Abstract
Methotrexate (MTX) is the cornerstone of chemotherapy for primary central nervous system lymphoma, yet how the blood-brain barrier (BBB) efflux transporters ABCG2 and ABCC4 influence the required high-dose therapy is unknown. To evaluate their role, we used four mouse strains, C57BL/6 (wild-type; WT), Abcg2−/−, Abcc4−/−, and Abcg2−/−;Abcc4−/− (double knockout; DKO) to conduct brain microdialysis studies after single intravenous MTX doses of 50 mg/kg. When the area under the concentration-time curve for plasma (AUCplasma) was used to assess systemic exposure to MTX, the rank order was Abcc4−/− < WT < Abcg2−/− < Abcg2−/−Abcc4−/−. Only the DKO exposure was significantly higher than that of the WT group (P < 0.01), a reflection of the role of Abcg2 in biliary excretion and Abcc4 in renal excretion. MTX brain interstitial fluid concentrations obtained by microdialysis were used to calculate the area under the concentration-time curve for the brain (AUCbrain), which found the rank order of exposure to be WT < Abcc4−/− < Abcg2−/− < Abcg2−/−Abcc4−/− with the largest difference being 4-fold: 286.13 ± 130 μg*min/ml (DKO) versus 66.85 ± 26 (WT). Because the transporters affected the systemic disposition of MTX, particularly in the DKO group, the ratio of the AUCbrain/AUCplasma or the brain/plasma partition coefficient Kp was calculated, revealing that the DKO strain had a significantly higher value (0.23 ± 0.09) than the WT strain (0.11 ± 0.05). Both Abcg2 and Abcc4 limited BBB penetration of MTX; however, only when both drug efflux pumps were negated did the brain accumulation of MTX significantly increase. These findings indicate a contributory role of both ABCG2 and ABCC4 to limiting MTX distribution in patients.
Introduction
The blood-brain barrier (BBB) is composed of capillaries lined by endothelial cells with tight junctions to limit passive diffusion of drugs, and protein pumps that when directed toward the blood act as efflux transporters that limit the distribution of drugs into the central nervous system (CNS) (Deeken and Loscher, 2007). Primary BBB efflux pumps, notably Abcb1 and Abcg2, are members of the ABC family of transporters and have been implicated in restricting the BBB penetration of several drugs (Schinkel et al., 1996). The consequence of limited brain access for CNS-active drugs is a lower therapeutic index, which could prevent use of the drug. It is essential to determine the role of the BBB and its efflux transporters in mediating the brain distribution of drugs, both to characterize the mechanisms of CNS disposition and to offer a rational means to overcome limited brain distribution.
Methotrexate (MTX) is the standard of care and a component of numerous experimental regimens for primary CNS lymphoma (DeAngelis, 2003; Zhu et al., 2009), a disease that accounts for 2% and 5% of all primary brain tumors (Gerstner and Batchelor, 2007). MTX is a folate antagonist with limited uptake in the brain, which likely contributes to the need for high-dose therapy (Nierenberg et al., 1991; Wolff et al., 2011). Studies in rodents (Devineni et al., 1996) and patients (Blakeley et al., 2009) that used brain microdialysis to measure the brain interstitial fluid MTX concentrations attest to its limited distribution in the normal brain, with a brain-to-plasma ratio 0.051 ± 0.032 and 0.032 ± 0.094 in rodents and humans, respectively. MTX has been shown to be a substrate for breast cancer resistance protein (BCRP) (Volk and Schneider, 2003; Li et al., 2013) and multidrug resistance protein 4 (MRP4) (Chen et al., 2002) using in vitro membrane vesicle systems. Abcg2 has been implicated in limiting the brain distribution of several drugs (Agarwal et al., 2010, 2011; Kodaira et al., 2010), and Abcc4 has been shown to prevent the brain distribution of topotecan and oseltamivir (Leggas et al., 2004; Ose et al., 2009). However, the role of these transporters in limiting the distribution of MTX at the BBB has not been adequately explored, hence our investigation in Abcg2−/−, Abcc4−/−, and Abcg2−/−;Abcc4−/− transgenic mice. To assess the role of these transporters in the brain penetration of MTX, we employed brain microdialysis in conjunction with serial plasma sampling after single intravenous dose administrations.
