Abstract
Impaired hepatic bile acid export may contribute to development of cholestatic drug-induced liver injury (DILI). The multidrug resistance-associated proteins (MRP) 3 and 4 are postulated to be compensatory hepatic basolateral bile acid efflux transporters when biliary excretion by the bile salt export pump (BSEP) is impaired. BSEP inhibition is a risk factor for cholestatic DILI. This study aimed to characterize the relationship between MRP3, MRP4, and BSEP inhibition and cholestatic potential of drugs. The inhibitory effect of 88 drugs (100 μM) on MRP3- and MRP4-mediated substrate transport was measured in membrane vesicles. Drugs selected for investigation included 50 BSEP non-inhibitors (24 non-cholestatic; 26 cholestatic) and 38 BSEP inhibitors (16 non-cholestatic; 22 cholestatic). MRP4 inhibition was associated with an increased risk of cholestatic potential among BSEP non-inhibitors. In this group, for each 1% increase in MRP4 inhibition, the odds of the drug being cholestatic increased by 3.1%. Using an inhibition cutoff of 21%, which predicted a 50% chance of cholestasis, 62% of cholestatic drugs inhibited MRP4 (P < 0.05); in contrast, only 17% of non-cholestatic drugs were MRP4 inhibitors. Among BSEP inhibitors, MRP4 inhibition did not provide additional predictive value of cholestatic potential; almost all BSEP inhibitors were also MRP4 inhibitors. Inclusion of pharmacokinetic predictor variables (e.g., maximal unbound concentration in plasma) in addition to percent MRP4 inhibition in logistic regression models did not improve cholestasis prediction. Association of cholestasis with percent MRP3 inhibition was not statistically significant, regardless of BSEP-inhibition status. Inhibition of MRP4, in addition to BSEP, may be a risk factor for the development of cholestatic DILI.
Introduction
Drug-induced liver injury (DILI) is a frequent and serious side effect of drug therapy and a major concern in drug discovery and clinical development. DILI is one of the leading causes of acute liver failure and was the most frequent reason for withdrawal of approved drugs from the US market between 1975 and 2000 (Lasser et al., 2002; Lee, 2003).
The term DILI describes different manifestations of liver toxicity following drug exposure ranging from asymptomatic elevation of liver enzymes to hepatic failure. Cholestatic and hepatocellular liver injury are the two major types of DILI. Unfortunately, at present, the pathophysiological mechanisms of hepatotoxicity are not well defined. Hypothesized mechanisms include apoptosis of hepatocytes, immune-mediated mechanisms, mitochondrial disruption, and bile duct injury, as well as inhibition of transport proteins. One proposed mechanism of cholestatic DILI is inhibition of bile acid transport, leading to necrotic and/or apoptotic cell death due to increased hepatocellular concentrations of bile acids (Hofmann, 1999; Wagner et al., 2009).
Hepatocytes are polarized cells that have specialized transport systems in the canalicular/apical and sinusoidal/basolateral membrane to maintain hepatic bile acid homeostasis. Under physiologic conditions, bile acids are excreted across the canalicular membrane into bile, where they form micelles with other bile components such as phospholipids or cholesterol. The bile salt export pump (BSEP), an ATP-dependent export protein located in the canalicular membrane, transports bile acids from the hepatocyte into bile (Noe et al., 2002). Because of BSEP’s central role in the hepatic excretion of bile acids, functional impairment of BSEP has been hypothesized to play a role in the development of liver injury. For example, patients with mutations in the ABCB11/BSEP gene that result in reduced expression levels or function of BSEP [e.g., progressive familial intrahepatic cholestasis type II] exhibit reduced bile acid excretion compared with normal patients, and rapidly develop liver injury due to hepatocellular accumulation of toxic bile acids (Jansen et al., 1999; Jansen and Muller, 2000; Perez and Briz, 2009). Several hepatotoxic drugs, including troglitazone, erythromycin, and bosentan, inhibit BSEP function in in vitro systems such as sandwich-cultured hepatocytes or BSEP membrane vesicle assays (Stieger et al., 2000; Fattinger et al., 2001; Kostrubsky et al., 2003). Recently, several large studies systematically investigated the relationship between DILI and BSEP inhibition using BSEP membrane vesicles (Morgan et al., 2010; Dawson et al., 2012; Morgan et al., 2013). These studies provided compelling evidence for an association between BSEP inhibition and hepatotoxicity. Furthermore, drugs with mixed hepatocellular and cholestatic liver injury exhibited increased potency to inhibit BSEP compared with drugs that only exhibited hepatocellular injury, or drugs that were not hepatotoxic (Dawson et al., 2012). However, the studies by Morgan et al. and Dawson et al. also clearly demonstrated that not all cholestatic drugs are BSEP inhibitors, suggesting that additional independent factors may be involved in the development of cholestatic liver injury.
In addition to apically localized BSEP, the basolateral multidrug resistance-associated proteins (MRP) 3 (ABCC3) and MRP4 (ABCC4) are involved in ATP-dependent bile acid export (Zeng et al., 2000; Rius et al., 2003; Zelcer et al., 2003b). Under normal physiologic conditions, the hepatic expression of MRP3 and MRP4 is low, whereas upregulation under cholestatic conditions (e.g., inhibition of BSEP, genetic BSEP variants) has been observed (Gradhand et al., 2008; Chai et al., 2012). Therefore, these basolateral proteins are hypothesized to provide a compensatory backup system for bile acid efflux from the hepatocyte into sinusoidal blood when the normal vectorial transport of bile acids from the hepatocyte into bile is compromised (Scheffer et al., 2002; Teng and Piquette-Miller, 2007; Gradhand et al., 2008; Chai et al., 2012). The contribution of Mrp4 to bile acid homeostasis and its role in the development of liver injury are highlighted by elevated liver concentrations of specific bile acids and increased liver toxicity in bile duct–ligated Mrp4 knockout compared with wild-type mice (Mennone et al., 2006).
As previous studies demonstrated, the prediction of cholestatic potential of a compound based on BSEP inhibitor status alone is characterized by a high incidence of false negatives (i.e., cholestatic compounds that do not inhibit BSEP) and false positives (i.e., BSEP inhibitors that are non-cholestatic). In the present study, 88 non-cholestatic as well as cholestatic drugs classified as BSEP inhibitors or non-inhibitors, and the relationship between inhibition of MRP3, MRP4, and the risk of cholestatic DILI, were investigated. This study was designed to test the hypothesis that the prediction of cholestatic DILI could be improved by information about the compound’s ability to inhibit the basolateral bile acid transporters, MRP3 and MRP4, in addition to BSEP.
Materials and Methods
Materials.
[3H]-Dehydroepiandrosterone-sulfate (DHEAS; 63 Ci/mmol), [3H]-estradiol-17β-glucuronide (E217G; 50.3 Ci/mmol), and Microscint-O liquid scintillation fluid were purchased from Perkin Elmer Life and Analytical Sciences (Waltham, MA). Compounds were purchased from Sigma-Aldrich (St. Louis, MO), unless otherwise indicated. Cell culture supplies were obtained from Gibco (Life Technologies, Grand Island, NY). Dimethylsulfoxide (DMSO) was purchased from Fisher Scientific (Fairlawn, NJ).
Compound Selection.
A total of 88 structurally diverse compounds from different therapeutic groups was selected to study MRP3 and MRP4 inhibition. Compounds were classified as BSEP non-inhibitors or inhibitors, and then cross-classified as non-cholestatic or cholestatic. Cholestatic classification relied on Thompson’s Micromedex DRUGDEX index (available at http://www.micromedex.com/products/drugdex/) and the Lexicomp database (available at http://www.lexi.com/) as well as literature information. Compounds were classified as cholestatic if cholestatic liver injury or case reports of cholestasis had been reported. Compounds classified as non-cholestatic comprised a mixed population that included drugs with no potential for liver injury or drugs for which the liver damage was hepatocellular with no reports of cholestasis. The compounds were classified further as BSEP inhibitors. Using receiver operator characteristic curve analysis of 85 compounds, Dawson et al. (2012) reported 300 µM as the optimal IC50 cutoff to separate drugs that exhibited cholestatic/mixed liver injury from drugs that caused hepatocellular injury or no DILI. However, because the maximal IC50 value reported for taurocholate transport in BSEP membrane vesicles by Morgan et al. (2010) was 135 µM, this lower value was chosen for classification of BSEP inhibitors in the present study to reduce the constraint of substrate selection (Morgan et al., 2010; Dawson et al., 2012). Information on hepatic metabolism, the primary routes of excretion, and clinical parameters were retrieved from Thompson’s Micromedex DRUGDEX index, Lexicomp database, the AHFS Drug Information textbook (American Society of Health-System Pharmacists, 2012), and PubMed.
