Abstract
The apoptosis-associated speck-like protein (ASC) is a key component of multimeric protein complexes that mediate inflammation and host defense. Composed of a Pyrin (PYD) domain and a caspase activation and recruitment domain (CARD), ASC functions downstream of nucleotide-binding domain, leucine-rich repeat containing receptors (NLRs) and absent in melanoma 2 (AIM2) through the formation of supramolecular structures termed inflammasomes. However, the mechanism underlying ASC signaling and its dependency on oligomeric arrangements in inflammasome formation remain poorly understood. When expressed in cells, ASC forms discrete foci (called “specks”) typically with one speck per cell. We employed a bimolecular fluorescence complementation (BiFC) system to investigate and visualize ASC foci formation in living cells. We demonstrate that the CARD of ASC plays a central role in ASC inflammasome assembly, representing the minimal unit capable of forming foci in conjunction with Caspase-1 CARD. Mutational studies point to multiple surfaces on the ASC CARD and two predominant areas on the Caspase-1 CARD mediating the formation of ASC/Caspase-1 foci. The lack of foci formation for ASC CARD mutants correlates with a loss of IL-1β processing in response to NLRP3 or AIM2 agonists in RAW264.7 cell reconstitution assays. Analogously, we show that productive formation of the Salmonella typhimurium-induced NLRC4 inflammasome is dependent on ASC-CARD-mediated platform formation. Thus, our results depict a central role of CARDs in the formation of ASC signaling platforms and provide an important tool for investigation of CARD-dependent networks.
Keywords: signaling platform formation, death domains, inflammasome, CARD, innate immunity, Caspase-1, inflammatory cytokines, Nod-like receptors (NLRs), NLRC4, Salmonella
INTRODUCTION
Innate immunity comprises the first line of defense against invading pathogens. The innate immune response is initiated by Toll-like receptors (TLRs), by NLRs, and other sensors such as AIM2, which recognize pathogen-associated molecular patterns (PAMPs) and damage associated molecular patterns (DAMPs). Members of the NLR family are potent inducers of key defense pathways, including initiation of the NF-κB response, activation of inflammatory caspases, and release of pro-inflammatory cytokines [1, 2]. Failure of correct NLR signaling results in various autoimmune disorders including Crohn's disease (NOD2) [3], Blau syndrome (NOD2) [4], Vitiligo (NLRP1) [5, 6], Muckle-Wells Syndrome (NLRP3) [7], neonatal onset multisystem inflammatory disease (NOMID) (NLRP3) [8], and familial cold autoinflammatory syndrome (NLRP3) [7, 9].
The adaptor apoptosis speck-like protein (ASC) represents the central link between NLRs/AIM2 and their downstream signaling target Caspase-1. Its bipartite Pyrin-CARD architecture allows ASC to simultaneously interact with the Pyrin domain (PYD) of NLRs/AIM2 and the CARD of Caspase-1, ultimately leading to the processing of inflammatory cytokines such as pro-IL-1γ and pro-IL-18 [10]. Activation of Caspase-1 is strongly dependent on the formation of highly oligomeric ASC dependent structures usually referred to as foci [11]. A variety of NLR stimuli as well as ASC overexpression [12, 13] lead to the formation of foci, which are therefore generally regarded to represent the active form of inflammasomes [13-15]. While established on a biological level, the functional mechanism of ASC-mediated foci formation and particularly the role of its adaptor domains in this process remain enigmatic. Commonly, the formation of ASC foci is considered to be mediated by its PYD domain, since mutations in this domain lead to the suppression of ASC auto-oligomerization [16]. The proposed role of the CARD has so far been restricted to a mere recruitment of Caspase-1 CARD and a function beyond this, such as participation in ASC oligomerization, has not previously been proposed.
Another layer of intricacy to CARD-mediated ASC inflammasome signaling comes from studies involving NLRC4, a key mediator of innate immune response to Salmonella typhimurium infection. As a CARD-containing NLR, the NLRC4 protein is in principle capable of directly interacting with the CARD of Caspase-1, omitting the need for an adaptor protein [17]. Yet, an amplifying function for ASC has been proposed in the context of responses to Salmonella infection [15, 18], further underlying the necessity to obtain insight into the CARD-dependent signaling mechanism of ASC.
To gain insight into the role of the CARD system in ASC-mediated innate immunity, we adapted the bimolecular fluorescence complementation (BiFC) assay [19], allowing us to investigate and visualize productive ASC foci formation (Figure 1A) in living cells. Briefly, the BiFC technology uses complementary fragments of Venus, which represents an improved yellow fluorescent protein. These fragments do not reconstitute spontaneously, but when fused to interacting proteins, the split fragments associate and restore a functional fluorescent signal. Using this assay, we were able to distinguish between non-functional assemblies, which show complementation of the fluorescent signal but lack of platform formation, and functional assemblies representing intact inflammasome-like foci. In this study, we show that the PYD domain of ASC is not sufficient for ASC foci formation, but also requires the presence of the CARD. Unexpectedly, when expressed in combination with Caspase-1 CARD, the CARD of ASC is sufficient to support productive foci assembly. We furthermore identified surface residues on the CARDs of ASC and Caspase-1, mutations of which interfered with ASC inflammasome formation. We then tested the effect of ASC mutants on inflammasome formation in RAW264.7 murine macrophage cells to delineate the importance of CARD dependent oligomerization in NLRP3 and AIM2 signaling pathways as well as in the NLRC4 response to Salmonella infection.
