Abstract
Recent genome-wide association studies have pointed to single-nucleotide polymorphisms (SNPs) in genes encoding the neuronal calcium channel CaV1.2 (CACNA1C; rs1006737) and the presynaptic active zone protein Piccolo (PCLO; rs2522833) as risk factors for affective disorders, particularly major depression. Previous neuroimaging studies of depression-related endophenotypes have highlighted the role of the subgenual cingulate cortex (CG25) in negative mood and depressive psychopathology. Here, we aimed to assess how recently associated PCLO and CACNA1C depression risk alleles jointly affect memory-related CG25 activity as an intermediate phenotype in clinically healthy humans. To investigate the combined effects of rs1006737 and rs2522833 on the CG25 response, we conducted three functional magnetic resonance imaging studies of episodic memory formation in three independent cohorts (N=79, 300, 113). An epistatic interaction of PCLO and CACNA1C risk alleles in CG25 during memory encoding was observed in all groups, with carriers of no risk allele and of both risk alleles showing higher CG25 activation during encoding when compared with carriers of only one risk allele. Moreover, PCLO risk allele carriers showed lower memory performance and reduced encoding-related hippocampal activation. In summary, our results point to region-specific epistatic effects of PCLO and CACNA1C risk variants in CG25, potentially related to episodic memory. Our data further suggest that genetic risk factors on the SNP level do not necessarily have additive effects but may show complex interactions. Such epistatic interactions might contribute to the ‘missing heritability' of complex phenotypes.
Keywords: CACNA1C, epistatisis, imaging genetics, memory, Piccolo, subgenual cingulate
Introduction
The etiology of human affective disorders is highly multifactorial with the estimated genetic contribution being as high as 85 percent for bipolar disorder (BPD)1 and around 30 to 40 percent for unipolar major depressive disorder (MDD).2 Despite the recent advances in large-scale genomic investigations of the etiology of complex human diseases, including psychiatric disorders, there is a considerable gap between the relative genetic contribution estimated from twin and sibling studies and the actual contribution from identified genetic risk variants. This unexplained variance of genetic contributions to human disease is commonly referred to as the missing heritability.3,4 Several biological processes have been suggested to underlie the phenomenon of missing heritability, including gene–environment interactions, gene–gene interactions, but also the heterogeneity of the phenotype of interest itself. To help overcome the latter problem, the concept of intermediate phenotypes, or endophenotypes, has been introduced and widely applied in psychiatric genetics. An endophenotype is defined as a measurable trait along the pathway from a genotype to a complex disease and commonly shows stronger heritability than the disease phenotype itself.5 Over the past decade, several potential endophenotypes related to neuropsychiatric disorders have been identified using structural and functional neuroimaging techniques.6
Neuroimaging studies in depressed patients suggest that a depression-related endophenotype might have its neural underpinnings in dysregulation of the function of the subgenual anterior cingulate cortex (ACC) (Brodmann Area 25; CG25). Previous studies show that cerebral metabolism in CG25 is tonically increased in depressed patients,7 although earlier studies have reported decreased CG25 metabolism or blood flow in depressed patients.8 One explanation for this discrepancy might be partial volume effects resulting from CG25 volume reduction, which has also been reported in depression (for a review, see Drevets et al.9,10). Compatible with the observed (volume-adjusted) increased CG25 metabolism in depression, the subgenual cingulate also shows increased functional connectivity with the default mode network in depressed patients at rest,11 and it has been suggested that CG25 might be part of a brain network involved in rumination, a hallmark symptom of depression.12 Moreover, task-related dysfunctional connectivity patterns of CG25 with prefrontal and temporal brain structures have been observed in acute and remitted depressed patients,13,14 and deep brain stimulation in CG25 has been demonstrated to alleviate symptoms in therapy-resistant MDD.15,16 Physiologically, the subgenual cingulate—as well as its rodent homolog, the infralimbic cortex—is involved in extinction learning, value representation and emotion regulation.17, 18, 19 The subgenual cingulate has also been suggested to participate in episodic memory formation by integrating information distributed in the limbic system and suppressing irrelevant representations.20 Recent studies highlight the potential role of CG25 in linking episodic memory and negative affect. In MDD patients, subgenual cingulate activity during encoding of emotional stimuli has been associated with negative recall bias, and this association is modulated by genetic variability of the HPA axis.21 Furthermore, co-activation of CG25 and the hippocampus during memory encoding has been linked to symptom improvement in posttraumatic stress disorder, a severe psychiatric condition characterized by symptoms of depression and anxiety and by repetitive involuntary intrusions of aversive memory traces.22
Although earlier candidate gene studies of mood disorders, particularly BPD and MDD, have largely focused on genes related to monoaminergic neurotransmission, recent genome-wide association studies (GWAS) have provided converging evidence for genes encoding components of glutamatergic synapses in the etiology of affective disorders. These GWAS results are in line with further evidence for glutamatergic synaptic dysfunction in depression, such as altered glutamate metabolism as assessed with [1H] MR spectroscopy23 and rapid antidepressant effects of the NMDA-type glutamate receptor antagonist ketamine.24 Depression risk genes related to glutamatergic synapse function include CACNA1C, which encodes the α-subunit of the postsynaptic L-type voltage-dependent calcium channel Cav1.2, and PCLO, which encodes the presynaptic active zone protein Piccolo (for a schematized illustration, see Supplementary Figure S3).