Cell Culture and Overexpression of MRP3 and MRP4.
Human MRP3 plasmid (pcDNA3.1−-MRP3) and MRP4 plasmid (pcDNA3.1+-MRP4) were kindly provided by Dr. Susan Cole (Queen’s University, Kingston, Canada) and Dr. Dietrich Keppler (German Cancer Research Center, Heidelberg, Germany), respectively. Human embryonic kidney 293T (HEK293T) cells were cultured in Dulbecco’s modified essential medium supplemented with 10% fetal bovine serum in a humidified incubator (5% CO2, 37°C). For MRP4, a stable transfected cell line was developed by transfecting HEK293T cells with the plasmid pcDNA3.1+-MRP4 using the Fugene-6 transfection kit (Roche Diagnostics, Mannheim, Germany). Single colonies were selected by treatment with hygromycin (150 µg/ml) and characterized for MRP4 protein expression using immunofluorescence and immunoblot analysis. Control cells using the vector pcDNA3.1+ were established using the same method. For membrane vesicle preparation, HEK293T-MRP4–overexpressing cells and the control cell line were trypsinated and centrifuged at 900g for 5 minutes at 4°C. The cell pellet was washed twice in 10 ml of Tris-sucrose buffer (TSB; 250 mM sucrose/50 mM Tris, pH 7.4) containing 0.25 mM CaCl2 using centrifugation conditions described above. The final cell pellet was overlaid with 10 ml of TSB containing 0.25 mM CaCl2 and protease inhibitors (complete mini EDTA-free; Roche Diagnostics), snap frozen in liquid nitrogen, and stored at −80°C.
For MRP3, transient transfection of HEK293T cells with X-tremeGENE 9 DNA transfection reagent (Roche Diagnostics) was performed according to the manufacturer’s instructions using a ratio of 3:1 of X-tremeGENE 9 and pcDNA−-MRP3 plasmid DNA. Seventy-two hours after transfection, the cells were harvested as described above for MRP4. Nontransfected cells were used to generate control membrane vesicles for the MRP3 assay.
Membrane Vesicle Preparation.
Membrane vesicles were prepared, as described previously (Ghibellini et al., 2008). Briefly, frozen cell pellets were thawed, resuspended in TSB, and exploded by N2 cavitation (300 psi, 5 minutes). After addition of EDTA (final concentration: 1 mM), the suspension was centrifuged (800g, 10 minutes, 4°C) and the supernatant was collected. The pellet was resuspended in TSB with 0.5 mM EDTA (final concentration) and centrifuged (800g, 10 minutes, 4°C). The resulting supernatants were overlaid over 35% (w/w) sucrose/50 mM Tris buffer (pH 7.4) in a high-speed centrifuge tube. After centrifugation (100,000g, 90 minutes, 4°C), the interphase was collected and added to a new high-speed centrifuge tube with 25 mM sucrose/50 mM Tris buffer (pH 7.4). After additional centrifugation (100,000g, 45 minutes, 4°C), the pellet was resuspended in 1 ml of TSB. The suspension was added to a high-speed centrifuge tube and centrifuged (100,000g, 20 minutes, 4°C). Thereafter, the supernatant was discarded and the pellet was resuspended in TSB to a concentration of ∼3–6 mg/ml. Subsequently, the suspension was homogenized using a 27-gauge needle (15 strokes). The membrane vesicle suspension was divided into aliquots, snap frozen in liquid nitrogen, and stored at −80°C.
Membrane Vesicle Assay.
Compound screening for interaction with MRP3 and MRP4 was performed using a high-throughput method (Supplemental Fig. 1). Briefly, membrane vesicles (10 µg) were incubated at 37°C with test compound (100 µM) or DMSO (vehicle control) in TSB containing MgCl2 (10 mM), creatine phosphate (10 mM), creatine kinase (100 µg/ml), ATP (4 mM), and [3H]-E217G (0.4 µCi/ml; 10 µM) for MRP3, or [3H]-DHEAS (0.7 µCi/ml; 2 µM) for MRP4, in a final volume of 50 µl. The test compound stock solutions were prepared at 10 mM in 100% DMSO, resulting in a final concentration of 1% DMSO. For control reactions, ATP was replaced with 4 mM AMP. MK571 (100 µM) was used as a positive control for inhibition. Vehicle control and positive control reactions were performed in triplicate on each 96-well plate. Incubations with test compounds were performed in a minimum of three separate experiments. After incubation for 10 minutes (MRP3) or 3.5 minutes (MRP4), the reaction was stopped by addition of 150 µl of ice-cold TSB and the entire sample was immediately filtered through GF/B Unifilters (Perkin Elmer; presoaked in 3 mM reduced glutathione/10 mM dithiothreitol in TSB overnight). Under aspiration, the wells were washed three times with ice-cold TSB using a vacuum filtration system. Microscint-20 (75 µl) was added to each well before counting radioactivity using a TopCount scintillation counter (Perkin Elmer, Waltham, MA). The ATP-dependent uptake of substrate was calculated by subtracting substrate uptake in the presence of AMP from substrate uptake in the presence of ATP. MRP-dependent substrate transport was calculated by subtracting ATP-dependent substrate accumulation in control or nontransfected membrane vesicles from substrate uptake in membrane vesicles prepared from MRP-transfected cells. Data are presented as percentage of vehicle-treated membrane vesicles (mean ± S.D.; n = 3). Kinetic parameters for E217G (MRP3) and DHEAS (MRP4) transport were estimated using the Michaelis-Menten equation. IC50 values were estimated by nonlinear regression (Prism 5.0; GraphPad Software Inc., La Jolla, CA).
Statistical Analysis Strategy.
Consistent with the study design, the primary results were obtained via a BSEP-stratified case-control analysis to evaluate the association between cholestasis and inhibition of MRP3 or MRP4. Cases were defined as compounds with a documented history of cholestatic DILI. Logistic regression models for cholestatic status were used to evaluate the predictive value of MRP3 inhibition and, separately, of MRP4 inhibition. Because BSEP inhibition is a known susceptibility factor for DILI (Morgan et al., 2010; Dawson et al., 2012), the logistic regression analyses were performed separately for BSEP non-inhibitors and BSEP inhibitors. The fitted models also were used to estimate odds ratios (with 95% confidence intervals) representing the increase in risk of cholestasis per unit increase in MRP3 or MRP4 percent inhibition. A corresponding null hypothesis, no association between cholestasis and MRP3 (or MRP4) inhibition, was tested using a Wald χ2 test procedure of size α = 0.05.
Additional analyses were performed to evaluate the robustness of these primary results to reasonable perturbations of assumptions and statistical methods used. For example, 1) the association between MRP3 (or MRP4) inhibition and cholestasis was evaluated by comparing non-cholestatic with cholestatic compounds in regard to median MRP3 (or MRP4) inhibition, followed by a Wilcoxon rank sum test procedure, and 2) association of cholestasis with MRP inhibition was evaluated in logistic regression models using dichotomized versions of MRP3 and MRP4 percent inhibition.
Additional analyses were conducted to generate new hypotheses to define MRP dichotomizations and other statistical models that might best predict cholestasis. For example, based on the primary logistic regression models, cutoffs defining MRP3 and MRP4 dichotomizations of interest included the following: 1) the MRP3 or MRP4 inhibition value that predicted a 50% chance of cholestasis, and 2) cutoffs suggested by receiver operating characteristic curve analysis. Logistic regression models of interest included predictors such as MRP4 percent inhibition, maximal unbound concentration in plasma (Cmax,u), maximum daily dose (MDD), average daily dose, protein binding, route of excretion, and extent of metabolism. In all cases, statistical computations were performed using SAS (SAS version 9.3; SAS Institute, Cary, NC) or Prism 5.0 (GraphPad Software Inc.).
Results
Characterization of Membrane Vesicles Expressing MRP3 and MRP4.
