Abstract
Prior studies have demonstrated that the ion channel transient receptor potential vanilloid 4 (TRPV4) is functionally expressed in airway smooth muscle cells and that TRPV4 single nucleotide polymorphisms are associated with airflow obstruction in patients with chronic obstructive pulmonary disease. We sought to use isometric tension measurements in ex vivo airways to determine whether short-term pharmacological activation of TRPV4 with the potent agonist GSK1016790 [N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide] would constrict human bronchial tissue. As predicted, transient receptor potential vanilloid 4 activation in the human airway produces contractions that are blocked by the nonselective transient receptor potential channel blocker ruthenium red. Moreover, the novel TRPV4-selective blocker GSK2334775 [(R)-6-(methylsulfonyl)-3-((4-(pyrrolidin-1-yl)piperindin-1-yl)methyl)-N-(2,2,2,-trifluoro-1-phenylethyl)-2-(3-(trifluoromethyl)phenyl)quinoline-4-carboxamide] inhibited these contractions over a concentration range consistent with its in vitro potency against recombinant and native TRPV4-containing channels. Surprisingly, TRPV4-dependent contractions were also blocked by a 5-lipoxygenase inhibitor and two structurally distinct cysteinyl leukotriene 1 receptor antagonists. In aggregate, our results fail to support the hypothesis that TRPV4 in airway smooth muscle cells regulates airway contractility short term. Rather, we provide pharmacological evidence that TRPV4 activation causes human airway constriction that is entirely dependent upon the production of cysteinyl leukotrienes. Together, these data identify a novel mechanism by which TRPV4 activation may contribute to pathologic remodeling and inflammation, in addition to airflow obstruction, in the diseased human respiratory tract.
Introduction
In many respiratory disorders, exaggerated responses to irritant inhalation result in airflow obstruction. Although multiple factors contribute to this net response, including tissue remodeling, luminal mucus plugging, and central nervous system–dependent parasympathetic reflexes, fundamental alterations in airway smooth muscle contractility are thought to be critical modulators of airway reactivity. Because airway smooth muscle function depends on cytosolic Ca2+ handling, a considerable amount of research has focused on identifying mechanisms that may restore airway smooth muscle homeostasis by limiting cytosolic Ca2+ elevations.
Inhibitors of voltage-dependent Ca2+ channels efficaciously inhibit human airway smooth muscle constriction caused by KCl-dependent depolarization, although constriction driven by physiologic mechanisms such as G protein–coupled receptor ligands and IgE crosslinking are comparatively resistant to voltage-dependent Ca2+ channel block (Gorenne et al., 1998). The molecular identity of the Ca2+-permeant channel facilitating this physiologically relevant extracellular Ca2+ influx has remained elusive; however, recent studies suggested that members of the transient receptor potential superfamily of ion channels may serve this role. In particular, transient receptor potential vanilloid 4 (TRPV4) has emerged as one strong candidate based on several observations: 1) the TRPV4 message is present in human cultured airway smooth muscle cells (Jia et al., 2004); 2) 4α-phorbol 12,13-didecanoate, stretch (Ito et al., 2008), and hypotonic solutions, which can each activate heterologously expressed TRPV4 channels (Vriens et al., 2004), cause extracellular Ca2+ influx into human airway smooth muscle cells that is blocked by the TRPV channel blocker ruthenium red but not other blockers of cytosolic Ca2+ mobilization (Ito et al., 2008); and 3) the robust constriction of human airway smooth muscle caused by hypotonic solutions is extracellular Ca2+ dependent (Jongejan et al., 1990). Furthermore, TRPV4 single nucleotide polymorphisms have been linked to airflow obstruction in chronic obstructive pulmonary disease (Zhu et al., 2009). On the basis of these results, we aimed to test the hypothesis that TRPV4 causes constriction of human isolated airways.
Using the potent and efficacious TRPV4 activator GSK1016790 [N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide] (Willette et al., 2008), we demonstrate that TRPV4 activation produces robust constriction of human and guinea pig (but not rat or mouse) isolated airway preparations. We further demonstrate that both the nonselective TRPV blocker ruthenium red and the novel TRPV4-selective blocker GSK2334775 [(R)-6-(methylsulfonyl)-3-((4-(pyrrolidin-1-yl)piperindin-1-yl)methyl)-N-(2,2,2,-trifluoro-1-phenylethyl)-2-(3-(trifluoromethyl)phenyl)quinoline-4-carboxamide] (Brooks et al., 2011) abolish these contractions, providing compelling pharmacological evidence that TRPV4 ion channel function is critical for this response. Surprisingly, further experiments failed to support the hypothesis that TRPV4 activation or blockade has any detectable acute or direct effect on airway smooth muscle contractility. Rather, TRPV4 activation causes contractions that are effectively abolished by inhibition of 5-lipoxygenase (5-LO) activity or antagonizing cysteinyl leukotriene (cysLT) 1 receptors, identifying TRPV4 channel activity as a novel mechanism that produces cysLT-dependent constriction of human airways.
Materials and Methods
Tissue Acquisition.
All procedures were performed in accredited facilities in accordance with Universal Precautions for Handling Human Blood, Body Fluids, and Tissue (Biohazardous Agent Registration 88-06-22-060) and institutional guidelines, including the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH publication 85-23). Human lungs from organ donors were obtained from the National Disease Research Interchange (Philadelphia, PA; www.ndriresource.org). Human bronchoalveolar lavage (BAL) samples were obtained from Dr. Steve Kelsen (Temple University Lung Center, Philadelphia, PA). All applicable institutional review board approvals were obtained. All human biologic samples were sourced ethically, and their research use was in accordance with the terms of informed consent. Animal experiments were performed at Association for Assessment and Accreditation of Laboratory Animal Care–accredited facilities in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the institution where the work was performed.