Calcium influx through Cav1.2 channels has an important role in hippocampus-dependent learning and memory processes as well as amygdala-dependent fear conditioning. In humans, the A allele of CACNA1C single-nucleotide polymorphism (SNP) rs1006737 has been repeatedly associated with higher risk for BPD in GWAS,25 and rs1006737 A has also been linked to increased risk for unipolar depression.26 Healthy carriers of the A variant exhibit reduced activation of bilateral hippocampus and of CG25 during episodic memory recall27 and atypical amygdala activation during processing of aversive stimuli.28 Recently, we could provide evidence that the modulation of hippocampal and CG25 activity by CACNA1C genotype might indeed reflect an endophenotype for affective disorders, as healthy relatives of patients with either MDD or BPD exhibited reduced activation of these areas during episodic recall irrespective of genotype, similar to carriers of the rs1006737 A allele.29
The presynaptic active zone protein Piccolo, together with the closely related protein Bassoon, is involved in the fine-tuning of neurotransmitter release at glutamatergic, GABAergic and dopaminergic synaptic terminals and has been suggested to have an important role in synaptic development and maintenance.30,31 In a GWAS of unipolar MDD, rs2522833, located within the PCLO gene and leading to a serine to alanine substitution in the C2A calcium sensor domain of the protein, came out as a top hit with the C allele (Ala) being the risk variant.32 Although not reaching genome-wide significance, further studies have suggested that rs2522833 or a variant in high linkage disequilibrium may indeed contribute to the genetic risk of MDD33 and to HPA axis dysregulation in MDD patients.34 Furthermore, healthy carriers of the C variant have been demonstrated to exhibit more pronounced depression-related personality traits and an increased amygdala response to negative emotional expressions. In mice, overexpression of the C2A domain has been linked to increased depression-related behavior.35
Here we had aimed to explore potential interaction effects of genetic risk factors for MDD on subgenual cingulate response as an intermediate phenotype. Because of our hypothesis that genes involved in glutamatergic synapse function support mechanisms that constitute endophenotypes for depression, we chose two well-supported candidate genes coding for synaptic proteins, namely CACNA1C and PCLO. We first investigated how depression risk alleles of CACNA1C rs1006737 and PCLO rs252283 might jointly affect CG25 activation during memory formation in a self-referential encoding task. When we unexpectedly observed a complex epistatic interaction of the two risk variants during self-referential episodic encoding, we aimed to replicate this finding in two additional episodic encoding tasks, conducted in two further independent cohorts of healthy participants.
Materials and methods
We investigated the potential interactions of CACNA1C and PCLO depression risk alleles in three functional magnetic resonance imaging studies, using different episodic memory encoding tasks. Previous studies have demonstrated that individual differences in memory processes are relatively stable36 and that particularly prefrontal and hippocampal activation differences are reliably modulated by genes involved in episodic memory.37, 38, 39, 40 Experiment 1 and 3 were carried out at the University of Magdeburg, Germany and at the Magdeburg campus of the Helmholtz Center for Neurodegenerative Diseases, and Experiment 2 took place within a multicenter study performed in Berlin, Bonn and Mannheim. We focused our analyses on the subgenual cingulate/CG25 and on the hippocampus, using the same a priori defined regions of interest (ROIs) in all three experiments. Detailed methodology of all three experiments is provided as Supplementary Information online.
Experiment 1: Self-referential memory encoding
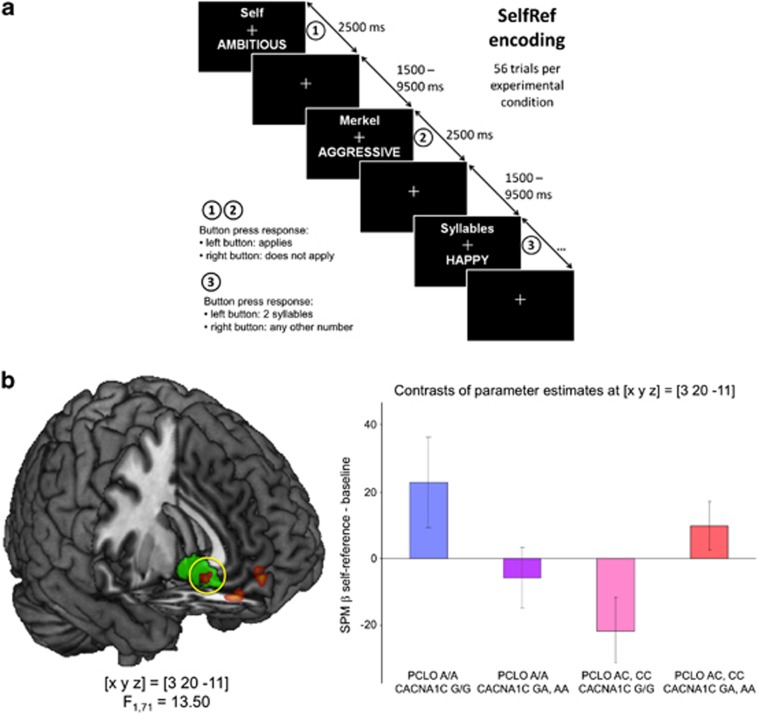
In a first experiment, a cohort of 79 young, healthy participants from Magdeburg, Germany (see Supplementary Information for details on methods) performed a self-referential memory encoding task.41 Participants were presented with adjectives describing personality traits and requested to either judge whether the trait applied to themselves (self-reference), whether it applied to a famous person (the German federal chancellor Angela Merkel; other-reference) or whether it had two or any other number of syllables (control condition). The experimental paradigm is depicted in Figure 1a.
Figure 1.
Effects of CACNA1C and PCLO depression risk variants on self-referential memory encoding (Experiment 1). (a) Experimental paradigm. Participants studied adjectives describing personality traits and performed either a self-reference task (‘Self'), an other-reference task (‘Merkel') or a control task (syllable counting). Stimuli were presented in a pseudo-randomized order with a near-exponential temporal jitter to optimize estimation of trial-specific BOLD responses. (b) Interaction of CACNA1C and PCLO depression risk variants on subgenual cingulate activation. Carriers of both high-risk alleles (CACNA1C rs1006737 A and PCLO rs2522833 C) exhibited comparable CG25 activation as participants homozygous for both low-risk alleles (CACNA1C rs1006737 G and PCLO rs2522833 A), whereas CG25 activity was drastically reduced in carriers of either one high-risk allele. The activation difference in CG25 was significant after family-wise error (FWE) correction for the ROI volume. Bar plots depict contrasts of parameter estimates (SPM betas) scaled to the global mean, ±s.e. ROI, region of interest; SPM, statistical parametric mapping.
