Abstract
The UDP-glucuronosyltransferase (UGT) 2B subfamily of enzymes plays an important role in the metabolism of numerous endogenous and exogenous compounds, including various carcinogens present in tobacco smoke. The goal of the present study was to examine the levels of expression of individual UGT2B genes in various tissues that are targets for tobacco carcinogenesis. Using MT-ATP6 as the experimentally validated housekeeping gene, the highest extrahepatic expression of UGT2B genes was observed in human tonsil, with UGT2B expression levels similar to that observed in human liver. UGT2B17 exhibited high relative expression in most tissues examined, including lung, most tissues of the aerodigestive tract, and pancreas. UGT2B7 expression was highest in pancreas but low or undetectable in most other tissues examined. UGT2B10 expression was high in both tonsil and tongue. There was wide variability between individuals in the magnitude of expression in each tissue site, and there were strong correlations between UGT2B expression levels in different individuals within many of the tissue sites, suggesting coordinated regulation of UGT2B gene expression in extrahepatic tissues. In the liver, UGTs 2B4, 2B7, 2B10, and 2B15 were significantly correlated with each other (all r2 > 0.70, P < 0.0001). In all examined tissues of the aerodigestive tract, UGTs 2B10, 2B11, and 2B17 exhibited a strong correlation with each other (all r2 > 0.75, P < 0.05). UGTs 2B7 and 2B10 exhibited a strong inverse correlation in the pancreas (r2 = –0.95, P < 0.01). These data suggest that specific UGT2B enzymes important in tobacco carcinogen metabolism are expressed and coordinately regulated in various target sites for tobacco-related cancers.
Introduction
The UDP-glucuronosyltransferase (UGT) family of phase II metabolic enzymes play a central role in the excretion of numerous endogenous compounds, including bilirubin (Bosma et al., 1994) and steroid hormones (Belanger et al., 1998, 2003), as well as exogenous compounds, including various drugs (Nagar and Remmel, 2006) and chemotherapeutic agents (Kemp et al., 2002; Sun et al., 2006, 2007, 2010; Balliet et al., 2009). The UGTs also play an important role in the metabolism and detoxification of multiple tobacco carcinogens, including tobacco-specific nitrosamines like 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and its major metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) (Ren et al., 2000; Wiener et al., 2004a; Chen et al., 2008b), and polycyclic aromatic hydrocarbons like benzo(a)pyrene [B(a)P]-7,8-diol and dibenzo(a,l)pyrene-11,12-diol [DB(a,l)P] (Fang et al., 2002; Olson et al., 2011). The UGTs primarily consist of two large subfamilies, the UGT1As and the UGT2Bs. The UGT1A family, located on chromosome 2q37, codes for nine functional protein isoforms sharing alternative 1st exons spliced to common exons 2–5. The UGT2B family members, located on chromosome 4q13, are expressed as individual genes encoded by six exons each with a unique 3′-untranslated region (Mackenzie et al., 2005; Nagar and Remmel, 2006). The UGTs are generally highly expressed in liver, the primary organ of metabolism, but also extrahepatically in tissues such as lung, the aerodigestive tract, and pancreas, all of which are important targets for tobacco-induced cancers (Beaulieu et al., 1996, 1998; Levesque et al., 1997, 1999; Strassburg et al., 1997, 1998; Tukey and Strassburg, 2001; Turgeon et al., 2001; Nakamura et al., 2008b; Court et al., 2012). Several previous studies have shown differential expression of the UGT1A family of genes, with UGTs 1A1, 1A3, 1A4, 1A5, 1A6 and 1A9 being the primary hepatic isoforms while UGTs 1A7, 1A8, and 1A10 are exclusively expressed extrahepatically (Nagar and Remmel, 2006; Nakamura et al., 2008b; Izukawa et al., 2009; Jones et al., 2012). UGT2B genes are expressed both hepatically and in several extrahepatic tissues (Tukey and Strassburg, 2000; Aueviriyavit et al., 2007; Izukawa et al., 2009; Ohno and Nakajin, 2009; Court et al., 2012), but few studies have performed a quantitative assessment of the expression of individual UGT2B isoforms in tissues that are targets for tobacco carcinogenesis, including sites within the aerodigestive and respiratory tracts. In addition, extensive interindividual variability of UGT2B expression has been described for individual UGT2B genes within organ sites, and this may contribute to variability in patient response and toxicity (Court et al., 2001; Hoskins et al., 2007; Peterkin et al., 2007), but few studies have examined UGT2B expression in tissues from multiple individuals. The goals of the present study were to examine the expression of UGT2B genes in tobacco target tissues including tissues of the aerodigestive and respiratory tracts and to examine the interindividual variability of expression of UGT2B genes in these tobacco-related target tissues.
Materials and Methods
Tissue Specimens.
The 114 histologically confirmed normal human tissue specimens used to evaluate UGT2B gene expression levels included the same tissues used to examine endogenous control gene expression, and included liver (n = 27), lung (n = 22), larynx (n = 7), tongue (n = 3), floor of mouth (n = 3), tonsil (n = 23), esophagus (n = 13), and pancreas (n = 16). All tissue specimens were from Caucasian individuals and were obtained from the Penn State College of Medicine Tissue Bank, where they had been quick frozen within 2 hours postsurgery. All protocols involving the collection and analysis of tissue specimens were approved by the institutional review board at Penn State University College of Medicine and were in accordance with assurances filed with and approved by the United States Department of Health and Human Services.
RNA Collection and cDNA Synthesis.
RNA was extracted from all tissue specimens with the Qiagen RNeasy Mini Kit (Valencia, CA). Samples were subjected to DNase I digestion during extraction to prevent genomic DNA contamination. RNA concentrations were determined using a NanoDrop ND-1000 spectrophotometer, and RNA purity was assessed by absorbance ratios A260/A280 (>1.9) and A260/A230 (>1.8). RNA integrity was determined using an Agilent 2100 Bioanalyzer with Agilent RNA 6000 Nano chips (Santa Clara, CA). All RNA samples used for analysis had RNA integrity numbers (RINs) greater than 5.0. Reverse transcription was performed using the Invitrogen Superscript First Strand cDNA synthesis kit (Carlsbad, CA) with oligo(dT) primers and 1 μg of starting RNA per sample. A negative control without RNA and a negative control without reverse transcriptase enzyme were analyzed in parallel.
Determination of Real-Time Polymerase Chain Reaction Kinetics for MT-ATP6 Gene and UGT2B Target Genes.
