Abstract
Cocaine dependence remains a challenging public health problem with relapse cited as a major determinant in its chronicity and severity. Environmental contexts and stimuli become reliably associated with its use leading to durable conditioned responses (‘cue reactivity') that can predict relapse as well as treatment success. Individual variation in the magnitude and influence of cue reactivity over behavior in humans and animals suggest that cue-reactive individuals may be at greater risk for the progression to addiction and/or relapse. In the present translational study, we investigated the contribution of variation in the serotonin (5-HT) 5-HT2C receptor (5-HT2CR) system in individual differences in cocaine cue reactivity in humans and rodents. We found that cocaine-dependent subjects carrying a single nucleotide polymorphism (SNP) in the HTR2C gene that encodes for the conversion of cysteine to serine at codon 23 (Ser23 variant) exhibited significantly higher attentional bias to cocaine cues in the cocaine-word Stroop task than those carrying the Cys23 variant. In a model of individual differences in cocaine cue reactivity in rats, we identified that high cocaine cue reactivity measured as appetitive approach behavior (lever presses reinforced by the discrete cue complex) correlated with lower 5-HT2CR protein expression in the medial prefrontal cortex and blunted sensitivity to the suppressive effects of the selective 5-HT2CR agonist WAY163909. Our translational findings suggest that the functional status of the 5-HT2CR system is a mechanistic factor in the generation of vulnerability to cocaine-associated cues, an observation that opens new avenues for future development of biomarker and therapeutic approaches to suppress relapse in cocaine dependence.
Keywords: attentional bias, cocaine, cue reactivity, 5-HT2C receptor, prefrontal cortex, serotonin
Introduction
Cocaine dependence remains a challenging public health problem with relapse cited as a major determinant in its chronicity and severity.1 With a history of cocaine use, environmental contexts and stimuli (for example, paraphernalia) become reliably associated with its use leading to durable conditioned responses (‘cue reactivity') that can predict relapse as well as treatment success.2, 3, 4, 5 Drug cue reactivity is the attentional orientation toward such drug-associated cues that are measurable as conditioned physiological effects (for example, heart rate), subjective properties (for example, craving), appetitive approach behaviors (for example, drug-seeking) and activation of specific corticostriatal subcircuits.1,5,6 Individual variation in the magnitude and influence of cue reactivity over behavior in humans7,8 and animals9,10 suggest that cue-reactive individuals may be at greater risk for the progression to addiction and/or relapse.8,11,12 A greater understanding of the neural underpinnings of cocaine cue reactivity promises to shed light on therapeutic approaches to effectively intervene in cocaine dependence and improve recovery outcomes.
The distributed corticostriatal circuitry that controls the incentive-motivational properties of drug-associated cues involves a key modulatory role for dopamine neurotransmission.13 Serotonin (5-HT) innervation of these interlooping pathways is also prominent14,15 and evidence suggests a modulatory role for 5-HT neurotransmission in cue reactivity processes (for review16). The 5-HT2C receptor (5-HT2CR) is one of fourteen 5-HT-receptive proteins in brain and is prominently localized to corticostriatal subregions including the medial prefrontal cortex (mPFC) in rodents,17 a homolog of the orbitofrontal cortex in humans.18 This cortical region is a critical component within the circuit responsive to cocaine-associated cues in humans19 and animals.20,21 Stimulation of the 5-HT2CR localized to the mPFC suppressed cocaine-seeking in rats,22 an observation that recapitulates the efficacy of systemic administration of selective 5-HT2CR agonists (RO 60-0175, WAY163909) to consistently reduce cue- and cocaine-primed drug-seeking.23, 24, 25, 26, 27 This 5-HT2CR agonist-induced functional antagonism of cocaine cue reactivity is reversed by the selective 5-HT2CR antagonist SB242084 and occurs at doses of the 5-HT2CR agonists that do not significantly alter general behaviors (for example, locomotor activity).23, 24, 25, 26, 27 Consistent with this behavioral profile, SB242084 also increased cocaine-seeking although inter-individual variability in its efficacy was observed.25,28, 29, 30 Finally, we recently demonstrated that cocaine cue reactivity was significantly elevated in rats following virally mediated loss of the 5-HT2CR in mPFC,31 establishing that reduced mPFC 5-HT2CR function is a neurobiological mediator of cocaine cue reactivity.
Natural variation within the 5-HT2CR system through single nucleotide polymorphisms (SNPs) could contribute to individual differences in sensitivity to reward-associated cues in humans. The single nucleotide variant Cys23Ser (rs6318) in the human 5-HT2CR gene (HTR2C) results in the substitution of a serine for a cysteine in the extracellular N-terminus of the receptor.32 This SNP is predicted to alter protein structure and/or stability32,33 which would be expected to alter the ability of a ligand to bind to the receptor and initiate downstream signal transduction.34,35 In support of this concept, there is evidence that the Ser23 variant has been associated with lower sensitivity to the effects of 5-HT2CR agonists in human studies.36, 37, 38 As a putative reduced-function SNP in the HTR2C in humans, we tested the hypothesis that the Ser23 variant may associate with higher cocaine cue reactivity31 measured as attentional bias (attentional orienting response in a computerized cocaine-word Stroop task).6 Alignment of the human and rat 5-HT2CR gene shows no sequence homology at the rs6318 position.39,40 However, given our recent finding that knockdown of the 5-HT2CR in the mPFC resulted in vulnerability to the expression of cocaine cue reactivity in rats,31 we tested the hypothesis that individual differences in cocaine cue reactivity as measured as appetitive approach behavior [lever presses reinforced by the discrete cue complex (for example, stimulus light, pump)]6 would associate with reduced 5-HT2CR protein expression and sensitivity to a selective 5-HT2CR agonist.
Materials and methods
Assessment of 5-HT2CR genotype and cue reactivity in cocaine-dependent subjects
Subjects
Subjects (n=114) who met DSM-IV criteria for current cocaine dependence were recruited within three ongoing studies measuring cue reactivity using the same diagnostic, psychometric and advertising procedures. Subjects were recruited via newspaper advertisements, screened for psychiatric disorders using the structured clinical interview for DSM-IV (SCID-I),41 and completed a medical history and physical examination. All subjects were tested for urine cocaine (benzoylecgonine), tetrahydrocannabinol, opioids, amphetamine, methamphetamine and benzodiazepines using the integrated E-Z split key cup II (Innovacon Company, San Diego, CA, USA) on each visit. All subjects had at least one cocaine-positive urine during screening; did not meet DSM-IV current dependence criteria for abused drugs other than cocaine, marijuana, nicotine or alcohol; did not have current or past medical disorders affecting the central nervous system; and did not have axis I disorders other than substance abuse or dependence. The subjects included non-treatment-seekers (n=21) and treatment-seekers (n=93). All subjects were tested during the baseline period of the studies. All subjects were free of alcohol at the time of testing as determined by a breathalyzer (Intoximeters, St Louis, MO, USA). Female subjects with a positive urine pregnancy test were excluded from the study. All data were collected in the Center for Neurobehavioral Research on Addictions at the University of Texas Health Science Center at Houston. All subjects were provided with written informed consent after being fully informed of the nature of the research in accordance with the Declaration of Helsinki. The consent form included agreement to participate in the genetic study. The study was approved by the Committees for the Protection of Human Subjects, which are the Internal Review Board of the University of Texas Health Science Center at Houston and the Baylor College of Medicine.
Cocaine-word Stroop task
The cocaine-word Stroop task was designed to measure attentional bias to cocaine-related stimuli.42, 43, 44 It is a widely used implicit task45 in which the participant is presented with words printed in color, and asked to discriminate the color of each stimulus; the participant is instructed to ignore the meaning of the words and concentrate only on responding to the color in which the word is written. The stimuli presented include neutral words and words that are related to the concerns or pathology under study, in this case, cocaine dependence. Slowness in responding to a color suggests distraction from color discrimination due to attention being ‘captured' by the meaning of the stimulus (that is, cocaine) word.46 Each analyzed session began with a block of 60 practice trials, followed by 30-trial blocks of test trials.43,44 The test trials included two blocks of 30 trials with cocaine-related words, and two blocks of 30 trials with neutral words. Within each block type, each word was randomly presented three times in three different colors. Block type was alternated within each session (for example, cocaine, neutral, cocaine, neutral), and the order of block type was counterbalanced across subjects. Trials with correct responses and reaction times larger than 200 msec were used to calculate mean reaction times.43,44 Attentional bias was operationalized as the difference between the reaction times (in msec) observed in trials with cocaine-related words and trials with neutral words, calculated for each subject and averaged across subjects. This calculation corrects for any difference in overall reaction times between cocaine-dependent and control subjects.46 A correct response was defined as responding to the word color on an appropriately colored response button. Accuracy was assessed as the ratio of correct trials to total trials within each block type.
