Abstract
Purpose
To evaluate the morphological and functional changes following intravitreal Ozurdex (dexamethasone implant) injections in patients with macular oedema (MO) secondary to retinal vascular diseases.
Design
This is a single centre, exploratory phase III, prospective, open-label clinical study.
Methods
Thirty patients with MO secondary to retinal vascular disorders underwent assessments for best corrected visual acuity, contrast sensitivity, microperimetry, chromatic sensitivity, macular thickness, and morphology using spectral domain optical coherence tomography (SD-OCT) and fluorescein angiography at baseline. They were treated with intravitreal Ozurdex at baseline and monitored monthly with visual acuity and SD-OCT assessments up to 36 weeks. Re-treatment was permitted from 16 to 24 weeks according to pre-defined criteria. All visual function tests were repeated at 24 weeks.
Results
The mean change in central sub-field thickness (CST) from baseline was significant at all visits up to 32 weeks. The lowest mean CST was recorded at 8 weeks and the highest mean ETDRS score was achieved at 12 weeks. All visual functions except contrast sensitivity improved significantly by 24 weeks. The study showed that the ideal re-treatment time point based on functional and structural outcomes and known side-effects of Ozurdex treatment is at 20 weeks.
Conclusion
Ozurdex therapy has a rapid and dramatic effect on the macula for about 8 weeks followed by a sustained modest effect up to week 32. The optimal re-treatment time point is at 20 weeks.
Keywords: macular oedema, visual outcomes, Ozurdex, optical coherence tomography, macular thickness
Introduction
Macular oedema (MO) is a common vision-threatening sequel of a variety of ocular conditions including diabetic retinopathy, central and branch retinal vein occlusion, and following cataract extraction. Hypo-reflective spaces representing focal coalescence of exudative fluid may appear localized to the inner or outer retina but frequently span the entire retinal thickness on optical coherence tomography (OCT). Chronic or severe MO may lead to temporary or permanent visual loss depending on the degree of strain or disruption of the microscopic intraretinal neural connections (transmission defects) and the intracellular damage suffered by the visual elements (transduction defects).1
Steroids inhibit the formation of both prostaglandins and leukotrienes and decrease intracellular and extracellular oedema by suppression of macrophage activity, vasoconstrictive effect, reduction of lymphokine production,2 and downregulation of production of vascular endothelial growth factor (VEGF).3 Several randomized clinical trials have reported good short-term efficacy data on intravitreal triamcinolone acetonide both in terms of improving visual acuity and reducing central retinal thickness (CRT) in patients with MO because of various retinal vascular diseases.4, 5, 6 However, the effect is short-lived and risks outweigh benefits. Intravitreal injections of triamcinolone are associated with elevated intraocular pressure (IOP) in up to 50% of injected eyes, cataract formation in 40% as well as sterile endophthalmitis.7, 8
A number of corticosteroid-based intravitreal implants have been developed to provide a sustained release of drug obviating the need of repeated intravitreal injections. The Ozurdex (Allergan Inc., Irvine, CA, USA) drug delivery system is a sustained-release formulation for posterior-segment delivery of 700 μg dexamethasone, made of a polylacticglycolic acid matrix. It received its market authorization in the European Union in 2010 for MO secondary to retinal vein occlusions. The recommended dose is one Ozurdex implant to be administered intravitreally to the affected eye and repeat doses to be considered when a patient experiences a response to treatment followed subsequently by a loss in visual acuity and in the physician's opinion may benefit from retreatment without being exposed to significant risk. The GENEVA trial was a 12-month study on Ozurdex for MO secondary to retinal vascular occlusions.9, 10 The visual outcome of the study suggested that the effect of the drug waned after 12 weeks. The effect of Ozurdex on the macular morphology was not monitored every month in the study so it is difficult to draw conclusions on the anatomical impact of the drug. As visual acuity does not always correlate with clinical severity of MO,11 other visual functions such as contrast sensitivity, colour threshold, and retinal sensitivity may provide a better understanding of the effect of Ozurdex on MO.
The primary objective of this study was to evaluate the monthly changes in macular thickness with OCT in eyes treated with intravitreal Ozurdex for MO secondary to retinal vascular diseases, over 36 weeks, to evaluate the best re-treatment plan for Ozurdex. The secondary objectives were to assess the change from baseline in various visual functions and OCT parameters at 24 weeks.
Setting
The study was conducted in the Laser and Retinal Research Unit of King's College Hospital, London.
Materials and methods
This interventional, prospective, exploratory study (ISRCTN - 66216819) was conducted following approval from the Institutional Review Board Ethics Committee (11/H0718/6; NRES committee London Central) and all participants gave written informed consent. The study adhered to the principles of the Declaration of Helsinki.