As described in the Supplemental Materials and Methods to the present study, the real-time polymerase chain reaction (qPCR) kinetics of the MT-ATP6 gene was tested and compared with several (n = 31) putative target genes. MT-ATP6 was shown to exhibit the lowest percent covariance within the majority of tissues (6 out of 10 tissue sites) and the lowest percent covariance between different tissue sites (4.2%) (Supplemental Table 1). The range of Ct values for MT-ATP6 was less than 3.0 cycles, suggesting that it was the most stably expressed control gene analyzed (Supplemental Fig. 1). For this reason, MT-ATP6 was chosen as the experimentally validated housekeeping gene for the current study. For a more complete discussion of housekeeping gene analysis and selection, please refer to the Supplemental Data file (includes Supplemental Materials and Methods, Results, and Discussion). For developing UGT2B and MT-ATP6 standard curves, 1 μg RNA from pooled human liver was reverse-transcribed as described above and the resulting cDNA was serially diluted. ABI gene expression assays (Life Technologies, Grand Island, NY) were used to amplify MT-ATP6, UGT2B4, UGT2B7, UGT2B10, UGT2B11, UGT2B15, and UGT2B17. The resulting average Ct values were plotted against the cDNA dilution, and the qPCR efficiency values for each of the real-time PCR assays was calculated from the slope of these standard curves using the formula, Ex = [2(–1/slope) – 1], where Ex is the amplification efficiency of the given qPCR gene expression assay. These curves were also used to determine the Ct value at which UGT2B gene expression assays become unreliable. For most, this occurred near a Ct value of 35; samples with Ct values greater than 35 were deemed below the limit of quantification (BLQ), even if there was a trace amount of transcript present.
Determination of UGT2B Expression Levels in Human Tissue Specimens via Real-Time Polymerase Chain Reaction.
Amplification by qPCR was carried out using a 25-ng RNA equivalent of cDNA. Expression levels were normalized to the expression of the MT-ATP6 gene. Quadruplicate qPCR reactions were performed for each cDNA sample using a 10-μl final reaction volume containing 5 μl of 2× TaqMan Universal PCR Master Mix, 4.5 μl of diluted cDNA, and 0.5 μl of gene expression assay. Reactions were performed in 384-well plates using the ABI Prism 7900 HT Sequence Detection System (Life Technologies) under the following conditions: 1 cycle at 50°C for 2 minutes, 1 cycle at 95°C for 10 minutes, and 40 cycles of 95°C for 15 seconds and 60°C for 1 minute.
The relative quantitation (RQ) of expression was calculated using the ΔΔCt method. Briefly, ΔCt was calculated as the Ct value of the target gene minus the Ct of the control gene (MT-ATP6). The ΔΔCt was then calculated as the ΔCt of the sample minus the ΔCt of a calibrator sample, in this case the geometric mean of the highest expressed UGT2B isoform in a given tissue. The RQ was determined with the equation (1 + Ex)–ΔΔCt.
Data Analysis.
For qPCR data, statistical analyses were performed using GraphPad Prism version 5.00. When calculating the mean RQ values, only samples that displayed detectable levels of expression were included in the analysis. Spearman’s rank method was used for correlative analysis of expressed UGT2B isoforms in all of the tissues except for tongue and mouth (only three specimens from each site precluded them from being analyzed in this manner).
Results
Relative Expression of UGT2B Genes in Human Tissues.
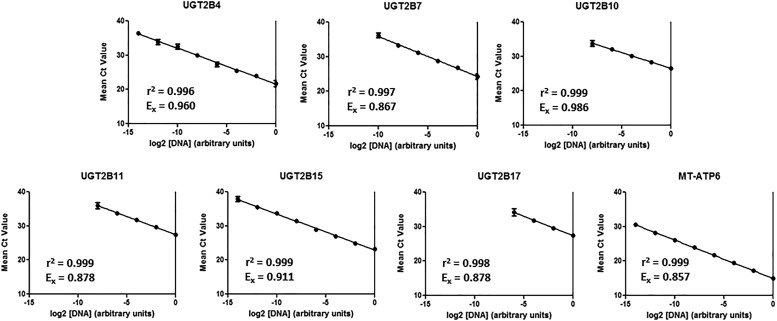
Standard curves were used to calculate real-time PCR efficiency values for the UGT2B genes and MT-ATP6 (Fig. 1). The slopes of the various Ct versus log 2[cDNA] curves were different for each gene, and the calculated efficiency values ranged from 85.7% to 98.6%. The relative efficiencies were used to correct the RQ values using the formula described under Materials and Methods. As the standard curves for each gene became unreliable beyond 35 cycles, 35 cycles was determined to be the quantitation limit of the real-time PCR assays. In subsequent experiments, any gene that amplified with a mean Ct value > 35 cycles was determined to be BLQ.
Fig. 1.
UGT2B and MT-ATP6 qPCR standard curves. Pooled cDNA from three liver samples was subjected to a serial 4-fold dilution prior to real-time PCR amplification. The efficiency values of each of the real-time PCR assays was calculated with the formula Ex=[2(–1/slope) – 1] as described in Materials and Methods. Average Ct values were calculated by taking the arithmetic mean of four replicate data points. Data are reported as mean ± standard deviation.
The mean relative expression of UGT2B genes in different tissues is shown in Table 1. All UGT2B isoforms were detected in all human liver tissue specimens examined, as previously reported by other groups (Nakamura et al., 2008a; Izukawa et al., 2009; Ohno and Nakajin, 2009; Court et al., 2012). Only UGT2B17 exhibited no expression in four of the liver specimens examined, likely because those specimens were from subjects exhibiting the homozygous genotype for the prevalent (30% in Caucasians) UGT2B17 whole-gene deletion allele (Wilson et al., 2004; Lazarus et al., 2005; Gallagher et al., 2007). The relative levels of hepatic expression were highest for UGT2B4, followed by UGT2B7 > UGT2B10 > UGT2B17 > UGT2B15 > UGT2B11 (Table 1). The mean hepatic expression of UGT2B4 was 57% of the total UGT2B expression in human liver, followed by UGT2B7 (32%), UGT2B10 (7.6%), UGT2B17 (2.8%), UGT2B15 (0.9%), and UGT2B11 (0.5%). Interindividual differences in hepatic expression ranged by at least 98-fold for all UGT2B genes, with UGT2B17 exhibiting the greatest interindividual differences (4689-fold; not including the four specimens with no expression), followed by UGT2B7 (3039-fold), UGT2B10 (549-fold), UGT2B4 (148-fold), UGT2B15 (102-fold), and UGT2B11 (98-fold) (Fig. 2). While UGT2B28 amplified in several liver samples, this occurred at Ct values outside the range of the standard curve (i.e., >35 cycles); the expression of UGT2B28 was BLQ for all of the other specimens examined in this study (results not shown).
TABLE 1.