DNA preparation
Venous blood (10 ml) from each subject was centrifuged at 2000 r.p.m. for 30 min (Eppendorf North America, New York, NY, USA). The buffy coat was removed and stored in 2.0 ml cryogenic vials at −80 °C. DNA was isolated from the buffy coat using the Puregene Kit (Qiagen, CA, USA) according to manufacturer's recommendation. Purified DNA for each subject was dissolved in 0.25 ml DNA hydration solution.
HTR2C genotyping
The HTR2C has been localized to chromosome X, band q24 (female genotypes: CC, CG or GG; male genotypes: C or G).32 All samples were assayed in duplicate on an Applied Biosystems ViiA 7 Real-Time PCR System (Applied Biosystems, Grand Island, NY, USA). Genotyping was performed with 10 ng of DNA, 1.5 μl of Taqman Genotyping Master Mix, 0.03 μl of pre-designed TaqMan primer-probe set (Applied Biosystems; Assay ID C_2270166_10) and 2.47 μl of water. PCR amplification consisted of 10 min at 95 °C, 40 cycles of 15 s at 92 °C and 1 min at 60 °C.
Statistical analyses
Reaction times and accuracy on the cocaine-word Stroop task were analyzed with a paired Student's t-test. Differences in age, sex, race, years of cocaine use, percent treatment-seekers, percent positive urine cocaine screens, percent alcohol abuse and percent cannabis abuse among subjects with different HTR2C genotypes were analyzed using one-way analysis of variance (ANOVA; age and years of cocaine use) or Fisher's exact test (sex, race, percent treatment-seekers, percent positive urine cocaine screens, percent alcohol abuse, percent cannabis abuse). Differences in attentional bias among subjects with different HTR2C genotypes were analyzed using one-way analysis of covariance (ANCOVA) with gene polymorphism as the independent variable and sex or race as the covariate in a general linear model. All reported P values for post hoc comparisons were Tukey–Kramer adjusted for multiple comparisons. To determine the population structure, genotypic data for ancestral informative markers for our cohort was compared against Centre d'Etude du Polymorphisme Humain–Human Genome Diversity Panel (CEPH–HGDP) samples (1035 subjects of 51 populations).47,48 The obtained values were similar to those calculated without correction for these covariates. The alpha level for all analyses was set at P=0.05.
Assessment of the 5-HT2CR system and associated cocaine cue reactivity in rodents
Animals
Experimentally naive, male, Sprague–Dawley rats (n=105) weighing 225–250 g at arrival were housed two per cage under a 12-h light-dark cycle at constant temperature (21–23 °C) and humidity (40–50%). Food and water were available ad libitum. All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (2011) and with the approval of the Institutional Animal Care and Use Committee at the University of Texas Medical Branch.
Drugs
(–)-Cocaine (National Institute on Drug Abuse) was dissolved in 0.9% NaCl. WAY163909 ((7bR, 10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]diazepino [6,7,1hi]indole; a gift from Pfizer, New York, NY, USA) was dissolved in 0.9% NaCl.
Cocaine self-administration and cue reactivity assessments
Implantations of intravenous catheters with back mounts were performed under anesthesia with a cocktail containing xylazine (8.6 mg kg−1), acepromazine (1.5 mg kg−1) and ketamine (43 mg kg−1) in bacteriostatic saline. Self-administration took place in standard operant chambers equipped with two retractable levers, a stimulus light above each lever, and a houselight housed within ventilated and sound-attenuating chambers (Med-Associates, St Albans, VT, USA). Cocaine infusions were delivered by a syringe attached to an infusion pump located outside the chamber. Daily flushes with a solution of bacteriostatic saline containing heparin sodium (10 U ml−1), streptokinase (0.67 mg ml−1) and ticarcillin disodium (66.67 mg ml−1) were performed to maintain catheter patency.
Self-administration consisted of 14 days of 180-min sessions, during which rats were trained to lever press to obtain a cocaine infusion (0.75 mg kg−1per 0.1 ml infusion) on a fixed ratio (FR) 1 schedule before progressing to an FR5.23,27,31,49 Schedule completions on the active lever resulted in delivery of cocaine over a 6-s period along with simultaneous illumination of the house and stimulus lights and activation of the infusion pump (discrete cue complex paired with delivery of cocaine); responses on the inactive lever were recorded but had no scheduled consequences. After cocaine delivery, the pump and stimulus light were inactivated simultaneously. The house light remained illuminated for a 20-s timeout period, during which lever presses had no scheduled consequences. Following stable self-administration on an FR5 (seven infusions per hour for at least three sessions with <10% variation in the number of infusions received for three consecutive sessions), cocaine-trained rats were subjected to a probe trial on self-administration day 12 to stratify individual rats as high cue reactive (HCR) or low cue reactive (LCR). During this 60-min probe trial, responses on the active lever were reinforced by presentation of the discrete cue complex (stimulus light, pump) previously associated with cocaine delivery. Self-administration was reinstated immediately following the end of the probe trial followed by an additional two self-administration sessions. The number of previously active lever presses during the probe trial was used to stratify rats within the HCR or LCR phenotype; a median split was used. The probe session did not interfere with the stability of self-administration as performance on the post-probe sessions was identical to the stable baseline established before the probe trial (data not shown).
Rats were returned to their home cage after 14 days of cocaine self-administration. In Experiment 1, rats (n=12 rats per phenotype) were reintroduced to the self-administration chambers 24 h later and assayed in a test session comprised of two sequential components. The first component evaluated whether HCR and LCR rats would exhibit differential levels of lever presses when placed in the context in the absence of the discrete cue complex. To this end, responses on both levers on an FR1 schedule were recorded but no discrete cues (for example, stimulus light, pump) were present nor delivered during the initial 10 min of the session. The second component was signaled by a single, non-response contingent delivery of the discrete cue complex presented at the termination of the first 10-min component. To assess cocaine cue reactivity, presses on the previously-active lever in the 60-min (second component) were reinforced by the discrete cue complex on an FR1; inactive lever presses were recorded but produced no scheduled consequences.31
In Experiment 2, rats were stratified as HCR or LCR on the basis of their performance on the probe trial (above) and returned to the self-administration chambers at 24 h of withdrawal. To assess cocaine cue reactivity, presses on the previously-active lever were reinforced by the discrete cue complex on an FR1 during a 60-min session;31 inactive lever presses were recorded but produced no scheduled consequences. For ex vivo neurochemical studies, rats were sacrificed immediately after the cue reactivity test session [HCR (n=5), LCR (n=6)] or upon removal from their home cage at the expected time of that test session without re-exposure to the self-administration chambers [HCR (n=6), LCR (n=6)] this second group of rats served as control for the behavioral experience during the cue reactivity session. For pharmacological analyses, a cohort of HCR (n=16 per treatment) and LCR rats (n=16 per treatment) were administered vehicle (saline, 1 ml kg−1, intraperitoneal) or WAY163909 (0.5 mg kg−1, intraperitoneal) 15 min before the start of the cue reactivity test session.