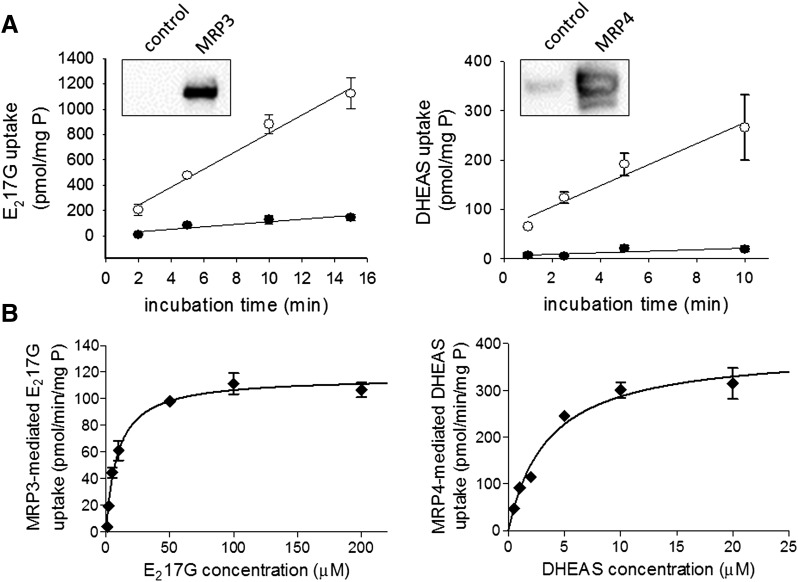
Membrane vesicles strongly expressed MRP3 and MRP4, as measured by Western blot analysis, compared with membrane vesicles from the non- or control-transfected parental cell line (Fig. 1). ATP-dependent uptake of 10 µM E217G (MRP3) and 2 µM DHEAS (MRP4) was approximately linear up to 15 and 10 minutes, respectively. MRP3-dependent E217G and MRP4-dependent DHEAS transport were concentration dependent and well described by Michaelis-Menten kinetics (Fig. 1). Km values of 9.1 ± 0.9 µM and 3.5 ± 0.5 µM, and Vmax values of 116 ± 2.7 pmol/min/mg protein and 387 ± 18 pmol/min/mg protein for MRP3 and MRP4, respectively, were estimated by nonlinear least-square regression.
Fig. 1.
Characterization of MRP3 and MRP4 membrane vesicles and substrate transport. (A) Time course of ATP-dependent substrate uptake. ATP-dependent uptake of [3H]-E217G (10 µM; MRP3, left panel) or [3H]-DHEAS (2 µM; MRP4, right panel) in plasma membrane vesicles (10 and 5 µg, respectively) prepared from control cells (●) or MRP3- or MRP4-overexpressing cells (○) was measured, as described in Materials and Methods. Insert, Western blot of MRP3 and MRP4, respectively, in membrane vesicles prepared from MRP-overexpressing or control cells. (B) Concentration-dependent transport. The rates of MRP3-mediated [3H]-E217G transport (left panel) and MRP4-mediated [3H]-DHEAS transport (right panel) were measured in membrane vesicles from MRP3- or MRP4-overexpressing cells or the respective control cells in the presence of 4 mM ATP or AMP. MRP-mediated ATP-dependent uptake was calculated, as described in Materials and Methods. Each point represents mean ± S.D. [n = 1 in triplicate for time dependency (A); representative mean ± S.D. data of two independent experiments performed in triplicate for concentration dependency (B)].
Inhibition of MRP Transport Activity in Membrane Vesicles by the Test Compounds.
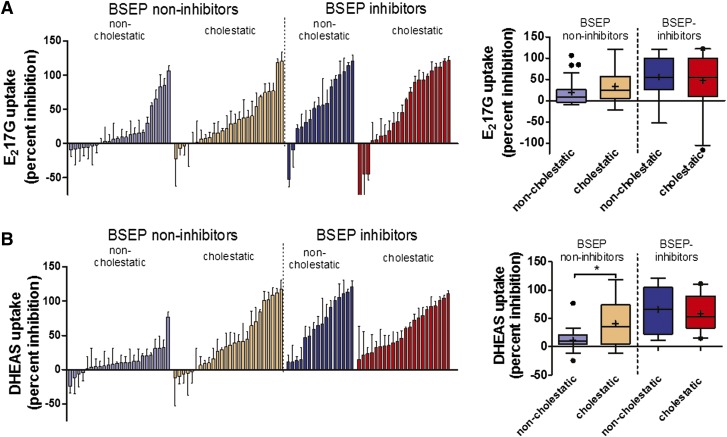
Eighty-eight drugs including 50 BSEP non-inhibitors (24 non-cholestatic, 26 cholestatic) and 38 BSEP inhibitors (16 non-cholestatic, 22 cholestatic) (Morgan et al., 2010; Dawson et al., 2012) were screened for inhibition of MRP3- and MRP4-mediated substrate transport in membrane vesicles. A wide range of inhibition values was observed in all groups. For MRP3, pioglitazone, rosiglitazone, ticlopidine, and praziquantel stimulated, rather than inhibited, substrate transport by more than 40% (Fig. 2; Table 1). Correlation between MRP3 and MRP4 percent inhibition was observed among BSEP non-inhibitors and BSEP inhibitors (Spearman’s r = 0.53 and r = 0.40, respectively; both P values ≤0.05; data not shown). The box-and-whisker plots (Fig. 2, right panel) show that among BSEP non-inhibitors, median MRP4 (and MRP3) percent inhibition was higher for cholestatic compounds compared with non-cholestatic compounds, whereas similar median percent MRP4 (and MRP3) inhibition was observed for non-cholestatic and cholestatic compounds among BSEP inhibitors (Fig. 2).
Fig. 2.
Inhibitory potency of non-cholestatic (n = 40) and cholestatic drugs (n = 48) on MRP3-(A) and MRP4-(B) mediated transport. Compounds were classified based on cholestatic potential and further classified as BSEP non-inhibitors versus BSEP inhibitors using an IC50 cutoff of 135 µM based on taurocholate transport in BSEP membrane vesicles. The Left panel indicates ATP-dependent E217G (MRP3) (A) and DHEAS (MRP4) (B) uptake measured in the presence of 100 µM compound or vehicle. Data are presented as percent inhibition compared with vehicle-treated control; values show mean ± S.D. of three independent experiments. Compounds are ordered by the magnitude of percent inhibition, and the order does not necessarily match for MRP3 and MRP4 inhibition. Pioglitazone, a cholestatic BSEP inhibitor, was omitted from the MRP3 figure for presentation purposes (mean percent inhibition −166% ± 66%). Right panel indicates box-and-whisker plots of percent MRP3 and MRP4 inhibition by type of liver injury for BSEP non-inhibitors and BSEP inhibitors. The boxes show median (−), mean (+), and upper and lower quartiles. The whiskers indicate the trimmed range of values defined by 1.5 times the interquartile range, whereas values outside the trimmed range are highlighted (●).
TABLE 1.
Inhibitory effect of 88 compounds on MRP3-mediated E217G transport and MRP4-mediated DHEAS transport, and reported BSEP inhibition
Values in bold indicate that the compounds are defined as MRP3 and MRP4 inhibitors at a concentration of 100 μM based on a cutoff of 19% and 21% for inhibition of MRP3- and MRP4-mediated transport to predict cholestatic potential, respectively.
