Abstract
The effect of inhalational anesthetics on myocardial contraction and energetics in type 2 diabetes mellitus is unknown. We investigated the effect of isoflurane (ISO) on force and intracellular Ca2+ transient (iCa), myocardial oxygen consumption (MVo2), and energetics/redox behavior in trabecular muscles from Zucker diabetic fatty (ZDF) rats. At baseline, force and corresponding iCa were lower in ZDF trabeculae than in controls. ISO decreased force in both groups in a dose-dependent manner. ISO did not affect iCa amplitude in controls, but ISO > 1.5% significantly reduced iCa amplitude in ZDF trabeculae. ISO-induced force depression fully recovered as a result of increased iCa when external Ca2+ was raised in controls. However, both force and iCa remained low in ZDF muscle at elevated external Ca2+. In controls, force, iCa, and MVo2 increased when stimulation frequency was increased from 0.5 to 1.5 Hz. ZDF muscles, however, exhibited blunted responses in force and iCa and decreased MVo2. Oxidative stress levels were unchanged in control muscles but increased significantly in ZDF muscles after exposure to ISO. Finally, the depressive effect of ISO was prevented by 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl (Tempol) in ZDF muscles. These findings suggest that ISO dose-dependently attenuates force in control and ZDF muscles with differential effect on iCa. The mechanism of force depression by ISO in controls is mainly decreased myofilament Ca2+ sensitivity, whereas in ZDF muscles the ISO-induced decrease in contraction is due to worsening oxidative stress, which inhibits iCa and force development.
Introduction
Type 2 diabetes has become a common health hazard worldwide, especially in developed countries. Patients with diabetes experience many complications, including coronary artery disease and heart failure. Often, these patients undergo general anesthesia for a variety of surgical procedures, and rates of perioperative complications are known to be higher in patients with longstanding diabetes than nondiabetic patients (Lee et al., 1999; Hoeks et al., 2009). For example, the postoperative mortality of diabetic patients after both cardiac and noncardiac surgery is significantly higher than that of nondiabetic patients (Thourani et al., 1999; Axelrod et al., 2002; Juul et al., 2004; Ganesh et al., 2005), and the long-term outcome in diabetic patients after noncardiac surgery is frequently associated with stroke and early death (Halm et al., 2009). Often, the increased postoperative mortality is the result of cardiovascular complications (Juul et al., 2004). This fact is not surprising given that diabetic patients have a higher prevalence of cardiovascular comorbidities (Booth et al., 2006). Although the exact reason for the higher perioperative complications in diabetic patients is not well understood (Hoeks et al., 2009), the impact of anesthesia during surgery should not be overlooked.
At a cellular level, the diabetic heart undergoes metabolic remodeling, as highlighted by increased mitochondrial dysfunction and uncoupling (Flarsheim et al., 1996; Bugger and Abel, 2010; Heather and Clarke, 2011). Additionally, myofilament function is impaired in both humans and animal models of diabetes (Dai and McNeill, 1992; Okayama et al., 1994; Zhou et al., 2000; Jweied et al., 2005; Song et al., 2008). However, whether volatile agents worsen contractile function during anesthesia and surgery is not known. Given that volatile agents are known to depress cardiac contractility in nondiabetic myocardium, regardless of functional state (i.e., normal and failing) (Rusy and Komai, 1987; Pagel et al., 1996; Vivien et al., 1997; Hanley et al., 2004; Preckel et al., 2004; Ding et al., 2011), they are expected to affect cardiac contraction of the diabetic heart. However, previous studies have shown conflicting results. One study showed that isolated papillary muscles from rat hearts with streptozotocin-induced diabetes (type 1) were less sensitive than control muscles to volatile anesthetics, whereas another study showed that they exhibited increased sensitivity (Hattori et al., 1987; David et al., 2004). Halothane similarly depressed contraction and intracellular Ca2+ (iCa) in isolated myocytes from both normal rats and streptozotocin-induced diabetic rats (Rithalia et al., 2004). Although these differences may be explained in part by differences in experimental conditions, the effect of volatile anesthetics on myocardial contraction in diabetes is yet unclear. Moreover, the effect of volatile anesthetics on myocardial contraction in type 2 diabetes is not well studied.
In the present study, we investigated the effect of isoflurane (ISO) on myocardial contraction in isolated, intact trabecular muscles from control hearts and hearts of Zucker diabetic fatty (ZDF) rats, which recapitulates the traits of type 2 diabetes [i.e., obesity, hyperglycemia, and insulin resistance and (possible) cardiomyopathy]. In addition, we measured myocardial oxygen consumption (MVo2) and oxidative stress levels in these intact muscles. We hypothesize that isoflurane exerts differential effect on contraction and energetics in ZDF cardiac muscles as compared with normal ones. The aims of the study were as follows: 1) to confirm that ISO exerts a negative inotropic effect in ZDF muscles in which loaded contraction and corresponding iCa are measured simultaneously; 2) to determine whether the negative inotropic effect is due to limited energy provision; mitochondrial respiration (i.e., MVo2) will be quantified under ISO exposure; and (3) to determine levels of oxidative stress under these experimental conditions and its impact on contraction of ZDF muscles in the presence of ISO.
Materials and Methods
Animals.
Both Zucker lean (control) and ZDF adult rats (14–17 weeks old; Charles River Laboratories, Wilmington, MA) were used in the study. The care of the animals and the experimental protocol were approved by the Animal Care and Use Committee of The Johns Hopkins University.
Trabecular Muscle Preparation.
The rats were anesthetized by intra-abdominal injection with pentobarbital (180 mg/kg). The heart then was exposed by mid-sternotomy, rapidly excised, and placed in a dissection dish. The aorta was cannulated, and the heart was perfused in a retrograde fashion (∼15 ml/min) with dissecting Krebs-Henseleit (K-H) solution equilibrated with 95% O2 and 5% CO2. The dissecting K-H solution was composed of 120 mM NaCl, 20 mM NaHCO3, 5 mM KCl, 1.2 mM MgCl, 10 mM glucose, 0.5 mM CaCl2, and 20 mM 2,3-butanedione monoxime (BDM) [pH 7.35–7.45 at room temperature (21–22°C)]. Trabecular muscle from the right ventricle of the heart was dissected and mounted between a force transducer and a motor arm, superfused with K-H solution without BDM at a rate of ∼10 ml/min, and stimulated at 0.5 Hz.