Experiment 2: Face-profession associative encoding
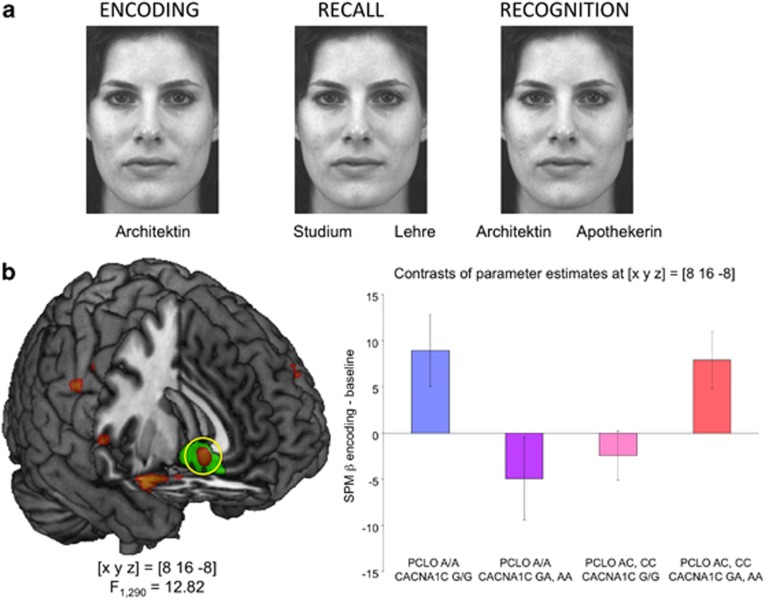
From the first experiment, the specificity of the interaction effect could not be determined, as the condition of self-referential encoding was compared with a low-level baseline condition and could thus reflect both self-reference and episodic memory formation. We therefore attempted to replicate our result in Experiment 2 using a blocked design associative memory encoding task (Figure 2a) that was part of a larger imaging genetics battery.27 A total of 300 healthy participants of broad age range (18 to 50) studied face-profession associations using an elaborate encoding strategy (imagining the person at work). Encoding was followed by recall and recognition tasks to verify encoding success.
Figure 2.
Effects of CACNA1C and PCLO depression risk variants on associative memory encoding (Experiment 2). (a) Experimental paradigm. The task was divided into three phases (encoding, recall, recognition). During encoding, participants studied face–profession associations (left). In the recall task, participants were asked to recall the profession associated with the face and decide whether this profession required academic studies or vocational training (center). During recognition, participants performed a two-alternative forced choice task deciding which profession had been presented with the face (right). Translations: Architektin—architect; Studium—academic studies; Lehre—vocational training; Apothekerin—pharmacist. Stimuli were presented as blocked design. (b) Interaction of CACNA1C and PCLO depression risk variants on subgenual cingulate activation. Carriers of both high-risk alleles exhibited comparable CG25 activation as participants homozygous for both low-risk alleles, whereas CG25 activation was lower in carriers of either one high-risk allele. The CACNA1C by PCLO genotype interaction in CG25 was significant after family-wise error (FWE) correction for the ROI volume. Bar plots depict contrasts of parameter estimates (SPM betas), ±standard error.
Experiment 3: Encoding of novel scenes
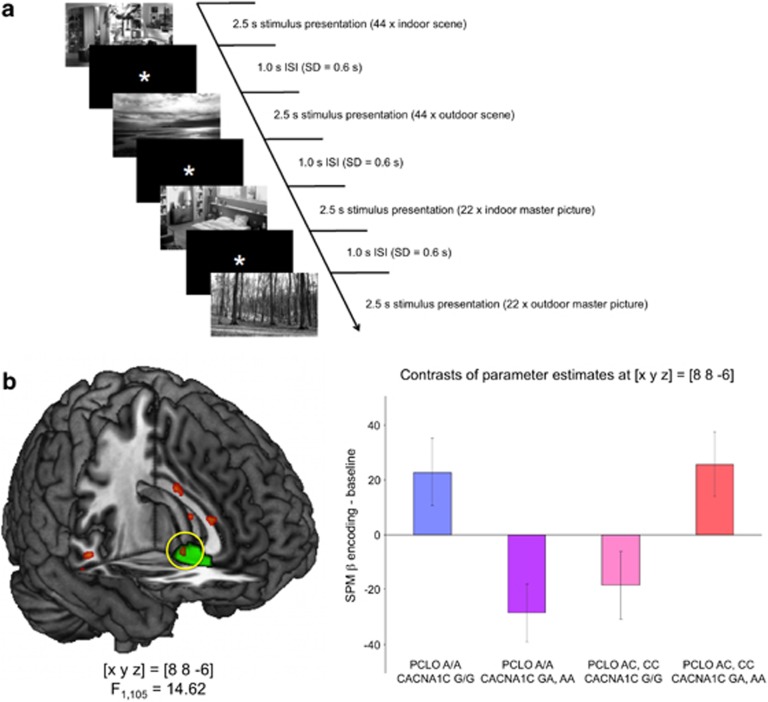
Experiments 1 and 2, although both being episodic encoding tasks, also shared, to some degree, their ‘social' element as the task in Experiment 2, albeit differing from that in Experiment 1, also required social mentalizing. In an attempt to further replicate and generalize our findings, we investigated a cohort of 120 young, healthy participants during encoding of novel scenes using a modified version of a previously described encoding paradigm in which participants studied indoor and outdoor scenes, and novelty was assessed relative to previously familiarized ‘master' scenes42 (Figure 3a). Six participants were excluded from data analysis because they had also participated in Experiment 1, and one participant was excluded owing to incomplete data.