Meana relative UGT2B expression levels in human tissue specimens
| Tissue Site | UGT2B4 | UGT2B7 | UGT2B10 | UGT2B11 | UGT2B15 | UGT2B17 | |
|---|---|---|---|---|---|---|---|
| Liver | Mean | 1.0E+00 | 5.6E-01 | 1.4E-01 | 9.6E-03 | 1.6E-02 | 5.0E-02 |
| S.E. | 5.9E-01 | 3.9E-01 | 1.1E-01 | 7.6E-03 | 8.8E-03 | 4.4E-02 | |
| % Total | 56.6% | 31.5% | 7.6% | 0.5% | 0.9% | 2.8% | |
| # BLQ | 0 of 27 | 0 of 27 | 0 of 27 | 0 of 27 | 0 of 27 | 4 of 27 | |
| Lung | Mean | 4.2E-03 | 1.6E-03 | 1.7E-03 | 2.4E-02 | 2.3E-03 | 1.5E-02 |
| S.E. | 1.6E-03 | 1.8E-03 | 1.5E-03 | 2.7E-02 | 2.0E-03 | 1.0E-02 | |
| % Total | 8.8% | 3.4% | 3.5% | 49.2% | 4.9% | 30.3% | |
| # BLQ | 0 of 22 | 11 of 22 | 8 of 22 | 1 of 22 | 9 of 22 | 3 of 22 | |
| Larynx | Mean | 7.0E-03 | BLQ | 6.9E-03 | 9.3E-03 | 1.2E-03 | 1.5E-02 |
| S.E. | 6.1E-03 | — | 6.2E-03 | 9.1E-03 | 1.0E-03 | 7.8E-03 | |
| % Total | 17.6% | — | 17.4% | 23.7% | 3.2% | 38.1% | |
| # BLQ | 0 of 7 | 7 of 7 | 0 of 7 | 0 of 7 | 0 of 7 | 0 of 7 | |
| Esophagus | Mean | 8.3E-04 | 9.4E-04 | 9.3E-04 | 1.7E-03 | 2.9E-03 | 2.4E-03 |
| S.E. | 5.7E-04 | 1.8E-03 | 7.1E-04 | 1.2E-03 | 2.8E-03 | 2.2E-03 | |
| % Total | 8.6% | 9.6% | 9.5% | 17.7% | 30.3% | 24.3% | |
| # BLQ | 1 of 13 | 10 of 13 | 3 of 13 | 1 of 13 | 3 of 13 | 0 of 13 | |
| Tonsil | Mean | 5.5E-02 | BLQ | 4.3E-02 | 6.0E-02 | 1.5E-02 | 1.0E-01 |
| S.E. | 9.0E-02 | — | 7.6E-02 | 7.7E-02 | 2.2E-02 | 5.5E-02 | |
| % Total | 20.2% | — | 15.8% | 22.0% | 5.3% | 36.8% | |
| # BLQ | 0 of 22 | 22 of 22 | 1 of 22 | 0 of 22 | 5 of 22 | 0 of 22 | |
| Floor of mouth | Mean | 3.5E-03 | BLQ | 3.4E-03 | 5.2E-03 | 5.9E-04 | 7.1E-03 |
| S.E. | 6.0E-03 | — | 5.6E-03 | 8.6E-03 | 9.5E-04 | 4.7E-03 | |
| % Total | 17.6% | — | 17.3% | 26.1% | 3.0% | 36.0% | |
| # BLQ | 0 of 3 | 3 of 3 | 0 of 3 | 0 of 3 | 0 of 3 | 0 of 3 | |
| Tongue | Mean | 3.1E-03 | BLQ | 2.5E-03 | 2.1E-03 | BLQ | 1.6E-03 |
| S.E. | 6.5E-03 | — | 4.6E-03 | 3.4E-03 | — | 1.5E-03 | |
| % Total | 33.3% | — | 26.9% | 22.8% | — | 17.0% | |
| # BLQ | 0 of 3 | 3 of 3 | 1 of 3 | 0 of 3 | 3 of 3 | 0 of 3 | |
| Pancreas | Mean | 1.7E-03 | 3.4E-02 | 1.2E-03 | 1.7E-03 | 3.1E-02 | 1.8E-02 |
| S.E. | 9.2E-04 | 2.9E-02 | 2.5E-04 | 8.9E-04 | 4.3E-02 | 3.6E-02 | |
| % Total | 2.0% | 38.5% | 1.4% | 1.9% | 35.5% | 20.7% | |
| # BLQ | 8 of 16 | 1 of 16 | 10 of 16 | 5 of 16 | 0 of 16 | 3 of 16 |
BLQ, below the limit of quantification.
Arithmetic mean of all samples in a given tissue, normalized to the mean hepatic expression of UGT2B4.
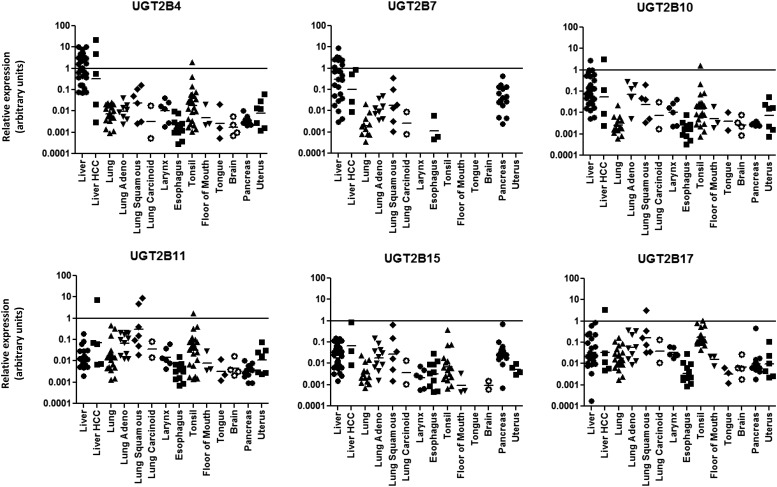
Fig. 2.
Relative expression levels of UGT2B genes in various tissues. UGT2B mRNA expression levels were assessed in 113 normal tissue specimens. The log of the relative expression levels for the UGT2B genes is shown. The relative abundance was calculated via the 2–ΔΔCt method with the geometric mean of the highest expressing isoform set as the calibrator.
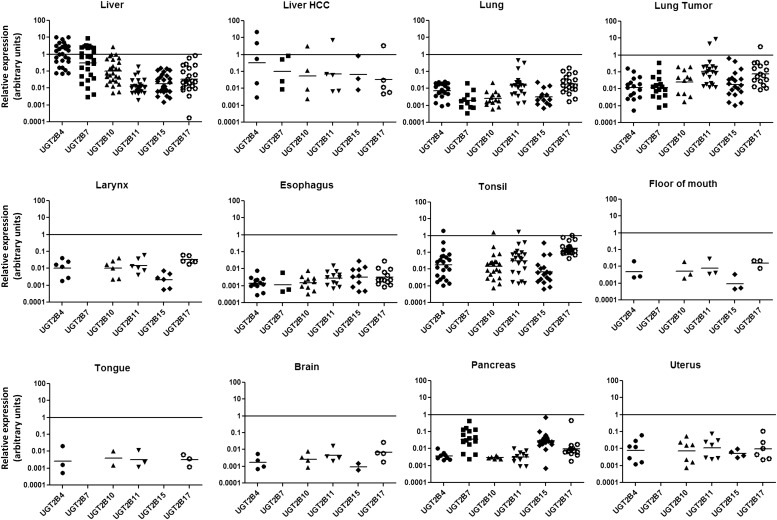
In human lung tissue, UGT2B11 was found to be expressed the highest of all UGT2B family members, followed by UGT2B17 > UGT2B4 > UGT2B15 > UGT2B10 > UGT2B7 (Table 1). In the 22 human lung tissues analyzed, all six of the UGT2B genes were detected in at least half of the individual specimens. There were significant interindividual differences in expression for UGT2B genes in lung, ranging from 379-fold for UGT2B11 to 27-fold for UGT2B4 (Fig. 2; not including those specimens in which a given UGT2B gene exhibited no expression). UGT2B11 was the only UGT2B gene that was expressed at higher levels (by 2.5-fold) in lung versus liver, with UGT2B17 exhibiting expression levels that were 3.4-fold lower than that observed in human liver.