| Drug | Pharmacology | DILI Type | DILI Severity | MRP3 % Inhibition | MRP4 % Inhibition | BSEP Inhibition | |
|---|---|---|---|---|---|---|---|
| (Mean ± S.D.)a | (Mean ± S.D.)a | IC50 (µM)b | IC50 (µM)c | ||||
| Non-cholestatic BSEP Non-inhibitors | |||||||
| 5-Fluorouracil | Antineoplastic | −d | −6 ± 12 | 1 ± 26 | >135 | ||
| Alprenolol | Antihypertensive | −d | 17 ± 3 | 10 ± 40 | >135 | ||
| Antipyrine | Analgesic/Antipyretic | − | −8 ± 23 | −5 ± 15 | >135 | ||
| Aspirin | NSAID | HCe | 9 ± 11 | 9 ± 42 | >135 | ||
| Caffeine | Stimulant | −d | 3 ± 10 | 5 ± 9 | >135 | ||
| Chlorpheniramine | Antihistamine | −d | 0 ± 7 | 20 ± 10 | >135 | ||
| Clopamide | Diuretic | −d | 6 ± 24 | 10 ± 16 | >135 | ||
| Dexamethasone | Antiinflammatory/Immunosuppressant | −d | −9 ± 9 | 5 ± 34 | 137.4 | >135 | |
| Diphenhydramine | Antihistamine | −d | 7 ± 2 | 31 ± 9 | >135 | ||
| Doxorubicin | Antineoplastic | HCe | −7 ± 19 | 12 ± 12 | >135 | ||
| Etoposide | Antineoplastic | HCe | 107 ± 7 | 33 ± 21 | >135 | ||
| Fluorescein | Diagnostic | −d | 84 ± 17 | 5 ± 10 | >135 | ||
| Metoclopramide | Antiemetic | − f | 3 ± 21 | −12 ± 22 | >135 | ||
| Nadolol | Antianginal/Antihypertensive | −d | 30 ± 9 | −25 ± 24 | >135 | ||
| Naloxone | Opioid antagonist | −d | 9 ± 9 | −7 ± 24 | >135 | ||
| Phenformin | Antidiabetic | −d | 15 ± 9 | 20 ± 50 | >135 | ||
| Probenecid | Antigout agent | HCg | 65 ± 13 | 8 ± 28 | 564.8 | ||
| Quinidine | Antiarrhythmic | HCe | 55 ± 4 | 77 ± 8 | >135 | ||
| Tacrine | Colinesterase inhibitor | HCe | −5 ± 17 | 6 ± 12 | >1000 | >135 | |
| Terbutaline | Sympathicomimetic | −f | −2 ± 11 | 20 ± 8 | >135 | ||
| Theophylline | Antiasthmatic | −g | 13 ± 19 | 4 ± 8 | >135 | ||
| Timolol | Antianginal/Antihypertensive | −d,f | 16 ± 18 | 12 ± 13 | >135 | ||
| Triamterene | Diuretic | −g | −3 ± 27 | 31 ± 21 | >135 | ||
| Vinblastine |
Antineoplastic |
HCe |
85 ± 11 |
10 ± 19 |
>135 |
||
| Cholestatic BSEP Non-inhibitors | |||||||
| Bezafibrate | Antilipemic | Cg | 2 ± 31 | 41 ± 6 | 231.7 | ||
| Carbamazepine | Antiepileptic | Cg | −22 ± 45 | −3 ± 14 | >1000 | ||
| Chloramphenicol | Antibiotic | Ce | 22 ± 20 | 6 ± 25 | >135 | ||
| Chlorpromazine | Antipsychotic | Cg | 77 ± 12 | 84 ± 4 | 147.6 | >135 | |
| Chlorpropamide | Antidiabetic | Ce | 15 ± 15 | −12 ± 44 | >1000 | >135 | |
| Cimetidine | Histamine H2 antagonist | Ce | 0 ± 16 | 0 ± 13 | >135 | ||
| Desipramine | Antidepressant | Ce | 38 ± 27 | 27 ± 9 | >135 | ||
| D-Penicillamine | Antiinflammatory | Cg | 9 ± 8 | −10 ± 13 | >1000 | ||
| Famotidine | Histamine H2 antagonist | Cd | 19 ± 3 | 16 ± 10 | >1000 | >135 | |
| Fluoxetine | Antidepressant | Cf | 69 ± 3 | 70 ± 15 | >135 | ||
| Furosemide | Diuretic | Cd | 29 ± 14 | 109 ± 20 | >1000 | >135 | |
| Haloperidol | Antipsychotic | Ce | 36 ± 10 | 34 ± 32 | >135 | ||
| Ibuprofen | NSAID | Cg | −1 ± 33 | 39 ± 15 | 598.6 | >135 | |
| Maprotiline | Antidepressant | Cg | 40 ± 21 | 29 ± 1 | >135 | ||
| Metformin | Antidiabetic | Cd | BBW | −3 ± 14 | −6 ± 33 | >135 | |
| Nitrofurantoin | Antibiotic | Cg | −7 ± 11 | 101 ± 11 | >1000 | ||
| Nortriptyline | Antidepressant | Ce | 54 ± 20 | 36 ± 10 | >135 | ||
| Promethazine | Antiemetic | C (P.I.) | 29 ± 19 | 64 ± 8 | >135 | ||
| Quinine | Antimalarial | Cg | 7 ± 17 | 41 ± 21 | >135 | ||
| Ranitidine | Histamine H2 antagonist | Ce | 7 ± 5 | 10 ± 10 | >1000 | >135 | |
| Sulfasalazine | Antiinflammatory | Cd | 119 ± 6 | 118 ± 13 | >135 | ||
| Sulindac | NSAID | Ce | 75 ± 13 | 112 ± 9 | 226 | >135 | |
| Tamoxifen | Antiestrogen | Ce | 120 ± 14 | 102 ± 7 | >135 | ||
| Tolbutamide | Antidiabetic | Ce | 38 ± 11 | −5 ± 23 | >1000 | >135 | |
| Trimethoprim | Antibiotic | Ce | 15 ± 17 | 9 ± 17 | >135 | ||
| Verapamil |
Antiarrhythmic |
Ce |
77 ± 11 |
44 ± 7.4 |
>135 |
||
| Non-cholestatic BSEP Inhibitors | |||||||
| Alpidem | Anxiolytic | HCb | Withdrawn | 55 ± 13 | 47 ± 15 | 9.2 | |
| Benzbromarone | Antigout agent | HCd | Withdrawn | 121 ± 8 | 111 ± 4 | 17.5 | |
| Buspirone | Anxiolytic | −d | −9 ± 25 | 13 ± 6 | 104.5 | ||
| Clobetasol propionate | Corticosteroid | −d | 83 ± 10 | 101 ± 23 | 8.5 | ||
| Finasteride | 5α-Reductase inhibitor | −d | 22 ± 5 | 49 ± 8 | 28.2 | ||
| Flupirtine | Nonopioid analgesic agent | HCh | 31 ± 17 | 11 ± 13 | 35.5 | ||
| Glafenine | NSAID | HCe | 59 ± 20 | 105 ± 9 | 22.3 | ||
| Lopinavir | Antiretroviral | HCd | 105 ± 20 | 76 ± 11 | 17.3 | ||
| Mibefradil | Antihypertensive | −d | 95 ± 5 | 91 ± 18 | <135 | ||
| Oxybutynin | Antispasmodic | −d | 51 ± 10 | 67 ± 19 | 27.4 | ||
| Praziquantel | Anthelmintic | −b | −52 ± 11 | 59 ± 16 | 96.8 | ||
| Primaquine | Antiprotozoal | HCe | 35 ± 13 | 11 ± 23 | 32.7 | ||
| Sorafenib | Tyrosine kinase inhibitor | HCd | 114 ± 5 | 121 ± 17 | 8 | ||
| Taxol | Antineoplastic | HCf | 57 ± 38 | 14 ± 26 | 15 | ||
| Tolcapone | COMT inhibitor | HCi | BBW | 101 ± 20 | 113 ± 17 | 119.6 | 34.5 |
| Valinomycin |
Antibiotic |
24 ± 19 |
65 ± 13 |
1.6 |
|||
| Cholestatic BSEP Inhibitors | |||||||
| Acitretin | Antipsoriatic retinoid | Cj | BBW | 4 ± 26 | 33 ± 14 | 18.2 | |
| Clozapine | Antipsychotic | Ce | 30 ± 16 | 25 ± 5 | 126.1 | >135 | |
| Cyclosporin A | Immunosuppressant | Ce | 106 ± 14 | 23 ± 12 | 0.5 | 0.9 | |
| Dicloxacillin | Antibiotic | Cg | 90 ± 9 | 41 ± 18 | 69.7 | <135 | |
| Erythromycin Estolate | Antibiotic | Cg | 2 ± 25 | 79 ± 6 | 4.1 | 13 | |
| Fenofibrate | Antilipemic | Ce | 5 ± 8 | 39 ± 3 | 15.3 | ||
| Fluvastatin | Antilipemic | Cd | 93 ± 11 | 62 ± 2 | 36.1 | ||
| Glyburide | Antidiabetic | Ce | 98 ± 4 | 93 ± 1 | 5.3 | 6.1 | |
| Indinavir | Antiretroviral | Cd | 75 ± 7 | 15 ± 24 | 21.2 | ||
| Indomethacin | NSAID | Ce | 64 ± 2 | 111 ± 18 | 42 | ||
| Nifedipine | Antianginal, antihypertensive | Cg | 32 ± 14 | 46 ± 6 | 30.7 | ||
| Nitrendipine | Antihypertensive | Cd | 45 ± 11 | 93 ± 11 | <135 | ||
| 19-Norethindrone | Contraceptive | Cd | 18 ± 14 | 33 ± 19 | <135 | ||
| Omeprazole | Proton pump inhibitor | Cd | 12 ± 16 | 21 ± 17 | <135 | ||
| Pioglitazone | Antidiabetic | Ck | −116 ± 48 | 34 ± 16 | 0.3 | 0.3 | |
| Rifampicin | Antibiotic | Cg | 93 ± 15 | 60 ± 14 | 11.3 | 25.3 | |
| Rifamycin SV | Antibiotic | Cg | 111 ± 6 | 75 ± 9 | 6.3 | ||
| Ritonavir | Antiretroviral | Cd | 112 ± 6 | 72 ± 3 | 2.2 | ||
| Rosiglitazone | Antidiabetic | Ck | −44 ± 9 | 88 ± 10 | 6.4 | 4.4 | |
| Simvastatin | Antilipemic | Cg | 122 ± 5 | 111 ± 8 | 24.7 | ||
| Ticlopidine | Antiplatelet | Cg | −45 ± 34 | 35 ± 12 | 74 | ||
| Troglitazone | Antidiabetic | Cl | Withdrawn | 121 ± 4 | 105 ± 5 | 2.7 | 5.9 |
BBW, black box warning; C, cholestatic; COMT, catechol-O-methyl transferase; HC, hepatocellular; NSAID, nonsteroidal anti-inflammatory drug; P.I., package insert; −, no reports of liver injury.
