Abstract
Objectives
Chronic rhinosinusitis (CRS) in children has been associated with a variety of disorders including atopic disease, cystic fibrosis, immunologic disorders and ciliary dyskinesia. Although a strong association, or even cause and effect relationship, between allergic rhinitis (AR) and CRS is commonly assumed, the epidemiologic relationship between these disorders has not yet been defined in children.
Methods
A retrospective review of all children diagnosed with CRS on otolaryngology or allergy office evaluation at a large tertiary-care pediatric hospital over a ten-year period was performed. Demographic data and concomitant diagnoses of AR, cystic fibrosis, immunologic disorders and primary ciliary dyskinesia were analyzed for relationships with CRS.
Results
A total of 4,044 children with an average age of 8.9 years and a slight male predominance (53.8%) with CRS were identified. Of these children, 0.2% had primary ciliary dyskinesia, 4.1% had cystic fibrosis, 12.3% had an immunologic disorder, and 26.9% had AR. A concomitant asthma diagnosis was positively associated with a diagnosis of AR (OR = 6.24, 95% CI: 5.27–7.39, P<0.001), whereas a concomitant cystic fibrosis diagnosis was negatively associated (OR = 0.12, 95% CI: 0.06–0.26, P<0.001).
Conclusions
AR is more prevalent than the other comorbidities combined in children with CRS, and is independently associated with the presence of asthma. Formal allergy testing, guided by clinical history and regional allergen sensitivity prevalence, should be strongly considered in all children with CRS, in particular those with reactive airway disease.
Keywords: allergic rhinitis, hypersensitivity, sinusitis, pediatrics, asthma, chronic rhinosinusitis
1. Introduction
Chronic rhinosinusitis (CRS) can be the ultimate manifestation of various disease processes[1,2] that cause sinonasal inflammation[3]. Whereas acute rhinosinusitis is common in the pediatric population, occurring as the sequela of six to eight percent of viral upper respiratory tract infections[4,5], chronic rhinosinusitis is comparatively rare. CRS in the pediatric population is defined as 90 days or more of persistent purulent rhinorrhea and nasal congestion[6]. The management of CRS in children consists primarily of medical treatment to eradicate bacterial infection and reduce underlying sinonasal inflammation[7]. Surgical interventions, such as adenoidectomy and endoscopic sinus surgery, are reserved for patients who fail medical management. Such interventions are designed to both eradicate potential bacterial reservoirs and enhance sinonasal aeration and drainage[7-9].
Regardless of treatment modality, the management of CRS requires an understanding of the underlying causes of sinonasal inflammation on a patient-by-patient basis. Because of the heterogeneous nature of CRS, clinical evaluation is required to uncover comorbidities that must be addressed, in addition to the specific interventions necessary to eradicate the sinus disease. Cystic fibrosis, immunodeficiency and ciliary dyskinesia are distinct conditions which contribute to the development and persistence of CRS symptoms in both children and adults[10,11]. The contribution of allergic rhinitis to the pathogenesis of CRS in children is more difficult to ascertain because, similar to CRS, allergic rhinitis is also characterized by sinonasal inflammation[12-15]. Although allergic rhinitis is commonly assumed to be associated with or a have a cause and effect relationship with CRS, the prevalence of allergic rhinitis in pediatric CRS has not to date been well characterized. In this study, a large cohort of pediatric patients with CRS is evaluated for the prevalence of allergic rhinitis. Moreover, the prevalence of allergic rhinitis is characterized in subpopulations of pediatric CRS who have concurrent cystic fibrosis, immunodeficiency or ciliary dyskinesia. Characterizing the relative prevalence of allergic rhinitis in comparison to other comorbid conditions associated with the pathogenesis of CRS will hopefully provide a greater understanding of the potential role of AR and inform subsequent treatment strategies[11,16].
2. Materials and Methods
2.1 Patient selection
Approval for this study was obtained from the Boston Children's Hospital Institutional Review Board. A consecutive series of patients (N = 4044) aged less than or equal to 18 years evaluated in the otolaryngology or allergy and immunology clinic with the diagnosis of chronic rhinosinusitis between August 2002 and August 2012 was identified based on associated ICD-9 code (473.*). ICD-9 codes were also utilized to screen for concomitant diagnoses of allergic rhinitis (477.*), asthma (493.*), immunity disorders (279.*), cystic fibrosis (277.*) and primary ciliary dyskinesia (759.*). Demographic data consisting of age at the time of presentation as well as gender were recorded.