Figure 3.
Effects of CACNA1C and PCLO depression risk variants on encoding of novel scenes (Experiment 3). (a) Experimental paradigm. Participants studied novel scenes intermixed with previously familiarized ‘master' scenes and were asked to respond via button press whether an indoor or outdoor scene was shown. Stimuli were presented in a pseudo-randomized order with a near-exponential temporal jitter to optimize estimation of trial-specific BOLD responses. (b) Interaction of CACNA1C and PCLO depression risk variants on subgenual cingulate activation. Carriers of both high-risk alleles exhibited comparable CG25 activation as participants homozygous for both low-risk alleles, whereas CG25 activation was lower in carriers of either one high-risk allele. The CACNA1C by PCLO genotype interaction in CG25 was significant after family-wise error (FWE) correction for the ROI volume. Bar plots depict contrasts of parameter estimates (SPM betas), ±s.e. ROI, region of interest; SPM, statistical parametric mapping.
Data analysis
Data analysis was performed using Statistical Parametric Mapping (SPM8, Wellcome Department of Imaging Neuroscience). After image preprocessing (correction for acquisition delay and head motion, spatial transformation to the MNI standard reference frame, spatial smoothing; see Supplementary Information for detailed protocols), a two-stage general linear model analysis was carried out. At the first stage, time courses of the experimental conditions were convolved with a canonical hemodynamic response function and submitted to a restricted maximum likelihood fit, with movement parameters as covariates of no interest. At the second stage, the encoding contrasts (Experiment 1: self-referential encoding versus baseline; Experiment 2: associative encoding condition compared with a low-level baseline;27 Experiment 3: successful encoding of novel scenes compared with a previously familiarized standard picture42) were submitted to a random effects analysis of covariance (ANCOVA) model with CACNA1C and PCLO genotypes as fixed factors (wild-type homozygotes vs risk allele carriers) and age, sex as covariates of no interest. In Experiment 2, a multicenter study, study site was additionally modeled as covariates of no interest. Because of our a priori hypotheses regarding the hippocampus and the subgenual cingulate, the significance level was set to P<0.05, family-wise error (FWE)-corrected for the respective ROIs.
Results
Behavioral results
In all three experiments, participants performed the task with high accuracy and successful memory performance. Detailed behavioral data are displayed in Supplementary Tables 4, 5 and 6. In Experiment 2, PCLO rs2522833 was significantly associated with memory performance during both recall and recognition, with C carriers showing lower memory performance than A homozygotes (see Supplementary Results for details). No significant effect of either PCLO or CACNA1C on memory performance was observed in experiments 1 and 3, but it should be noted that the lack of significance might result from insufficient statistical power (see Supplementary Results and Discussion).
Genotype-independent neural correlates of episodic encoding
In all three experiments, successful memory formation was associated with robust activation of the hippocampal formation. Additionally, we observed task-related activations in brain networks more specifically related to the respective encoding tasks.
Experiment 1
When comparing the self-reference and control conditions, all participants, irrespective of genotype group, reliably activated the default mode network (medial prefrontal cortex (mPFC), rACC, posterior cingulate, precuneus) and a distributed network of limbic structures during self-reference, replicating previous results41 (Supplementary Figure S1A). The inferior mPFC cluster of activation extended into CG25.
Experiment 2
Across the entire study cohort, encoding relative to the perceptual baseline condition was associated with activation of default mode network structures (mPFC/rACC including CG25, posterior cingulate), extrastriate visual areas as well as limbic structures, particularly the hippocampus (Supplementary Figure S1B). As in experiment 1, the results were comparable with previous studies using this paradigm.27
Experiment 3
As in the original study42 and replicating results of studies using similar paradigms,40 encoding of novel scenes was associated with activation of an extensive occipito–parietal network encompassing ventral and dorsal visual stream structures as well as bilateral hippocampal activation, irrespective of genotype (Supplementary Figure S1C). A small medial OFC cluster in the vicinity of the subgenual cingulate was also found to be activated as a function of novelty.
Effects of CACNA1C and PCLO genotype on subgenual cingulate activation
In all the three experiments, we could observe a complex interaction of PCLO and CACNA1C genotypes in the subgenual cingulate cortex.
Experiment 1
In a two-way random effects analysis of variance (ANOVA) model, we tested for the effects of CACNA1C and PCLO risk alleles by comparing homozygous and heterozygous carriers of the risk alleles with wild-type homozygous participants. Because we were primarily interested in the genetic effects on subgenual cingulate function, we performed a region of interest (ROI)-based analysis using a pre-defined combined anatomical and literature-based ROI of CG25 (see Supplementary Material). We observed a complex gene–gene interaction of CACNA1C rs1006737 and PCLO rs2522833 in CG25, with participants homozygous for the low-risk allele of both SNPs and carriers of both high-risk alleles showing higher CG25 activation during self-reference when compared with carriers of only one high-risk allele (either CACNA1C rs1006737 A or PCLO rs2522833 C, but not both; F1,71=13.50; P=0.034, FWE-corrected for CG25 ROI volume; see Figure 1b).
Experiment 2
As in Experiment 1, a two-way random effects ANOVA testing for the influence of CACNA1C rs1006737 and PCLO rs2522833 genotypes on CG25 activation during encoding revealed a complex gene–gene interaction with similar CG25 activation in participants homozygous for both low-risk alleles and in carriers of both high-risk alleles, whereas high-risk allele carriers of one but not both SNPs showed a relative deactivation in the subgenual cingulate (F1,290=12.82; P=0.025, FWE-corrected for CG25 ROI volume; Figure 2b).