UGT2B gene expression was detected in individual larynx specimens. The highest expression was seen for UGT2B17, followed by UGT2B11 > UGT2B4 > UGT2B10 > UGT2B15 (Table 1). UGT2B7 was not detected in any of the individual larynx samples examined. Interindividual differences in expression for UGT2B genes in larynx were less than that observed in lung, ranging from 22-fold for UGT2B4 to 4-fold for UGT2B17 (Fig. 2). UGTs 2B11 and 2B17 were the only UGT2B genes that were expressed at comparable levels in larynx versus liver (1.1- and 3-fold lower, respectively, in larynx).
In the 13 human esophagus specimens analyzed in this study, several of the UGT2B genes were expressed at very low levels, with UGT2B15 the highest expressed followed by UGT2B17 > UGT2B11 > UGT2B10 > UGT2B4 > UGT2B7 (Table 1). Compared with human liver tissue, UGT2B genes ranged from 1198-fold lower for UGT2B4 to 21- and 5.4-fold lower for UGTs 2B17 and 2B15, respectively, as compared with that observed in human liver (Fig. 2); UGT2B7 was only detected in three of the 13 specimens and was not included in the range estimate. While expression was low for several genes, there was still significant interindividual differences in expression for UGT2B genes, ranging from 63-fold for UGT2B15 to 13-fold for UGT2B7 (Fig. 2).
With the exception of UGT2B7, UGT2B genes were found to be well-expressed in the majority of human tonsil specimens analyzed. The expression was highest for UGT2B17, followed by UGT2B11 > UGT2B4 > UGT2B10 > UGT2B15 (Table 1). Compared with their expression in human liver tissue, two UGT2B genes (UGT2B11 and UGT2B17) were expressed at higher levels (6.3- and 2.0-fold higher, respectively), with UGT2B10 and UGT2B15 exhibiting expression levels that were only 3.1- and 1.1-fold lower, respectively (Fig. 3). Relative to its expression in liver, UGT2B4 expression was relatively low in tonsil (5.5-fold lower). There were significant levels of interindividual difference in UGT2B expression in human tonsil for several UGT2B genes ranging from 2162-fold for UGT2B10 to 577-fold for UGT2B15 (Fig. 2).
Fig. 3.
Relative expression levels of individual UGT2B genes across different tissues. UGT2B mRNA expression levels were assessed in 113 normal tissue specimens. The log of the relative expression levels for the UGT2B genes is shown. The relative abundance was calculated via the 2–ΔΔCt method with the geometric mean of the highest expressing tissue set as the calibrator.
The three floor-of-mouth tissue specimens examined in the present study showed expression of all UGT2B isoforms except UGT2B7. The highest expression observed was for UGT2B17, followed by UGT2B11 > UGT2B4 > UGT2B10 > UGT2B15 (Table 1). Compared with human liver tissue, UGT2B4 was 288-fold lower, UGT2B10 was 40-fold lower, UGT2B15 was 27-fold lower, UGT2B17 was 7-fold lower, and UGT2B11 was 1.9-fold lower (Fig. 3). There were relatively low levels of interindividual difference in expression for the three samples, ranging from 10-fold for UGT2B10 to 2.9-fold for UGT2B17 (Fig. 2).
In human tongue tissue, detectable levels of expression were observed with UGT2B4 > UGT2B10 > UGT2B11 > UGT2B17 (Table 1), with their expression ranging from 322-fold lower for UGT2B4 to 4.5-fold lower for UGT2B11 as compared with that observed for human liver (Fig. 3). Similar to that observed for other upper aerodigestive tract tissues (larynx, tonsil, floor of mouth), no expression of UGT2B7 was detected in any of the tongue tissues analyzed. The interindividual difference in expression ranged from 38-fold for UGT2B4 to 5.3-fold for UGT2B17 (Fig. 2).
The UGT2B genes were detected in the 16 pancreas specimens, with UGT2B7 > UGT2B17 > UGT2B15 > UGT2B4 > UGT2B11 > UGT2B10 (Table 1). While significant interindividual differences in expression were observed for UGT2B15 (1043-fold), UGT2B17 (252-fold), and UGT2B7 (181-fold), stable expression levels (1.7-fold differences) were observed for UGT2B10 between pancreas samples (Fig. 2). UGTs 2B11 (2.0-fold higher) and 2B17 (2.7-fold lower) exhibited expression levels that were comparable to that observed in human liver tissue (Fig. 3).
Correlation between Human UGT2B Gene Expression Levels in Human Tissues.
Correlative analysis of UGT2B expression in eight of the tissue sites (for whom at least 4 individual specimens were analyzed) is shown in Table 2. In liver, there was a significant correlation between the expression of UGTs 2B4, 2B7, 2B10, and 2B15 (all P < 0.01). Hepatic UGT2B11 and UGT2B17 expression levels were not significantly correlated with any of the other UGT2B genes. In lung, the expression of all UGT2B genes except UGT2B4 were significantly (P < 0.01) correlated with each other. In the larynx and tonsil, all of the expressed UGT2B genes were significantly (P < 0.05) correlated with each other with the exception of UGT2B7, which was not expressed at detectable levels in any of the samples. In esophagus, UGTs 2B4, 2B10, 2B11, and 2B17 were significantly (P < 0.01) correlated with each other, but not with 2B15. Unlike that observed for other tissues examined in this study, none of the expressed isoforms were significantly correlated with each other in pancreas, though there was a strong inverse correlation between UGT2B7 and UGT2B10 expression (P < 0.01).
TABLE 2.
Correlation between UGT2B mRNA expression levels in different tissuesa
| UGT2B4 | UGT2B7 | UGT2B10 | UGT2B11 | UGT2B15 | UGT2B17 | |
|---|---|---|---|---|---|---|
| Liver | ||||||
| UGT2B4 | 0.74**** | 0.73**** | −0.25 | 0.70**** | 0.05 | |
| UGT2B7 | 0.94**** | −0.25 | 0.82**** | 0.21 | ||
| UGT2B10 | −0.14 | 0.84**** | −0.08 | |||
| UGT2B11 | −0.17 | 0.06 | ||||
| UGT2B15 | −0.06 | |||||
| UGT2B17 |
||||||
| Lung | ||||||
| UGT2B4 | 0.19 | 0.18 | 0.34 | 0.34 | 0.39 | |
| UGT2B7 | 0.93**** | 0.98**** | 0.98**** | 0.87** | ||
| UGT2B10 | 0.95**** | 0.97**** | 0.93**** | |||
| UGT2B11 | 0.87*** | 0.45 | ||||
| UGT2B15 | 0.80** | |||||
| UGT2B17 |
||||||
| Larynx | ||||||
| UGT2B4 | N/A | 0.97** | 0.96** | 0.99*** | 0.86* | |
| UGT2B7 | N/A | N/A | N/A | N/A | ||
| UGT2B10 | 0.96** | 0.99**** | 0.90* | |||
| UGT2B11 | 0.97** | 0.90* | ||||
| UGT2B15 | 0.89* | |||||
| UGT2B17 |
||||||
| Esophagus | ||||||
| UGT2B4 | N/A | 0.96**** | 0.94**** | −0.19 | 0.87*** | |
| UGT2B7 | N/A | N/A | N/A | N/A | ||
| UGT2B10 | 0.92*** | −0.21 | 0.86** | |||
| UGT2B11 | −0.07 | 0.91**** | ||||
| UGT2B15 | −0.11 | |||||
| UGT2B17 |
||||||
| Tonsil | ||||||
| UGT2B4 | N/A | 0.99**** | 0.99**** | 0.99**** | 0.79**** | |
| UGT2B7 | N/A | N/A | N/A | N/A | ||
| UGT2B10 | 0.98**** | 0.99**** | 0.75**** | |||
| UGT2B11 | 0.99**** | 0.82**** | ||||
| UGT2B15 | 0.83**** | |||||
| UGT2B17 |
||||||
| Pancreas | ||||||
| UGT2B4 | −0.17 | 0.42 | −0.05 | −0.19 | −0.40 | |
| UGT2B7 | −0.95** | 0.28 | 0.20 | −0.06 | ||
| UGT2B10 | 0.72 | −0.52 | −0.86 | |||
| UGT2B11 | 0.17 | −0.25 | ||||
| UGT2B15 | 0.04 | |||||
| UGT2B17 |
p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Data presented are r2 values from correlation analyses of expressed isoforms using the Spearman’s rank method.
