Abstract
Chronic cholestasis results in liver injury and eventually liver failure. Although ursodeoxycholic acid (UDCA) showed limited benefits in primary biliary cirrhosis, there is an urgent need to develop alternative therapy for chronic cholestatic disorders. Previous studies from our laboratory demonstrated that all-trans-retinoic acid (atRA) is a potent suppressor of CYP7A1, the rate-limiting enzyme in bile acid synthesis. atRA also repressed the expression of tumor growth factor-β and collagen 1A1 in activated primary human stellate cells and LX2 cells. When administered together with UDCA to bile duct–ligated rats, this combined therapy significantly reduced the bile acid pool size and improved liver conditions. To further examine whether atRA alone or in combination with UDCA has greater beneficial effects than UDCA treatment alone, we assessed this treatment in two additional chronic cholestatic rodent models: α-naphthylisothiocyanate (ANIT)–treated rats and the Mdr2−/− (Abcb4−/−) knockout mouse. atRA alone significantly reduced bile duct proliferation, inflammation, and hydroxyproline levels in ANIT-treated rats, whereas the combination of atRA and UDCA significantly reduced plasma bile salt level compared with UDCA treatment. atRA alone or in combination with UDCA significantly reduced plasma levels of alkaline phosphatase and bile salts in 12-week-old Mdr2−/− mice. Reduced bile duct proliferation and inflammation were also observed in the livers of these mice. Together, atRA alone or in combination with UDCA significantly reduced the severity of liver injury in these two animal models, further supporting the combination treatment of atRA and UDCA as a potential new therapy for patients with chronic cholestatic liver disease who have not responded fully to UDCA.
Introduction
Chronic cholestasis results in bile duct proliferation, liver fibrosis, cirrhosis, and eventually liver failure. Ursodeoxycholic acid (UDCA) is currently the only effective medical treatment of primary biliary cirrhosis (PBC) but is limited to early stages of the disease. By contrast, there is no accepted medical treatment for primary sclerosing cholangitis (PSC) except liver transplantation. Therefore, there is an urgent need to develop alternative therapy for these chronic cholestatic disorders (Carey and Lindor, 2012).
Previous studies from our laboratory using primary human hepatocytes and HepG2 cells determined that all-trans-retinoic acid (atRA) is a potent suppressor of CYP7A1, the rate-limiting enzyme in bile acid synthesis (Cai et al., 2010). atRA also repressed the expression of tumor growth factor-β and collagen 1A1 in activated primary human stellate cells and LX2 cells (He et al., 2011). When administered together with UDCA to a cholestatic rat model induced by bile duct ligation for 14 days, this combined therapy significantly reduced the bile acid pool size, liver cell necrosis, inflammation, hepatic fibrosis, and bile duct proliferation (He et al., 2011). These dramatic findings suggest that atRA in combination with UDCA might be an effective alternative therapy for patients with chronic cholestasis who have not fully responded to UDCA. atRA is already a U.S. Food and Drug Administration–approved medicine for treating patients with acne and acute promyelocytic leukemia (Reichrath et al., 2007; Baljevic et al., 2011).
To further examine whether atRA alone or in combination with UDCA has greater beneficial effects than UDCA treatment alone, we assessed this treatment in two additional chronic cholestatic rodent models: α-naphthylisothiocyanate (ANIT)–treated rats (Ferreira et al., 2003) and Mdr2−/− (Abcb4−/−) knockout mice (Smit et al., 1993; Mauad et al., 1994; Fickert et al., 2004, 2006). The Mdr2−/− knockout mouse is a rodent model reminiscent of sclerosing cholangitis in humans. Our results demonstrate that atRA alone or in combination with UDCA also significantly reduced the severity of liver injury in these two animal models, further supporting the combination treatment of atRA and UDCA as a potential new therapy for patients with chronic cholestatic liver disease who have not responded fully to UDCA.
Materials and Methods
Animal Experiments.