Force was measured by a force transducer system (KG7; Scientific Instruments, Heidelberg, Germany) and expressed in millinewtons per square millimeter of cross-sectional area. The muscles underwent isometric contractions with the resting muscle length set such that resting force was ~15% of total force development (i.e., optimal muscle length). Intracellular Ca2+ concentration was measured by using the free acid form of fura-2, as described previously (Gao et al., 1994, 2012; Dai et al., 2007; Ding et al., 2011). All experiments were performed at room temperature (20–22°C). Although several experiments were attempted at 37°C, such experiments were technically inadequate due to rapid loss of the Ca2+ indicator and deterioration of contractile function. ISO was delivered via an isoflurane-specific vaporizer (calibrated by a vapor analyzer) to the K-H solution along with the O2 (95%)/CO2 (5%) gas mixture at a constant flow rate (1.0 l/min). The K-H solution was bubbled through a fine-porosity gas distribution tube with desired doses (vol%) of ISO for at least 15 minutes before use. Because of the volatile nature of ISO, the K-H solution was constantly bubbled with gas mixture saturated with ISO and the reservoir was covered to maintain the desired percentage of ISO throughout the experiments. In this design, the muscles were perfused with K-H buffer that was saturated with the desired percentage of ISO.
Measurement of MVo2.
MVo2 was determined with a fiberoptic, spectrometer-coupled chemical sensor (Ocean Optics, Largo, FL) that provides full spectral analysis of dissolved oxygen pressure, as described previously (Cortassa et al., 2006; Cortassa et al., 2009). The tip of the oxygen-sensitive fiber-optic sensor (diameter: 300 µm) contains the O2-sensitive compound ruthenium (which fluorescence is quenched by O2) embedded in a resin. The fluorescence emitted at 600 nm is a function of O2 dissolved in the surroundings of the tip. During measurement, the sensor tip was positioned within 100 μm of the muscle. MVo2 was measured as the slope of the O2 decline upon brief cessation of flow of the perfusate during a 45-second to 1-minute interval. Under these conditions, we recorded a linear change in the fluorescent signal for which the slope is proportional to MVo2 of the muscle. As expected, the respiratory rate increased with increased stimulation frequency and with increased Ca2+ concentrations in the buffer bathing the muscle (see Results). The measurements refer to the maximal slope in the presence of the uncoupler trifluorocarbonylcyanide phenylhydrazone (1 μM). MVo2 was also normalized to each individual muscle and expressed as micromolars of O2 × minute−1 × gram wet tissue−1.
Measurement of NADH and Oxidative Stress Levels.
NADH was quantified by using the autofluorescence signal of the muscles with excitation at 360 nm and emission at 450 nm. The NADH signal was calibrated at the end of the experiment by perfusing the muscle with 1 µM trifluorocarbonylcyanide phenylhydrazone and 5 mM cyanide, which were added successively after BDM (20 mM). NADH level was expressed as the ratio of NADH fluorescence (NADHF) during treatment to baseline NADH fluorescence (NADHF0), which was measured right after the stabilization period at 0.5 Hz.
Oxidative stress levels were measured by using fluorescent signal of probes 5-(6)-chloromethyl-2,7-dichloro-hydrofluorescein (CM-DCF) and MitoSOX (C43H43N3IP). Both CM-DCF and MitoSox are general indicators of oxidative stress (i.e., not specific reactive oxygen species indicators) within the cell. Fluorescent probes were loaded into the trabeculae by chemical loading. The loading solution consisted of K-H with the diacetate forms of CM-DCF (CM-H2DCFDA, C27H19Cl3O8, 7 µM) and MitoSOX (2 µM), which were dissolved in 0.4% Pluronic F-127 (EO100PO65EO100), 1% dimethylsulfoxide, 5 g/l Cremophor, and 1 µM N,N,N′,N′ tetrakis(2-pyridylmethyl)ethylenediamine. The trabecula was exposed to the loading solution for 20 minutes at room temperature. After loading, the fluorescent signals from the muscle were acquired at 520 nm (CM-DCF) and 580 nm (MitoSox), respectively. The fluorescence measured following the stabilization period at 0.5 Hz was considered baseline (F0) for all subsequent fluorescence signal measurements. The signals of CM-DCF and MitoSOX were expressed as the ratio of fluorescence during treatment (F) to that at baseline (F0). Levels of CM-DCF and MitoSOX were normalized with respect to NADH autofluorescence measured simultaneously and were expressed as CM-DCFF/F0/NADHF/F0 and MitoSOXF/F0/NADHF/F0, respectively.
Statistics.
Student’s t test and multivariate ANOVA (MNOVA) were used for statistical analysis of the data (Systat 10.2.01; Systat Software, San Jose, CA). A value of P < 0.05 was considered to indicate significant differences between groups. Unless otherwise indicated, pooled data are expressed as means ± S.E.M.
Results
Effect of ISO on Force Development and Intracellular Ca2+ Transients.
