Abstract
Psychostimulants, such as cocaine and amphetamines, act primarily through the monoamine neurotransmitters dopamine (DA), norepinephrine, and serotonin. Although stimulant addiction research has largely focused on DA, medication development efforts targeting the dopaminergic system have thus far been unsuccessful, leading to alternative strategies aimed at abating stimulant abuse. Noradrenergic compounds have shown promise in altering the behavioral effects of stimulants in rodents, nonhuman primates, and humans. In this review, we discuss the contribution of each adrenergic receptor (AR) subtype (α1, α2, and β) to five stimulant-induced behaviors relevant to addiction: locomotor activity, conditioned place preference, anxiety, discrimination, and self-administration. AR manipulation has diverse effects on these behaviors; each subtype profoundly influences outcomes in some paradigms but is inconsequential in others. The functional neuroanatomy and intracellular signaling mechanisms underlying the impact of AR activation/blockade on these behaviors remain largely unknown, presenting a new frontier for research on psychostimulant–AR interactions.
Introduction
For many years, medication development efforts for psychostimulant abuse therapies revolved around understanding and modifying dopamine (DA) transmission. Because DA mediates the primary rewarding/reinforcing effects of psychostimulants, the focus on DA was understandable. However, after decades of research, dopaminergic compounds have failed to gain Food and Drug Administration approval or general acceptance as treatments for stimulant dependence. Several reasons likely contribute to this lack of efficacy. For example, dopaminergic drugs showed abuse liability themselves (Mariani and Levin, 2012). In addition, experienced drug abusers often report that, although the drug may no longer produce a subjective euphoric effect, they continue to use the drug for other reasons, rendering medications that alter the positive subjective effects of psychostimulants impotent. Accordingly, recent research has shifted focus from altering primary reward/reinforcement to preventing relapse. Although DA transmission does contribute to relapse-like behavior, other neurotransmitter systems have been implicated and may actually be more influential, revealing new possibilities for therapeutic targets (Weinshenker and Schroeder, 2007).
Although it is generally accepted that the abuse-related effects of psychostimulants occur primarily through dopaminergic activity, this class of drugs alters several neurotransmitter systems. In particular, both cocaine and amphetamine-like compounds also increase extracellular levels of norepinephrine (NE) and serotonin by preventing reuptake by their respective plasma membrane transporters (i.e., DAT, NET, and SERT) and/or inducing release. On the basis of recent studies implicating NE in relapse-like behavior, interest in the contribution of this neurotransmitter to addictive processes has reemerged (Weinshenker and Schroeder, 2007; Gaval-Cruz and Weinshenker, 2009). Some noradrenergic compounds have already shown promise in human laboratory studies and initial clinical trials (Gaval-Cruz and Weinshenker, 2009; Fox et al., 2012; Haile et al., 2012; Newton et al., 2012; Shorter et al., 2013; K. Cunningham, personal communication). Yet, our knowledge of how the NE system reacts to, and interacts with, psychostimulants is remarkably incomplete, and the neurobiological mechanisms underlying the effects of these compounds are not well understood.
Because the contribution of DAT, NET, and SERT to stimulant-induced behaviors has been extensively discussed elsewhere (e.g., Sora et al., 1998; Hall et al., 2004), we will focus this review on how adrenergic receptors (ARs) influence responses to psychostimulants, and how these systems could be targeted for novel addiction therapies. We will also highlight the nearly complete absence of data concerning the relevant neuroanatomical substrates and the intracellular signaling components downstream of AR activation within the context of psychostimulant responses, which severely hampers our current understanding of these processes and represents a fertile frontier for future research.
Adrenergic Receptor Subtypes and Compounds
ARs are G protein–coupled receptors that bind, and are activated by, NE and its derivative transmitter epinephrine. Because epinephrine levels in the brain are very low (Mefford, 1988), it is likely that NE mediates most of the effects discussed in this review, although some evidence for epinephrine regulation of motor activity exists (Stone et al., 2003). Included in this family of receptors are 9 subtypes encoded by separate genes: three α1ARs (α1a, α1b, and α1d), three α2ARs (α2a, α2b, and α2c), and three βARs (β1, β2, and β3). α1ARs are Gαq coupled, and their activation stimulates phospholipase C activity to cleave phosphatidylinositol 4,5-biphosphate and increase inositol triphosphate and diacylglycerol, causing an increase of intracellular calcium and activation of protein kinase C. βARs are typically Gαs-coupled and activate protein kinase A (PKA) via stimulation of adenylate cyclase activity and cAMP production. α2ARs are Gαi-coupled and function as inhibitory autoreceptors on noradrenergic neurons, although both pre- and postsynaptic α2AR heteroreceptors on NE target neurons are also abundant in the brain. Activation of these receptors decreases PKA activation by suppressing cAMP production by adenylate cyclase. In addition, the Gβγ protein complex associated with ARs is capable of modulating intracellular signaling molecules and ion channels, including phospholipase C, G protein receptor kinase, inwardly rectifying potassium channels, and calcium channels, among others (Lin and Smrcka, 2011). However, the functional consequences of βγ signaling in the context of stimulant-induced behaviors have not been well investigated.
Although ARs typically signal via these molecules, several noncanonical AR signaling pathways that are independent of G proteins and cAMP exist. For example, β2AR activation stimulates a glycogen synthase kinase 3β/Akt pathway via β-arrestins, which previously were thought only to be important for G protein–coupled receptor internalization and sensitization but are now known to be scaffolds for multiprotein complexes and signaling (Yamamoto et al., 2007; Beaulieu et al., 2009). Additionally, β1ARs can signal through Gαolf to activate a receptor tyrosine kinase, which in turn stimulates the Ras/Raf/mitogen-activated protein kinase kinase/mitogen-activated protein kinase/mitogen- and stress-activated protein kinase pathway to produce cAMP response element–binding protein phosphorylation and gene transcription (Meitzen et al., 2011). β2ARs can switch coupling from Gαs to Gαi via PKA-mediated phosphorylation of the receptor, leading to mitogen-activated protein kinase pathway activation (Daaka et al., 1997). β2AR activation can cause G protein receptor kinase phosphorylation, which activates a β-arrestin and phosphodiesterase-4 feedback circuit to decrease cAMP activity and PKA phosphorylation of the receptor (Baillie et al., 2003). Without this β-arrestin/phosphodiesterase-4 feedback, an enhanced switch from Gαs to Gαi coupling is observed. This β2AR–Gαi signaling also occurs in the brain and is reported to mediate learning and memory, potentially via phospholipase C (Schutsky et al., 2011a,b; Ouyang et al., 2012). The mechanisms underlying ligand-induced activation of these pathways are understudied and could provide critical insights into AR-mediated effects.
Pharmacologic Compounds Targeting Adrenergic Receptors
Because many compounds targeting these ARs with varying degrees of selectivity for one subtype versus another are used to determine the roles of each receptor in stimulant-induced behaviors, we provide a brief description of these compounds.
The prototypical α1AR antagonist is prazosin, but it is limited by its equal affinity for each of the α1AR subtypes (Zhong and Minneman, 1999). Terazosin is similar to prazosin but is favored in intracranial infusion studies because it is more soluble in artificial cerebrospinal fluid, which is often used as a vehicle for these experiments (Stone et al., 1999). WB-4101 [2-(2,6-dimethoxyphenoxyethyl)aminomethyl-1,4-benzodioxane hydrochloride] was the first subtype-selective α1AR antagonist, with an affinity for the α1aAR approximately 20-fold greater than the α1bAR; however its binding affinity does not differentiate the α1dAR (Morrow and Creese, 1986). Two compounds that are selective for α1a over α1b and α1d are 5-methylurapidil and (+)-niguldipine (Boer et al., 1989; Hanft and Gross, 1989). BMY 7378 [8-(2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl)-8-azaspiro[4.5]decane-7,9-dione] has a 100-fold greater affinity for α1d than α1a or α1b but also acts as a partial 5HT1a receptor agonist (Goetz et al., 1995; Zhong and Minneman, 1999). Epinephrine activates α1ARs with the highest affinity, followed by NE and phenylephrine, respectively (Morrow and Creese, 1986). As measured by radioligand binding, epinephrine, NE, and phenylephrine show the highest affinities for α1d (Minneman et al., 1994), and only phenylephrine binds with a greater affinity for α1a than α1b (Morrow and Creese, 1986). However, when measuring intracellular responses in recombinant human embryonic kidney 293 cells expressing only one subtype, similar potencies are found for epinephrine, NE, and phenylephrine regardless of the receptor subtype expressed (Minneman et al., 1994). Methoxamine, ST 587 [2-(2-chloro-5-trifluoromethylphenylimino)imida-zolidine], and SDZ NVI 085 [(−)-(4aR, 10aR)-3,4,4a,5,10,10a-hexahydro-6-methoxy-4-methyl-9-methylthio-2H-naphth[2,3-b]-1,4-oxazine hydrogen malonate] also activate α1ARs (Spealman, 1995; Munzar and Goldberg, 1999).
Agonists at α2ARs include clonidine, UK 14304 [5-bromo-N-(2-imidazolin-2-yl)-6-quinoxaline, 5-bromo-N-(4,5-dihydro-1H-imidazol-2-yl)-6-quinoxalinamine], lofexidine, guanabenz, and dexmedetomidine (Aghajanian and VanderMaelen, 1982; Carter, 1997; Kleven and Koek, 1997; Sallinen et al., 1997; Erb et al., 2000). Guanabenz/guanfacine is preferential for α2a, but clonidine and dexmedetomidine have equal affinity at α2 subtypes (Gobert et al., 1998). α2AR antagonists include yohimbine, efaroxan, BRL-44408 (2-[(4,5-dihydro-1H-imidazol-2-yl)methyl]-2,3-dihydro-1-methyl-1H-isoindole), dexefaroxan, idazoxan, and atipamezole (Dickinson et al., 1988; Villegier et al., 2003; Juhila et al., 2005; Jimenez-Rivera et al., 2006; Doucet et al., 2013). Yohimbine and atipamezole show equal affinities at all three receptor subtypes, but atipamezole has a 200-fold greater selectivity than yohimbine for the α2AR over the α1AR (Schwartz and Clark, 1998). Because yohimbine interacts with a number of non-noradrenergic systems in addition to acting at the α2AR, its effects should be interpreted with caution (Feuerstein et al., 1985; Millan et al., 2000; Conrad et al., 2012).
