Abstract
Nonalcoholic fatty liver disease is a prevalent form of chronic liver disease that can progress to the more advanced stage of nonalcoholic steatohepatitis (NASH). NASH has been shown to alter drug transporter regulation and may have implications in the development of adverse drug reactions. Several experimental rodent models have been proposed for the study of NASH, but no single model fully recapitulates all aspects of the human disease. The purpose of the current study was to determine which experimental NASH model best reflects the known alterations in human drug transporter expression to enable more accurate drug disposition predictions in NASH. Both rat and mouse NASH models were used in this investigation and include the methionine and choline deficient (MCD) diet model, atherogenic diet model, ob/ob and db/db mice, and fa/fa rats. Pathologic scoring evaluations demonstrated that MCD and atherogenic rats, as well as ob/ob and db/db mice, developed NASH. Liver mRNA and protein expression analyses of drug transporters showed that in general, efflux transporters were induced and uptake transporters were repressed in the rat MCD and the mouse ob/ob and db/db models. Lastly, concordance analyses suggest that both the mouse and rat MCD models as well as mouse ob/ob and db/db NASH models show the most similarity to human transporter mRNA and protein expression. These results suggest that the MCD rat and mouse model, as well as the ob/ob and db/db mouse models, may be useful for predicting altered disposition of drugs with similar kinetics across humans and rodents.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a complex, multifaceted disease that encompasses a spectrum of liver pathologies, including simple fatty liver (hepatic steatosis) and nonalcoholic steatohepatitis (NASH). NASH is the more pathologically advanced stage of the disease and is characterized by increased hepatocellular damage, chronic liver inflammation, and fibrosis (Ali and Cusi, 2009; Feldstein, 2010; Masuoka and Chalasani, 2013). Recently, NAFLD has quickly increased in prevalence and is now considered the most common form of chronic liver disease in Western society (Ali and Cusi, 2009). Current epidemiologic data estimate that NAFLD affects approximately 30% to 50% of the adult population, while the prevalence of NASH is predicted to be 5.7% to 17% (McCullough, 2006; Ali and Cusi, 2009; Lomonaco et al., 2013). Alarmingly, the prevalence of NAFLD can be as high as 90% in morbidly obese patients, and with the continual rise in obesity and type 2 diabetes in the general population, NAFLD prevalence rates are expected to increase to near epidemic proportions in the future (Ali and Cusi, 2009; Lomonaco et al., 2013).
The development of oxidative stress, mitochondrial dysfunction, and an increase in proinflammatory cytokine production are important features that characterize NASH pathology (McCullough, 2006). Consequently, hepatocellular damage is sustained throughout the progressive stages of NAFLD, leading to perturbations in gene regulation and liver function (Lake et al., 2011, 2013). Among the many functions of the liver, it is a central mediator in governing the detoxification and elimination of both endo- and xenobiotics via a diverse array of biotransformation and transport mechanisms (Keogh, 2012). As a result of the significant hepatocellular damage caused by oxidative stress and chronic inflammation in NASH, hepatic biotransformation and transport mechanisms are dysregulated, which can potentially alter the absorption, distribution, metabolism, and excretion (ADME) of xenobiotics and lead to altered drug exposure (Fisher et al., 2008, 2009b; Hardwick et al., 2010, 2012). It is well established that drug transporter mRNA and protein expression alterations in NASH cause perturbations in the disposition of pharmaceutical agents and environmental toxicants (Lickteig et al., 2007; Fisher et al., 2009a; Hardwick et al., 2010, 2011, 2012; Canet et al., 2012). In the clinic, these NASH-associated changes in pharmacokinetics may impact drug efficacy and/or toxicity, potentially requiring greater pharmacovigilance. Therefore, identifying experimental models that more accurately reflect the pharmacokinetic parameters of the human disease, such as transporter expression, is critical in predicting drug disposition in human NASH.
Due to ethical and practical limitations, rodent models are used to further understand and characterize the functional aberrations of xenobiotic disposition in NASH. Dietary models, where rodents are fed specialized diets, are the most common NASH models because they may accurately reproduce the clinical and/or histopathologic features of the disease (Schattenberg and Galle, 2010; Hebbard and George, 2011). These models include the methionine and choline deficient (MCD) diet as well as a modified high fat diet with supplemented cholate and cholesterol (atherogenic diet). Both of these diets are capable of recapitulating the histopathologic features of NASH; however, the MCD diet, in contrast to the atherogenic diet, fails to fully capture the metabolic disorders that frequently accompany NASH, such as insulin resistance, dyslipidemia, and type 2 diabetes (Matsuzawa et al., 2007; Larter and Yeh, 2008). In addition to dietary models, genetically obese rodents that carry deficiencies in leptin signaling, such as ob/ob and db/db mice and fa/fa rats, are also used as NASH models. Due to their inherent leptin dysregulation, these animals are hyperphagic, obese, and develop insulin resistance and therefore are often considered as better models that provide a full spectrum of the clinical morbidities that frequently accompany NAFLD (Larter and Yeh, 2008; Takahashi et al., 2012).
The purpose of this current study is to determine which of the experimental NASH models best recapitulates the mRNA and protein expression profiles of clinically relevant drug transporters altered in human NASH. The MCD and atherogenic diet were used as dietary NASH models, and the ob/ob and db/db mice and fa/fa rat were used as genetic NASH models. Clinical biomarkers of metabolic syndrome were measured, and histologic analyses were conducted to confirm NASH. Additionally, rodent mRNA and protein expression of hepatic drug transporters were measured and compared with previously published human NASH expression profiles using concordance and effect size statistical analyses.
Materials and Methods
Tris-HCl, EDTA, sodium chloride (NaCl), glycerol, potassium phosphate (KPO4), potassium chloride (KCl), sodium pyrophosphate (decahydrate), and Nonidet P-40 were obtained from Sigma-Aldrich (St. Louis, MO). Neutral buffered formalin (10%) was obtained from Fisher Scientific (Pittsburgh, PA).
Animals.