Immunoblotting
The HCR or LCR rats stratified on the probe test in Experiment 2 were evaluated for cue reactivity at 24 h of withdrawal and sacrificed immediately following the cue reactivity test session or remained in their home cages and sacrificed at 24 h of withdrawal. Rats were anesthetized [chloral hydrate solution (400 mg kg−1)] and decapitated; the mPFC was microdissected and flash frozen in liquid nitrogen and stored at −80 °C for subsequent crude synaptosomal protein extraction and immunoblotting.50,51 Equal amounts of protein were separated by SDS–PAGE using 4–12% Bis-Tris gels and transferred to a PVDF membrane for immunoblotting with 5-HT2CR antibody (D-12, sc-17797, Santa Cruz, Dallas, TX, USA; 1:100) or pan-cadherin antibody (AB6528, Abcam, Cambridge, MA, USA; 1:10 000). Membranes were incubated with mouse IgG IRDye (1:10 000) for detection by Odyssey Imaging System (LI-COR Biosciences, Lincoln, NE, USA). The integrated intensity of each band (arbitrary units) was analyzed with the Odyssey Software. The ratio of the intensity of the 5-HT2CR-immunoreactive band to the cadherin-immunoreactive band was determined for normalization.
Statistical analyses
A one-way ANOVA (SAS 9.3) for repeated measures was used to analyze the dependent measures of the total number of active and inactive lever presses per session over the last three sessions of the self-administration phase. Student's t-test was employed to compare HCR and LCR rats on the total number of responses (previously active and inactive levers) and the latency to respond on the previously-active lever during the probe trial, the context-associated and cue reactivity test sessions as well as the density of 5-HT2CR protein expression. For pharmacological analyses, a two-way ANOVA for the factors of phenotype and treatment was conducted; a priori comparisons between the total number of responses on the previously-active and inactive levers as well as the latency to respond on the previously active lever during the test session were made using Student's t-test. The experimentwise alpha level was set at P=0.05.
Results
Assessment of 5-HT2CR genotype and cue reactivity in cocaine-dependent subjects
The demographics of the study population are presented in Table 1. Cocaine-dependent subjects were stratified into three groups, those homozygous (CC; females) and hemizygous (C; males) for the C allele which encodes for the Ser23 variant, those homozygous (GG; females) and hemizygous (G; males) for the G allele or heterozygous (CG; females only) which encodes for the Cys variant. Genotype was not associated with the distribution of age (F2,111=0.78; NS) or race (Fisher's exact test, NS), but was associated with sex (Fisher's exact test, P<0.01) (Table 1). Genotype was not associated with years of cocaine use (F2,111=0.15, NS), percent positive cocaine urine screens (Fisher's exact test, NS), percent alcohol abuse (Fisher's exact test, NS), percent cannabis abuse (Fisher's exact test, NS), or percent treatment-seekers (Fisher's exact test, NS) (Table 1).
Table 1. Demographic and clinical characteristics of cocaine-dependent subjects by HTR2C genotype.
|
Genotype |
|||
|---|---|---|---|
| C/CC | CG | G/GG | |
| Subjects (n) | 27 | 6 | 81 |
| Age (years±s.e.m.) | 45.37±1.50 | 41.33±2.32 | 43.64±0.93 |
| Sexa | 26 M, 1 F | 6 F | 71 M, 10 F |
| Race | 24 AA, 3 Cau | 6 AA | 54 AA, 18 Cau, 9 O |
| Cocaine (years±s.e.m.) | 15.56±1.5 | 17.50±2.2 | 15.25±0.9 |
| %Positive cocaine | 78 | 75 | 74 |
| %Alcohol abuse | 31 | 17 | 25 |
| %Cannabis abuse | 22 | 33 | 10 |
| %Treatment-seekers | 70 | 67 | 86 |
Abbreviations: F, female; M, male;
AA, African-American; Cau, Caucasian; O, other races (Hispanic, Asian).
Fisher's exact test, P<0.01.
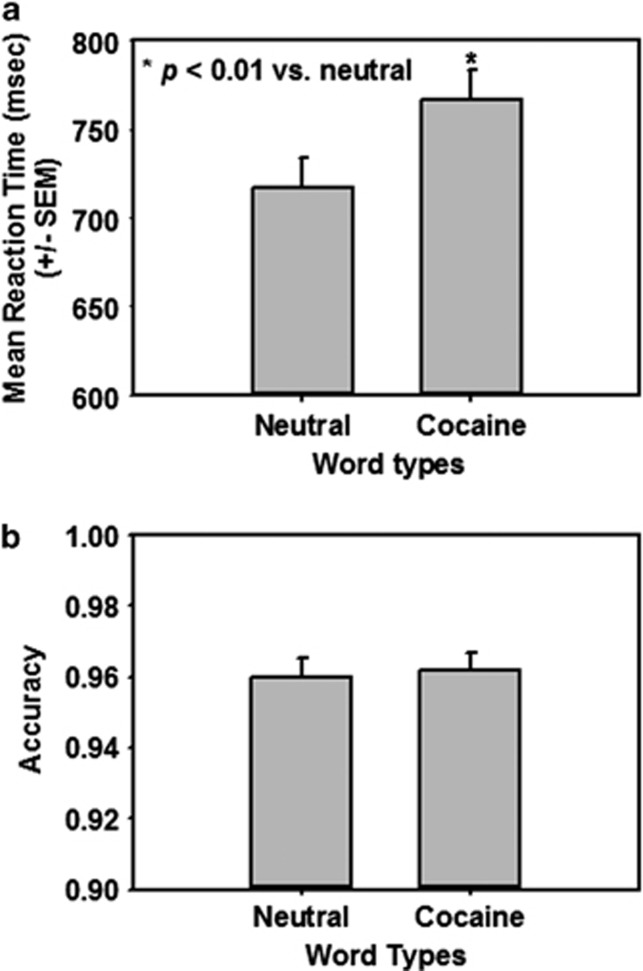
The response to presentation of cocaine-associated cues (‘cue reactivity') was measured as attentional bias in the cocaine-word Stroop task in cocaine-dependent subjects. Several studies have reported that cocaine-dependent subjects show attentional bias to cocaine-related words whereas healthy control subjects do not.42,44 Here, cocaine-dependent subjects had significantly longer reaction times to indicate the word color in trials with cocaine-related words than in trials with neutral words (Figure 1a; t=6.96; P<0.01). Accuracy did not differ between cocaine-related and neutral word trials (Figure 1b; t=−0.49; NS). Attentional bias did not differ between cocaine-dependent treatment-seekers (48.18±8.2 msec) and non-treatment-seekers (52.97±14.2 msec; t=0.29; NS). Our effect size is consistent with other published studies of attentional bias conducted in drug-dependent subjects.52
Figure 1.
Cocaine-dependent subjects exhibit attentional bias toward cocaine-related words on the cocaine-word Stroop task. The difference in mean reaction times between trials with cocaine-related words and those with neutral words was used as a measure of attentional bias toward cocaine-related words. (a) Cocaine-dependent subjects displayed longer reaction times (msec; mean±s.e.m.) to indicate the word color in trials with cocaine-related words vs trials with neutral words (P<0.01 vs neutral words). (b) Accuracy (ratio of correct responses to total trials on either neutral or cocaine-related word trials) did not differ between cocaine-related and neutral word trials in cocaine-dependent subjects.