Control is defined as uptake in presence of 1% DMSO, no inhibitor (100% transport).
Micromedex.
Lexicomp.
BSEP Non-Inhibitors: Association of Cholestasis with MRP Inhibition.
Current in vitro BSEP inhibition assays aim to predict cholestasis at an early stage in drug development. The observed high false-negative rate poses a significant attrition risk due to the unpredicted cholestatic potential. To test the hypothesis that information on MRP3/MRP4 inhibition can reduce this false-negative rate, analyses were performed for drugs that do not inhibit BSEP.
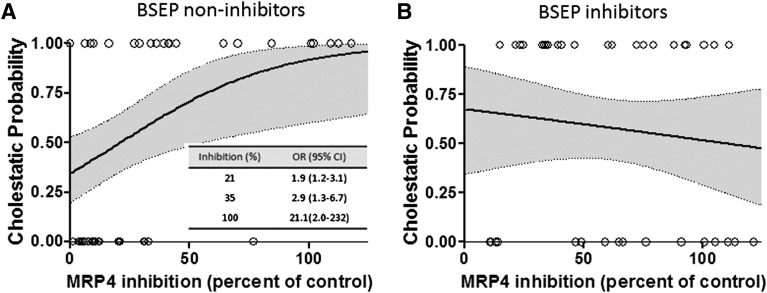
Among the 50 BSEP non-inhibitors, a statistically significant association was detected between cholestasis and percent MRP4 inhibition using logistic regression analysis (Fig. 3). The odds that a drug was cholestatic increased by 3.1% (95% confidence interval: 0.7% to 5.6%) for each 1% increase in MRP4 inhibition. In other words, as MRP4 percent inhibition increased from 0% to 21% to 100%, the probability that a drug is cholestatic increased from 35% to 50% to 92%. Using MRP4 percent inhibition ≥21%, which predicted a 50% chance of cholestasis, as a criterion for dichotomization (cutoff), 20 of the 50 BSEP non-inhibitors were classified as MRP4 inhibitors for purposes of further descriptive and exploratory analyses. As expected from the primary logistic regression analysis, this classification was predictive of cholestatic potential using a χ2 test (Table 2). Using this dichotomized approach, in the group of BSEP non-inhibitors, 62% of the cholestatic drugs (16 of 26) were MRP4 inhibitors, whereas only 17% of the non-cholestatic drugs (4 of 24) were MRP4 inhibitors (Table 2). Furthermore, among BSEP non-inhibitors, 16 of 20 (80%) MRP4 inhibitors were correctly classified as cholestatic; however, 10 of 26 (38%) cholestatic drugs were not MRP4 inhibitors.
Fig. 3.
Logistic regression curves for estimated probability of cholestatic potential based on the percent MRP4 inhibition among BSEP non-inhibitors (A) and BSEP inhibitors (B). The bold curves show the estimated probability of cholestasis, and the shaded areas represent 95% confidence intervals. The symbols denote the observed percent MRP4 inhibition values for cholestatic (values = 1.00) and non-cholestatic (values = 0.00) drugs. The inset shows the estimated odds ratios with 95% confidence intervals for different MRP4 inhibition values (e.g., a compound exhibiting 35% MRP4 inhibition has 2.9-fold greater odds of being cholestatic).
TABLE 2.
Association of MRP4 inhibition and type of liver injury
Compounds were defined as MRP4 inhibitors at a concentration of 100 μM based on a cutoff of 21% for inhibition of MRP4-mediated transport to predict cholestatic potential. χ2 test P values are shown.
| MRP4 Inhibition | |||
| BSEP Non-inhibitors (n = 50) | |||
| Cholestatic |
Non-cholestatic |
P value |
|
| MRP4 non-inhibitors | 10 | 20 | |
| 0.0016 | |||
|
MRP4 inhibitor |
16 |
4 |
|
| BSEP Inhibitors (n = 38) | |||
| Cholestatic |
Non-cholestatic |
P value |
|
| MRP4 non-inhibitors | 1 | 4 | |
| 0.14 | |||
| MRP4 inhibitor | 21 | 12 | |
A cutoff value of 19% predicted a 50% chance of cholestasis for MRP3, using the method described for MRP4. However, the primary logistic regression analysis of an association between percent MRP3 inhibition and cholestatic potential was not statistically significant. Furthermore, MRP3 and MRP4 inhibition were strongly correlated. Therefore, additional exploratory analyses described below with clinical and pharmacokinetic parameters were performed only for MRP4, but not MRP3, inhibitor status (using a cutoff of 21%).
BSEP Inhibitors: Association of Cholestasis with MRP Inhibition.
As previously noted, some drugs that inhibit BSEP in vitro are not cholestatic in vivo (Dawson et al., 2012). Categorizing compounds as potentially cholestatic based on BSEP inhibition status alone may result in the erroneous exclusion of drug candidates in the drug development process. In the present study, BSEP inhibitors were analyzed further to address whether MRP3 or MRP4 inhibition could help discriminate between cholestatic and non-cholestatic drugs in this group. Among the 38 BSEP inhibitors, association of cholestasis with percent inhibition of MRP3- or MRP4-mediated transport was not detected in the primary logistic regression analysis (Fig. 3). For further analyses, MRP4 percent inhibition was dichotomized using the same cutoff values that had been developed for BSEP non-inhibitors (Table 2; Fig. 3). Using this approach, almost all BSEP inhibitors also were classified as MRP4 inhibitors. There was a slight enrichment of MRP4 inhibitors among cholestatic compounds (95%, 21 of 22) compared with non-cholestatic drugs (75%, 12 of 16); however, this did not reach statistical significance.
Association of Clinical Parameters with BSEP and MRP4 Inhibition.
Our data demonstrate that MRP4 inhibition is able to predict cholestatic potential among compounds that are BSEP non-inhibitors; however, 38% of the cholestatic drugs in this group are not inhibitors of MRP4. Furthermore, we demonstrated that, even with information on the percent of MRP4 inhibition (and MRP4 inhibitor status), we are not able to discriminate between cholestatic and non-cholestatic drugs among BSEP inhibitors. These findings suggest that, in addition to BSEP and MRP4, other factors need to be considered to improve the prediction of liver injury. In an attempt to identify contributing elements, pharmacokinetic data were collected as described in Materials and Methods (Supplemental Table 1). Robust statistical analyses suggest that there is little or no association between the type of DILI and extent of metabolism or route of excretion, regardless of the BSEP inhibitor status (Supplemental Tables 2 and 3 with odds ratios).