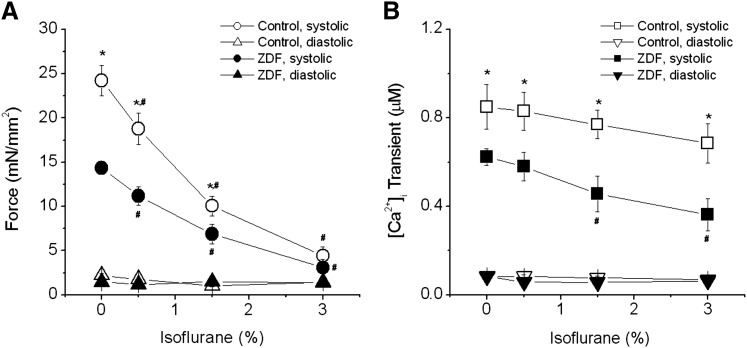
The body weight and blood glucose levels of the animals used in the study are shown in Table 1. ZDF rats had significantly higher body weight and blood glucose levels than did control lean rats. These differences are consistent with full development of type 2 diabetes in ZDF rats. Even without ISO, both force development and iCa were already depressed in ZDF muscles, suggesting impaired excitation-contraction in ZDF muscles (Fig. 1). This decreased contraction is consistent with echocardiographic findings of lower fractional shortening in hearts from ZDF rats (Zhou et al., 2000; van den Brom et al., 2009). In normal cardiac muscle, ISO concentrations up to 3% depressed force without affecting amplitudes of iCa, as we have shown previously (Ding et al., 2011). In ZDF muscles, force development decreased in a similar dose-dependent manner as in lean ones, but iCa behaved differently from controls (Fig. 1). In lean control muscles, iCa remained largely unchanged at any given doses of ISO tested, although it tended to decrease slightly at high doses (P > 0.05 versus at 0% ISO), indicating that ISO decreased myofilament Ca2+ sensitivity. In ZDF muscles, however, iCa was significantly decreased from baseline at 1.5% ISO (P < 0.05 versus at 0% ISO) and at 3% ISO (P < 0.05 versus at 0% ISO). This finding indicates that ISO-induced force depression at concentrations greater than 1.5% is due mainly to decreased iCa.
TABLE 1.
Body weight and blood glucose levels of control (lean) and ZDF rats
ZDF, Zucker diabetic fatty rat.
P < 0.01 versus lean group (n = 6).
Fig. 1.
Effect of isoflurane on force (A) and iCa (B) in cardiac muscles from control and ZDF rats. (A) Pooled data show that systolic force is lower in trabeculae from ZDF rats than in those from control rats at baseline. Isoflurane decreased systolic force similarly in control and ZDF muscles. (B) Pooled data show iCa of control and ZDF muscles. In control muscles, amplitudes of iCa remained unchanged at all tested isoflurane concentrations (up to 3%). However, in ZDF muscles, iCa amplitudes were significantly reduced compared with baseline at doses of 1.5% and higher. There were no changes in diastolic forces and resting Ca2+ levels in both groups. *P < 0.05 versus ZDF; #P < 0.05 versus 0% isoflurane within each group; n = 4–5 each group. Extracellular [Ca2+] = 1.0 mM.
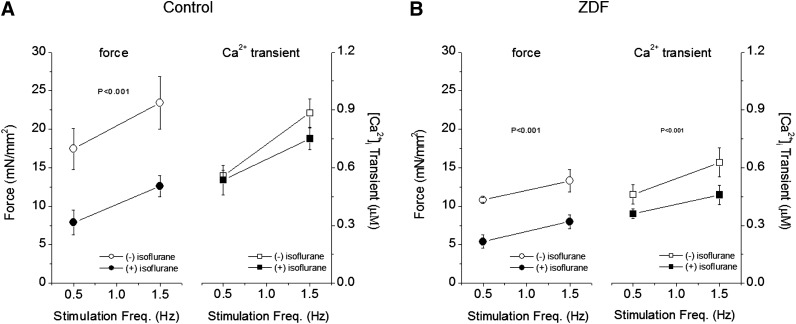
We also determined the effect of stimulation frequency on force and iCa for each group in the presence of ISO (Fig. 2). ISO (1.5%) significantly depressed force development at each stimulation frequency but did not affect the slope of the frequency response in control muscles (Fig. 2A). Additionally, the amplitude iCa in response to stimulation frequency of control muscles was not affected by ISO either. In muscles from ZDF rats, the response to stimulation rate was blunted compared with that of control muscles (Fig. 2B). Moreover, unlike in control muscles, ISO depressed the increases of iCa as the stimulation rate increased to 1.5 Hz, an effect that most likely underlay the blunted force development. Thus, the effect of ISO on the responses of force and iCa to a higher stimulation rate appears to result from decreased myofilament Ca2+ responsiveness in control muscles but depressed iCa in ZDF muscles.
Fig. 2.
Effect of isoflurane on force and iCa at different stimulation frequencies in cardiac muscles from control (A) and ZDF (B) rats. (A) In control muscles, the positive response to stimulation frequency was not affected by isoflurane despite significant reduction in force development (left panel) (P < 0.001, MNOVA). The responses of iCa were not affected by isoflurane. (B) In ZDF muscles, the responses of both force and iCa to increased stimulation rate were blunted before isoflurane. Isoflurane depressed both force and iCa (P < 0.001, MNOVA). N = 5; extracellular [Ca2+] = 1.0 mM.
Effect of External Ca2+ on Force and Intracellular Ca2+ Transient in the Presence of ISO.
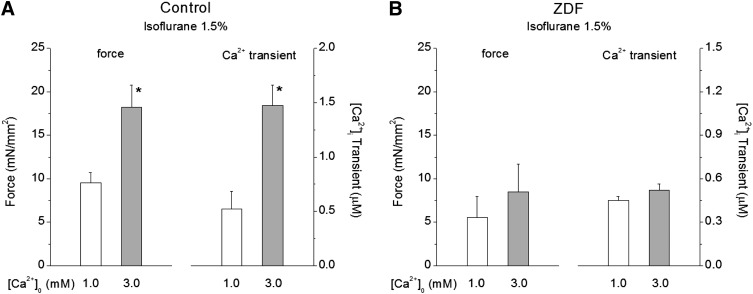
We have previously shown that the decreased force development in the presence of ISO could be recovered to pre-exposure level by raising external Ca2+, which in turn doubles (or triples) iCa in normal cardiac muscle (Ding et al., 2011). In the next series of experiments, we increased external Ca2+ concentration and compared the effect of Ca2+ on force and iCa in control and ZDF muscles exposed to ISO. Figure 3A shows that in control muscles exposed to 1.5% ISO, force increased significantly when external Ca2+ concentration was raised from 1.0 to 3.0 mM. The increased force development was accompanied by significant increases in iCa. These data are consistent with the premise that, in control muscles, ISO reduces myofilament Ca2+ responsiveness. In contrast, Fig. 3B shows in ZDF muscles exposed to 1.5% ISO neither force nor iCa responded to an increase in external Ca2+. These results indicate that ISO inhibited increases in iCa, thus inhibiting force development when external Ca2+ concentration was raised.