The prototypical βAR antagonist is propranolol, and like prazosin, it is not selective for any of the β subtypes. Timolol and nadolol act equally at β1 and β2 ARs, but nadolol cannot cross the blood-brain barrier (Colussi-Mas et al., 2005). Selective β1AR antagonists include atenolol, which also only acts peripherally, and betaxolol (Harris et al., 1996; Bernardi et al., 2009). ICI-118,551 [3-(isopropylamino)-1-[(7-methyl-4-indanyl)oxy]butan-2-ol] has high affinity for the β2AR (O’Donnell and Wanstall, 1980; Bilski et al., 1983). SR58611A {ethyl [[(7S)-7-[[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl]amino]-5,6,7,8-tetrahydronaphthalen-2-yl]oxy]acetate} and SR59230A [(2S)-1-(2-ethylphenoxy)-3-[[(1S)-1,2,3,4-tetrahydronaphthalen-1-yl]amino]propan-2-ol] are selective agonists and antagonists, respectively, for the β3AR (Consoli et al., 2007).
Although not an exhaustive list of AR activators and inhibitors, these are some of the most commonly used compounds in psychostimulant studies that we will refer to later in this review.
Stimulant-Induced Locomotor Activity
A characteristic trait of stimulant drugs, such as cocaine and d-amphetamine, is the ability to increase locomotor activity in rodents. This hyperactivity is robust and provides a reliable metric for assessing the contribution of different systems to simple drug effects. In these studies, subjects are placed in an open field-like chamber, and activity is measured via a grid of infrared photobeams across the chamber. The animal’s position is monitored by beam breaks or by visual tracking software that uses contrast between the animal and the floor to identify location and movement. When initially placed in the chamber, animals will typically show an increased level of locomotion induced by the novelty of the chamber that is subject to habituation. Drug administration can occur either prior to this exploratory period or after habituation. Additionally, repeated administration of stimulants leads to behavioral sensitization in which the same dose results in greater levels of activity. Although the face validity of this behavioral measure for drug addiction per se is poor, locomotor activity can be a predictor of abuse liability (Marinelli and White, 2000; Simmons et al., 2013), and it has been suggested that the sensitization paradigm reflects the incentive salience value of drugs and models drug craving (Robinson and Berridge, 2001).
Manipulations of NE receptor subtypes indicate opposing roles of α1AR and α2AR, with blockade of α1AR decreasing and antagonism of α2AR increasing the acute locomotor response to stimulants. Numerous studies have shown that α1ARs antagonists such as prazosin, terazosin, and WB-4101 decrease drug-induced motor activity and behavioral sensitization (Snoddy and Tessel, 1985; Dickinson et al., 1988; Blanc et al., 1994; Darracq et al., 1998; Drouin et al., 2002; Weinshenker et al., 2002; Wellman et al., 2002; Vanderschuren et al., 2003; Auclair et al., 2004; Salomon et al., 2006; Alsene et al., 2010). Importantly, the effects seen with α1AR antagonism appear to be specific to drugs with abuse liability because prazosin did not impair basal locomotion or hyperactivity induced by the muscarinic antagonist scopolamine (Blanc et al., 1994; Wellman et al., 2002; Alsene et al., 2010). Compared with the wild type, mice genetically lacking the α1bAR subtype (α1b KO) had a decrease in acute and sensitized responses to amphetamine and cocaine despite normal basal dopaminergic function and DA receptor populations (Auclair et al., 2002, 2004; Drouin et al., 2002; Villegier et al., 2003). Furthermore, the effects of prazosin on drug-induced hyperactivity were abolished in the α1b KO mice, indicating that the α1b subtype is the most important mediator of this psychostimulant response (Drouin et al., 2002). α1d KO mice showed a decreased locomotor response to amphetamine, suggesting a contribution of this subtype. However, spontaneous wheel running and novelty-induced rearing were also reduced in these animals, indicating a nonspecific effect on motor activity (Sadalge et al., 2003). Intracerebroventricular administration of the α1a receptor antagonist, 5-methylurapidil, failed to suppress cocaine hyperlocomotion (Clifford et al., 2007). The location of the α1ARs regulating stimulant-induced activity appears to be the prefrontal cortex (PFC; Blanc et al., 1994; Darracq et al., 1998) and nucleus accumbens shell (Mitrano et al., 2012), because local infusions of α1AR antagonists into these regions reduced cocaine and/or amphetamine-induced locomotion.
Antagonism of α2AR, on the other hand, which facilitates NE transmission by blocking autoreceptor function, increased both acute stimulant-induced locomotion (Dickinson et al., 1988; Villegier et al., 2003; Jimenez-Rivera et al., 2006) and sensitized responses (Doucet et al., 2013) in mice and rats. Conversely, the α2AR agonist clonidine, which suppresses NE release via autoreceptor stimulation, produced a decreased acute response to cocaine (Vanderschuren et al., 2003; Jimenez-Rivera et al., 2006) and prevented amphetamine sensitization (Doucet et al., 2013). None of these studies provided evidence for the neuroanatomical substrates mediating these α2AR responses.
Fewer studies have examined the role of βARs on locomotor responses to stimulants. Propranolol, a nonselective βAR blocker, increased the acute effects of cocaine in rats (Harris et al., 1996) but not mice (Al-Hasani et al., 2013). Mixed results have been reported with amphetamine; low doses of propranolol (1.0–3.0 mg/kg) increased activity induced by amphetamine (1.0 mg/kg) in rats (Vanderschuren et al., 2003), but much higher doses of both drugs (30 mg/kg propranolol and 3.2 mg/kg amphetamine) produced decreased locomotion compared with mice treated with amphetamine alone (Snoddy and Tessel, 1985). Administration of a centrally acting βAR antagonist blocked the development of sensitization to amphetamine or cocaine, whereas peripherally acting antagonists did not (Colussi-Mas et al., 2005; Bernardi and Lattal, 2012a). These studies implicated the bed nucleus of the stria terminalis (BNST), which displayed induction of the immediate early gene c-fos after amphetamine administration, and intra-BNST infusion of the βAR antagonist timolol prevented sensitization (Colussi-Mas et al., 2005).
In summary, it appears that NE transmission has an overall facilitatory effect on stimulant-induced locomotion, and blocking postsynaptic ARs attenuates this behavior.
Place Preference and Aversion
The conditioned place preference (CPP) procedure is a popular paradigm used to measure the rewarding effects of addictive drugs (Tzschentke, 2007; Aguilar et al., 2009). This procedure uses a two- or three-compartment apparatus in which daily conditioning sessions pair the effects of a drug to one compartment and vehicle to the other. In the three-chamber version of the CPP paradigm, the third compartment is a neutral middle partition that is not paired with any stimuli and can be used as a “start box.” To determine whether the animal has an initial bias toward one side, a preconditioning test occurs during which the subject can freely explore all compartments. Ideally, the paradigm follows a balanced design in which no initial side preference is observed. If the subjects show a preconditioning preference, the experimental design can be described as “biased” or “unbiased.” In a biased design, the drug pairing is made with regards to the animals’ initial preference. For example, to increase the probability of observing a place preference on the final test, drug is paired with the compartment each animal prefers less during preconditioning. In the preferred unbiased design, the drug-compartment pairing is random: some animals receive the drug on the “preferred” side, others on the “nonpreferred” side. Alternatively, one compartment can be designated as the drug-paired chamber for all animals regardless of initial preference. After repeated pairings, a test session is used to assess the rewarding or aversive properties of the drug in question by allowing the animal to freely move between the compartments in a drug-free state. An increased amount of time spent in the drug-paired chamber is thought to reflect drug reward, whereas decreased time indicates a drug aversion. Because the paradigm requires learning, the conditioned effect can be extinguished, is subject to retrieval and reconsolidation processes, and can be reinstated after a drug-prime or stress exposure, which are believed to model aspects of relapse in human drug abusers.
Place Preference Induction.
In general, drugs that modulate NE activity specifically are ineffective at creating conditioned preferences or aversions on their own. Neither the α1AR agonist phenylephrine nor the α1AR antagonist prazosin supported the formation of a place preference (Zarrindast et al., 2002; Sahraei et al., 2004). Similarly, α2AR antagonists have also showed either no effect (Morales et al., 2001; Sahraei et al., 2004; Tahsili-Fahadan et al., 2006) or a conditioned aversion (File, 1986). In contrast, the α2AR agonist clonidine elicited a CPP (Asin and Wirtshafter, 1985; Cervo et al., 1993), but this effect was only observed at specific doses and when paired with the less preferred compartment, and other α2AR agonists have not mimicked this effect (Sahraei et al., 2004; Tahsili-Fahadan et al., 2006). The βAR antagonist timolol also did not produce a preference on its own (Robledo et al., 2004). These results indicate that neither AR agonists nor antagonists per se have rewarding properties or abuse liability.
Despite a thorough search of the literature, surprisingly few studies were found that examined the effects of AR antagonists on the development of a psychostimulant CPP. One study reported that propranolol (10 mg/kg) failed to alter cocaine CPP (Al-Hasani et al., 2013), and two others showed that mice lacking the α2aAR or both the β1AR and β2AR had normal amphetamine and cocaine CPP (Juhila et al., 2005; Vranjkovic et al., 2012). Surprisingly, no published studies have examined the influence of α1ARs on stimulant CPP. Considering the substantial evidence indicating a role for α1AR signaling in stimulant-induced locomotor activity, discussed above, and certain aspects of stimulant self-administration, discussed below, future efforts to determine the influence of α1ARs on the rewarding effects of cocaine and the neuroanatomy underlying any such findings are warranted.
Retrieval, Reconsolidation, and Extinction.
Although the effects of NE on the extinction of fear conditioning have been thoroughly investigated (Mueller and Cahill, 2010), only a few studies have examined the role of AR signaling in the extinction of conditioned drug effects. Because the neurobiological events mediating memory retrieval, reconsolidation, and extinction impact the ability to express or extinguish a CPP, care must be taken in the design and interpretation of studies to address the distinction between these processes. For the purposes of this review, we will be using the terms retrieval, reconsolidation, and extinction as the authors employed them to describe their work.
