Abstract
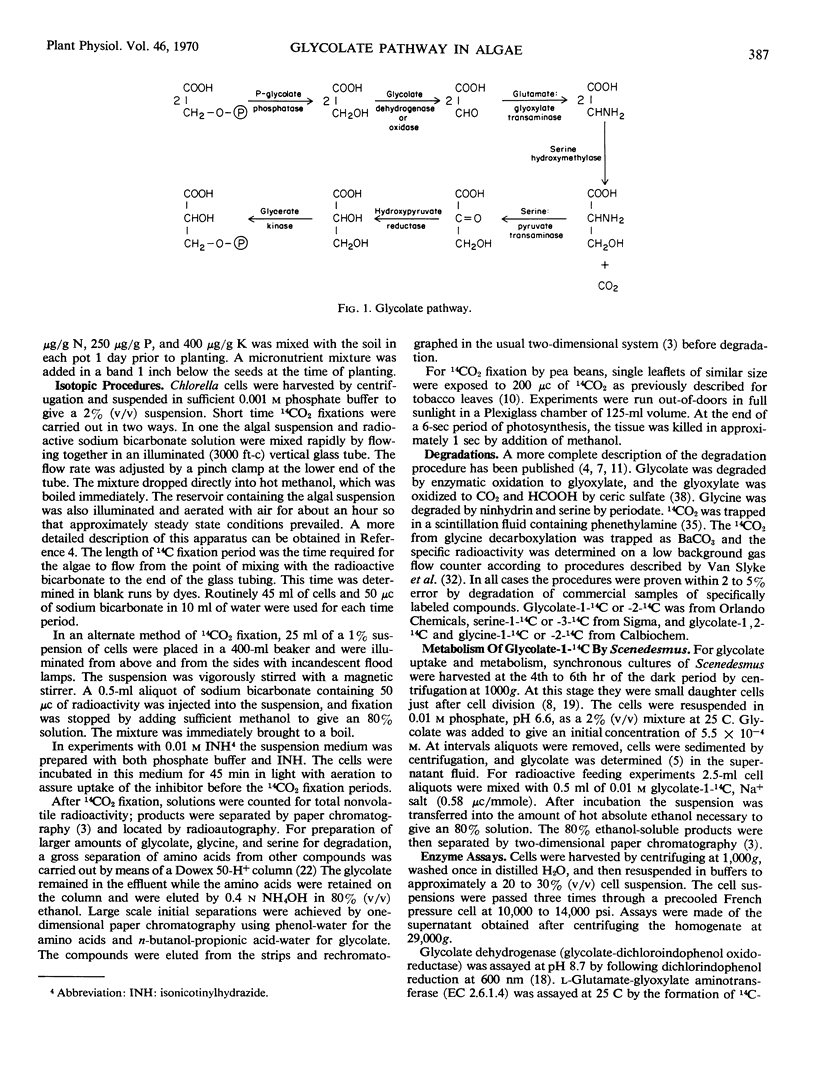
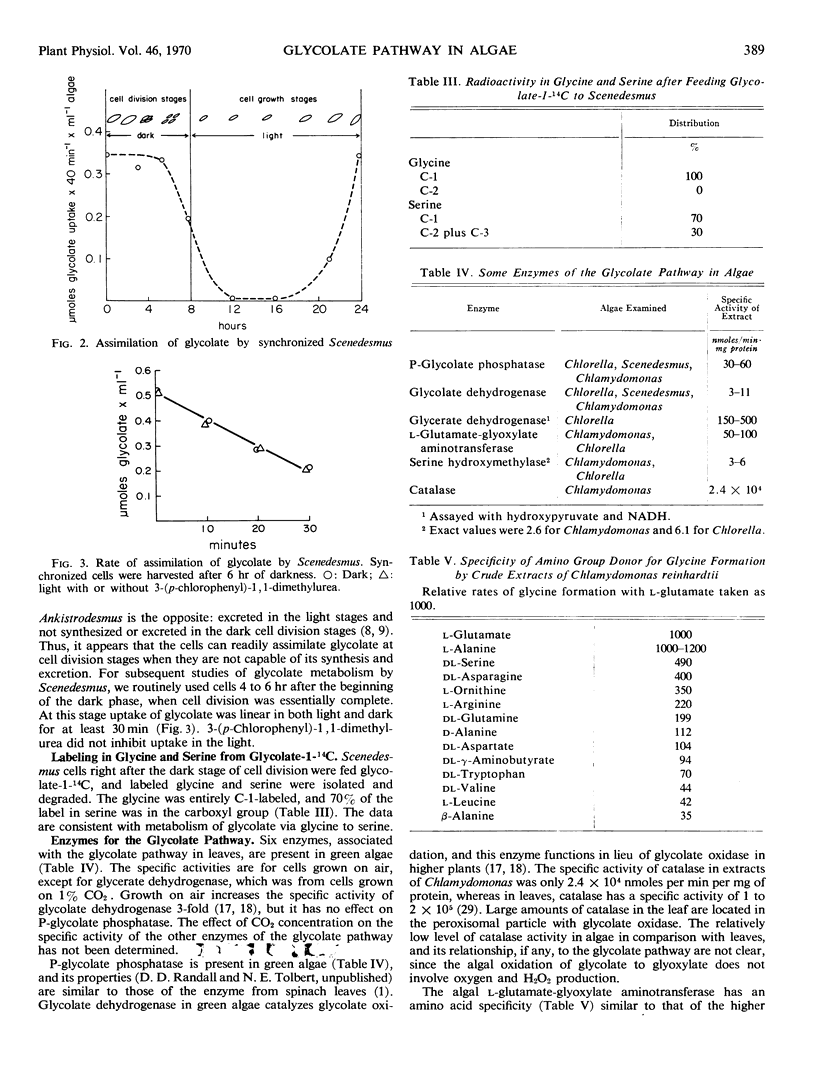
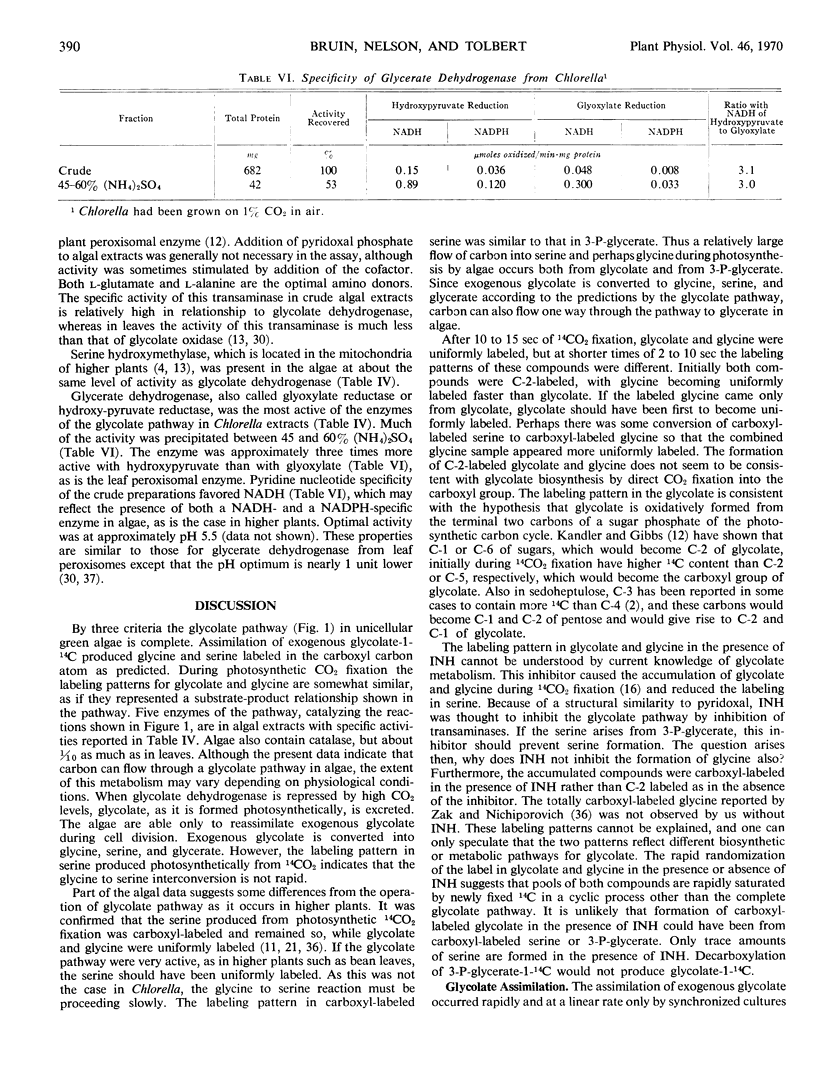
By three criteria, the glycolate pathway of metabolism is present in unicellular green algae. Exogenous glycolate-1-14C was assimilated and metabolized to glycine-1-14C and serine-1-14C. During photosynthetic 14CO2 fixation the distributions of 14C in glycolate and glycine were similar enough to suggest a product-precursor relationship. Five enzymes associated with the glycolate pathway were present in algae grown on air. These were P-glycolate phosphatase, glycolate dehydrogenase (glycolate:dichloroindophenol oxidoreductase), l-glutamate:glyoxylate aminotransferase, serine hydroxymethylase, and glycerate dehydrogenase. Properties of glycerate dehydrogenase and the aminotransferase were similar to those from leaf peroxisomes. The specific activity of glycolate dehydrogenase and serine hydroxymethylase in algae was 1/5 to 1/10 that of the other enzymes, and both these enzymes appear ratelimiting for the glycolate pathway.
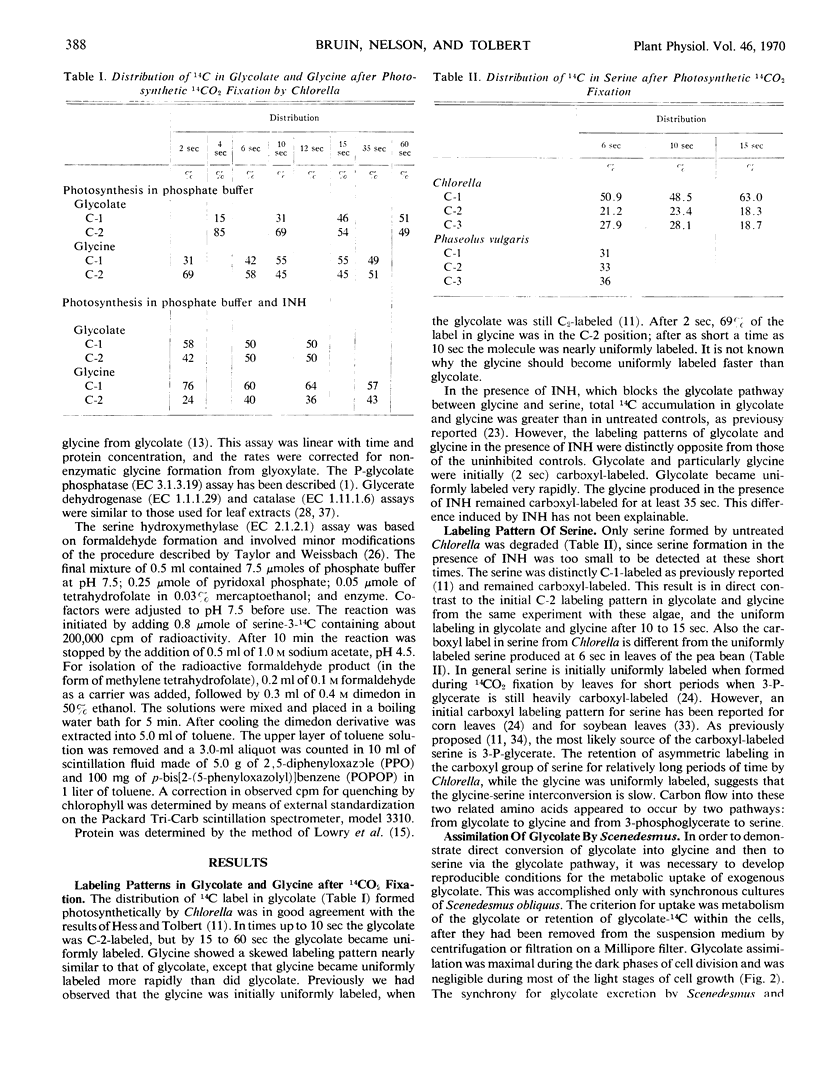
Labeling patterns for products of the glycolate pathway during 14CO2 fixation are not the same as those obtained with higher plants. In higher plants glycolate, glycine, and serine are uniformly labeled at shortest time periods. In algae, serine was predominately carboxyl-labeled, similarly to 3-phosphoglycerate. This result, plus the lower