Fig. 3.
Effect of extracellular Ca2+ concentration on the recovery of force and iCa in the presence of isoflurane. (A) In control muscles, developed force recovered to over 80% of the preisoflurane level with concomitant increase in iCa (∼200% preisoflurane treatment). *P < 0.05 versus values at extracellular [Ca2+] = 1.0 mM; n = 4. (B) In ZDF muscles, increasing [Ca2+]o to 3.0 mM increased force and iCa minimally in the presence of isoflurane; n = 4.
Effect of ISO on MVo2.
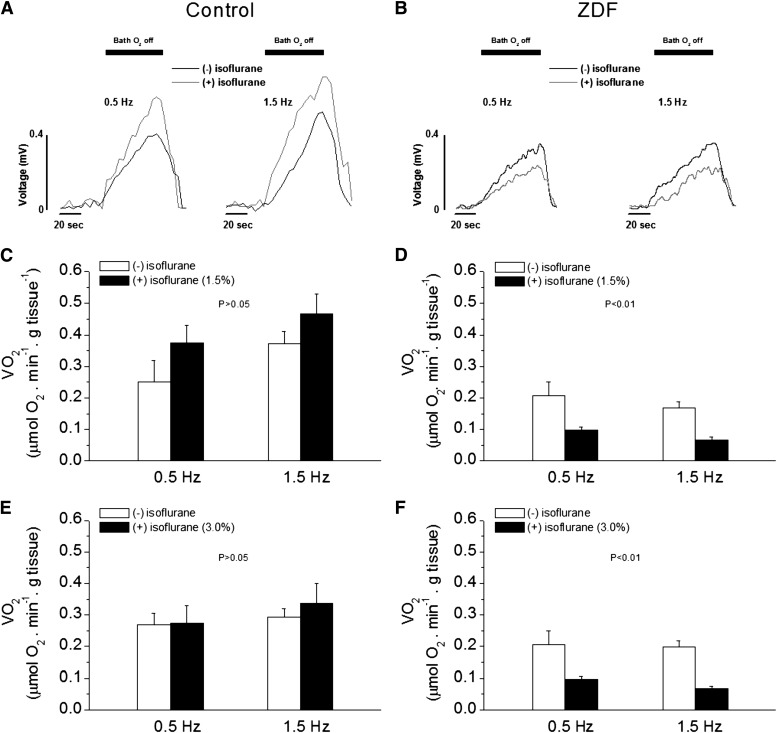
Diabetic hearts are believed to have altered energetics resulting from abnormal mitochondrial respiration and substrate metabolism (Bugger and Abel, 2010; Heather and Clarke, 2011). Notably, mitochondrial respiration is uncoupled, and its energetic transition is compromised (Boudina et al., 2007; Tocchetti et al., 2012). These changes lead to mechanical dysfunction and altered O2 consumption, respectively (Golfman et al., 2005; Boardman et al., 2009). To investigate whether ISO affects mitochondrial respiration in diabetes, especially when coupled with mechanical performance, we measured MVo2 in working muscles from ZDF rats. As compared with control muscles, ZDF muscles had a slightly lower MVo2 at baseline (P > 0.05 versus control) (Fig. 4). In control muscles, MVo2 increased as stimulation frequency increased, consistent with increases in force development (i.e., Fig. 3A) and indicating mitochondrial respiration was stimulated by increased demand (Cortassa et al., 2009). Within control muscles, ISO (1.5%) increased MVo2 slightly (Fig. 4, A and C) (P > 0.05, MNOVA) at the tested stimulation frequencies. In contrast, muscles from ZDF rats showed no changes (or slight decrease) in MVo2 as stimulation frequency increased and ISO decreased MVo2 at both stimulation frequencies (Fig. 4, B and D) (P < 0.01, MNOVA). At higher dose of ISO (3%), there were no differences in MVo2 in control muscles (Fig. 4E), whereas it remained depressed in ZDF muscles (Fig. 4F). The responses of MVo2 to stimulation frequencies and to ISO between control and ZDF muscles were significantly different (P < 0.01, MNOVA). The differential responses of MVo2 to stimulation frequencies and to ISO between control and ZDF muscles indicate that myocardial energetics are significantly altered in ZDF muscle in the presence of ISO.
Fig. 4.
Effect of isoflurane on oxygen consumption (MVo2) of intact cardiac muscle from control (A, C, and E) and ZDF (B, D, and F) rats. (A) Raw tracings of voltage changes from the O2 electrode as levels of O2 surrounding the muscle decreased at stimulation rates of 0.5 Hz (left) and 1.0 Hz (right). MVo2 increased as stimulation frequency increased. Isoflurane also increased MVo2 slightly. Black bars indicate a period in which the muscle was allowed to consume O2 contained only in the bath solution (see text for details). (B) Raw tracings of voltage changes from the O2 electrode as levels of O2 surrounding the muscle decreased at stimulation rates of 0.5 Hz (left) and 1.0 Hz (right). MVo2 remained unchanged or decreased as stimulation frequency increased. (C) Pooled data of the effect of isoflurane (1.5%) on MVo2 in control muscles. (D) Pooled data showing that isoflurane decreased MVo2 at any given stimulation frequency in ZDF muscles (P < 0.01 versus without isoflurane, MNOVA); n = 6. Extracellular [Ca2+] = 1.0 mM. (E) Pooled data of the effect of isoflurane (3.0%) on MVo2 in control muscles. (F) Pooled data of the effect of isoflurane (3.0%) on MVo2 in ZDF muscles. MVo2 was decreased at both stimulation rates in the presence of isoflurane (P < 0.01, MNOVA). n = 6, extracellular [Ca2+] = 1.0 mM. n = 6, extracellular [Ca2+] = 1.0 mM.
Effect of ISO on Oxidative Stress.