The retrieval of a cocaine CPP memory was blocked by presession administration of β-receptor antagonists (Otis and Mueller, 2011). These effects were localized to βARs in the PFC or dorsal hippocampus but not basolateral amygdala (BLA; Otis et al., 2013, 2014). Furthermore, βARs, specifically β2ARs, mediate the reconsolidation of cocaine CPP as administration of propranolol and ICI-118,551 (β2 antagonist), but not betaxolol (β1 antagonist), immediately after retrieval impaired the expression of CPP in subsequent sessions (Bernardi et al., 2006, 2009; Fricks-Gleason and Marshall, 2008). Similar effects were observed with a high dose of prazosin, but not a lower dose (Bernardi et al., 2009), yet how and where these βAR-mediated effects occur were not determined.
In contrast to retrieval, the effects of βAR activation on reconsolidation were localized to the BLA; c-fos immunoreactivity was increased in the BLA after reconsolidation and local infusions of β antagonists blocked the effect (Bernardi et al., 2009; Otis et al., 2013).
Regarding extinction of CPP, a possible role of αARs has been identified, but the data are not clear-cut. Mice treated with prazosin immediately after daily drug-free test sessions extinguished at normal rates, yet reacquired cocaine CPP with a single re-exposure session that was ineffective in vehicle-treated animals (Bernardi and Lattal, 2010). However, when the data were reanalyzed to control for initial preference, a high dose of prazosin accelerated extinction in animals with a high initial preference score (Bernardi and Lattal, 2012b). Yohimbine impaired the extinction of cocaine CPP, although this effect was not replicated with a selective α2AR antagonist and may be mediated by orexin rather than NE (Davis et al., 2008; Conrad et al., 2012).
Reinstatement.
After extinction of a drug–environment association, place preference can be reinstated with a noncontingent drug prime. Cocaine-primed reinstatement of CPP was unchanged by administration of propranolol, prazosin, or clonidine just prior to the cocaine prime (Mantsch et al., 2010; Al-Hasani et al., 2013), yet βAR antagonism during retrieval and extinction sessions prevented subsequent reinstatement (Fricks-Gleason and Marshall, 2008; Otis and Mueller, 2011; Otis et al., 2014). The mechanisms by which these compounds alter memory to prevent future cocaine-primed reinstatement but fail to impact the acute priming effects of cocaine should be further investigated.
Stress exposure can reinstate stimulant CPP in an AR-dependent manner. Stress-induced reinstatement of CPP depended on β2AR activity but was preserved after α1AR blockade (Mantsch et al., 2010). Stress-induced reinstatement could also be blocked by a cannabinoid receptor (CB1) antagonist, and a subthreshold dose of the α2AR antagonist BRL-44408 reinstated cocaine CPP when combined with a CB1 agonist (Vaughn et al., 2012), indicating an interaction of the NE and cannabinoid systems in this paradigm. Moreover, β2AR agonists and α2AR antagonists produced reinstatement on their own (Mantsch et al., 2010; Vranjkovic et al., 2012). κ-Opioid receptors were necessary for stress-induced reinstatement, and κ-agonist–induced reinstatement of cocaine CPP required κ expression in the noradrenergic locus coeruleus and was enhanced by clonidine, propranolol, or betaxolol, but not ICI-118,551, at doses that did not reinstate on their own (Al-Hasani et al., 2013). These results indicate that diverse stress-inducing compounds act via noradrenergic mechanisms, specifically β2AR activation, to facilitate reinstatement of a CPP.
Drug-Induced Anxiety
In addition to the rewarding effects of stimulants, drugs like cocaine also have anxiogenic properties. The elevated plus maze can be used as a behavioral readout of anxiety-like behavior. In this task, an animal is placed in a plus-shaped apparatus raised a few feet from the floor. Two of the four arms are enclosed with walls, and the other two arms are open. Time spent in the closed arms is thought to represent anxiety-like behavior because (1) restriction to the open arms causes greater behavioral and physiologic responses consistent with anxiety than closed arm restriction, (2) compounds that cause anxiety in humans increase time spent in the closed arms, and (3) clinically effective anxiolytic drugs selectively increase time spent in the open arms (Pellow et al., 1985).
With the elevated plus maze, mice injected with cocaine decreased the amount of time spent in the open arms. This anxiety-like behavior was blocked by propranolol, but not prazosin or yohimbine, implicating βARs (Schank et al., 2008). Thus, the noradrenergic system may act as a brake to limit the intake of cocaine by inducing negative side effects and suggests a therapeutic avenue to decrease drug use.
Additionally, withdrawal from chronic cocaine induced anxiety-like behavior in the elevated plus maze and the defensive burying task, another preclinical measure of anxiety, that was attenuated by the β1AR antagonist betaxolol and the α2AR agonist guanfacine, respectively (Rudoy and Van Bockstaele, 2007; Buffalari et al., 2012). β1AR protein levels and corticotropin-releasing factor transcription in the amygdala were increased during cocaine withdrawal and reduced after betaxolol administration, suggesting that a β1AR-mediated change in corticotropin-releasing factor production is important for this behavior (Rudoy and Van Bockstaele, 2007; Rudoy et al., 2009). Furthermore, betaxolol returned intracellular PKA catalytic subunit abundance after cocaine withdrawal to levels of drug-naive animals and prevented cocaine-induced cAMP response element–binding protein phosphorylation (Rudoy et al., 2009). Similarly, propranolol blocked cocaine withdrawal-induced anxiety in rats and also clinically in patients dealing with severe withdrawal symptoms (Harris and Aston-Jones, 1993; Kampman et al., 2001). Because drug-dependent individuals continue taking drugs to decrease the aversive effects of withdrawal, targeting the receptors responsible for this anxiety could have substantial therapeutic efficacy.
Drug Discrimination
Drug discrimination is a measure of the interoceptive effects of a drug. In this procedure, animals are trained to respond on one operandum (drug appropriate) after an experimenter-administered injection of a training drug and another operandum (vehicle appropriate) after vehicle administration. Responses on the appropriate lever are reinforced by food, water, shock termination, or other stimuli that maintain high, stable rates of behavior, but typically appetitive reinforcement is used. Once animals meet training criteria in which responses are made on the appropriate operandum with high selectivity (typically greater than 80–90%), a session occurs to test whether various doses of the training drug, other drugs, or pretreatments plus the training drug can alter the discriminative stimulus properties. When the test treatment produces interoceptive effects similar to the training drug, the subject responds predominantly on the drug-appropriate lever. When the test treatment produces interoceptive effects that are distinct from the training drug, the subject responds predominantly on the vehicle-appropriate lever. In this way, one can interrogate whether a test treatment produces a state that “feels” like that produced by the training drug. For example, when trained with cocaine, the psychostimulants amphetamine and methylphenidate engendered responding on the cocaine-appropriate lever, whereas nonstimulant drugs such as fenfluramine and mescaline elicited responding on the vehicle lever (McKenna and Ho, 1980).
The discriminative stimulus effects of psychostimulants are largely dependent on DA activity and only partially mediated by αARs, yet βARs have greater influence. Cocaine and amphetamine cross-generalized, and their effects could be abolished after administration of the DA D2 antagonist haloperidol (Schechter and Cook, 1975; McKenna and Ho, 1980). Regarding αARs and discrimination, a mixed bag of results has been reported with some studies indicating effects and others failing to find significance depending on the training drug and species used. The αAR antagonist phenoxybenzamine failed to alter the discriminative effects of cocaine or amphetamine (Schechter and Cook, 1975; McKenna and Ho, 1980). Similarly, it was recently reported that the nonselective αAR antagonist phentolamine had no effect on the ability of amphetamine or ephedrine to substitute for amphetamine in pigeons (Ercil and France, 2003). The α1AR antagonist dibenamine also did not change cocaine’s discriminative stimulus effects in rats (Colpaert et al., 1976). Prazosin has been the most thoroughly examined αAR antagonist, yet it lacks a clear, consistent role in the interoceptive effects of stimulants. It failed to profoundly shift the dose-response curves of cocaine (Kleven and Koek, 1998), methamphetamine (Munzar and Goldberg, 1999), or amphetamine (West et al., 1995) in rats, yet produced rightward shifts in the curves of cocaine in pigeons (Johanson and Barrett, 1993) and squirrel monkeys (Spealman, 1995; Rowlett et al., 2004), methamphetamine in pigeons (Sasaki et al., 1995), and amphetamine in mice (Snoddy and Tessel, 1985). No effect on cocaine or methamphetamine discrimination was observed after α1AR agonist administration in rats or monkeys (Spealman, 1995; Kleven and Koek, 1997; Munzar and Goldberg, 1999). Because no consistent, cross-species effects of αAR signaling has been observed, it appears that activity at these receptors is largely unnecessary for the interoceptive effects of stimulants.
A similar hodgepodge of results has been reported with compounds targeting α2AR. Whereas the α2AR agonist UK 14304 failed to alter the discriminative stimulus effects of cocaine (Spealman, 1995; Kleven and Koek, 1997), clonidine partially substituted for methamphetamine, amphetamine, and cocaine in rats (D’Mello, 1982; Wood et al., 1985; Munzar and Goldberg, 1999) and cocaine in pigeons (Johanson and Barrett, 1993) but not cocaine in squirrel monkeys (Spealman, 1995). Oddly, when tested in combination with methamphetamine doses higher than the training dose, clonidine decreased drug-appropriate responding (Munzar and Goldberg, 1999). Cocaine discrimination was unaffected by yohimbine or efaroxan, another α2AR antagonist (Wood et al., 1985; Spealman, 1995; Kleven and Koek, 1997). Thus, the data on α2AR compounds are inconsistent and confusing. These differential effects could be explained, at least in part, by the specificity of these drugs at different doses. At low doses, α2AR agonists and antagonists preferentially interact with the α2AR inhibitory autoreceptor, whereas at higher doses, these drugs can also engage α2AR heteroceptors on target neurons, as well as α1ARs (Gobert et al., 1998).