Male 8–10-week-old C57BL/6J, B6.Cg-Lep<ob>/J (ob/ob) and B6.BKS(D)-Lepr<db>/J (db/db) mice were obtained from Jackson Laboratories (Bar Harbor, ME). Male 8–10-week-old, Sprague Dawley and Crl:ZUC-Lepr<fa> fatty (fa/fa) rats were obtained from Charles River Laboratories (Wilmington, MA). All animals were acclimated in 12-hour light and dark cycles in a University of Arizona Association for Assessment and Accreditation of Laboratory Animal Care–certified animal facility for at least 1 week prior to initiation of experiments and were given access to standard chow and water ad libitum. Housing and experimental procedures were in accordance with National Institutes of Health guidelines for the care and use of experimental animals and were approved by the University of Arizona Institutional Animal Care and Use Committee. To model NASH, C57BL/6J mice and Sprague Dawley rats (n = 4–7) were fed either a methionine- and choline-deficient diet (#518810) (Dyets, Inc., Bethlehem, PA), or an atherogenic diet (#D06061401) (Research Diets Inc., New Brunswick, NJ) for 8 weeks. As a control, C57BL/6J mice (n = 4–7) and Sprague Dawley rats (n = 4–7) were fed a methionine and choline resupplemented diet (#518754) (Dyets, Inc.). The ob/ob (n = 4) and db/db (n = 4–7) mice were fed an MCD diet for 4 weeks to induce NASH. The fa/fa rats were provided a modified high fat diet (#101447) for 8 weeks (Dyets, Inc.). Animals were weighed prior to diet start to record a baseline body weight.
Tissue Harvesting.
At the conclusion of dietary feeding, the animals were weighed to record a final body weight then euthanized using CO2 asphyxiation. Terminal blood was collected via cardiac puncture and plasma was extracted by centrifugation at 9,500 × g for 5 minutes using a tabletop centrifuge (4°C). The resulting plasma was stored at −20°C until analysis. The liver was immediately harvested and weighed, and a small portion was fixed for 2 days in 10% neutral buffered formalin (4°C), followed by tissue processing and paraffin-embedding at the University of Arizona Histology Core Facility. The remaining tissue was snap frozen in liquid nitrogen and stored at −80°C for future analyses.
Plasma Chemistries.
Rodent plasma samples were submitted to the pathology laboratory at the University Animal Care facility, University of Arizona Health Science Center for determination of plasma alanine aminotransferase and glucose levels. Plasma insulin was determined using a rodent enzyme-linked immunosorbent assay (Millipore, St. Charles, MO) per the manufacturer’s protocol.
Tissue Staining and Evaluations.
Hematoxylin and eosin (H&E) stains were performed on formalin fixed, paraffin-embedded liver sections at the University of Arizona Histology Core according to the facility’s common practice. Masson’s trichrome staining was performed using the Masson Trichrome Stain Kit (Sigma Aldrich) according to the manufacture’s protocol. H&E-stained liver sections were submitted to the Arizona Health Sciences Center Animal Facility for pathologic scoring evaluations according to a previously validated NASH scoring system (Kleiner et al., 2005). All samples were evaluated blindly and excluded disease and animal information.
RNA Purification.
Total RNA was extracted and isolated from rat and mouse liver using RNAzol B reagent (Tel-Test Inc., Friendswood, TX) per the manufacturer's protocol. RNA concentrations were determined using UV spectrophotometry, and the integrity of the RNA was confirmed by ethidium bromide staining after agarose gel electrophoresis.
Branched Chain DNA Analysis.
Branched chain DNA (bDNA) analysis was used to determine mRNA transcript levels of transporter genes and has been previously shown to be a highly specific method for mRNA quantification with a high degree of accuracy and reproducibility (Lee et al., 2008; Lu et al., 2009; Hardwick et al., 2010, 2011). Specific oligonucleotide probes for multidrug resistance–associated protein (Mrp) 1–4, multidrug resistance protein (Mdr) 1a and 1b, breast cancer resistance protein (Bcrp), and organic anion transporting polypeptide (Oatp) 1a1, 1a4, 1b2, and 2b1 were diluted in lysis buffer supplied by the Quantigene HV Signal Amplification Kit (Genospectra, Fremont, CA). Substrate solution, lysis buffer, capture hybridization buffer, amplifier, and label probe buffer used in the analysis were all obtained from the Quantigene Discovery Kit (Genospectra). The assay was performed in 96-well format with 10 µg of total RNA added to the capture hybridization buffer and 50 µl of the diluted probe set. The total RNA was then allowed to hybridize to the probe set overnight at 53°C. Hybridization steps were performed per the manufacturer's protocol the following day. Luminescence of the samples was measured with a Quantiplex 320 bDNA luminometer interfaced with Quantiplex Data Management Software, version 5.02 (Bayer, Walpole, MA).
Protein Preparations.
Whole cell lysate preparations of mouse and rat liver were prepared from ∼200 mg of tissue homogenized in NP-40 buffer (20 mM Tris HCl, 137 mM NaCl, 10% glycerol, 1% Nonidet P-40, and 2 mM EDTA) with one Protease Inhibitor Cocktail Tablet (Roche, Indianapolis, IN) per 25 ml at 4°C. Homogenized tissue was then agitated at 4°C for 2 hours, centrifuged at 10,000 × g for 30 minutes, and the supernatant transferred to a clean collection tube. Liver microsomal fractions were prepared from ∼200 mg of frozen tissue. Briefly, tissue was homogenized in buffer A (50 mM Tris HCl pH 7.4, 1 mM EDTA, and 154 mM KCl) with added Protease Inhibitor Cocktail Tablet (Roche) per 25 ml at 4°C. The resulting homogenate was centrifuged at 10,000 × g for 30 minutes at 4°C and the supernatant was collected into ultracentrifuge tubes and centrifuged at 100,000 × g for 70 minutes at 4°C. The resulting pellet was resuspended in 600 µl of buffer B (100 mM sodium pyrophosphate pH 7.4 and 0.1 mM EDTA) and subjected to a second 100,000 × g centrifugation for 70 minutes at 4°C. The resulting pellet was resuspended in 100 µl of buffer C (10 mM KPO4 pH 7.4, 1 mM EDTA, and 20% glycerol). Protein concentrations for both whole cell and microsomal fractions were determined using the Pierce BCA Protein Quantitation Assay (Thermo Scientific, Rockford, IL) per the manufacturer’s protocol and stored at −80°C until further analysis.
Immunoblot Protein Analysis.
