovascular disease, post-delivery follow-up is recommended.
OBJECTIVE
To determine predictors of post-delivery primary and obstetric care utilization in women with and without medical complications.
RESEARCH DESIGN
Five-year retrospective cohort study using commercial and Medicaid insurance claims in Maryland.
SUBJECTS
7,741 women with a complicated pregnancy (GDM, hypertensive disorders and pregestational diabetes mellitus [DM]) and 23,599 women with a comparison pregnancy.
MEASURES
We compared primary and postpartum obstetric care utilization rates in the 12 months after delivery between the complicated and comparison pregnancy groups. We conducted multivariate logistic regression to assess the association between pregnancy complications, sociodemographic predictor variables and utilization of care, stratified by insurance type.
RESULTS
Women with a complicated pregnancy were older at delivery (p < 0.001), with higher rates of cesarean delivery (p < 0.0001) and preterm labor or delivery (p < 0.0001). Among women with Medicaid, 56.6 % in the complicated group and 51.7 % in the comparison group attended a primary care visit. Statistically significant predictors of receiving a primary care visit included non-Black race, older age, preeclampsia or DM, and depression. Among women with commercial health insurance, 60.0 % in the complicated group and 49.5 % in the comparison group attended a primary care visit. Pregnancy complication did not predict a primary care visit among women with commercial insurance.
CONCLUSIONS
Women with pregnancy complications were more likely to attend primary care visits post-delivery compared to the comparison group, but overall visit rates were low. Although Medicaid expansion has potential to increase coverage, innovative models for preventive health services after delivery are needed to target women at higher risk for chronic disease development.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-013-2744-2) contains supplementary material, which is available to authorized users.
KEY WORDS: pregnancy, utilization of care, primary care, gestational diabetes mellitus, hypertension
INTRODUCTION
Pregnancy complications, including gestational diabetes mellitus (GDM) and hypertensive disorders of pregnancy, are known risk factors for both type 2 diabetes and cardiovascular disease.1–4 The public health burden of diabetes attributable to GDM is significant, as 10–31 % of parous women diagnosed with type 2 diabetes have a history of GDM.5 Because diagnoses of GDM and preeclampsia are made early in a woman’s life and at a time of intensive medical treatment, pregnancy and postpartum period provide critical opportunities for risk stratification, implementation of long-term prevention strategies and continued physician monitoring.6,7
For women with recent GDM, several national organizations and consensus conferences8–10 recommend screening for diabetes at 6-weeks postpartum, 1 year and then periodically thereafter. The Working Group on High Blood Pressure in Pregnancy recommends that women with a recent hypertensive disorder in pregnancy (defined as chronic hypertension, gestational hypertension, preeclampsia and eclampsia) be reevaluated postpartum, and counseled with respect to future pregnancies and elevated personal risk of cardiovascular disease.11
Among women with recent GDM, it is well-documented that not all women attend their postpartum visits and even fewer receive screening tests for type 2 diabetes.12–16 According to Hunt and colleagues, the women at highest risk for developing T2DM received the fewest services.17 Post-delivery primary care provides an opportunity for patient education about risk for diabetes and cardiovascular disease, and to promote weight loss and pregnancy spacing. Understanding predictors of primary care utilization in the first year after delivery could inform health services interventions aimed at long-term disease prevention, to ultimately reduce cardiovascular disease and diabetes.
We conducted a retrospective cohort analysis of claims data from a commercial health insurance plan and a Medicaid Managed Care Organization in Maryland to compare primary care utilization in the 12 months following delivery between women with complications in pregnancy and women with pregnancies without these complications. We hypothesized that overall, primary care follow-up within 12 months would be low and that pregnancy complications would predict the occurrence of a primary care visit. Including both commercial and Medicaid insurance plans enabled comparisons within and between these two different insured populations. We also hypothesized that women with commercial health insurance who had greater access to continuous coverage would have higher rates of primary care and obstetric follow-up.
METHODS
Data Source
We obtained claims data between 1 July 2003 and 31 December 2009 from two insurance plans managed by Johns Hopkins HealthCare, LLC: 1) a Medicaid Managed Care Organization in Maryland, and 2) a self-insured commercial health benefit plan that provides health insurance to 85 % of Johns Hopkins University employees. The data set included outpatient, inpatient, pharmacy and laboratory claims and met the Health Insurance Portability and Accountability Act’s definition of a limited data set. The study was approved by the Johns Hopkins University School of Medicine Institutional Review Board.
Study Sample Selection
We required female sex, age > 12 and < 45, and a delivery, defined using the National Center for Quality Assurance’s (NCQA) Healthcare Effectiveness Data and Information Set (HEDIS) measures for assessing the quality of prenatal and postpartum care for Medicaid and The Children’s Health Insurance Program18 (Supplemental Table A, available online). We further modified the definition of delivery to include the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for the stillborn and multiple gestation births. We conducted a sensitivity analysis broadening the definition to include the addition of multiple other ICD-9-CM and Current Procedural Terminology (CPT) codes that indicated a pregnancy or delivery, which yielded only 4 % more deliveries. We used the modified HEDIS definition for simplicity and comparability with other studies (Supplemental Table A, available online).
We estimated the date of conception as 280 days prior to the delivery and included all claims 6 months prior to conception (preconception), during pregnancy and 12 months post-delivery. To ensure we had the correct delivery date, we required ≥ 1 insurance claim within 2 weeks of the assigned delivery date. We applied two insurance coverage requirements, pregnancy coverage ≥ 100 days and ≥ 42 days after delivery.
Classification of Women into Complicated and Comparison Pregnancy Groups
Using previously developed definitions,19,20 women were assigned to the “complicated pregnancy group” if they had one or more of the following medical complications: GDM, pregestational diabetes (DM), or hypertensive disorders in pregnancy (preeclampsia, eclampsia, gestational hypertension, chronic hypertension and preeclampsia superimposed on chronic hypertension) (Supplemental Table A, available online).19 We elected to include pregestational Type 1 and 2 diabetes along with GDM in the “complicated pregnancy group” for several reasons: 1) we had decided to include chronic hypertension, also a pre-pregnancy diagnosis, because it was part of the definition for hypertensive disorders in pregnancy11; 2) newer evidence supports “diabetes in pregnancy” as a continuum of glucose intolerance with many pregnant patients having undiagnosed type 2 diabetes21; and 3) we designed our analyses to enable separate assessments between diagnoses of GDM and DM. The first pregnancy or first complicated pregnancy was selected as the index, and if a woman had multiple complications in the same pregnancy, she was classified into each of these groups for comparisons. The comparison group contained women without any of the above defined pregnancy complications in any of her pregnancies.