All animal experimental protocols were approved by the local Animal Care and Use Committee, according to criteria outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication 86-23, revised 1985). Male Sprague-Dawley rats (200–230 g) were obtained from Charles River Laboratories (Wilmington, MA). ANIT (dissolved in corn oil) was administered to 24 male rats (80 mg/kg s.c. weekly) for 8 weeks. After 4 weeks, when pilot experiments indicated that ANIT had produced chronic liver injury, the animals were divided into four groups. Group A received phosphate-buffered saline (the treatment control and solvent), group B received 15 mg/kg UDCA (suspended in phosphate-buffered saline), group C received 5 mg/kg atRA, and group D received combinations of UDCA (15 mg/kg) and atRA (5 mg/kg) daily by gavage for the additional 4 weeks. For experiments with Mdr2−/− mice, 2-month-old male mice were randomly divided into four groups (n = 6–8) and fed diets containing 100 mg/kg UDCA and/or 33 mg/kg atRA or a control diet for 1 month. In addition, age-matched Mdr2+/+ and Mdr2+/− male mice were also fed the control diet as additional healthy control groups (n = 6 each). Body weight was measured weekly. The animals were killed in random order between 9:00 AM and 11:00 AM after an overnight fast. Samples of plasma, liver, kidney, urine (from bladder), and feces were collected for further analyses.
Plasma Biochemistry and Liver Histology.
Plasma liver alanine aminotransferase (ALT) and alkaline phosphatase (ALP) enzymes were analyzed by the Analytical Core in the Mouse Metabolic Phenotyping Center at Yale University (New Haven, CT). 3α-Hydroxy bile salt concentrations were determined in plasma, urine, feces, and liver tissue using a kit from Trinity BioTech (Newark, NJ) or Diazyme Laboratories (Poway, CA). Formalin-fixed tissue was embedded in paraffin and sections were stained with hematoxylin and eosin and Sirius Red. Liver histology was blindly assessed for inflammation, necrosis, bile duct proliferation, and fibrosis on a scale from 1 to ≥4, as previously described (He et al., 2011). Liver hydroxyproline content was measured as described (Soroka et al., 2010).
Immunohistochemistry.
Immunohistochemistry was performed using an antibody against cytokeratin 19 (CK19) (Troma-III) developed by R. Kemler and obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institutes of Health Eunice Kennedy-Shriver National Institute of Child Health and Human Development and maintained by the University of Iowa Department of Biologic Sciences (Iowa City, IA) using a 3,3′-diaminobenzidine peroxidase kit (Vector Laboratories, Burlingame, CA). Images were acquired using an Olympus BX2 microscope (Olympus Corporation, Center Valley, PA). Quantitation of CK19 labeling was performed using ImageJ software (National Institutes of Health, Bethesda, MD) with thresholding. Data are presented as a percentage of the total area that is positive for CK19.
Quantitative Real-Time Polymerase Chain Reaction and Western Blot Analysis.
Gene mRNA expression was detected using TaqMan real-time polymerase chain reaction in an ABI7500 system (Applied Biosystems, Foster City, CA), and protein was analyzed by Western blotting, as previously described (Soroka et al., 2010). The glyceraldehyde-3-phosphate dehydrogenase gene was used as a reference to normalize data. The TaqMan primer/probes and antibodies are as described in our previous publications (Soroka et al., 2010; He et al., 2011).
Statistical Analysis.
Data are presented as the means ± S.D. Differences between experimental groups were assessed for significance using one-way analysis of variance. The two-tailed t test was used to calculate the P value. A P value < 0.05 was considered statistically significant.
Results
There was no significant difference in body weight and liver/body ratio among groups after 4-week treatment with or without atRA and/or UDCA in ANIT-treated rats. All four groups had normal levels of plasma ALT and γ-glutamyltransferase.