Whole cell lysate or microsomal proteins (50 μg/well) were prepared in Laemmli sample buffer (Bio-Rad Laboratories, Hercules, CA) with or without β-mercaptoethanol and heated at 37°C for 30 minutes prior to separation by SDS-PAGE on 7.5% gels. Resolved protein was transferred to polyvinylidene fluoride membranes for 70 minutes at 350 mAmps at 4°C. Following transfer, the membranes were blocked in 5% nonfat dry milk diluted in phosphate-buffered saline–Tween 20 for 1 hour at room temperature. To determine relative protein levels the following primary antibodies were used: Mrp2, sc-5770; p-glycoprotein (P-gp), sc-8313; Mrp3, sc-5775; Oatp1b2, sc-376904 (rat), and sc-47270 (mouse) (Santa Cruz Biotechnology, Santa Cruz, CA); Mrp4, ab15602 (Abcam, Cambridge, MA); Oatp1a4, OATP21-A (Alpha Diagnostics Intl., Inc., San Antonio, TX); Bcrp, MC-981 (Kamiya Biomedical Co., Seattle, WA). The blots were incubated with primary antibody overnight at 4°C with constant rocking. The following HRP-conjugated secondary antibodies were used: anti-rat (sc-2065), anti-rabbit (sc-2004), anti-goat (sc-2350), and anti-mouse (sc-2005) (Santa Cruz Biotechnology). Quantification of relative protein expression was determined using image processing and analysis with Image J software (National Institutes of Health, Bethesda, MD) and normalized to β-actin protein (whole cell lysate) (sc-47778, Santa Cruz Biotechnology) or pan-cadherin (microsomal fraction) (Ab16505, Abcam).
mRNA and Protein Concordance Analysis across Human and Rodent Models.
Concordance analyses to compare human and rodent mRNA and protein expression in NASH were performed by measuring the effect size of NASH versus control for each gene. The effect size (estimated by Glass’s Δ) is the standardized mean difference between two populations and can be calculated using the following equation:
 |
where  and
and  are the sample means for two groups, and Sp is the pooled standard deviation for both. Data from bDNA analysis were used for mRNA comparisons, whereas normalized densitometry data were used for protein comparisons. All raw human data (mRNA and protein) used in this analysis has been previously published (Hardwick et al., 2011) (Clarke et al., submitted manuscript). The analyses were performed using R version 3.0.2 (http://www.r-project.org/).
are the sample means for two groups, and Sp is the pooled standard deviation for both. Data from bDNA analysis were used for mRNA comparisons, whereas normalized densitometry data were used for protein comparisons. All raw human data (mRNA and protein) used in this analysis has been previously published (Hardwick et al., 2011) (Clarke et al., submitted manuscript). The analyses were performed using R version 3.0.2 (http://www.r-project.org/).
Statistical Analysis.
Data were analyzed using one-way analysis of variance to determine significant differences between model groups with a Tukey’s post-hoc analysis. A significance level of P ≤ 0.05 was used for all analyses. All analyses were carried out using GraphPad Prism software Version 5 (GraphPad Software, Inc., La Jolla, CA).
Results
Rodent Body Weights, Tissue Weights, and Clinical Chemistries.
To determine and confirm the clinical features that are normally associated with NASH, body and tissue weight as well as plasma chemistry profiles were measured. Body weight, tissue weight, and liver-to-body-weight ratios for rats and mice are shown in Tables 1 and 2, respectively. No significant change in body weight compared with control was observed among the rat models, although the MCD rats trended toward a decrease whereas the fa/fa and atherogenic models trended toward an increase in body weight (Table 1). In contrast, the MCD and atherogenic mice had a significant reduction in body weight, while the ob/ob mice had increased in body weight compared with control mice (Table 2).
TABLE 1.
Rat body weight, liver weight, and plasma chemistries
Body weight, weight gain, liver weight, liver-to-body-weight ratios, and plasma chemistries are shown for rat NASH models. Data represent the mean ± S.E.M. from three to seven rats. Weight gain and loss are indicated by plus (+) and minus (−) signs, respectively.
| Parameter | Control | MCD | Atherogenic | fa/fa |
|---|---|---|---|---|
| Terminal body weight (g, n = 3) | 522.7 ± 9.0 | 308.0 ± 2.7 | 594.0 ± 24.2 | 733.3 ± 10.2 |
| Weight gain (g, n = 3) | +169.3 ± 7.5 | −47.0 ± 6.3* | +270.7 ± 18.0 | +205.0 ± 6.6 |
| Liver weight (g, n = 3) | 21.2 ± 0.3 | 16.1 ± 0.3 | 43.6 ± 1.9* | 38.3 ± 0.4* |
| Liver /body weight (%, n = 3) | 4.1 ± 0.1 | 5.2 ± 0.1 | 7.3 ± 0.0* | 5.2 ± 0.1 |
| Glucose (mg/dl, n = 4) | 157.5 ± 4.7 | 112.1 ± 7.3* | 159.3 ± 10.5 | 199.8 ± 11.1* |
| Insulin (ng/ml, n = 3) | 3.7 ± 0.9 | 1.3 ± 0.6 | 9.0 ± 2.5 | 28.6 ± 1.2* |
| ALT (U/l, n = 7) | 19.1 ± 1.1 | 136.1 ± 15.0* | 51.9 ± 5.5* | 53.5 ± 4.9* |
ALT, alanine aminotransferase.
P ≤ 0.05 versus control rats.
TABLE 2.
Mouse body weight, liver weight, and plasma chemistries
Body weight, liver weight, liver-to-body-weight ratios, and plasma chemistries are shown for mouse NASH models. Data represent the mean ± S.E.M. from three to seven mice. Weight gain and loss are indicated by plus (+) and minus (−) signs, respectively.
| Parameter | Control | MCD | Atherogenic | ob/ob | db/db |
|---|---|---|---|---|---|
| Terminal body weight (g, n = 3) | 35.2 ± 0.8 | 17.5 ± 0.6* | 26.8 ± 1.2* | 45.8 ± 1.2* | 39.0 ± 1.1 |
| Weight gain (g, n = 3) | +9.5 ± 1.0 | −10.8 ± 1.7* | +1.8 ± 0.1* | −5.8 ± 1.4* | −6.7 ± 0.6* |
| Liver weight (g, n = 3) | 2.0 ± 0.09 | 0.8 ± 0.0* | 1.7 ± 0.0 | 2.8 ± 0.4 | 3.1 ± 0.1* |
| Liver/body weight (%, n = 3) | 5.8 ± 0.2 | 4.6 ± 0.1 | 6.5 ± 0.3 | 6.1 ± 0.8 | 8.0 ± 0.4* |
| Glucose (mg/dl, n =3) | 121.1 ± 56.5 | 53.1 ± 27.7 | 111.8 ± 59.3 | 182.9 ± 6.4 | 137.4 ± 19.5 |
| Insulin (ng/ml, n = 3) | 3.2 ± 0.3 | 0.5 ± 0 | 2.5 ± 1.4 | 3.8 ± 0.9 | 3.9 ± 0.4 |
| ALT (U/l, n = 7) | 46.02 ± 7.7 | 211.9 ± 15.2* | 90.14 ± 22.4 | 274.99 ± 48.4* | 343.457 ± 33.0 * |
ALT; alanine aminotransferase.