Outcome Definitions for Utilization of Care
We used CPT codes to define our main outcome as ≥ 1 ambulatory visits with a primary care provider within 12 months following delivery (Supplemental Table A, available online). Our secondary outcome was an obstetric postpartum visit within 3 months following delivery (Supplemental Table A, available online). To provide a broader context of post-delivery care and compare utilization between different health care services, we also described utilization in the 3 months preconception, during and after pregnancy for emergency room (ER) visits, receipt of early prenatal care,18 glucose testing, and medications (Supplemental Table A, available online).
Other Covariate Definitions
We used ICD9-CM codes to define other delivery and medical complications (Supplemental Table A, available online). Race and ethnicity information was provided for Medicaid. We used zip code at delivery to link our data set with the 2000 Census information from the American Community Survey to obtain neighborhood characteristics. We converted ZIP Code Tabulation Areas (ZCTAs) in the census to ZIP codes using files from the Dartmouth Atlas of Healthcare.22
Statistical Analysis
We assessed the proportion who utilized each visit type within pre-specified time periods: pre-conception, pregnancy, and within 3 and 12 months post-delivery. For these proportions, we calculated the denominator as the number of women with insurance coverage for at least half of the time period (i.e. 45 days and 6 months, respectively). We used multivariate logistic regression models to assess the associations between the primary care utilization within 12 months after delivery and postpartum obstetric care utilization within 3 months after delivery, and pregnancy complications and sociodemographic factors. Analyses were performed using (SAS, version 9.1; SAS Institute Inc., Cary, NC).
RESULTS
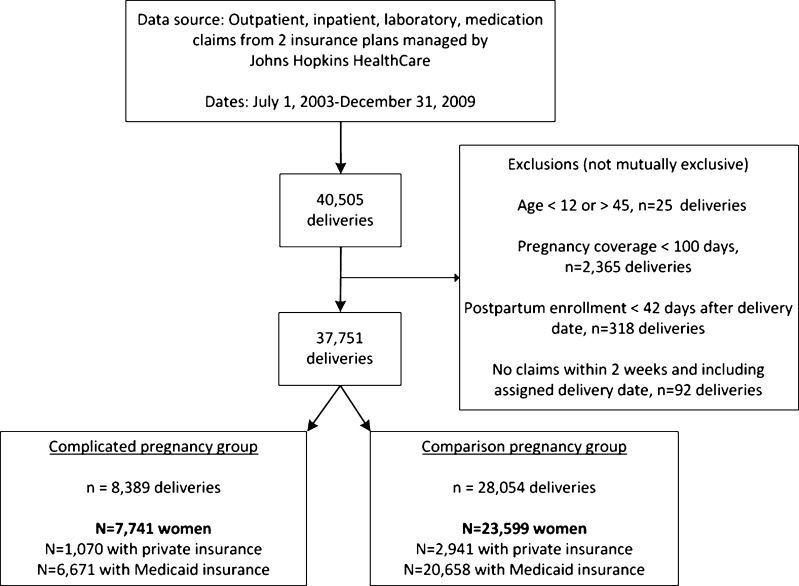
We included 37,751 deliveries, which accounted for 8,389 complicated (7,741 women) and 28,054 comparison pregnancies (23,559 women) (Fig. 1). More women in the complicated pregnancy group lived in neighborhoods with higher proportions of African American residents (p < 0.001) and residents with incomes below the federal poverty level (p = 0.001). Rates of hypertensive disorders, GDM and DM were 17.0 %, 9.1 % and 1.4 %, respectively. (Table 1).
Figure 1.
Selection of the sample of women with Medicaid and commercial health insurance in complicated and comparison pregnancy groups, 2003–2009.
Table 1.
Characteristics of Pregnant Women by Complicated and Comparison Pregnancy Groups
| Characteristic | Overall | Complicated pregnancy | Comparison pregnancy | p value |
|---|---|---|---|---|
| N = 31,340 | N = 7,741 | N = 23,599 | ||
| Mean age at delivery, years (SD) | 25.2 (6.1) | 26.5 (6.5) | 24.8 (6.0) | < 0.001 |
| Medicaid insurance (vs. private), n (%) | 87.2 % | 86.2 % | 87.5 % | 0.002 |
| Medicaid eligible because of pregnancy*, % | 63.9 % | 61.7 % | 64.6 % | < 0.001 |
| Race/ethnicity | < 0.001 | |||
| African American, % | 44.3 % | 48.4 % | 42.9 % | |
| White, % | 33.4 % | 31.6 % | 33.9 % | |
| Hispanic, % | 4.8 % | 4.5 % | 4.9 % | |
| Other race, % | 17.6 % | 15.5 % | 18.2 % | |
| Neighborhood level sociodemographics† | ||||
| Mean proportion of African American residents, % (SD %) | 32.1 % (26.9 %) | 33.1 % (27.5 %) | 31.8 % (26.7 %) | < 0.001 |
| Mean proportion without high school diploma among age > 25, % (SD %) | 20.7 % (9.9 %) | 21.1 % (10.2 %) | 20.5 % (9.8 %) | < 0.001 |
| Mean proportion of residents below federal poverty, % (SD %) | 9.3 % (7.6 %) | 9.5 % (7.9 %) | 9.2 % (7.5 %) | 0.001 |
| Mean proportion urban, % (SD %) | 81.8 % (30.4 %) | 82.5 % (29.9 %) | 81.6 % (30.6 %) | 0.02 |
| Pregnancy and delivery complications‡ | ||||
| Any hypertensive disorder in pregnancy, % | 17.0 % | 68.8 % | 0.0 % | n/a |
| Preeclampsia, % (mild or severe) | 8.2 % | 33.1 % | 0.0 % | n/a |
| Eclampsia, % | 0.5 % | 2.0 % | 0.0 % | n/a |
| Chronic hypertension in pregnancy, % | 6.4 % | 25.9 % | 0.00 % | n/a |
| Gestational hypertension | 7.1 % | 28.9 % | 0.0 % | n/a |
| Gestational diabetes mellitus, % | 9.1 % | 37.0 % | 0.0 % | n/a |
| Pregestational diabetes, % | 1.4 % | 5.5 % | 0.0 % | n/a |
| Cesarean delivery, % | 27.9 % | 38.0 % | 24.6 % | < 0.001 |
| Preterm labor or delivery, % | 9.4 % | 13.7 % | 8.0 % | < 0.001 |