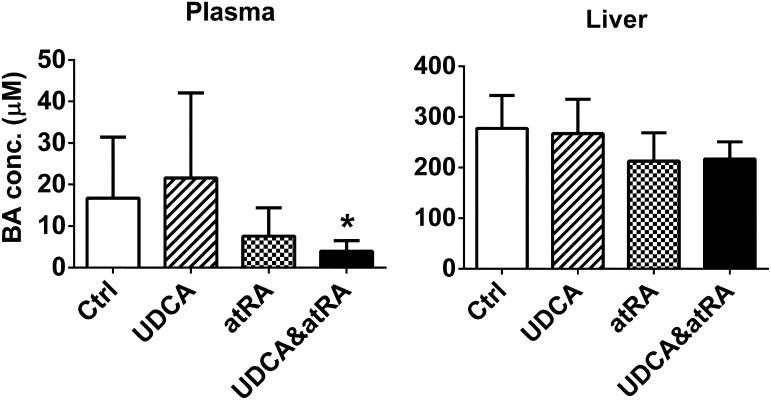
UDCA and atRA in Combination Significantly Reduced Plasma Bile Salt Concentrations in ANIT-Treated Rats.
Significantly lower levels of plasma bile acid were detected in rats treated with both atRA and UDCA compared with the UDCA-treated group, although the liver bile acid levels were not significantly different (Fig. 1). In addition, urine bile acid levels were undetectable in all four groups and fecal bile acids did not differ among these groups.
Fig. 1.
UDCA and atRA in combination significantly reduced plasma bile salt concentrations in ANIT-treated rats. Data are the mean ± S.D. compared with controls (n = 6). *P < 0.05. BA, bile acid; conc, concentration; Ctrl, control.
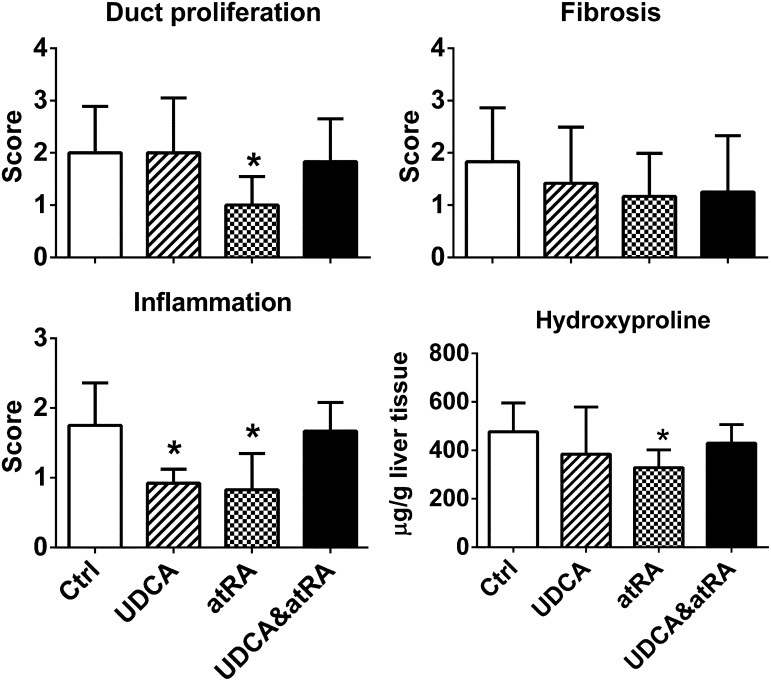
atRA Treatment Alone Reduced Bile Duct Proliferation, Inflammation, and Liver Hydroxyproline in ANIT-Treated Rat Livers.
Blinded examination of liver histology revealed that treatment with atRA alone significantly reduced bile duct proliferation and inflammation. No histologic differences in fibrosis were noted given that the liver injury was relatively mild in rats treated with ANIT for 8 weeks (Fig. 2). However, hepatic levels of hydroxyproline were significantly less after atRA compared with the control group. Liver histology also showed less inflammation in the UDCA-treated group, although this difference was not maintained in the group treated with atRA plus UDCA. Histologic evidence of necrosis was absent in all four groups.
Fig. 2.
atRA treatment alone but not in combination with UDCA reduced bile duct proliferation, inflammation, and liver hydroxyproline in ANIT-treated rat livers. Data are the mean ± S.D. (compared with controls; n = 6). *P < 0.05. To note, a paired t test indicates that hydroxyproline values correlate with histologic scores of fibrosis (P = 0.034). Ctrl, control.