2.2 Statistical analysis
All analysis and descriptive statistics were performed with the statistical software R (www.r-project.org). Statistical significance between the prevalence of binary characteristics between different cohorts of patients was performed using Fisher's exact test, while differences between continuous variables were performed using a Student's t-test. Associations between the presence of allergic rhinitis in the cohort of children with CRS and predictor variables (age, gender, and presence of comorbid conditions including asthma, cystic fibrosis, disorders of the immune system and primary ciliary dyskinesia) were determined by logistic regression using the lrm() function from the Regression Modeling Strategies (rms) package [18]. Univariate logistic regression was performed for each predictor variable. Multivariate logistical analysis was performed using all predictor variables. In the multivariate model, significant predictors were identified via backwards elimination, using a P-value cut-off of 0.100. Cross validation was performed through bootstrapping of the dataset using the validate() function from the rms package over 100 iterations. For each variable retained in the final model, a P-value and a log-odds ratio were calculated. P values less than 0.05 were considered significant in the analysis of associations.
3. Results
Over the ten year span from which patients were screened, 4,044 children with the diagnosis of chronic rhinosinusitis (CRS) were identified. The clinical and demographic characteristics of these children are outlined in Table 1. The average age of these children was 8.9 years (SD: 4.9 years, range: 0.3 – 18.9 years) with a 53.8% male and 46.2% female gender breakdown.
Table 1. Clinical and Demographic Characteristics of Children with CRS.
| Number (%) |
Age Years |
Gender | Asthma % |
||
|---|---|---|---|---|---|
|
| |||||
| Male % | Female % | ||||
|
| |||||
| All CRS patients | 4,044 (100%)* | 8.9 | 53.8% | 46.2% | 18.1% |
| with AR | 1,086 (26.9%) | 8.9 | 56.5% | 43.5% | 40.7% |
| without AR | 2958 (73.1%) | 8.9 | 52.8% | 47.2% | 9.8% |
|
| |||||
| CRS patients with | |||||
|
| |||||
| Cystic Fibrosis (CF) | 165 (4.1%)* | 10.9 | 49.7% | 50.3% | 4.8% |
| with AR | 6 (3.6%) | 8.2 | 33.3% | 66.7% | 16.7% |
| without AR | 159 (96.4%) | 11.0 | 50.3% | 49.7% | 4.4% |
|
| |||||
| Immunodeficiency (ID) | 496 (12.3%)* | 7.7 | 54.6% | 44.4% | 31.7% |
| with AR | 184 (37.1%) | 8.4 | 52.2% | 47.8% | 41.8% |
| without AR | 312 (62.9%) | 7.2 | 56.1% | 43.9% | 25.6% |
|
| |||||
| Primary Ciliary Dyskinesia (PCD) | 10 (0.2%)* | 9.6 | 60.0% | 40.0% | 10.0% |
| with AR | 1 (10%) | 13.7 | 0.0% | 100.0% | 0.0% |
| without AR | 9 (90%) | 9.2 | 66.7% | 33.3% | 11.1% |
|
| |||||
| Uncomplicated CRS | 3,376 (83.5%)* | 8.9 | 53.9% | 46.1% | 16.7% |
| with AR | 896 (26.5%) | 9.0 | 57.6% | 42.4% | 40.6% |
| without AR | 2,480 (73.5%) | 8.9 | 52.5% | 47.5% | 8.1% |
Relative to the total number of children with CRS (4,044)
3.1 Prevalence of related comorbidities in children with chronic rhinosinusitis
Of the 4,044 children diagnosed with CRS, 165 (4.1%) also carried a diagnosis of CF, 496 (12.3%) a diagnosis consistent with an immune system disorder, and 10 (0.2%) a diagnosis of PCD. Three children with CRS were found to have a diagnosis of CF and an immune system disorder. For the purposes of subsequent analyses, these children were considered to be in both the CF and the immune system disorders cohorts.