| Multiple gestation, % | 1.7 % | 2.7 % | 1.3 % | < 0.001 |
| Stillborn birth, % | 0.3 % | 0.3 % | 0.3 % | 0.90 |
| Multiple gestation, % | 1.7 % | 2.7 % | 1.3 % | < 0.001 |
| Drug use and smoking in pregnancy | ||||
| Any drug use | 4.1 % | 4.4 % | 4.0 % | 0.08 |
| Alcohol use | 0.5 % | 0.5 % | 0.4 % | 0.38 |
| Smoking | 26.9 % | 31.8 % | 25.3 % | < 0.001 |
| Other comorbid illnesses in pregnancy | ||||
| Depression | 5.4 % | 6.4 % | 5.0 % | < 0.001 |
| Other mental disorders | 14.9 % | 15.6 % | 14.7 % | 0.054 |
| Thyroid disease | 2.9 % | 4.6 % | 2.4 % | < 0.001 |
| HIV | 0.5 % | 0.6 % | 0.4 % | 0.015 |
| Asthma | 11.4 % | 13.7 % | 10.7 % | < 0.001 |
| Obesity | 9.6 % | 18.3 % | 6.7 % | < 0.001 |
SD standard deviation
*Medicaid eligibility based on pregnancy is a Medicaid enrollee category called SOBRA (Medicaid for pregnant women and children) that covers the pregnancy and up to 90 days postpartum
†Definitions according to 2000 U.S. Census, reported by ZIP code
‡Conditions are not mutually exclusive and many overlap—in the complicated pregnancy group, 8.9 % had both a hypertensive disorder and GDM and 2.4 % had both a hypertensive disorder and pregestational diabetes
The majority of women with Medicaid were eligible because of pregnancy (63.9 %) (Table 1). Women in this eligibility group were more likely to be White (38.5 % vs. 24.3 %) and had fewer comorbid health conditions, lower rates of pregnancy and delivery complications (except for GDM [9.30 % vs. 7.5 %]), as compared to women who were eligible for Medicaid independent of pregnancy. However, women eligible for Medicaid for a non-pregnancy indication had greater preconception and early pregnancy insurance coverage (Supplemental Table B, available online). Among all women with Medicaid insurance 51.8 % (29.7 % with eligibility based on pregnancy, 88.2 % with other eligibility) continued to have coverage for 6 or more months after delivery, compared with 89.7 % of women with commercial insurance (Supplemental Table B, available online). For women with Medicaid, post-delivery coverage rates were highest for women with hypertensive disorders (57.0 %) and DM (66.9 %), but lower for women with GDM (47.4 %) (Table 2).
Table 2.
Rates of Utilization of Care During Preconception, Pregnancy and Post-Delivery, Stratified by Medicaid and Commercial Health Insurance Plans
| Utilization in each time period | Complicated pregnancy | Comparison pregnancy | |||
|---|---|---|---|---|---|
| GDM | DM | HDP | Total | Total | |
| Medicaid health insurance | N = 2,367 | N = 344 | N = 4,690 | N = 6,671 | N = 20,658 |
| Utilization of post-delivery care, % | |||||
| Postpartum obstetric visit | 67.4 % | 65.1 % | 63.8 % | 65.0 % | 61.5 % |
| Emergency room visit in 3 months post-delivery | 12.2 % | 16.9 % | 14.2 % | 13.6 % | 11.7 % |
| Glucose testing* in 3 months post-delivery | 5.7 % | 14.8 % | 2.1 % | 3.3 % | 0.5 % |
| Insurance coverage ≥ 6 months after delivery, % | 47.4 % | 66.9 % | 57.0 % | 54.2 % | 49.7 % |
| Primary care visit in 12 months post-delivery | 55.0 % | 69.6 % | 57.0 % | 56.6 % | 51.7 % |
| Emergency room visit in 12 months post-delivery | 44.6 % | 57.8 % | 50.3 % | 49.1 % | 45.8 % |
| Glucose testing* in 12 months post-delivery | 15.2 % | 49.1 % | 8.7 % | 11.1 % | 3.5 % |
| Insulin† in 12 months after delivery | 1.2 % | 26.7 % | 1.3 % | 1.8 % | 0.0 % |
| Oral diabetes medication† in 12 months post-delivery | 1.0 % | 13.1 % | 0.9 % | 1.1 % | 0.0 % |
| Antihypertensive medication† in 12 months post-delivery | 3.9 % | 14.5 % | 9.8 % | 7.1 % | 0.4 % |
| Utilization of pregnancy and preconception care, % | |||||
| Receipt of early‡ prenatal care | 37.7 % | 51.9 % | 36.4 % | 37.5 % | 34.2 % |
| Emergency room visit | 15.9 % | 28.4 % | 20.0 % | 19.2 % | 17.5 % |
| Insurance coverage ≥ 3 months before delivery§, % | 22.1 % | 44.2 % | 33.6 % | 30.3 % | 27.5 % |
| Primary care visit | 30.8 % | 42.8 % | 32.6 % | 32.5 % | 29.1 % |
| Obstetrician/gynecology visit | 26.8 % | 22.4 % | 20.4 % | 22.3 % | 16.9 % |
| Emergency room visit | 40.0 % | 51.3 % | 37.8 % | 38.8 % | 35.4 % |
| Commercial health insurance | N = 499 | N = 78 | N = 637 | N = 1070 | N = 2941 |
| Utilization of post-delivery care, % | |||||
| Postpartum obstetric visit | 48.7 % | 56.4 % | 52.6 % | 50.7 % | 44.6 % |
| Emergency room visit in 3 months post-delivery | 4.6 % | 10.3 % | 6.0 % | 5.5 % | 3.8 % |