Patients
Patients aged 18 years or above with a clinical diagnosis of MO secondary to diabetes, branch and central retinal vein occlusion, or pseudophakic or post-inflammatory cystoid MO and confirmed on Spectralis spectral domain-OCT (SD-OCT) and best corrected visual acuity (BCVA) in the study eye between 37 and 68 ETDRS letters were deemed eligible for the study. Patients with any other eye disease which could mask or contribute to MO, or any ocular condition in the study eye that in the opinion of the investigator would prevent a 15-letter improvement in visual acuity (eg, severe macular ischemia, extensive macular laser scarring, or atrophy) were excluded. Patients with advanced glaucoma not adequately controlled by medicinal products alone, history of IOP elevation in response to steroid treatment in either eye that resulted in ≥10 mm Hg increase in IOP from baseline with an absolute IOP ≥25 mm Hg, or required therapy with three or more anti-glaucoma medications were also excluded from the study. Systemic conditions that precluded trial entry included known uncontrolled systemic disease or current immunosuppressive disease, initiation of medical therapy for diabetes, or a change from oral hypoglycaemic agents to insulin therapy within 4 months before the screening visit and renal failure requiring haemodialysis or peritoneal dialysis within 6 months before screening visit. Women in the child bearing age underwent a pregnancy test and were advised to avoid conception during the 36 weeks of the study.
Patients were recruited into the trial from the retinal clinics in King's College Hospital NHS Foundation Trust from June 2011 to March 2012 and the last patient exited from the trial in December 2012.
Assessments
After written informed consent, patients underwent baseline examination of BCVA, dilated ophthalmic examination, tonometry, and Spectralis SD-OCT. The OCT volume scan was performed at every monthly visit on a 20 × 20 degree cube, with 49 raster lines, each containing 1064 pixels, separated by 125 μm. Macular stereo and four field fundus colour photographs and fundus fluorescein angiography were performed at baseline and week 24 only but could be repeated at the investigator's discretion.
All visual function tests were performed at baseline and repeated at week 24. These included BCVA, contrast sensitivity, chromatic sensitivity, reading vision, and microperimetry. All patients were refracted by a certified examiner and BCVA for each eye was measured using standard ETDRS protocol at 4 m distance with a modified ETDRS distance chart. Visual acuity was scored as the total number of ETDRS letters read correctly.
Contrast sensitivity measurement was performed after visual acuity measurements, with the Pelli-Robson chart (Clement Clarke Inc., Harlow, UK) at a distance of 1 m and chart luminance of 80–120 cd/m2.
After refraction, reading performance was measured by the certified examiner using a standardized protocol with the MNREAD acuity charts (Optelec, Vista, CA, USA). The reading acuity, maximum reading speed, and critical print size were calculated based on pre-defined formulae.12
Chromatic sensitivity was performed monocularly using the colour assessment and diagnosis test. This is a sensitive test that yields small colour detection thresholds. The test employs direction-specific, moving, chromatic stimuli embedded in a background of random, dynamic, luminance contrast noise. A four-alternative, forced-choice procedure is employed to measure the subject's thresholds for detection of colour signals in 16 directions in colour space, while ensuring that the subject cannot make use of any residual luminance contrast signals.13
The Nidek Microperimeter (Nidek Technologies, Padova, Italy) was used to quantify macular sensitivity and fixation. It incorporates an eye tracker to compensate for eye movements and allows automated follow-up examination at the same retinal loci and also allows colour fundus image registration by infra-red camera (45° field of view). The pupils were dilated before this test and the subject was dark adapted for 15 min before the test. The test was conducted according to pre-defined protocol: room darkened; a briefing trial test carried out initially; 5 min gap given between tests on each eye; all patients to have a 30 s fixation test; a 2° red cross was used as fixation target, the test stimulus colour is white, Goldmann III size (26 min arc or 0.4°), and duration was 200 ms. The background illumination was set at 1.27 cd/m2. The intensity of the stimulus ranges from 0 to 20 db where ‘0' represents the brightest luminance (127 cd/m2). The perimetry strategy of the MP-1 starts at an initially defined threshold level (12 dB) for each stimulus. A 18 loci grid covering central 20° was manually centered on fovea. We used the 4-2 threshold strategy. Fixation was measured for a period of 30 s once patients had located the cross.
The pattern of fixation was classified based on location and stability using the MP-1 software as recommended by Fujii et al.14
Treatment
All patients were treated at the baseline visit with intravitreal implant of 700 μg dexamethasone, Ozurdex (Allergan Inc.) under aseptic precautions as per the protocol by a qualified investigator in a designated treatment room.