Experiment 3
When testing for a gene–gene interaction using a random effects two-way ANOVA model of the novelty encoding contrasts (subsequently recognized novel scenes compared with the master images), we could replicate the complex interaction of CACNA1C rs1006737 and PCLO rs2522833 observed in the first two experiments, with carriers of either no or both high-risk alleles showing higher CG25 activation relative to carriers of either rs1006737 A or rs2522833 C alleles, but not both (F1,105=14.62; P=0.039, FWE-corrected for CG25 ROI volume; Figure 3b).
Effects of PCLO genotype on hippocampal activation and memory performance
Guided by the observed lower memory performance in PCLO rs2522833 C carriers in experiment 2 (see Supplementary Results for details), we hypothesized that the C allele of rs2522833 might also be associated with decreased hippocampal activation. In experiments 1 and 2, encoding relative to baseline was associated with reduced activation of the left hippocampus in C carriers. There was also a trend for lower encoding-related right hippocampal activation in C carriers in Experiment 3. Moreover, in participants of experiment 2, we also observed lower performance in the Verbal Learning and Memory Test in rs2522833 C allele carriers (for detailed results on memory performance and hippocampal activation as a function of rs2522833 genotype, see Supplementary Results and Supplementary Figure S2). CACNA1C rs1006737 genotype was not associated with memory performance in any of the three experiments.
Discussion
Summarizing the results of the three experiments, we could observe a gene–gene interaction between CACNA1C rs1006737 and PCLO rs2522833 in CG25 where carriers of no risk allele and carriers of both risk alleles exhibited higher activation relative to carriers of only one risk variant during episodic encoding relative to baseline. Furthermore, we found evidence for an association of PCLO rs2522833 with memory performance and memory-related hippocampal activation.
An epistatic interaction of depression risk alleles
In three functional magnetic resonance imaging studies experiments using three different memory encoding tasks, we convergingly observed comparable patterns of a complex, epistatic interaction of CACNA1C and PCLO depression risk alleles in three independent samples.
Although the multigenic nature of depression and related phenotypes is well established, the ‘multiplicative' interaction type of two risk alleles is an unexpected finding. The fact that all three experiments were carried out in neurologically and psychiatrically healthy individuals raises the possibility that a higher load of genetic risk variants for depression might co-occur with the presence of resilience factors that might be genetic or environmental or both. It should be pointed out, however, that the frequency of the risk alleles is considerably higher than the point prevalence of major depression, making it unlikely that our observation purely results from a sampling bias in a healthy population. Given the contribution of both SNPs to the genetic risk for depression, a predicted outcome of our study might have been that carriers of both risk alleles would show an additive or even synergistic effect of risk allele load on CG25 activity. Unexpectedly, however, the pattern of genotype-related CG25 activation observed in the present study points to a complex gene–gene interaction. Specifically, participants homozygous for the low-risk alleles of both rs1006737 and rs2522833 and carriers of both high-risk alleles showed comparable levels of subgenual cingulate activation, whereas risk allele carriers of either one high-risk variant exhibited relatively reduced activation in this region. Of note, we were able to replicate this unexpected pattern in two further cohorts, using different episodic encoding task.
A broad definition of the term epistasis has been used to describe gene–gene interactions with non-additive effects that can be either positive or negative.43 Epistatic interactions on neuroimaging endophenotypes have been previously observed for genes related to the dopaminergic system,44,45 but also for genes involved in different neurotransmitter systems.46,47 The phenotypic patterns observed in those studies, however, could be readily explained by the known molecular effects of the genetic variations investigated, with either both genes investigated influencing the related parameters (for example, cortical versus subcortical dopamine availability) or both variants of interest affecting neurotransmitter systems with known interactions. In the present study, we demonstrated a complex interaction of two SNPs previously identified in GWAS. On the other hand, the underlying molecular and cellular mechanisms remain elusive thus far.
The finding that two genetic variations previously linked to depression exert a reliable epistatic effect on a depression-related endophenotype could potentially provide new insights into the problem of missing heritability.3,4 The fact that carriers of both risk alleles showed similar levels of subgenual cingulate activation as low-risk allele homozygotes for both variants raises the possibility that putative genetic risk variants might, despite their individual contribution to disease risk, attenuate their respective effects when present simultaneously. Yet, one caveat in the present study remains that, although the observed pattern could be reliably replicated in three independent healthy cohorts, we can thus far only make inferences regarding a neural endophenotype, but not concerning clinical risk for depression. Future studies are warranted that specifically target potential interactions of previously identified genetic risk factors for neuropsychiatric disorders at the level of actual risk to develop a disorder. Such epistatic interactions might be difficult to detect in standard GWAS analyses, but at a chromosomal level, epistatic interaction between distant loci has already been described for the genetic risk for BPD,48 and with the recent advance in computational biology, genome-wide interactome studies might soon become feasible and help to systematically uncover such complex genetic contributions to the risk for neuropsychiatric disorders.