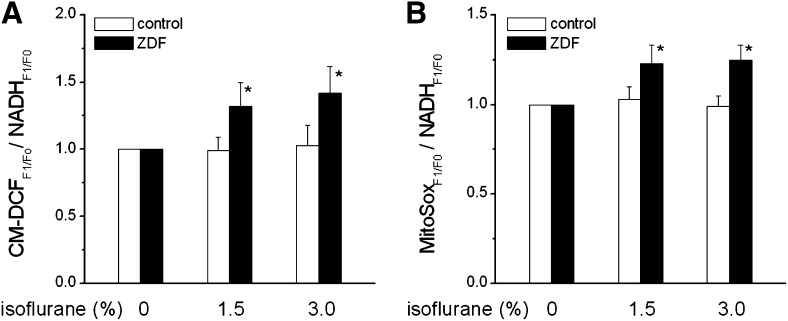
To investigate the underlying mechanism by which ISO affects force, iCa, and MVo2, we measured oxidative stress levels in real time in control and ZDF muscles in the presence and absence of ISO. Specifically, we compared levels of NADH, CM-DCF, and MitoSox before and after isoflurane exposure. Because NADH levels were comparable before and after ISO, we determined oxidative stress levels by normalizing fluorescent signals of CM-DCF and MitSox from each muscle to NADH signals in the same muscle. In the absence of ISO, oxidative stress levels did not differ between control and ZDF groups (Fig. 5). However, In the presence of ISO, oxidative stress increased in ZDF muscles but remained unchanged in controls.
Fig. 5.
Effect of isoflurane (1.5% and 3.0%) on oxidative stress levels in cardiac muscles from control and ZDF rats. (A) Effect of isoflurane on CM-DCF fluorescence in control and ZDF muscles. CM-DCF fluorescence was normalized to NADH fluorescence. (B) Effect of isoflurane on MitoSox fluorescence in control and ZDF muscles. MitoSox fluorescence was normalized to NADH fluorescence. *P < 0.05 versus before isoflurane; P < 0.01 versus control muscles, MNOVA; n = 5. Extracellular [Ca2+] = 1.0 mM.
Effect of Tempol on Force Development in the Presence of Isoflurane.
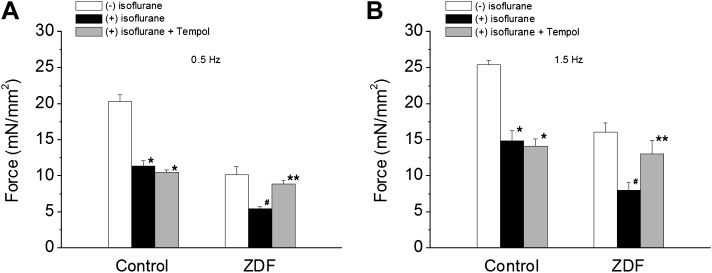
If increased oxidative stress were responsible for the ISO action in ZDF muscles, relieving oxidative stress would prevent decreases in iCa and force development. We tested this hypothesis by investigating the effect of antioxidant, 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl (Tempol), on force development in the presence of ISO (1.5%). Tempol is a cell membrane–permeable amphilite that decreases oxidative stress by dismutating superoxide, facilitating hydrogen peroxide metabolism, and limiting the formation of hydroxyl radicals. It is broadly effective in detoxifying reactive oxygen species (ROS) in cells (Wilcox and Pearlman, 2008). Tempol did not affect force development (tested up to 2 mM) at baseline (results not shown). Figure 6 shows that Tempol (0.4 mM) prevented decreases in force development in ZDF muscles when they were exposed to ISO. While in control muscles, Tempol failed to stop ISO-induced force depression. These data further support that the decreased contraction in the presence of ISO is due to increased oxidative stress in ZDF muscles.
Fig. 6.
Effect of Tempol (0.4 mM) on force development in the presence of isoflurane in control and ZDF muscles at stimulation frequency of 0.5 Hz (A) and 1.5 Hz (B). Isoflurane decreased force development in both control and ZDF muscles. Tempol did not affect the isoflurane-induced force depression in control muscles. However, Tempol inhibited the force-depressing effect of isoflurane in ZDF muscles. *P < 0.01 versus in the absence of isoflurane in the control group; #P < 0.01 versus in the absence of isoflurane in ZDF group; **P < 0.05 versus in the absence of Tempol in the ZDF group. N = 4, extracellular [Ca2+] = 1.0 mM.
Discussion
This study investigated the effects of ISO on cardiac mechanics and energetics in isolated intact trabecular muscles from ZDF and control rats. ZDF rats share many features of human type 2 diabetes, such as obesity, hyperglycemia, insulin resistance, and cardiomyopathy. Thus, our study is clinically relevant to patients with type 2 diabetes. In the intact trabecular muscles, we were not only able to measure force directly and reliably but also to assess mitochondrial respiration simultaneously. It is noteworthy that we can couple mechanical activity to MVo2 (i.e., mitochondrial respirations) within each individual muscle. We found that force development was lower in muscles from diabetic rats, most likely due to lower baseline iCa. Additionally, whereas ISO decreased force similarly in muscles from control and diabetic rats, it differentially affected iCa. In control muscle, ISO had little effect on iCa at concentrations up to 3%, but, in ZDF muscles, it significantly depressed iCa at concentrations of 1.5% and above. ISO also increased oxidative stress in diabetic muscles, and muscles from diabetic rats that were exposed to ISO exhibited little recovery of force or iCa when external Ca2+ was increased. In contrast, relieving oxidative stress prevented ISO-induced force depression. These results indicate that ISO worsened the abnormal cardiac excitation-contraction coupling in diabetic myocardium by increasing oxidative stress.
Our finding that force development in ZDF muscles was decreased at baseline is consistent with reports in previous studies (Zhou et al., 2000; Golfman et al., 2005; van den Brom et al., 2009). Cardiac contraction has also been shown to be depressed in myocytes and hearts from type 2 diabetic mice (db/db and ob/ob) (Belke et al., 2004; Dong et al., 2006; Pereira et al., 2006; Boudina et al., 2007) and skinned myocytes from humans with type 2 diabetes (Jweied et al., 2005). Although depressed cardiac contraction seems to be a cardinal feature of type 2 diabetes, the mechanism underlying the depressed state is not well understood. Mitochondrial dysfunction appears to contribute substantially to cardiac dysfunction (Flarsheim et al., 1996; Bugger and Abel, 2010; Heather and Clarke, 2011), but some studies have suggested that decreased myofilament Ca2+ responsiveness may also underlie contractile dysfunction in diabetes (Jweied et al., 2005; Ramirez-Correa et al., 2008). In this study, we found that both force and iCa were decreased, exhibited a flat response to increased stimulation rate, and failed to respond to elevated external Ca2+. These data suggest that the lower force development is the result of reduced activator Ca2+. However, it remains unclear whether myofilament Ca2+ responsiveness is altered in ZDF muscles, as has been shown in human type 2 diabetes (Jweied et al., 2005).