Discussion
In the present study, UGT2B genes were shown to be expressed in tobacco-related tissues. UGT2B17 was shown to be well-expressed relative to other UGT2B genes in most of the tissues examined including larynx, tonsil, esophagus, floor of mouth, pancreas, and lung, all of whom are noted targets for tobacco-induced carcinogenesis (Viswanath et al., 2010). With the exception of human tongue, UGT2B17 was expressed at the highest or second-highest level of any of the UGT2B genes. UGT2B17 exhibits the highest activity of all UGTs in forming the O-glucuronide of NNAL (Lazarus et al., 2005), and the UGT2B17 gene deletion is associated with lung adenocarcinoma risk (Gallagher et al., 2007), a pattern consistent with the high levels of UGT2B17 expression observed in lung in the present study and with NNAL’s induction of lung adenocarcinoma in rodent models (Rivenson et al., 1988; Hecht, 1998). While no studies have examined the role of the UGT2B17 deletion in cancer susceptibility within the aerodigestive tract, relatively high levels of UGT2B17 expression in larynx, tonsil, esophagus, and floor of mouth suggests that the UGT2B17 deletion may play a similarly important etiologic role in the induction of cancers within these tissues.
Previous studies showed that UGT2B7 was found to be active against multiple tobacco carcinogens including NNAL (Ren et al., 2000; Wiener et al., 2004a) and polycyclic aromatic hydrocarbons like B(a)P and DB(a,l)P (Fang et al., 2002; Olson et al., 2011). In the present study, UGT2B7 was shown to exhibit low or undetectable levels of expression in most tissues examined except for pancreas, where it exhibited highest expression. A UGT2B7 codon 268 single-nucleotide polymorphism resulting in a His > Tyr amino acid change was associated with functional differences in UGT2B7 activity against NNAL (Wiener et al., 2004b) and other substrates (Blevins-Primeau et al., 2009). Studies are required to examine the role of this and other functional UGT2B7 variants in pancreatic cancer susceptibility.
UGT2B10 was shown to be the primary enzyme responsible for the formation of NNAL-N-gluc (Chen et al., 2008b). Relative to other UGT2B genes, UGT2B10 mRNA was expressed at the second-highest level of all UGT2B genes in tongue tissue and could play an important role in tobacco carcinogen detoxification at this site. In addition, like several UGT2B genes, UGT2B10 was expressed in tonsil at levels comparable to that observed in human liver, a pattern suggesting that UGT2B genes, including UGT2B10, play an important role in the glucuronidation of substrates within this tissue. A knockout missense single-nucleotide polymorphism at codon 67 resulting in an aspartic acid to tyrosine amino acid change has been previously characterized for UGT2B10 (Chen et al., 2008a, 2010), and this variant could play an important role in tobacco-related cancer risk in these tissues.
Hepatic expression of UGT2B family members has been described (Izukawa et al., 2009; Ohno and Nakajin, 2009; Court et al., 2012). The highest expressions observed in liver were for UGT2B4 and UGT2B7 followed by UGT2B10, which was largely consistent with these previous studies. UGT2B15 expression in the present study was found to be in concordance with the findings in the Court et al. study, but much lower than the UGT2B15 expression in the Ohno and Nakajin study. In the latter study pooled RNA from three specimens was used for analysis. Given the well-reported interindividual variability for UGT genes, a single outlier specimen with high UGT2B15 expression could have skewed the observed results. UGT2B4 is known as a steroid- and bile acid–glucuronidating UGT, so it is needed in high abundance in the liver (Fournel-Gigleux et al., 1989; Pillot et al., 1993; Levesque et al., 1999). In contrast, UGTs 2B15 and 2B11 were the lowest expressed UGT2B genes in human liver. UGT2B15 is known primarily for its activity against C19 steroids (Beaulieu et al., 1996; Levesque et al., 1997), a characteristic shared by the hepatically abundant UGT2B17, while UGT2B11 exhibits limited substrate specificity against metabolites of arachidonic and linoleic acids (Turgeon et al., 2003).
UGT2B28 expression was below the limit of quantification in the tissue specimens examined in this study, including several liver specimens. While low levels of hepatic UGT2B28 expression were observed in a previous study (Court et al., 2012), the amplification observed for liver specimens in the present study occurred at Ct values outside the range of the standard curve (>35 cycles). The data from the present study are also consistent with previous studies that observed minimal UGT2B28 expression in other human tissues with the notable exceptions being breast and adipose tissue, neither of which was included in the present study.
Interindividual differences in UGT2B expression were large in the tissues examined in this study, making comparisons between individuals difficult. The wide range of expression differences agrees well with previous publications that have assessed this phenomenon (Aueviriyavit et al., 2007; Izukawa et al., 2009). The high interindividual differences in expression may be due to multiple genetic, epigenetic, and epidemiologic factors. Tobacco smoke is itself known to contain a number of agents that can induce UGT activity, and thus an individual’s smoking habits are likely to influence UGT gene expression levels and enzymatic activity (Villard et al., 1998; Elovaara et al., 2007). However, as only basic demographic information was available for the subjects from whom these specimens were obtained and the smoking habits of the individuals were unknown, deductions regarding the potential influence of tobacco smoke induction of UGT genes could not be performed. In addition, the high variability may in part be due to the fact that most tissues contain diverse mixtures of cell types; the fact that laser-capture microdissection was not performed, particularly for heterogeneous tissues like lung and pancreas, is a limitation of the present study.
In the liver, the high correlation between the expression of UGT2B4, UGT2B7, UGT2B10, and UGT2B15, but not UGT2B17, is in agreement with previous studies (Izukawa et al., 2009). This is likely due to a common mechanism of hepatic transcriptional regulation. One potential transcription factor that may explain the correlation between these isoforms is hepatocyte nuclear factor (HNF)1α, which has been shown to regulate several UGT2B genes (Bernard et al., 1999; Ishii et al., 2000; Gregory, 2004; Barbier et al., 2005; Gardner-Stephen and Mackenzie, 2007). In addition to liver-enriched transcription factors that regulate basal expression, hepatic UGT expression could potentially be modulated via a number of receptors (Zhou et al., 2005). For example, UGT2B4 was shown to be regulated by peroxisome proliferator-activated receptor as a mechanism for regulating cholesterol and bile acid metabolism (Barbier et al., 2003), while UGT2B7 can be induced by the antioxidant-responsive nrf2 pathway (Nakamura et al., 2008a).