P ≤ 0.05 versus control mice.
To assess the magnitude of either weight gain or loss, change in body weight from start to finish of the study was measured. The magnitude of weight change between MCD and control rats was significantly different due to the loss in body weight in the MCD rats. The atherogenic and fa/fa rats tended to increase in weight more than controls but the magnitude of change was not statistically significant (Table 1). In contrast, the magnitude of weight change that occurred in the MCD, atherogenic, ob/ob, and db/db mice was significantly different from control mice (Table 2). Liver weight was measured and the atherogenic and fa/fa rats, as well as the db/db mice, had increased liver mass compared with controls (Tables 1 and 2). Liver-to-body-weight ratios indicate that the atherogenic rat model and db/db mice had increased liver mass in relation to body mass.
NASH is clinically associated with a variety of metabolic disorders, including hyperglycemia and diabetes. Therefore, blood glucose and insulin were measured to determine if these experimental NASH models parallel the conditions typically present in the human NASH condition. Of the rat models, only the fa/fa rats developed hyperglycemia and hyperinsulinemia compared with controls (Table 1). In contrast, the rat MCD model demonstrated significantly reduced plasma glucose levels compared with control. No significant changes were identified in plasma glucose or insulin across the mouse models, although glucose levels tended to increase in the ob/ob and db/db mice (Table 2). Alanine aminotransferase plasma levels were significantly increased in the rat MCD, atherogenic, fa/fa, as well as the mouse MCD, ob/ob, and db/db models.
NASH Histology and Pathologic Assessment in Rodent Models.
H&E stained liver sections as well as Masson’s trichrome staining from mouse and rat NASH animals are shown in Fig. 1. Macrovesicular steatosis, a common pathologic lesion that accompanies NASH, is clearly present in the livers of rat MCD as well as mouse MCD, ob/ob, and db/db models (Fig. 1,A and B, black arrowhead). To determine the extent of liver fibrosis, Masson’s trichrome staining was used on formalin-fixed, paraffin-embedded tissue samples. The results clearly show significant branching fibrosis (blue staining, arrow) in rat MCD liver (Fig. 1C). Masson’s trichrome stain in mouse livers did not reveal any fibrotic tissue (Fig. 1D).
Fig. 1.
Liver histopathology of rodent NASH models. Representative hematoxylin and eosin stained liver sections from rat (A) and mouse (B) NASH models. Macrovesicular steatotic deposits, a distinguishable lesion seen in NASH, are shown by the black arrowhead. Masson’s trichrome staining in rat (C) and mouse (D) NASH models. Branching fibrosis is indicated by the black arrow. Images were taken at 20x magnification.
To quantify the severity of NASH within each model, H&E stained samples were evaluated according to a previously validated NASH pathology scoring rubric. The total sum of the scores measured for characteristic NASH lesions yields a total NASH activity score, with scores at or above 4 being defined as NASH (Table 3). This assessment shows that rat MCD and atherogenic as well as mouse db/db models have NASH (Table 3). Interestingly, although the ob/ob model has severe pathology (macrovesicular lipid deposits and inflammation) this model fails to fully develop advanced NASH due to lower levels of inflammation and the absence of fibrosis.
TABLE 3.
Liver pathology scoring of NASH rodent models
NASH activity scores (NAS) were tabulated by summing the numerical grades of steatosis (0–3), inflammation (0–2), hepatocyte ballooning (0–2), and fibrosis (0–4) present within the liver. A total NAS score above 4 is a positive NASH diagnosis.
| Steatosis | Inflammation | Fibrosis | Ballooning | Total NAS | ||
|---|---|---|---|---|---|---|
| Rats | Control | 0 | 0.25 ± 0.06 | 0 | 0 | 0.25 ± 0.25 |
| MCD | 3 | 1 | 0.75 ± 0.06 | 0 | 4.75 ± 0.25 | |
| Atherogenic | 1.25 ± 0.06 | 1.25 ± 0.06 | 0.5 ± 0.07 | 0 | 4 ± 0.58 | |
| fa/fa | 1 | 0 | 0 | 0 | 1 | |
| Mice | Control | 1 ± 0.14 | 0 | 0 | 0 | 1 ± 0.58 |
| MCD | 2 | 1.25 ± 0.06 | 0 | 0 | 3.25 ± 0.25 | |
| Atherogenic | 2 | 0.75 ± 0.06 | 0.25 | 0 | 3 ± 0.41 | |
| ob/ob | 3 | 0.5 ± 0.07 | 0 | 0 | 3.5 ± 0.29 | |
| db/db | 3 | 1 | 0.5 ± 0.07 | 0 | 4.5 ± 0.29 |
Hepatic mRNA Expression of Drug Transporters in Experimental NASH Models.
Previous studies have shown that mRNA expression of drug transporters is altered in the liver of both human and MCD-diet-induced rodent NASH (Fisher et al., 2009a; Hardwick et al., 2010, 2011). To determine if gene expression is altered in other experimental NASH models, the mRNA of clinically relevant drug transporters was measured via bDNA analysis (Fig. 2). mRNA expression of the efflux transporters Mrp1, Mrp2, Mrp3, Mrp4, Bcrp, Mdr1a, and Mdr1b were all significantly induced in the rat MCD model, whereas only Mrp2 and Mdr1b were induced in the atherogenic rat model (Fig. 2A). Of the mouse NASH models, the efflux transporters Mrp1 (db/db), Mrp2 (MCD, atherogenic, ob/ob, and db/db), Mrp3 (ob/ob and db/db), Mrp4 (MCD, ob/ob and db/db), and Mdr1a (MCD, ob/ob, and db/db) were induced at a significant level (Fig. 2B).
Fig. 2.
Liver mRNA expression of drug transporters in rodent NASH. mRNA expression of rat efflux (A) and uptake (C) transporters as well as mouse efflux (B) and uptake (D) transporters in rodent NASH models via branched DNA gene analysis. Data represent the mean ± S.E.M. from four animals. *P ≤ 0.05 versus control within each group.