Re-treatment was allowed at weeks 16, 20, and 24 if the following criteria were met after an initial improvement (reduction) of macular thickness of at least 50 μm, average macular thickness increased by 100 μm or more from the last visit and at least a five-letter drop in BCVA score from the previous visit, provided the patient had not experienced raised IOP above 30 mm Hg following their first injection. Each patient was allowed to have a maximum of two treatments in this study.
Outcome measures
The primary outcome measure was the mean change in central subfield thickness (CST) at 4 weekly time points. Secondary OCT outcome measures included mean change in CRT and macular volume. Responders at each time point were defined in three ways: proportion of patients with CST <300 μm, those with a decrease of CST by at least 100 μm, or 15% from baseline. Recurrence of oedema was defined as proportion of patients with an increase in CST by 100 μm or 15% from lowest recorded CST. Rebound oedema was defined as an increase of at least 100 μm from baseline after exposure to Ozurdex therapy. Non-responders were defined as those who persistently had CST at ≥300 μm throughout the study.
Secondary visual function outcome measures included mean change in BCVA (ETDRS letters), proportion of patients with gain of 0, 5, 10, and 15 ETDRS letters, or more (improvement) and proportion with loss of <15 ETDRS letters (stabilization), at weeks 24 and 36. The change in contrast sensitivity, colour vision thresholds, reading vision, microperimetry thresholds at 4° and 12°, and change in fixation on microperimetry at 24 weeks were also recorded.
All serious adverse events (SAEs) and adverse events were recorded between baseline and week 36.
Analysis
This is an exploratory study on changes in macular thickness after Ozurdex implant. So with an expected drop out rate of 5%, we were expected to recruit 32 patients to have 30 patients with complete follow-up to fulfill our objectives. Efficacy assessment was performed on the full analysis set (the intent-to-treat population), which included all patients who received at least one application of study treatment and had at least one post-baseline assessment for BCVA. Missing data were imputed using the last-observation-carried-forward approach. Treatment-emergent adverse events were reported for all patients who received at least one application of study treatment and had at least one post-baseline safety assessment. Descriptive statistics for absolute values and changes from baseline were reported for each end point. For the variables, mean and standard deviations were used for continuous variables and counts and percentages for categorical variables. Analysis of variance was used for continuous, normally distributed variables. The categorical variables were examined using Fisher exact test or Pearson's χ2. Data were assumed significant if P<0.05 (SPSS V.17.0, SPSS, Chicago, IL, USA).
Results
Demographics and baseline characteristics
A total of 32 patients were evaluated and 30 patients were enrolled in the study. The baseline characteristics are shown in Table 1. All patients completed the follow-up and were included in the analysis. However, there were seven study appointments that were outside the window of the study visit. One patient did not have the second treatment despite being eligible for it because he was due for a surgery on the same day and preferred not to have his eye injection.
Table 1. Patient demographics and baseline characteristics.
| Mean age (SD), years | 62±3.54 |
| Range, years | 41–91 |
| Male, n (%) | 16 (53.3) |
| Race, n (%) | |
| Caucasian | 20 (66.7) |
| Afrocaribbean | 8 (26.7) |
| Asian | 2 (6.7) |
| Causes of macular edema, n (%) | |
| Diabetic macular edema | |
| Mild and moderate NPDR | 10 (33.3) |
| Severe NPDR | 7 (23.3) |
| Treated proliferative diabetic retinopathy | 7 (23.3) |
| Central retinal vein occlusion | 0 |
| Branch retinal vein occlusion | 5 (16.7) |
| Inflammatory | 0 |
| Pseudophakic | 1 (3.3) |
| Smokers, n (%) | 11 (36.7) |
| Mean HbA1C (SD) in diabetics | |
| ≤8%, n (%) | 15 (50) |
| >8%, n (%) | 9 (30) |
| Hypertensives on treatment, n (%) | 12 (40) |
| Previous glaucoma medications | 0 |
| Pseudophakia, n (%) | 8 (26.7) |
| Previous macular laser, n (%) | 18 (60) |
| Mean number of laser treatments, n (SD) | 2.3±1.6 |
| Mean ETDRS letter score (SD) | 56.1±9.65 |
| <54 letters, n (%) | 12 (40) |
| ≥54 letters, n (%) | 18 (60) |
Abbreviations: ETDRS, early treatment diabetic retinopathy study; HbA1C, glycosylated haemoglobin; NPDR, non-proliferative diabetic retinopathy.
Change in OCT parameters
The mean decrease in CST and CRT from baseline was significant at all 4 weekly time points up to week 32 with steepest reduction observed at week 8 (Table 2). The lowest mean CST was observed at 8 weeks. The CST remained 100 μm less than baseline until week 16. Although the macular volume decreased from baseline at all time points, the change was minimal from week 16. The lowest mean macular volume was achieved at week 12. The changes in these parameters were no different when the DMO sub-group was analysed as a sub-group.