Materials and Methods
Methotrexate hydrate (molecular weight: 454.44), 7-hydroxy methotrexate (7-OH MTX; molecular weight: 470.44), and aminopterin were purchased from Sigma-Aldrich (St. Louis, MO). All other chemicals were reagent grade or high-performance liquid chromatography grade and were purchased from Sigma-Aldrich. Guide cannulas (5-mm shaft) and MetaQuant probes (regenerated cellulose, dialysis tip length 2 mm, mol. wt. cutoff 13) were purchased from Brainlink (Groningen, The Netherlands).
Animals.
C57BL/6 wild-type (WT) and transgenic mice with genetic deletions of either the Abcg2 transporter (Abcg2 knockout, Abcg2−/−), Abcc4 transporter (Abcc4 knockout, Abcc4−/−) or both transporters (double knockout or DKO, Abcg2−/−;Abcc4−/−) were derived in the laboratory of Dr. Gary Kruh (deceased) (Belinsky et al., 2007; Wang et al., 2011), previously at Fox Chase Cancer Center and finally at the University of Illinois at Chicago, and then transferred and maintained at Charles River Laboratories (Wilmington, MA) through an agreement with Mount Sinai School of Medicine. The mice were transferred to Mount Sinai approximately 7 days before entry into the pharmacokinetic studies and were maintained on a 12-hour light/dark cycle with unlimited access to food and water. All animal studies were approved by the Institutional Animal Care and Use Committee at Mount Sinai School of Medicine. All mice used in the experiment were male, were 10 to 12 weeks of age, and weighed 25 to 30 g.
Microdialysis Preparation.
Anesthetized mice underwent a surgical microdialysis and vascular cannula implantation procedure, as previously described elsewhere (Guo et al., 2007), 3 days before the pharmacokinetic study with the modification that a MetaQuant probe system was located at 0.74 mm anterior and 2.0 mm lateral to the bregma with the guide cannula lowered to a depth of 2.5 mm. The Meta-Quant microdialysis system is designed to increase analyte recovery by perfusion of the dialysis membrane at a low flow rate (0.1 µl/min) while simultaneously using a higher makeup flow rate (0.9 µl/min) to eliminate long sample collection periods (Cremers et al., 2009). The animals were placed on a heating pad during recovery, and when fully ambulatory were provided with food and water ad libitum. The food was removed 2 hours before the pharmacokinetic study.
The guide cannulas were replaced by the microdialysis probes and calibrated by a retrodialysis method, and the recovery was calculated as previously described elsewhere (Guo et al., 2007). At the completion of the retrodialysis calibration, the animals were administered a dose of 50 mg/kg MTX hydrate as an i.v. bolus through a tail vein. Serial dialysate (∼20 µl) samples were collected every 20 minutes up to 8 hours after the dose using a refrigerated sample collector (CMA 470; Harvard Apparatus, Holliston, MA), and they were stored directly at −80°C until analysis. Serial blood samples of 20 μl were collected at 5, 15, and 30 minutes, and 1, 2, 3, 4, 6, and 8 hours after the dose. Saline (10 µl) was injected through the carotid artery cannula to avoid volume depletion. The samples were then centrifuged, and the resultant plasma was stored at −80°C until the analysis with liquid chromatography/tandem mass spectrometry (LC-MS/MS) according to a previously published method (Guo et al., 2007).
Sample preparation was performed as described elsewhere (Guo et al., 2007). We precipitated 2 µl of plasma and 10 µl of brain dialysate with 20 µl of acetonitrile, which was then used for quantification. The method was modified by changing the flow rate for high-performance liquid chromatography as follows: a mobile phase of 0.5 mM ammonium formate with 0.1% formic acid and acetonitrile using gradient elution, 2% acetonitrile for the first 0.5 minutes, then 80% acetonitrile from 0.8 to 2.0 minutes, then 2% acetonitrile from 2.5 minutes to 7 minutes at a flow rate of 0.3 ml/min.
Data Analysis.