Experiments using βAR drugs have revealed some interesting results. Several studies found no effect of propranolol on the discriminative stimulus effects of amphetamine (Schechter and Cook, 1975; Snoddy and Tessel, 1985; Ercil and France, 2003), methamphetamine (Munzar and Goldberg, 1999), or cocaine (Spealman, 1995). However, propranolol and cocaine partially substituted for each other (Colpaert et al., 1979; Young and Glennon, 2009). Furthermore, in a discrimination test between 2.5 and 10 mg/kg cocaine, propranolol, tertatolol, and the β2AR antagonist ICI-118,551, but not the peripherally limited βAR antagonist nadolol nor the β1AR antagonist betaxolol, enhanced the ability of the low dose of cocaine to engender responding on the 10 mg/kg cocaine-associated lever (Kleven and Koek, 1997). When pretreated with prazosin, the enhancing effect of propranolol was blocked (Kleven and Koek, 1998; Young and Glennon, 2009). These results suggest a role for central β2ARs in cocaine’s discriminative stimulus effects, particularly when low doses of cocaine are used, that is modulated by α1AR activity.
In summary, the interoceptive effects of stimulants are, at best, modestly susceptible to alteration by AR agonists or antagonists. Specifically, the clearest evidence supports the ability of βAR antagonists to influence the discriminative stimulus effects of psychostimulants in an α1AR-dependent fashion.
Self-Administration
The gold standard for assessing the reinforcing properties of a drug is the operant self-administration paradigm, in which an animal performs a behavior reinforced by the delivery (intravenous, oral, etc.) of a drug that is usually paired with a sensory cue (e.g., light or tone). Psychostimulants and other drugs that are abused by humans are readily self-administered by animals and produce behavioral patterns that are reminiscent of aspects of human addiction. First, in the “acquisition” phase, the animal learns the operant task (e.g., lever press, nose poke, etc.) that results in reinforcer presentation, and the “maintenance” phase commences once the behavioral rates and drug intake stabilize. Maintenance responding is thought to model ongoing drug taking in humans, and alterations in this phase have been used to determine the neurobiological basis of addiction and to test potential interventions. A variety of schedules can be employed during the maintenance phase to address specific aspects of reinforcer efficacy. For example, a fixed ratio (FR) schedule, in which the completion of a set number of responses (e.g., every response in an FR1, every 5th response in an FR5, etc.) delivers a reinforcer, is a simple schedule frequently used to determine whether an animal will self-administer a compound. By comparison, a progressive ratio schedule, in which the response requirement increases exponentially during the course of the session until a “breakpoint” is reached when the subject stops responding to earn reinforcers, determines the relative reinforcing efficacy of the drug (Richardson and Roberts, 1996). More recently, interest has piqued in two subsequent phases, “extinction” and “reinstatement.” During extinction, the drug is replaced with a nonreinforcing vehicle (e.g., saline, water). The animal learns that the operant task no longer precipitates reward presentation, and the conditioned behavior declines to low levels. Once the behavior is extinguished, administration of a drug prime, restoration of cues previously associated with the drug, or stress (e.g., mild electric foot shock or pharmacological stressor like yohimbine) can “reinstate” the operant behavior to rates comparable to maintenance levels even though the operant behavior is not reinforced by drug presentation. Thus, reinstatement represents drug seeking and is thought to model relapse behaviors of human addicts. Because many addicts try repeatedly to quit but have difficulty staying drug-free, this phase has become a prime target for recent medication development efforts.
Maintenance.
Most noradrenergic compounds have not shown reinforcing properties on their own (e.g., Risner and Jones, 1976), although some, such as βAR agonists/antagonists, have not been tested. The one exception is the α2AR agonist clonidine, which was self-administered by rats (Davis and Smith, 1977; Shearman et al., 1981), macaques (Woolverton et al., 1982), and baboons (Weerts and Griffiths, 1999). Interestingly, rats self-administered clonidine even at doses that resulted in toxicity and occasionally death (Davis and Smith, 1977). However, methadone-dependent patients did not self-administer clonidine (Preston et al., 1985). Therefore, it appears that, at least in this clinical setting, the abuse liability of noradrenergic compounds is inconsequential.
With regards to the primary reinforcing effects of stimulants, AR activation or blockade is largely ineffective in altering the maintenance phase of intravenous self-administration, yet subtle effects were reported for α1ARs and βARs in oral consumption. In monkeys, the αAR antagonists phentolamine, phenoxybenzamine, and prazosin failed to alter cocaine self-administration across various schedules of reinforcement (Wilson and Schuster, 1974; Woolverton, 1987; Howell and Byrd, 1991). A similar lack of effect was observed in dogs self-administering amphetamine (Risner and Jones, 1976) or cocaine (Risner and Jones, 1980). Infusion of prazosin directly into the PFC or ventral tegmental area likewise did not alter cocaine intake in rats (Ecke et al., 2012). Given the technical difficulties of intravenous self-administration in mice, the importance of ARs has not been tested in this species, although genetic ablation of the α1bAR reduced cocaine consumption in an oral self-administration paradigm (Drouin et al., 2002). α2AR agonists had no effect on amphetamine (Yokel and Wise, 1978), heroin/cocaine “speedball” (Highfield et al., 2001), or cocaine (Wee et al., 2008) intravenous self-administration. βAR antagonists also failed to impact long-access (6 hour/session) cocaine responding (Wee et al., 2008) but have been reported to decrease cocaine self-administration in 3-hour sessions with rats (Harris et al., 1996) and in 100-minute sessions in squirrel monkeys (Goldberg and Gonzalez, 1976). However, food-maintained responding also decreased after propranolol administration in rats, suggesting a nonspecific suppression of operant behavior (Harris et al., 1996). Combined, these results indicate that stimulants maintain their reinforcing effects through mechanisms other than ARs. With the abundance of research identifying DA as the neurotransmitter mediating the primary reinforcing effects of stimulants, these data are not surprising.
Oral self-administration of amphetamine is vastly different than intravenous self-administration, because rats develop an aversion to oral amphetamine and consume mostly water in a two bottle choice procedure. Propranolol, but not haloperidol, increased the intake of amphetamine, indicating that the aversive effects of oral amphetamine are mediated by βAR signaling but not DA (Kongyingyoes et al., 1988). Additional support for a role of βARs in the negative effects of stimulants was observed using a runway model of self-administration in which rats must walk down an alley to a goal box to receive intravenous cocaine infusions. In this model, approach and retreat behaviors indicate the reinforcing and aversive effects, respectively, of cocaine. Combined administration of betaxolol and ICI-118,551 infused in the central amygdala or BNST decreased retreat behaviors (Wenzel et al., 2014). Therefore, it appears that the influence of NE on stimulant self-administration depends on the sensitivity of the operant task to the NE-dependent, aversive effects of the drug. Perhaps, this NE-dependent increase in the aversive effects of stimulants can be advantageous if used as a strategy to develop noradrenergic therapies that would counteract the euphoria experienced by drug abusers.
An important contribution of α1AR signaling emerged during various paradigms of escalated stimulant self-administration. For example, rats that underwent a cocaine pre-exposure regimen showed escalated cocaine self-administration that was abolished when prazosin was coadministered with cocaine during the sensitization phase (Zhang and Kosten, 2007). Furthermore, prazosin decreased breakpoint on a progressive ratio schedule of cocaine self-administration in rats under 6-hour long‐access conditions that typically showed escalated drug intake (Wee et al., 2008). α1AR abundance was decreased in the BNST by high levels of cocaine exposure (Wee et al., 2008), which the authors speculated occurred as a compensatory response to inflated NE overflow during prolonged self-administration and produced antagonist sensitivity. However, the behavioral consequences of intra-BNST prazosin infusions remain to be tested in this paradigm.
Thus, it appears that chronic drug exposure recruits α1AR-dependent pathways that are necessary for escalated drug taking in experimental animals. Because these escalation procedures are believed to more closely resemble addiction and binge drug taking in humans compared with short-access maintenance schedules, these effects of α1AR signaling may have clinical relevance. Only two human studies have examined the clinical utility of α1ARs in stimulant dependence, and the findings parallel the escalated intake effects in the rodent literature. For example, the α1AR antagonist doxazosin decreased self-report of “high,” “stimulating,” and “like cocaine” after cocaine administration (20 mg/kg i.v.; Newton et al., 2012) and increased cocaine-negative urines in treatment-seeking cocaine-dependent people compared with placebo under some dosing regimens (Shorter et al., 2013). These results indicate a need for further investigation of the promising therapeutic capabilities of α1AR antagonists.
Extinction.
Only a few studies have examined the role of NE in the extinction of stimulant self-administration. Stemming from evidence that extinction of drug self-administration and fear conditioning requires activity in the infralimbic cortex, one study dissected the contribution of NE to the extinction of cocaine self-administration via microinfusions of GABAergic, glutamatergic, and noradrenergic compounds (LaLumiere et al., 2010). Silencing activity in the region with GABA agonists impaired extinction learning. Intrainfralimbic administration of the β2AR agonist clenbuterol immediately after extinction sessions enhanced the retention of extinction learning, whereas ICI-188,551 infused immediately prior to extinction sessions impaired extinction. In another study, repeated exposure to yohimbine during the first few extinction sessions slowed the rate of extinction (Kupferschmidt et al., 2009). Clearly, further studies examining other AR subtypes are needed.
Reinstatement.
The most profound effects of AR signaling manipulations occur during the reinstatement phase of stimulant self-administration, which is thought to reflect relapse-like drug-seeking behavior in humans. Stress, cues previously associated with the drug, or the noncontingent administration of the drug itself can trigger drug-seeking behaviors. Interestingly, NE alone has been shown to reinstate cocaine seeking when administered intracerebroventricularly (Brown et al., 2009). The authors attributed this phenomenon to a stress effect because it was associated with activation of neurons in the BNST and central amygdala that are part of the brain’s stress pathway (Brown et al., 2011).
Yohimbine, an α2AR antagonist that increases NE release by blocking the primary noradrenergic inhibitory autoreceptor, is an anxiogenic drug that reinstates psychostimulant-seeking behavior in rats and monkeys (Lee et al., 2004; Shepard et al., 2004; Schroeder et al., 2013). However, it is unclear whether the effects of yohimbine occur through the noradrenergic system at all. In monkeys, the reinstating effects of yohimbine were blocked by clonidine and replicated with a selective α2AR antagonist (Lee et al., 2004), implicating the α2AR. Conversely, clonidine had no effect on yohimbine-primed reinstatement in rats (Brown et al., 2009), suggesting a contribution of nonadrenergic receptors, although the preferential α2aAR-selective agonist guanfacine did reduce yohimbine-primed reinstatement in this species (Buffalari et al., 2012).