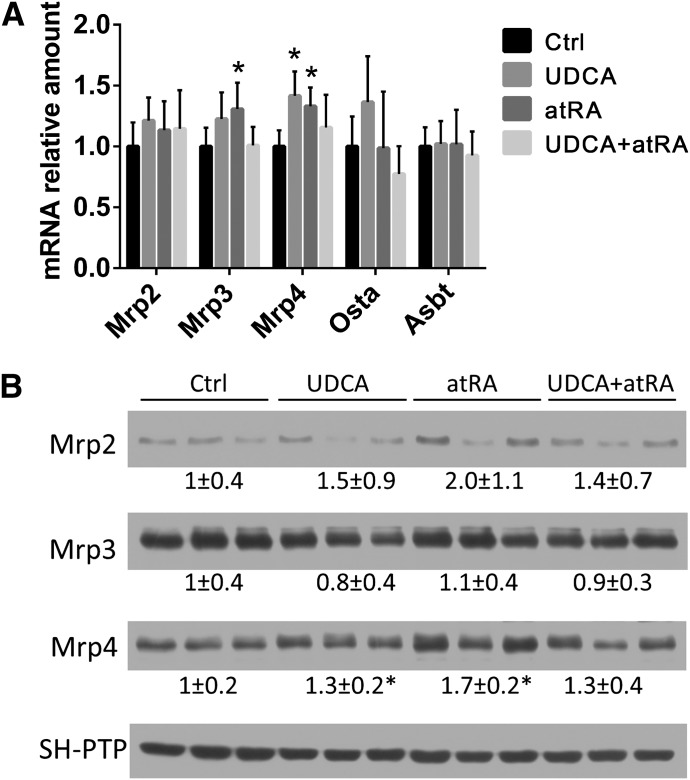
Expression of Bile Acid Transporter Genes in the Liver and Kidney of ANIT-Treated Rats.
Analyses of mRNA and/or protein expression of liver genes involved in bile acid synthesis and transport revealed no significant differences among the four groups, including expression levels of Bsep (Abcb11), Mrp2 (Abcc2), Mrp3 (Abcc3), Mrp4 (Abcc4), Ntcp (Slc10a1), Oatp2 (Slco1a2), Ostα, and Cyp7a1 (data not shown). There were also no differences in mRNA expression of liver transforming growth factor-β, tumor necrosis factor-α, and interleukin-1β (data not shown). By contrast, both atRA and UDCA single treatments significantly increased the expression of Mrp4 mRNA and protein in the kidney, whereas only atRA increased the expression of Mrp3 mRNA in the kidney (Fig. 3).
Fig. 3.
atRA altered the expression of bile salt transporters in kidney. (A) Mrp3 and Mrp4 mRNA expression were significantly increased in atRA-treated rat kidneys compared with untreated controls (n = 6). (B) Western blots demonstrate that Mrp4 protein expression was significantly increased in atRA- and UDCA-treated rat kidneys (n = 6) compared with untreated controls. *P < 0.05. Ctrl, control.
atRA Treatment Alone or in Combination with UDCA Significantly Reduced Plasma Levels of ALP and Bile Salts in 12-Week-Old Mdr2−/− Mice.
Previous studies have established the Mdr2−/− mouse as an animal model for PSC because the typical histologic features of onion skin fibrosis develop around the bile ducts in these mice at approximately 2 months of age (Fickert et al., 2004). To test whether atRA alone or in combination with UDCA improved liver injury in these mice, we treated 2-month-old Mdr2−/− male mice for 4 weeks. Analysis of plasma biochemistry confirmed that the homozygous knockout (Mdr2−/−) mice developed cholestatic liver injury because levels of ALT, ALP, and bile acids (including liver levels) were elevated compared with age-matched wild-type (Mdr2+/+) and Mdr2+/− heterozygotes (Table 1). Interestingly, atRA treatment alone or in combination with UDCA significantly lowered the plasma levels of ALP but not ALT (Table 1), suggesting less injury to the bile duct epithelium in the groups treated with atRA alone or in combination with UDCA. Lower plasma bile acid levels were also detected in animals treated with UDCA and atRA alone and in combination, suggesting that these treated mice were less cholestatic than the control groups.