Children with CRS who also had CF tended to be slightly older with an average age of 10.9 years (SD: 4.7 years) in comparison to those CRS children with PCD (average age 9.6 years [SD: 5.7 years]) or immunologic disorders (average age of 7.7 years [SD: 4.7 years]). The difference in age between the children with CF and immunologic disorders was found to be statistically significant (P < 0.001 by ANOVA and P < 0.001 by t-test). There were no statistically significant differences in the gender composition of children with these three comorbid conditions.
3.2 Prevalence of allergic rhinitis in children with chronic rhinosinusitis
The prevalence of allergic rhinitis was characterized in various populations of children with CRS (Table 1). Of all children with CRS, 1,086 children (26.9%) were diagnosed with allergic rhinitis (AR). The average age of these children with both CRS and AR was 8.9 years (range: 0.7-18.9 years). The gender composition of these children with CRS and AR was similar to those children with CRS who did not have AR, likewise demonstrating a slight male predominance. Children without CF, immunologic disorders or PCD—termed “uncomplicated” CRS—comprised 83.5% (3,376 patients) of all children with CRS. The prevalence of AR in this “uncomplicated” CRS group was 26.5%. There was no difference in average age or gender composition in those “uncomplicated” CRS children with AR compared to those without AR (P = 0.815, P = 0.160, respectively).
3.3 Prevalence of allergic rhinitis in children with chronic rhinosinusitis and comorbid conditions
Children with CRS were divided into subsets according to a concomitant diagnosis of cystic fibrosis (CF), diseases of the immune system, and primary ciliary dyskinesia (PCD) (Table 1). Of the 165 children with CRS and CF, only 6 (3.6%) children were also diagnosed with AR. The average age of these six children with CF, AR and CRS was 8.2 years, while children with CF and CRS alone tended to be older with mean age of 11.0 years; this difference, however, was not statistically significant (P = 0.226). Of the 496 children with CRS and immunologic disorders, 184 (37.1%) were also diagnosed with AR, with an average age of 8.4 years. In comparison, those children with CRS and an immunologic disorder but not AR tended to be younger with an average age of 7.2 years (P = 0.008). Only one (10%) of the 10 children with PCD and CRS was diagnosed with AR. This patient was 13.7 years of age.
3.4 Prevalence of asthma in children with allergic rhinitis and chronic rhinosinusitis
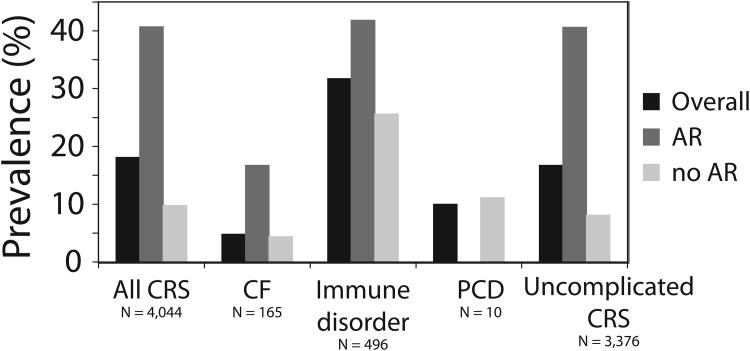
The prevalence of asthma in children with CRS was evaluated (Figure 1 and Table 1). Of all children with CRS, 18.1% also carried a diagnosis of asthma. There was a considerably higher prevalence of asthma in the population of CRS children with AR (40.7%) compared to those children without AR (9.8%); this was statistically significant (OR = 4.16, 95% CI: 3.53 – 4.92, P < 0.001). In children with CRS and a concomitant immunologic disorder, 31.7% of children also carried a diagnosis of asthma. A higher prevalence of asthma was similarly seen in those children with AR and an immunologic disorder (41.8%) compared to those without AR (25.6%) (OR = 1.63, 95% CI: 1.12 – 2.38, P = 0.009). In children with CRS and CF, the overall prevalence of asthma was low (4.8%). Although a higher prevalence of asthma (16.7%) was observed in the children with AR, CRS and CF compared to those without AR (4.4%), this was not statistically significant (OR = 3.74, 95% CI: 0.08 – 38.6, P = 0.286). Only one patient with CRS and PCD had asthma, corresponding to a prevalence of 10% in this group. This prevalence was somewhat lower than the prevalence of asthma in the overall pediatric CRS cohort.