Hepatic Oatp transporter mRNA expression was measured and shown in Fig. 2, C and D. Oatp1a1 was significantly down-regulated in the fa/fa rat model as well as the mouse MCD, ob/ob, and db/db models, whereas the MCD rat and db/db mouse models had a significant down-regulation of the Oatp1b2 isoform (Fig. 2, C and D). Conversely, hepatic Oatp1a4 displayed an opposite effect and was significantly induced in the MCD and atherogenic rat models as well as the MCD and db/db mice.
Hepatic Protein Expression of Drug Transporters in Experimental NASH Models.
To verify whether the observed alterations to transporter mRNA expression translate to altered protein expression, Western blot analyses were performed to determine relative protein expression levels of select hepatic transporters across the NASH models (Fig. 3). Protein expression of Mrp2, Mrp3, Mrp4, and Pgp were all up-regulated in the MCD rat model (Fig. 3, A and B). In contrast, hepatic Oatp1a4 protein expression was down-regulated in all models, whereas Oatp1b2 protein expression was down-regulated in the MCD and atherogenic models but induced in fa/fa rats.
Fig. 3.
Liver protein expression of drug transporters in rodent NASH. Representative Western blot of rat (A) and mouse (C) transporters in rodent NASH models. Densitometry analysis of Western blot data of rat (B) and mouse (D) blots is shown. β-actin was used a loading control for whole cell lysates, whereas pan-cadherin was used for microsomal preparations. Data represent the mean ± S.E.M. from four animals. *P ≤ 0.05 versus control within each group.
Among the mouse models, Mrp3 and P-gp were significantly induced in the ob/ob mice, whereas Mrp4 was significantly induced in the MCD mouse model (Fig. 3, C and D). The ob/ob and db/db mice trended toward an induction of Mrp4 protein, but this did not reach statistical significance. Alternatively, Oatp1b2 was down-regulated in the MCD, ob/ob, and db/db models. No change in Bcrp protein expression was observed across all rodent models.
Concordance Analysis across Human and Rodent mRNA and Protein Expression in NASH.
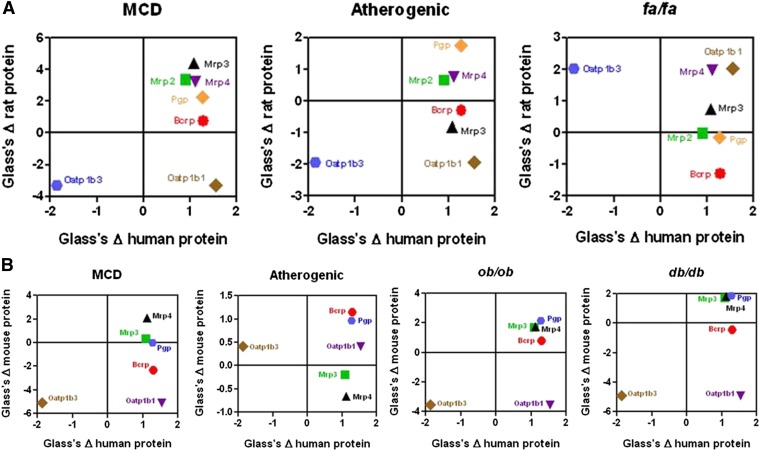
To determine how well the rodent NASH models recapitulate human NASH mRNA and protein expression changes, a concordance analysis was performed using the data derived from this study with previously published data from human NASH mRNA and protein expression data (Hardwick et al., 2011)(Clarke et al., submitted manuscript). Due to the lack of orthology in human OATP1B1 and OATP1B3 to rodents, rodent Oatp1b2 was compared with both human OATP1B1 and OATP1B3 separately. Additionally, human MDR1 (P-gp) mRNA expression was compared with rodent Mdr1a and Mdr1b orthologs separately. Figs. 4 and 5 show human mRNA and protein data as a function of rodent mRNA and protein effect sizes, respectively. Effect sizes in the same direction (positive or negative) represent similar trends in the direction of gene expression, whereas the magnitude of change corresponds to the statistical power in detecting a difference in expression in NASH versus control. The rat and mouse MCD model, as well as mouse ob/ob and db/db, have the most abundant transporter genes that share a positive effect size across both human and rodent (top right quadrant of graphs, Fig. 4, A and B). Human OATP1B3 shares a negative effect size (down-regulation), which is also present in rat and mouse MCD, along with mouse ob/ob and db/db models (lower left quadrant of graphs). In contrast, the mouse and rat atherogenic models, along with the rat fa/fa model, show opposite effect sizes compared with human mRNA expression for several transporter genes, including Mdr1a (rat atherogenic), Mrp2 (fa/fa), Mrp4 (fa/fa), and Mrp1 (mouse atherogenic) (Fig. 4, A and B).
Fig. 4.
Effect size analysis of human and rodent NASH mRNA expression of drug transporters. Effect size of human transporter mRNA expression as a function of rat (A) and mouse (B) mRNA expression in NASH. Positive effect changes reflect induction of gene expression, whereas negative effect changes reflect down-regulation of gene expression. The magnitude of the effect change (positive or negative) reflects the power of the disease (human or rodent model) to detect a change in gene expression over control. Values were calculated by the method described in the Materials and Methods section.
Fig. 5.
Effect size analysis of human and rodent NASH protein expression of drug transporters. Effect size of human transporter protein expression as a function of rat (A) and mouse (B) protein expression in NASH is shown. Positive effect changes reflect induction of protein expression, whereas negative effect changes reflect repression in protein expression. The magnitude of the effect change (positive or negative) reflects the power of the disease (human or rodent model) to detect a change in protein expression over control. Values were calculated by the method described in the Materials and Methods section.
Fig. 5 shows the effect sizes of human protein as a function of rat (Fig. 5A) and mouse (Fig. 5B) transporter protein expression. The rat MCD model shows a similar effect size distribution compared with human for all transporters except OATP1B1, which is up-regulated in human but Oatp1b2 is down-regulated in rat MCD (Fig. 5A). Similarly, the mouse ob/ob model shares positive effect size changes compared with human for all transporters except for OATP1B1, which is induced in human NASH and down-regulated in the ob/ob model (Fig. 5B). The mouse db/db and MCD models do share a similar effect change to human protein expression for all transporters except for Bcrp, which shows a negative effect size in these rodent models whereas a positive effect size is observed in human NASH (Fig. 5B). Similar to the mRNA effect size comparison, rodent atherogenic, as well as fa/fa rats do not share similar protein effect size changes to human NASH across all transporters investigated. For a list of raw effect size data see Supplemental Tables 1–4.