TABLE 1.
Plasma biochemistry in wild-type and Mdr2 knockout mice
Data are the mean ± S.D.
|
Mdr2+/+ Mice |
Mdr2+/− Mice |
Mdr2−/− Mice |
||||
|---|---|---|---|---|---|---|
| Control Diet (n = 7) | Control Diet (n = 7) | Control Diet (n = 12) | UDCA (n = 11) | atRA (n = 12) | UDCA and atRA (n = 12) | |
| ALT (U/L) | 42 ± 7 | 52 ± 17 | 294 ± 86a | 376 ± 134a | 238 ± 99a | 273 ± 34a |
| ALP (U/L) | 72 ± 6 | 78 ± 8 | 204 ± 29a | 190 ± 38a | 172 ± 24a,b | 173 ± 24a,b |
| Plasma bile acid (µM) | 17 ± 15 | 12 ± 9 | 46 ± 16a | 23 ± 2a,b | 24 ± 4a,b | 20 ± 9a,b |
| Liver bile acid (µM) | 118 ± 94 | 102 ± 72 | 329 ± 47a | 236 ± 36a,b | 299 ± 115a | 235 ± 74a,b |
P < 0.05 to Mdr2+/+ wild type
P < 0.05 to Mdr2−/− control diet.
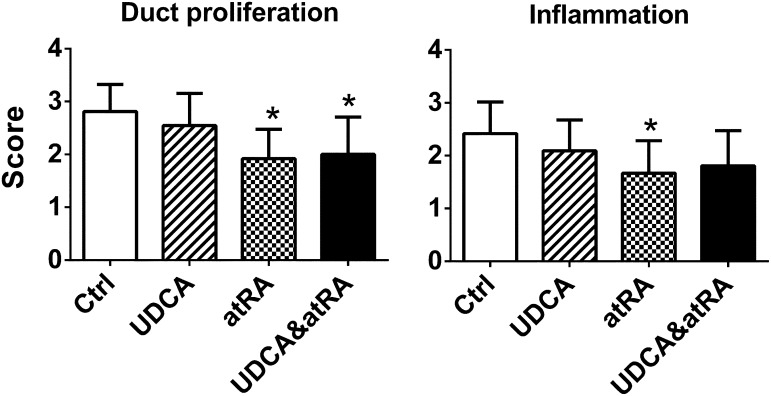
atRA Treatment Alone or in Combination with UDCA Reduced Bile Duct Proliferation and Inflammation in Mdr2−/− Livers.
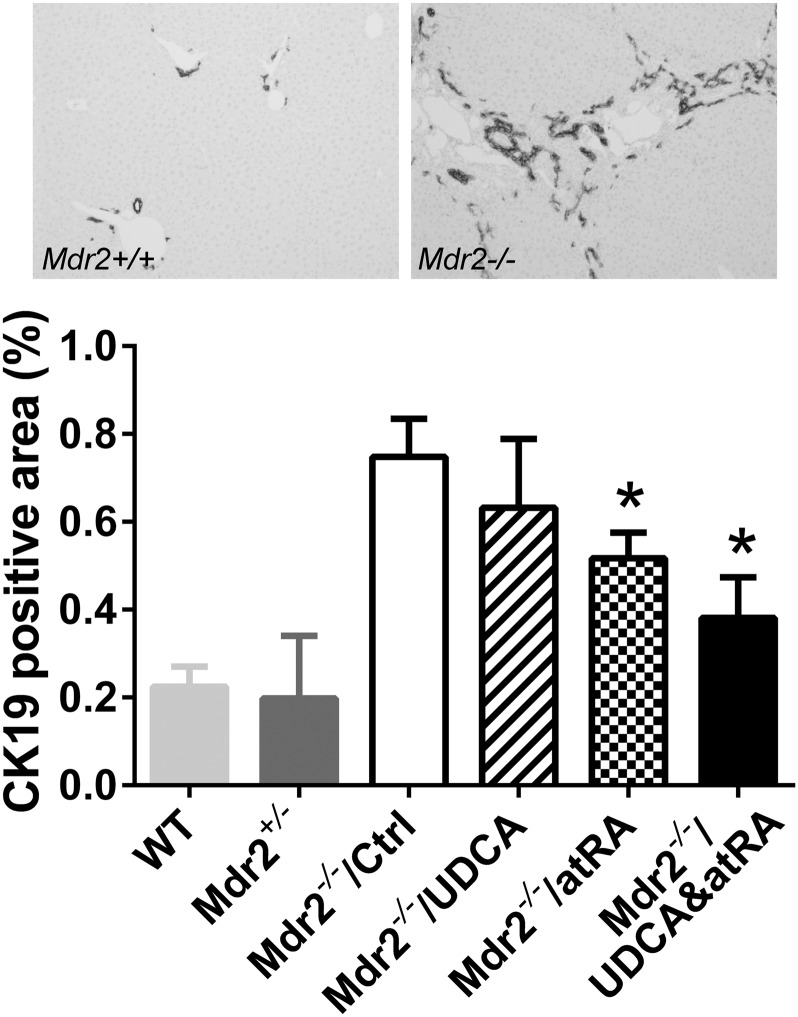
Blinded examination of liver histology detected less bile duct proliferation in the liver of Mdr2−/− groups treated with atRA alone or in combination with UDCA (Fig. 4). Less inflammation was also observed in the atRA-treated group. No significant difference was detected in liver fibrosis among the Mdr2−/− groups. This was also confirmed by measuring liver hydroxyproline content. There was also no histologic evidence of necrosis in the livers of these mice. To further confirm the hematoxylin and eosin histologic assessment of bile duct proliferation, we performed immunohistochemical labeling of CK19, a cytokeratin specifically