Abstract
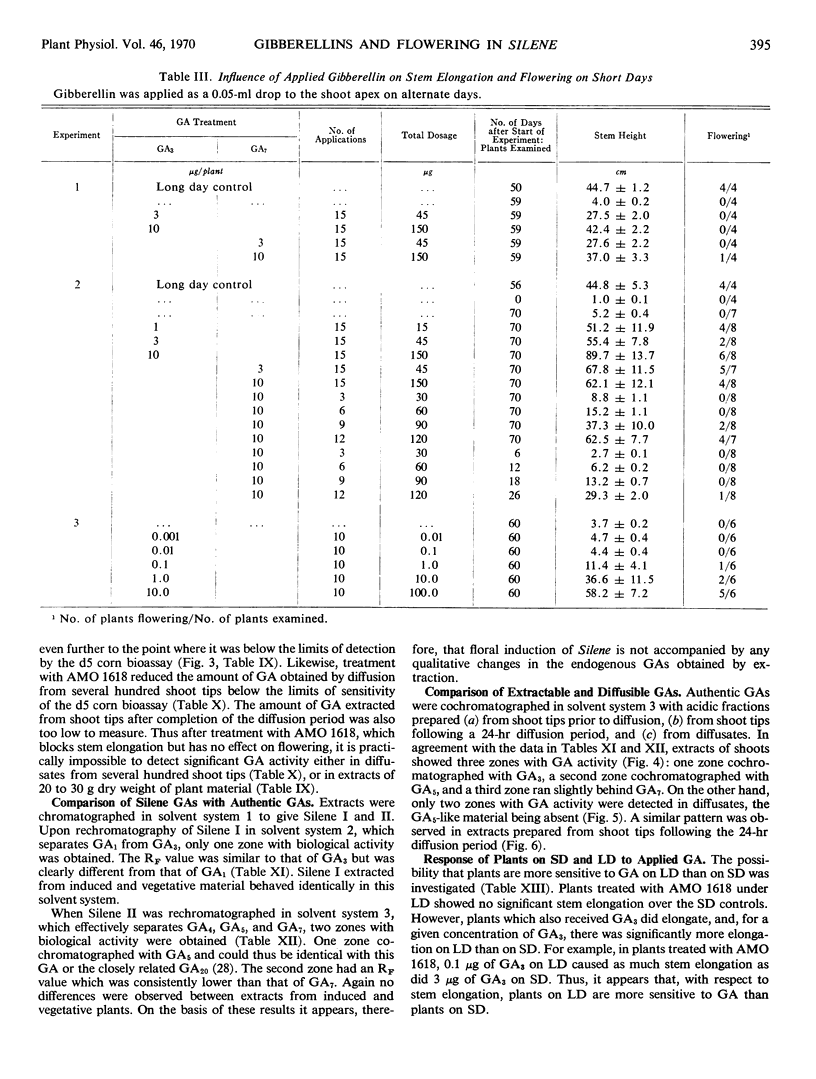
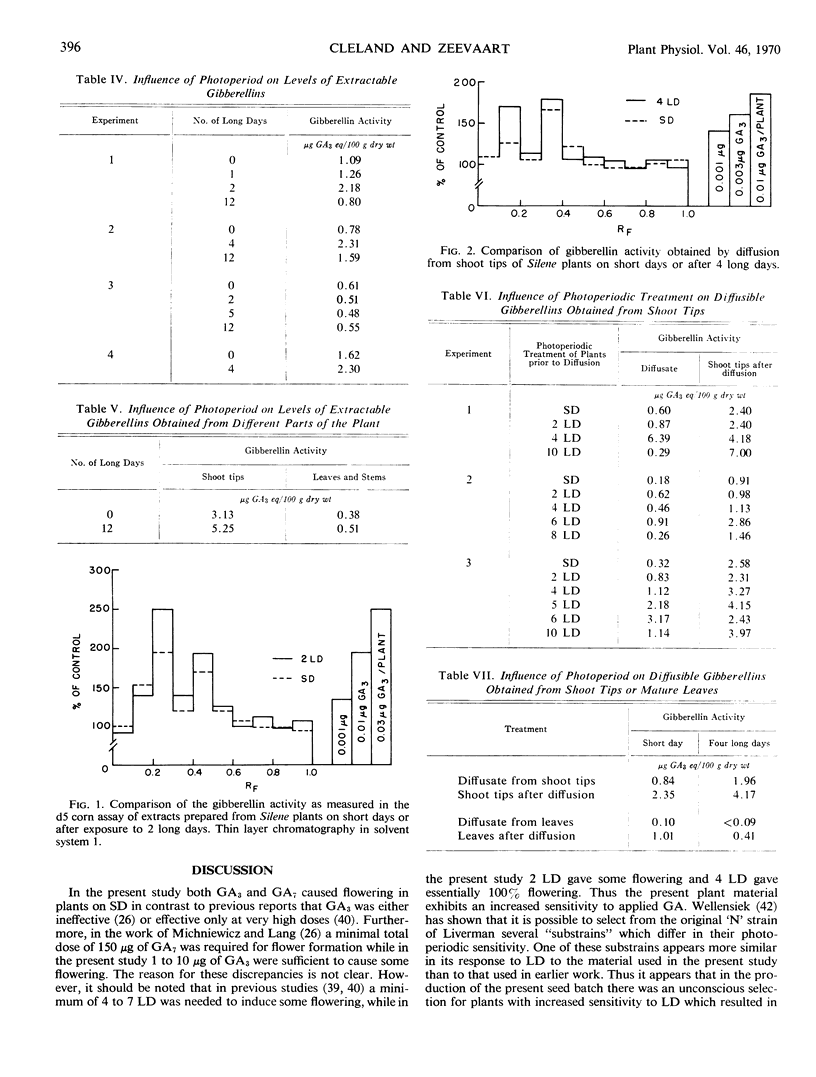
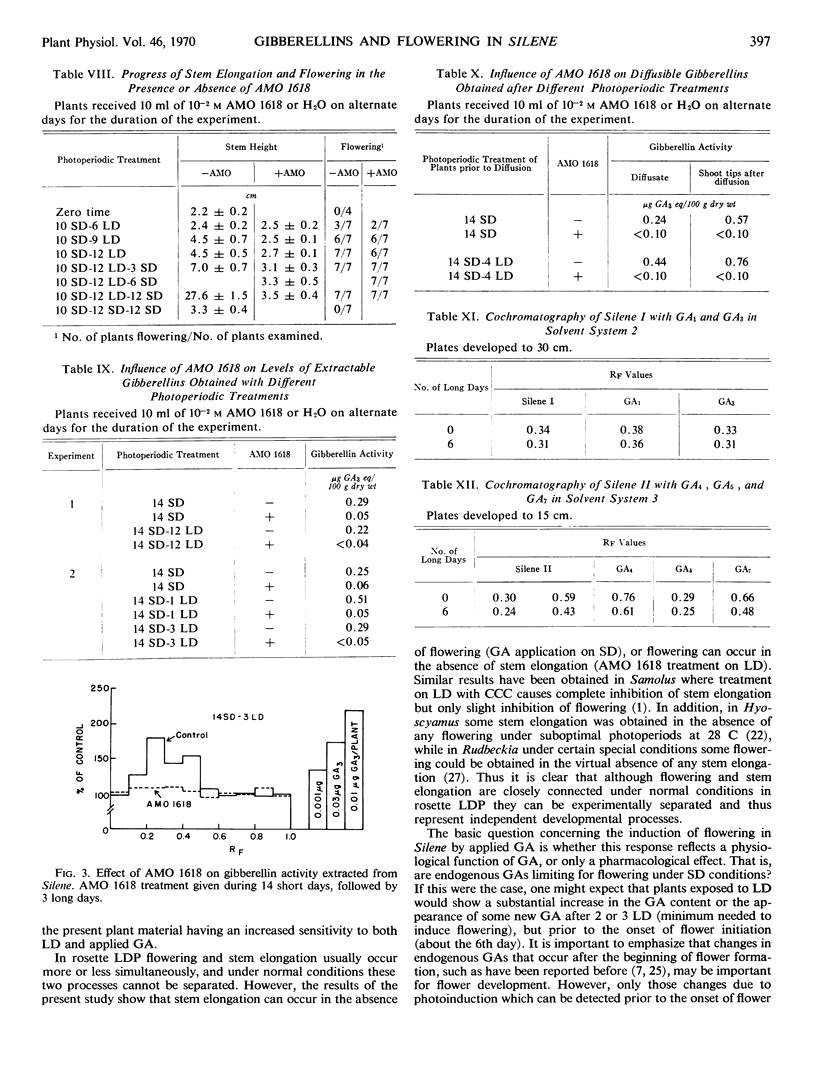
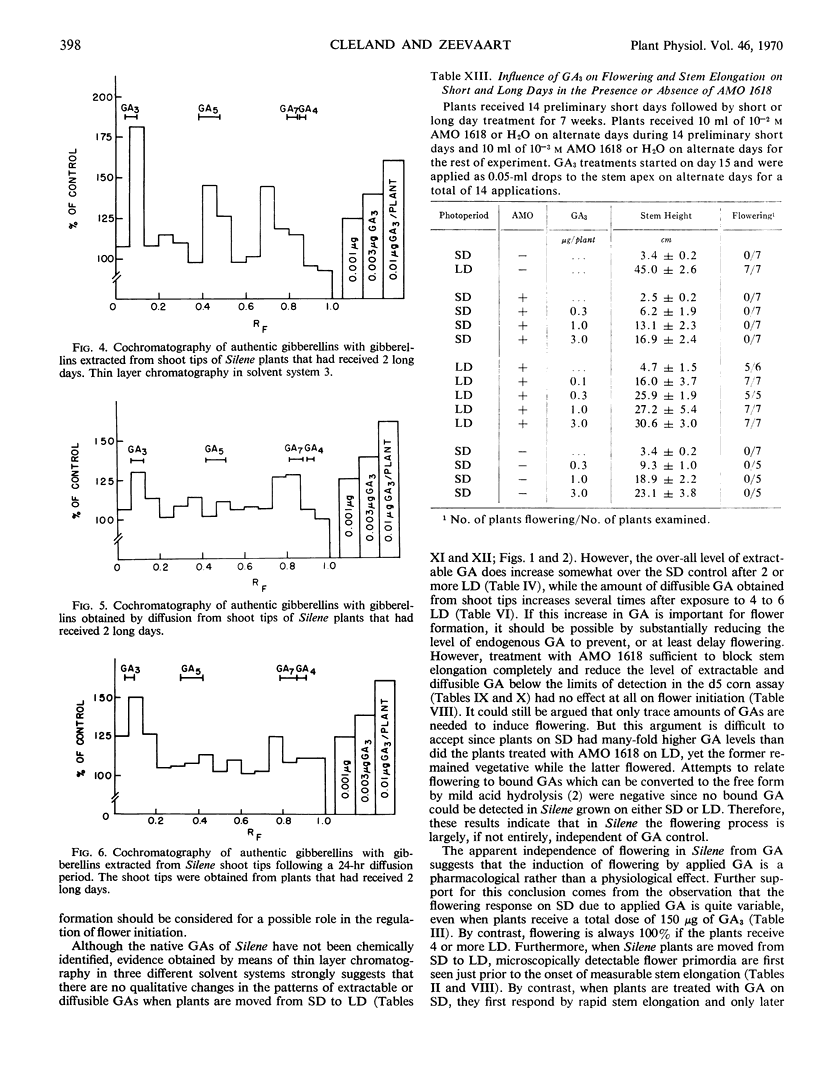
Two long days induced some flowering and 4 or more long days caused 100% flowering in Silene armeria. On long days microscopically detectable flower primordia were first seen after 6 days, which is at least 1 day before the start of stem elongation. Both gibberellin A3 and A7 caused flowering on short days, but the results were variable and flowering was never 100%. Three different gibberellins were detected in Silene extracts. The pattern of gibberellins extracted from plants on short and long days was qualitatively the same, but on long days gibberellin content was up to 100% higher than on short days. Only small amounts of diffusible gibberellins were obtained from Silene shoot tips (including very young leaves) on short days. However, on long days the diffusible gibberellins increased by as much as 10-fold after 4 to 6 long days but then declined somewhat after 10 long days. The gibberellins extracted from the shoot tips at the completion of the diffusion period also increased under long days, although the increase was not as large as for the diffusible gibberellins. An A5-like gibberellin present in extracts was not detected in diffusates.