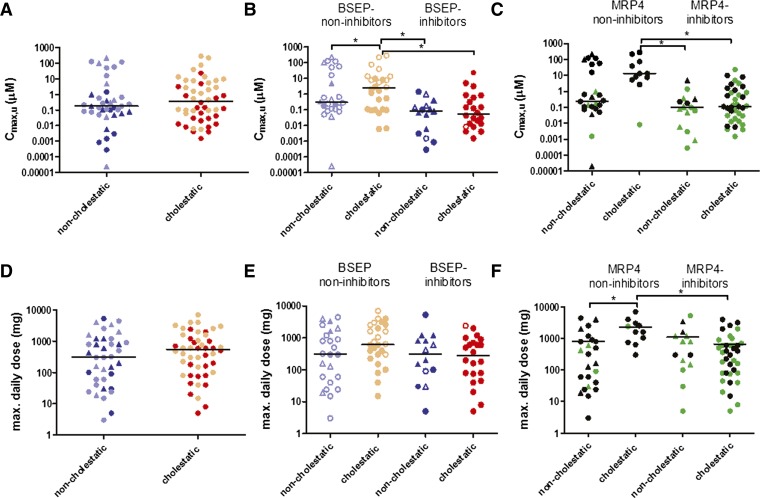
Furthermore, as observed previously by Dawson et al. (Dawson et al., 2012), there was no statistically significant difference between non-cholestatic and cholestatic compounds with regard to the Cmax,u or the MDD (Fig. 4, A and D). However, cholestatic BSEP non-inhibitors were characterized by higher median Cmax,u values compared with cholestatic BSEP inhibitors and non-cholestatic drugs. Similarly, higher median Cmax,u values were observed in cholestatic MRP4 non-inhibitors compared with both cholestatic and non-cholestatic MRP4 inhibitors (P < 0.05) (Fig. 4; Supplemental Table 4). Cholestatic MRP4 non-inhibitors had a higher median MDD compared with cholestatic MRP4 inhibitors and non-cholestatic MRP4 non-inhibitors. However, inclusion of Cmax,u, MDD, and average daily dose, protein binding, route of excretion, and extent of metabolism as predictor variables in addition to MRP4 percent inhibition in logistic regression equations did not improve the prediction of cholestasis.
Fig. 4.
Comparison of the maximal unbound concentration in plasma (Cmax,u) (A–C) and the maximal daily dose (MDD) (D–F) for non-cholestatic and cholestatic compounds. Each point represents an individual drug. The lines show the median. Statistical significance (*P < 0.05) is noted for a Kruskal-Wallis test, followed by Dunn’s multiple comparison test. (A and D) Values for Cmax,u and MDD of non-cholestatic and cholestatic compounds. Light symbols represent BSEP non-inhibitors; dark symbols represent BSEP inhibitors. (B and E) Cmax,u and MDD of non-cholestatic and cholestatic compounds are stratified by BSEP inhibition. Open symbols represent MRP4 non-inhibitors; closed symbols represent MRP4 inhibitors. Drugs known to cause hepatocellular liver toxicity are depicted as triangles. (C and F) Cmax,u and MDD of non-cholestatic and cholestatic compounds are stratified by MRP4 inhibition. Black symbols represent BSEP non-inhibitors; green symbols represent BSEP inhibitors. Drugs known to cause hepatocellular liver toxicity are depicted as triangles.
Discussion
Inhibition of BSEP, which transports bile acids from the hepatocyte into bile, correlates with the risk of cholestatic DILI in humans (Morgan et al., 2010; Dawson et al., 2012). However, comprehensive studies indicate that not all cholestatic compounds are BSEP inhibitors, and not all BSEP inhibitors cause cholestasis, suggesting that factors in addition to BSEP inhibition are involved in the development of cholestatic liver injury. The present study examined the relationship between inhibition of MRP3 and MRP4 and the cholestatic potential of drugs based on the putative role of these proteins as transporters that protect hepatocytes from the accumulation of potentially toxic bile acids.
A 96-well high-throughput membrane vesicle assay was developed to measure MRP3 and MRP4 inhibition; high protein expression and transport of the model substrates E217G and DHEAS were observed in MRP3 and MRP4 membrane vesicles, respectively. The Km values estimated for MRP3 (9.1 µM) and MRP4 (3.5 µM) were consistent with previously reported Km values of 17–43 µM for MRP3 (Zelcer et al., 2001; Akita et al., 2002), and 2 µM for MRP4 (Zelcer et al., 2003a) (Fig. 1).
In the present study, a few compounds exhibited >100% inhibition of MRP3 or MRP4, suggesting a more complex interaction with substrate transport in membrane vesicles (e.g., inhibition of ATP-independent transport processes, or allosteric interactions). Interestingly, many of these compounds (e.g., troglitazone, sorafenib, rifampicin, ritonavir) are also potent inhibitors of other transport proteins. In addition, a few drugs stimulated MRP3 or MRP4 transport activity (<0% inhibition). Although stimulation has been observed for other drug transporters such as MRP2 and organic anion transporting polypeptides (OATPs), the physiologic consequences are not known. At least in our study, the compounds that stimulated MRP transport do not appear to protect against hepatotoxicity; a similar percentage of these compounds was present in the non-cholestatic and cholestatic groups.
For BSEP non-inhibitors, a substantial association was detected between the extent of inhibition of MRP4-mediated transport and cholestatic potential, as follows: (1) for each 1% increase in MRP4 inhibition, the odds of the drug being cholestatic increased by 3.1%, and (2) if the drug inhibited MRP4 by at least 21%, there was at least a 50% chance of cholestasis (Fig. 3). Using the 21% inhibition as a cutoff for classification of MRP4 inhibition, a strong enrichment of MRP4 inhibitors among cholestatic compared with non-cholestatic BSEP non-inhibitors was observed (62% versus 17%) (Table 2). These findings highlight that screening for MRP4 inhibition may reduce the false-negative rate (i.e., cholestatic compounds that do not inhibit BSEP) observed when predicting cholestatic DILI based on in vitro BSEP inhibition data.
As many BSEP inhibitors are not associated with cholestatic liver injury, we hypothesized that knowledge about MRP3 and MRP4 inhibition also could provide additional information to discriminate between cholestatic and non-cholestatic drugs in the group of BSEP inhibitors. Interestingly, BSEP inhibitors tended to yield higher levels of MRP4 inhibition (Fig. 2), and we observed that almost all BSEP inhibitors also inhibited MRP4 (Table 2). Consequently, for BSEP inhibitors, the extent of MRP4 inhibition did not provide additional information as a predictor of cholestatic potential. Although there was a slight, but not statistically significant, enrichment of MRP4 inhibitors among cholestatic compounds, these data suggest that MRP4 inhibition alone is unable to distinguish between cholestatic and non-cholestatic drugs among BSEP inhibitors.
MRP3 inhibition (regardless of BSEP-inhibitor status) was not predictive of cholestasis based on the present data. The importance of MRP4 over MPR3 in cholestatic conditions is supported by studies using knockout mice. Increased liver toxicity, decreased serum bile acid concentrations, and elevated liver concentrations of specific bile acids were reported after bile duct ligation in Mrp4 knockout mice, but not in Mrp3 knockout mice compared with wild-type mice (Belinsky et al., 2005; Mennone et al., 2006; Zelcer et al., 2006).
Unfortunately, preclinical models currently fail to reliably predict hepatotoxicity. This may be due to: 1) differences in bile acid disposition/composition between species; 2) disparate intracellular concentrations of hepatotoxic drugs due to species differences in binding, metabolism, and/or excretion; and/or 3) species-dependent interactions of compounds with drug transporters, either as a substrate or an inhibitor. An accurate method to identify compounds with hepatotoxic potential early in development is urgently needed. Testing the inhibitory potential of compounds for interactions with human hepatic bile acid transporters using appropriate in vitro systems could help prioritize compounds and identify molecules that would require further investigations (e.g., bile acid profiling, special screens for liver toxicity during phase II/III trials). Limitations of membrane vesicle assays include the lack of metabolic activity, the absence of subcellular organelles that may play an important role in trafficking, sequestration and excretion of compounds and derived metabolites within hepatocytes, and lack of interaction with other transport proteins compared with the in vivo situation. Polarized hepatocyte systems may provide more useful information. Of course, these in vitro systems in isolation cannot predict critical information about the pharmacokinetic properties of the compound, or the contribution of hepatic clearance to overall elimination. Use of metabolites in the membrane vesicle screening approach might improve predictability of cholestatic liver injury, although human-specific metabolites are not always available or even known. Especially during early development, when information on metabolites might not be readily available, use of S9 fractions in combination with the membrane vesicle assay could bypass this limitation.
In this study, we used E217G and DHEAS as surrogate model substrates to assess the inhibition potential toward MRP3 and MRP4, respectively. Consistent with previous findings by Morgan et al. (2013), bile acids could not be used as reliable assay substrates to accurately predict MRP inhibitors. It is unknown whether bile acids would be more sensitive substrates to predict cholestasis. Nevertheless, our data demonstrate that the MRP4 inhibition potential of a compound toward the surrogate substrate can improve prediction of the cholestatic potential.