| Glucose testing* in 3 months post-delivery | 11.4 % | 26.9 % | 4.7 % | 8.2 % | 0.5 % |
| Insurance coverage ≥ 6 months after delivery, % | 91.2 % | 88.5 % | 88.7 % | 88.4 % | 89.8 % |
| Primary care visit in 12 months post-delivery | 57.4 % | 68.1 % | 63.9 % | 60.0 % | 49.5 % |
| Emergency room visit in 12 months post-delivery | 16.0 % | 30.4 % | 22.8 % | 20.3 % | 12.5 % |
| Glucose testing* in 12 months post-delivery | 20.9 % | 53.6 % | 11.1 % | 16.3 % | 2.7 % |
| Insulin† in 12 months post-delivery | 1.0 % | 23.1 % | 1.9 % | 2.1 % | 0.0 % |
| Oral diabetes medication† in 12 months post-delivery | 0.6 % | 14.1 % | 1.6 % | 1.3 % | 0.0 % |
| Antihypertensive medication† in 12 months post-delivery | 4.4 % | 16.7 % | 17.1 % | 10.6 % | 0.9 % |
| Utilization of pregnancy and preconception care, % | |||||
| Receipt of early‡ prenatal care | 71.1 % | 77.9 % | 67.2 % | 69.5 % | 66.3 % |
| Emergency room visit | 9.4 % | 14.3 % | 11.8 % | 10.6 % | 8.2 % |
| Insurance coverage ≥ 3 months before delivery§, % | 85.6 % | 92.3 % | 85.2 % | 85.8 % | 81.0 % |
| Primary care visit | 38.6 % | 41.7 % | 43.3 % | 40.8 % | 31.1 % |
| Obstetrician/gynecology visit | 41.2 % | 54.2 % | 40.9 % | 41.8 % | 33.8 % |
| Emergency room visit | 11.0 % | 11.1 % | 13.6 % | 11.3 % | 8.7 % |
BP blood pressure; CPT current procedural terminology; DM pregestational diabetes mellitus (type 1 or 2 DM); GDM gestational diabetes mellitus; HDP hypertensive disorders of pregnancy
*Glucose testing includes any laboratory test with a glucose value (see Supplemental Table A, available online, for listing of included CPT codes)
†Medication use was defined as ≥ 90 days of filled prescriptions
‡Early prenatal care is defined by NCQA HEDIS measure as a prenatal care visit in the first 104 days of pregnancy18
§Insurance coverage in the 3 months prior to conception. Conception date was estimated by assuming a 40 week pregnancy, and was based on the delivery date minus 40 weeks
Utilization of Primary Care in the 12 Months Following Delivery
Among women with Medicaid insurance, the following proportions attended a primary care visit within 12 months post-delivery: 55.0 % with GDM, 69.6 % with DM, 57.0 % with hypertensive disorders, compared with 51.7 % in the comparison pregnancy group (Table 2). Rates of primary care visit attendance were similar in women with commercial health insurance compared to those with Medicaid: 57.4 % with GDM, 68.1 % with DM and 63.9 % with a hypertensive disorder in pregnancy attended a primary care visit, compared with 49.5 % of women in the comparison pregnancy group (Table 2).
Table 3 shows the results of the multivariate analyses to determine predictors of receiving primary care after delivery. Among women with Medicaid coverage, statistically significant sociodemographic predictors of primary care after delivery were White race (OR 1.37, p < 0.001) or Hispanic ethnicity (OR 1.23, p = 0.05), older age (age 25-34: OR 1.28, p < 0.001; age > 35: OR 1.42, p < 0.001 [reference = age 18–24]) and living in a neighborhood with a higher proportion of people without a high school diploma (Tertile 3: OR 1.28, p < 0.001) (Table 3). Among women with Medicaid preeclampsia (OR 1.24, p = 0.03), DM (OR 1.70, p < 0.001) (but not GDM), thyroid disease (OR 1.96, p < 0.001), asthma (OR 1.35, p < 0.001) and depression (OR 1.25, p = 0.01) were predictors. Current smoking (OR 0.89, p = 0.004) and Medicaid eligibility based on pregnancy (OR 0.90, p = 0.09) negatively predicted primary care utilization, but having any preconception insurance coverage positively predicted primary care (OR 1.12, p = 0.01) (Table 3).
Table 3.
Predictors* of Attending a Primary Care Visit Within 12 Months of Delivery, Stratified by Medicaid and Commercial Health Insurance
| Characteristic | Medicaid insurance | Commercial insurance | ||||
|---|---|---|---|---|---|---|
| OR | 95 % CI | p value | OR | 95 % CI | p value | |
| N = 12,341 | N = 3,475 | |||||
| Race/ethnicity† (Black = ref) | ||||||
| White | 1.37 | 1.24–1.50 | < 0.001 | N/A | N/A | N/A |
| Hispanic | 1.23 | 1.00–1.50 | 0.05 | N/A | N/A | N/A |
| Other races | 0.86 | 0.60–1.23 | 0.41 | N/A | N/A | N/A |
| Age (Age 18–24 = ref) | ||||||
| Age ≤ 17 | 0.98 | 0.87–1.11 | 0.78 | 0.28 | 0.02–3.36 | 0.32 |
| Age 25–34 | 1.28 | 1.17–1.39 | < 0.001 | 0.88 | 0.66–1.17 | 0.38 |
| Age > 35 | 1.42 | 1.21–1.67 | < 0.001 | 0.89 | 0.65–1.22 | 0.47 |