Figure 1.
Prevalence of asthma in all children with CRS as well as in children with CRS and CF, immune disorders, PCD or no associated comorbidities (uncomplicated CRS). For each group, the prevalence of asthma with respect to allergic rhinitis status is also depicted.
3.5 Clinical and demographic factors associated with allergic rhinitis in children with chronic rhinosinusitis
Clinical and demographic characteristics associated with a diagnosis of AR in children with CRS were determined. In the entire cohort of children with CRS, multiple characteristics were associated with the presence of AR (Table 2). On univariate analysis, younger age was very slightly associated with AR (OR = 0.99, 95% CI: 0.99 – 1.00, P = 0.011), while a male gender was positively associated with AR (OR = 1.16, 95% CI: 1.01 – 1.33, P = 0.033). A diagnosis of CF was quite negatively associated with AR (OR = 0.09, 95% CI: 0.05 – 0.20, P< 0.001), while a positive association was detected for either a diagnosis of asthma (OR = 6.47, 95% CI: 5.47 – 7.65, P<0.001) or an immunologic disorder (OR = 1.60, 95% CI: 1.33 – 1.94, P<0.001). When considering only children with uncomplicated CRS (Table 3), a positive association was detected between male gender (OR = 1.21, 95% CI: 1.04 – 1.41, P = 0.012) and a diagnosis of asthma (OR = 8.09, 95% CI: 6.66 – 9.83, P<0.001) with AR on univariate analysis.
Table 2. Clinical and Demographic Characteristics Associated with AR in Children with CRS.
| Univariate Odds Ratio | Univariate P-value | Multivariate Odds Ratio | Multivariate P-value | |
|---|---|---|---|---|
| Age (yrs) | 0.99 (0.99 – 1.00) | 0.011 | 1.01 (1.00 – 1.01) | 0.750 |
| Gender (male) | 1.16 (1.01 – 1.33) | 0.033 | 1.09 (0.94 – 1.26) | 0.264 |
| Cystic fibrosis | 0.09 (0.05 – 0.20) | < 0.001 | 0.12 (0.06 – 0.26) | < 0.001 |
| Immunodeficiency | 1.60 (1.33 – 1.94) | < 0.001 | 1.15 (0.93 – 1.42) | 0.187 |
| Primary ciliary dyskinesia | 0.51 (0.11 – 2.30) | 0.380 | 0.60 (0.13 – 2.87) | 0.775 |
| Asthma | 6.47 (5.47 – 7.65) | < 0.001 | 6.24 (5.27 – 7.39) | < 0.001 |
Table 3. Clinical and Demographic Characteristics Associated with AR in Children with Uncomplicated CRS*.
| Univariate Odds Ratio | Univariate P-value | Multivariate Odds Ratio | Multivariate P-value | |
|---|---|---|---|---|
| Age (yrs) | 0.99 (0.99 – 1.00) | 0.428 | 1.00 (0.99 – 1.00) | 0.047 |
| Gender (male) | 1.21 (1.04 – 1.41) | 0.012 | 1.15 (0.98 – 1.36) | 0.095 |
| Asthma | 8.09 (6.66 – 9.83) | < 0.001 | 8.25 (6.77 – 10.04) | < 0.001 |
Without cystic fibrosis, immunodeficiency and primary ciliary dyskinesia
On multivariate analysis, a diagnosis of CF remained negatively associated with AR (OR = 0.12, 95% CI: 0.06 – 0.26, P<0.001), and asthma remained positively associated with AR (OR = 6.24, 95% CI: 5.27 – 7.39, P<0.001) in all children with CRS. In children with uncomplicated CRS, multivariate analysis revealed positive associations between the diagnosis of AR and younger age (OR = 0.99, 95% CI: 0.99 – 1.00, P = 0.047) and asthma (OR = 8.25, 95% CI: 6.77 – 10.04, P<0.001).