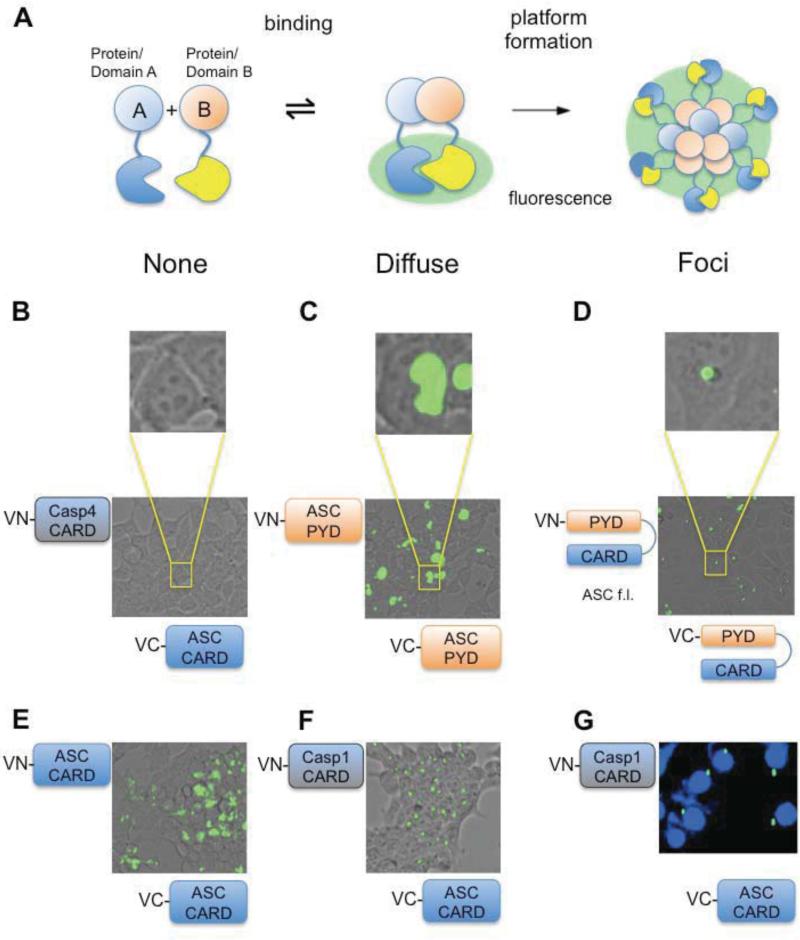
Figure 1. Oligomeric foci formation visualized by the BiFC system.
A. Schematics of BiFC assay. Lack of interaction between proteins bearing complementary fragments (VC, VN) of Venus-GFP leads to a lack of fluorescence. Diffuse fluorescence indicates proximity of binding partners due to binding or loose assemblies. Foci formation indicates defined productive oligomerization and hence signaling platform formation. B-D. BiFC assay results exemplifying different outcomes in correspondence to above depicted schematics. HEK293T cells were transiently transfected with VN- and VC-fusions. Cells were visualized for fluorescence 24 hours post transfection. B. Example for lack of interaction using VC ASC-CARD and VN-Caspase-4 CARD. C. Diffuse fluorescence observed after co-transfection of VN-ASC PYD and VC-ASC PYD. D. Foci formation observed after co-transfection of VN-ASC full-length and VC-ASC full-length. E. Fluorescent microscopy images showing BiFC of VN-ASC CARD and VC-ASC CARD leading to a diffuse fluorescence pattern. F. Fluorescent microscopy images showing BiFC of VC-ASC CARD and VN-Caspase-1 CARD resulting in foci formation. G. HeLa cells were transfected with VN-Caspase-1 CARD and VC-ASC CARD. Fluorescent microscopy images showing perinuclear localization of ASC CARD/Caspase-1 CARD foci with nuclei visualized by DAPI staining. For each BiFC experiment outlined here and below all VCASC constructs were tested for auto-activation by co-expression with empty VN-vector and VNCaspase-1 constructs by co-expression with empty VC-vectors, respectively.
EXPERIMENTAL
Models
A model for the CARD of Caspase-1 was created using MODELLER [20] based on the structure of the Iceberg CARD from PDB 1DGN. Figures for the Caspase-1 CARD model and for ASC CARD (based on the ASC structure from PDB 2KN6) were prepared using Pymol (www.pymol.org).
Construction of plasmids and mutagenesis
CARDs of human Caspase-1 and -4 were fused to N-terminal Venus fragments of pVNN vector. CARDs of human ASC and NLRC4 were fused to C-terminal Venus fragments of pVCC vector. pVNN and pVCC vectors were provided by Dr. Gordon Mills’ Lab (MD Anderson Cancer Center). Point mutations in Caspase-1 and ASC CARD were generated using QuikChange site-directed mutagenesis (Stratagene). All mutants were verified by DNA sequencing analysis.
BiFC assay
Human embryonic kidney (HEK) 293T or HeLa cells were transfected with VN- and VC-fusion constructs using lipofectamine 2000 according to manufacturer's protocol. Cells were incubated at 37°C for 20 h. Fluorophore formation in living cells was imaged using fluorescence microscopy. Images were acquired using an AMG EVOS digital inverted fluorescence microscope. For DNA staining, cells were fixed with 4% formaldehyde, washed three times with 1xPBS, permeabilized with 0.1% Triton X-100 and labeled with DAPI.
Cell culture and stable cell lines
Human embryonic kidney (HEK) 293T, HeLa, and RAW264.7 cells were cultured in Dulbecco's modified Eagle's medium (GIBCO) supplemented with glutamine, antibiotics, and 10% fetal bovine serum (FBS). Stable wild type (WT) ASC and mutant ASC RAW264.7 cells were generated using lentiviral infection. Full-length WT ASC and mutant ASC were subcloned into the pLEX expression plasmid. Lentivirus was produced by co-expression of pLEX with the packaging plasmids pM2.G and psPAX2 in 10 cm dishes of (HEK) 293T cells. Clarified culture supernatants were used to infect 106 RAW264.7 cells in 6 well plates for 24 hours at 37°C, followed by puromycin selection.