Table 2. Changes in OCT parameters and visual acuity per month.
| Mean CST | CST change from baseline | P value | CST change from previous visit | P value | Mean visual acuity | Change in visual acuity from baseline | P value | Change in visual acuity from previous visit | P value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 527.4+47.5 | 56.10+9.7 | ||||||||
| 4 Weeks | 337.1+28.6 | −190.3+54.6 | 0.000 | −190.33 | 0.000 | 64.28+11.6 | 8.18±5.5 | 0.000 | 8.18+5.5 | 0.000 |
| 8 weeks | 301+27 | −226.4+53.4 | 0.000 | −36.07 | 0.001 | 64.93+12.7 | 8.83±5.8 | 0.004 | 0.65+2.2 | 0.804 |
| 12 Weeks | 347.1+49.4 | −180+68 | 0.000 | 46.07 | 0.020 | 65.34+14.2 | 9.2±6.2 | 0.005 | 0.41+3.1 | 0.841 |
| 16 Weeks | 416.9+61.7 | −110+68.5 | 0.002 | 69.83 | 0.000 | 61.55+14.5 | 5.45±6.4 | 0.096 | −3.79+2.4 | 0.005 |
| 20 Weeks | 408.1+60 | −94.3+75.7 | 0.003 | 19.31 | 0.184 | 60.21+15.3 | 4.1±6.6 | 0.224 | −1.34+3.7 | 0.132 |
| 24 Weeks | 406.1+58.2 | −121+73.8 | 0.002 | −16.54 | 0.600 | 61.67+14.9 | 5.5±6.5 | 0.092 | 1.46+2.6 | 0.458 |
| 28 Weeks | 419.2+53 | −108.1+69.5 | 0.003 | −0.43 | 0.983 | 64.13+13.9 | 8+6.1 | 0.012 | 2.46+2.7 | 0.016 |
| 32 Weeks | 437.4+53 | −89.6+69.9 | 0.013 | 18.48 | 0.224 | 61.82+12.9 | 3.58±7.2 | 0.328 | −2.31+1.2 | 0.008 |
| 36 Weeks | 457.3+57 | −70.1+73 | 0.059 | 19.58 | 0.091 | 60.33+13.2 | 4.2±6 | 0.163 | −1.49+2.5 | 0.790 |
P<0.05 is given in bold.
Abbreviation: CST, central sub-field thickness.
Change in visual acuity
The mean change in visual acuity from baseline was significant until week 12 (Table 2). The maximal mean visual acuity improvement was observed at 12 weeks. The number of patients with a gain of 0, 5, 10, or 15 letters or more at 24 weeks were 20 (66%), 17 (56.6%), 12 (40%), and 6 (20%), respectively, and the corresponding proportions at 36 weeks were 17 (56.6%), 14 (46.6%), 11 (36.6%), and 3 (10%). The number of patients who lost less than 15 letters were 26 (86.6%) and 28 (93.3%) at 24 and 36 weeks, respectively. One patient lost 26 letters at 20 weeks due to anterior uveitis and then recovered to baseline visual acuity by 36 weeks. Another patient developed vitreous haemorrhage because of posterior vitreous detachment at 32 weeks, but improved to 41 letters by 36 weeks. In the DMO sub-group, the mean change in visual acuity from baseline was significant until week 20. The maximal visual acuity gain was achieved at 12 weeks as in the whole cohort, but the mean visual acuity gain was 1.2 letters more (10.4 vs 9.2) and was sustained up to 20 weeks (data not shown).
Change in IOP
The IOP increased from mean value of 15.6+2.8 mm Hg at baseline to 17.1+2.8 mm Hg at 4 weeks, 18.1+1 mm Hg at 8 weeks, 18.1+2 mm Hg at 12 weeks, 16.7+3 mm Hg at 16 weeks, and 16.1+2 mm Hg by 20 weeks. The IOP showed a rise to 17+2 mm Hg by 24 and 28 weeks and falling to 16+2 mm Hg by 36 weeks
Responder profile
Table 3 shows the proportion of patients that may be defined as responders based on the three definitions of responders. The maximum number of responders was observed at 8 weeks irrespective of the definitions used to define responders. Proportion of patients with a CST of less than 300 μm reduced significantly by 16 weeks. However, if a reduction of 15% or 100 μm is used to define response, approximately 45% qualify as responders until week 28. Rebound oedema from 20 weeks was observed in less than 10% of the sample. Recurrence of oedema based on an increase in CST by 100 μm or by 15% from lowest recorded CST was noted as early as 12 weeks and continued to increase until 36 weeks. The number of patients with a decrease of five letters in BCVA gradually increased to 46.7% by 20 weeks.