| Pregnancy and delivery complications | ||||||
| Any hypertensive disorder in pregnancy | 0.92 | 0.70–1.20 | 0.53 | 0.94 | 0.51–1.71 | 0.83 |
| Chronic hypertension in pregnancy | 1.14 | 0.88–1.47 | 0.33 | 1.62 | 0.90–2.90 | 0.11 |
| Preeclampsia or eclampsia | 1.24 | 1.03–1.51 | 0.03 | 1.28 | 0.83–1.98 | 0.27 |
| Gestational hypertension | 1.17 | 0.91–1.51 | 0.23 | 1.44 | 0.80–2.59 | 0.22 |
| Gestational diabetes mellitus | 1.00 | 0.87–1.15 | 0.99 | 1.18 | 0.95–1.45 | 0.13 |
| Pregestational diabetes | 1.70 | 1.27–2.28 | < 0.001 | 1.65 | 0.96–2.85 | 0.07 |
| Preterm delivery | 0.91 | 0.81–1.02 | 0.11 | 1.05 | 0.81–1.35 | 0.74 |
| Cesarean delivery | 1.01 | 0.93–1.10 | 0.78 | 1.14 | 0.98–1.32 | 0.08 |
| Other medical illnesses | ||||||
| Thyroid disease | 1.96 | 1.53–2.50 | < 0.001 | 1.79 | 1.37–2.34 | < 0.001 |
| HIV | 0.90 | 0.57–1.42 | 0.65 | 3.35 | 0.35–31.78 | 0.29 |
| Asthma | 1.35 | 1.22–1.49 | < 0.001 | 1.43 | 1.12–1.84 | 0.01 |
| Substance use and mental health | ||||||
| Either drug or alcohol use | 1.07 | 0.92–1.26 | 0.38 | 0.65 | 0.17–2.46 | 0.52 |
| Current smoking | 0.89 | 0.82–0.96 | 0.004 | 1.31 | 1.08–1.59 | 0.01 |
| Depression | 1.25 | 1.06–1.47 | 0.01 | 1.17 | 0.83–1.66 | 0.37 |
| Other mental disorders complicating pregnancy | 1.01 | 0.90–1.13 | 0.89 | 1.60 | 1.17–2.20 | 0.003 |
| Insurance coverage | ||||||
| Any preconception coverage | 1.12 | 1.03–1.21 | 0.01 | N/A | N/A | N/A |
| Medicaid eligibility based on pregnancy | 0.90 | 0.83–0.98 | 0.09 | N/A | N/A | N/A |
| Neighborhood characteristics (tertile 2 = ref) | ||||||
| % Black residents | ||||||
| Tertile 1 (lowest) | 1.05 | 0.95–1.16 | 0.36 | 0.99 | 0.81–1.21 | 0.95 |
| Tertile 3 (highest) | 0.96 | 0.86–1.06 | 0.41 | 0.75 | 0.62–0.89 | 0.001 |
| % age > 25 without HS diploma | ||||||
| Tertile 1 (lowest) | 1.03 | 0.92–1.16 | 0.56 | 0.71 | 0.60–0.85 | < 0.001 |
| Tertile 3 (highest) | 1.28 | 1.15–1.41 | < 0.001 | 1.09 | 0.86–1.37 | 0.48 |
| % residents below federal poverty level | ||||||
| Tertile 1 (lowest) | 0.93 | 0.83–1.04 | 0.19 | 1.17 | 0.96–1.44 | 0.13 |
| Tertile 3 (highest) | 1.12 | 1.00–1.26 | 0.06 | 1.22 | 0.96–1.56 | 0.11 |
CI confidence interval; HS high school; OR odds ratio; N/A not available in the dataset for commercial insurance plan
*Models adjusted for listed variables, as well as other medical complications of pregnancy, drug use and smoking, neighborhood characteristics, access to care during pregnancy and preconception
†Race/ethnicity information available in Medicaid data only
Among women with commercial health insurance, statistically significant positive predictors of primary care included thyroid disease or asthma, but not pregnancy complications. In contrast to women with Medicaid insurance, current smoking was a positive predictor (OR 1.31, p = 0.01) of primary care. Living in a neighborhood with a lower proportion of people without a high school diploma (Tertile 1: OR 0.76, p = 0.001) and a higher proportion of Black residents (Tertile 3: OR 0.75, p = 0.001) negatively predicted primary care utilization (Table 3).
Utilization of Postpartum Obstetric Care in the 3 Months Following Delivery
Among women with Medicaid, 65.0 % with a complicated pregnancy (67.4 % with GDM, 65.1 % with DM, and 63.8 % with hypertensive disorders), compared to 61.5 % in the comparison group, attended a postpartum obstetric visit within 3 months of delivery (Table 2). Women with commercial health insurance had lower rates of receiving a postpartum obstetric visit compared to women with Medicaid insurance (50.8 % in the complicated pregnancy 44.6 % in the comparison pregnancy groups) (Table 2).
Table 4 shows the results of the multivariate analyses to determine predictors of receiving a postpartum obstetric visit. Among women with Medicaid insurance, statistically significant predictors of a postpartum obstetric visit included White race (OR 1.22, p < 0.001) or Hispanic ethnicity (OR 1.48, p < 0.001), chronic hypertension (OR 1.28, p = 0.02), preeclampsia (OR 1.30, p = 0.001), GDM (OR 1.14, p = 0.01), cesarean delivery (OR 1.29, p < 0.001), depression (OR 1.22, p = 0.003) and Medicaid eligibility based on pregnancy (OR 1.16, p < 0.001). Negative predictors of receiving a postpartum visit included other mental disorders in pregnancy (OR 0.81, p < 0.001) and drug or alcohol use (OR 0.71, p < 0.001) (Table 4).
Table 4.