Discussion
With the increasing dependency on pharmacotherapy to manage symptoms associated with disease, adverse drug reactions (ADRs) have become a significant cause for morbidity and mortality worldwide. In the United States alone, ADRs are one of the top 10 causes of death, accounting for ∼100,000 deaths annually and over 700,000 hospitalizations per year (Lazarou et al., 1998; Wooten, 2010; Valente and Murray, 2011). The causes for ADRs are multifaceted and include idiosyncratic drug reactions as well as interindividual variations in the metabolism and elimination of drugs (Valente and Murray, 2011; Shepherd et al., 2012). It is well established that genetic polymorphisms that exist within drug transporters and drug metabolizing enzymes have a role in determining the pharmacokinetics of drugs, thereby impacting the development of clinical ADRs (Clarke and Cherrington, 2012; Daly, 2012; Yiannakopoulou, 2013). However, genetic polymorphisms within genes that mediate ADME processes are estimated to account for less than 20% of ADRs, suggesting that other host factors, such as diseases, may be significant in the development of ADRs (Ingelman-Sundberg and Rodriguez-Antona, 2005). Therefore, it is important to investigate patients with diseases such as NASH as being an at-risk population for developing drug-induced ADRs.
The purpose of this study was to examine experimental models of NASH and determine which of these models accurately represents the expression patterns of hepatic drug transporters in human NASH. This information will allow for meaningful predictions of drug disposition in NASH that could identify potential ADRs in preclinical studies. However, rodent NAFLD models vary dramatically in their ability to reproduce both the clinical and histopathological features of the disease, making selection of the appropriate model difficult. For example, the MCD diet model is criticized for failing to recapitulate the natural progression of the disease, along with lacking common aberrant clinical features such as obesity and hyperglycemia (Rinella and Green, 2004; Tahan et al., 2004). Our data confirm that mice and rats fed a MCD diet fail to develop metabolic aberrations such as obesity and hyperglycemia, suggesting that metabolic alterations are not a consequence of MCD feeding. However, both rats and mice fed an MCD diet develop histopathological features associated with NASH, such as lobular inflammation, macrovesicular steatosis, and varying degrees of fibrosis, which is consistent with previous findings (Leclercq et al., 2000; Fisher et al., 2009a). Interestingly, however, our results suggest that rats are more sensitive to the effects of MCD feeding than mice. MCD rats scored higher in steatosis and fibrosis grades compared with mice in addition to histologic evaluations confirming these findings by the appearance of a greater number of macrovesicular lipid and collagen deposits. A previous study showed that Wistar rats develop more pronounced steatotic deposits in the liver compared with the C57BL/6 mouse strain (Kirsch et al., 2003). Our results are in agreement with these findings, but in contrast to our observation that rats developed more severe NASH, Kirsch et al. reported that mice were more sensitive to the MCD diet. A possible explanation for this discrepancy may lie in the differences in pathologic markers measured (NAS scoring versus lipid peroxidation biproducts and mitochondrial injury) or the duration of MCD feeding (4 weeks versus 8 weeks). Certainly, the longer diet regimen used in our study may drive NASH to a more advanced pathology that resembles human disease.
In response to the criticism that the MCD diet model fails to represent the spectrum of clinical features of NASH, several other rodent NASH models have been developed and investigated. Genetically obese rodents having dysregulated leptin signaling, such as the fa/fa rats as well as the ob/ob and db/db mice, have increased in popularity due to their inherent nature in developing clinical features associated with the metabolic syndrome, including obesity, dyslipidemia, and hyperglycemia (Bray and York, 1979; Carmiel-Haggai et al., 2005; Schattenberg and Galle, 2010). However, ob/ob and db/db mice do not develop NASH spontaneously and must be exposed to a “second hit” such as short-term MCD feeding to propagate the manifestation of NASH (Takahashi et al., 2012). In our study, ob/ob and db/db mice fed an MCD diet for 4 weeks develop histopathological features consistent with NASH such as macrovesicular steatosis and inflammation. In addition, the db/db mice had enlarged livers, increased liver-to-body-weight ratios, and were obese despite a reduction in body weight. As expected, MCD feeding failed to maintain the metabolic disturbances seen in these strains, such as hyperglycemia and hyperinsulinemia. These observations are consistent with previous findings and are a negative consequence of the MCD diet (Sahai et al., 2004; Yamaguchi et al., 2007). Interestingly, despite a previous report of fa/fa rats developing NASH upon high-fat diet feeding (Carmiel-Haggai et al., 2005), our study did not find the full development of NASH in these animals. While these rats are significantly obese and clinical markers are suggestive of the presence of the metabolic syndrome, the histopathological analysis reveals a lack of NASH diagnostic markers present despite having hepatic steatosis.
Despite the differences in NASH manifestations, the ability to recapitulate human gene expression in the liver is most valuable in translational research in the ADME of pharmaceuticals and toxicants. Membrane drug transporters are important mediators of xenobiotic disposition (Klaassen and Aleksunes, 2010) and therefore alterations in the expression and/or function of transporters can impact the pharmacokinetics of drugs, potentially increasing the likelihood of developing ADRs. In the present investigation, we report both mRNA and protein expression profiles of clinically important hepatic drug transporters across several NASH rodent models. Our results suggest that the rat and mouse MCD, along with the db/db and ob/ob mouse models significantly alter the expression profiles of both uptake and efflux transporters in the liver that is consistent with human NASH. Specifically, efflux transporters belonging to the ATP-binding cassette family of transporters are generally induced, whereas uptake transporters belonging to the solute carrier family of transporters are repressed, which is consistent with previous analyses conducted in MCD-fed rodents (Lickteig et al., 2007; Fisher et al., 2009a; Hardwick et al., 2012). It is interesting to note that Oatp1a4 mRNA expression is induced in NASH, whereas protein expression is down-regulated in MCD rats, but for the purpose of this investigation, protein expression will be taken into greater consideration since protein levels will have a functional impact on ADME processes. Taken together, these uniform responses to hepatic injury by NASH suggest a coordinated response in the regulation of hepatic drug transporters similar to what is observed in human NASH (Hardwick et al., 2011; Lake et al., 2011). These results are consistent with previous findings suggesting that this may be a protective mechanism that limits further xenobiotic exposure to the liver by decreasing uptake and facilitating efflux (Lake et al., 2011). Interestingly, the MCD, ob/ob, and db/db models all share histopathological features that are consistent with NASH but lack clinical aberrations associated with the metabolic syndrome. This suggests that the pathologic lesions sustained by the liver in NASH, rather than the metabolic aberrations, may be the major driving force in regulating the transporter gene expression changes observed in the disease, although this needs to be examined further.