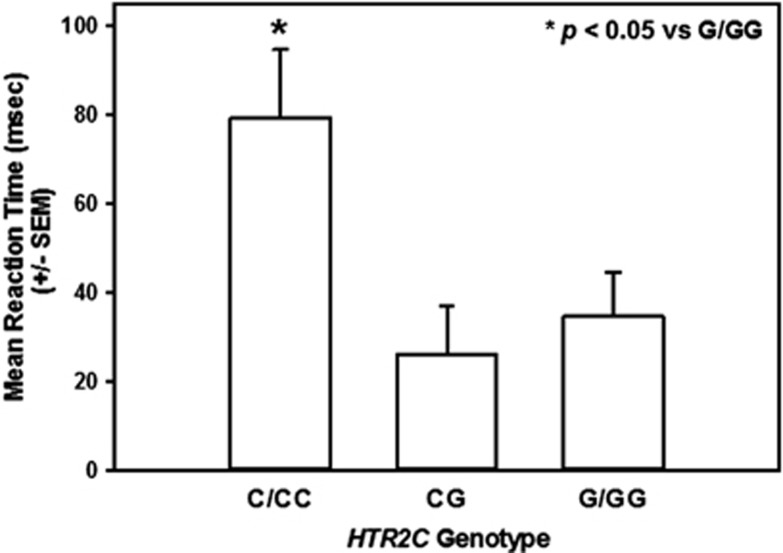
Cue reactivity was evaluated in subjects stratified for the HTR2C genotype. An ANCOVA demonstrated a main effect of genotype on attentional bias after adjusting for sex (Table 2; F2,108=3.79; P<0.05). Post hoccomparisons revealed that attentional bias for both sexes with the C or CC genotype was significantly greater than that for subjects with the G or GG genotype (Table 2; P<0.05). As 97 of 114 subjects were male and the HTR2C gene is X-linked, an ANOVA for male subjects only was performed. A main effect of genotype on attentional bias for male subjects was detected (Table 2; F1,93=4.91; P<0.05); male subjects with the C genotype displayed significantly higher attentional bias than male subjects with G genotype (Table 2; P<0.05). Post hoc comparisons indicated that attentional bias for females with the CG genotype was not different from those with the CC or GG genotype in this small sample of female subjects (Table 2, NS). An ANCOVA for all African-American (AA) subjects was also performed as 84 of 114 subjects were AA; a main effect of genotype on attentional bias was observed (Figure 2; F2,81=4.43; P<0.05). Post hoc comparisons demonstrated that attentional bias for AA subjects with the C/CC genotype was significantly higher than AA subjects with the G/GG genotype (Figure 2; P<0.05).
Table 2. Genotype, sex and attentional bias in cocaine-dependent subjects.
|
Genotype |
Malea | Female | Both | |
|---|---|---|---|---|
| Male | Female | |||
| C | CC | 75.88±14.13 (26)b | 152.83 (1) | 78.73±13.89 (27)c |
| CG | – | 26.03±11.04 (6) | 26.03±11.04 (6) | |
| G | GG | 37.80±9.02 (71) | 68.95±27.94 (10) | 41.65±8.64 (81) |
The numbers in the parenthesis indicate the subject number.
Data are presented as mean (±s.e.m.) attentional bias in msec calculated for each subject and averaged across subjects.
P<0.05 vs G (males only).
P<0.05 vs G/GG (both males and females).
Figure 2.
The highest attentional bias is seen in African-American cocaine-dependent subjects with the Ser23 protein variant. Mean reaction times (msec±s.e.m.) for African-American subjects with the C/CC genotype which encodes for the Ser23 protein variant were significantly greater than that for African-American subjects with the G/GG genotype (*P<0.05 vs G/GG genotype).
Assessment of cocaine cue reactivity in rodents
We tested the hypothesis that individual differences in HCR vs LCR rats would be observable within the context (self-administration chambers) or in the levels of cocaine cue reactivity (lever presses reinforced by the discrete cue complex). Rats in Experiment 1 readily acquired cocaine self-administration to stability; across the last three sessions (data not shown), there was no main effect of session for the number of active lever presses (F2,29=0.14; NS), inactive lever presses (F2,29=1.07; NS) or the number of infusions received (F2,29=0.05; NS). Rats were stratified (median split) as HCR or LCR on the basis of the number of lever presses for cocaine-associated cues during the probe session (data not shown; see Methods). Total cocaine intake did not differ between HCR rats (373.9±18.3 mg kg−1) and LCR rats (395.4±16.6 mg kg−1; NS). There was a positive correlation between previously active lever presses on the probe session with that seen on the cue reactivity test session for individual animals (r=0.304; P<0.05).
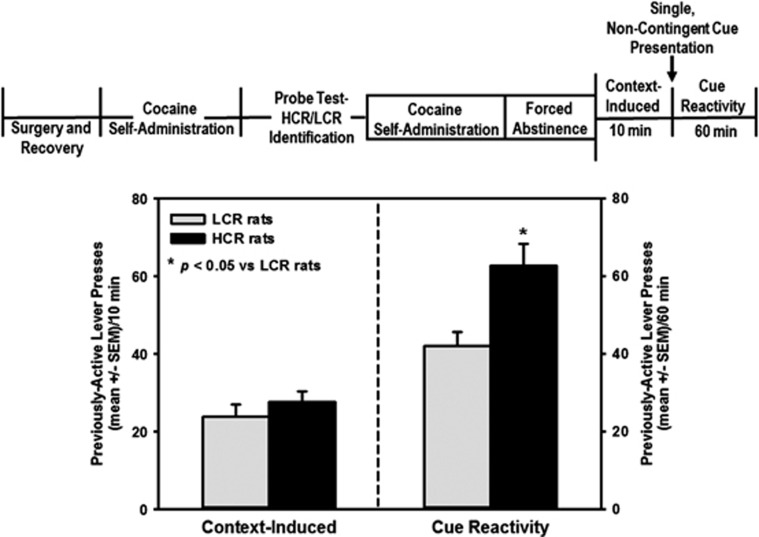
The levels of operant behavior within the cocaine-taking context only or reinforced by the discrete cue complex were assessed in HCR vs LCR rats at 24 h of withdrawal. Previously active lever presses did not differ between HCR and LCR rats upon exposure to the cocaine-taking context in the absence of the discrete cue complex (Figure 3, left; t=0.77; NS). HCR rats displayed significantly higher previously active lever presses that were reinforced by the discrete cue complex vs LCR rats (Figure 3, right; t=2.81; P<0.05). Inactive lever presses did not differ between HCR and LCR rats during the context only component (HCR=2.5±0.4; LCR=2±0.9; t=0.77; NS) or the cue reactivity component (HCR=8.4±1.5; LCR=6±1.3; t=1.17; NS). These data suggest that HCR and LCR rats exhibit distinct appetitive approach behavior when provided with the opportunity to deliver the discrete cue complex. The propensity to engage in appetitive behavior to deliver the discrete cue complex may represent a useful construct within which to investigate individual differences in cocaine cue reactivity.
Figure 3.
Individual differences in appetitive approach behavior in rats are driven by discrete cocaine-associated stimuli. The levels of operant behavior within the cocaine-associated context (left) or reinforced by the discrete cue complex (right) at 24 h of withdrawal were assessed in rats stratified as high (HCR) vs low cue reactive (LCR). Previously-active lever presses did not differ between HCR (n=12) and LCR (n=12) rats upon exposure to the context in the absence of the discrete cue complex (first 10 min of session; NS). HCR rats displayed significantly higher cue-reinforced lever presses (second 60 min of session) vs LCR rats (*P<0.05 vs LCR rats).
Assessment of the 5-HT2CR system and associated cue reactivity in rodents
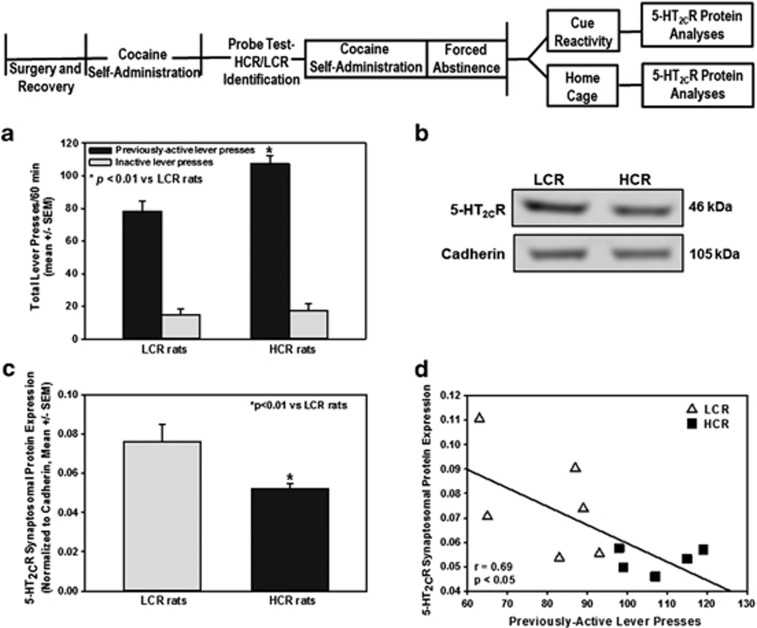
We tested the hypothesis in Experiment 2 that individual differences in levels of cue reactivity would correlate with the expression of 5-HT2CR ex vivo. HCR rats displayed significantly higher previously active lever presses for the discrete cue complex vs LCR rats (Figure 4a; t=3.65; P<0.01). Inactive lever presses (Figure 4a; t=0.5; NS) and the latency to the first lever press (data not shown; t=0.97; NS) were not different between HCR and LCR rats. A two-way, repeated measures ANOVA on the last three sessions of stable self-administration indicated no main effect of phenotype (F1,29=0.02; NS), day (F2,29=1.43; NS), and no phenotype × day interaction (F2,29=1.02; NS) for active lever presses, indicating that individual differences in cue reactivity in rats are unrelated to previous cocaine-taking history.