Predictors* of Attending a Postpartum Obstetric Visit Within 3 Months of Delivery, Stratified by Medicaid and Commercial Health Insurance
| Characteristic | Medicaid insurance | Commercial insurance | ||||
|---|---|---|---|---|---|---|
| OR | 95 % CI | p value | OR | 95 % CI | p value | |
| N = 23,692 | N = 3,877 | |||||
| Race/ethnicity† (Black = ref) | ||||||
| White | 1.22 | 1.14–1.31 | < 0.001 | N/A | N/A | N/A |
| Hispanic | 1.48 | 1.28–1.70 | < 0.001 | N/A | N/A | N/A |
| Other races | 1.67 | 1.32–2.12 | < 0.001 | N/A | N/A | N/A |
| Age (Age 18–24 = ref) | ||||||
| Age ≤ 17 | 1.06 | 0.96–1.18 | 0.26 | 1.64 | 0.15–18.46 | 0.69 |
| Age 25-34 | 1.00 | 0.94–1.06 | 0.90 | 0.84 | 0.65–1.10 | 0.21 |
| Age > 35 | 0.99 | 0.89–1.12 | 0.92 | 0.70 | 0.52–0.93 | 0.01 |
| Pregnancy and delivery complications | ||||||
| Any hypertensive disorder in pregnancy | 0.83 | 0.67–1.03 | 0.09 | 0.95 | 0.55–1.64 | 0.86 |
| Chronic hypertension in pregnancy | 1.28 | 1.05–1.57 | 0.02 | 1.13 | 0.67–1.92 | 0.65 |
| Preeclampsia or eclampsia | 1.30 | 1.11–1.52 | 0.001 | 1.38 | 0.94–2.02 | 0.10 |
| Gestational hypertension | 1.13 | 0.92–1.38 | 0.24 | 1.10 | 0.65–1.87 | 0.72 |
| Gestational diabetes | 1.14 | 1.03–1.26 | 0.01 | 1.12 | 0.92–1.36 | 0.26 |
| Pregestational diabetes | 1.06 | 0.84–1.33 | 0.65 | 1.45 | 0.90–2.32 | 0.13 |
| Preterm delivery | 0.92 | 0.84–1.02 | 0.10 | 1.13 | 0.89–1.44 | 0.30 |
| Cesarean delivery | 1.29 | 1.22–1.38 | < 0.001 | 1.11 | 0.97–1.28 | 0.12 |
| Other medical illnesses | ||||||
| Thyroid disease | 1.33 | 1.10–1.61 | 0.003 | 1.16 | 0.91–1.47 | 0.23 |
| HIV | 0.73 | 0.50–1.07 | 0.11 | 0.63 | 0.10–3.95 | 0.62 |
| Asthma | 0.96 | 0.89–1.04 | 0.36 | 0.99 | 0.79–1.25 | 0.95 |
| Substance use and mental health | ||||||
| Either drug or alcohol use | 0.71 | 0.62–0.81 | < 0.001 | 0.73 | 0.22–2.44 | 0.61 |
| Current smoking | 1.11 | 1.04–1.18 | 0.002 | 1.09 | 0.91–1.30 | 0.37 |
| Depression | 1.22 | 1.07–1.40 | 0.003 | 1.17 | 0.86–1.60 | 0.33 |
| Other mental disorders complicating pregnancy | 0.81 | 0.75–0.89 | < 0.001 | 1.43 | 1.08–1.88 | 0.01 |
| Insurance coverage | ||||||
| Any preconception coverage | 0.95 | 0.89–1.02 | 0.15 | N/A | N/A | N/A |
| Medicaid eligibility based on pregnancy | 1.16 | 1.09–1.24 | < 0.001 | N/A | N/A | N/A |
| Neighborhood characteristics (tertile 2 = ref) | ||||||
| % Black residents | ||||||
| Tertile 1 (lowest) | 1.15 | 1.07–1.23 | < 0.001 | 1.29 | 1.07–1.55 | 0.01 |
| Tertile 3 (highest) | 1.06 | 0.98–1.14 | 0.15 | 0.95 | 0.81–1.12 | 0.56 |
| % age > 25 without HS diploma | ||||||
| Tertile 1 (lowest) | 1.02 | 0.94–1.10 | 0.68 | 0.76 | 0.65–0.89 | 0.001 |
| Tertile 3 (highest) | 0.70 | 0.65–0.76 | < 0.001 | 0.94 | 0.76–1.17 | 0.60 |
| % residents below federal poverty | ||||||
| Tertile 1 (lowest) | 0.97 | 0.89–1.05 | 0.46 | 0.97 | 0.80–1.18 | 0.76 |
| Tertile 3 (highest) | 0.96 | 0.88–1.04 | 0.31 | 1.02 | 0.81–1.29 | 0.85 |
CI confidence interval; HS high school; OR odds ratio; N/A not available in the data set for commercial insurance plan
*Models adjusted for listed variables, as well as other medical complications of pregnancy, drug use and smoking, neighborhood characteristics, access to care during pregnancy and preconception
†Race/ethnicity information available in Medicaid data only
Among women with commercial health insurance, we identified fewer statistically significant predictors of postpartum obstetric care, but the point estimates for pregnancy complications had similar magnitude and direction to those in the Medicaid group.
Utilization of Other Health Care Services During Pregnancy and After Delivery
Among women with Medicaid, both the complicated and comparison pregnancy groups had high emergency room utilization in the 12 months post-delivery (49.1 % in the complicated pregnancy group and 45.8 % in the comparison pregnancy group), but rates were lower among women with commercial health insurance (20.3 % in the complicated and 12.5 % in the comparison groups). Few women with complicated pregnancies were taking diabetes medications after delivery, except for those with DM (13.1 %). Antihypertensive medication use after delivery was highest for women with DM (14.5 %), followed by those with a hypertensive disorder (9.8 %) and GDM (3.9 %), compared to 0.4 % in the comparison pregnancy group (Table 2).
DISCUSSION
In this population of women with Medicaid and commercial health insurance between 2003 and 2009, 56.6 % of women with a pregnancy complication, compared with 51.7 % of women with a comparison pregnancy, attended a primary care visit within 1 year of delivery, despite current recommendations that they receive monitoring and preventive health screenings. Among women with Medicaid insurance, major predictors of primary care within 12 months of delivery included a diagnosis of preeclampsia and DM, but not GDM. Among women with commercial insurance, predictors of primary care within 12 months of delivery included other medical conditions like thyroid disease or asthma, but not pregnancy complications. Our findings suggest that despite pregnancy complications, which are known risk factors for diabetes and cardiovascular disease, women are not consistently following-up with primary care providers.