Using our previously published mRNA and protein expression data from human livers diagnosed as healthy or NASH, we performed a statistical analysis comparing the mean effect size of gene expression changes between each of the rodent models investigated and our published human data. The results from this analysis suggest that the rat and mouse MCD models as well as the mouse ob/ob and db/db models had the highest power in detecting gene expression changes that reflect the alterations in human transporters. The atherogenic models, as well as the rat fa/fa model share similar changes in transporter expression with human NASH in both direction and magnitude, but other transporters are inconsistent and fail to parallel human NASH transporter expression. Interestingly, the models that used MCD feeding share the most similarity to drug transporter expression in human NASH. It is well known that MCD feeding causes significant induction of oxidative stress as well as the release of proinflammatory cytokines, both of which are mediators of drug transporter gene regulation (Leclercq et al., 2000; Chowdhry et al., 2010; Cherrington et al., 2013; Ikemura et al., 2013). Additionally, hepatic oxidative stress is increased in humans with NASH leading to aberrations in oxidative stress-mediated gene regulation (Hardwick et al., 2010). These observations further suggest that the pathologic consequences sustained throughout the progressive stages of NASH likely play a major role in the dysregulation of ADME in NASH, whereas metabolic perturbations are less influential.
In conclusion, the rat and mouse MCD as well as the mouse ob/ob and db/db NASH models best represent the drug transporter expression changes seen in the livers of humans with NASH. Future investigations into the effects of NASH on the disposition of xenobiotics that share similar transporter kinetic profiles across humans and rodents are encouraged to use these models. However, a better understanding on global gene expression changes across these models is still warranted for more accurate predictions on translating drug disposition changes in humans with NASH.
Supplementary Material
Acknowledgments
The authors thank Dr. David Besselsen for performing the pathological evaluations on all liver histology samples and Dr. Zhenqiang Lu for performing the statistical concordance analyses.
Abbreviations
- ADME
absorption, distribution, metabolism, and excretion
- ADR
adverse drug reaction
- Bcrp
breast cancer resistance protein
- bDNA
branched chain DNA
- MCD
methionine and choline deficient
- Mdr
multidrug resistance protein
- Mrp
multidrug resistance–associated protein
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- Oatp
organic anion transporting polypeptide
- P-gp
p-glycoprotein
Authorship Contributions
Participated in research design: Canet, Cherrington.
Conducted experiments: Canet, Hardwick, Lake, Dzierlenga, Clarke.
Performed data analysis: Canet, Cherrington.
Wrote or contributed to the writing of the manuscript: Canet, Cherrington.
Footnotes
This work was supported by the National Institutes of Health [Grants AI083927, ES006694, and HD062489]; the National Institute of Environmental Health Science Toxicology Training Grant [ES007091]; and The National Science Foundation of Arizona.
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Ali R, Cusi K. (2009) New diagnostic and treatment approaches in non-alcoholic fatty liver disease (NAFLD). Ann Med 41:265–278 [DOI] [PubMed] [Google Scholar]
- Bray GA, York DA. (1979) Hypothalamic and genetic obesity in experimental animals: an autonomic and endocrine hypothesis. Physiol Rev 59:719–809 [DOI] [PubMed] [Google Scholar]
- Canet MJ, Hardwick RN, Lake AD, Kopplin MJ, Scheffer GL, Klimecki WT, Gandolfi AJ, Cherrington NJ. (2012) Altered arsenic disposition in experimental nonalcoholic fatty liver disease. Drug Metab Dispos 40:1817–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmiel-Haggai M, Cederbaum AI, Nieto N. (2005) A high-fat diet leads to the progression of non-alcoholic fatty liver disease in obese rats. FASEB J 19:136–138 [DOI] [PubMed] [Google Scholar]
- Cherrington NJ, Estrada TE, Frisk HA, Canet MJ, Hardwick RN, Dvorak B, Lux K, Halpern MD. (2013) The hepatic bile acid transporters Ntcp and Mrp2 are downregulated in experimental necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 304:G48–G56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhry S, Nazmy MH, Meakin PJ, Dinkova-Kostova AT, Walsh SV, Tsujita T, Dillon JF, Ashford ML, Hayes JD. (2010) Loss of Nrf2 markedly exacerbates nonalcoholic steatohepatitis. Free Radic Biol Med 48:357–371 [DOI] [PubMed] [Google Scholar]
- Clarke JD, Cherrington NJ. (2012) Genetics or environment in drug transport: the case of organic anion transporting polypeptides and adverse drug reactions. Expert Opin Drug Metab Toxicol 8:349–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly AK. (2012) Genetic polymorphisms affecting drug metabolism: recent advances and clinical aspects. Adv Pharmacol 63:137–167 [DOI] [PubMed] [Google Scholar]
- Feldstein AE. (2010) Novel insights into the pathophysiology of nonalcoholic fatty liver disease. Semin Liver Dis 30:391–401 [DOI] [PubMed] [Google Scholar]
- Fisher CD, Jackson JP, Lickteig AJ, Augustine LM, Cherrington NJ. (2008) Drug metabolizing enzyme induction pathways in experimental non-alcoholic steatohepatitis. Arch Toxicol 82:959–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CD, Lickteig AJ, Augustine LM, Oude Elferink RP, Besselsen DG, Erickson RP, Cherrington NJ. (2009a) Experimental non-alcoholic fatty liver disease results in decreased hepatic uptake transporter expression and function in rats. Eur J Pharmacol 613:119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CD, Lickteig AJ, Augustine LM, Ranger-Moore J, Jackson JP, Ferguson SS, Cherrington NJ. (2009b) Hepatic cytochrome P450 enzyme alterations in humans with progressive stages of nonalcoholic fatty liver disease. Drug Metab Dispos 37:2087–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RN, Fisher CD, Canet MJ, Lake AD, Cherrington NJ. (2010) Diversity in antioxidant response enzymes in progressive stages of human nonalcoholic fatty liver disease. Drug Metab Dispos 38:2293–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RN, Fisher CD, Canet MJ, Scheffer GL, Cherrington NJ. (2011) Variations in ATP-binding cassette transporter regulation during the progression of human nonalcoholic fatty liver disease. Drug Metab Dispos 39:2395–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RN, Fisher CD, Street SM, Canet MJ, Cherrington NJ. (2012) Molecular mechanism of altered ezetimibe disposition in nonalcoholic steatohepatitis. Drug Metab Dispos 40:450–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbard L, George J. (2011) Animal models of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 8:35–44 [DOI] [PubMed] [Google Scholar]