expressed in bile duct cells in liver. As shown in Fig. 5, CK19-positive staining was significantly reduced in the Mdr2−/− mice treated with atRA alone or in combination with UDCA, in agreement with our histologic assessment. Lower liver bile acid levels were also detected in these animals treated with the combination of atRA and UDCA, as well as UDCA alone, but not with atRA alone due to greater variation in these data.
Fig. 4.
atRA treatment alone or with UDCA reduced bile duct proliferation and inflammation in Mdr2−/− mouse livers. Data are the mean ± S.D. compared with controls (n = 11–13). *P < 0.05. Ctrl, control.
Fig. 5.
Reduced bile duct proliferation in Mdr2−/− mice by atRA and combination treatment was confirmed using CK19 labeling. Data are the mean ± S.D. compared with controls (n = 6–8). *P < 0.05. Ctrl, control; WT, wild type.
Hepatic Expression of Genes Involved in Inflammation, Bile Acid Synthesis, and Transport in Mdr2−/− Mice.
No significant changes in mRNA expression of Bsep, Mrp2, Mrp3, Mrp4, Ntcp, and Cyp7a1 were noted in any of the groups. There was also no difference in expression levels of hepatic tumor necrosis factor-α and interleukin-1β mRNA. Liver CK19 mRNA levels in the mice treated with atRA alone or the combination groups tended to be lower, but did not reach statistical significance due to great variation in the data among animals in each treatment group (data not shown). Western blot analysis also did not detect differences in liver protein expression of Bsep, Mrp2, and Oatp2 (data not shown).