4. Discussion
CRS represents a heterogeneous set of disease processes which phenotypically converge with respect to chronic mucosal inflammation of the paranasal sinuses[1,2]. Cystic fibrosis[19,20], immune deficiencies[21] and primary ciliary dyskinesia[22] have all been independently associated with the pathogenesis of CRS, each likely through different mechanisms[11,16]. Allergic rhinitis is also suspected to contribute to the development of CRS by inflammation of the mucosa of the sinonasal cavity[11,16]. The epidemiology of AR in the setting of CRS has not been as fully characterized as these other comorbidities.
In this study, a comprehensive screen of all children evaluated for and diagnosed with CRS over a ten year period in the otolaryngology and allergy clinics of a tertiary-care pediatric hospital was performed. The prevalence of AR was determined in this large cohort of children with CRS and compared to the prevalence of CF, immune disorders and PCD. The prevalence of AR was also characterized in subpopulations of children with CRS who also had concurrent CF, immune disorders and PCD. Such a characterization of AR in pediatric CRS has not been previously described. Moreover, the diagnosis of AR in children with CRS was characterized through associations with demographic factors.
The limitations of this study are largely centered on the dependence upon ICD-9 codes as the sole basis to screen for patients with certain diagnoses. It is possible that miscoding may confound the selected cohort of patients, although the effect of any such miscoding is minimized by screening patients from otolaryngology and allergy clinics that are accustomed to evaluating patients with CRS and the associated comorbidities. Physician diagnostic accuracy was assumed and, as such, confirmation of diagnostic studies such as sweat chloride testing, quantitative and qualitative immunologic testing, nasal mucosal biopsy and pulmonary function testing was not performed.
While CF, PCD, and immunologic disorders are associated with and potentially contribute to the development CRS in children, the present study demonstrates that children diagnosed with these comorbidities are in the minority, accounting together for less than 20% of the CRS population. In contrast, 27% of the CRS children were documented to have AR. Because AR in the pediatric population is diagnosed by a wide range of clinicians with a wide variety of diagnostic tools ranging from clinical history alone to the use of skin and serological allergy testing, the true prevalence of AR in the general pediatric population is hard to absolutely ascertain. To date, the prevalence of AR in the general pediatric population has been estimated from ten to forty percent [23,24]. The 27% prevalence of AR established here in children with CRS notably falls within this range.
The overall prevalence of AR did not substantially change whether considering children with CRS in the absence of associated comorbid conditions or all children with CRS including those with CF, immune disorders or PCD [25]. However, the prevalence of AR in children with CRS was noted to vary in certain comorbid disease subgroups. A strong negative association was detected between a diagnosis of CF and a diagnosis of AR in children with CRS. This is not an expected finding in light of the characteristics of CRS in the setting of CF [26-28]. Asthma was additionally found to be present in 16-18% of all children with CRS. A concomitant diagnosis of asthma was several times more common in children with CRS who also had AR. In fact, a strong positive association was detected between a diagnosis of asthma and AR in children with CRS, which was irrespective of CF, immunologic or PCD status. This finding is consistent with the well-established frequent coexistence of AR and asthma [29]. Consistent with a previously reported association between asthma and CRS in adults [30], the prevalence of asthma in children with CRS reported here was slightly higher than the general pediatric population [23].
These results provide novel insights with important implications for the care of children with CRS. Although sweat chloride testing and immunologic profiling are commonly considered and often performed in children with CRS, formal allergy testing may not be undertaken as routinely [25]. The results of this study show the prevalence of AR to be comparatively greater than that of CF, immunologic disorders and PCD combined in children with CRS, calling into question the relative clinical priorities. Children with CRS, by definition, experience daily sinonasal symptoms such as congestion and purulent rhinorrhea driven by persistent sinonasal inflammation which may be clinically inseparable from the manifestations of allergic rhinitis. That over a quarter of these children would have a medically manageable allergic contribution to their sinonasal inflammation argues for routine consideration of aeroallergen sensitivity testing in these children. This is particular true of children with asthma who may be up to eight times more likely to have AR in the setting of CRS.
Acknowledgments
Funding: WP was supported in part by NIH grant K24 AI106822.