In lung tissue, the correlation of UGT2B17 with other UGT2B genes suggests a coordinated mechanism of regulation in this tissue, and that transcriptional regulation is occurring by different mechanisms in lung versus liver. In the larynx, esophagus, and tonsil, all of the expressed isoforms are well-correlated with each other, suggesting a common regulatory mechanism in these tissues. The lack of correlation of different UGT2B genes in the pancreas suggests a different mechanism of UGT2B regulation in pancreas compared with other tissues examined. Future studies are needed to determine whether individuals with uniformly high or low UGT2B mRNAs display similar correlations at the protein levels. UGT quantitation at the protein level has been problematic due to the high degree of UGT homology, but a recent study demonstrated that the various UGT protein isoforms can be quantified using nano-ultra-high-performance liquid chromatography–tandem mass spectrometry with selected reaction monitoring (Fallon et al., 2013). However, correlative analyses between the various UGT protein isoforms were not performed in that study.
While previous studies examining UGT mRNA expression have used commonly used housekeeping control genes like 18S rRNA (Court et al., 2012) or GAPDH (Ohno and Nakajin, 2009), the MT-ATP6 gene was used as the control gene in the present study. Data from the present study indicated that MT-ATP6 exhibited the least variability of all potential control genes tested when comparing different individuals and tissue sites. These data suggest that MT-ATP6 may be an excellent candidate as a normalization gene for assessing gene expression profiles across multiple tissues from many individuals. The concordance observed in the hepatic UGT2B expression patterns between this and previous quantitative studies, in which either 18S rRNA (Court et al., 2012) or GAPDH (Ohno and Nakajin, 2009) were assessed, suggests that additional genes other than MT-ATP6 could be used as effective housekeeping genes in liver. A comparison of control gene effectiveness for other tissue sites could not be performed between this and the study by Court et al. (2012), since UGT2B expression was analyzed for only single lung and pancreas specimens in that study. In the study by Ohno and Nakajin (2009), only UGT2B17 was observed in lung, and only UGTs 2B4 and 2B15 were expressed in pancreas, suggesting significant differences in sensitivity between this and the present study, making direct comparisons difficult. No previous studies have examined UGT2B expression in individual sites within the aerodigestive tract.
In summary, specific UGT2B genes important in tobacco carcinogen metabolism were shown to be expressed in various target sites for tobacco-related cancers, with UGTs 2B7, 2B10, and 2B17 well-expressed in at least several of the tissues examined in this study. These studies also demonstrate a high degree of correlation between UGT2B genes in both hepatic and extrahepatic tissues, suggesting that there are distinct mechanisms of coordinated regulation in extrahepatic tissues, which may have important therapeutic implications.
Supplementary Material
Acknowledgments
The authors thank the Penn State College of Medicine Tissue Bank for providing tissue specimens and the Penn State College of Medicine Functional Genomics Core Facility for real-time PCR services. The authors also thank Gang Chen for scientific input and technical advice.
Abbreviations
- BLQ
below the limit of quantification
- MT-ATP6
mitochondrially encoded ATP synthase 6
- NNAL
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol
- qPCR
real-time polymerase chain reaction
- RQ
relative quantitation
- UGT
UDP glucuronosyltransferase
Authorship Contributions
Participated in research design: Jones, Lazarus.
Conducted experiments: Jones.
Performed data analysis: Jones, Lazarus.
Wrote or contributed to writing of the manuscript: Jones, Lazarus.
Footnotes
This work was supported by the National Institutes of Health National Institute of Dental and Craniofacial Research [Grant R01-DE13158 (to P.L.)]; and the Pennsylvania Department of Health’s Health Research Formula Funding Program [Grant 4100038714 (to P.L.)].
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Aueviriyavit S, Furihata T, Morimoto K, Kobayashi K, Chiba K. (2007) Hepatocyte nuclear factor 1 alpha and 4 alpha are factors involved in interindividual variability in the expression of UGT1A6 and UGT1A9 but not UGT1A1, UGT1A3 and UGT1A4 mRNA in human livers. Drug Metab Pharmacokinet 22:391–398 [DOI] [PubMed] [Google Scholar]
- Balliet RM, Chen G, Gallagher CJ, Dellinger RW, Sun D, Lazarus P. (2009) Characterization of UGTs active against SAHA and association between SAHA glucuronidation activity phenotype with UGT genotype. Cancer Res 69:2981–2989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier O, Duran-Sandoval D, Pineda-Torra I, Kosykh V, Fruchart JC, Staels B. (2003) Peroxisome proliferator-activated receptor alpha induces hepatic expression of the human bile acid glucuronidating UDP-glucuronosyltransferase 2B4 enzyme. J Biol Chem 278:32852–32860 [DOI] [PubMed] [Google Scholar]
- Barbier O, Girard H, Inoue Y, Duez H, Villeneuve L, Kamiya A, Fruchart JC, Guillemette C, Gonzalez FJ, Staels B. (2005) Hepatic expression of the UGT1A9 gene is governed by hepatocyte nuclear factor 4alpha. Mol Pharmacol 67:241–249 [DOI] [PubMed] [Google Scholar]
- Beaulieu M, Lévesque E, Hum DW, Bélanger A. (1996) Isolation and characterization of a novel cDNA encoding a human UDP-glucuronosyltransferase active on C19 steroids. J Biol Chem 271:22855–22862 [DOI] [PubMed] [Google Scholar]
- Beaulieu M, Lévesque E, Hum DW, Bélanger A. (1998) Isolation and characterization of a human orphan UDP-glucuronosyltransferase, UGT2B11. Biochem Biophys Res Commun 248:44–50 [DOI] [PubMed] [Google Scholar]
- Bélanger A, Hum DW, Beaulieu M, Lévesque E, Guillemette C, Tchernof A, Bélanger G, Turgeon D, Dubois S. (1998) Characterization and regulation of UDP-glucuronosyltransferases in steroid target tissues. J Steroid Biochem Mol Biol 65:301–310 [DOI] [PubMed] [Google Scholar]
- Bélanger A, Pelletier G, Labrie F, Barbier O, Chouinard S. (2003) Inactivation of androgens by UDP-glucuronosyltransferase enzymes in humans. Trends Endocrinol Metab 14:473–479 [DOI] [PubMed] [Google Scholar]