Smooth Muscle Tension Measurements.
Sections of bronchus were removed from the lung and cleaned of adherent connective, parenchymal, and fatty tissue. Bronchial strips approximately 3 to 4 mm in width were prepared and placed into modified Krebs-Henseleit solution composed of 113.0 mM NaCl, 4.8 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25.0 mM NaHCO3, and 11.0 mM dextrose and were equilibrated with 95% O2/5% CO2 and maintained at 37°C. Unless otherwise noted, saturating concentrations of a nonselective cyclooxygenase (COX) inhibitor (either 1 μM meclofenamic acid or 3 μM indomethacin) were included in the buffer solution to eliminate confounding effects of endogenously released COX products. The human bronchi were evaluated under a static tissue bath condition as well as in superfusion conditions. In all cases, the tissues were suspended under an optimal resting tension of 1.5g. After an incubation period to reach stable basal tone, tissues were exposed to the muscarinic agonist carbamylcholine chloride (carbachol, 10 μM) to confirm viability. After tension reached a plateau, the carbachol was washed free from the tissue and the bronchi relaxed back to baseline. Antagonists or vehicle were added to the tissue for at least 60 minutes, after which the TRPV4 opener GSK1016790 (100 nM, unless otherwise noted) was then added to the buffer solution. After the GSK1016790-evoked tension reached a plateau, carbachol (10 μM) was administered again to establish a reference maximal contraction. All responses to GSK1016790 were expressed as a percentage of this reference carbachol contraction. When multiple airway segments were used in a single experimental condition from the same donor, the responses from these segments were averaged and the response was evaluated as a single n value. We found no significant difference in the percentage of contraction evoked by GSK1016790 in the superfusion or static tissue bath design; therefore, we pooled the data from the two methods.
For animal studies, the trachea was removed from male Hartley guinea pigs (weight range, 450–650 g; Charles River, Portage, MI), male mice (C56BL/6J; The Jackson Laboratory, Bar Harbor, ME), and male rats (Sprague Dawley; Charles River). For guinea pigs and rats, the epithelium of the trachea was removed and strips were cut, approximately two cartilage rings in width. For mice, the whole trachea was studied. Individual tissues were suspended under optimal tension and equilibrated for 60–90 minutes before the addition of GSK1016790. The contractions were monitored and expressed as a percentage of the maximal contractions observed with carbamylcholine. The rodent tracheas that failed to respond to GSK1016790 all responded strongly to the muscarinic agonist (e.g., mouse trachea contracted 2.3 ± 0.2 g; n = 4)
Calcium Imaging Experiments.
Calcium imaging experiments used a FLIPR5 fluorometric imaging plate reader (Molecular Devices, Sunnyvale, CA). Experiments on recombinant human TRPV4 channels were conducted according to previously published procedures (Huh et al., 2012; Thorneloe et al., 2012). To study native human TRPV4-containing channels, BAL cells from healthy human volunteers (male or female never-smokers, aged 27–52 years, FEV1 (forced expiratory volume in 1 second) >80% of predicted values) were isolated from roughly 30-ml samples. BAL fluid, which was obtained in divalent cation-free phosphate-buffered saline containing 200 U/ml penicillin, 200 μg/ml streptomycin, and 1 μg/ml amphotericin B, was filtered to remove mucus or other aggregates, centrifuged at 4°C (700g, 10 minutes), counted using trypan blue to exclude nonviable cells, and resuspended at a concentration of 4 × 105 cells/ml in Dulbecco’s modified Eagle’s medium high glucose with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. BAL cells were plated in 96-well black-wall clear-bottom plates (Packard View) at 4 × 104 cells per well. Adherent macrophage selection was achieved by repeatedly washing away nonadherent cells 30–120 minutes after plating, after which cell culture media were replenished and cells were incubated no longer than 24 hours. After incubation, the media were aspirated and replaced with 100 ml of loading medium containing Eagle’s minimal essential medium with Earl’s salts and l-glutamine, 0.1% bovine serum albumin (Millipore, Billerica, MA), 4 μM Fluo-4-acetoxymethyl ester fluorescent indicator dye (Fluo-4 AM; Invitrogen, Carlsbad, CA), and 2.5 mM probenecid. Cells were then incubated for 1 hour at 37°C.
After aspirating the dye-containing media, the cells were washed three times with 100 ml Krebs-Ringers-Henseleit assay buffer (120 mM NaCl, 4.6 mM KCl, 1.03 mM KH2PO4, 25 mM NaHCO3, 1.0 mM CaCl2, 1.1 mM MgCl2, 11 mM glucose, 20 mM HEPES, 0.1% gelatin, 2.5 mM probenecid, pH 7.4). To evaluate antagonist effects of GSK2334775, 100 ml Krebs-
Ringers-Henseleit assay buffer, containing 0.1% dimethylsulfoxide, GSK2334775 (10 or 100 nM), or ruthenium red (10 μM), was added to the wells and the plate warmed to 37°C for 15 minutes before dye-loaded cells were exposed to excitation light (488 nm) from a 6 W Argon laser. After the basal emission fluorescence measurements were taken, the cellular response to a concentration range of TRPV4 opener GSK1016790 (0.3–1000 nM) was monitored for 10 minutes at 516 nm emission fluorescence intensity. Results from each well were converted to the percentage peak GSK1016790 response, because this was always >75% of the reading produced by adding ionomycin (1 μM) at the end of the experiment (not shown). The data were then transferred to GraphPad Prism software (GraphPad Software Inc., La Jolla, CA) and plotted. Shifts of GSK1016790 EC50 values in the presence of antagonist compared with vehicle were used to determine antagonist potency using standard Schild analysis.