Table 3. Responder profile.
|
Responders (%) |
Recurrence (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Time | A | B | C | D | E | F | G | H |
| Week 4 | 12/30 (40) | 20/30 (66.7) | 18/30 (60) | 0 | 0 | 4/30 (13.3) | 4/30 (13.3) | 0 |
| Week 8 | 19/30 (63.3) | 24/30 (80) | 21/30 (70) | 0 | 0 | 5/30 (16.6) | 6/30 (20) | 0 |
| Week 12 | 14/30(46.6) | 19/30 (63.3) | 17/30 (56.7) | 5/30 (16.7) | 4/30 (13.3) | 4/30 (13.3) | 8/30 (26.7) | 0 |
| Week 16 | 5/30 (16.6) | 14/30 (46.7) | 14/30 (46.7) | 12/30 (40) | 9/30 (30) | 7/30 (23.3) | 15/30 (50) | 8/30 (26.7) |
| Week 20 | 3/30 (10) | 14/30 (46.7) | 11/30 (36.7) | 16/30 (53.3) | 12/30 (40) | 9/30 (30) | 20/30 (66.7) | 13/30 (43.3) |
| Week 24 | 6/30 (20) | 15/30 (50) | 12/30 (40) | 16/30 (53.3) | 13/30 (43.3) | 7/30 (23.3) | 18/30 (60) | 11/30 (36.7) |
| Week 28 | 7/30 (23.3) | 14/30 (46.7) | 14/30 (46.7) | 14/30 (46.7) | 9/30 (30) | 7/30 (23.3) | 14/30 (46.7) | 8/30 (26.7) |
| Week 32 | 4/30 (13.3) | 11/30 (36.7) | 10/30 (33.3) | 15/30 (50 | 12/30 (40) | 10/30 (33.3) | 17/30 (56.7) | 9/30 (30) |
| Week 36 | 6/30 (20) | 11/30 (36.7) | 8/30 (26.7) | 19/30 (63.3) | 15/30 (50) | 11/30 (36.7) | 22/30 (73.3) | 16/30 (53.3) |
(A) Proportion of patients with CST (central sub-field thickness) <300 μm (responder).
(B) Proportion of patients with decrease of CST by 100 μm from baseline (responder).
(C) Proportion of patients that showed a decrease of CST by at least 15% from baseline (responders).
(D) Proportion of patients with increase CST by 100 μm from lowest recorded CST (recurrence).
(E) Proportion of patients with increase CST by 15% from lowest recorded CST (recurrence).
(F) Proportion of patients with drop of BCVA (best corrected visual acuity) by 5 letters or more from baseline.
(G) Proportion of patients with drop of BCVA by 5 letters or more from best recorded BCVA.
(H) Proportion of patients with a drop of BCVA by 5 letters or more from best recorded BCVA and an increase CST by 100 μm from lowest recorded CST.
Other visual function changes at 24 weeks
All visual functions except contrast sensitivity improved significantly at 24 weeks compared with baseline (Table 4).
Table 4. Secondary visual function outcomes at 24 weeks.
| Baseline | 24 Weeks | Mean change/SD | P value | |
|---|---|---|---|---|
| Contrast sensitivity | 29.8+3.7 | 30.7+5.3 | 0.9+1.6 | 0.149 |
| Reading acuity | 0.51+0.19 | 0.41+0.28 | 0.1+0.12 | 0.034 |
| MRS | 164.3+53.8 | 185.9+71.9 | 21.6+18.1 | 0.039 |
| CPS | 0.988+0.19 | 0.873+0.26 | 0.115+0.15 | 0.035 |
| Ret sensitivity 4° | 4.8+4.5 | 9.2+5.8 | 4.4+4.1 | <0.0001 |
| Ret sensitivity 12° | 6.1+4.5 | 10.2+5.5 | 4.1+4.3 | <0.0001 |
| Mean tritan threshold (SD) | 20.6+11.7 | 16.4+11.6 | 4.2+10.1 | 0.012 |
| Mean protan threshold (SD) | 14.7+5.1 | 12.9+6.6 | 1.8+5 | 0.073 |
| Fixation location, n | 28 | 28 | ||
| PC, n (%) | 13 (46.4) | 12 (40) | >0.05 | |
| POC, n (%) | 3 (10.7) | 6 (20) | ||
| PE, n (%) | 12 (40) | 10 (33) | ||
| Fixation stability, n | 28 | 28 | ||
| Stable, n (%) | 19 (67.9) | 16 (53.3) | ||
| RUS, n (%) | 7 (25) | 11 (36.6) | >0.05 | |
| Unstable, n (%) | 2 (7.1) | 1 (3.3) |
Abbreviations: CPS, critical print size; MRS, mean reading speed; PC, predominantly central; PE, predominantly eccentric; POC, poor central; RUS, relatively unstable.