Noncompartmental analysis was performed on the both the plasma and brain concentration–time profiles for each mouse in all four genotypes using Kinectica (version 5.1; Thermo Fisher Scientific, Pittsburgh, PA). The terminal rate constants were calculated using the data points that best described the terminal elimination phase. The areas under the concentration-time curve for plasma (AUCplasma) and brain (AUCbrain) from time 0 to infinity were calculated using the log-linear method, with the AUC from the last measured time point to infinity estimated by dividing the last measured concentration by the elimination rate constant. In all cases, the contribution of the extrapolated AUC to the time infinity values was 8% or less. The brain-to-plasma partition coefficient (Kp,brain) of MTX was calculated as a ratio of AUCbrain/AUCplasma.
Statistical Analysis.
GraphPad Prism 5 (GraphPad Software, La Jolla, CA) was used to determine differences between genotypes by nonparametric one-way analysis of variance using a Kruskal-Wallis test followed by Dunn’s post test to compare between strains. P < 0.05 was considered statistically significant.
Results and Discussion
Given the extensive use of MTX in the treatment of primary and secondary CNS tumors, it is essential that the role of BBB in limiting its brain distribution be examined in detail. However, in so doing, it is important to also evaluate the influence of the transporters on the systemic disposition of MTX by assessing changes in the drug clearance, because clearance (CL)—the inverse of the AUC—will determine plasma drug concentrations and influence tissue distribution. Both the Abcg2−/− and Abcg2−/−Abcc4−/− strains had reduced total clearance values (Table 1, Fig. 1 A) compared with the wild-type and the Abcc4−/− mice; however, only the DKO strain reached statistical significance, 2-fold lower than the wild-type strain (P = 0.004). The absence of Abcc4 did not alter the clearance of MTX in mice (Kitamura et al., 2008), consistent with our results. This indicates that the reduction in total clearance in the DKO strain is largely due to reduced biliary clearance—a major route of elimination of MTX in rodents (Bremnes et al., 1989)—and the lack of Abcg2 on bile canaliculi (Zamek-Gliszczynski et al., 2006). Nonetheless, a role for Abcc4 in the renal clearance of MTX could be inferred, because the total clearance in the DKO was quite a bit lower than in the single Abcg2 knockout strain. This potential role of MRP4—localized to the renal proximal tubule (van Aubel et al., 2002)—could be more important in humans where renal clearance of MTX is dominant.
TABLE 1.
Pharmacokinetic parameters in the plasma for MTX and 7-OH MTX and in the brain for MTX determined by noncompartmental analysis after a 50-mg/kg intravenous bolus dose of MTX in wild-type, Abcg2−/−, Abcc4−/−, and Abcg2−/−Abcc4−/− mice
| Parameters | Wild Type n = 6 | Abcg2−/− n = 5 | Abcc4−/− n = 5 | Abcg2−/−;Abcc4−/− n = 6 |
|---|---|---|---|---|
| Plasma | ||||
| CL (ml/min) | 1.96 ± 0.38 | 1.58 ± 0.52 | 2.00 ± 0.40 | 1.00 ± 0.20* |
| Vd (l) | 0.54 ± 0.40 | 0.28 ± 0.14 | 0.73 ± 0.29 | 0.18 ± 0.09* |
| Half-life(MTX,plasma) (min) | 195 ± 171 | 124 ± 28 | 247 ± 81 | 116 ± 28* |
| AUCMTX(0–inf) (min*μg/ml) | 633 ± 121 | 836.9 ± 208 | 631 ± 185 | 1259 ± 289** |
| AUC7-OH-MTX(0–inf) (min*μg/ml) | 26.1 ± 8.7 | 59.9 ± 23.7 | 22.2 ± 6.2 | 81.5 ± 23.9** |
| Half-life7OH MTX (min) | 224 ± 131 | 237 ± 74.7 | 242 ± 96 | 200 ± 74 |
| AUC7OH/AUCMTX (%) | 4.16 ± 1.35 | 7.80 ± 1.59 | 3.55 ± 0.6 | 6.52 ± 1.10 |
| Brain | ||||
| AUCbrain (min*μg/ml) | 66.85 ± 26 | 134 ± 54.80 | 118.12 ± 60.50 | 286.13 ± 130** |
| Half-lifebrain (min) | 151 ± 69 | 104 ± 44 | 62.3 ± 22.7 | 88 ± 15 |
| Cmax (μg/ml) | 1.2 ± 0.5 | 1.8 ± 0.9 | 1.5 ± 0.6 | 3.7 ± 2.2 |
| Kp,brain | 0.11 ± 0.05 | 0.17 ± 0.08 | 0.19 ± 0.09 | 0.23 ± 0.09* |
P < 0.05; **P < 0.01.