Results
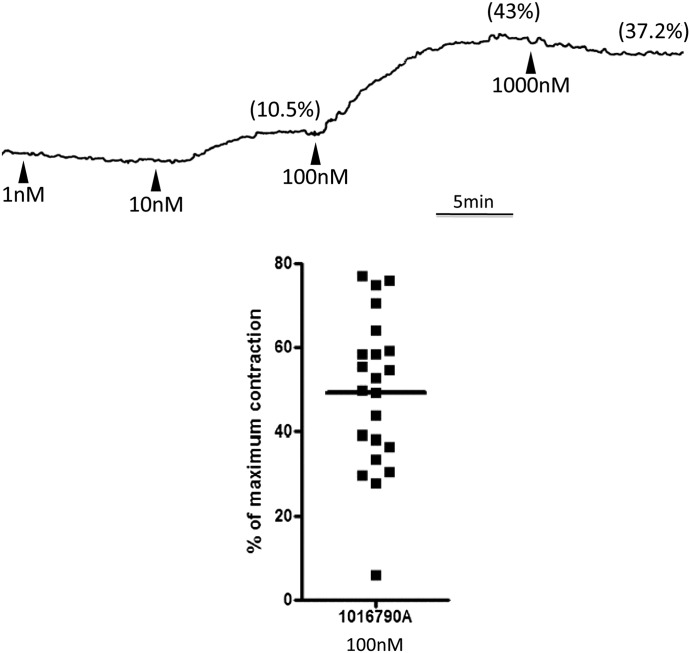
To test our hypothesis that activation of the TRPV4 channel would constrict the human bronchus, we exposed ex vivo human bronchial tissue to the potent and efficacious TRPV4 opener GSK1016790. Consistent with our predictions, GSK1016790 caused contractions of human bronchi. In two preliminary experiments, we found that the threshold concentration for GSK1016790 was 10 nM and the maximum response was obtained at 100 nM (see representative concentration-response curve in Fig. 1). For all further studies, we evaluated the response to 100 nM of the TRPV4 opener. When bronchi from 22 donors were evaluated, GSK1016790 caused a robust (>25% of the maximum response evoked by the muscarinic agonist carbachol added at the end of the experiment) contraction in all but one tissue (Fig. 1) that averaged 50% ± 3% of the maximum. We tested the ability of the nonselective TRPV blocker ruthenium red to inhibit this response in bronchi from four donors, in which the contraction to 100 nM GSK1016790 averaged 45% ± 13%. As predicted, ruthenium red (1 μM) abolished the response (0% ± 0%) but did not alter the carbachol reference contraction at the end of the experiment.
Fig. 1.
The TRPV4 opener GSK1016790 constricts human bronchi ex vivo. (A) Representative trace demonstrating the concentration-dependent contractile effect of the TRPV4 opener GSK1016790. Arrowheads represent addition to organ bath of the indicated concentration of GSK1016790. Values in parentheses above the trace denote the percentage of maximum contraction evoked by the indicated concentration. (B) Data points from individual human airways demonstrating the ability of GSK1016790 to efficaciously constrict human bronchi. The horizontal bar denotes the mean of values from 22 donors.
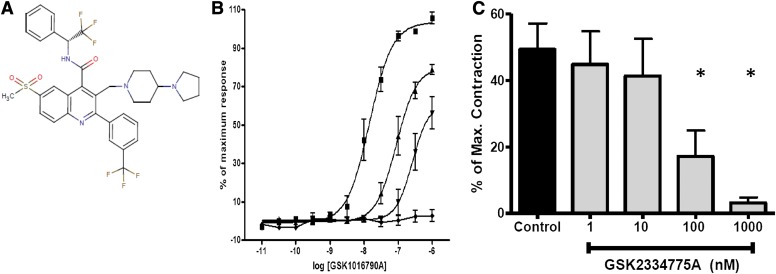
To increase confidence that TRPV4 channel activity causes constriction of the human airway, we identified the novel compound GSK2334775, a close structural relative of the previously identified potent and selective TRPV4 blocker GSK2193874A [7-bromo-N-(1-phenylcyclopropyl)-3-[(4-piperidin-1-ylpiperidin-1-yl) methyl]-2-[3-(trifluoromethyl)phenyl]quinoline-4-carboxamide] (Huh et al., 2012; Thorneloe et al., 2012) (Fig. 2A). In fluorescent imaging plate reader assays that measure intracellular Ca2+ levels, our novel blocker potently blocked agonist-induced Ca2+ fluxes through recombinant human TRPV4 channels (pIC50 = 8.0 + 0.1; n = 10), and with comparable potency in attachment-selected BAL cells from healthy volunteers (pA2 = 8.3 + 0.1; n = 4; Fig. 2B). This cell population, which predominantly contains alveolar macrophages, expresses native TRPV4-containing channels in mice (Hamanaka et al., 2010), and our data provide evidence that these findings are translatable to humans. Thus, GSK2334775 efficaciously blocks both recombinant and native TRPV4-containing channels with comparable potency.
Fig. 2.
The novel TRPV4 blocker GSK2334775 potently blocks TRPV4-containing channels in vitro and inhibits GSK1016790A-induced constriction of the human bronchus. (A) Chemical structure of GSK2334775 (see the text for full chemical name). (B) Summary data demonstrating the inhibition of the GSK1016790A-induced Ca2+ mobilization in human attachment-selected BAL cells by GSK2334775 (▪, control; ▴, 10 nM; ▾, 100 nM), and its abolition by ruthenium red (♦, 10 μM, mean ± S.E.M. of 3–5 experiments). (C) Summary data demonstrating that GSK1016790A-dependent contractions of human bronchi are inhibited in a concentration-dependent manner by the novel TRPV4 blocker GSK2334775 (mean ± S.E.M. of five to six experiments). Asterisks denote statistically significant differences from the control (P < 0.05, one-way analysis of variance with Dunnett’s multiple comparison test).