To our knowledge, this is the first study to describe primary care follow-up rates and predictors of care among women with complicated and comparison pregnancies. Our study confirmed findings in prior studies, which have reported low rates of obstetric postpartum visit attendance and receipt of screening for type 2 diabetes among women with GDM.13,17,23 No studies have previously assessed receipt of post-delivery follow-up, such as blood pressure screenings for women with preeclampsia, who are at risk for hypertension, cardiovascular and renal disease.2,24 For all women, postpartum obstetric visit attendance has been recognized18 as an important indicator of the quality of health care delivery, but it has been difficult to improve through active outreach and incentives.16,25,26
Our study highlights several important target areas for improving women’s preventive health after delivery. First, we described insurance coverage gaps after delivery among women with Medicaid insurance. The majority of women with Medicaid were eligible because of the pregnancy and only 30 % continued insurance coverage beyond 6 months after delivery, compared to 88 % of those eligible for Medicaid because of a non-pregnancy indication. Second, despite continuous insurance coverage, less than 60 % of women with Medicaid or commercial health insurance coverage received primary care within 1 year after delivery, even among those with chronic illnesses or pregnancy complications. Disparities in utilizing primary care were particularly evident for African Americans compared to Whites. Although the Affordable Care Act (ACA) and Medicaid expansion provide an opportunity to improve women’s access to insurance coverage before and after delivery, our results show that coverage alone may not be sufficient to improve women’s utilization of appropriate interconception and preventive primary care services. Third, most women (> 60 %) did not access any preconception care. Among those women with a preconception primary care visit, we found higher rates of primary care utilization in the 1 year after delivery (75 % in the complicated and 65 % in the comparison groups). These results indicate that we need to improve outreach prior to conception in women at risk of having a complicated pregnancy because of obesity or an underlying medical diagnosis, to optimize care and reduce pregnancy complications.27 In addition, our study highlights a missed opportunity to improve coordination and communication between obstetricians and primary care providers for women who already had connections with a provider before their pregnancy, as well as for those who should establish care with a new provider. Fourth, we showed an unexpected finding of high ER utilization in the 12 months post-delivery among women with Medicaid. Future research to understand the reasons for post-delivery ER use could inform how we target interventions to improve both acute and preventive services in these populations.
There are several strengths to this study. We had access to two large Maryland claims databases with 5 years of data and over 30,000 pregnant women, enabling us to make comparisons across complication types as well as to assess differences between Medicaid and commercial health insurances. We were able to capture both outpatient diagnoses such as GDM, as well as predominantly inpatient diagnoses like preeclampsia. Finally, we developed strategies to describe the preconception, prenatal and post-delivery insurance coverage of our population to accurately estimate utilization rates without including those without insurance coverage in the denominator.
Although we had a large study population, we recognize several limitations. First, claims data are susceptible to both over-coding and under-coding. If a pregnancy complication was over-coded as a “rule-out” diagnosis, our results could be biased towards detecting fewer differences in follow-up rates between the two pregnancy groups. However, we used previously tested definitions of pregnancy complications,19,20 and the prevalence rates of GDM and hypertensive disorders were similar to national prevalence rates.28–30 Other diagnoses such as obesity and preterm labor may have been under-coded in our data set, as our rate for preterm labor was lower than anticipated,31 and there is evidence that obesity is frequently under recognized and under-coded.32 Second, we do not have information about utilization of primary care after coverage loss, and thus we may have overestimated overall rates of health care post-delivery. Third, claims data do not provide important clinical or sociodemographic data, such as glucose levels, blood pressures or urinalysis results. We linked our data set with the 2000 Census data in order to have neighborhood sociodemographic characteristics. Fourth, we identified fewer statistically significant predictors of post-delivery in part because of the smaller sample size and reduced power with many covariates. Generally, the direction and magnitude of the odds ratios were similar between commercial health and Medicaid insurances.
Our results have important clinical, public health and policy implications for women’s preventive health care after delivery. Post-delivery care for women with pregnancy complications provides a critical opportunity to impact lifelong preventive health behaviors for women and their families, including breastfeeding promotion, postpartum weight loss and providing reliable forms of birth control. Pregnancy has often been labeled a “teachable moment,”15 because women are motivated to make significant health behavioral changes. A smooth transition into primary care could reinforce these changes through a longitudinal primary care relationship. As the ACA is implemented, we anticipate greater numbers of women to have access to insurance coverage. In fact, the U.S. Department of Health and Human Services adopted the Institute of Medicine’s recommendations in “Clinical Preventive Services for Women: Closing the Gap” for expanded insurance coverage of preventive health services insurance.33 However, our results indicate that even among women with insurance, rates of follow-up care are lower than recommended, regardless of pregnancy complications and chronic disease risk factors. These findings are timely and actionable, as the ACA provides the opportunity to innovate and re-design the provision of women’s interconception and long-term preventive health services, and to target women at highest risk. For example, clinic-based post-delivery preventive care may not be feasible or productive for women who are overwhelmed with new family responsibilities and do not perceive urgent risks for themselves.23 Because women may be more focused on the care for their infants, linking maternal care with other family care opportunities, such as in pediatric settings,34 has potential to increase attendance. In addition, a provision of the ACA authorized the creation of the home visitation program, which may provide an opportunity to re-frame post-delivery women’s preventive health within the context of family health services provided within the home, reducing barriers to receiving care.35
CONCLUSIONS
Compared to women without pregnancy complications, insured women with hypertensive disorder in pregnancy or DM (but not GDM) were more likely to attend primary care visits within 1 year after delivery, but rates were still lower than recommended. Although Medicaid expansion through the ACA has the potential to improve women’s insurance coverage, our results highlight that coverage is not sufficient to increase utilization among women at risk for chronic disease. Innovative postpartum health care delivery models have potential to target women at highest risk, improve utilization of recommended post-delivery preventive health services, and ultimately, to reduce women’s development of obesity, diabetes and heart disease.
Electronic Supplementary Material
(PDF 96 kb)
Acknowledgements
Funding
Dr. Wendy Bennett is supported by a career development award from the National Heart, Lung, and Blood Institute, 5K23HL098476– 02.
Conflict of Interest
The authors declare that they do not have any conflicts of interest.
Ethical Approval
The study was approved by the Institutional Review Board of the Johns Hopkins University School of Medicine.