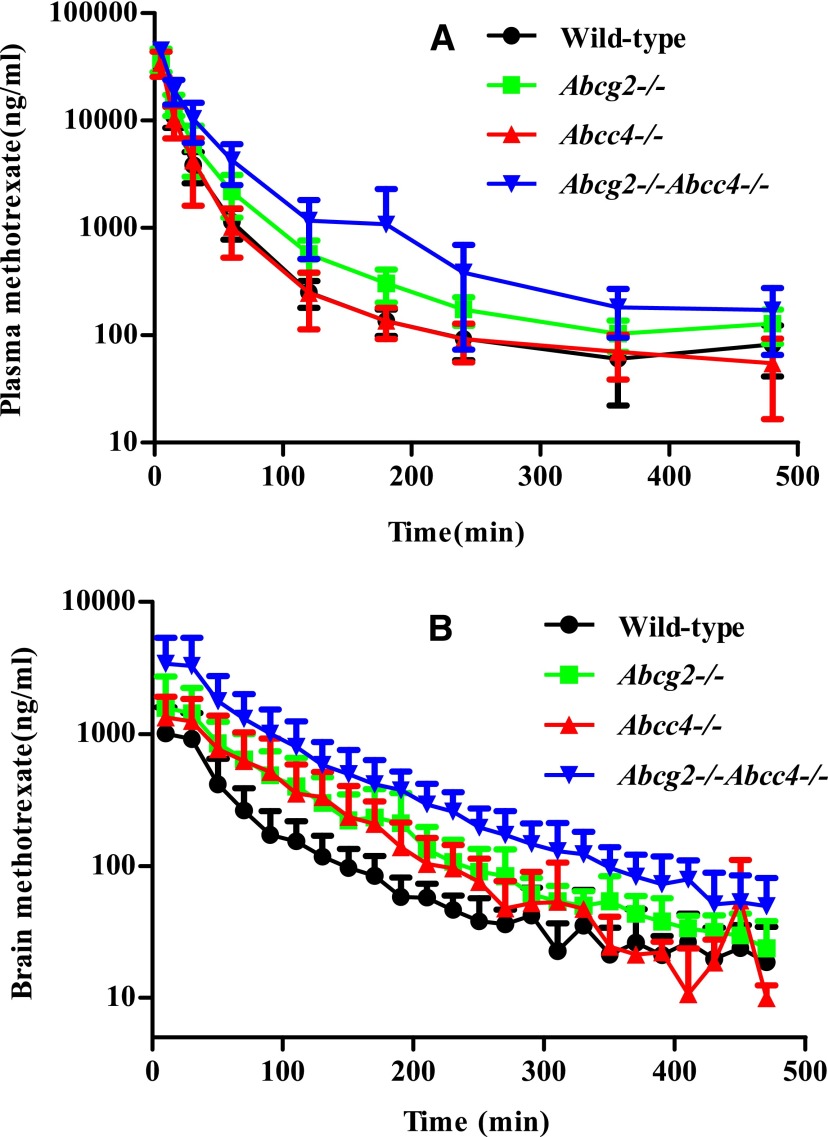
Fig. 1.
(A) Plasma concentrations of methotrexate in wild-type, Abcg2−/−, Abcc4−/−, and Abcg2−/−Abcc4−/− mice after a 50-mg/kg dose of i.v. methotrexate hydrate. Mean ± S.D. (n = 4 or 5). Plasma concentrations in the Abcg2−/−Abcc4−/− mice are consistently higher than those observed in the wild type. (B) Corresponding brain concentrations of methotrexate in wild-type, Abcg2−/−, Abcc4−/−, and Abcg2−/−Abcc4−/− mice after a 50-mg/kg dose of i.v. methotrexate hydrate. Mean ± S.D. (n = 4 or 5). Brain concentrations in the Abcg2−/− and Abcg2−/−Abcc4−/− mice are higher than those observed in the wild type.