Treatment with AMO 1618 (2-isopropyl-4-dimethylamino-5-methylphenyl-1-piperidine-carboxylate methyl chloride) completely inhibited stem elongation on long days but had no effect on flowering. In addition, treatment with AMO 1618 caused at least an 80% decrease in the level of extractable gibberellin, while the diffusible gibberellin was reduced below the limits of detection in the d5 corn bioassay. When endogenous gibberellin levels were suppressed by pretreatment with AMO 1618 on short days, gibberellin A3 caused more stem elongation in plants moved to long days than in plants left on short days. Thus the sensitivity of Silene plants to gibberellin with respect to stem growth is affected by photoperiod. It is concluded that in Silene endogenous gibberellins are a controlling factor for stem elongation but apparently are not required for flower formation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barendse G. W., Kende H., Lang A. Fate of radioactive gibberellin a(1) in maturing and germinating seeds of peas and Japanese morning glory. Plant Physiol. 1968 May;43(5):815–822. doi: 10.1104/pp.43.5.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland C. F., Briggs W. S. Gibberellin and CCC Effects on Flowering and Growth in the Long-day Plant Lemna gibba G3. Plant Physiol. 1969 Apr;44(4):503–507. doi: 10.1104/pp.44.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis D. T., Upper C. D., West C. A. An enzymic site of inhibition of gibberellin biosynthesis by Amo 1618 and other plant growth retardants. Plant Physiol. 1965 Sep;40(5):948–952. doi: 10.1104/pp.40.5.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. L., Lang A. Extractable and Diffusible Gibberellins From Light- and Dark-grown Pea Seedlings. Plant Physiol. 1968 Apr;43(4):629–634. doi: 10.1104/pp.43.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. L., Phillips I. D. Organs of gibberellin synthesis in light-grown sunflower plants. Plant Physiol. 1966 Oct;41(8):1381–1386. doi: 10.1104/pp.41.8.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H., Lang A. Gibberellins and Light Inhibition of Stem Growth in Peas. Plant Physiol. 1964 May;39(3):435–440. doi: 10.1104/pp.39.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang A. THE EFFECT OF GIBBERELLIN UPON FLOWER FORMATION. Proc Natl Acad Sci U S A. 1957 Aug 15;43(8):709–717. doi: 10.1073/pnas.43.8.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liverman J. L., Lang A. Induction of Flowering in Long Day Plants by Applied Indoleacetic Acid. Plant Physiol. 1956 Mar;31(2):147–150. doi: 10.1104/pp.31.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musgrave A., Kende H. Radioactive gibberellin a(5) and its metabolism in dwarf peas. Plant Physiol. 1970 Jan;45(1):56–61. doi: 10.1104/pp.45.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEMBDNER G., GROSS R., SCHREIBER K. [Thin layer chromatography of gibberellins]. Experientia. 1962 Dec 15;18:584–585. doi: 10.1007/BF02172196. [DOI] [PubMed] [Google Scholar]
- Suge H., Rappaport L. Role of gibberellins in stem elongation and flowering in radish. Plant Physiol. 1968 Aug;43(8):1208–1214. doi: 10.1104/pp.43.8.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart J. A. Effects of the Growth Retardant CCC on Floral Initiation and Growth in Pharbitis nil. Plant Physiol. 1964 May;39(3):402–408. doi: 10.1104/pp.39.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart J. A. Reduction of the Gibberellin Content of Pharbitis Seeds by CCC and After-Effects in the Progeny. Plant Physiol. 1966 May;41(5):856–862. doi: 10.1104/pp.41.5.856. [DOI] [PMC free article] [PubMed] [Google Scholar]