Immunoblotting
Processed IL-1β released into the culture supernatant was detected by immunoblotting. Supernatants were collected and samples were concentrated by chloroform-methanol precipiation. Precipitated protein pellets were resuspended in Laemmli sample buffer and separated by SDS-PAGE. Immunoblots were probed with polyclonal anti-mouse IL-1β antibody (R&D Systems, AF-401-NA) at 1:1000 dilution. Cell lysates were probed with monoclonal anti-ß-actin antibody (Sigma-Aldrich, A5441) at 1:1000 dilution as a loading control. For detection of lower order assemblies, lysates from transfection experiments were resolved by native PAGE (4-16%) using the NativePAGE Novex Bis-Tris gel system from Invitrogen. The presence of ASC CARD and Caspase-1 CARD assemblies was revealed by using polyclonal anti-ASC antibody (Calbiochem, ST1121) and polyclonal anti-GFP, which selectively recognizes the N-terminal fragment of Venus (Santa Cruz Biotechnology, sc-8334) at 1:1000 dilution.
Salmonella typhimurium infection
Wild-type serovar Enteritidis LK5 was used for infection experiments (gift of S. Maloy, San Diego State University, San Diego, Calif.). Prior to infection, serovar Enteritidis strains were grown overnight in high-salt LB broth (LB broth plus 300 mM NaCl) and then subcultured 1:4 and allowed to grow for an additional 2 to 3 h. Bacteria were washed twice in phosphate-buffered saline and then resuspended in OptiMem growth media. RAW cells were washed in phosphate-buffered saline and then overlaid with bacterial suspension such that the multiplicity of infection was 2, 20 or 200 bacteria per mammalian cell. Supernatants were collected at 2 h for IL-1β secretion measurement by ELISA. Secreted IL-1β was measured using mouse IL-1β Ready-SET-Go! ELISA kits from eBioscience (San Diego, CA).
RESULTS
The CARD of ASC is crucial for the formation of ASC inflammasomes
The ability of ASC to assemble into so-called “foci” is key for inflammasome activation. Due to its widely known insolubility upon overexpression in bacterial systems the use of recombinant ASC or its domains for monitoring oligomer formation in vitro is limited. We therefore adapted the bimolecular fluorescence complementation (BiFC) assay for qualitatively investigating the mechanism of ASC foci formation in living cells. The BiFC assay is based on two split fragments of the Venus fluorescence protein. The fragments termed VN and VC (Venus -N and -C terminal fragment, respectively) are fused to proteins, which constitute potential binding partners. Upon interaction between the binding partners, the fluorescent fragments are brought in close proximity restoring a functional fluorescent signal (Figure 1A) to result in what we term a “diffuse” fluorescence signal (Figure 1C and E). However, if the proteins not only undergo interaction but form productive signaling platforms, this diffuse signal condenses to intense speck-like foci (compare Figure panels C and D), representing a clear readout to observe and test this event. Therefore this system is appropriate to investigate ASC foci formation, which was previously observed to result in focused fluorescent platforms when ASC was fused to full-length GFP [21]. Furthermore, this assay has the advantage of not only following foci formation of full-length ASC, but also visualizing productive platform formation in the light of specific domains and combinations thereof. When the BiFC technology was applied to full-length VNASC and VC-ASC, we indeed observed the formation of foci (Figure 1D), making this approach an attractive tool to investigate the CARD system and its implications in ASC-mediated inflammatory responses.
We next examined the role of each domain of the bi-partite adaptor ASC in the process of inflammasome formation. Commonly, only the PYD domain of ASC is regarded as the driving force for ASC inflammasome formation [13], while the role of the ASC CARD is thought to be restricted to recruitment and binding of Caspase-1 via homotypic CARD-CARD interactions. However, when we investigated this in the BiFC system, we observed that a combination of VN-and VC-ASC PYD resulted in a diffuse fluorescent signal (Figure 1C). This result was surprising as it indicates that the PYD domains exhibit affinity towards each other, but fail to form minimal ASC platforms. Similarly, VN-and VC-ASC CARD showed diffuse fluorescence, indicating a CARD-CARD attraction (Figure 1E). The observed foci formation in the context of full-length ASC, and absence of foci when either domain was expressed alone, points to a necessity for both domains and thus an active role of the CARD in driving ASC-dependent inflammasome formation.
Given the importance of the ASC CARD in inflammasome assembly, we further investigated the relation to its downstream target Caspase-1 CARD, particularly with regard to foci formation. Surprisingly, co-expression of VC-ASC CARD with VN-Caspase-1 CARD resulted in platform formation (Figure 1F). The resulting foci were similar to foci formed in the BiFC assay using full-length ASC. Furthermore, these ASC-CARD/Caspase-1-CARD foci also showed the same attributes, namely the presence of one distinct perinuclear focus per cell (Figure 1G), as previously observed for endogenous ASC inflammasomes [12]. The formation of foci solely by the CARDs of these two proteins points to an active role of the Caspase-1 CARD in productive platform formation.