specific activity of serine hydroxymethylase, indicates that the glycine-serine interconversin in algae is slower than in plants. Initially (2 to 4 seconds) glycolate and glycine were more C-2 labeled. They rapidly became uniformly labeled, with glycine becoming uniformly labeled first. In the presence of isonicotinylhydrazide, labeled glycolate and glycine accumulated, and only a trace of serine-14C was detected. Then glycolate and glycine were initially carboxyl-labeled, and glycolate became uniformly labeled almost immediately and before glycine. These results suggest rapid metabolism of glycolate and glycine, in addition to the glycolate pathway.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chan H. W., Bassham J. A. Metabolism of 14C-labeled glycolic acid by isolated spinach chloroplasts. Biochim Biophys Acta. 1967 Jul 25;141(2):426–429. doi: 10.1016/0304-4165(67)90119-5. [DOI] [PubMed] [Google Scholar]
- Chang W. H., Tolbert N. E. Distribution of C in Serine and Glycine after CO(2) Photosynthesis by Isolated Chloroplasts. Modification of Serine-C Degradation. Plant Physiol. 1965 Nov;40(6):1048–1052. doi: 10.1104/pp.40.6.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W. H., Tolbert N. E. Excretion of Glycolate, Mesotartrate and Isocitrate Lactone by Synchronized Cultures of Ankistrodesmus braunii. Plant Physiol. 1970 Sep;46(3):377–385. doi: 10.1104/pp.46.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J. L., Tolbert N. E. Glycolate pathway in algae. Plant Physiol. 