NE signaling has a critical role in stress-induced, cue-induced, and drug-primed reinstatement in rats, but through distinct combinations of receptor subtypes. Either systemic administration of α2AR agonist (clonidine, lofexidine, or guanabenz) or a cocktail of β1 and β2AR antagonists infused directly into the BNST or central amygdala prevented foot shock stress induced reinstatement of cocaine seeking (Erb et al., 2000; Leri et al., 2002), clonidine prevented reinstatement induced by κ-opioid pharmacologic stressors (Valdez et al., 2007), prazosin attenuated cocaine-primed reinstatement (Zhang and Kosten, 2005), and a combination of prazosin and propranolol modestly reduced cue-induced reinstatement (Smith and Aston-Jones, 2011). Clonidine also blocked stress-induced, but not cue-induced reinstatement of cocaine + heroin “speedball” in rats (Highfield et al., 2001). Thus, βARs mediate the effects of NE on stress-induced reinstatement, α1ARs are required for cocaine-primed reinstatement, and α1ARs and βARs contribute to cue induced-reinstatement but via redundant pathways. α2AR agonists appear to diminish multiple forms of reinstatement by decreasing overall NE release via inhibitory autoreceptor activation.
A somewhat different picture emerges for primates. For example, adrenergic compounds had no effect on cocaine-primed reinstatement in squirrel monkeys (Platt et al., 2007). However, reinstatement paradigms differ between rats and nonhuman primates in important ways that may affect interpretation of these results. For instance, nonhuman primate self-administration typically involves a second-order schedule of reinforcement in which cues associated with cocaine serve as conditioned reinforcers. During “cocaine-primed reinstatement,” a combination of drug-prime and drug-associated cues drives cocaine-seeking behavior. Because prazosin, on its own, had no effect on cue-induced reinstatement in rodents, the lack of effect in the monkey cocaine + cue reinstatement paradigm is not entirely surprising. The only human studies germane to this subject found that clonidine and guanfacine decreased stress- or cue-induced craving for cocaine (Jobes et al., 2011; Fox et al., 2012).
Adrenergic Receptor Signaling Cascades and Functional Neuroanatomy: Where’s the Beef?
In summary, although all ARs appear to impact stimulant responses, the relative contributions of α1, α2, and βAR subtypes differ depending on the behavioral paradigm employed (Fig. 1). As discussed in the introduction, ARs are G protein–coupled receptors that signal through diverse downstream effector proteins to alter neurotransmission, cell excitability, and gene transcription. We anticipated devoting a section of this review to the roles of these AR signaling cascades but found that the cupboard is bare. Despite the profound effects of adrenergic compounds on many aspects of psychostimulant responses, a detailed knowledge of AR signaling molecules, and an armament of pharmacological and genetic tools to manipulate these cascades, a vast vacuum exists downstream of ARs within the context of psychostimulant-induced behaviors. There is only one published study that has even attempted to address this issue, which we discuss in other parts of this review (Rudoy et al., 2009). Knowledge of the functional neuroanatomy underlying the role of ARs in stimulant responses is also lacking for many paradigms. With the resurgence of interest in the contribution of NE to stimulant addiction, including recent and ongoing clinical trials investigating the efficacy of compounds that alter NE signaling, the intracellular mechanisms and neuroanatomical substrates by which adrenergic drugs could be acting must be elucidated. These studies would have important implications for the development of targeted therapeutics to treat stimulant, and potentially other, addictions.
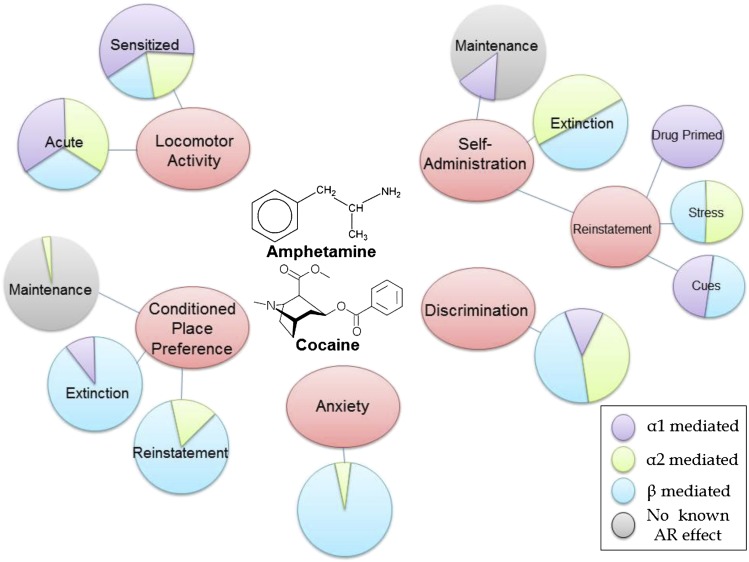
Fig. 1.
Relative contribution of adrenergic receptor subtypes to stimulant-induced behaviors. Stimulants, such as amphetamines and cocaine, produce behaviors including locomotor activity, conditioned place preference, drug-induced anxiety, drug discrimination, and self-administration. Various aspects of these behaviors are subject to control by AR signaling. Based on the available literature, it appears that each AR subtype exerts different relative effects on various stimulant-induced behaviors. Shown is a qualitative representation of the relative influences of α1 (lilac), α2 (lime), and β (cyan) AR subtypes to each stimulant-induced behavior, with greater influence represented by colored shading of a larger fraction of the pie graph. Gray shading indicates no known effect, which includes negative results as well as instances where the contribution of the receptor has never been tested.
Abbreviations
- AR
adrenergic receptor
- BLA
basolateral amygdala
- BMY 7378
8-(2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl)-8-azaspiro[4.5]decane-7,9-dione
- BNST
bed nucleus of the stria terminalis
- BRL-44408
2-[(4,5-dihydro-1H-imidazol-2-yl)methyl]-2,3-dihydro-1-methyl-1H-isoindole
- CPP
conditioned place preference
- DA
dopamine
- FR
fixed ratio
- ICI-118,551
3-(isopropylamino)-1-[(7-methyl-4-indanyl)oxy]butan-2-ol
- NE
norepinephrine
- PFC
prefrontal cortex
- PKA
protein kinase A
- SDZ NVI 085
(−)-(4aR, 10aR)-3,4,4a,5,10,10a-hexahydro-6-methoxy-4-methyl-9-methylthio-2H-naphth[2,3-b]-1,4-oxazine hydrogen malonate
- SR58611A
ethyl [[(7S)-7-[[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl]amino]-5,6,7,8-tetrahydronaphthalen-2-yl]oxy]acetate
- SR59230A
(2S)-1-(2-ethylphenoxy)-3-[[(1S)-1,2,3,4-tetrahydronaphthalenyl]amino]propan-2-ol
- ST 587
2-(2-chloro-5-trifluoromethylphenylimino)imida-zolidine
- UK 14304
5-bromo-6-(2-imidazolin-2-yl)-6-quinoxaline, 5-bromo-N-(4,5-dihydro-1H-imidazol-2-yl)-6-quinoxalinamine
- WB-4101
2-(2,6-dimethoxyphenoxyethyl)aminomethyl-1,4-benzodioxane hydrochloride
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Schmidt, Weinshenker.
Footnotes
This work was supported by the National Institutes of Health National Institute of Drug Abuse [Grants R01-DA027535, R03-DA034867, R01-DA017963]; and the National Institutes of Health National Institute of Mental Health [Grant 5T32-MH087977-05 to K.T.S.].