REFERENCES
- 1.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 2.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. BMJ. 2007;335(7627):974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carr DB, Newton KM, Utzschneider KM, et al. Preeclampsia and risk of developing subsequent diabetes. Hypertens Pregnancy. 2009:1-13. [DOI] [PubMed]
- 4.Fraser A, Nelson SM, Macdonald-Wallis C, et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the avon longitudinal study of parents and children. Circulation. 2012;125(11):1367–1380. doi: 10.1161/CIRCULATIONAHA.111.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung NW, Byth K. Population health significance of gestational diabetes. Diabetes Care. 2003;26(7):2005–2009. doi: 10.2337/diacare.26.7.2005. [DOI] [PubMed] [Google Scholar]
- 6.Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? BMJ. 2002;325(7356):157–160. doi: 10.1136/bmj.325.7356.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rich-Edwards JW, McElrath TF, Karumanchi SA, Seely EW. Breathing life into the lifecourse approach: pregnancy history and cardiovascular disease in women. Hypertension. 2010;56(3):331–334. doi: 10.1161/HYPERTENSIONAHA.110.156810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gestational diabetes mellitus. Diabetes Care. 2004;27(1):S88–S90. [DOI] [PubMed]
- 9.American College of Obstetricians-Gynecologists Practice Bulletin Clinical Management guidelines for obstetrician-gynecologists. Number 30, September 2001 (replaces technical bulletin number 200, Dec 1994). Gestational Diabetes. Obstet Gynecol. 2001;98(3):525–538. [PubMed] [Google Scholar]
- 10.Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the fifth international workshop-conference on gestational diabetes mellitus. Diabetes care. 2007;30(S):251–260. doi: 10.2337/dc07-s225. [DOI] [PubMed] [Google Scholar]
- 11.Report of the national high blood pressure education program working group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183(1):S1–S22. [PubMed]
- 12.Smirnakis KV, Chasan-Taber L, Wolf M, Markenson G, Ecker JL, Thadhani R. Postpartum diabetes screening in women with a history of gestational diabetes. Obstet Gynecol. 2005;106(6):1297–1303. doi: 10.1097/01.AOG.0000189081.46925.90. [DOI] [PubMed] [Google Scholar]
- 13.Kim C, Tabaei BP, Burke R, et al. Missed opportunities for type 2 diabetes mellitus screening among women with a history of gestational diabetes mellitus. Am J Public Health. 2006;96(9):1643–1648. doi: 10.2105/AJPH.2005.065722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russell MA, Phipps MG, Olson CL, Welch HG, Carpenter MW. Rates of postpartum glucose testing after gestational diabetes mellitus. Obstet Gynecol. 2006;108(6):1456–1462. doi: 10.1097/01.AOG.0000245446.85868.73. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara A, Ehrlich SF. Strategies for diabetes prevention before and after pregnancy in women with GDM. Curr Diabetes Rev. 2011;7(2):75–83. doi: 10.2174/157339911794940738. [DOI] [PubMed] [Google Scholar]
- 16.Ferrara A, Hedderson MM, Ching J, Kim C, Peng T, Crites YM. Referral to telephonic nurse management improves outcomes in women with gestational diabetes. Am J Obstet Gynecol. 2012;206(6):491.e1–491.e5. doi: 10.1016/j.ajog.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunt KJ, Conway DL. Who returns for postpartum glucose screening following gestational diabetes mellitus? Am J Obstet Gynecol. 2008;198(4):404.e1–404.e6. doi: 10.1016/j.ajog.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Committee for Quality Assurance (NCQA)0.1994-2009 Prenatal and postpartum care (PPC) definitions. Available at: http://www.ncqa.org/portals/0/Prenatal%20Postpartum%20Care.pdf. Accessed October 10, 2013
- 19.Bennett WL, Gilson MM, Jamshidi R, et al. Impact of bariatric surgery on hypertensive disorders in pregnancy: retrospective analysis of insurance claims data. BMJ. 2010;340:c1662. doi: 10.1136/bmj.c1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burke AE, Bennett WL, Jamshidi RM, et al. Reduced incidence of gestational diabetes with bariatric surgery. J Am Coll Surg. 2010;211(2):169–175. doi: 10.1016/j.jamcollsurg.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 21.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67–S74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The Dartmouth Atlas of Healthcare. Available at: http://www.dartmouthatlas.org/tools/downloads.aspx?tab=37. Accessed October 10, 2013
- 23.Bennett WL, Ennen CS, Carrese JA, et al. Barriers to and facilitators of postpartum follow-up care in women with recent gestational diabetes mellitus: a qualitative study. J Womens Health (Larchmt). 2011;20(2):239–245. doi: 10.1089/jwh.2010.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM. Preeclampsia and the risk of end-stage renal disease. N Engl J Med. 2008;359(8):800–809. doi: 10.1056/NEJMoa0706790. [DOI] [PubMed] [Google Scholar]
- 25.Clark HD, Graham ID, Karovitch A, Keely EJ. Do postal reminders increase postpartum screening of diabetes mellitus in women with gestational diabetes mellitus? A randomized controlled trial. Am J Obstet Gynecol. 2009;200(6):634.e1–634.e7. doi: 10.1016/j.ajog.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 26.National Committee for Quality Assurance (NCQA). 2012 NCQA summary table of measures, product lines and changes. Available at: http://www.ncqa.org/LinkClick.aspx?fileticket=O-31v4G27sU%3d&tabid=1415. Accessed October 10, 2013.
- 27.Johnson K, Posner SF, Biermann J, et al. Recommendations to improve preconception health and health care--united states. A report of the CDC/ATSDR preconception care work group and the select panel on preconception care. MMWR Recomm Rep. 2006;55(RR-6):1–23. [PubMed] [Google Scholar]
- 28.Lawrence JM, Contreras R, Chen W, Sacks DA. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999–2005. Diabetes Care. 2008;31(5):899–904. doi: 10.2337/dc07-2345. [DOI] [PubMed] [Google Scholar]
- 29.Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, united states, 1987-2004. Am J Hypertens. 2008;21(5):521–526. doi: 10.1038/ajh.2008.20. [DOI] [PubMed] [Google Scholar]
- 30.Kim SY, Saraiva C, Curtis M, Wilson HG, Troyan J, Sharma AJ. Fraction of gestational diabetes mellitus attributable to overweight and obesity by race/ethnicity, california, 2007-2009. Am J Public Health. 2013;103(10):e65–e72. doi: 10.2105/AJPH.2013.301469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruser CB, Sanders L, Brescia GR, et al. Identification and management of overweight and obesity by internal medicine residents. J Gen Intern Med. 2005;20(12):1139–1141. doi: 10.1111/j.1525-1497.2005.0263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.U.S. Department of Health and Human Services Health Resources and Services Administration. Women’s Preventive Services: Required Health Plan Coverage Guidelines. Available at: http://www.hrsa.gov/womensguidelines/. Accessed October 10, 2013.
- 34.Cheng TL, Solomon BS. Translating life course theory to clinical practice to address health disparities. Matern Child Health J. 2013. [DOI] [PMC free article] [PubMed]
- 35.U.S. Department of Health and Human Services. "HHS announces expansion of Maternal, Infant, and Early Childhood Home Visiting". Available at: http://www.hhs.gov/news/press/2013pres/09/20130906a.html. Accessed October 10, 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 96 kb)