After identifying GSK2334775 as a compound suitable for studying TRPV4 pharmacology, we sought to determine whether it would prevent GSK1016790-induced constriction of human bronchi. Indeed, GSK2334775 produced a concentration-dependent inhibition of the GSK1016790 contractions with an IC50 value consistent with its pA2 values obtained against native TRPV4-containing channels (Fig. 2C). Inhibition of the response by both a known nonselective cationic pore blocker of TRPV channels and a novel small molecule TRPV4 inhibitor provides compelling pharmacological evidence that TRPV4 channel activity constricts human bronchi and may limit airflow in patients with obstructive airways disease.
We noted that GSK1016790-evoked constrictions evolved relatively slowly, with a 3- to 10-minute t1/2. In preliminary experiments, we also noted that GSK1016790-evoked contractions were very slowly reversible (data not shown). Our interpretation of these data was that GSK1016790 may constrict human airway smooth muscle via indirect mechanisms that result in the production of slowly reversible/lipophilic mediators. Our experiments were conducted in maximally efficacious concentrations of nonselective COX inhibitors that would preclude the production of bronchoconstricting prostaglandins or thromboxanes. Of note, when we evaluated the effect of GSK1016790 in bronchi from two donors in the absence of COX inhibitors, those contracted 32.7% and 30.4% of maximum, which was not appreciably different than what we observed when COX was blocked.
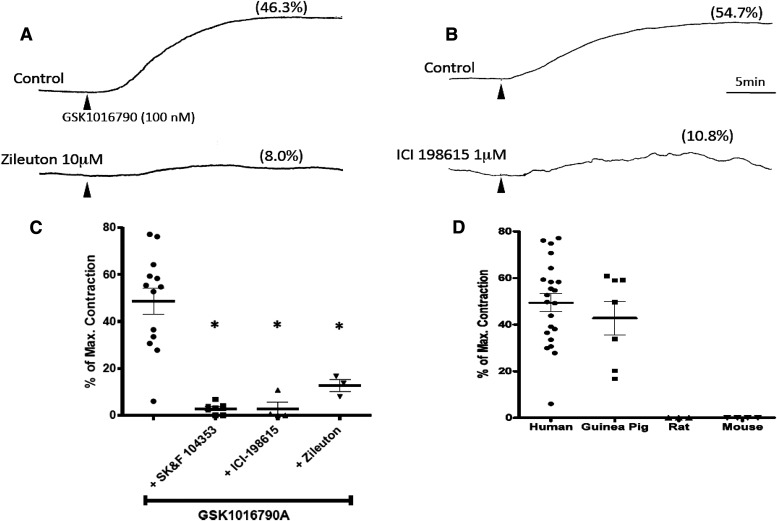
On the basis of the slow kinetics and COX-independent nature of the contractile response, we hypothesized that GSK1016790 contractions may be dependent upon the production of cysLTs, the only other well characterized lipophilic and slowly reversible constrictor of human airways. If cysLTs were necessary mediators of GSK1016790-induced contractions, the contractions should be sensitive to antagonists of cysLT1 receptors, the receptor subtype known to mediate the actions of cysLTs on human airway smooth muscle (Panettieri et al., 1998). We tested this hypothesis by pretreating tissues with maximally inhibitory concentrations of ICI-198615 [1-[[2-methoxy-r-[[(phenylsulfonyl)amino]carbonyl]phenyl]methyl]-1H-indazol-6-yl] carbamic acid cyclopentyl ester; 1 μM] or SK&F 104353 [2(S)-hydroxy-3(R)-[(2-carboxyethyl)thio]-3-[2-(8-phenyloctyl)pheny l]-propanoic acid; 10 μM], structurally unrelated cysLT1 receptor antagonists (Hay et al., 1987; Snyder et al., 1987). Consistent with our hypothesis, both of these molecules virtually prevented GSK1016790-mediated contractions (Fig. 3C).
Fig. 3.
Representative traces of human airways constricted by GSK1016790 (100 nM, added at time marked by arrowhead) in the presence or absence (control) of the 5-LO inhibitor zileuton in (A) or the cysLT1 antagonist ICI-198615 in (B). (C) Summary data and individual values of GSK1016790 (100 nM) effects alone or in the presence of cysLT1 receptor antagonism (SK&F 104353 and ICI-198615) or 5-LO inhibition (zileuton). Data are expressed as the mean ± S.E.M. (n = 14) and asterisks denote statistically significant differences (P < 0.05, one-way analysis of variance with Dunnett’s multiple comparison test) between GSK1016790 only and the indicated treatments. (D) Human bronchi and guinea pig trachea, which constrict in response to cysLTs, constrict robustly to GSK1016790 (100 nM), whereas rat and mouse trachea, which do not constrict upon exposure to cysLTs, are not constricted by GSK1016790. Human airway data in (D) are the same data presented in Fig. 1B and the GSK1016790-only data in (C) represent a subset of the same data points from experiments in which LT-based manipulations were performed.
To address the possibility that the cysLT1 antagonists were blocking TRPV4, we further evaluated the effect of 5-LO inhibition on the GSK1016790-mediated contractions. CysLTs are synthesized by 5-LO, an enzyme that catalyzes the oxygenation of arachidonic acid. The well characterized 5-LO inhibitor zileuton (10 μM) markedly inhibited GSK1016790-evoked contractions (Fig. 3, A and C), further substantiating our hypothesis that de novo synthesis of cysLTs is an obligate downstream process required for GSK1016790-mediated contractions of the human airway.