The AUC for the 7-hydroxy metabolite of methotrexate was higher in Abcg2−/− and reached statistical significance in Abcg2−/−Abcc4−/− groups (59.9 ± 23.7 and 81.5 ± 23.9 µg*min/ml, respectively, P = 0.0022) as compared with the wild-type mice (26.13 ± 8.7 µg*min/ml). This was consistent with the work of Vlaming et al. (2009) in Abcg2−/− mice; they concluded that the increased AUC7-OH MTX is likely due to enhanced retention of MTX in the liver and its subsequent metabolism rather to than any transporter effect on the metabolite.
BCRP and MRP4 are among the most highly expressed transport proteins at the mouse and human BBB (Carl et al., 2010; Agarwal et al., 2012). Unbound brain concentrations and not total brain concentrations are indicative of interactions with target receptors and determine CNS efficacy (Liu et al., 2009). Therefore, because brain microdialysis provides a measure of the unbound drug concentration, it is the ideal technique to indicate the amount of drug available to the intracellular compartment for drug-receptor binding as well as to indicate the influence of drug efflux pumps at the BBB.
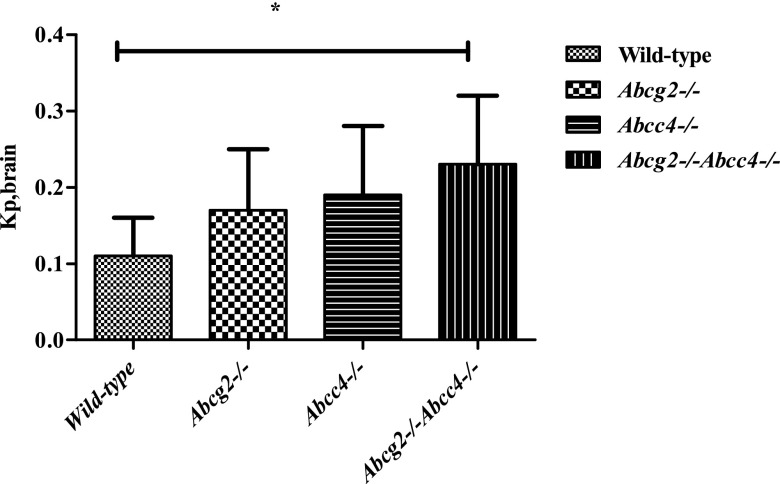
The brain concentrations in the DKO mice were higher than those observed in the wild-type mice or either single-knockout mice (Fig. 1B), which yielded corresponding changes in the AUCbrain (see Table 1). However, to accurately assess the role of the transporters on regional brain distribution, it is important to use the brain partition coefficient (Kp)—the ratio AUCbrain/AUCplasma—which accounts for changes in the systemic exposure of MTX. Specifically, the brain partition coefficient was 2-fold greater (Kp = 0.23 ± 0.09, see Table 1) in the DKO mice compared with the WT strain (Kp = 0.11 ± 0.05), which represented the largest difference among all strains (P = 0.017) (Fig. 2). The brain partition coefficient in Abcg2 knockouts (0.17 ± 0.08) was not significantly different from that in wild-type mice (0.11 ± 0.05) and partially reflects the increased plasma exposure. On the other hand, the Kp in Abcc4 knockout mice (0.19 ± 0.09), although not statistically significantly different from the WT strain and analogous to that in the Abcg2 knockout mice, suggests a local BBB effect due to deletion of the transporter; that is, the AUCplasma values in the Abcc4 knockout and wild-type groups were virtually identical. MTX is a low-affinity substrate for human BCRP, having a Km of about 680 μM to 1.3 mM (Chen et al., 2003; Volk and Schneider, 2003), compared with 220 µM for human MRP4 as determined by in vitro studies (Chen et al., 2002). The expression of BCRP is almost 2-fold higher than MRP4 at the mouse BBB (Agarwal et al., 2012) and nearly 40-fold higher in humans (Uchida et al., 2011). If we assume that the transporter affinity (Km) for MTX in mice is similar to the in vitro values, and that the expression of the transporters at the BBB is reflective of the Vmax for the respective transporters, then the clearance out of the brain (ratio of Vmax to Km, assuming linearity) for both transporters could be comparable. Although this extrapolation is speculative, it could explain why the single deletion of either Bcrp or Mrp4 had nearly similar effects on the brain distribution of MTX. Not unexpectedly, the simultaneous absence of Abcg2 and Abcc4, the DKO strain, caused the greatest increase in the Kp values of MTX, which supports the cooperative action of these transporters at the BBB. A similar study with topotecan also revealed cooperative behavior of MRP4 with BCRP and P-glycoprotein at the mouse BBB (Lin et al., 2013).