ASC and Caspase-1 CARD platforms are highly oligomeric and involve multiple surfaces on ASC and two main areas on Caspase-1 CARD
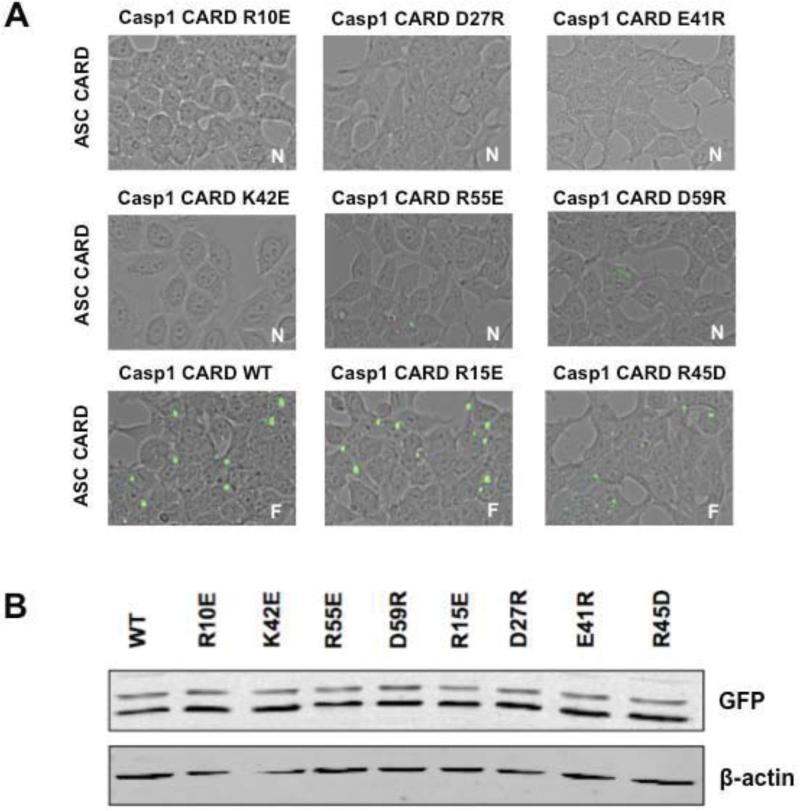
The observed foci formed by ASC/Caspase-1 CARDs in our BiFC assay allowed us to interrogate surfaces both on ASC and Caspase-1 CARDs with respect to platform assembly. Using the structure of ASC CARD [22] and a model of Caspase-1 CARD based on the Iceberg NMR structure [23] (supplement Figure 1A and 1B), we mutated several charged, solvent exposed residues covering all surfaces on both domains. In a first set of experiments we tested Caspase-1 CARD mutants using the BiFC assay and transiently expressed WT and mutant VNCaspase-1 CARD together with WT VC-ASC CARD to investigate their ability to form functional platforms. A subset of mutants (R10E, D27R, E41R, K42E, R55E, and D59R) of the Caspase-1 CARD not only abolished foci formation, but also resulted in loss of fluorescence, indicating abrogation of interaction (Figure 2A). This loss does not stem from reduced expression levels of the mutants due to misfolding or other factors as shown in Figure 2B. These experiments constitute a broad extension of a previous study, which reported that the D27G mutation of Caspase-1 CARD interrupts ASC/Caspase-1 CARD signaling [24]. We furthermore show that all mutants leading to a loss of foci formation and interaction reside in two main surfaces (Figure, 2A). The first key surface is created by helices 1, 3, and 4 (R10, K42, R55, D59) while the second area required for foci formation is located in helices 2 and 3 (D27, K42) of the Caspase-1 CARD. Mutation of residues E8R, R15E on helix 1, R33E in the loop connecting helices 2 and 3, R45D in helix 3 and Q67R, Y75E and E78R on helix 5 showed no effect on foci formation (Figure 2A and supplement Figure 1C).
Figure 2. Mutational analysis of Caspase-1 CARD surfaces with respect to foci formation with WT ASC CARD.
A. BiFC results of HEK293T cells transfected with either WT or mutant VN-Caspase-1 CARD together with WT VC-ASC CARD. Either lack of fluorescence or foci formation was observed for different Caspase-1 mutants. Mutants R10E, D27R, E41R, K42E, R55E, and D59R lead to loss of binding, whereas mutants R15E and R45D showed no effect on foci formation (F=Foci, N=no interaction). B. Cell lysates were immunoblotted for VN-GFP (depicting Caspase-1 CARD) and ß-actin as a loading control showing similar expression levels of mutant Caspase-1 constructs compared to WT Caspase-1.
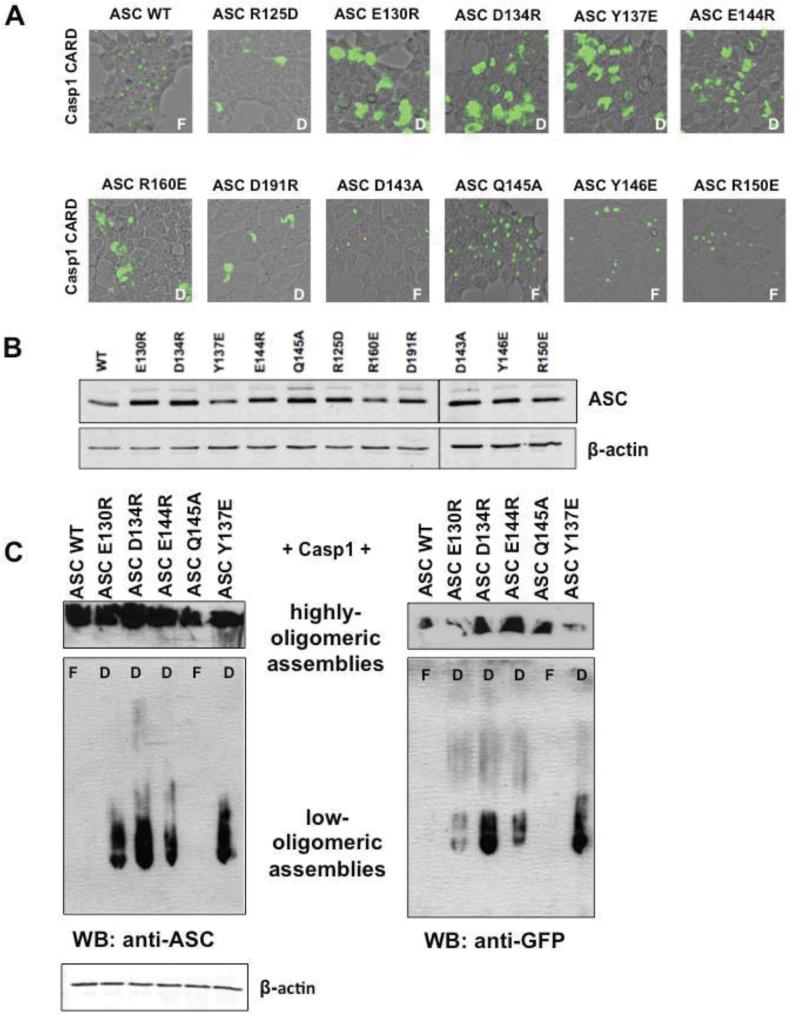
Next, VC-ASC CARD WT or mutants were co-transfected with WT VN-Caspase-1 CARD. Mutants covering several surfaces (R125D, E130R, D134R, Y137E, E144R, R160E and D191R) lost the ability to form foci, whereas mutants D143A, Q145A, Y146E, and R150E showed no effect (Figure 3A). Again, as in the case for Caspase-1 CARD, all ASC CARD mutants show similar or greater expression compared to the wild type CARD (Figure 3B). This points to the involvement of multiple surfaces both on ASC and Caspase-1 (supplement Figures 1A and B). However, in the case of ASC mutants, diffuse fluorescence was still observed, suggesting low order associations, but failure to propagate productive platform formation.