P<0.05 is shown in bold.
Re-treatment criteria
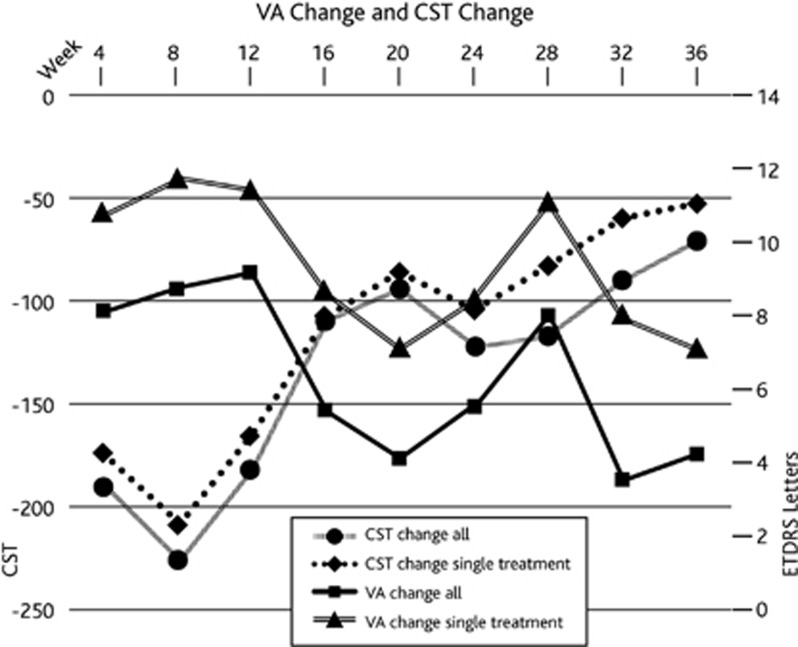
Proportion of patients that required a second injection based on our re-treatment criteria was 16.6% (5/30). They were all patients with DMO. Two patients each were treated at 16 weeks and 24 weeks and one at 20 weeks. The changes in visual acuity and CST for the whole cohort were compared with those who received only a single treatment at baseline. The patterns of changes in visual acuity and CST over time were similar as shown in Figure 1. However, we can infer that patients who required second treatment had poorer potential for visual gain.
Figure 1.
Graph showing the change in central sub-field thickness (CST) and visual acuity (VA) over time in the whole study cohort and those that received a single Ozurdex injection only.
Safety events
Two patients (6.7%) required IOP-lowering medications and one patient developed vitreous haemorrhage secondary to posterior vitreous detachment. A fifth of the study cohort suffered serious SAEs but none were related to the study medication. Six patients had SAEs, none of them were related to the Ozurdex injection. These included fracture wrist, fracture leg, surgery for carpal tunnel syndrome, scheduled surgery for aortic valve replacement, hyperkalaemia in one patient, and injection (insulin) site infection in another patient (Table 5). One patient lost 26 letters at 20 weeks because of anterior uveitis and then recovered to baseline visual acuity by 36 weeks.
Table 5. Adverse events.
| Any AE related to treatment, n (%) | 2 (6.7) |
| Any SAE, n (%) | 6 (20) |
| Any SAE related to treatment, n (%) | 0 |
| Proportion requiring IOP lowering medication, n (%) | |
| 1 IOP-lowering medication | 2 (6.7) |
| 2 IOP-lowering medications | 0 |
| Proportion requiring cataract surgery | 0 |
| Other selected adverse events, n (%) | |
| Endophthalmitis | 0 |
| Anterior uveitis | 1 |
| Vitreous haemorrhage | 1 (3.3%) |
| Retinal detachment | 0 |
| Systemic adverse events | |
| Deaths | 0 |
| Non-fatal MI/CVA | 0 |
Abbreviations: AE, adverse event; IOP, intraocular pressure; SAE, serious adverse event.
Discussion
This study shows that the maximal efficacy in terms of lowest recorded mean CST after a single dose of Ozurdex was at 8 weeks. The most dramatic reduction in mean CST of approximately 200 μm was noted at 4 weeks. The optimal re-treatment time point was at 20 weeks.
The pharmacokinetic effects of Ozurdex have been studied in normal monkey eyes. The pharmacokinetics of Ozurdex in the vitreous and retina of monkey eyes demonstrated two distinct phases that corresponded to the observed fragmentation of the implant. A high initial drug concentration was observed for 60 days followed by a period of low concentration up to 210 days. The drug was not detected from days 240 to 270.15 The use of central macular thickness to model changes of intraocular drug concentrations has been previously described. Aubren et al demonstrated a population pharmacokinetic–pharmacodynamic modelling to understand the elimination rate of triamcinolone based on the changes in central macular thickness.16
In comparison, in this study, if the reduction of CST from baseline is considered a surrogate marker for the overall durability of the drug concentration in the vitreous and retina from baseline, the findings of this study mirror the effects in normal monkey eyes in that the initial high drug concentration phase lasts 8 weeks. The CST then remains below baseline until about week 36, suggesting that a second phase of lower concentration of Ozurdex continues to provide a sustained drying effect of the retina.