1967 Mar;42(3):371–379. doi: 10.1104/pp.42.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J. L., Tolbert N. E. Glycolate, glycine, serine, and glycerate formation during photosynthesis by tobacco leaves. J Biol Chem. 1966 Dec 10;241(23):5705–5711. [PubMed] [Google Scholar]
- Kandler O., Gibbs M. Asymmetric Distribution of C in the Glucose Phosphates Formed During Photosynthesis. Plant Physiol. 1956 Sep;31(5):411–412. doi: 10.1104/pp.31.5.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisaki T., Tolbert N. E. Glycolate and glyoxylate metabolism by isolated peroxisomes or chloroplasts. Plant Physiol. 1969 Feb;44(2):242–250. doi: 10.1104/pp.44.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lord M. J., Merrett M. J. Glycollate oxidase in Chlorella pyrenoidosa. Biochim Biophys Acta. 1968 Jul 9;159(3):543–544. doi: 10.1016/0005-2744(68)90140-x. [DOI] [PubMed] [Google Scholar]
- Marker A. F., Whittingham C. P. The photoassimilation of glucose in Chlorella with reference to the role of glycollic acid. Proc R Soc Lond B Biol Sci. 1966 Oct 11;165(1001):473–485. doi: 10.1098/rspb.1966.0078. [DOI] [PubMed] [Google Scholar]
- Nelson E. B., Tolbert N. E., Hess J. L. Glycolate stimulation of oxygen evolution during photosynthesis. Plant Physiol. 1969 Jan;44(1):55–59. doi: 10.1104/pp.44.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E. B., Tolbert N. E. The regulation of glycolate metabolism in Chlamydomonas reinhardtii. Biochim Biophys Acta. 1969 Jul 30;184(2):263–270. doi: 10.1016/0304-4165(69)90028-2. [DOI] [PubMed] [Google Scholar]