Figure 4.
High cue reactive (HCR) rats exhibit lower 5-HT2CR protein expression in medial prefrontal cortex (mPFC) relative to low cue reactive (LCR) rats. (a) Mean total lever presses (±s.e.m.) on the previously-active and inactive levers are presented for the cue reactivity test session. Each previously-active lever press resulted in the presentation of the discrete cue complex in the absence of cocaine delivery on an FR1. Rats identified as HCR (n=5) displayed significantly higher lever presses for cocaine-associated cues vs LCR rats (n=6; *P<0.01). Inactive lever presses did not differ between HCR and LCR rats. (b) Qualitative and (c) quantitative data demonstrate phenotypic differences in mPFC 5-HT2CR synaptosomal protein expression. HCR rats displayed lower cortical synaptosomal 5-HT2CR protein levels relative to LCR rats (*P<0.05). (d) An inverse correlation was observed between mPFC 5-HT2CR synaptosomal protein and responses on the previously-active lever for cocaine-associated cues in individual rats (r=0.815; P<0.01). The differential protein expression observed in HCR (0.051±0.002 arbitrary units) and LCR (0.076±0.008 arbitrary units) rats was not related to the cue reactivity test itself as comparable 5-HT2CR mPFC protein levels were observed in HCR (n=6; 0.049±0.01 arbitrary units) and LCR rats (n=6; 0.087±0.02 arbitrary units) that remained in their home cage until sacrifice.
Figure 4b depicts representative immunoblots for mPFC synaptosomal protein from HCR and LCR rats sacrificed immediately following the cue reactivity test session. HCR rats displayed significantly lower 5-HT2CR synaptosomal protein levels in the mPFC vs LCR rats (Figure 4c; t=−3.75; P<0.01); an inverse correlation was observed between mPFC 5-HT2CR synaptosomal protein and responses on the previously-active lever for the discrete cue complex in individual rats (Figure 4d; r=0.69; P<0.05). Because these rats underwent the cue reactivity session, such exposure could account in part for the observed changes in mPFC 5-HT2CR protein levels. Thus, 5-HT2CR protein levels were assessed in a cohort of cocaine-trained rats that were retained in their home cage and sacrificed 24 h after termination of cocaine self-administration sessions (that is, not tested for cue reactivity). The differential protein expression observed in HCR (0.051±0.002 arbitrary units) and LCR (0.076±0.008 arbitrary units) (Figure 4) rats (stratified on the probe session) is not related to the cue reactivity test itself as comparable 5-HT2CR mPFC protein levels were observed in HCR (0.049±0.01 arbitrary units) and LCR rats (0.087±0.02 arbitrary units) that were not exposed to the cue reactivity test session. These data suggest that high levels of cue reactivity are associated with lower 5-HT2CR expression in the mPFC supporting our hypothesis that differential 5-HT2CR neurobiology may contribute to individual differences in cocaine cue reactivity.
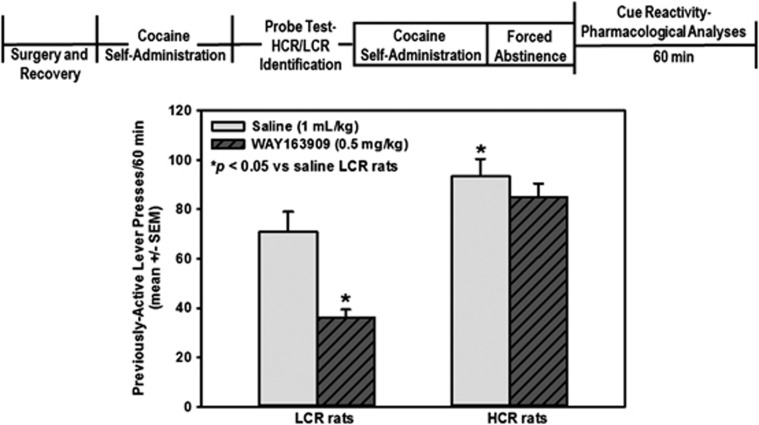
We then tested the hypothesis that HCR and LCR rats during early withdrawal would exhibit differential pharmacological sensitivity to the suppressive effects of the selective 5-HT2CR agonist WAY163909 over cocaine cue reactivity (Figure 5). A main effect of phenotype (F1,41=30.93; P<0.0001), treatment (F1,41=11.34; P<0.01), and a phenotype × treatment interaction (F1,41=4.23; P<0.05) for previously active lever presses was observed. Saline-treated HCR rats displayed higher previously-active lever presses vs saline-treated LCR rats (Figure 5; P<0.05). LCR rats treated with WAY163909 exhibited lower previously-active lever presses vs saline-treated LCR rats (Figure 5; P<0.05); WAY163909 did not significantly alter previously-active lever presses vs saline in HCR rats (Figure 5; NS). WAY163909 (0.5 mg kg−1) suppressed previously-active lever presses ~48% in LCR rats and ~12% in HCR rats. No main effect of phenotype (F1,41=1.1; NS), treatment (F1,41=0.01; NS), and no phenotype × treatment interaction (F1,41=1.37; NS) for inactive lever presses during the cue reactivity test session was observed. Mean (±s.e.m.) inactive lever presses did not differ between saline-treated HCR (14.9±4.3; NS) and LCR rats (10.8±4.6), between saline- and WAY163909-treated HCR rats (13.9±6.9; NS), or saline- and WAY163909-treated LCR rats (13.2±4.7; NS). No main effect of phenotype (F1,41=3.11; NS), treatment (F1,41=0.25; NS), and no phenotype × treatment interaction (F1,41=1.34; NS) for latency to the first lever press during the cue reactivity test session was observed. Mean (±s.e.m.) latency (sec) did not differ between saline-treated LCR (29.4±8.1) and HCR rats (24.5±9.6; NS), saline- and WAY163909-treated LCR rats (42.9±7.1; NS), or saline-and WAY163909-treated HCR rats (19.1±6.4; NS). Taken together, these data demonstrate that HCR rats are less sensitive than LCR rats to the suppressive effects of WAY163909 on cue reactivity at 24 h of withdrawal.
Figure 5.
High cue reactive (HCR) rats exhibit lower sensitivity to the suppressive effects of the selective 5-HT2CR agonist WAY163909 relative to low cue reactive (LCR) rats. Mean active lever presses (±s.e.m.) are presented for the cue reactivity test session. Each previously active lever press resulted in the presentation of the discrete cue complex in the absence of cocaine delivery on an FR1. Rats identified as HCR (n=16 rats per treatment) and LCR rats (n=16 rats per treatment) were injected with saline (15 min; 1 ml kg−1, intraperitoneal) or WAY163909 (15 min; 0.5 mg kg−1; intraperitoneal) prior to a cocaine cue reactivity test session on FA day 1. WAY163909 significantly attenuated lever presses for cocaine-associated cues in LCR, but not HCR, rats (*P<0.05 vs saline-treated LCR rats). FA, forced abstinence.
Discussion
The present study demonstrated that cocaine-dependent subjects who carry the less-common Ser23 variant of the HTR2C exhibit significantly higher cocaine cue reactivity than did those who carry the Cys23 variant, adding the HTR2C to handful of genes potentially identified as candidates involved in cocaine cue reactivity.53,54 Likewise, in a model of individual differences in cocaine cue reactivity in rats, we identified that high cocaine cue reactivity correlated with lower levels of mPFC 5-HT2CR protein expression and a blunted sensitivity to the suppressive effects of the selective 5-HT2CR agonist WAY163909. Interestingly, we discovered that individual differences in drug-seeking were evident when rats were given the opportunity to deliver the discrete cue complex but not when given the opportunity to simply press levers in the cocaine-taking context, supporting the incentive-motivational value of the discrete cue complex as a key defining characteristic in provoking cocaine-seeking.55 Together with our previous observation that knockdown of the mPFC 5-HT2CR resulted in vulnerability to the expression of cocaine cue reactivity in rats,31 we propose that the functional status of the 5-HT2CR system is a mechanistic driver in the generation of vulnerability to cocaine-associated cues.