Figure 3. Mutational analysis of ASC CARD surfaces with respect to foci formation with WT Caspase-1 CARD.
A. HEK293T cells were transiently transfected with VN-Caspase-1 CARD and VC-ASC CARD WT or ASC CARD mutants, respectively. 24 hours after transfection, foci formation was analyzed using fluorescence microscopy. Representative images from fluorescent microscopy are shown. ASC mutants R125D, E130R, D134R, Y137E, E144R, R160E, and D191R lead to diffuse phenotype, whereas mutants D134A, Y146E, Q145A, and R150E resulted in foci formation (F= Foci, D=diffuse staining). B. Immunostaining of total cell lysates showing proper expression of fusion proteins. Cell lysates were probed for ASC and for ß-actin as a loading control. C. Cells transfected with either WT or mutants (E130R, D134R, E144R, Q145A, Y137E) VC-ASC CARD and WT VN-Caspase-1 CARD were analyzed in the BiFC assay 24 h post transfection and then lysed by adding Native PAGE lysis buffer (Invitrogen) and sonication. Total lysates were applied to Native PAGE and immunoblotted for ASC and VN-GFP (depicting Caspase-1 CARD). Only the mutants causing a “diffuse” phenotype showed the presence of low order ASC-Caspase-1 CARD assemblies, which were able to enter the gel-matrix. Highly-oligomeric assemblies with very low mobility are shown above. Cell lysates were also probed for ß-actin as a loading control (bottom).
To further investigate the nature of the assemblies showing diffuse fluorescence, we lysed cells transfected with VC-ASC (WT and mutants) and VN-Caspase-1 constructs and subjected them to native PAGE. For this purpose, cells were resuspended in lysis buffer and subjected to sonication. Notably, whole cell lysates were used for native PAGE, since centrifugation leads to precipitation of intact ASC foci (data not shown). Immunoblotting for ASC and Caspase-1 constructs showed that only cells containing “diffuse” ASC mutants showed a presence of lowoligomeric assemblies migrating in the native PAGE, whereas cells containing functional foci of WT ASC-CARD and Caspase-1 CARD completely lacked these low-oligomeric arrangements (Figure 3C). Thus, low-oligomeric assemblies retaining solubility, likely do not represent active signaling platforms.
CARD-dependent ASC foci correlate with Caspase-1-mediated IL-1β processing downstream of both NLRP3 and AIM2
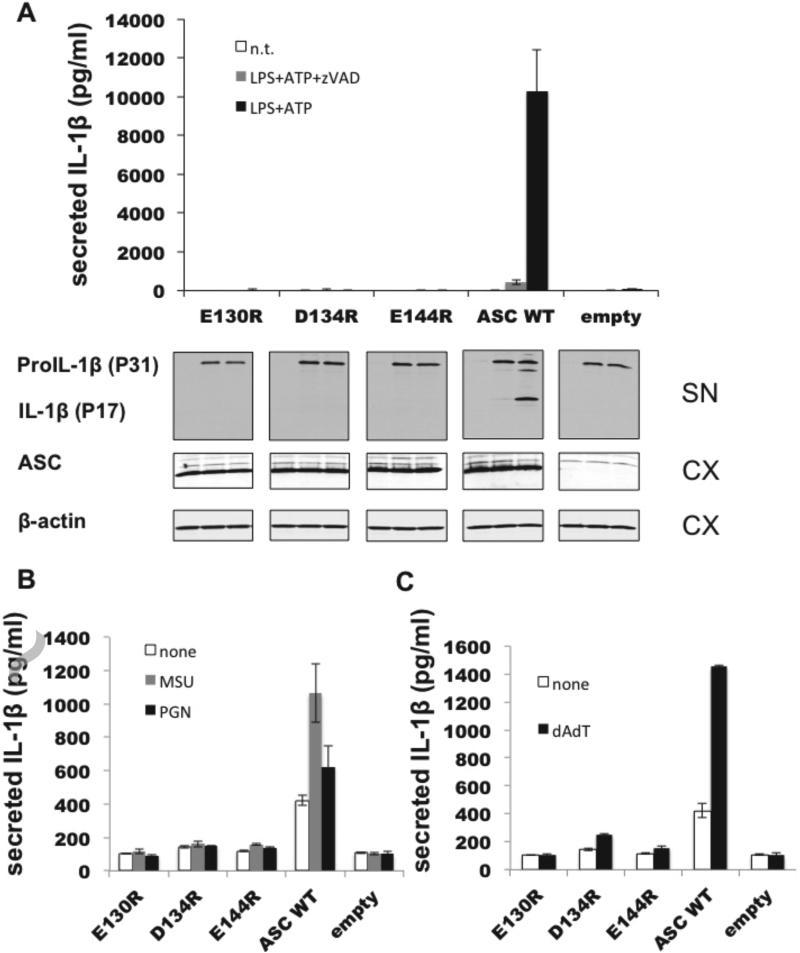
To verify our findings from the BiFC assay and to further investigate the importance of CARD-mediated oligomerization in inflammasome activation, we tested WT and ASC mutants for their ability to mediate NLRP3 and AIM2 signaling under more physiological conditions. We chose the RAW264.7 macrophage cell line, which lacks endogenous ASC, and generated stable cell lines reconstituted with either full-length WT ASC or ASC mutants. Stable cell lines were challenged using divergent stimuli that have been shown to trigger NLRP3 inflammasome formation consisting of (i) ATP, a danger signal that triggers potassium release and Ca2+ mobilization [25]; (ii) peptidoglycan (PGN), a known pathogen-associated molecular pattern (PAMP) derived from bacterial cell walls [26]; and (iii) monosodium urate (MSU) mimicking the reported effect of urea crystals in gout [27]. As expected, RAW264.7 cells expressing WT ASC resulted in inflammasome activation and subsequent release of processed IL-1β upon treatment with LPS and ATP (Figure 4A). Addition of zVAD-fmk prevented IL-1β processing showing the caspase-dependency of this process. In contrast, RAW cells expressing ASC mutants E130R, D134R, or E144R, which led to the diffuse phenotype lacking productive foci formation, produced levels of IL-1β similar to the negative control (RAW cells lacking ASC) following treatment with all NLRP3 stimuli (Figure 4A and B). This defect did not result from a difference in pro-IL-1β synthesis, since immunoblotting showed equivalent pro-IL-1β levels in all cell lines (Figure 4A). These findings confirm the crucial role of the ASC CARD for NLRP3-ASC inflammasome signaling and Caspase-1 activation in a cellular context.