Fig. 2.
The partition coefficient for methotrexate in the brain (Kp,brain) in wild-type, Abcg2−/−, Abcc4−/−, and Abcg2−/−Abcc4−/− mice. The Kp,brain in Abcc4−/−, Abcg2−/− mice is 2-fold higher than in the wild-type mice (*P < 0.05).
In this study, we show conclusively that the brain exposure of MTX is limited by efflux due to BCRP and MRP4 acting in a cooperative manner. This is an important effect to be considered, as MTX is a standard of care in primary CNS lymphoma and in metastatic brain tumors (Lassman et al., 2006; Jacot et al., 2010). Although the breakdown of the BBB in a tumor can result in a higher brain concentration of MTX (Blakeley et al., 2009), many brain tumors are invasive and diffuse behind an intact BBB (DeAngelis, 2003). The results from this study reinforce the idea that the BBB and the transporters at the BBB play an important role in the disposition of MTX and that its efficacy could be limited by their presence.
Abbreviations
- AUC
area under the curve
- BBB
blood-brain barrier
- BCRP
breast cancer resistance protein
- CL
clearance
- CNS
central nervous system
- DKO
double knockout
- Kp
brain partition coefficient
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- MRP4
multidrug resistance protein 4
- MTX
methotrexate
- 7-OH MTX
7-hydroxy methotrexate
- WT
wild-type
Authorship Contributions
Participated in research design: Wu, Gallo.
Conducted experiments: Wu, Zhang.
Contributed new reagents or analytic tools: Zhang, Wu.
Performed data analysis: Sane, Gallo.
Wrote or contributed to the writing of the manuscript: Sane, Gallo.
Footnotes
This work was supported by the National Institutes of Health National Cancer Institute [Grant CA073728].
References
- Agarwal S, Sane R, Gallardo JL, Ohlfest JR, Elmquist WF. (2010) Distribution of gefitinib to the brain is limited by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2)-mediated active efflux. J Pharmacol Exp Ther 334:147–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Sane R, Ohlfest JR, Elmquist WF. (2011) The role of the breast cancer resistance protein (ABCG2) in the distribution of sorafenib to the brain. J Pharmacol Exp Ther 336:223–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Uchida Y, Mittapalli RK, Sane R, Terasaki T, Elmquist WF. (2012) Quantitative proteomics of transporter expression in brain capillary endothelial cells isolated from P-glycoprotein (P-gp), breast cancer resistance protein (Bcrp), and P-gp/Bcrp knockout mice. Drug Metab Dispos 40:1164–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belinsky MG, Guo P, Lee K, Zhou F, Kotova E, Grinberg A, Westphal H, Shchaveleva I, Klein-Szanto A, Gallo JM, et al. (2007) Multidrug resistance protein 4 protects bone marrow, thymus, spleen, and intestine from nucleotide analogue-induced damage. Cancer Res 67:262–268 [DOI] [PubMed] [Google Scholar]
- Blakeley JO, Olson J, Grossman SA, He X, Weingart J, Supko JG, New Approaches to Brain Tumor Therapy (NABTT) Consortium (2009) Effect of blood brain barrier permeability in recurrent high grade gliomas on the intratumoral pharmacokinetics of methotrexate: a microdialysis study. J Neurooncol 91:51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremnes RM, Slørdal L, Wist E, Aarbakke J. (1989) Dose-dependent pharmacokinetics of methotrexate and 7-hydroxymethotrexate in the rat in vivo. Cancer Res 49:6359–6364 [PubMed] [Google Scholar]