References
- Aghajanian GK, VanderMaelen CP. (1982) alpha 2-adrenoceptor-mediated hyperpolarization of locus coeruleus neurons: intracellular studies in vivo. Science 215:1394–1396 [DOI] [PubMed] [Google Scholar]
- Aguilar MA, Rodríguez-Arias M, Miñarro J. (2009) Neurobiological mechanisms of the reinstatement of drug-conditioned place preference. Brain Res Brain Res Rev 59:253–277 [DOI] [PubMed] [Google Scholar]
- Al-Hasani R, McCall JG, Foshage AM, Bruchas MR. (2013) Locus coeruleus kappa-opioid receptors modulate reinstatement of cocaine place preference through a noradrenergic mechanism. Neuropsychopharmacology 38:2484–2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsene KM, Fallace K, Bakshi VP. (2010) Ventral striatal noradrenergic mechanisms contribute to sensorimotor gating deficits induced by amphetamine. Neuropsychopharmacology 35:2346–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asin KE, Wirtshafter D. (1985) Clonidine produces a conditioned place preference in rats. Psychopharmacology (Berl) 85:383–385 [DOI] [PubMed] [Google Scholar]
- Auclair A, Cotecchia S, Glowinski J, Tassin JP. (2002) D-amphetamine fails to increase extracellular dopamine levels in mice lacking alpha 1b-adrenergic receptors: relationship between functional and nonfunctional dopamine release. J Neurosci 22:9150–9154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auclair A, Drouin C, Cotecchia S, Glowinski J, Tassin JP. (2004) 5-HT2A and alpha1b-adrenergic receptors entirely mediate dopamine release, locomotor response and behavioural sensitization to opiates and psychostimulants. Eur J Neurosci 20:3073–3084 [DOI] [PubMed] [Google Scholar]
- Baillie GS, Sood A, McPhee I, Gall I, Perry SJ, Lefkowitz RJ, Houslay MD. (2003) beta-Arrestin-mediated PDE4 cAMP phosphodiesterase recruitment regulates beta-adrenoceptor switching from Gs to Gi. Proc Natl Acad Sci USA 100:940–945 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Beaulieu J-M, Gainetdinov RR, Caron MG. (2009) Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol 49:327–347 [DOI] [PubMed] [Google Scholar]
- Bernardi RE, Lattal KM. (2010) A role for alpha-adrenergic receptors in extinction of conditioned fear and cocaine conditioned place preference. Behav Neurosci 124:204–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi RE, Lattal KM. (2012a) Post-conditioning propranolol disrupts cocaine sensitization. Pharmacol Biochem Behav 102:515–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi RE, Lattal KM. (2012b) Prazosin differentially affects extinction of cocaine conditioned place preference on the basis of dose and initial preference. Neuroreport 23:1048–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi RE, Lattal KM, Berger SP. (2006) Postretrieval propranolol disrupts a cocaine conditioned place preference. Neuroreport 17:1443–1447 [DOI] [PubMed] [Google Scholar]
- Bernardi RE, Ryabinin AE, Berger SP, Lattal KM. (2009) Post-retrieval disruption of a cocaine conditioned place preference by systemic and intrabasolateral amygdala beta2- and alpha1-adrenergic antagonists. Learn Mem 16:777–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilski AJ, Halliday SE, Fitzgerald JD, Wale JL. (1983) The pharmacology of a beta 2-selective adrenoceptor antagonist (ICI 118,551). J Cardiovasc Pharmacol 5:430–437 [DOI] [PubMed] [Google Scholar]
- Blanc G, Trovero F, Vezina P, Hervé D, Godeheu AM, Glowinski J, Tassin JP. (1994) Blockade of prefronto-cortical alpha 1-adrenergic receptors prevents locomotor hyperactivity induced by subcortical D-amphetamine injection. Eur J Neurosci 6:293–298 [DOI] [PubMed] [Google Scholar]
- Boer R, Grassegger A, Schudt C, Glossmann H. (1989) (+)-Niguldipine binds with very high-affinity to CA-2+ channels and to a subtype of alpha1-1-adrenoceptors. Eur J Pharmacol 172:131–145 [DOI] [PubMed] [Google Scholar]
- Brown ZJ, Nobrega JN, Erb S. (2011) Central injections of noradrenaline induce reinstatement of cocaine seeking and increase c-fos mRNA expression in the extended amygdala. Behav Brain Res 217:472–476 [DOI] [PubMed] [Google Scholar]
- Brown ZJ, Tribe E, D’souza NA, Erb S. (2009) Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 203:121–130 [DOI] [PubMed] [Google Scholar]
- Buffalari DM, Baldwin CK, See RE. (2012) Treatment of cocaine withdrawal anxiety with guanfacine: relationships to cocaine intake and reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 223:179–190 [DOI] [PubMed] [Google Scholar]
- Carter AJ. (1997) Hippocampal noradrenaline release in awake, freely moving rats is regulated by alpha-2 adrenoceptors but not by adenosine receptors. J Pharmacol Exp Ther 281:648–654 [PubMed] [Google Scholar]
- Cervo L, Rossi C, Samanin R. (1993) Clonidine-induced place preference is mediated by alpha 2-adrenoceptors outside the locus coeruleus. Eur J Pharmacol 238:201–207 [DOI] [PubMed] [Google Scholar]
- Clifford PS, Davis KW, Elliott AE, Wellman PJ. (2007) Effects of ICV administration of the alpha1A-adrenoceptor antagonist 5-methylurapidil on concurrent measures of eating and locomotion after cocaine in the rat. Life Sci 81:1059–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpaert FC, Niemegeers CJE, Janssen PAJ. (1976) Cocaine cue in rats as it relates to subjective drug effects: a preliminary report. Eur J Pharmacol 40:195–199 [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Niemegeers CJE, Janssen PAJ. (1979) Discriminative stimulus properties of cocaine: neuropharmacological characteristics as derived from stimulus generalization experiments. Pharmacol Biochem Behav 10:535–546 [DOI] [PubMed] [Google Scholar]
- Colussi-Mas J, Panayi F, Scarna H, Renaud B, Bérod A, Lambás-Señas L. (2005) Blockade of beta-adrenergic receptors prevents amphetamine-induced behavioural sensitization in rats: a putative role of the bed nucleus of the stria terminalis. Int J Neuropsychopharmacol 8:569–581 [DOI] [PubMed] [Google Scholar]
- Conrad KL, Davis AR, Silberman Y, Sheffler DJ, Shields AD, Saleh SA, Sen N, Matthies HJG, Javitch JA, Lindsley CW, et al. (2012) Yohimbine depresses excitatory transmission in BNST and impairs extinction of cocaine place preference through orexin-dependent, norepinephrine-independent processes. Neuropsychopharmacology 37:2253–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consoli D, Leggio GM, Mazzola C, Micale V, Drago F. (2007) Behavioral effects of the beta3 adrenoceptor agonist SR58611A: is it the putative prototype of a new class of antidepressant/anxiolytic drugs? Eur J Pharmacol 573:139–147 [DOI] [PubMed] [Google Scholar]
- Daaka Y, Luttrell LM, Lefkowitz RJ. (1997) Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature 390:88–91 [DOI] [PubMed] [Google Scholar]
- Darracq L, Blanc G, Glowinski J, Tassin JP. (1998) Importance of the noradrenaline-dopamine coupling in the locomotor activating effects of D-amphetamine. J Neurosci 18:2729–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AR, Shields AD, Brigman JL, Norcross M, McElligott ZA, Holmes A, Winder DG. (2008) Yohimbine impairs extinction of cocaine-conditioned place preference in an alpha2-adrenergic receptor independent process. Learn Mem 15:667–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis WM, Smith SG. (1977) Catecholaminergic mechanisms of reinforcement: direct assessment by drug-self-administration. Life Sci 20:483–492 [DOI] [PubMed] [Google Scholar]
- Dickinson SL, Gadie B, Tulloch IF. (1988) Alpha 1- and alpha 2-adrenoreceptor antagonists differentially influence locomotor and stereotyped behaviour induced by d-amphetamine and apomorphine in the rat. Psychopharmacology (Berl) 96:521–527 [DOI] [PubMed] [Google Scholar]
- D’Mello GD. (1982) Comparison of the discriminative stimulus properties of clonidine and amphetamine in rats. Neuropharmacology 21:763–769 [DOI] [PubMed] [Google Scholar]
- Doucet EL, Bobadilla A-C, Houades V, Lanteri C, Godeheu G, Lanfumey L, Sara SJ, Tassin J-P. (2013) Sustained impairment of α2A-adrenergic autoreceptor signaling mediates neurochemical and behavioral sensitization to amphetamine. Biol Psychiatry 74:90–98 [DOI] [PubMed] [Google Scholar]
- Drouin C, Darracq L, Trovero F, Blanc G, Glowinski J, Cotecchia S, Tassin JP. (2002) Alpha1b-adrenergic receptors control locomotor and rewarding effects of psychostimulants and opiates. J Neurosci 22:2873–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecke LE, Elmer GI, Suto N. (2012) Cocaine self-administration is not dependent upon mesocortical α1 noradrenergic signaling. Neuroreport 23:325–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. (2000) Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology 23:138–150 [DOI] [PubMed] [Google Scholar]
- Ercil NE, France CP. (2003) Amphetamine-like discriminative stimulus effects of ephedrine and its stereoisomers in pigeons. Exp Clin Psychopharmacol 11:3–8 [DOI] [PubMed] [Google Scholar]
- Feuerstein TJ, Hertting G, Jackisch R. (1985) Endogenous noradrenaline as modulator of hippocampal serotonin (5-HT)-release. Dual effects of yohimbine, rauwolscine and corynanthine as alpha-adrenoceptor antagonists and 5-HT-receptor agonists. Naunyn Schmiedebergs Arch Pharmacol 329:216–221 [DOI] [PubMed] [Google Scholar]
- File SE. (1986) Aversive and appetitive properties of anxiogenic and anxiolytic agents. Behav Brain Res 21:189–194 [DOI] [PubMed] [Google Scholar]
- Fox HC, Seo D, Tuit K, Hansen J, Kimmerling A, Morgan PT, Sinha R. (2012) Guanfacine effects on stress, drug craving and prefrontal activation in cocaine dependent individuals: preliminary findings. J Psychopharmacol 26:958–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricks-Gleason AN, Marshall JF. (2008) Post-retrieval beta-adrenergic receptor blockade: effects on extinction and reconsolidation of cocaine-cue memories. Learn Mem 15:643–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaval-Cruz M, Weinshenker D. (2009) Mechanisms of disulfiram-induced cocaine abstinence: antabuse and cocaine relapse. Mol Interv 9:175–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobert A, Rivet JM, Audinot V, Newman-Tancredi A, Cistarelli L, Millan MJ. (1998) Simultaneous quantification of serotonin, dopamine and noradrenaline levels in single frontal cortex dialysates of freely-moving rats reveals a complex pattern of reciprocal auto- and heteroreceptor-mediated control of release. Neuroscience 84:413–429 [DOI] [PubMed] [Google Scholar]