Figure 4. “Diffuse“ ASC mutants fail to induce IL-1ß response upon NLRP3 and AIM2 inflammasome stimulation.
A. RAW264.7 cells stably expressing WT ASC, mutant ASC (E130R, D134R, E144R) or empty vector were primed for pro-IL-1ß production using 1 μg/ml LPS for four hours and subsequently stimulated with 5 mM ATP in the presence and absence of zVAD for 30 minutes. Supernatants were analyzed for IL-1ß production indicating inflammasome dependent Caspase-1 activation using ELISA and immunoblotting. Equivalent expression levels of ASC WT and mutants as well as ß-actin control in lysates were analyzed by immunoblotting. SN supernatant, CX cell extract. B. Analogous to A, RAW264.7 cells stably expressing WT ASC, mutant ASC or empty vector were stimulated with NLRP3 agonists MSU (100 μg/ml) and PGN (5 μg/ml) post LPS treatment in a 96 well format. Supernatant was collected four hours post-stimuli and analyzed for IL-1ß secretion using ELISA (mean±SD; n=3). C. Equivalent to A, B except the AIM2 stimulus poly(dA:dT) (1 μg/ml) was applied post LPS priming.
To further expand our investigation we included the PAMP-sensing factor AIM2, which also forms ASC-dependent Caspase-1 activating inflammasomes. AIM2 contains an N-terminal PYD domain, but in contrast to NLR proteins, features a C-terminal HIN200 domain that senses double stranded DNA in response to bacterial or viral infection [28-30]. Most importantly, AIM2 lacks the NACHT domain typical for NLRs, which is thought to form AAA+ type ring structures and was reported to serve as an oligomerization unit [31] that drives inflammasome formation. Thus, AIM2 inflammasomes constitute an attractive tool to further investigate the role of CARD-dependent ASC oligomerization in this inflammatory signaling pathway.
Therefore, we tested the effect of ASC CARD mutants on AIM2 signaling using poly-dAdT, a dsDNA analog and specific AIM2 activator. Our results show that the ASC mutants that led to a diffuse phenotype in the BiFC system also exhibited significant reduction of IL-1β production upon AIM2 activation, while WT ASC showed robust IL-1β secretion (Figure 4C). Thus, similar to the NACHT-containing NLRs, AIM2 signaling requires a functional ASC CARD, pointing to an important role of the CARD in driving the signaling process.
A crucial role of ASC CARD in NLRC4-mediated Salmonella typhimurium infection
Next we used the insights obtained about the role of the ASC CARD in the oligomerization process to further investigate the elusive function of ASC in Salmonella-induced cytokine activation mediated by NLCR4. In contrast to NLRP proteins, NLRC4 contains an N-terminal CARD, which in principle allows direct interaction with CARD-containing targets, omitting the need for a PYD/CARD containing ASC adaptor. NLRC4 is a known sensor for flagellin during Salmonella typhimurium and Legionella infections and mediates Caspase-1 activation resulting in IL-1β processing [32, 33]. The N-terminal CARD of NLRC4 has been shown to directly interact with the CARD of Caspase-1 [17]. Conversely, a recent study described the formation of NLRC4-dependent foci, which incorporate ASC and Caspase-1 upon Salmonella infection [15]. However, no evidence of a direct interaction between NLRC4 and ASC has been described and the exact role of ASC in the NLRC4 inflammasome complex is still not fully understood.