- Carl SM, Lindley DJ, Couraud PO, Weksler BB, Romero I, Mowery SA, Knipp GT. (2010) ABC and SLC transporter expression and pot substrate characterization across the human CMEC/D3 blood-brain barrier cell line. Mol Pharm 7:1057–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZS, Lee K, Walther S, Raftogianis RB, Kuwano M, Zeng H, Kruh GD. (2002) Analysis of methotrexate and folate transport by multidrug resistance protein 4 (ABCC4): MRP4 is a component of the methotrexate efflux system. Cancer Res 62:3144–3150 [PubMed] [Google Scholar]
- Chen ZS, Robey RW, Belinsky MG, Shchaveleva I, Ren XQ, Sugimoto Y, Ross DD, Bates SE, Kruh GD. (2003) Transport of methotrexate, methotrexate polyglutamates, and 17beta-estradiol 17-(β-d-glucuronide) by ABCG2: effects of acquired mutations at R482 on methotrexate transport. Cancer Res 63:4048–4054 [PubMed] [Google Scholar]
- Cremers TI, de Vries MG, Huinink KD, van Loon JP, v d Hart M, Ebert B, Westerink BH, De Lange EC. (2009) Quantitative microdialysis using modified ultraslow microdialysis: direct rapid and reliable determination of free brain concentrations with the MetaQuant technique. J Neurosci Methods 178:249–254 [DOI] [PubMed] [Google Scholar]
- DeAngelis LM. (2003) Primary central nervous system lymphoma: a curable brain tumor. J Clin Oncol 21:4471–4473 [DOI] [PubMed] [Google Scholar]
- Deeken JF, Löscher W. (2007) The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res 13:1663–1674 [DOI] [PubMed] [Google Scholar]
- Devineni D, Klein-Szanto A, Gallo JM. (1996) In vivo microdialysis to characterize drug transport in brain tumors: analysis of methotrexate uptake in rat glioma-2 (RG-2)-bearing rats. Cancer Chemother Pharmacol 38:499–507 [DOI] [PubMed] [Google Scholar]
- Gerstner E, Batchelor T. (2007) Primary CNS lymphoma. Expert Rev Anticancer Ther 7:689–700 [DOI] [PubMed] [Google Scholar]
- Guo P, Wang X, Liu L, Belinsky MG, Kruh GD, Gallo JM. (2007) Determination of methotrexate and its major metabolite 7-hydroxymethotrexate in mouse plasma and brain tissue by liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal 43:1789–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacot W, Gerlotto-Borne MC, Thezenas S, Pouderoux S, Poujol S, About M, Romieu G. (2010) Carmustine and methotrexate in combination after whole brain radiation therapy in breast cancer patients presenting with brain metastases: a retrospective study. BMC Cancer 10:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura Y, Hirouchi M, Kusuhara H, Schuetz JD, Sugiyama Y. (2008) Increasing systemic exposure of methotrexate by active efflux mediated by multidrug resistance-associated protein 3 (mrp3/abcc3). J Pharmacol Exp Ther 327:465–473 [DOI] [PubMed] [Google Scholar]
- Kodaira H, Kusuhara H, Ushiki J, Fuse E, Sugiyama Y. (2010) Kinetic analysis of the cooperation of P-glycoprotein (P-gp/Abcb1) and breast cancer resistance protein (Bcrp/Abcg2) in limiting the brain and testis penetration of erlotinib, flavopiridol, and mitoxantrone. J Pharmacol Exp Ther 333:788–796 [DOI] [PubMed] [Google Scholar]
- Lassman AB, Abrey LE, Shah GD, Panageas KS, Begemann M, Malkin MG, Raizer JJ. (2006) Systemic high-dose intravenous methotrexate for central nervous system metastases. J Neurooncol 78:255–260 [DOI] [PubMed] [Google Scholar]
- Leggas M, Adachi M, Scheffer GL, Sun D, Wielinga P, Du G, Mercer KE, Zhuang Y, Panetta JC, Johnston B, et al. (2004) Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol Cell Biol 24:7612–7621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Agarwal S, Elmquist WF. (2013) Brain efflux index to investigate the influence of active efflux on brain distribution of pemetrexed and methotrexate. Drug Metab Dispos 41:659–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Marchetti S, Pluim D, Iusuf D, Mazzanti R, Schellens JH, Beijnen JH, van Tellingen O. (2013) Abcc4 together with abcb1 and abcg2 form a robust cooperative drug efflux system that restricts the brain entry of camptothecin analogues. Clin Cancer Res 19:2084–2095 [DOI] [PubMed] [Google Scholar]