If we consider the rate of reduction of CST between visits as the marker of remaining drug concentration at each visit, the maximal effect of the drug is at 4 weeks with a dramatic mean reduction of 190 μm and a further 36 μm reduction at 8 weeks supporting the period of initial high drug concentration. The lowest recordable mean CST was also at 8 weeks.
Therefore, this study substantiates the pharmacokinetic and pharmcacodynamic studies of Ozurdex in normal monkeys by using three surrogate markers of drug concentration: change in CST from baseline, change in CST from previous visit, and lowest recordable CST. These observations suggest that unlike other biodegradeable implants with a triphasic response curve, Ozurdex has a biphasic response of an early burst because of high concentration of the drug that is followed by a plateau that last about 32 weeks.
The anatomical changes observed in this study highlight the strengths and weaknesses of Ozurdex in the treatment of MO because of retinal vascular diseases. The immediate drying effect of the macula was noted in all but one patient indicating that it may be useful not only as first-line drying agent but also as sequential or combination therapy with anti-VEGF therapy. However, the recurrence of oedema by 16–20 weeks is a disadvantage as early re-treatment may also cause increase rates of elevated IOP and cataract as reported with intravitreal triamcinolone. Given that the proportion of patients with a reduction of five letters or more in BCVA associated with an increase in CST of 100 μm or more from lowest recorded value was highest at 20 weeks, it seems optimal to state that a 5 monthly re-treatment is appropriate if the re-treatment criteria are met.
Another point observed in this study is the ideal choice of re-treatment criteria. Only five patients met the re-treatment criteria of more than five-letter loss with an increase in 100 μm from the previous visit. However, if an increase of 100 μm from lowest recorded CST was used as the re-treatment criteria approximately 50% of the participants would have been re-treated at 16–20 weeks. Given that 100 μm is an arbitrary figure, an individualized re-treatment criterion may be defined as an increase by 15% from lowest recorded CST. This re-treatment would mean that 40% of the participants would have required treatment at 20 weeks. However, if a five-letter drop in visual acuity and an increase in 100 μm from lowest recorded CST are taken into account, the optimal re-treatment time point is at 20 weeks. Earlier re-treatment based on changes in CST alone may be associated with higher prevalence of cataract and raised IOPs.
Rebound oedema after Ozurdex has been reported previously.17 This effect was minimal in this study with a maximum of three patients showing increased CST above baseline after an initial reduction. There was one patient with an increase in CST more than baseline with no evidence of any reduction in CST following treatment. This observation shows that the drying effect of Ozurdex is almost universal for most retinal vascular diseases despite the cause of the MO.
The maximum gain of 9.24 letters is observed at 12 weeks. This pattern of visual gain with Ozurdex closely resembles the effect of loading phase of 3 monthly anti-VEGF injections. Therefore, Ozurdex may be used as first-line treatment as a drying agent instead of three anti-VEGF injections. It is promising to observe that all visual functions tested in this study except contrast sensitivity also showed significant improvement at 24 weeks as this indicates a positive effect on the retinal neuronal function probably due to a realignment of neuronal structures induced by the drying effect of Ozurdex. A direct neurtrophic effect of Ozurdex cannot be ruled out.
In conclusion, this study shows that Ozurdex is an effective therapy to improve visual function and macular thickness in patients with MO because of retinal vascular disease up to 36 weeks. If re-treatment is indicated, the time point of 20 weeks seems to represent a reasonable compromise in terms of effectiveness and side-effects of Ozurdex.
Acknowledgments
We thank Rashmi Akshikar, Roopa Vemala, Matthew Richardson, Richard Leung for assistance in conducting the trial. We thank Allergan Ltd for funding this project and D J Simon for statistical support. Institutional Review Board Ethics Committee (11/H0718/6) (NRES committee London Central).
R Mathew has received travel grants and speaker fees from Allergan. S Sivaprasad has received research grants, travel grants, and participated in advisory boards of Novartis, Pfizer, Allergan, Alimera services, and Bayer. The remaining authors declared no conflict of interest.