- Bernard P, Goudonnet H, Artur Y, Desvergne B, Wahli W. (1999) Activation of the mouse TATA-less and human TATA-containing UDP-glucuronosyltransferase 1A1 promoters by hepatocyte nuclear factor 1. Mol Pharmacol 56:526–536 [DOI] [PubMed] [Google Scholar]
- Blevins-Primeau AS, Sun D, Chen G, Sharma AK, Gallagher CJ, Amin S, Lazarus P. (2009) Functional significance of UDP-glucuronosyltransferase variants in the metabolism of active tamoxifen metabolites. Cancer Res 69:1892–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma PJ, Seppen J, Goldhoorn B, Bakker C, Oude Elferink RP, Chowdhury JR, Chowdhury NR, Jansen PL. (1994) Bilirubin UDP-glucuronosyltransferase 1 is the only relevant bilirubin glucuronidating isoform in man. J Biol Chem 269:17960–17964 [PubMed] [Google Scholar]
- Chen G, Dellinger RW, Gallagher CJ, Sun D, Lazarus P. (2008a) Identification of a prevalent functional missense polymorphism in the UGT2B10 gene and its association with UGT2B10 inactivation against tobacco-specific nitrosamines. Pharmacogenet Genomics 18:181–191 [DOI] [PubMed] [Google Scholar]
- Chen G, Dellinger RW, Sun D, Spratt TE, Lazarus P. (2008b) Glucuronidation of tobacco-specific nitrosamines by UGT2B10. Drug Metab Dispos 36:824–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Giambrone NE, Jr, Dluzen DF, Muscat JE, Berg A, Gallagher CJ, Lazarus P. (2010) Glucuronidation genotypes and nicotine metabolic phenotypes: importance of functional UGT2B10 and UGT2B17 polymorphisms. Cancer Res 70:7543–7552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court MH, Duan SX, von Moltke LL, Greenblatt DJ, Patten CJ, Miners JO, Mackenzie PI. (2001) Interindividual variability in acetaminophen glucuronidation by human liver microsomes: identification of relevant acetaminophen UDP-glucuronosyltransferase isoforms. J Pharmacol Exp Ther 299:998–1006 [PubMed] [Google Scholar]
- Court MH, Zhang X, Ding X, Yee KK, Hesse LM, Finel M. (2012) Quantitative distribution of mRNAs encoding the 19 human UDP-glucuronosyltransferase enzymes in 26 adult and 3 fetal tissues. Xenobiotica 42:266–277 [DOI] [PubMed] [Google Scholar]
- Elovaara E, Mikkola J, Stockmann-Juvala H, Luukkanen L, Keski-Hynnilä H, Kostiainen R, Pasanen M, Pelkonen O, Vainio H. (2007) Polycyclic aromatic hydrocarbon (PAH) metabolizing enzyme activities in human lung, and their inducibility by exposure to naphthalene, phenanthrene, pyrene, chrysene, and benzo(a)pyrene as shown in the rat lung and liver. Arch Toxicol 81:169–182 [DOI] [PubMed] [Google Scholar]
- Fallon JK, Neubert H, Hyland R, Goosen TC, Smith PC. (2013) Targeted quantitative proteomics for the analysis of 14 UGT1As and -2Bs in human liver using NanoUPLC-MS/MS with selected reaction monitoring. J Proteome Res 12:4402–4413 [DOI] [PubMed] [Google Scholar]
- Fang JL, Beland FA, Doerge DR, Wiener D, Guillemette C, Marques MM, Lazarus P. (2002) Characterization of benzo(a)pyrene-trans-7,8-dihydrodiol glucuronidation by human tissue microsomes and overexpressed UDP-glucuronosyltransferase enzymes. Cancer Res 62:1978–1986 [PubMed] [Google Scholar]
- Fournel-Gigleux S, Jackson MR, Wooster R, Burchell B. (1989) Expression of a human liver cDNA encoding a UDP-glucuronosyltransferase catalysing the glucuronidation of hyodeoxycholic acid in cell culture. FEBS Lett 243:119–122 [DOI] [PubMed] [Google Scholar]
- Gallagher CJ, Muscat JE, Hicks AN, Zheng Y, Dyer AM, Chase GA, Richie J, Lazarus P. (2007) The UDP-glucuronosyltransferase 2B17 gene deletion polymorphism: sex-specific association with urinary 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol glucuronidation phenotype and risk for lung cancer. Cancer Epidemiol Biomarkers Prev 16:823–828 [DOI] [PubMed] [Google Scholar]
- Gardner-Stephen DA, Mackenzie PI. (2007) Isolation of the UDP-glucuronosyltransferase 1A3 and 1A4 proximal promoters and characterization of their dependence on the transcription factor hepatocyte nuclear factor 1alpha. Drug Metab Dispos 35:116–120 [DOI] [PubMed] [Google Scholar]
- Gregory RT. (2004) Presidential address: The state of the union of vascular surgery, 2004. Vascular 12:354–358 [DOI] [PubMed] [Google Scholar]
- Hecht SS. (1998) Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol 11:559–603 [DOI] [PubMed] [Google Scholar]
- Hoskins JM, Goldberg RM, Qu P, Ibrahim JG, McLeod HL. (2007) UGT1A1*28 genotype and irinotecan-induced neutropenia: dose matters. J Natl Cancer Inst 99:1290–1295 [DOI] [PubMed] [Google Scholar]
- Ishii Y, Hansen AJ, Mackenzie PI. (2000) Octamer transcription factor-1 enhances hepatic nuclear factor-1alpha-mediated activation of the human UDP glucuronosyltransferase 2B7 promoter. Mol Pharmacol 57:940–947 [PubMed] [Google Scholar]
- Izukawa T, Nakajima M, Fujiwara R, Yamanaka H, Fukami T, Takamiya M, Aoki Y, Ikushiro S, Sakaki T, Yokoi T. (2009) Quantitative analysis of UDP-glucuronosyltransferase (UGT) 1A and UGT2B expression levels in human livers. Drug Metab Dispos 37:1759–1768 [DOI] [PubMed] [Google Scholar]
- Jones NR, Sun D, Freeman WM, Lazarus P. (2012) Quantification of Hepatic UDP glucuronosyltransferase 1A splice variant expression and correlation of UDP glucuronosyltransferase 1A1 variant expression with glucuronidation activity. J Pharmacol Exp Ther 342:720–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp DC, Fan PW, Stevens JC. (2002) Characterization of raloxifene glucuronidation in vitro: contribution of intestinal metabolism to presystemic clearance. Drug Metab Dispos 30:694–700 [DOI] [PubMed] [Google Scholar]
- Lazarus P, Zheng Y, Runkle EA, Muscat JE, Wiener D. (2005) Genotype-phenotype correlation between the polymorphic UGT2B17 gene deletion and NNAL glucuronidation activities in human liver microsomes. Pharmacogenet Genomics 15:769–778 [DOI] [PubMed] [Google Scholar]
- Lévesque E, Beaulieu M, Green MD, Tephly TR, Bélanger A, Hum DW. (1997) Isolation and characterization of UGT2B15(Y85): a UDP-glucuronosyltransferase encoded by a polymorphic gene. Pharmacogenetics 7:317–325 [DOI] [PubMed] [Google Scholar]
- Lévesque E, Beaulieu M, Hum DW, Bélanger A. (1999) Characterization and substrate specificity of UGT2B4 (E458): a UDP-glucuronosyltransferase encoded by a polymorphic gene. Pharmacogenetics 9:207–216 [PubMed] [Google Scholar]
- Mackenzie PI, Bock KW, Burchell B, Guillemette C, Ikushiro S, Iyanagi T, Miners JO, Owens IS, Nebert DW. (2005) Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenet Genomics 15:677–685 [DOI] [PubMed] [Google Scholar]