Selection of the appropriate concentration for transport inhibition studies is the subject of much debate. Theoretically, the unbound hepatic cytosolic inhibitor concentration would be the relevant concentration to select for these studies. As discussed in the recently published International Transporter Consortium white paper (Chu et al., 2013), intracellular unbound concentrations may be similar to, or orders of magnitude higher than, plasma concentrations, depending on the intrinsic hepatic uptake and elimination clearance values. The concentration selected for investigation in the present study (100 µM) was in the same range as recently published data showing a correlation between BSEP inhibition and the cholestatic potential of drugs in which IC50 cutoff values of ∼130 µM or even 300 µM were used to predict cholestatic versus non-cholestatic drugs, despite generally low unbound plasma concentrations (Morgan et al., 2010; Dawson et al., 2012). Further work is needed to measure relevant hepatocyte concentrations of drugs and their metabolites, and evaluate the association of unbound intracellular concentrations with the occurrence of cholestatic DILI. This approach also may help identify compounds that are BSEP inhibitors in vitro but are not cholestatic in vivo.
Factors other than MRP3, MRP4, and BSEP inhibition that may improve the prediction of liver injury also were examined in the present study. Robust statistical regression analyses suggested that the extent of hepatic metabolism was not associated with the risk of cholestatic liver injury (Supplemental Table 2). Consistent with our findings, Lammert et al. observed no statistically significant relationship between the extent of metabolism and drugs that caused jaundice, which often is associated with cholestasis. In contrast, these authors did detect a relationship between the extent of hepatic metabolism and some types of hepatic injury such as liver failure or fatal DILI (Lammert et al., 2010). However, the intracellular concentration of metabolite(s) that inhibits bile acid transport rather than the extent of overall metabolism is more likely the important factor associated with liver injury.
Similarly, the primary route of excretion was not associated with risk of cholestatic liver injury (Supplemental Table 3). This is contrary to the results of a previous study showing that jaundice was associated with biliary excretion (Lammert et al., 2010). Interestingly, statistical analyses revealed an apparent association between biliary excretion and BSEP inhibition (data not shown). This is surprising because BSEP has been reported to have limited ability to transport drugs; only pravastatin and vinblastine have been identified as substrates of human or rodent BSEP (Lecureur et al., 2000; Hirano et al., 2005). These results suggest that close proximity to the canalicular membrane is important to exert BSEP inhibition in vivo, or that there is a strong overlap between substrates of canalicular drug transporters such as MRP2 or BCRP, and BSEP inhibitors.
Daily dose is an important factor for development of DILI; drugs with daily doses >50 mg demonstrate an increased risk of causing liver failure (Lammert et al., 2008; Lucena et al., 2009). Our study did not detect an association between the type of liver injury (non-cholestatic no-liver injury versus cholestatic) and the maximal or average daily dose or Cmax,u (Fig. 4; Supplemental Table 4). Notably, higher median Cmax,u values were observed for cholestatic drugs that were not BSEP or MRP4 inhibitors compared with cholestatic drugs that were BSEP or MRP4 inhibitors, respectively. Cholestatic drugs that are BSEP or MRP4 non-inhibitors may have different physicochemical properties that also influence pharmacokinetic parameters.
While the present work was under review, Morgan et al. (2013) published new findings supporting the hypothesis that the risk of DILI may be even greater if the compound inhibits one or more of the other hepatic bile acid transporters (MRP2, MRP3, and/or MRP4) in addition to BSEP. They recommend that if the compound is a BSEP inhibitor, the inhibitory potency on MRP2, MRP3, and MRP4 also should be evaluated to improve the correlation with liver injury compared with inhibition of BSEP alone. Adding to these findings, our data emphasize that even among BSEP non-inhibitors, MRP4 inhibition is associated with an increased risk of cholestatic potential. Thus, screening for MRP4 inhibition can reduce the rate of false negatives (i.e., cholestatic compounds that do not inhibit BSEP).
In conclusion, this work highlights that inhibition of MRP4, a basolateral bile acid transporter, is a risk factor for the development of cholestatic DILI. This result is consistent with the hypothesis that inhibition of bile acid transporters contributes to the development of cholestatic DILI. Although inhibition of hepatic bile acid transport is a susceptibility factor for the development of liver injury, other environmental, genetic, immunologic, as well as drug-specific factors may influence the overall risk for a patient to develop DILI. The present data strongly suggest that, in addition to BSEP inhibition, evaluating the interaction potential of drug candidates with human MRP4 could aid in identifying compounds with reduced liability of DILI at a much earlier stage in drug development.
Supplementary Material
Acknowledgments
The authors thank Dr. Susan Cole (Queen’s University, Kingston, Canada) and Dr. Dietrich Keppler (German Cancer Research Center, Heidelberg, Germany) for kindly providing the MRP3 (pcDNA3.1−-MRP3) and MRP4 (pcDNA3.1+-MRP4) expression vectors, respectively.
Abbreviations
- BSEP
bile salt export pump
- Cmax,u
maximal unbound concentration in plasma
- DHEAS
dehydroepiandrosterone sulfate
- DILI
drug-induced liver injury
- DMSO
dimethylsulfoxide
- E217G
estradiol-17β-glucuronide
- MDD
maximal daily dose
- MRP
multidrug resistance-associated protein
- TSB
Tris-sucrose buffer
Authorship Contributions
Participated in research design: Köck, Ferslew, Netterberg, Yang, Urban, Swaan, Stewart, Brouwer.
Conducted experiments: Köck, Ferslew, Netterberg, Yang.
Performed data analysis: Köck, Ferslew, Netterberg, Stewart.
Wrote or contributed to the writing of the manuscript: Köck, Ferslew, Yang, Urban, Swaan, Stewart, Brouwer.
Footnotes
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant R01 GM041935 to K.L.R.B. and a NIGMS Collaborative Science Supplement to K.L.R.B, T.J.U. and P.W.Sw.] and by Deutsche Forschungsgemeinschaft [Grant Ko4186/1-1 to K.K.]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This work was presented, in part, as a poster at the Society of Toxicology 51st Annual Meeting; 2012 Mar 10–15; San Francisco, CA, and at the American Association for the Study of Liver Disease 63rd Annual Meeting; 2012 Nov 9–13; Boston, MA.
 This article has supplemental materials available at dmd.aspetjournals.org.
This article has supplemental materials available at dmd.aspetjournals.org.
References
- AHFS Drug Information. (2012) American Society of Health-System Pharmacists, Bethesda, MD.