Subgenual cingulate function in depression endophenotypes and memory
Converging evidence suggests that structural and functional alterations of the subgenual cingulate might constitute an endophenotype for depression.27,49 Physiologically, in healthy humans, CG25 activity has been linked to negative emotional states like grief17 or social exclusion,50 but also to the cognitive control of negative emotions18,19 as well as to the regulation of emotion-induced autonomic responses51 and successfully overcoming fear.52 Compatibly, the infralimbic cortex, the rodent homolog of the subgenual cingulate has been shown to contribute to fear extinction and regulation of corticosteroid release,53 and chronic glucocorticoid exposure leads to dendritic remodeling in the infralimbic cortex and impaired fear conditioning.54 Although the stimulus material used in our experiments was not designed to be emotionally arousing, it could be argued that the self-reference condition in experiment 1 and the social mentalizing required for the encoding task in experiment 2 might functionally engage CG25 to a certain extent, and, indeed, we observed genotype-independent activation of the ventromedial PFC in these two experiments, which extended into the subgenual cingulate (see Supplementary Figure S1). Although the tasks of the first two experiments also engaged social cognition, the scenes presented in experiment 3 did not contain recognizable human faces, thus minimizing a potential social component of the task. A unifying feature, however, was the episodic nature of the tasks, in all of which complex associative information was encoded into hippocampus-dependent memory (all three tasks were followed by retrieval tasks that confirmed successful encoding; see Supplementary Information for details). The subgenual cingulate/infralimbic cortex is extensively interconnected with the hippocampus and other limbic structures like the amygdala and the ventral striatum, and it has been suggested that the subgenual ACC and ventromedial PFC might functionally integrate distributed information within the limbic system and also suppress irrelevant limbic information.20 With respect to the present study, gene variants linked to risk for depression might exert a complex epistatic effect on subgenual cingulate-dependent processing of episodic information. We could previously demonstrate that CACNA1C genotype was associated with alterations of hippocampal and CG25 activity during the recall phase of experiment 2 and that genetically mediated activation differences in these regions correlated with depression-related psychopathology,27,29 but not memory performance. In the present study, genetically mediated differences in memory performance have been observed for PCLO only and associated with reduced hippocampal, but not CG25 recruitment (see Supplementary Results and Discussion). A limitation of the current study is that no uniform measures of psychopathology were available across the three cohorts, and we could therefore not test for a relationship between functional magnetic resonance imaging studies responses or memory performance and depression-related traits in our participants. It can therefore not be excluded that the observed effects might be primarily related to memory processing and affect depression-related phenotypes indirectly, although previous studies point to a role for dysfunctional memory processes such as negativity bias and depressive illness.21,55 Future studies will therefore be required to further assess the relationship between memory processing and pathomechanisms of depression.
Synaptic dysfunction and psychiatric endophenotypes
Earlier genetic association studies and investigations of endophentypes of psychiatric disorders have largely focused on the monoaminergic neurotransmitter systems and other neuromodulators like BDNF. GWAS results, on the other hand, highlight the role of genetic variations in components of the glutamatergic synapse in psychiatric, particularly affective disorders. For example, the CACNA1C gene product constitutes the alpha subunit of the L-type calcium channel CaV1.2, an important postsynaptic calcium channel, which is co-clustered with AMPA- and NMDA-type glutamate receptors.56 Piccolo is a large presynaptic active zone protein and has an important role in the maintenance of presynaptic integrity and coordination of neurotransmitter release31,57 (for a schematized illustration see Supplementary Figure S3). It is tempting to speculate that molecular changes in the presynapse (like dysregulation of neurotransmitter release) and the postsynaptic spine or dendrite (like a blunted Ca2+ response) might counterbalance each other in certain limits, thus affecting the interpretation of the results. In other words, a symmetric change of pre- and postsynapse in the same individual may have less severe or even no consequence as compared with a genetic condition asymmetrically affecting only one synaptic compartment. Additional structure and adapter molecules of the glutamatergic synapse have recently been identified as potential genetic modifiers of risk for affective disorders, such as the postsynaptic adapter protein Homer158 and the synaptic extracellular matrix proteoglycan neurocan.59,60 Moreover, endophenotypes relevant to psychiatric disorders such as social cognition and aggression have been linked to genetic variability of synaptic proteins like PSD-9561 or AKAP79/150.62 In line with those results, altered glutamate levels in the rostral ACC have been associated with symptom severity in depressed patients.23 The present study cautions, however, that, at least at the level of endophenotypes, the excitatory synaptic contribution to risk for affective disorders might not be linear, maybe reflecting the complexity of the synaptic machinery.
Conclusions
In three independent cohorts, using three different memory encoding paradigms, a complex epistatic interaction between two depression risk alleles coding for synaptic proteins could be reproducibly demonstrated in the subgenual cingulate. The finding that carriers of both risk alleles exhibit similar activation patterns as carriers of no risk allele points to epistatic gene–gene interactions at the level of endophenotypes for psychiatric disorders. Future studies should systematically address such interactions and thereby possibly uncover a potentially underestimated contributor to missing heritability.