- Nagar S, Remmel RP. (2006) Uridine diphosphoglucuronosyltransferase pharmacogenetics and cancer. Oncogene 25:1659–1672 [DOI] [PubMed] [Google Scholar]
- Nakamura A, Nakajima M, Higashi E, Yamanaka H, Yokoi T. (2008a) Genetic polymorphisms in the 5′-flanking region of human UDP-glucuronosyltransferase 2B7 affect the Nrf2-dependent transcriptional regulation. Pharmacogenet Genomics 18:709–720 [DOI] [PubMed] [Google Scholar]
- Nakamura A, Nakajima M, Yamanaka H, Fujiwara R, Yokoi T. (2008b) Expression of UGT1A and UGT2B mRNA in human normal tissues and various cell lines. Drug Metab Dispos 36:1461–1464 [DOI] [PubMed] [Google Scholar]
- Ohno S, Nakajin S. (2009) Determination of mRNA expression of human UDP-glucuronosyltransferases and application for localization in various human tissues by real-time reverse transcriptase-polymerase chain reaction. Drug Metab Dispos 37:32–40 [DOI] [PubMed] [Google Scholar]
- Olson KC, Sun D, Chen G, Sharma AK, Amin S, Ropson IJ, Spratt TE, Lazarus P. (2011) Characterization of dibenzo[a,l]pyrene-trans-11,12-diol (dibenzo[def,p]chrysene) glucuronidation by UDP-glucuronosyltransferases. Chem Res Toxicol 24:1549–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkin VC, Bauman JN, Goosen TC, Menning L, Man MZ, Paulauskis JD, Williams JA, Myrand SP. (2007) Limited influence of UGT1A1*28 and no effect of UGT2B7*2 polymorphisms on UGT1A1 or UGT2B7 activities and protein expression in human liver microsomes. Br J Clin Pharmacol 64:458–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillot T, Ouzzine M, Fournel-Gigleux S, Lafaurie C, Radominska A, Burchell B, Siest G, Magdalou J. (1993) Glucuronidation of hyodeoxycholic acid in human liver. Evidence for a selective role of UDP-glucuronosyltransferase 2B4. J Biol Chem 268:25636–25642 [PubMed] [Google Scholar]
- Ren Q, Murphy SE, Zheng Z, Lazarus P. (2000) O-Glucuronidation of the lung carcinogen 4-(methylnitrosamino)-1- (3-pyridyl)-1-butanol (NNAL) by human UDP-glucuronosyltransferases 2B7 and 1A9. Drug Metab Dispos 28:1352–1360 [PubMed] [Google Scholar]
- Rivenson A, Hoffmann D, Prokopczyk B, Amin S, Hecht SS. (1988) Induction of lung and exocrine pancreas tumors in F344 rats by tobacco-specific and Areca-derived N-nitrosamines. Cancer Res 48:6912–6917 [PubMed] [Google Scholar]
- Strassburg CP, Oldhafer K, Manns MP, Tukey RH. (1997) Differential expression of the UGT1A locus in human liver, biliary, and gastric tissue: identification of UGT1A7 and UGT1A10 transcripts in extrahepatic tissue. Mol Pharmacol 52:212–220 [DOI] [PubMed] [Google Scholar]
- Strassburg CP, Manns MP, Tukey RH. (1998) Expression of the UDP-glucuronosyltransferase 1A locus in human colon. Identification and characterization of the novel extrahepatic UGT1A8. J Biol Chem 273:8719–8726 [DOI] [PubMed] [Google Scholar]
- Strassburg CP, Kneip S, Topp J, Obermayer-Straub P, Barut A, Tukey RH, Manns MP. (2000) Polymorphic gene regulation and interindividual variation of UDP-glucuronosyltransferase activity in human small intestine. J Biol Chem 275:36164–36171 [DOI] [PubMed] [Google Scholar]
- Sun D, Chen G, Dellinger RW, Duncan K, Fang JL, Lazarus P. (2006) Characterization of tamoxifen and 4-hydroxytamoxifen glucuronidation by human UGT1A4 variants. Breast Cancer Res 8:R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Sharma AK, Dellinger RW, Blevins-Primeau AS, Balliet RM, Chen G, Boyiri T, Amin S, Lazarus P. (2007) Glucuronidation of active tamoxifen metabolites by the human UDP glucuronosyltransferases. Drug Metab Dispos 35:2006–2014 [DOI] [PubMed] [Google Scholar]
- Sun D, Chen G, Dellinger RW, Sharma AK, Lazarus P. (2010) Characterization of 17-dihydroexemestane glucuronidation: potential role of the UGT2B17 deletion in exemestane pharmacogenetics. Pharmacogenet Genomics 20:575–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey RH, Strassburg CP. (2000) Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol 40:581–616 [DOI] [PubMed] [Google Scholar]
- Tukey RH, Strassburg CP. (2001) Genetic multiplicity of the human UDP-glucuronosyltransferases and regulation in the gastrointestinal tract. Mol Pharmacol 59:405–414 [DOI] [PubMed] [Google Scholar]
- Turgeon D, Carrier JS, Lévesque E, Hum DW, Bélanger A. (2001) Relative enzymatic activity, protein stability, and tissue distribution of human steroid-metabolizing UGT2B subfamily members. Endocrinology 142:778–787 [DOI] [PubMed] [Google Scholar]
- Turgeon D, Chouinard S, Belanger P, Picard S, Labbe JF, Borgeat P, Belanger A. (2003) Glucuronidation of arachidonic and linoleic acid metabolites by human UDP-glucuronosyltransferases. J Lipid Res 44:1182–1191 [DOI] [PubMed] [Google Scholar]
- Villard PH, Herber R, Sérée EM, Attolini L, Magdalou J, Lacarelle B. (1998) Effect of cigarette smoke on UDP-glucuronosyltransferase activity and cytochrome P450 content in liver, lung and kidney microsomes in mice. Pharmacol Toxicol 82:74–79 [DOI] [PubMed] [Google Scholar]
- Viswanath K, Herbst RS, Land SR, Leischow SJ, Shields PG, Writing Committee for the AACR Task Force on Tobacco and Cancer (2010) Tobacco and cancer: an American Association for Cancer Research policy statement. Cancer Res 70:3419–3430 [DOI] [PubMed] [Google Scholar]
- Wiener D, Doerge DR, Fang JL, Upadhyaya P, Lazarus P. (2004a) Characterization of N-glucuronidation of the lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in human liver: importance of UDP-glucuronosyltransferase 1A4. Drug Metab Dispos 32:72–79 [DOI] [PubMed] [Google Scholar]
- Wiener D, Fang JL, Dossett N, Lazarus P. (2004b) Correlation between UDP-glucuronosyltransferase genotypes and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone glucuronidation phenotype in human liver microsomes. Cancer Res 64:1190–1196 [DOI] [PubMed] [Google Scholar]
- Wilson W, 3rd, Pardo-Manuel de Villena F, Lyn-Cook BD, Chatterjee PK, Bell TA, Detwiler DA, Gilmore RC, Valladeras IC, Wright CC, Threadgill DW, et al. (2004) Characterization of a common deletion polymorphism of the UGT2B17 gene linked to UGT2B15. Genomics 84:707–714 [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhang J, Xie W. (2005) Xenobiotic nuclear receptor-mediated regulation of UDP-glucuronosyl-transferases. Curr Drug Metab 6:289–298 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.