- Akita H, Suzuki H, Hirohashi T, Takikawa H, Sugiyama Y. (2002) Transport activity of human MRP3 expressed in Sf9 cells: comparative studies with rat MRP3. Pharm Res 19:34–41 [DOI] [PubMed] [Google Scholar]
- Belinsky MG, Dawson PA, Shchaveleva I, Bain LJ, Wang R, Ling V, Chen ZS, Grinberg A, Westphal H, Klein-Szanto A, et al. (2005) Analysis of the in vivo functions of Mrp3. Mol Pharmacol 68:160–168 [DOI] [PubMed] [Google Scholar]
- Chai J, He Y, Cai SY, Jiang Z, Wang H, Li Q, Chen L, Peng Z, He X, Wu X, et al. (2012) Elevated hepatic multidrug resistance-associated protein 3/ATP-binding cassette subfamily C 3 expression in human obstructive cholestasis is mediated through tumor necrosis factor alpha and c-Jun NH2-terminal kinase/stress-activated protein kinase-signaling pathway. Hepatology 55:1485–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu X, Korzekwa K, Elsby R, Fenner K, Galetin A, Lai Y, Matsson P, Moss A, Nagar S, Rosania GR, et al. International Transporter Consortium. (2013) Intracellular drug concentrations and transporters: measurement, modeling, and implications for the liver. Clin Pharmacol Ther 94(1):126–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson S, Stahl S, Paul N, Barber J, Kenna JG. (2012) In vitro inhibition of the bile salt export pump correlates with risk of cholestatic drug-induced liver injury in humans. Drug Metab Dispos 40:130–138 [DOI] [PubMed] [Google Scholar]
- Fattinger K, Funk C, Pantze M, Weber C, Reichen J, Stieger B, Meier PJ. (2001) The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther 69:223–231 [DOI] [PubMed] [Google Scholar]
- Floyd JS, Barbehenn E, Lurie P, Wolfe SM. (2009) Case series of liver failure associated with rosiglitazone and pioglitazone. Pharmacoepidemiol Drug Saf 18:1238–1243 [DOI] [PubMed] [Google Scholar]
- Ghibellini G, Leslie EM, Pollack GM, Brouwer KLR. (2008) Use of tc-99m mebrofenin as a clinical probe to assess altered hepatobiliary transport: integration of in vitro, pharmacokinetic modeling, and simulation studies. Pharm Res 25:1851–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradhand U, Lang T, Schaeffeler E, Glaeser H, Tegude H, Klein K, Fritz P, Jedlitschky G, Kroemer HK, Bachmakov I, et al. (2008) Variability in human hepatic MRP4 expression: influence of cholestasis and genotype. Pharmacogenomics J 8:42–52 [DOI] [PubMed] [Google Scholar]
- Hirano M, Maeda K, Hayashi H, Kusuhara H, Sugiyama Y. (2005) Bile salt export pump (BSEP/ABCB11) can transport a nonbile acid substrate, pravastatin. J Pharmacol Exp Ther 314:876–882 [DOI] [PubMed] [Google Scholar]
- Hofmann AF. (1999) The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med 159:2647–2658 [DOI] [PubMed] [Google Scholar]
- Jansen PL and Muller M (2000) Genetic cholestasis: lessons from the molecular physiology of bile formation. Can J Gastroenterol 14:233-238. [DOI] [PubMed]
- Jansen PL, Strautnieks SS, Jacquemin E, Hadchouel M, Sokal EM, Hooiveld GJ, Koning JH, De Jager-Krikken A, Kuipers F, Stellaard F, et al. (1999) Hepatocanalicular bile salt export pump deficiency in patients with progressive familial intrahepatic cholestasis. Gastroenterology 117:1370–1379 [DOI] [PubMed] [Google Scholar]
- Kaplowitz N and DeLeve LD (2007) Drug-Induced Liver Disease, 2nd ed, CRC Press, New York. [Google Scholar]
- Kostrubsky VE, Strom SC, Hanson J, Urda E, Rose K, Burliegh J, Zocharski P, Cai H, Sinclair JF, and Sahi J (2003) Evaluation of hepatotoxic potential of drugs by inhibition of bile-acid transport in cultured primary human hepatocytes and intact rats. Toxicol Sci 76:220-228. [DOI] [PubMed]
- Kreiss C, Amin S, Nalesnik MA, Chopra K, Shakil AO. (2002) Severe cholestatic hepatitis in a patient taking acitretin. Am J Gastroenterol 97:775–777 [DOI] [PubMed] [Google Scholar]
- Lammert C, Bjornsson E, Niklasson A, Chalasani N. (2010) Oral medications with significant hepatic metabolism at higher risk for hepatic adverse events. Hepatology 51:615–620 [DOI] [PubMed] [Google Scholar]
- Lammert C, Einarsson S, Saha C, Niklasson A, Bjornsson E, Chalasani N. (2008) Relationship between daily dose of oral medications and idiosyncratic drug-induced liver injury: search for signals. Hepatology 47:2003–2009 [DOI] [PubMed] [Google Scholar]
- Lasser KE, Allen PD, Woolhandler SJ, Himmelstein DU, Wolfe SM, Bor DH. (2002) Timing of new black box warnings and withdrawals for prescription medications. JAMA 287:2215–2220 [DOI] [PubMed] [Google Scholar]
- Lecureur V, Sun D, Hargrove P, Schuetz EG, Kim RB, Lan LB, Schuetz JD. (2000) Cloning and expression of murine sister of P-glycoprotein reveals a more discriminating transporter than MDR1/P-glycoprotein. Mol Pharmacol 57:24–35 [PubMed] [Google Scholar]
- Lee WM. (2003) Acute liver failure in the United States. Semin Liver Dis 23:217–226 [DOI] [PubMed] [Google Scholar]
- Lucena MI, Andrade RJ, Kaplowitz N, García-Cortes M, Fernández MC, Romero-Gomez M, Bruguera M, Hallal H, Robles-Diaz M, Rodriguez-González JF, et al. Spanish Group for the Study of Drug-Induced Liver Disease (2009) Phenotypic characterization of idiosyncratic drug-induced liver injury: the influence of age and sex. Hepatology 49:2001–2009 [DOI] [PubMed] [Google Scholar]
- Mayoral W, Lewis JH, Zimmerman H. (1999) Drug-induced liver disease. Curr Opin Gastroenterol 15:208–216 [DOI] [PubMed] [Google Scholar]
- Mennone A, Soroka CJ, Cai SY, Harry K, Adachi M, Hagey L, Schuetz JD, Boyer JL. (2006) Mrp4-/- mice have an impaired cytoprotective response in obstructive cholestasis. Hepatology 43:1013–1021 [DOI] [PubMed] [Google Scholar]
- Morgan RE, Trauner M, van Staden CJ, Lee PH, Ramachandran B, Eschenberg M, Afshari CA, Qualls CW Jr, Lightfoot-Dunn R, and Hamadeh HK (2010) Interference with bile salt export pump function is a susceptibility factor for human liver injury in drug development. Toxicol Sci 118:485-500. [DOI] [PubMed]
- Morgan RE, van Staden CJ, Chen Y, Kalyanaraman N, Kalanzi J, Dunn RT 2nd, Afshari CA, and Hamadeh HK (2013) A multifactorial approach to hepatobiliary transporter assessment enables improved therapeutic compound development. Toxicol Sci [published ahead of print]. [DOI] [PubMed]
- Noé J, Stieger B, Meier PJ. (2002) Functional expression of the canalicular bile salt export pump of human liver. Gastroenterology 123:1659–1666 [DOI] [PubMed] [Google Scholar]
- Perez MJ, Briz O. (2009) Bile-acid-induced cell injury and protection. World J Gastroenterol 15:1677–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puls F, Agne C, Klein F, Koch M, Rifai K, Manns MP, Borlak J, Kreipe HH. (2011) Pathology of flupirtine-induced liver injury: a histological and clinical study of six cases. Virchows Arch 458:709–716 [DOI] [PubMed] [Google Scholar]
- Rius M, Nies AT, Hummel-Eisenbeiss J, Jedlitschky G, Keppler D. (2003) Cotransport of reduced glutathione with bile salts by MRP4 (ABCC4) localized to the basolateral hepatocyte membrane. Hepatology 38:374–384 [DOI] [PubMed] [Google Scholar]
- Scheffer GL, Kool M, de Haas M, de Vree JM, Pijnenborg AC, Bosman DK, Elferink RP, van der Valk P, Borst P, and Scheper RJ (2002) Tissue distribution and induction of human multidrug resistant protein 3. Lab Invest 82:193-201. [DOI] [PubMed]
- Stieger B, Fattinger K, Madon J, Kullak-Ublick GA, Meier PJ. (2000) Drug- and estrogen-induced cholestasis through inhibition of the hepatocellular bile salt export pump (Bsep) of rat liver. Gastroenterology 118:422–430 [DOI] [PubMed] [Google Scholar]
- Stricker BHC (1992) Drug-Induced Hepatic Injury, 2nd ed., Elsevier, Amsterdam, New York. [Google Scholar]
- Teng S, Piquette-Miller M. (2007) Hepatoprotective role of PXR activation and MRP3 in cholic acid-induced cholestasis. Br J Pharmacol 151:367–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M, Zollner G, Trauner M. (2009) New molecular insights into the mechanisms of cholestasis. J Hepatol 51:565–580 [DOI] [PubMed] [Google Scholar]
- Zelcer N, Reid G, Wielinga P, Kuil A, van der Heijden I, Schuetz JD, Borst P. (2003a) Steroid and bile acid conjugates are substrates of human multidrug-resistance protein (MRP) 4 (ATP-binding cassette C4). Biochem J 371:361–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelcer N, Saeki T, Bot I, Kuil A, Borst P. (2003b) Transport of bile acids in multidrug-resistance-protein 3-overexpressing cells co-transfected with the ileal Na+-dependent bile-acid transporter. Biochem J 369:23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelcer N, Saeki T, Reid G, Beijnen JH, Borst P. (2001) Characterization of drug transport by the human multidrug resistance protein 3 (ABCC3). J Biol Chem 276:46400–46407 [DOI] [PubMed] [Google Scholar]
- Zelcer N, van de Wetering K, de Waart R, Scheffer GL, Marschall HU, Wielinga PR, Kuil A, Kunne C, Smith A, van der Valk M, et al. (2006) Mice lacking Mrp3 (Abcc3) have normal bile salt transport, but altered hepatic transport of endogenous glucuronides. J Hepatol 44:768–775 [DOI] [PubMed] [Google Scholar]
- Zeng H, Liu G, Rea PA, Kruh GD. (2000) Transport of amphipathic anions by human multidrug resistance protein 3. Cancer Res 60:4779–4784 [PubMed] [Google Scholar]
- Zimmermann HJ (1999) Hepatotoxicity: the Adverse Effects of Drugs and Other Chemicals on the Liver Lippincott Williams & Wilkins; Philadelphia [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.