Acknowledgments
We would like to thank Josephine Klaembt, Kerstin Möhring, Ilona Wiedenhöft, Katja Neumann and Claus Tempelmann for help with functional magnetic resonance imaging studies data acquisition and Gusalija Behnisch for expert technical assistance with genotyping. Funding for this study was provided by the German Ministry for Education and Research (BMBF) grant NGFNplus MooDS to AM-L, AH and HW by the German Research Foundation (DFG, SFB 636-B7 to AM-L, SFB 779-A8 to BHS and CS, SFB 779-A7 to ED, and SFB 779-B9 to EDG) and the BMBF ERANet-Neuron (AMRePACELL to EDG). AA, AB and MK were fellows of the Leibniz Graduate School LGS Synaptogenetics. The funding agencies had no role in the design of the experiments or in the interpretation of the results.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry. 2003;60:497–502. doi: 10.1001/archpsyc.60.5.497. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Maher B. Personal genomes: the case of the missing heritability. Nature. 2008;456:18–21. doi: 10.1038/456018a. [DOI] [PubMed] [Google Scholar]
- Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney RE, Joormann J, Eugene F, Dennis EL, Gotlib IH. Neural correlates of rumination in depression. Cogn Affect Behav Neurosci. 2010;10:470–478. doi: 10.3758/CABN.10.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CG, Yucel M, Allen NB, Harrison BJ. Task-related deactivation and functional connectivity of the subgenual cingulate cortex in major depressive disorder. Front Psychiatry. 2012;3:14. doi: 10.3389/fpsyt.2012.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S, Lambon Ralph MA, Moll J, Deakin JF, Zahn R. Guilt-selective functional disconnection of anterior temporal and subgenual cortices in major depressive disorder. Arch Gen Psychiatry. 2012;69:1014–1021. doi: 10.1001/archgenpsychiatry.2012.135. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Schlapfer TE, Bewernick BH. Deep brain stimulation for psychiatric disorders—state of the art. Adv Tech Stand Neurosurg. 2009;34:37–57. doi: 10.1007/978-3-211-78741-0_2. [DOI] [PubMed] [Google Scholar]
- O'Connor MF, Irwin MR, Wellisch DK. When grief heats up: pro-inflammatory cytokines predict regional brain activation. Neuroimage. 2009;47:891–896. doi: 10.1016/j.neuroimage.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Walter H, von Kalckreuth A, Schardt D, Stephan A, Goschke T, Erk S. The temporal dynamics of voluntary emotion regulation. PLoS One. 2009;4:e6726. doi: 10.1371/journal.pone.0006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis IL, Takashima A. The role of the ventromedial prefrontal cortex in memory consolidation. Behav Brain Res. 2011;218:325–334. doi: 10.1016/j.bbr.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Mickey BJ, Langenecker SA, Heitzeg MM, Love TM, Wang H, et al. Variation in the corticotropin-releasing hormone receptor 1 (CRHR1) gene influences fMRI signal responses during emotional stimulus processing. J Neurosci. 2012;32:3253–3260. doi: 10.1523/JNEUROSCI.5533-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickie EW, Brunet A, Akerib V, Armony JL. Neural correlates of recovery from post-traumatic stress disorder: a longitudinal fMRI investigation of memory encoding. Neuropsychologia. 2011;49:1771–1778. doi: 10.1016/j.neuropsychologia.2011.02.055. [DOI] [PubMed] [Google Scholar]
- Horn DI, Yu C, Steiner J, Buchmann J, Kaufmann J, Osoba A, et al. Glutamatergic and resting-state functional connectivity correlates of severity in major depression—the role of pregenual anterior cingulate cortex and anterior insula. Front Syst Neurosci. 2010;4 doi: 10.3389/fnsys.2010.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavalali ET, Monteggia LM. Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am J Psychiatry. 2012;169:1150–1156. doi: 10.1176/appi.ajp.2012.12040531. [DOI] [PubMed] [Google Scholar]
- Ferreira MA, O'Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008;40:1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green EK, Grozeva D, Jones I, Jones L, Kirov G, Caesar S, et al. The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Mol Psychiatry. 2010;15:1016–1022. doi: 10.1038/mp.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erk S, Meyer-Lindenberg A, Schnell K, Opitz von Boberfeld C, Esslinger C, Kirsch P, et al. Brain function in carriers of a genome-wide supported bipolar disorder variant. Arch Gen Psychiatry. 2010;67:803–811. doi: 10.1001/archgenpsychiatry.2010.94. [DOI] [PubMed] [Google Scholar]
- Jogia J, Ruberto G, Lelli-Chiesa G, Vassos E, Maieru M, Tatarelli R, et al. The impact of the CACNA1C gene polymorphism on frontolimbic function in bipolar disorder. Mol Psychiatry. 2011;16:1070–1071. doi: 10.1038/mp.2011.49. [DOI] [PubMed] [Google Scholar]
- Erk S, Meyer-Lindenberg A, Schmierer P, Mohnke S, Grimm O, Garbusow M, et al. Hippocampal and frontolimbic function as intermediate phenotype for psychosis: evidence from healthy relatives and a common risk variant in CACNA1C Biol Psychiatry, in press. [DOI] [PubMed]
- Ziv NE, Garner CC. Cellular and molecular mechanisms of presynaptic assembly. Nat Rev Neurosci. 2004;5:385–399. doi: 10.1038/nrn1370. [DOI] [PubMed] [Google Scholar]
- Waites CL, Leal-Ortiz SA, Okerlund N, Dalke H, Fejtova A, Altrock WD, et al. Bassoon and Piccolo maintain synapse integrity by regulating protein ubiquitination and degradation. EMBO J. 2013;32:954–969. doi: 10.1038/emboj.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, de Geus EJ, Willemsen G, James MR, Smit JH, Zandbelt T, et al. Genome-wide association for major depressive disorder: a possible role for the presynaptic protein piccolo. Mol Psychiatry. 2009;14:359–375. doi: 10.1038/mp.2008.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hek K, Mulder CL, Luijendijk HJ, van Duijn CM, Hofman A, Uitterlinden AG, et al. The PCLO gene and depressive disorders: replication in a population-based study. Hum Mol Genet. 2010;19:731–734. doi: 10.1093/hmg/ddp529. [DOI] [PubMed] [Google Scholar]
- Minelli A, Scassellati C, Cloninger CR, Tessari E, Bortolomasi M, Bonvicini C, et al. PCLO gene: its role in vulnerability to major depressive disorder. J Affect Disord. 2012;139:250–255. doi: 10.1016/j.jad.2012.01.028. [DOI] [PubMed] [Google Scholar]