References
- Pelosini L, Hull CC, Boyce JF, McHugh D, Stanford MR, Marshall J. Optical coherence tomography may be used to predict visual acuity in patients with macular edema. Invest Ophthalmol Vis Sci. 2011;52 (5:2741–2748. doi: 10.1167/iovs.09-4493. [DOI] [PubMed] [Google Scholar]
- Abe T, Hayasaka S, Nagaki Y, Kadoi C, Matsumoto M, Hayasaka Y. Pseudophakic cystoid macular edema treated with high-dose intravenous methylprednisolone. J Cataract Refract Surg. 1999;25 (9:1286–1288. doi: 10.1016/s0886-3350(99)00159-5. [DOI] [PubMed] [Google Scholar]
- Binz N, Graham CE, Simpson K, Lai YK, Shen WY, Lai CM, et al. Long-term effect of therapeutic laser photocoagulation on gene expression in the eye. Faseb J. 2006;20 (2:383–385. doi: 10.1096/fj.05-3890fje. [DOI] [PubMed] [Google Scholar]
- Scott IU, Ip MS, VanVeldhuisen PC, Oden NL, Blodi BA, Fisher M, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular Edema secondary to branch retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6. Arch Ophthalmol. 2009;127 (9:1115–1128. doi: 10.1001/archophthalmol.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martidis A, Duker JS, Greenberg PB, Rogers AH, Puliafito CA, Reichel E, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002;109 (5:920–927. doi: 10.1016/s0161-6420(02)00975-2. [DOI] [PubMed] [Google Scholar]
- Young S, Larkin G, Branley M, Lightman S. Safety and efficacy of intravitreal triamcinolone for cystoid macular oedema in uveitis. Clin Exp Ophthalmol. 2001;29 (1:2–6. doi: 10.1046/j.1442-9071.2001.00360.x. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Kreissig I, Sofker A, Degenring RF. Intravitreal injection of triamcinolone for diffuse diabetic macular edema. Arch Ophthalmol. 2003;121 (1:57–61. [PubMed] [Google Scholar]
- Loewenstein A, Goldstein M. Intravitreal triamcinolone acetonide for diabetic macula edema. Isr Med Assoc J. 2006;8 (6:426–427. [PubMed] [Google Scholar]
- Haller JA, Bandello F, Belfort R, Jr., Blumenkranz MS, Gillies M, Heier J, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117 (6:1134–1146. doi: 10.1016/j.ophtha.2010.03.032. [DOI] [PubMed] [Google Scholar]
- Haller JA, Bandello F, Belfort R, Jr, Blumenkranz MS, Gillies M, Heier J, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011;118 (12:2453–2460. doi: 10.1016/j.ophtha.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Browning DJ, Glassman AR, Aiello LP, Beck RW, Brown DM, Fong DS, et al. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114 (3:525–536. doi: 10.1016/j.ophtha.2006.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legge GE, Ross JA, Luebker A, LaMay JM. Psychophysics of reading. VIII. The Minnesota Low-Vision Reading Test. Optom Vis Sci. 1989;66 (12:843–853. doi: 10.1097/00006324-198912000-00008. [DOI] [PubMed] [Google Scholar]
- Connolly DM, Barbur JL, Hosking SL, Moorhead IR. Mild hypoxia impairs chromatic sensitivity in the mesopic range. Invest Ophthalmol Vis Sci. 2008;49 (2:820–827. doi: 10.1167/iovs.07-1004. [DOI] [PubMed] [Google Scholar]
- Fujii GY, De Juan E, Jr, Humayun MS, Sunness JS, Chang TS, Rossi JV. Characteristics of visual loss by scanning laser ophthalmoscope microperimetry in eyes with subfoveal choroidal neovascularization secondary to age-related macular degeneration. Am J Ophthalmol. 2003;136 (6:1067–1078. doi: 10.1016/s0002-9394(03)00663-9. [DOI] [PubMed] [Google Scholar]
- Chang-Lin JE, Attar M, Acheampong AA, Robinson MR, Whitcup SM, Kuppermann BD, et al. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Invest Ophthalmol Vis Sci. 2011;52 (1:80–86. doi: 10.1167/iovs.10-5285. [DOI] [PubMed] [Google Scholar]
- Audren F, Tod M, Massin P, Benosman R, Haouchine B, Erginay A, et al. Pharmacokinetic-pharmacodynamic modeling of the effect of triamcinolone acetonide on central macular thickness in patients with diabetic macular edema. Invest Ophthalmol Vis Sci. 2004;45 (10:3435–3441. doi: 10.1167/iovs.03-1110. [DOI] [PubMed] [Google Scholar]
- Querques L, Querques G, Lattanzio R, Gigante SR, Del Turco C, Corradetti G, et al. Repeated intravitreal dexamethasone implant (Ozurdex(R)) for retinal vein occlusion. Ophthalmologica. 2013;229 (1:21–25. doi: 10.1159/000342160. [DOI] [PubMed] [Google Scholar]