Our new finding that the Cys23Ser SNP aligns with cue reactivity in cocaine-dependent subjects supports the concept that inherent variability in 5-HT2CR neurobiology may contribute to the liability of individuals to cocaine cues and cue-related relapse phenomena. The manner in which the Ser23 variant impacts baseline 5-HT2CR function is not yet fully defined. The replacement of the cysteine in the extracellular N-terminus of the 5-HT2CR is predicted to eliminate the formation of a disulfide bond, which would be expected to destabilize the receptor structure.32,33 The impact of the Ser23 SNP on the structural integrity of the 5-HT2CR protein could include alterations in ligand binding and downstream signaling responsivity. In COS-7 cells, the Ser23 variant exhibited lower high-affinity, but not low-affinity, binding to the 5-HT2CR and the agonist response in these cells was more markedly desensitized relative to the Cys23 variant.34 The 5-HT2CR encoded by the Ser23 variant localized predominantly to the cell surface in HEK293 cells and was aligned with faster recovery of 5-HT-evoked cellular signaling following prolonged exposure to an inverse agonist.35 It is possible that aberrant 5-HT2CR-mediated functions in Ser23 carriers may exhibit differential responsivity to stress56,57 or pharmacological triggers, including 5-HT2CR agonists.36, 37, 38 However, there have been no experimental evaluations in animal models in vivo which would be valuable to tease apart the mechanisms by which the Cys23Ser SNP may drive 5-HT2CR neurobiology and its impact on cocaine cue reactivity. Such studies are vital as a recent publication found that the Ser23 and Cys23 variants behaved indistinguishably in HEK293 and NIH-3T3 cells.58 Thus, although there is in vitro evidence that the Ser23 variant leads to altered cellular responses to stimuli, definitive information remains to be collected to best understand the association reported here between expression of the Ser23 variant and enhanced cocaine cue reactivity, as well as in the clinical course of some psychiatric disorders (for review59,60).
There are reports of altered 5-HT2CR responsivity after cocaine exposure in humans61,62 and experimenter-delivered cocaine in animals.63 Our observations that mPFC 5-HT2CR expression and pharmacological sensitivity to a selective 5-HT2CR agonist associate with individual variations in levels of cue reactivity in rodents are consistent with the possibility that reduced mPFC 5-HT2CR function is a neurobiological mediator of cocaine cue reactivity.31 These findings may be related to pre-existing neurochemical vulnerabilities specific for reward-predicting cues31,64 or to the cyclical variations in 5-HT efflux consequent to cocaine self-administration.65 It is currently unknown whether the difference in cortical 5-HT2CR expression observed here translates directly to differential functional output of the receptor to manifest cue reactivity, however, high cue reactive rats were less sensitive to the suppressive effects of WAY163909. The composition of the cellular microenvironment (that is, protein-binding partners) also contributes to 5-HT2CR-mediated signaling and agonist responsiveness.66 We have reported that the 5-HT2CR is localized to the postsynaptic density in PFC50 and thus positioned to directly modulate synaptic plasticity in cortical neurons; the 5-HT2CR agonist MK212 is reported to enhance long-term potentiation in forebrain.67 Taken together, these biochemical and behavioral data suggest that high cocaine cue reactivity (but not sucrose cue reactivity) (Swinford-Jackson and Cunningham, unpublished)27 may be governed by a blunted response capacity of the 5-HT2CR. The discovery that individual differences in cue reactivity coexist concomitantly with distinct 5-HT2CR expression patterns in the synaptosomal compartment indicates that balance in the cortical 5-HT2CR functional status may be the key to shaping the neural state that contributes to cocaine-associated cue reactivity during abstinence.
Some limitations of this study should be noted. With the small number of female subjects in the human data set in this study and the exclusion of females in the rodent data set, the findings of this study cannot be extrapolated to women. As the HTR2C is X-linked, future studies should investigate the role of 5-HT2CR neurotransmission in sex differences observed in cocaine cue reactivity as sex may be a factor that contributes to cocaine cue-related neurobiology.68 The direct translatability of the studies presented herein is somewhat limited as there are key discrepancies in cocaine exposure patterns and cocaine use history between humans and rodents. The human data set included subjects with extensive cocaine histories, whereas the rodent data set included animals with shorter exposures to cocaine self-administration. Further, the Cys23Ser SNP has not been identified in rodents nor has the Cys23Ser SNP been tied directly to frontocortical activation patterns in response to drug-associated cues or the cortical 5-HT2CR functional status in cocaine-dependent subjects. Nonetheless, the inclusion of the rodent study allowed for the experimental test of the hypothesis that individual differences in cocaine cue reactivity during early abstinence are associated with differential measures of cortical 5-HT2CR neural integrity.
Our translational findings cumulatively suggest that susceptibility to cocaine cue reactivity may be related to inter-individual variation within the 5-HT2CR system. Although other studies have examined the association of genotype with cue reactivity in cocaine users,53,54,69 our study employed the largest sample size to date, and we are the first to have examined the association of the HTR2C genotype in experimentally measured cue reactivity. The rodent studies suggest that a differential 5-HT2CR functional status, marked by lower cortical 5-HT2CR synaptosomal protein expression and reduced pharmacological sensitivity, associates with greater reactivity to cocaine-associated cues. Future studies are required to expand on our observations to consider the 5-HT2CR system as a risk factor or predictor of cocaine cue reactivity, and perhaps explore as a biological marker of propensity toward craving and relapse in cocaine dependence.
Acknowledgments
We thank Dr David Goldman (Laboratory of Neurogenetics, National Institute on Alcohol Abuse and Alcoholism) for thoughtful discussions and comments on the manuscript. We thank Ms Sonja J Stutz (UTMB) for assistance with rat self-administration surgeries and procedures. This work was supported by the National Institute on Drug Abuse grants K99 DA033374 (NCA), P20 DA024157 (KAC), K05 DA020087 (KAC), K02 DA000403 (FGM), P50 DA009262 (FGM), MD Anderson's Cancer Center Support Grant DA026120 (SL) and the Center for Addiction Research at the University of Texas Medical Branch. The work was also supported in part by the Toomim Family Fund (DAN). This material is the result of work supported in part by resources and the use of facilities at the Michael E DeBakey VA Medical Center, Houston, TX, USA.
Dr Moeller is a consultant for Boehringer-Ingelheim. Dr Cunningham is a consultant for Arena Pharmaceuticals and an editor of Neuropsychopharmacology Reviews for which she receives compensation from the American College of Neuropsychopharmacology. The remaining authors declare no conflict of interest.