- Ikemura K., Nakagawa E, Kurata T, Iwamoto T, Okuda M. (2013) Altered pharmacokinetics of cimetidine caused by down-regulation of renal rat organic cation transporter 2 (rOCT2) after liver ischemia-reperfusion injury. Drug Metab Pharmacokinet 28:504–509 [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M, Rodriguez-Antona C. (2005) Pharmacogenetics of drug-metabolizing enzymes: implications for a safer and more effective drug therapy. Philos Trans R Soc Lond B Biol Sci 360:1563–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh JP. (2012) Membrane transporters in drug development. Adv Pharmacol 63:1–42 [DOI] [PubMed] [Google Scholar]
- Kirsch R, Clarkson V, Shephard EG, Marais DA, Jaffer MA, Woodburne VE, Kirsch RE, Hall PdeL. (2003) Rodent nutritional model of non-alcoholic steatohepatitis: species, strain and sex difference studies. J Gastroenterol Hepatol 18:1272–1282 [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Aleksunes LM. (2010) Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol Rev 62:1–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, et al. Nonalcoholic Steatohepatitis Clinical Research Network (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41:1313–1321 [DOI] [PubMed] [Google Scholar]
- Lake AD, Novak P, Fisher CD, Jackson JP, Hardwick RN, Billheimer DD, Klimecki WT, Cherrington NJ. (2011) Analysis of global and absorption, distribution, metabolism, and elimination gene expression in the progressive stages of human nonalcoholic fatty liver disease. Drug Metab Dispos 39:1954–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake AD, Novak P, Shipkova P, Aranibar N, Robertson D, Reily MD, Lu Z, Lehman-McKeeman LD, Cherrington NJ. (2013) Decreased hepatotoxic bile acid composition and altered synthesis in progressive human nonalcoholic fatty liver disease. Toxicol Appl Pharmacol 268:132–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larter CZ, Yeh MM. (2008) Animal models of NASH: getting both pathology and metabolic context right. J Gastroenterol Hepatol 23:1635–1648 [DOI] [PubMed] [Google Scholar]
- Lazarou J, Pomeranz BH, Corey PN. (1998) Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 279:1200–1205 [DOI] [PubMed] [Google Scholar]
- Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. (2000) CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest 105:1067–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AC, Dai Z, Chen B, Wu H, Wang J, Zhang A, Zhang L, Lim TM, Lin Y. (2008) Electrochemical branched-DNA assay for polymerase chain reaction-free detection and quantification of oncogenes in messenger RNA. Anal Chem 80:9402–9410 [DOI] [PubMed] [Google Scholar]
- Lickteig AJ, Fisher CD, Augustine LM, Aleksunes LM, Besselsen DG, Slitt AL, Manautou JE, Cherrington NJ. (2007) Efflux transporter expression and acetaminophen metabolite excretion are altered in rodent models of nonalcoholic fatty liver disease. Drug Metab Dispos 35:1970–1978 [DOI] [PubMed] [Google Scholar]
- Lomonaco R, Sunny NE, Bril F, Cusi K. (2013) Nonalcoholic fatty liver disease: current issues and novel treatment approaches. Drugs 73:1–14 [DOI] [PubMed] [Google Scholar]
- Lu B, Maqsodi B, Yang W, McMaster GK, Perner S, Regan M, Bubley GJ, Balk SP, Rubin M, Sanda MG. (2009) Detection of TMPRSS2-ERG fusion gene expression in prostate cancer specimens by a novel assay using branched DNA. Urology 74:1156–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuoka H C, Chalasani N. (2013). Nonalcoholic fatty liver disease: an emerging threat to obese and diabetic individuals. Ann N Y Acad Sci 1281:106–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa N, Takamura T, Kurita S, Misu H, Ota T, Ando H, Yokoyama M, Honda M, Zen Y, Nakanuma Y, et al. (2007) Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet. Hepatology 46:1392–1403 [DOI] [PubMed] [Google Scholar]
- McCullough AJ. (2006) Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroenterol 40 (Suppl 1):S17–S29 [DOI] [PubMed] [Google Scholar]
- Rinella ME, Green RM. (2004) The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. J Hepatol 40:47–51 [DOI] [PubMed] [Google Scholar]
- Sahai A, Malladi P, Pan X, Paul R, Melin-Aldana H, Green RM, Whitington PF. (2004) Obese and diabetic db/db mice develop marked liver fibrosis in a model of nonalcoholic steatohepatitis: role of short-form leptin receptors and osteopontin. Am J Physiol Gastrointest Liver Physiol 287:G1035–G1043 [DOI] [PubMed] [Google Scholar]
- Schattenberg JM, Galle PR. (2010) Animal models of non-alcoholic steatohepatitis: of mice and man. Dig Dis 28:247–254 [DOI] [PubMed] [Google Scholar]
- Shepherd G, Mohorn P, Yacoub K, May DW. (2012) Adverse drug reaction deaths reported in United States vital statistics, 1999-2006. Ann Pharmacother 46:169–175 [DOI] [PubMed] [Google Scholar]
- Tahan V, Yavuz D, Imeryuz N, Avsar E, Tozun N. (2004) Oral glucose tolerance deteriorates in rats fed with methionine choline deficient diet. J Hepatol 41:352, author reply 353. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Soejima Y, Fukusato T. (2012) Animal models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol 18:2300–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente S, Murray LP. (2011) Creative strategies to improve patient safety: allergies and adverse drug reactions. J Nurses Staff Dev 27:E1–E5, quiz E6–E7 [DOI] [PubMed] [Google Scholar]
- Wooten JM. (2010) Adverse drug reactions: Part I. South Med J 103:1025–1028, quiz 1029 [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, Bhanot S, Monia BP, Li YX, Diehl AM. (2007) Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology 45:1366–1374 [DOI] [PubMed] [Google Scholar]
- Yiannakopoulou ECh. (2013) Pharmacogenomics of phase II metabolizing enzymes and drug transporters: clinical implications. Pharmacogenomics J 13:105–109 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.