In control muscles, ISO depressed force development without affecting iCa, indicating decreased myofilament responsiveness. However, in diabetic muscles, both force and iCa were decreased, suggesting that the decreased force resulted from decreased iCa (Fig. 1). Thus, Ca2+ availability is further reduced by ISO in diabetic muscles. The function of L-type sarcolemmal Ca2+ channels has been found to be reduced, leading to diminished Ca2+ entry and hence reduced Ca2+ availability, which was further exacerbated by a decrease in ryanodine receptor Ca2+ channel density and sarcoplasmic reticulum Ca2+ load (Pereira et al., 2006) and by defective sarcoplasmic reticulum Ca2+ ATPase function (Belke et al., 2004). Apparently, ISO affects all of these processes and leads to further decreases in iCa. This notion is also supported by our data, which showed that raising the external Ca2+ concentration failed to augment force or iCa in the presence of ISO (Fig. 3). The mechanism for ISO-induced decreases in force and iCa is most likely due to worsening oxidative stress (Fig. 5), which inhibits key processes involved in excitation-contraction coupling (i.e., sarcoplasic reticulum ATPase, ryanodine receptors, etc.) (Sag et al., 2013).
Myocardial energetics also differed between control and diabetic muscles exposed to ISO. In the presence of ISO, MVo2 was unchanged (or slightly increased) despite significantly decreased force development in control muscles (Figs. 1 and 4). Because the main source of MVo2 is mitochondrial respiration (Cortassa et al., 2009), the lack of significant changes in MVo2 suggests ISO has a negligible effect on mitochondrial respiration in normal muscles. Nevertheless, it cannot be ignored that ISO did produce a trend toward higher MVo2 in control muscles, an effect that may be due to mild mitochondrial uncoupling (Ljubkovic et al., 2007). In diabetic muscles, baseline force development and iCa were lower than those of control muscles, but MVo2 was unchanged, indicating that diabetic muscles are less efficient, which is consistent with increased mitochondrial uncoupling (Bugger and Abel, 2010). Unlike in control muscles, in diabetic muscles, ISO attenuated mitochondrial respiration, as proven by reduced MVo2 (Fig. 4) via increased oxidative stress (Bhatt et al., 2012). This reduction might impede a variety of energy-requiring processes in excitation-contraction coupling and thereby lead to reduced iCa and force development in diabetic muscles.
What is the mechanism that underlies ISO inhibition of mitochondrial respiration in diabetic muscles? Our finding that ISO augments oxidative stress in diabetic muscles suggests that ROS plays an important role in ISO-induced inhibition of mitochondrial respiration. This notion is supported by the following evidence: 1) impaired mitochondrial respiration predisposes diabetic myocardium to (or even promotes) the generation of ROS via electron transport chain (Boudina et al., 2007; Tocchetti et al., 2012), and 2) ISO can directly stimulate mitochondrial production of ROS (Hirata et al., 2011). Although ISO-stimulated mitochondrial production of ROS is protective in normal myocardium [i.e., anesthetic preconditioning (Kersten et al., 1997)], it is likely that this process becomes maladaptive in diabetic myocardium (i.e., worsening oxidative stress), thus leading to inhibition of mitochondrial respiration.
The worsened oxidative stress of diabetic muscles upon exposure to ISO not only inhibited force development and iCa but also hindered their recovery when extracellular Ca2+, a common positive inotropic agent, was increased (Fig. 3). In contrast, antioxidant Tempol prevented the decreases in force development by decreasing ROS in ZDF muscles (Fig. 6). This observation has important implications. First, patients with diabetes may respond poorly to inotropic agents when being treated for ISO-induced low contractility. Second, reducing oxidative stress should be considered as part of the treatment regimen when managing ISO-induced depression of contractility.
One limitation in this study is that the experiments were performed at room temperature with slower pacing rates (0.5–1.5 Hz). The special requirements of the techniques and preparations prevented us from performing these extremely difficult experiments successfully at body temperature and normal heart rate. Therefore, one should be cautioned that extrapolating and implicating these in vitro findings is limited. Clearly, in vivo experiments at physiologic conditions are needed. Changes in diastolic properties in diabetes, especially in type 2 diabetes, are expected. We did not focus our investigation on these in this study because of the extent of our current experimental protocols. A separate study is needed to investigate these important properties in diabetic hearts.
In conclusion, ISO inhibits myocardial contraction by decreasing iCa in trabeculae from ZDF diabetic rats. The reduced Ca2+ availability results from impaired mitochondrial energetics and decreased oxygen usage caused by increased ROS production. In addition, the increased oxidative stress during ISO exposure retards the ability of positive inotropic agents to reverse ISO-induced myocardial depression in ZDF muscles, and relieving oxidative stress by antioxidants prevents ISO-induced force depression. Whereas these results from our study are stimulating, it remains to be seen whether antioxidant therapy will indeed attenuate ISO-induced myocardial depression action in diabetic hearts under physiologic conditions and in patients clinically.
Acknowledgments
The authors thank Claire Levine for editing this article.
Abbreviations
- BDM
2,3-butanedione monoxime
- CM-DCF
5-(6)-chloromethyl-2,7-dichloro-hydrofluorescein
- iCa
intracellular Ca2+ transient
- ISO
isoflurane
- K-H
Krebs-Henseleit
- MNOVA
multivariate ANOVA
- MVo2
myocardial oxygen consumption
- ROS
reactive oxygen species
- Tempol
4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl
- ZDF
Zucker diabetic fatty
Authorship Contributions
Participated in research design: Shen, Bhatt, Cortassa, Gao.
Conducted experiments: Shen, Bhatt, Gao.