Discussion
The pathogenesis of cholestasis varies greatly among patients with different cholestatic disorders, but there are some common features, including elevated levels of plasma bile acids and ALP as well as histologic evidence of bile duct proliferation and liver fibrosis. Unfortunately, there is no single animal model that fully resembles all of the clinical and pathologic features seen in cholestatic patients. We previously demonstrated that the combination of atRA and UDCA significantly improved plasma biochemistry and histologic features of cholestasis compared with UDCA or atRA alone in an obstructive model of 14-day bile duct ligation in rats, in which reduced bile acid pool size, duct proliferation, liver necrosis, and fibrosis were observed (He et al., 2011). In this study, we tested these treatments in two additional chronic cholestatic rodent models. We found that the combination treatment of atRA and UDCA significantly reduced plasma bile acid levels in ANIT-treated rats (a chemical-induced liver injury model; Fig. 1), whereas plasma ALT and γ-glutamyltransferase levels remained in the normal range in all groups (indicating that the liver injury was relatively mild in these animals). Gene expression analyses also did not detect changes in liver bile salt transporters. However, an increase in kidney Mrp4 expression was observed in atRA-treated rats (Fig. 3). This adaptation might be beneficial because it could result in enhanced renal excretion of toxic compounds, including conjugated bile salts.
In the Mdr2−/− knockout mice, both atRA alone and in combination with UDCA significantly reduced plasma levels of bile acid and ALP. Reduced plasma bile acid levels were also seen in UDCA-treated animals (Table 1). Liver histology revealed that atRA alone and in combination with UDCA reduced bile duct proliferation. This reduction was further confirmed by CK19 immunohistochemical labeling, also indicating that the liver injury was improved in groups treated with atRA alone or in combination with UDCA in the Mdr2−/− mice.
The reductions in plasma bile acids and bile duct proliferation seen in these two experimental animal models are consistent with our earlier findings that atRA has a profound inhibitory effect on bile acid synthesis in both human and rodent hepatocytes (He et al., 2011). Although atRA might be expected to have anti-inflammatory effects as well (as seen in the Mdr2−/− mice; Fig. 4), the primary beneficial effects in these animal models seem to be related to the reduction in the bile acid pool size as reflected in the reduced plasma and liver bile acid levels seen with atRA and UDCA.
When we compared the findings of this study with the beneficial effects of atRA alone or in combination with UDCA treatment seen in the liver of bile duct–ligated rats (He et al., 2011), there was a less dramatic effect on features of cholestasis seen in these ANIT-treated rats and Mdr2−/− knockout mice. Biochemical and histologic features of cholestasis in these two models were also much less severe than those seen after 14 days of bile duct ligation in the rat; thus, less overall benefit might be expected. However, to our surprise, there were no additive beneficial effects in the combination therapy in both ANIT-treated rats and Mdr2−/− knockout mice, as was seen in bile duct–ligated rats. It is possible that the stimulation of bile flow that is associated with UDCA resulted in increased clearance of atRA because vitamin A and its metabolites are cleared from the blood into bile (Boyer, 2013). This would reduce the systemic effects of atRA when combined with UDCA in these two cholestatic rodent models in which bile flow is not obstructed, in contrast with the situation after bile duct ligation. This also may explain why lower plasma bile acid levels were detected with combination therapy in the current two rodent models, even though there were no differences in Cyp7A1 expression among the different treatment groups.
In summary, atRA alone or in combination with UDCA treatment reduced plasma levels of ALP and bile acids and histologic features of bile duct proliferation in chronic cholestatic rodent models due to ANIT treatment in rats and the Mdr2−/− mouse. These findings support our prior findings in bile duct–ligated rats and further suggest that atRA alone or in combination with UDCA may be an effective alternative therapy for patients with chronic cholestatic liver disease.
Acknowledgments
The authors thank Kathy Harry for technical assistance.
Abbreviations
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- ANIT
α-naphthylisothiocyanate
- atRA
all-trans-retinoic acid
- CK19
cytokeratin 19
- PBC
primary biliary cirrhosis
- PC
phosphatidylcholine
- PSC
primary sclerosing cholangitis
- UDCA
ursodeoxycholic acid
Authorship Contributions
Participated in research design: Cai, Boyer.
Conducted experiments: Cai, Mennone, Soroka, Boyer.
Performed data analysis: Cai, Mennone, Soroka, Boyer.
Wrote or contributed to the writing of the manuscript: Cai, Soroka, Boyer.