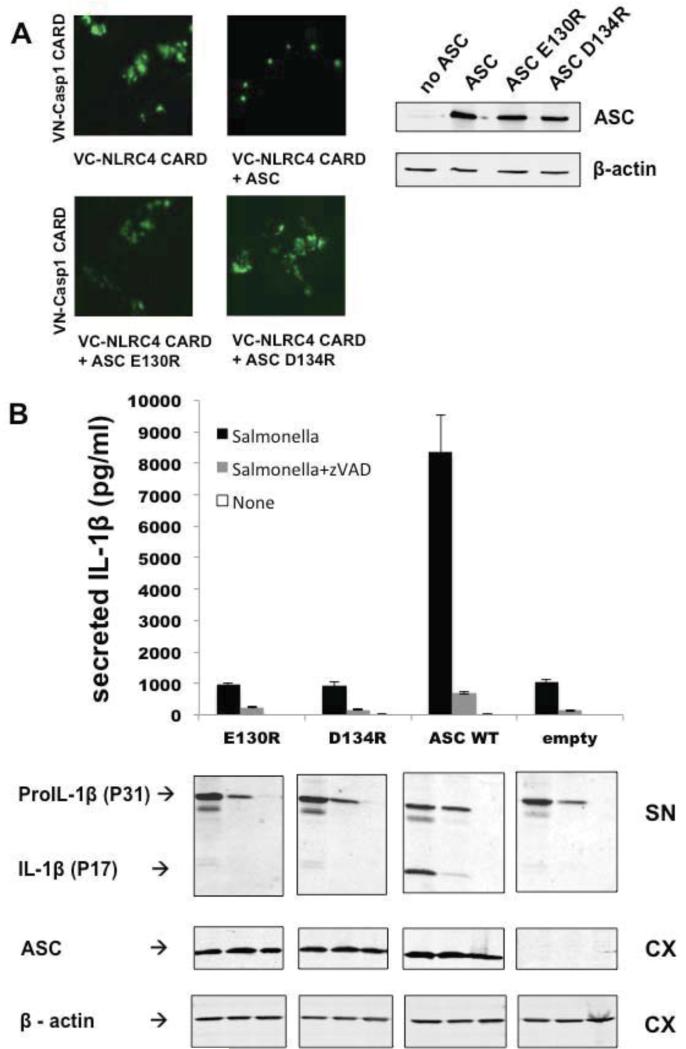
Therefore we tested the effect of ASC on the interaction of VN-NLRC4 CARD and VC-Caspase-1 CARD. To this end, we expressed the CARDs of VN-NLRC4 and VC-Caspase-1 in the presence or absence of full-length ASC. Indeed, co-expression of full-length myc-tagged ASC together with the VN-NLRC4 CARD and VC-Caspase-1 CARD caused a change from diffuse staining to punctate fluorescent foci (Figure 5A). We then tested the ASC mutants E130R and D134R, which are CARD mutants we found to cause diffuse fluorescence patterns in the preceding sections, therefore lacking the ability to propagate productive platform formation. Indeed, when used to replace WT ASC in co-expression experiments with the VN-/VC- tagged CARDs of NLRC4 and Caspase-1, the foci described above were lost and a diffuse pattern was observed (Figure 5A). Taken together, these findings indicate and visualize that the ASC platform is crucial for productive NLRC4 inflammasome foci formation, and support the idea that efficient IL-1β processing is ASC dependent. To verify and extend the function of ASC as pertains to NLRC4 inflammasome signaling, we infected RAW264.7 cells stably expressing WT or ASC mutants (E130R, D134R) with Salmonella typhimurium. In line with foci formation observed in the BiFC assay, WT ASC led to robust IL-1β release upon Salmonella infection (Figure 5B). Consistent with the fact that ASC platform formation is required for efficient Caspase-1 activation and thus cleavage of pro-IL-1β, RAW cells expressing ASC mutants E130R and D134R showed an approximately ten-fold reduction in the response to Salmonella infection, similar to the response observed for the empty vector control (Figure 5B). This reduction did not result from a difference in pro-IL-1β synthesis, since immunoblotting revealed equivalent pro-IL-1β levels in all cell lines (Figure 5B). Furthermore, the requirement of ASC for NLRC4-mediated IL-1β secretion was seen for different multiplicities of infection (supplement Figure 2). Thus, in line with the results of the BiFC assay, the infection experiments also revealed a key role of ASC and its CARD in NLRC4-mediated response to Salmonella infection.
Figure 5. ASC CARD is required for foci formation between NLRC4 CARD and Caspase-1 CARD and for NLRC4 inflammasome activation upon Salmonella infection.
A. BiFC bridge assay. HEK293T cells were transfected with VC-NLRC4 CARD and VNCaspase-1 CARD. Addition of WT ASC full-length led to a change from diffuse to punctate staining, whereas ASC full-length mutants E130R and D134R were incapable of inducing foci formation (left). Immunostaining for ASC and ß-actin showing proper expression levels for WT ASC and mutants (right). B. RAW264.7 cells stably expressing WT ASC, mutant ASC or empty vector were infected with Salmonella (MOI 20) for 3 hours. Supernatants were analyzed for IL-1ß production using ELISA (mean±SD; n=3) and immunoblotting using anti-IL-1ß antibody. Cell lysates were also analyzed by immunoblotting, showing equivalent expression levels of ASC WT and ASC mutants. Cell lysates were probed for ß-actin as a loading control.
DISCUSSION
In this study we apply a combination of a fluorescence-based protein complementation assay technology and physiological readouts to depict the role of the ASC CARD in ASC-mediated inflammasome signaling pathways. We show a significant role of the ASC CARD in ASC foci formation and find that the PYD is insufficient to drive platform formation. Moreover, we show that the ASC CARD in isolation is capable of forming foci in combination with the CARD of its target Caspase-1. This unexpected involvement in foci formation may point to a general role of the Caspase-1 CARD or other death domain family proteins in augmenting signaling platform assembly in a “target feedback mechanism”. Additionally, due to their ability of solely forming foci, CARDs may also directly interact with other factors driving inflammasome formation such as scaffold proteins [14]. To our knowledge our NLRC4/ASC/Caspase-1 BiFC assay is the first system visualizing a direct interaction of ASC with NLRC4/Caspase-1 as well as its absolute requirement for foci formation.