- Furukawa-Hibi Y, Nitta A, Fukumitsu H, Somiya H, Furukawa S, Nabeshima T, et al. Overexpression of piccolo C2A domain induces depression-like behavior in mice. Neuroreport. 2010;21:1177–1181. doi: 10.1097/WNR.0b013e3283411685. [DOI] [PubMed] [Google Scholar]
- Miller MB, Van Horn JD, Wolford GL, Handy TC, Valsangkar-Smyth M, Inati S, et al. Extensive individual differences in brain activations associated with episodic retrieval are reliable over time. J Cogn Neurosci. 2002;14:1200–1214. doi: 10.1162/089892902760807203. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quervain DJ, Papassotiropoulos A. Identification of a genetic cluster influencing memory performance and hippocampal activity in humans. Proc Natl Acad Sci USA. 2006;103:4270–4274. doi: 10.1073/pnas.0510212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Rubino V, Sambataro F, Blasi G, Latorre V, Fazio L, et al. Prefrontal-hippocampal coupling during memory processing is modulated by COMT val158met genotype. Biol Psychiatry. 2006;60:1250–1258. doi: 10.1016/j.biopsych.2006.03.078. [DOI] [PubMed] [Google Scholar]
- Schott BH, Seidenbecher CI, Richter S, Wustenberg T, Debska-Vielhaber G, Schubert H, et al. Genetic variation of the serotonin 2a receptor affects hippocampal novelty processing in humans. PLoS One. 2011;6:e15984. doi: 10.1371/journal.pone.0015984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Duzel E, Schutze H, Yonelinas AP, Heinze HJ. Functional phenotyping of successful aging in long-term memory: preserved performance in the absence of neural compensation. Hippocampus. 2011;21:803–814. doi: 10.1002/hipo.20834. [DOI] [PubMed] [Google Scholar]
- Phillips PC. Epistasis—the essential role of gene interactions in the structure and evolution of genetic systems. Nat Rev Genet. 2008;9:855–867. doi: 10.1038/nrg2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacubian J, Sommer T, Schroeder K, Glascher J, Kalisch R, Leuenberger B, et al. Gene-gene interaction associated with neural reward sensitivity. Proc Natl Acad Sci USA. 2007;104:8125–8130. doi: 10.1073/pnas.0702029104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prata DP, Mechelli A, Fu CH, Picchioni M, Toulopoulou T, Bramon E, et al. Epistasis between the DAT 3′ UTR VNTR and the COMT Val158Met SNP on cortical function in healthy subjects and patients with schizophrenia. Proc Natl Acad Sci USA. 2009;106:13600–13605. doi: 10.1073/pnas.0903007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan HY, Chen Q, Sust S, Buckholtz JW, Meyers JD, Egan MF, et al. Epistasis between catechol-O-methyltransferase and type II metabotropic glutamate receptor 3 genes on working memory brain function. Proc Natl Acad Sci USA. 2007;104:12536–12541. doi: 10.1073/pnas.0610125104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Goldman AL, Verchinski BA, Chen G, Kolachana BS, et al. Evidence of biologic epistasis between BDNF and SLC6A4 and implications for depression. Mol Psychiatry. 2008;13:709–716. doi: 10.1038/mp.2008.32. [DOI] [PubMed] [Google Scholar]
- Abou Jamra R, Fuerst R, Kaneva R, Orozco Diaz G, Rivas F, Mayoral F, et al. The first genomewide interaction and locus-heterogeneity linkage scan in bipolar affective disorder: strong evidence of epistatic effects between loci on chromosomes 2q and 6q. Am J Hum Genet. 2007;81:974–986. doi: 10.1086/521690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz JB, Drevets WC. Imaging phenotypes of major depressive disorder: genetic correlates. Neuroscience. 2009;164:300–330. doi: 10.1016/j.neuroscience.2009.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, McNealy K, Pfeifer JH, Dapretto M. Subgenual anterior cingulate responses to peer rejection: a marker of adolescents' risk for depression. Dev Psychopathol. 2011;23:283–292. doi: 10.1017/S0954579410000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Nili U, Goldberg H, Weizman A, Dudai Y. Fear thou not: activity of frontal and temporal circuits in moments of real-life courage. Neuron. 2010;66:949–962. doi: 10.1016/j.neuron.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- Sperduti M, Martinelli P, Kalenzaga S, Devauchelle AD, Lion S, Malherbe C, et al. Don't be too strict with yourself! rigid negative self-representation in healthy subjects mimics the neurocognitive profile of depression for autobiographical memory. Front Behav Neurosci. 2013;7:41. doi: 10.3389/fnbeh.2013.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev. 2009;89:411–452. doi: 10.1152/physrev.00029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee K, Yang X, Gerber SH, Kwon HB, Ho A, Castillo PE, et al. Piccolo and bassoon maintain synaptic vesicle clustering without directly participating in vesicle exocytosis. Proc Natl Acad Sci USA. 2010;107:6504–6509. doi: 10.1073/pnas.1002307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel M, Mattheisen M, Frank J, Treutlein J, Degenhardt F, Breuer R, et al. Genome-wide association-, replication-, and neuroimaging study implicates HOMER1 in the etiology of major depression. Biol Psychiatry. 2010;68:578–585. doi: 10.1016/j.biopsych.2010.05.038. [DOI] [PubMed] [Google Scholar]
- Cichon S, Muhleisen TW, Degenhardt FA, Mattheisen M, Miro X, Strohmaier J, et al. Genome-wide association study identifies genetic variation in neurocan as a susceptibility factor for bipolar disorder. Am J Hum Genet. 2011;88:372–381. doi: 10.1016/j.ajhg.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miro X, Meier S, Dreisow ML, Frank J, Strohmaier J, Breuer R, et al. Studies in humans and mice implicate neurocan in the etiology of mania. Am J Psychiatry. 2012;169:982–990. doi: 10.1176/appi.ajp.2012.11101585. [DOI] [PubMed] [Google Scholar]
- Feyder M, Karlsson RM, Mathur P, Lyman M, Bock R, Momenan R, et al. Association of mouse Dlg4 (PSD-95) gene deletion and human DLG4 gene variation with phenotypes relevant to autism spectrum disorders and Williams' syndrome. Am J Psychiatry. 2010;167:1508–1517. doi: 10.1176/appi.ajp.2010.10040484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter S, Gorny X, Marco-Pallares J, Kramer UM, Machts J, Barman A, et al. A Potential Role for a Genetic Variation of AKAP5 in Human Aggression and Anger Control. Front Hum Neurosci. 2011;5:175. doi: 10.3389/fnhum.2011.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.