Footnotes
This research was supported by the American Liver Foundation Primary Biliary Cirrhosis Fund for the Cure and PSC Partners Seeking a Cure (to S.Y.C.) and the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grants P30-DK34989 (to Yale Liver Center) and R37-DK25636 (to J.L.B.)].
This work was partially presented at the following meetings: Cai S-Y, Mennone A, and Boyer JL (2010) Retinoic acid treatment improves ANIT-induced chronic cholestatic liver injury in the rat; 2010 Annual Meeting of the American Association for the Study of Liver Diseases, 2010 Oct 31–Nov 4; San Francisco, CA; and Cai S-Y, He H, Mennone A, Assis D, and Boyer JL (2012) All trans-retinoic acid +/− urosodeoxycholic acid has antifibrotic and anti-inflammatory effects in cholestatic liver injury; Keystone Symposia on Molecular and Cellular Biology: Fibrosis: Translation of Basic Research to Human Disease and Novel Therapeutics; 2012 Mar 30–Apr 4; Big Sky, MT.
References
- Baljevic M, Park JH, Stein E, Douer D, Altman JK, Tallman MS. (2011) Curing all patients with acute promyelocytic leukemia: are we there yet? Hematol Oncol Clin North Am 25:1215–1233, viii viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JL. (2013) Bile formation and secretion. Compr Physiol 3:1035–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai SY, He H, Nguyen T, Mennone A, Boyer JL. (2010) Retinoic acid represses CYP7A1 expression in human hepatocytes and HepG2 cells by FXR/RXR-dependent and independent mechanisms. J Lipid Res 51:2265–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey EJ, Lindor KD. (2012) Current pharmacotherapy for cholestatic liver disease. Expert Opin Pharmacother 13:2473–2484 [DOI] [PubMed] [Google Scholar]
- Ferreira FM, Oliveira PJ, Rolo AP, Santos MS, Moreno AJ, da Cunha MF, Seiça R, Palmeira CM. (2003) Cholestasis induced by chronic treatment with alpha-naphthyl-isothiocyanate (ANIT) affects rat renal mitochondrial bioenergetics. Arch Toxicol 77:194–200 [DOI] [PubMed] [Google Scholar]
- Fickert P, Fuchsbichler A, Wagner M, Zollner G, Kaser A, Tilg H, Krause R, Lammert F, Langner C, Zatloukal K, et al. (2004) Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology 127:261–274 [DOI] [PubMed] [Google Scholar]
- Fickert P, Wagner M, Marschall HU, Fuchsbichler A, Zollner G, Tsybrovskyy O, Zatloukal K, Liu J, Waalkes MP, Cover C, et al. (2006) 24-norUrsodeoxycholic acid is superior to ursodeoxycholic acid in the treatment of sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology 130:465–481 [DOI] [PubMed] [Google Scholar]
- He H, Mennone A, Boyer JL, Cai SY. (2011) Combination of retinoic acid and ursodeoxycholic acid attenuates liver injury in bile duct-ligated rats and human hepatic cells. Hepatology 53:548–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauad TH, van Nieuwkerk CM, Dingemans KP, Smit JJ, Schinkel AH, Notenboom RG, van den Bergh Weerman MA, Verkruisen RP, Groen AK, Oude Elferink RP, et al. (1994) Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am J Pathol 145:1237–1245 [PMC free article] [PubMed] [Google Scholar]
- Reichrath J, Lehmann B, Carlberg C, Varani J, Zouboulis CC. (2007) Vitamins as hormones. Horm Metab Res 39:71–84 [DOI] [PubMed] [Google Scholar]
- Smit JJ, Schinkel AH, Oude Elferink RP, Groen AK, Wagenaar E, van Deemter L, Mol CA, Ottenhoff R, van der Lugt NM, van Roon MA, et al. (1993) Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell 75:451–462 [DOI] [PubMed] [Google Scholar]
- Soroka CJ, Mennone A, Hagey LR, Ballatori N, Boyer JL. (2010) Mouse organic solute transporter alpha deficiency enhances renal excretion of bile acids and attenuates cholestasis. Hepatology 51:181–190 [DOI] [PMC free article] [PubMed] [Google Scholar]