- Orth G. M., Tolbert N. E., Jimenez E. Rate of Glycolate Formation During Photosynthesis at High pH. Plant Physiol. 1966 Jan;41(1):143–147. doi: 10.1104/pp.41.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRITCHARD G. G., WHITTINGHAM C. P., GRIFFIN W. J. Effect of isonicotinyl hydrazide on the path of carbon in photosynthesis. Nature. 1961 May 6;190:553–554. doi: 10.1038/190553a0. [DOI] [PubMed] [Google Scholar]
- RABSON R., TOLBERTNE, KEARNEY P. C. Formation of serine and glyceric acid by the glycolate pathway. Arch Biochem Biophys. 1962 Jul;98:154–163. doi: 10.1016/0003-9861(62)90161-3. [DOI] [PubMed] [Google Scholar]
- TOLBERT N. E., ZILL L. P. Excretion of glycolic acid by algae during photosynthesis. J Biol Chem. 1956 Oct;222(2):895–906. [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Kisaki T., Hageman R. H., Yamazaki R. K. Peroxisomes from spinach leaves containing enzymes related to glycolate metabolism. J Biol Chem. 1968 Oct 10;243(19):5179–5184. [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Yamazaki R. K., Hageman R. H., Kisaki T. A survey of plants for leaf peroxisomes. Plant Physiol. 1969 Jan;44(1):135–147. doi: 10.1104/pp.44.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Yamazaki R. K. Leaf peroxisomes and their relation to photorespiration and photosynthesis. Ann N Y Acad Sci. 1969 Dec 19;168(2):325–341. doi: 10.1111/j.1749-6632.1969.tb43119.x. [DOI] [PubMed] [Google Scholar]