We took advantage of known species differences in airway smooth muscle pharmacology to provide further evidence that TRPV4 activation causes bronchial constriction entirely via cysLTs. Airways of guinea pigs (McAlexander et al., 1998), similar to humans, constrict in response to cysLTs, whereas airways of rats and mice do not (Held et al., 1999; Richter and Sirois, 2000). Because GSK1016790 activates both rat and mouse TRPV4 (Willette et al., 2008), we reasoned that if GSK1016790 constricted human and guinea pig—but not rat or mouse—airways, this would provide additional evidence that GSK1016790 contractions are cysLT dependent. Indeed, GSK1016790 did not constrict rat or mouse trachea (Fig. 3D). Of note, in two additional preliminary experiments, a 10-fold larger concentration of GSK1016790 (1 μM) also did not constrict mouse airways (not shown). In marked contrast, 100 nM GSK1016790 contracted guinea pig trachea with efficacy comparable with that seen in human tissues (Fig. 4). Moreover, these guinea pig airway contractions were blocked by 1 μM ruthenium red (3% ± 3% of carbachol maximum versus 46% ± 10% in tissues treated with GSK1016790 alone; n = 4). The responses in two guinea pig tracheae were essentially abolished by the cysLT1 antagonist SK&F 104353 (10 μM; 34% and 61% versus 0.4% and 0%). This finding that GSK1016790 only constricts airway tissues of species known to constrict to cysLTs is consistent with our human bronchus data demonstrating that multiple structurally unrelated modulators of cysLT production or action inhibit the GSK1016790 response.
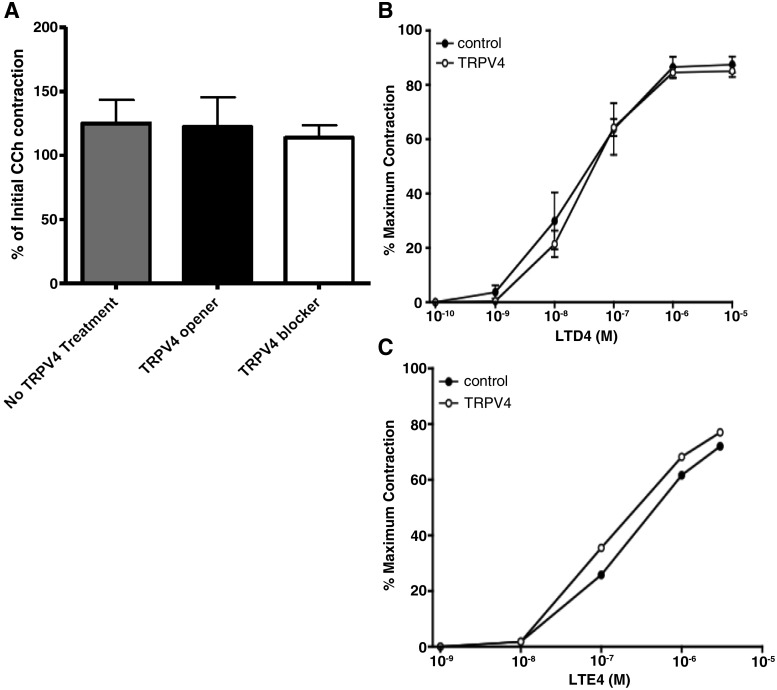
Fig. 4.
(A) The response to carbachol (CCh) in the presence of the treatments as a percentage of the initial response (mean ± SEM, n = 24–29). (B) With the 5-LO inhibitor zileuton present to prevent cysLT synthesis, exposure to GSK1016790 (100 nM, ○) does not alter the concentration-response relationship between exogenously applied LTD4 concentration-response curves for human bronchial contractions (n = 4 donors), or in the LTE4 concentration-response curves (n = 2 donors) (C).
Because TRPV4 channels are functionally expressed in human airway smooth muscle cells in vitro (Jia et al., 2004), we predicted that TRPV4 activation might enhance airway smooth muscle contractility. To investigate this possibility, we first pretreated bronchial tissues with the 5-LO inhibitor zileuton, which served to prevent any potentially confounding endogenous production of cysLTs, and we then examined the concentration-response relationships of exogenously applied leukotriene (LT) D4 in the absence and presence of the TRPV4 opener GSK1016790 (100 nM). Under conditions of 5-LO blockade, consistent with our aforementioned results, GSK1016790 had no effect on baseline tension, nor did it influence the concentration-response characteristics of LTD4 (Fig. 4A). In bronchi from two additional donors, we also noted that GSK1016790 had no effect on the concentration-response curves for LTE4 (Fig. 4C). In addition, we noted that neither GSK1016790 nor GSK2334775 had any effect on the contractile efficacy of the maximum response to carbachol used as our standard (P > 0.1; Fig. 4A). These data fail to provide any evidence that TRPV4 activation can acutely alter human airway smooth muscle contractility independent of cysLT production.
Mast cells situated near the airway smooth muscle are a likely source of the cysLTs causing the GSK1016790-induced contractions. We compared the response to GSK1016790 with a maximal mast cell–mediated response that could be evoked with anti-IgE. In experiments with paired bronchi from eight donors, we found the TRPV4 agonist causes a contraction that is approximately 50% of the “mast cell maximum” response obtained with 1 μg/ml anti-IgE (59.5% ± 4.4% versus 33.5% ± 8.7%; n = 8).