References
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, deB FB, Chuzi S, Pachas G, et al. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Hartwell K, DeSantis SM, Saladin M, Rae-Clark AL, Price KL, et al. Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug Alcohol Depend. 2010;106:21–27. doi: 10.1016/j.drugalcdep.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Niaura RS, Childress AR, Abrams DB, Monti PM. Cue reactivity in addictive behaviors: theoretical and treatment implications. Int J Addict. 1990;25 ((7A-8A:957–993. doi: 10.3109/10826089109071030. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Cue-reactivity and the future of addiction research. Addiction. 1999;94:349–351. [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97 ((1-2:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Modesto-Lowe V, Kranzler HR. Using cue reactivity to evaluate medications for treatment of cocaine dependence: a critical review. Addiction. 1999;94:1639–1651. doi: 10.1046/j.1360-0443.1999.941116393.x. [DOI] [PubMed] [Google Scholar]
- Mahler SV, de Wit H. Cue-reactors: individual differences in cue-induced craving after food or smoking abstinence. PLoS One. 2010;5:e15475. doi: 10.1371/journal.pone.0015475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, et al. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Res Rev. 2008;58:121–135. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot CS, Witkiewitz K, George WH, Marlatt GA. Relapse prevention for addictive behaviors. Subst Abuse Treat Prev Policy. 2011;6:17. doi: 10.1186/1747-597X-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisciandaro JJ, Myrick H, Henderson S, Rae-Clark AL, Brady KT. Prospective associations between brain activation to cocaine and no-go cues and cocaine relapse. Drug Alcohol Depend. 2013;131 ((1-2:44–49. doi: 10.1016/j.drugalcdep.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Alia-Klein N, Honorio CJ, Maloney T, Woicik PA, et al. Dopaminergic response to drug words in cocaine addiction. J Neurosci. 2009;29:6001–6006. doi: 10.1523/JNEUROSCI.4247-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidov HGW, Grzanna R, Molliver ME. The serotonin innervation of the cerebral cortex in the rat: An immunohistochemical analysis. Neuroscience. 1980;5:207–227. doi: 10.1016/0306-4522(80)90099-8. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Linley SB.Efferent and afferent connections of the dorsal and median raphe nuclei in the rat Monti JM, Pandi-Perumal SR, Jacobs BL, Nutt DJ.(eds).Serotonin and Sleep: Molecular, Functional and Clinical Aspects Birkhäuser Verlag: Basel, Switzerland; pp. 69–102., 2008. [Google Scholar]
- Cunningham KA, Anastasio NC. Serotonin at the nexus of impulsivity and cue ceactivity in cocaine addiction. Neuropharmacology. 2013;76 ((Pt B:460–478. doi: 10.1016/j.neuropharm.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: Comparison between 5-HT2A and 5-HT2C receptors. Mol Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex. Behav Brain Res. 2003;146 ((1-2:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Rajaram S, Cottone LA, Zhang L, Maloney T, et al. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience. 2007;144:1153–1159. doi: 10.1016/j.neuroscience.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeg EH, Jackson ME, Jedema HP, Bradberry CW. Orbitofrontal and anterior cingulate cortex neurons selectively process cocaine-associated environmental cues in the rhesus monkey. J Neurosci. 2009;29:11619–11627. doi: 10.1523/JNEUROSCI.3206-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Chefer S, Kurup PK, Guillem K, Vaupel DB, Ross TJ, et al. fMRI response in the medial prefrontal cortex predicts cocaine but not sucrose self-administration history. Neuroimage. 2012;62:1857–1866. doi: 10.1016/j.neuroimage.2012.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentkowski NS, Duke FD, Weber SM, Pockros LA, Teer AP, Hamilton EC, et al. Stimulation of medial prefrontal cortex serotonin 2C 5-HT2C receptors attenuates cocaine-seeking behavior. Neuropsychopharmacology. 2010;35:2037–2048. doi: 10.1038/npp.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Anastasio NC, Fox RG, Stutz SJ, Bubar MJ, Swinford SE, et al. Synergism between a serotonin 5-HT2A receptor (5-HT2AR) antagonist and 5-HT2CR agonist suggests new pharmacotherapeutics for cocaine addiction. ACS Chem Neurosci. 2013;4:110–121. doi: 10.1021/cn300072u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Acosta JI. Stimulation of 5-HT2C receptors attenuates cue and cocaine-primed reinstatement of cocaine-seeking behavior in rats. Behav Pharmacol. 2007;18:791–800. doi: 10.1097/FBP.0b013e3282f1c94b. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Grottick AJ, Higgins GA. Differential effects of the 5-HT2A receptor antagonist M100,907 and the 5-HT2C receptor antagonist SB242,084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology. 2002;27:576–586. doi: 10.1016/S0893-133X(02)00342-1. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Fletcher PJ, Higgins GA. Studies to investigate the role of 5-HT(2C) receptors on cocaine- and food-maintained behavior. J Pharmacol Exp Ther. 2000;295:1183–1191. [PubMed] [Google Scholar]
- Cunningham KA, Fox RG, Anastasio NC, Bubar MJ, Stutz SJ, Moeller FG, et al. Selective serotonin 5-HT2C receptor activation suppresses the reinforcing efficacy of cocaine and sucrose but differentially affects the incentive-salience value of cocaine- vs sucrose-associated cues. Neuropharmacology. 2011;61:513–523. doi: 10.1016/j.neuropharm.2011.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manvich DF, Kimmel HL, Cooper DA, Howell LL. The serotonin 2C receptor antagonist SB 242084 exhibits abuse-related effects typical of stimulants in squirrel monkeys. J Pharmacol Exp Ther. 2012;342:761–769. doi: 10.1124/jpet.112.195156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloux Y, Dilleen R, Economidou D, Theobald D, Everitt BJ. Reduced forebrain serotonin transmission is causally involved in the development of compulsive cocaine seeking in rats. Neuropsychopharmacology. 2012;37:2505–2514. doi: 10.1038/npp.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manvich DF, Kimmel HL, Howell LL. Effects of serotonin 2C receptor agonists on the behavioral and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 2012;341:424–434. doi: 10.1124/jpet.111.186981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasio NC, Stutz SJ, Fox RG, Sears RM, Emeson RB, DiLeone RJ, et al. Functional status of the serotonin 5-HT2C receptor (5-HT2CR) drives interlocked phenotypes that precipitate relapse-like behaviors in cocaine dependence. Neuropsychopharmacology. 2014;39:370–382. doi: 10.1038/npp.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappalainen J, Zhang L, Dean M, Oz M, Ozaki N, Yu DH, et al. Identification, expression, and pharmacology of a Cys23-Ser23 substitution in the human 5-HT2c receptor gene (HTR2C) Genomics. 1995;27:274–279. doi: 10.1006/geno.1995.1042. [DOI] [PubMed] [Google Scholar]
- Piva F, Giulietti M, Baldelli L, Nardi B, Bellantuono C, Armeni T, et al. Bioinformatic analyses to select phenotype affecting polymorphisms in HTR2C gene. Hum Psychopharmacol. 2011;26:365–372. doi: 10.1002/hup.1214. [DOI] [PubMed] [Google Scholar]
- Okada M, Northup JK, Ozaki N, Russell JT, Linnoila M, Goldman D. Modification of human 5-HT(2C) receptor function by Cys23Ser, an abundant, naturally occurring amino-acid substitution. Mol Psychiatry. 2004;9:55–64. doi: 10.1038/sj.mp.4001357. [DOI] [PubMed] [Google Scholar]
- Walstab J, Steinhagen F, BRUSS M, Gothert M, Bonisch H. Differences between human wild-type and C23S variant 5-HT2C receptors in inverse agonist-induced resensitization. Pharmacol Rep. 2011;63:45–53. doi: 10.1016/s1734-1140(11)70397-8. [DOI] [PubMed] [Google Scholar]
- Brasch-Andersen C, Moller MU, Christiansen L, Thinggaard M, Otto M, Brosen K, et al. A candidate gene study of serotonergic pathway genes and pain relief during treatment with escitalopram in patients with neuropathic pain shows significant association to serotonin receptor2C (HTR2C) Eur J Clin Pharmacol. 2011;67:1131–1137. doi: 10.1007/s00228-011-1056-x. [DOI] [PubMed] [Google Scholar]
- Kuhn KU, Joe AY, Meyer K, Reichmann K, Maier W, Rao ML, et al. Neuroimaging and 5-HT2C receptor polymorphism: a HMPAO-SPECT study in healthy male probands using mCPP-challenge of the 5-HT2C receptor. Pharmacopsychiatry. 2004;37:286–291. doi: 10.1055/s-2004-832685. [DOI] [PubMed] [Google Scholar]