- VAN SLYKE D. D., STEELE R., PLAZIN J. Determination of total carbon and its radioactivity. J Biol Chem. 1951 Oct;192(2):769–805. [PubMed] [Google Scholar]
- VERNON L. P., ARONOFF S. Metabolism of soybean leaves. II. Amino acids formed during short-term photosynthesis. Arch Biochem. 1950 Nov;29(1):179–186. [PubMed] [Google Scholar]
- WOELLER F. H. Liquid scintillation counting of C-14-labelled CO2 with phenethylamine. Anal Biochem. 1961 Oct;2:508–511. doi: 10.1016/0003-2697(61)90056-2. [DOI] [PubMed] [Google Scholar]
- ZELITCH I. THE RELATION OF GLYCOLIC ACID SYNTHESIS TO THE PRIMARY PHOTOSYNTHETIC CARBOXYLATION REACTION IN LEAVES. J Biol Chem. 1965 May;240:1869–1876. [PubMed] [Google Scholar]
- ZELITCH I. The isolation and action of crystalline glyoxylic acid reductase from tobacco leaves. J Biol Chem. 1955 Oct;216(2):553–575. [PubMed] [Google Scholar]
- Zelitch I., Day P. R. Glycolate oxidase activity in algae. Plant Physiol. 1968 Feb;43(2):289–291. doi: 10.1104/pp.43.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I., Day P. R. Variation in photorespiration. The effect of genetic differences in photorespiration on net photosynthesis in tobacco. Plant Physiol. 1968 Nov;43(11):1838–1844. doi: 10.1104/pp.43.11.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]