Footnotes
Conflict of interest statement: The authors state that there are no disclosures and no conflicts of interest.
This study was presented at the Annual Meeting of the American Society of Pediatric Otolaryngology on April 27, 2013.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Van Crombruggen K, Zhang N, Gevaert P, et al. Pathogenesis of chronic rhinosinusitis: inflammation. J Allergy Clin Immunol. 2011 Oct;128(4):728–732. doi: 10.1016/j.jaci.2011.07.049. [DOI] [PubMed] [Google Scholar]
- 2.Payne SC, Borish L, Steinke JW. Genetics and phenotyping in chronic sinusitis. J Allergy Clin Immunol. 2011 Oct;128(4):710–20. doi: 10.1016/j.jaci.2011.05.022. quiz 721-2. [DOI] [PubMed] [Google Scholar]
- 3.Berger G, Kogan T, Paker M, et al. Pediatric chronic rhinosinusitis histopathology: differences and similarities with the adult form. Otolaryngol Head Neck Surg. 2011 Jan;144(1):85–90. doi: 10.1177/0194599810390443. [DOI] [PubMed] [Google Scholar]
- 4.Wald ER, Guerra N, Byers C. Upper respiratory tract infections in young children: duration of and frequency of complications. Pediatrics. 1991 Feb;87(2):129–133. [PubMed] [Google Scholar]
- 5.Revai K, Dobbs LA, Nair S, et al. Incidence of acute otitis media and sinusitis complicating upper respiratory tract infection: the effect of age. Pediatrics. 2007 Jun;119(6):e1408–12. doi: 10.1542/peds.2006-2881. [DOI] [PubMed] [Google Scholar]
- 6.American Academy of Pediatrics. Subcommittee on Management of Sinusitis and Committee on Quality Improvement. Clinical practice guideline: management of sinusitis. Pediatrics. 2001 Sep;108(3):798–808. doi: 10.1542/peds.108.3.798. [DOI] [PubMed] [Google Scholar]
- 7.Wu AW, Shapiro NL, Bhattacharyya N. Chronic rhinosinusitis in children: what are the treatment options? Immunol Allergy Clin North Am. 2009 Nov;29(4):705–717. doi: 10.1016/j.iac.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Sedaghat AR, Cunningham MJ. Does balloon catheter sinuplasty have a role in the surgical management of pediatric sinus disease? Laryngoscope. 2011 Oct;121(10):2053–2054. doi: 10.1002/lary.21929. [DOI] [PubMed] [Google Scholar]
- 9.Ramadan HH, Cost JL. Outcome of adenoidectomy versus adenoidectomy with maxillary sinus wash for chronic rhinosinusitis in children. Laryngoscope. 2008 May;118(5):871–873. doi: 10.1097/MLG.0b013e3181653422. [DOI] [PubMed] [Google Scholar]
- 10.Ryan WR, Ramachandra T, Hwang PH. Correlations between symptoms, nasal endoscopy, and in-office computed tomography in post-surgical chronic rhinosinusitis patients. Laryngoscope. 2011 Mar;121(3):674–678. doi: 10.1002/lary.21394. [DOI] [PubMed] [Google Scholar]
- 11.Ryan MW, Brooks EG. Rhinosinusitis and comorbidities. Curr Allergy Asthma Rep. 2010 May;10(3):188–193. doi: 10.1007/s11882-010-0098-y. [DOI] [PubMed] [Google Scholar]
- 12.Baroody FM, Mucha SM, deTineo M, et al. Evidence of maxillary sinus inflammation in seasonal allergic rhinitis. Otolaryngol Head Neck Surg. 2012 Jun;146(6):880–886. doi: 10.1177/0194599811435972. [DOI] [PubMed] [Google Scholar]
- 13.Sedaghat AR, Gray ST, Wilke CO, et al. Risk factors for development of chronic rhinosinusitis in patients with allergic rhinitis. Int Forum Allergy Rhinol. 2012 Sep-Oct;2(5):370–375. doi: 10.1002/alr.21055. [DOI] [PubMed] [Google Scholar]