- Liu X, Vilenski O, Kwan J, Apparsundaram S, Weikert R. (2009) Unbound brain concentration determines receptor occupancy: a correlation of drug concentration and brain serotonin and dopamine reuptake transporter occupancy for eighteen compounds in rats. Drug Metab Dispos 37:1548–1556 [DOI] [PubMed] [Google Scholar]
- Nierenberg D, Harbaugh R, Maurer LH, Reeder T, Scott G, Fratkin J, Newman E. (1991) Continuous intratumoral infusion of methotrexate for recurrent glioblastoma: a pilot study. Neurosurgery 28:752–761 [DOI] [PubMed] [Google Scholar]
- Ose A, Ito M, Kusuhara H, Yamatsugu K, Kanai M, Shibasaki M, Hosokawa M, Schuetz JD, Sugiyama Y. (2009) Limited brain distribution of [3R,4R,5S]-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate phosphate (Ro 64-0802), a pharmacologically active form of oseltamivir, by active efflux across the blood-brain barrier mediated by organic anion transporter 3 (Oat3/Slc22a8) and multidrug resistance-associated protein 4 (Mrp4/Abcc4). Drug Metab Dispos 37:315–321 [DOI] [PubMed] [Google Scholar]
- Schinkel AH, Wagenaar E, Mol CA, van Deemter L. (1996) P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest 97:2517–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y, Ohtsuki S, Katsukura Y, Ikeda C, Suzuki T, Kamiie J, Terasaki T. (2011) Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J Neurochem 117:333–345 [DOI] [PubMed] [Google Scholar]
- van Aubel RA, Smeets PH, Peters JG, Bindels RJ, Russel FG. (2002) The MRP4/ABCC4 gene encodes a novel apical organic anion transporter in human kidney proximal tubules: putative efflux pump for urinary cAMP and cGMP. J Am Soc Nephrol 13:595–603 [DOI] [PubMed] [Google Scholar]
- Vlaming ML, van Esch A, Pala Z, Wagenaar E, van de Wetering K, van Tellingen O, Schinkel AH. (2009) Abcc2 (Mrp2), Abcc3 (Mrp3), and Abcg2 (Bcrp1) are the main determinants for rapid elimination of methotrexate and its toxic metabolite 7-hydroxymethotrexate in vivo. Mol Cancer Ther 8:3350–3359 [DOI] [PubMed] [Google Scholar]
- Volk EL, Schneider E. (2003) Wild-type breast cancer resistance protein (BCRP/ABCG2) is a methotrexate polyglutamate transporter. Cancer Res 63:5538–5543 [PubMed] [Google Scholar]
- Wang Z, Zhou Q, Kruh GD, Gallo JM. (2011) Dose-dependent disposition of methotrexate in Abcc2 and Abcc3 gene knockout murine models. Drug Metab Dispos 39:2155–2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JE, Kortmann RD, Wolff B, Pietsch T, Peters O, Schmid HJ, Rutkowski S, Warmuth-Metz M, Kramm C. (2011) High dose methotrexate for pediatric high grade glioma: results of the HIT-GBM-D pilot study. J Neurooncol 102:433–442 [DOI] [PubMed] [Google Scholar]
- Zamek-Gliszczynski MJ, Nezasa K, Tian X, Kalvass JC, Patel NJ, Raub TJ, Brouwer KL. (2006) The important role of Bcrp (Abcg2) in the biliary excretion of sulfate and glucuronide metabolites of acetaminophen, 4-methylumbelliferone, and harmol in mice. Mol Pharmacol 70:2127–2133 [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Gerstner ER, Engler DA, Mrugala MM, Nugent W, Nierenberg K, Hochberg FH, Betensky RA, Batchelor TT. (2009) High-dose methotrexate for elderly patients with primary CNS lymphoma. Neuro-oncol 11:211–215 [DOI] [PMC free article] [PubMed] [Google Scholar]