- Goetz AS, King HK, Ward SDC, True TA, Rimele TJ, Saussy DL., Jr (1995) BMY 7378 is a selective antagonist of the D subtype of alpha 1-adrenoceptors. Eur J Pharmacol 272:R5–R6 [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Gonzalez FA. (1976) Effects of propranolol on behavior maintained under fixed-ratio schedules of cocaine injection or food presentation in squirrel monkeys. J Pharmacol Exp Ther 198:626–634 [PubMed] [Google Scholar]
- Hanft G, Gross G. (1989) Subclassification of alpha 1-adrenoceptor recognition sites by urapidil derivatives and other selective antagonists. Br J Pharmacol 97:691–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile CN, De La Garza R, 2nd, Mahoney JJ, 3rd, Nielsen DA, Kosten TR, Newton TF. (2012) The impact of disulfiram treatment on the reinforcing effects of cocaine: a randomized clinical trial. PLoS ONE 7:e47702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS, Sora I, Drgonova J, Li XF, Goeb M, Uhl GR. (2004) Molecular mechanisms underlying the rewarding effects of cocaine. Ann N Y Acad Sci 1025:47–56 [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. (1993) Beta-adrenergic antagonists attenuate withdrawal anxiety in cocaine- and morphine-dependent rats. Psychopharmacology (Berl) 113:131–136 [DOI] [PubMed] [Google Scholar]
- Harris GC, Hedaya MA, Pan WJ, Kalivas P. (1996) beta-adrenergic antagonism alters the behavioral and neurochemical responses to cocaine. Neuropsychopharmacology 14:195–204 [DOI] [PubMed] [Google Scholar]
- Highfield D, Yap J, Grimm JW, Shalev U, Shaham Y. (2001) Repeated lofexidine treatment attenuates stress-induced, but not drug cues-induced reinstatement of a heroin-cocaine mixture (speedball) seeking in rats. Neuropsychopharmacology 25:320–331 [DOI] [PubMed] [Google Scholar]
- Howell LL, Byrd LD. (1991) Characterization of the effects of cocaine and GBR 12909, a dopamine uptake inhibitor, on behavior in the squirrel monkey. J Pharmacol Exp Ther 258:178–185 [PubMed] [Google Scholar]
- Jimenez-Rivera CA, Feliu-Mojer M, Vazquez-Torres R. (2006) Alpha-noradrenergic receptors modulate the development and expression of cocaine sensitization. Ann N Y Acad Sci 1074:390–402 [DOI] [PubMed] [Google Scholar]
- Jobes ML, Ghitza UE, Epstein DH, Phillips KA, Heishman SJ, Preston KL. (2011) Clonidine blocks stress-induced craving in cocaine users. Psychopharmacology (Berl) 218:83–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE, Barrett JE. (1993) The discriminative stimulus effects of cocaine in pigeons. J Pharmacol Exp Ther 267:1–8 [PubMed] [Google Scholar]
- Juhila J, Honkanen A, Sallinen J, Haapalinna A, Korpi ER, Scheinin M. (2005) alpha(2A)-Adrenoceptors regulate d-amphetamine-induced hyperactivity and behavioural sensitization in mice. Eur J Pharmacol 517:74–83 [DOI] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, Mulvaney F, Alterman AI, Cornish J, Gariti P, Cnaan A, Poole S, Muller E, Acosta T, et al. (2001) Effectiveness of propranolol for cocaine dependence treatment may depend on cocaine withdrawal symptom severity. Drug Alcohol Depend 63:69–78 [DOI] [PubMed] [Google Scholar]
- Kleven MS, Koek W. (1997) Discriminative stimulus properties of cocaine: enhancement by beta-adrenergic receptor antagonists. Psychopharmacology (Berl) 131:307–312 [DOI] [PubMed] [Google Scholar]
- Kleven MS, Koek W. (1998) Discriminative stimulus properties of cocaine: enhancement by monoamine reuptake blockers. J Pharmacol Exp Ther 284:1015–1025 [PubMed] [Google Scholar]
- Kongyingyoes B, Jänicke B, Coper H. (1988) The influence of brain catecholamines on ‘drug taking behaviour’ relative to oral self-administration of d-amphetamine by rats. Drug Alcohol Depend 22:223–233 [DOI] [PubMed] [Google Scholar]
- Kupferschmidt DA, Tribe E, Erb S. (2009) Effects of repeated yohimbine on the extinction and reinstatement of cocaine seeking. Pharmacol Biochem Behav 91:473–480 [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Niehoff KE, Kalivas PW. (2010) The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn Mem 17:168–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. (2004) Pharmacological blockade of alpha2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology 29:686–693 [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. (2002) Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci 22:5713–5718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Smrcka AV. (2011) Understanding molecular recognition by G protein βγ subunits on the path to pharmacological targeting. Mol Pharmacol 80:551–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Weyer A, Vranjkovic O, Beyer CE, Baker DA, Caretta H. (2010) Involvement of noradrenergic neurotransmission in the stress- but not cocaine-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: role for β-2 adrenergic receptors. Neuropsychopharmacology 35:2165–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani JJ, Levin FR. (2012) Psychostimulant treatment of cocaine dependence. Psychiatr Clin North Am 35:425–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, White FJ. (2000) Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons. J Neurosci 20:8876–8885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna ML, Ho BT. (1980) The role of dopamine in the discriminative stimulus properties of cocaine. Neuropharmacology 19:297–303 [DOI] [PubMed] [Google Scholar]
- Mefford IN. (1988) Epinephrine in mammalian brain. Prog Neuropsychopharmacol Biol Psychiatry 12:365–388 [DOI] [PubMed] [Google Scholar]
- Meitzen J, Luoma JI, Stern CM, Mermelstein PG. (2011) β1-Adrenergic receptors activate two distinct signaling pathways in striatal neurons. J Neurochem 116:984–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Newman-Tancredi A, Audinot V, Cussac D, Lejeune F, Nicolas JP, Cogé F, Galizzi JP, Boutin JA, Rivet JM, et al. (2000) Agonist and antagonist actions of yohimbine as compared to fluparoxan at alpha(2)-adrenergic receptors (AR)s, serotonin (5-HT)(1A), 5-HT(1B), 5-HT(1D) and dopamine D(2) and D(3) receptors. Significance for the modulation of frontocortical monoaminergic transmission and depressive states. Synapse 35:79–95 [DOI] [PubMed] [Google Scholar]
- Minneman KP, Theroux TL, Hollinger S, Han CD, Esbenshade TA. (1994) Selectivity of agonists for cloned alpha 1-adrenergic receptor subtypes. Mol Pharmacol 46:929–936 [PubMed] [Google Scholar]
- Mitrano DA, Schroeder JP, Smith Y, Cortright JJ, Bubula N, Vezina P, Weinshenker D. (2012) α-1 Adrenergic receptors are localized on presynaptic elements in the nucleus accumbens and regulate mesolimbic dopamine transmission. Neuropsychopharmacology 37:2161–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales L, Perez-Garcia C, Alguacil LF. (2001) Effects of yohimbine on the antinociceptive and place conditioning effects of opioid agonists in rodents. Br J Pharmacol 133:172–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, Creese I. (1986) Characterization of alpha 1-adrenergic receptor subtypes in rat brain: a reevaluation of [3H]WB4104 and [3H]prazosin binding. Mol Pharmacol 29:321–330 [PubMed] [Google Scholar]
- Mueller D, Cahill SP. (2010) Noradrenergic modulation of extinction learning and exposure therapy. Behav Brain Res 208:1–11 [DOI] [PubMed] [Google Scholar]
- Munzar P, Goldberg SR. (1999) Noradrenergic modulation of the discriminative-stimulus effects of methamphetamine in rats. Psychopharmacology (Berl) 143:293–301 [DOI] [PubMed] [Google Scholar]
- Newton TF, De La Garza R, 2nd, Brown G, Kosten TR, Mahoney JJ, 3rd, Haile CN. (2012) Noradrenergic α₁ receptor antagonist treatment attenuates positive subjective effects of cocaine in humans: a randomized trial. PLoS ONE 7:e30854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell SR, Wanstall JC. (1980) Evidence that ICI 118, 551 is a potent, highly Beta 2-selective adrenoceptor antagonist and can be used to characterize Beta-adrenoceptor populations in tissues. Life Sci 27:671–677 [DOI] [PubMed] [Google Scholar]
- Otis JM, Dashew KB, Mueller D. (2013) Neurobiological dissociation of retrieval and reconsolidation of cocaine-associated memory. J Neurosci 33:1271–1281a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis JM, Fitzgerald MK, Mueller D. (2014) Inhibition of hippocampal β-adrenergic receptors impairs retrieval but not reconsolidation of cocaine-associated memory and prevents subsequent reinstatement. Neuropsychopharmacology 39:303–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis JM, Mueller D. (2011) Inhibition of β-adrenergic receptors induces a persistent deficit in retrieval of a cocaine-associated memory providing protection against reinstatement. Neuropsychopharmacology 36:1912–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang M, Young MB, Lestini MM, Schutsky K, Thomas SA. (2012) Redundant catecholamine signaling consolidates fear memory via phospholipase C. J Neurosci 32:1932–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. (1985) Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14:149–167 [DOI] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. (2007) Noradrenergic mechanisms in cocaine-induced reinstatement of drug seeking in squirrel monkeys. J Pharmacol Exp Ther 322:894–902 [DOI] [PubMed] [Google Scholar]
- Preston KL, Bigelow GE, Liebson IA. (1985) Self-administration of clonidine, oxazepam, and hydromorphone by patients undergoing methadone detoxification. Clin Pharmacol Ther 38:219–227 [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66:1–11 [DOI] [PubMed] [Google Scholar]
- Risner ME, Jones BE. (1976) Role of noradrenergic and dopaminergic processes in amphetamine self-administration. Pharmacol Biochem Behav 5:477–482 [DOI] [PubMed] [Google Scholar]
- Risner ME, Jones BE. (1980) Intravenous self-administration of cocaine and norcocaine by dogs. Psychopharmacology (Berl) 71:83–89 [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. (2001) Incentive-sensitization and addiction. Addiction 96:103–114 [DOI] [PubMed] [Google Scholar]
- Robledo P, Balerio G, Berrendero F, Maldonado R. (2004) Study of the behavioural responses related to the potential addictive properties of MDMA in mice. Naunyn Schmiedebergs Arch Pharmacol 369:338–349 [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Platt DM, Spealman RD. (2004) Cocaine-like discriminative stimulus effects of heroin: modulation by selective monoamine transport inhibitors. J Pharmacol Exp Ther 310:342–348 [DOI] [PubMed] [Google Scholar]