- 14.Sedaghat AR, Gray ST, Chambers KJ, et al. Sinonasal Anatomic Variants and Asthma are Associated with Faster Development of Chronic Rhinosinusitis in Patients with Allergic Rhinitis. Int Forum Allergy Rhinol. 2013 doi: 10.1002/alr.21163. [DOI] [PubMed] [Google Scholar]
- 15.Feng CH, Miller MD, Simon RA. The united allergic airway: connections between allergic rhinitis, asthma, and chronic sinusitis. Am J Rhinol Allergy. 2012 May-Jun;26(3):187–190. doi: 10.2500/ajra.2012.26.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Principi N, Esposito S. New insights into pediatric rhinosinusitis. Pediatr Allergy Immunol. 2007 Nov;18(18):7–9. doi: 10.1111/j.1399-3038.2007.00623.x. [DOI] [PubMed] [Google Scholar]
- 17.Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008 Mar;100(3 Suppl 3):S1–148. doi: 10.1016/s1081-1206(10)60305-5. [DOI] [PubMed] [Google Scholar]
- 18.Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- 19.Babinski D, Trawinska-Bartnicka M. Rhinosinusitis in cystic fibrosis: not a simple story. Int J Pediatr Otorhinolaryngol. 2008 May;72(5):619–624. doi: 10.1016/j.ijporl.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Raman V, Clary R, Siegrist KL, et al. Increased prevalence of mutations in the cystic fibrosis transmembrane conductance regulator in children with chronic rhinosinusitis. Pediatrics. 2002 Jan;109(1):E13. doi: 10.1542/peds.109.1.e13. [DOI] [PubMed] [Google Scholar]
- 21.Gross S, Blaiss MS, Herrod HG. Role of immunoglobulin subclasses and specific antibody determinations in the evaluation of recurrent infection in children. J Pediatr. 1992 Oct;121(4):516–522. doi: 10.1016/s0022-3476(05)81137-0. [DOI] [PubMed] [Google Scholar]
- 22.Leigh MW. Primary ciliary dyskinesia. Semin Respir Crit Care Med. 2003 Dec;24(6):653–662. doi: 10.1055/s-2004-815661. [DOI] [PubMed] [Google Scholar]
- 23.Meltzer EO, Blaiss MS, Derebery MJ, et al. Burden of allergic rhinitis: results from the Pediatric Allergies in America survey. J Allergy Clin Immunol. 2009 Sep;124(3 Suppl):S43–70. doi: 10.1016/j.jaci.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Wright AL, Holberg CJ, Martinez FD, et al. Epidemiology of physician-diagnosed allergic rhinitis in childhood. Pediatrics. 1994 Dec;94(6 Pt 1):895–901. [PubMed] [Google Scholar]
- 25.Leo G, Piacentini E, Incorvaia C, et al. Chronic rhinosinusitis and allergy. Pediatr Allergy Immunol. 2007 Nov;18(18):19–21. doi: 10.1111/j.1399-3038.2007.00626.x. [DOI] [PubMed] [Google Scholar]
- 26.Godoy JM, Godoy AN, Ribalta G, et al. Bacterial pattern in chronic sinusitis and cystic fibrosis. Otolaryngol Head Neck Surg. 2011 Oct;145(4):673–676. doi: 10.1177/0194599811407279. [DOI] [PubMed] [Google Scholar]
- 27.Wu X, Peters-Hall JR, Ghimbovschi S, et al. Glandular gene expression of sinus mucosa in chronic rhinosinusitis with and without cystic fibrosis. Am J Respir Cell Mol Biol. 2011 Sep;45(3):525–533. doi: 10.1165/rcmb.2010-0133OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Zele T, Claeys S, Gevaert P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006 Nov;61(11):1280–1289. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 29.Lack G. Pediatric allergic rhinitis and comorbid disorders. J Allergy Clin Immunol. 2001 Jul;108(1 Suppl):S9–15. doi: 10.1067/mai.2001.115562. [DOI] [PubMed] [Google Scholar]
- 30.Jarvis D, Newson R, Lotvall J, et al. Asthma in adults and its association with chronic rhinosinusitis: the GA2LEN survey in Europe. Allergy. 2012 Jan;67(1):91–98. doi: 10.1111/j.1398-9995.2011.02709.x. [DOI] [PubMed] [Google Scholar]