Discussion
Previous studies demonstrated that the nonselective cation channel TRPV4 is expressed and functional within the mammalian respiratory tract (Jia et al., 2004; Andrade et al., 2005; Yang et al., 2006; Jian et al., 2008; Zhu et al., 2009; Li et al., 2011; Delany et al., 2001) and multiple lines of evidence suggested that TRPV4 activation may influence airway smooth muscle function. TRPV4 single nucleotide polymorphisms, including one nonsynonymous one that behaves as a gain-of-function channel in human airway epithelial cells (Li et al., 2011), are associated with airflow limitation in chronic obstructive pulmonary disease (Zhu et al., 2009), and pharmacological studies have suggested that TRPV4 can influence airway smooth muscle Ca2+ signaling in vitro (Jia et al., 2004). Herein, we used potent and selective pharmacological tools to examine whether acute TRPV4 activation causes constriction of human isolated airways. Contrary to our initial hypothesis that TRPV4 directly influences airway smooth muscle contractility, we demonstrated that TRPV4 activation causes human airway constriction exclusively via the production of cysLTs. Thus, although we could detect no role of the TRPV4 ion channel in acute responses to bronchoconstrictive stimuli, our data offer novel evidence in support of a pathologic role of TRPV4 in human bronchial airways. Specifically, our data introduce cysLT synthesis as a novel mechanism by which TRPV4 channel activation by endogenous and/or inhaled factors may facilitate airway inflammation, pathologic tissue remodeling, and airflow obstruction.
In this study, we used a combination of potent and selective TRPV4 modulators. The agonist GSK1016790 was previously identified as a selective TRPV4 agonist capable of activating heterologously expressed human TRPV4 channels with considerably greater potency and efficacy than previously characterized TRPV4 activators, including the phorbol ester 4α-phorbol 12,13-didecanoate (Willette et al., 2008). Our finding that the contractions caused by GSK1016790 were secondary to TRPV4 activation is supported by the fact that they were blocked by the nonselective TRPV antagonist ruthenium red. Moreover, the contractions were blocked by the selective TRPV4 antagonist GSK2334775 at concentrations consistent with its apparent affinity for recombinant human TRPV4 channels. Incidentally, our preliminary characterization of this antagonist with adherence-selected BAL cells from healthy human volunteers provides evidence that this cell population, which predominantly contains alveolar macrophages, expresses TRPV4 channels. This novel finding is consistent with previous findings in mouse alveolar macrophages (Hamanaka et al., 2010) and offers the first evidence that this phenomenon translates to humans.
TRPV4 channel activity causes cation flux across biologic membranes, which causes depolarization of membrane potentials and elevations in cytosolic Ca2+. The obligatory role of cytosolic Ca2+ elevations in smooth muscle excitation-contraction coupling, combined with existing data that multiple chemical and physical stimuli that activate TRPV4 increase cytosolic Ca2+ concentrations in human airway smooth muscle cells (Jia et al., 2004; Ito et al., 2008), led us to hypothesize that chemical activation of TRPV4 with GSK1016790 would directly constrict human airway smooth muscle. Multiple lines of evidence contradict this hypothesis and instead support the hypothesis that TRPV4 stimulation in human bronchi indirectly activates smooth muscle via the production of cysLTs. First, the robust airway contractions caused by GSK1016790 were nearly abolished by two structurally unrelated cysLT1 receptor antagonists. Second, blocking 5-LO, an obligatory enzyme obligatory for cysLT production, virtually abolished the TRPV4-mediated contractions. Third, GSK1016790 constricted airway tissue from humans and guinea pigs, two species with airways that constrict in response to cysLTs, whereas GSK1016790 did not constrict airways from rats or mice, even though GSK1016790 activates their TRPV4 channels. Fourth, GSK1016790, in the presence of the 5-LO inhibitor zileuton, had no effect on the potency or efficacy of other contractile agonists such as LTD4, LTE4, or carbachol. The latter results demonstrate that acute TRPV4 modulation does not fundamentally alter the contractile efficacy of the human bronchus.
Intravenous administration of GSK1016790 to mice or rats is acutely lethal because of circulatory collapse precipitated by failure of the alveolar septal barrier (Willette et al., 2008). The rapid and profound nature of this response precludes investigation of mechanisms by which TRPV4 activity may modulate cartilaginous airway function in these species. Moreover, cysLTs do not constrict airways of mice and rats (Held et al., 1999). Thus, although TRPV4 activation is overtly toxic in the distal lung of multiple mammalian species, channel activity in the cartilaginous airways may cause a wider range of deleterious effects in human airways than in those of rodents. In this regard, our data expand upon and complement those of Li et al. (2011), who demonstrated that diesel exhaust can stimulate TRPV4 in human airway epithelial cells, leading to secretion of matrix metalloprotease 1, an enzyme involved in pathologic tissue remodeling that does not have a mouse ortholog.
Although these data do not support a role of TRPV4 in direct and acute constriction of human airway smooth muscle, they in no way exclude the possibility that TRPV4 may cause Ca2+ influx in human airway smooth muscle cells that results in pathologic remodeling (e.g., proliferation and/or release of inflammatory mediators) rather than constriction. To this point, whereas both TRPV4 and T-type Ca2+ channels cause cytosolic Ca2+ elevations in lung endothelium (Wu et al., 2009), the downstream responses that they trigger are functionally segregated because TRPV4-dependent Ca2+ influx increases vascular permeability, whereas the T-type Ca2+ channel-dependent pathway leads to upregulation of the adhesion molecule P-selectin (Wu et al., 2009).