- Rudoy CA, Reyes A-RS, Van Bockstaele EJ. (2009) Evidence for beta1-adrenergic receptor involvement in amygdalar corticotropin-releasing factor gene expression: implications for cocaine withdrawal. Neuropsychopharmacology 34:1135–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudoy CA, Van Bockstaele EJ. (2007) Betaxolol, a selective beta(1)-adrenergic receptor antagonist, diminishes anxiety-like behavior during early withdrawal from chronic cocaine administration in rats. Prog Neuropsychopharmacol Biol Psychiatry 31:1119–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadalge A, Coughlin L, Fu H, Wang B, Valladares O, Valentino R, Blendy JA. (2003) alpha 1d Adrenoceptor signaling is required for stimulus induced locomotor activity. Mol Psychiatry 8:664–672 [DOI] [PubMed] [Google Scholar]
- Sahraei H, Ghazzaghi H, Zarrindast MR, Ghoshooni H, Sepehri H, Haeri-Rohan A. (2004) The role of alpha-adrenoceptor mechanism(s) in morphine-induced conditioned place preference in female mice. Pharmacol Biochem Behav 78:135–141 [DOI] [PubMed] [Google Scholar]
- Sallinen J, Link RE, Haapalinna A, Viitamaa T, Kulatunga M, Sjoholm B, MacDonald E, PeltoHuikko M, Leino T, Barsh GS, Kobilka BK, Scheinin M. (1997) Genetic alteration of alpha(2C)-adrenoceptor expression in mice: influence on locomotor, hypothermic, and neurochemical effects of dexmedetomidine, a subtype-nonselective alpha(2)-adrenoceptor agonist. Mol Pharmacol 51:36–46 [DOI] [PubMed] [Google Scholar]
- Salomon L, Lanteri C, Glowinski J, Tassin JP. (2006) Behavioral sensitization to amphetamine results from an uncoupling between noradrenergic and serotonergic neurons. Proc Natl Acad Sci USA 103:7476–7481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki JE, Tatham TA, Barrett JE. (1995) The discriminative stimulus effects of methamphetamine in pigeons. Psychopharmacology (Berl) 120:303–310 [DOI] [PubMed] [Google Scholar]
- Schank JR, Liles LC, Weinshenker D. (2008) Norepinephrine signaling through beta-adrenergic receptors is critical for expression of cocaine-induced anxiety. Biol Psychiatry 63:1007–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter MD, Cook PG. (1975) Dopaminergic mediation of the interoceptive cue produced by d-amphetamine in rats. Psychopharmacology (Berl) 42:185–193 [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Alisha Epps S, Grice TW, Weinshenker D. (2013) The selective dopamine β-hydroxylase inhibitor nepicastat attenuates multiple aspects of cocaine-seeking behavior. Neuropsychopharmacology 38:1032–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutsky K, Ouyang M, Castelino CB, Zhang L, Thomas SA. (2011a) Stress and glucocorticoids impair memory retrieval via β2-adrenergic, Gi/o-coupled suppression of cAMP signaling. J Neurosci 31:14172–14181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutsky K, Ouyang M, Thomas SA. (2011b) Xamoterol impairs hippocampus-dependent emotional memory retrieval via Gi/o-coupled β2-adrenergic signaling. Learn Mem 18:598–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DD, Clark TP. (1998) Selectivity of atipamezole, yohimbine and tolazoline for alpha-2 adrenergic receptor subtypes: implications for clinical reversal of alpha-2 adrenergic receptor mediated sedation in sheep. J Vet Pharmacol Ther 21:342–347 [DOI] [PubMed] [Google Scholar]
- Shearman GT, Hynes M, Lal H. (1981) Self-administration of clonidine by the rat. Prog Clin Biol Res 71:259–276 [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. (2004) The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry 55:1082–1089 [DOI] [PubMed] [Google Scholar]
- Shorter D, Lindsay JA, Kosten TR. (2013) The alpha-1 adrenergic antagonist doxazosin for treatment of cocaine dependence: A pilot study. Drug Alcohol Depend 131:66–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DL, Mandt BH, Ng CMC, Richards TL, Yamamoto DJ, Zahniser NR, Allen RM. (2013) Low- and high-cocaine locomotor responding rats differ in reinstatement of cocaine seeking and striatal mGluR5 protein expression. Neuropharmacology 75:347–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. (2011) α(2) Adrenergic and imidazoline receptor agonists prevent cue-induced cocaine seeking. Biol Psychiatry 70:712–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoddy AM, Tessel RE. (1985) Prazosin: effect on psychomotor-stimulant cues and locomotor activity in mice. Eur J Pharmacol 116:221–228 [DOI] [PubMed] [Google Scholar]
- Spealman RD. (1995) Noradrenergic involvement in the discriminative stimulus effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther 275:53–62 [PubMed] [Google Scholar]
- Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R, Lesch KP, Murphy DL, Uhl GR. (1998) Cocaine reward models: conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc Natl Acad Sci USA 95:7699–7704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EA, Lin Y, Rosengarten H, Kramer HK, Quartermain D. (2003) Emerging evidence for a central epinephrine-innervated alpha 1-adrenergic system that regulates behavioral activation and is impaired in depression. Neuropsychopharmacology 28:1387–1399 [DOI] [PubMed] [Google Scholar]
- Stone EA, Zhang Y, Rosengarten H, Yeretsian J, Quartermain D. (1999) Brain alpha 1-adrenergic neurotransmission is necessary for behavioral activation to environmental change in mice. Neuroscience 94:1245–1252 [DOI] [PubMed] [Google Scholar]
- Tahsili-Fahadan P, Yahyavi-Firouz-Abadi N, Khoshnoodi MA, Motiei-Langroudi R, Tahaei SA, Ghahremani MH, Dehpour AR. (2006) Agmatine potentiates morphine-induced conditioned place preference in mice: modulation by alpha2-adrenoceptors. Neuropsychopharmacology 31:1722–1732 [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. (2007) Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol 12:227–462 [DOI] [PubMed] [Google Scholar]
- Valdez GR, Platt DM, Rowlett JK, Rüedi-Bettschen D, Spealman RD. (2007) Kappa agonist-induced reinstatement of cocaine seeking in squirrel monkeys: a role for opioid and stress-related mechanisms. J Pharmacol Exp Ther 323:525–533 [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Beemster P, Schoffelmeer ANM. (2003) On the role of noradrenaline in psychostimulant-induced psychomotor activity and sensitization. Psychopharmacology (Berl) 169:176–185 [DOI] [PubMed] [Google Scholar]
- Vaughn LK, Mantsch JR, Vranjkovic O, Stroh G, Lacourt M, Kreutter M, Hillard CJ. (2012) Cannabinoid receptor involvement in stress-induced cocaine reinstatement: potential interaction with noradrenergic pathways. Neuroscience 204:117–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villégier AS, Drouin C, Bizot JC, Marien M, Glowinski J, Colpaërt F, Tassin JP. (2003) Stimulation of postsynaptic alpha1b- and alpha2-adrenergic receptors amplifies dopamine-mediated locomotor activity in both rats and mice. Synapse 50:277–284 [DOI] [PubMed] [Google Scholar]
- Vranjkovic O, Hang S, Baker DA, Mantsch JR. (2012) β-adrenergic receptor mediation of stress-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: roles for β1 and β2 adrenergic receptors. J Pharmacol Exp Ther 342:541–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Mandyam CD, Lekic DM, Koob GF. (2008) Alpha 1-noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. Eur Neuropsychopharmacol 18:303–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerts EM, Griffiths RR. (1999) Evaluation of the intravenous reinforcing effects of clonidine in baboons. Drug Alcohol Depend 53:207–214 [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Miller NS, Blizinsky K, Laughlin ML, Palmiter RD. (2002) Mice with chronic norepinephrine deficiency resemble amphetamine-sensitized animals. Proc Natl Acad Sci USA 99:13873–13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D, Schroeder JP. (2007) There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology 32:1433–1451 [DOI] [PubMed] [Google Scholar]
- Wellman P, Ho D, Cepeda-Benito A, Bellinger L, Nation J. (2002) Cocaine-induced hypophagia and hyperlocomotion in rats are attenuated by prazosin. Eur J Pharmacol 455:117–126 [DOI] [PubMed] [Google Scholar]
- Wenzel JM, Cotton SW, Dominguez HM, Lane JE, Shelton K, Su ZI, Ettenberg A. (2014) Noradrenergic β-receptor antagonism within the central nucleus of the amygdala or the bed nucleus of the stria terminalis attenuates the negative/anxiogenic effects of cocaine. J Neurosci, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- West WB, Van Groll BJ, Appel JB. (1995) Stimulus effects of d-amphetamine II: DA, NE, and 5-HT mechanisms. Pharmacol Biochem Behav 51:69–76 [DOI] [PubMed] [Google Scholar]
- Wilson MC, Schuster CR. (1974) Aminergic influences on intravenous cocaine self-administration by Rhesus monkeys. Pharmacol Biochem Behav 2:563–571 [DOI] [PubMed] [Google Scholar]
- Wood DM, Lal H, Yaden S, Emmett-Oglesby MW. (1985) One-way generalization of clonidine to the discriminative stimulus produced by cocaine. Pharmacol Biochem Behav 23:529–533 [DOI] [PubMed] [Google Scholar]
- Woolverton WL. (1987) Evaluation of the role of norepinephrine in the reinforcing effects of psychomotor stimulants in rhesus monkeys. Pharmacol Biochem Behav 26:835–839 [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Wessinger WD, Balster RL. (1982) Reinforcing properties of clonidine in rhesus monkeys. Psychopharmacology (Berl) 77:17–23 [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Yoo SK, Nishita M, Kikuchi A, Minami Y. (2007) Wnt5a modulates glycogen synthase kinase 3 to induce phosphorylation of receptor tyrosine kinase Ror2. Genes Cells 12:1215–1223 [DOI] [PubMed] [Google Scholar]
- Yokel RA, Wise RA. (1978) Amphetamine- type reinforcement by dopaminergic agonists in the rat. Psychopharmacology (Berl) 58:289–296 [DOI] [PubMed] [Google Scholar]
- Young R, Glennon RA. (2009) S(-)Propranolol as a discriminative stimulus and its comparison to the stimulus effects of cocaine in rats. Psychopharmacology (Berl) 203:369–382 [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Bahreini T, Adl M. (2002) Effect of imipramine on the expression and acquisition of morphine-induced conditioned place preference in mice. Pharmacol Biochem Behav 73:941–949 [DOI] [PubMed] [Google Scholar]
- Zhang XY, Kosten TA. (2005) Prazosin, an alpha-1 adrenergic antagonist, reduces cocaine-induced reinstatement of drug-seeking. Biol Psychiatry 57:1202–1204 [DOI] [PubMed] [Google Scholar]
- Zhang XY, Kosten TA. (2007) Previous exposure to cocaine enhances cocaine self-administration in an alpha 1-adrenergic receptor dependent manner. Neuropsychopharmacology 32:638–645 [DOI] [PubMed] [Google Scholar]
- Zhong HY, Minneman KP. (1999) Differential activation of mitogen-activated protein kinase pathways in PC12 cells by closely related alpha1-adrenergic receptor subtypes. J Neurochem 72:2388–2396 [DOI] [PubMed] [Google Scholar]