Contributed new reagents or analytic tools: Aon, O’Rourke.
Performed data analysis: Shen, Bhatt, Xu, Meng, Gao.
Wrote or contributed to the writing of the manuscript: Shen, Bhatt, Xu, Aon, Cortassa, Berkowitz, Gao.
Footnotes
This work was supported in part by the National Institutes of Health National Heart, Lung, and Blood Institute [Grant R21-HL106054] (to S.C.); and the American Heart Association [Grant 13GRNT16920028] (to W.D.G.).
References
- Axelrod DA, Upchurch GR, Jr, DeMonner S, Stanley JC, Khuri S, Daley J, Henderson WG, Hayward R. (2002) Perioperative cardiovascular risk stratification of patients with diabetes who undergo elective major vascular surgery. J Vasc Surg 35:894–901 [DOI] [PubMed] [Google Scholar]
- Belke DD, Swanson EA, Dillmann WH. (2004) Decreased sarcoplasmic reticulum activity and contractility in diabetic db/db mouse heart. Diabetes 53:3201–3208 [DOI] [PubMed] [Google Scholar]
- Bhatt N, Aon MA, Shen X, O'Rourke B, Gao WD, and Cortassa S (2012) Bioenergetics of contractile function in heart trabeculae from diabetic rats (abstract). Biophys J 102:571a [Google Scholar]
- Boardman N, Hafstad AD, Larsen TS, Severson DL, Aasum E. (2009) Increased O2 cost of basal metabolism and excitation-contraction coupling in hearts from type 2 diabetic mice. Am J Physiol Heart Circ Physiol 296:H1373–H1379 [DOI] [PubMed] [Google Scholar]
- Booth GL, Kapral MK, Fung K, Tu JV. (2006) Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet 368:29–36 [DOI] [PubMed] [Google Scholar]
- Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, et al. (2007) Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes 56:2457–2466 [DOI] [PubMed] [Google Scholar]
- Bugger H, Abel ED. (2010) Mitochondria in the diabetic heart. Cardiovasc Res 88:229–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortassa S, Aon MA, O’Rourke B, Jacques R, Tseng HJ, Marbán E, Winslow RL. (2006) A computational model integrating electrophysiology, contraction, and mitochondrial bioenergetics in the ventricular myocyte. Biophys J 91:1564–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortassa S, O’Rourke B, Winslow RL, Aon MA. (2009) Control and regulation of mitochondrial energetics in an integrated model of cardiomyocyte function. Biophys J 96:2466–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S, McNeill JH. (1992) Myocardial performance of STZ-diabetic DOCA-hypertensive rats. Am J Physiol 263:H1798–H1805 [DOI] [PubMed] [Google Scholar]
- Dai T, Tian Y, Tocchetti CG, Katori T, Murphy AM, Kass DA, Paolocci N, Gao WD. (2007) Nitroxyl increases force development in rat cardiac muscle. J Physiol 580:951–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David JS, Tavernier B, Amour J, Vivien B, Coriat P, Riou B. (2004) Myocardial effects of halothane and sevoflurane in diabetic rats. Anesthesiology 100:1179–1187 [DOI] [PubMed] [Google Scholar]
- Ding W, Li Z, Shen X, Martin J, King SB, Sivakumaran V, Paolocci N, Gao WD. (2011) Reversal of isoflurane-induced depression of myocardial contraction by nitroxyl via myofilament sensitization to Ca2+. J Pharmacol Exp Ther 339:825–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong F, Zhang X, Yang X, Esberg LB, Yang H, Zhang Z, Culver B, Ren J. (2006) Impaired cardiac contractile function in ventricular myocytes from leptin-deficient ob/ob obese mice. J Endocrinol 188:25–36 [DOI] [PubMed] [Google Scholar]
- Flarsheim CE, Grupp IL, Matlib MA. (1996) Mitochondrial dysfunction accompanies diastolic dysfunction in diabetic rat heart. Am J Physiol 271:H192–H202 [DOI] [PubMed] [Google Scholar]
- Ganesh SP, Pietrobon R, Cecílio WA, Pan D, Lightdale N, Nunley JA. (2005) The impact of diabetes on patient outcomes after ankle fracture. J Bone Joint Surg Am 87:1712–1718 [DOI] [PubMed] [Google Scholar]
- Gao WD, Backx PH, Azan-Backx M, Marban E. (1994) Myofilament Ca2+ sensitivity in intact versus skinned rat ventricular muscle. Circ Res 74:408–415 [DOI] [PubMed] [Google Scholar]
- Gao WD, Murray CI, Tian Y, Zhong X, DuMond JF, Shen X, Stanley BA, Foster DB, Wink DA, King SB, et al. (2012) Nitroxyl-mediated disulfide bond formation between cardiac myofilament cysteines enhances contractile function. Circ Res 111:1002–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golfman LS, Wilson CR, Sharma S, Burgmaier M, Young ME, Guthrie PH, Van Arsdall M, Adrogue JV, Brown KK, Taegtmeyer H. (2005) Activation of PPARgamma enhances myocardial glucose oxidation and improves contractile function in isolated working hearts of ZDF rats. Am J Physiol Endocrinol Metab 289:E328–E336 [DOI] [PubMed] [Google Scholar]
- Halm EA, Tuhrim S, Wang JJ, Rockman C, Riles TS, Chassin MR. (2009) Risk factors for perioperative death and stroke after carotid endarterectomy: results of the New York carotid artery surgery study. Stroke 40:221–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley PJ, ter Keurs HE, Cannell MB. (2004) Excitation-contraction coupling in the heart and the negative inotropic action of volatile anesthetics. Anesthesiology 101:999–1014 [DOI] [PubMed] [Google Scholar]
- Hattori Y, Azuma M, Gotoh Y, Kanno M. (1987) Negative inotropic effects of halothane, enflurane, and isoflurane in papillary muscles from diabetic rats. Anesth Analg 66:23–28 [PubMed] [Google Scholar]
- Heather LC, Clarke K. (2011) Metabolism, hypoxia and the diabetic heart. J Mol Cell Cardiol 50:598–605 [DOI] [PubMed] [Google Scholar]