The observation that TRPV4-dependent contractions of human airways are cysLT dependent was unexpected, based both on the previously discussed evidence for TRPV4 function in airway smooth muscle and the absence of any published link between TRPV4 and LT production. The cellular source of the cysLTs produced in these experiments has not yet been elucidated. Human airway epithelium (Jame et al., 2007) and fibroblasts (James et al., 2006) can produce cysLTs via 5-LO activity. The epithelium does not appear to influence this response, because epithelial cells were mechanically denuded in guinea pig airway preparations, yet the magnitude of guinea pig airway contractions in response to GSK1016790 were identical to humans. Although mouse alveolar macrophages express TRPV4 (Hamanaka et al., 2010) and our data confirm that human BAL cells do as well, respiratory tract macrophages are found predominantly in the more distal airspaces where they ingest material that is not removed by the physical routes of coughing and the mucociliary escalator. In addition, the presence of eosinophils and evidence for their function is sparse in nonasthmatic airways. We presently favor the hypothesis that mast cells situated near the bronchial smooth muscle are the source of the cysLTs. Adenosine is a stimulator of bronchial mast cells and leads to human bronchial contractions similar to what we observed with GSK1016790; in both cases, the responses are largely inhibited by an antagonist of cystLT1 receptors (Björck et al., 1992). Antigenic or anti-IgE stimulation of bronchial mast cells also constricts bronchial smooth muscle via a cysLT (and histamine)-dependent mechanism (Ellis et al., 1994). However, we observed no correlation in the bronchial constriction response to anti-IgE and GSK1016790.
In summary, our data demonstrate that a potent and selective chemical activator of TRPV4 causes robust constriction of human airways via an entirely cysLT-dependent mechanism. We provide further support that TRPV4 ion channel activity is responsible for this effect by demonstrating that constrictions caused by GSK1016790 are abolished not only by the nonselective TRPV channel blocker ruthenium red but also by the novel TRPV4-selective blocker GSK2334775. These data provide the first link between TRPV4 activation and the production of cysLTs and provide novel insight into how TRPV4 may contribute to airflow limitation, inflammation, and pathologic remodeling in human respiratory disease. In addition, these data identify GSK2334775 as a novel compound that can enable investigation of TRPV4 pharmacology in human tissue and potentially serve as a template for future therapeutics directed against TRPV4.
Acknowledgments
The authors thank Steve Kelsen of Temple University for performing bronchoscopy procedures and obtaining BAL samples from human volunteers.
Abbreviations
- 5-lipoxygenase
5-LO
- BAL
bronchoalveolar lavage
- COX
cyclooxygenase
- cysLT
cysteinyl leukotriene
- GSK1016790
N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide
- GSK 2193874
7-bromo-N-(1-phenylcyclopropyl)-3-[(4-piperidin-1-ylpiperidin-1-yl) methyl]-2-[3-(trifluoromethyl)phenyl]quinoline-4-carboxamide
- GSK2334775
(R)-6-(methylsulfonyl)-3-((4-(pyrrolidin-1-yl)piperindin-1-yl)methyl)-N-(2,2,2,-trifluoro-1-phenylethyl)-2-(3-(trifluoromethyl)phenyl)quinoline-4-carboxamide
- ICI-198615
[1-[[2-methoxy-r-[[(phenylsulfonyl)amino]carbonyl]phenyl]methyl]-1H-indazol-6-yl] carbamic acid cyclopentyl ester
- LT
leukotriene
- NIH
National Institutes of Health
- SK&F 104353
2(S)-hydroxy-3(R)-[(2-carboxyethyl)thio]-3-[2-(8-phenyloctyl)pheny l]-propanoic acid
- TRPV
transient receptor potential vanilloid
Authorship Contributions
Participated in research design: McAlexander, Undem.
Conducted experiments: Luttmann, Hunsberger.
Performed data analysis: Luttmann, Hunsberger.
Wrote or contributed to the writing of the manuscript: McAlexander, Undem.
Footnotes
This research was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grant 5R01-HL062296]; and GlaxoSmithKline Pharmaceuticals.
References
- Andrade YN, Fernandes J, Vázquez E, Fernández-Fernández JM, Arniges M, Sánchez TM, Villalón M, Valverde MA. (2005) TRPV4 channel is involved in the coupling of fluid viscosity changes to epithelial ciliary activity. J Cell Biol 168:869–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björck T, Gustafsson LE, Dahlén SE. (1992) Isolated bronchi from asthmatics are hyperresponsive to adenosine, which apparently acts indirectly by liberation of leukotrienes and histamine. Am Rev Respir Dis 145:1087–1091 [DOI] [PubMed] [Google Scholar]
- Brooks CA, Cheung M, Eidam HS, Fox RM, Hilfiker MA, Manas ES, Ye G (2011) inventors, GlaxoSmithKline, assignee. TRPV4 antagonists. PCT International Application WO2011119701 A1. 29 Sept 2011.