- Quested DJ, Whale R, Sharpley AL, McGavin CL, Crossland N, Harrison PJ, et al. Allelic variation in the 5-HT2C receptor (HTR2C) and functional responses to the 5-HT2C receptor agonist, m-chlorophenylpiperazine. Psychopharmacology (Berl) 1999;144:306–307. doi: 10.1007/s002130051010. [DOI] [PubMed] [Google Scholar]
- Saltzman AG, Morse B, Whitman MM, Ivanshchenko Y, Jaye M, Felder S. Cloning of the human serotonin 5-HT2 and 5-HT1C receptor subtypes. Biochem Biophys Res Commun. 1991;181:1469–1478. doi: 10.1016/0006-291x(91)92105-s. [DOI] [PubMed] [Google Scholar]
- Xie EZ, Zhu LY, Zhao LY, Chang LS. The human serotonin 5-HT2c receptor: Complete cDNA, genomic structure, and alternatively spliced variant. Genomics. 1996;35:551–561. doi: 10.1006/geno.1996.0397. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB.Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition Biometrics Research Department, New York State Psychiatric Institute: New York, NY, USA, 1996. [Google Scholar]
- Hester R, Dixon V, Garavan H. A consistent attentional bias for drug-related material in active cocaine users across word and picture versions of the emotional Stroop task. Drug Alcohol Depend. 2006;81:251–257. doi: 10.1016/j.drugalcdep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Liu S, Lane SD, Schmitz JM, Green CE, Cunningham KA, Moeller FG. Increased intra-individual reaction time variability in cocaine-dependent subjects: role of cocaine-related cues. Addict Behav. 2012;37:193–197. doi: 10.1016/j.addbeh.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Lane SD, Schmitz JM, Waters AJ, Cunningham KA, Moeller FG. Relationship between attentional bias to cocaine-related stimuli and impulsivity in cocaine-dependent subjects. Am J Drug Alcohol Abuse. 2011;37:117–122. doi: 10.3109/00952990.2010.543204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Munafo MR, Franken IH. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychol Bull. 2009;135:589–607. doi: 10.1037/a0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox WM, Fadardi JS, Pothos EM. The addiction-stroop test: Theoretical considerations and procedural recommendations. Psychol Bull. 2006;132:443–476. doi: 10.1037/0033-2909.132.3.443. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Wu G, Huang W, Harding MJ, Hamon SC, Lappalainen J, et al. Pharmacogenetic randomized trial for cocaine abuse: disulfiram and dopamine beta-hydroxylase. Biol Psychiatry. 2013;73:219–224. doi: 10.1016/j.biopsych.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Domingo CB, Hamon SC, Nielsen DA. DBH gene as predictor of response in a cocaine vaccine clinical trial. Neurosci Lett. 2013;541:29–33. doi: 10.1016/j.neulet.2013.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Fox RG, Stutz SJ, Rice KC, Cunningham KA. Blockade of the serotonin 5-HT2A receptor suppresses cue-evoked reinstatement of cocaine-seeking behavior in a rat self-administration model. Behav Neurosci. 2009;123:382–396. doi: 10.1037/a0014592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasio NC, Lanfranco MF, Bubar MJ, Seitz PK, Stutz SJ, McGinnis AG, et al. Serotonin 5-HT(2C) receptor protein expression is enriched in synaptosomal and post-synaptic compartments of rat cortex. J Neurochem. 2010;113:1504–1515. doi: 10.1111/j.1471-4159.2010.06694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Bubar MJ, Lanfranco MF, Hillman GR, Cunningham KA. Serotonin(2C) receptor localization in GABA neurons of the rat medial prefrontal cortex: Implications for understanding the neurobiology of addiction. Neuroscience. 2007;146:1667–1688. doi: 10.1016/j.neuroscience.2007.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AJ, Marhe R, Franken IH. Attentional bias to drug cues is elevated before and during temptations to use heroin and cocaine. Psychopharmacology (Berl) 2012;219:909–921. doi: 10.1007/s00213-011-2424-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Parvaz MA, Shumay E, Beebe-Wang N, Konova AB, Alia-Klein N, et al. Gene xabstinence effects on drug cue reactivity in addiction: multimodal evidence.J Neurosci 20133310027–10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smelson D, Yu L, Buyske S, Gonzalez G, Tischfield J, Deutsch CK, et al. Genetic association of GABA-A receptor alpha-2 and mu opioid receptor with cocaine cue-reactivity: evidence for inhibitory synaptic neurotransmission involvement in cocaine dependence. Am J Addict. 2012;21:411–415. doi: 10.1111/j.1521-0391.2012.00253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, et al. Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS One. 2012;7:e38987. doi: 10.1371/journal.pone.0038987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummett BH, Kuhn CM, Boyle SH, Babyak MA, Siegler IC, Williams RB. Cortisol responses to emotional stress in men: association with a functional polymorphism in the 5HTR2C gene. Biol Psychol. 2012;89:94–98. doi: 10.1016/j.biopsycho.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickey BJ, Sanford BJ, Love TM, Shen PH, Hodgkinson CA, Stohler CS, et al. Striatal dopamine release and genetic variation of the serotonin 2C receptor in humans. J Neurosci. 2012;32:9344–9350. doi: 10.1523/JNEUROSCI.1260-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fentress HM, Grinde E, Mazurkiewicz JE, Backstrom JR, Herrick-Davis K, Sanders-Bush E. Pharmacological properties of the Cys23Ser single nucleotide polymorphism in human 5-HT2C receptor isoforms. Pharmacogenomics J. 2005;5:244–254. doi: 10.1038/sj.tpj.6500315. [DOI] [PubMed] [Google Scholar]
- Drago A, Serretti A. Focus on HTR2C: A possible suggestion for genetic studies of complex disorders. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:601–637. doi: 10.1002/ajmg.b.30864. [DOI] [PubMed] [Google Scholar]
- Sanders-Bush E, Fentress H, Hazelwood L. Serotonin 5-HT2 receptors: Molecular and genomic diversity. Mol Interv. 2003;3:319–330. doi: 10.1124/mi.3.6.319. [DOI] [PubMed] [Google Scholar]
- Handelsman L, Kahn RS, Sturiano C, Rinaldi PJ, Gabriel S, Schmeidler JP, et al. Hostility is associated with a heightened prolactin response to meta-chlorophenylpiperazine in abstinent cocaine addicts. Psychiatry Res. 1998;80:1–12. doi: 10.1016/s0165-1781(98)00048-1. [DOI] [PubMed] [Google Scholar]
- Patkar AA, Mannelli P, Peindl K, Hill KP, Gopalakrishnan R, Berrettini WH. Relationship of disinhibition and aggression to blunted prolactin response to meta-chlorophenylpiperazine in cocaine-dependent patients. Psychopharmacology (Berl) 2006;185:123–132. doi: 10.1007/s00213-005-0261-7. [DOI] [PubMed] [Google Scholar]
- Filip M, Bubar MJ, Cunningham KA. Contribution of serotonin (5-hydroxytryptamine; 5-HT) 5-HT2 receptor subtypes to the hyperlocomotor effects of cocaine: Acute and chronic pharmacological analyses. J Pharmacol Exp Ther. 2004;310:1246–1254. doi: 10.1124/jpet.104.068841. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry. 2009;65:869–873. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LH, Koob GF, Weiss F. Extracellular serotonin is decreased in the nucleus accumbens during withdrawal from cocaine self-administration. Behav Brain Res. 1995;73 ((1-2:225–228. doi: 10.1016/0166-4328(96)00101-5. [DOI] [PubMed] [Google Scholar]
- Rajagopal S, Ahn S, Rominger DH, Gowen-MacDonald W, Lam CM, Dewire SM, et al. Quantifying ligand bias at seven-transmembrane receptors. Mol Pharmacol. 2011;80:367–377. doi: 10.1124/mol.111.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe K, Fujimoto T, Akaishi T, Misawa M. Stimulation of basolateral amygdaloid serotonin 5-HT(2C) receptors promotes the induction of long-term potentiation in the dentate gyrus of anesthetized rats. Neurosci Lett. 2009;451:65–68. doi: 10.1016/j.neulet.2008.12.023. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Hong KI, Lacadie CM, Fulbright RK, Tuit KL, Sinha R. Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. Am J Psychiatry. 2012;169:406–414. doi: 10.1176/appi.ajp.2011.11020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo MR, Johnstone EC, Mackintosh B. Association of serotonin transporter genotype with selective processing of smoking-related stimuli in current smokers and ex-smokers. Nicotine Tob Res. 2005;7:773–778. doi: 10.1080/14622200500259861. [DOI] [PubMed] [Google Scholar]