- Hirata N, Shim YH, Pravdic D, Lohr NL, Pratt PF, Jr, Weihrauch D, Kersten JR, Warltier DC, Bosnjak ZJ, Bienengraeber M. (2011) Isoflurane differentially modulates mitochondrial reactive oxygen species production via forward versus reverse electron transport flow: implications for preconditioning. Anesthesiology 115:531–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeks S, Flu WJ, van Kuijk JP, Bax J, Poldermans D. (2009) Cardiovascular risk assessment of the diabetic patient undergoing major noncardiac surgery. Best Pract Res Clin Endocrinol Metab 23:361–373 [DOI] [PubMed] [Google Scholar]
- Juul AB, Wetterslev J, Kofoed-Enevoldsen A. (2004) Long-term postoperative mortality in diabetic patients undergoing major non-cardiac surgery. Eur J Anaesthesiol 21:523–529 [DOI] [PubMed] [Google Scholar]
- Jweied EE, McKinney RD, Walker LA, Brodsky I, Geha AS, Massad MG, Buttrick PM, de Tombe PP. (2005) Depressed cardiac myofilament function in human diabetes mellitus. Am J Physiol Heart Circ Physiol 289:H2478–H2483 [DOI] [PubMed] [Google Scholar]
- Kersten JR, Schmeling TJ, Pagel PS, Gross GJ, Warltier DC. (1997) Isoflurane mimics ischemic preconditioning via activation of K(ATP) channels: reduction of myocardial infarct size with an acute memory phase. Anesthesiology 87:361–370 [DOI] [PubMed] [Google Scholar]
- Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, Sugarbaker DJ, Donaldson MC, Poss R, Ho KK, et al. (1999) Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 100:1043–1049 [DOI] [PubMed] [Google Scholar]
- Ljubkovic M, Mio Y, Marinovic J, Stadnicka A, Warltier DC, Bosnjak ZJ, Bienengraeber M. (2007) Isoflurane preconditioning uncouples mitochondria and protects against hypoxia-reoxygenation. Am J Physiol Cell Physiol 292:C1583–C1590 [DOI] [PubMed] [Google Scholar]
- Okayama H, Hamada M, Hiwada K. (1994) Contractile dysfunction in the diabetic-rat heart is an intrinsic abnormality of the cardiac myocyte. Clin Sci 86:257–262 [DOI] [PubMed] [Google Scholar]
- Pagel PS, Lowe D, Hettrick DA, Jamali IN, Kersten JR, Tessmer JP, Warltier DC. (1996) Isoflurane, but not halothane, improves indices of diastolic performance in dogs with rapid ventricular, pacing-induced cardiomyopathy. Anesthesiology 85:644–654 [DOI] [PubMed] [Google Scholar]
- Pereira L, Matthes J, Schuster I, Valdivia HH, Herzig S, Richard S, Gómez AM. (2006) Mechanisms of [Ca2+]i transient decrease in cardiomyopathy of db/db type 2 diabetic mice. Diabetes 55:608–615 [DOI] [PubMed] [Google Scholar]
- Preckel B, Müllenheim J, Hoff J, Obal D, Heiderhoff M, Thämer V, Schlack W. (2004) Haemodynamic changes during halothane, sevoflurane and desflurane anaesthesia in dogs before and after the induction of severe heart failure. Eur J Anaesthesiol 21:797–806 [DOI] [PubMed] [Google Scholar]
- Ramirez-Correa GA, Jin W, Wang Z, Zhong X, Gao WD, Dias WB, Vecoli C, Hart GW, Murphy AM. (2008) O-Linked GlcNAc modification of cardiac myofilament proteins: a novel regulator of myocardial contractile function. Circ Res 103:1354–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rithalia A, Qureshi MA, Howarth FC, Harrison SM. (2004) Effects of halothane on contraction and intracellular calcium in ventricular myocytes from streptozotocin-induced diabetic rats. Br J Anaesth 92:246–253 [DOI] [PubMed] [Google Scholar]
- Rusy BF, Komai H. (1987) Anesthetic depression of myocardial contractility: a review of possible mechanisms. Anesthesiology 67:745–766 [DOI] [PubMed] [Google Scholar]
- Sag CM, Wagner S, Maier LS. (2013) Role of oxidants on calcium and sodium movement in healthy and diseased cardiac myocytes. Free Radic Biol Med 63:338–349 [DOI] [PubMed] [Google Scholar]
- Song D, Kuo KH, Yao R, Hutchings SR, Pang CC. (2008) Inducible nitric oxide synthase depresses cardiac contractile function in Zucker diabetic fatty rats. Eur J Pharmacol 579:253–259 [DOI] [PubMed] [Google Scholar]
- Thourani VH, Weintraub WS, Stein B, Gebhart SS, Craver JM, Jones EL, Guyton RA. (1999) Influence of diabetes mellitus on early and late outcome after coronary artery bypass grafting. Ann Thorac Surg 67:1045–1052 [DOI] [PubMed] [Google Scholar]
- Tocchetti CG, Caceres V, Stanley BA, Xie C, Shi S, Watson WH, O’Rourke B, Spadari-Bratfisch RC, Cortassa S, Akar FG, et al. (2012) GSH or palmitate preserves mitochondrial energetic/redox balance, preventing mechanical dysfunction in metabolically challenged myocytes/hearts from type 2 diabetic mice. Diabetes 61:3094–3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brom CE, Huisman MC, Vlasblom R, Boontje NM, Duijst S, Lubberink M, Molthoff CF, Lammertsma AA, van der Velden J, Boer C, et al. (2009) Altered myocardial substrate metabolism is associated with myocardial dysfunction in early diabetic cardiomyopathy in rats: studies using positron emission tomography. Cardiovasc Diabetol 8:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivien B, Hanouz JL, Gueugniaud PY, Lecarpentier Y, Coriat P, Riou B. (1997) Myocardial effects of halothane and isoflurane in hamsters with hypertrophic cardiomyopathy. Anesthesiology 87:1406–1416 [DOI] [PubMed] [Google Scholar]
- Wilcox CS, Pearlman A. (2008) Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol Rev 60:418–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. (2000) Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci USA 97:1784–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]