- Delany NS, Hurle M, Facer P, Alnadaf T, Plumpton C, Kinghorn I, See CG, Costigan M, Anand P, Woolf CJ, et al. (2001) Identification and characterization of a novel human vanilloid receptor-like protein, VRL-2. Physiol Genomics 4:165–174 [DOI] [PubMed] [Google Scholar]
- Ellis JL, Hubbard WC, Meeker S, Undem BJ. (1994) Ragweed antigen E and anti-IgE in human central versus peripheral isolated bronchi. Am J Respir Crit Care Med 150:717–723 [DOI] [PubMed] [Google Scholar]
- Gorenne I, Labat C, Gascard JP, Norel X, Nashashibi N, Brink C. (1998) Leukotriene D4 contractions in human airways are blocked by SK&F 96365, an inhibitor of receptor-mediated calcium entry. J Pharmacol Exp Ther 284:549–552 [PubMed] [Google Scholar]
- Hamanaka K, Jian MY, Townsley MI, King JA, Liedtke W, Weber DS, Eyal FG, Clapp MM, Parker JC. (2010) TRPV4 channels augment macrophage activation and ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 299:L353–L362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay DW, Muccitelli RM, Tucker SS, Vickery-Clark LM, Wilson KA, Gleason JG, Hall RF, Wasserman MA, Torphy TJ. (1987) Pharmacologic profile of SK&F 104353: a novel, potent and selective peptidoleukotriene receptor antagonist in guinea pig and human airways. J Pharmacol Exp Ther 243:474–481 [PubMed] [Google Scholar]
- Held HD, Martin C, Uhlig S. (1999) Characterization of airway and vascular responses in murine lungs. Br J Pharmacol 126:1191–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh D, Leslie DC, Matthews BD, Fraser JP, Jurek S, Hamilton GA, Thorneloe KS, McAlexander MA, Ingber DE. (2012) A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci Transl Med 4:159ra147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Kume H, Naruse K, Kondo M, Takeda N, Iwata S, Hasegawa Y, Sokabe M. (2008) A novel Ca2+ influx pathway activated by mechanical stretch in human airway smooth muscle cells. Am J Respir Cell Mol Biol 38:407–413 [DOI] [PubMed] [Google Scholar]
- Jame AJ, Lackie PM, Cazaly AM, Sayers I, Penrose JF, Holgate ST, Sampson AP. (2007) Human bronchial epithelial cells express an active and inducible biosynthetic pathway for leukotrienes B4 and C4. Clin Exp Allergy 37:880–892 [DOI] [PubMed] [Google Scholar]
- James AJ, Penrose JF, Cazaly AM, Holgate ST, Sampson AP. (2006) Human bronchial fibroblasts express the 5-lipoxygenase pathway. Respir Res 7:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Wang X, Varty L, Rizzo CA, Yang R, Correll CC, Phelps PT, Egan RW, Hey JA. (2004) Functional TRPV4 channels are expressed in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 287:L272–L278 [DOI] [PubMed] [Google Scholar]
- Jian MY, King JA, Al-Mehdi AB, Liedtke W, Townsley MI. (2008) High vascular pressure-induced lung injury requires P450 epoxygenase-dependent activation of TRPV4. Am J Respir Cell Mol Biol 38:386–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongejan RC, De Jongste JC, Raatgeep RC, Bonta IL, Kerrebijn KF. (1990) Effects of changes in osmolarity on isolated human airways. J Appl Physiol (1985) 68:1568–1575 [DOI] [PubMed] [Google Scholar]
- Li J, Kanju P, Patterson M, Chew WL, Cho SH, Gilmour I, Oliver T, Yasuda R, Ghio A, Simon SA, Liedtke W. (2011) TRPV4-mediated calcium-influx into human bronchial epithelia upon exposure to diesel exhaust particles. Environ Health Perspect 119:784–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlexander MA, Myers AC, Undem BJ. (1998) Inhibition of 5-lipoxygenase diminishes neurally evoked tachykinergic contraction of guinea pig isolated airway. J Pharmacol Exp Ther 285:602–607 [PubMed] [Google Scholar]
- Panettieri RA, Tan EML, Ciocca V, Luttmann MA, Leonard TB, Hay DWP. (1998) Effects of LTD4 on human airway smooth muscle cell proliferation, matrix expression, and contraction in vitro: differential sensitivity to cysteinyl leukotriene receptor antagonists. Am J Respir Cell Mol Biol 19:453–461 [DOI] [PubMed] [Google Scholar]
- Richter M, Sirois P. (2000) Effects of eicosanoids, neuromediators and bioactive peptides on murine airways. Eur J Pharmacol 389:225–234 [DOI] [PubMed] [Google Scholar]
- Snyder DW, Giles RE, Keith RA, Yee YK, Krell RD. (1987) In vitro pharmacology of ICI 198,615: a novel, potent and selective peptide leukotriene antagonist. J Pharmacol Exp Ther 243:548–556 [PubMed] [Google Scholar]
- Thorneloe KS, Cheung M, Bao W, Alsaid H, Lenhard S, Jian MY, Costell M, Maniscalco-Hauk K, Krawiec JA, Olzinski A, et al. (2012) An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Sci Transl Med 4:159ra148. [DOI] [PubMed] [Google Scholar]
- Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. (2004) Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci USA 101:396–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willette RN, Bao W, Nerurkar S, Yue TL, Doe CP, Stankus G, Turner GH, Ju H, Thomas H, Fishman CE, et al. (2008) Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: Part 2. J Pharmacol Exp Ther 326:443–452 [DOI] [PubMed] [Google Scholar]
- Wu S, Jian MY, Xu YC, Zhou C, Al-Mehdi AB, Liedtke W, Shin HS, Townsley MI. (2009) Ca2+ entry via alpha1G and TRPV4 channels differentially regulates surface expression of P-selectin and barrier integrity in pulmonary capillary endothelium. Am J Physiol Lung Cell Mol Physiol 297:L650–L657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XR, Lin MJ, McIntosh LS, Sham JSK. (2006) Functional expression of transient receptor potential melastatin- and vanilloid-related channels in pulmonary arterial and aortic smooth muscle. Am J Physiol Lung Cell Mol Physiol 290:L1267–L1276 [DOI] [PubMed] [Google Scholar]
- Zhu G, Gulsvik A, Bakke P, Ghatta S, Anderson W, Lomas DA, Silverman EK, Pillai SG, ICGN Investigators (2009) Association of TRPV4 gene polymorphisms with chronic obstructive pulmonary disease. Hum Mol Genet 18:2053–2062 [DOI] [PubMed] [Google Scholar]