Using the BiFC assay as a qualitative tool for visualizing foci formation, we performed an extensive mutational screen covering surface residues on both ASC and Caspase-1 CARDs and find that multiple surfaces are involved in ASC CARD-dependent foci formation, while two main surfaces are required for the CARD of Caspase-1. Subsequently, we reconstituted a macrophage cell line that is naturally devoid of ASC with full-length ASC WT or CARD mutants, and observe that the lack of foci formation of ASC CARD mutants in the BiFC system correlated with loss of IL-1β secretion. This correlation was observed for both NLRP3- and AIM2-dependent activation of Caspase-1, emphasizing a critical role of the ASC CARD in functional inflammasome formation. Finally, our system enabled us to depict and visualize the elusive function of ASC in NLRC4 inflammasome formation upon Salmonella infection. Using the CARDs of NLRC4, ASC and Caspase-1 in a three-component BiFC “bridge” assay, we show that ASC is key for productive foci formation. When tested in a physiological context, ASC mutants that fail to propagate foci formation in the BiFC system, result in a ten-fold reduction of secreted IL-1β upon Salmonella infection similar to cells lacking ASC. These findings reveal an important bridging function of ASC in the Salmonella-mediated NLRC4-Caspase-1 inflammasome.
Taken together our study not only depicts a new key role for the CARD in the formation of highly oligomeric functional ASC inflammasomes, but also sheds new light on the function of ASC in NLRC4-dependent Salmonella infection. It furthermore represents a model for the investigation of signaling platforms that rely on productive oligomerization for signal transduction. We predict that the approach used here may be applicable to investigations of other large multimeric protein complexes such as plaque-forming disorders stemming from aberrant aggregation events, as described for Alzheimer's, Huntington's, and Parkinson's disease.
Supplementary Material
ACKNOWLEDGEMENT
This study was funded by NIH grants R01AA017238 and P01 ES01673 (to S.J.R) and NIH grant AI-56324 (to J.C.R), an Erwin-Schrödinger Postdoctoral Fellowship of the Austrian Science fund (to M.P.) and a Crohn's and Colitis Foundation (CCFA) Postdoctoral Fellowship (to M.G.). We thank Dr. Gordon Mill for sharing plasmids pVNN and pVCC.
Abbreviations used
- ASC
apoptosis speck-like protein
- CARD
caspase activation and recruitment domain
- PYD
Pyrin
- NLRs
nucleotide-binding domain, leucine-rich repeat containing receptors
- AIM2
absent in melanoma 2
- BiFC
bimolecular fluorescence complementation
Footnotes
AUTHOR CONTRIBUTION
Martina Proell, Motti Gerlic, John C. Reed, and Stefan J. Riedl designed experiments, Martina Proell and Motti Gerlic performed experiments, Martina Proell, Motti Gerlic, John C. Reed, Peter D. Mace, and Stefan J. Riedl analyzed data, Martina Proell, John C. Reed, and Stefan J. Riedl wrote the paper.
REFERENCES
- 1.Kersse K, Bertrand MJ, Lamkanfi M, Vandenabeele P. NOD-like receptors and the innate immune system: coping with danger, damage and death. Cytokine Growth Factor Rev. 2011;22:257–276. doi: 10.1016/j.cytogfr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13:325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nunez G, Cho JH. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 4.Miceli-Richard C, Lesage S, Rybojad M, Prieur AM, Manouvrier-Hanu S, Hafner R, Chamaillard M, Zouali H, Thomas G, Hugot JP. CARD15 mutations in Blau syndrome. Nat Genet. 2001;29:19–20. doi: 10.1038/ng720. [DOI] [PubMed] [Google Scholar]
- 5.D'Osualdo A, Reed JC. NLRP1, a regulator of innate immunity associated with vitiligo. Pigment Cell Melanoma Res. 2012;25:5–8. doi: 10.1111/j.1755-148X.2011.00942.x. [DOI] [PubMed] [Google Scholar]
- 6.Jin Y, Mailloux CM, Gowan K, Riccardi SL, LaBerge G, Bennett DC, Fain PR, Spritz RA. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med. 2007;356:1216–1225. doi: 10.1056/NEJMoa061592. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aksentijevich I, Nowak M, Mallah M, Chae JJ, Watford WT, Hofmann SR, Stein L, Russo R, Goldsmith D, Dent P, Rosenberg HF, Austin F, Remmers EF, Balow JE, Jr., Rosenzweig S, Komarow H, Shoham NG, Wood G, Jones J, Mangra N, Carrero H, Adams BS, Moore TL, Schikler K, Hoffman H, Lovell DJ, Lipnick R, Barron K, O'Shea JJ, Kastner DL, Goldbach-Mansky R. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 2002;46:3340–3348. doi: 10.1002/art.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. Nod-like proteins in immunity, inflammation and disease. Nat Immunol. 2006;7:1250–1257. doi: 10.1038/ni1412. [DOI] [PubMed] [Google Scholar]
- 10.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 11.Case CL, Roy CR. Asc modulates the function of NLRC4 in response to infection of macrophages by Legionella pneumophila. MBio. 2011;2 doi: 10.1128/mBio.00117-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shenoy AR, Wellington DA, Kumar P, Kassa H, Booth CJ, Cresswell P, MacMicking JD. GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science. 2012;336:481–485. doi: 10.1126/science.1217141. [DOI] [PubMed] [Google Scholar]
- 13.Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, Rosenberg S, Zhang J, Alnemri ES. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14:1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng J, Waite AL, Tkaczyk ER, Ke K, Richards N, Hunt AJ, Gumucio DL. Kinetic properties of ASC protein aggregation in epithelial cells. J Cell Physiol. 2010;222:738–747. doi: 10.1002/jcp.22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broz P, Newton K, Lamkanfi M, Mariathasan S, Dixit VM, Monack DM. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J Exp Med. 2010;207:1745–1755. doi: 10.1084/jem.20100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moriya M, Taniguchi S, Wu P, Liepinsh E, Otting G, Sagara J. Role of charged and hydrophobic residues in the oligomerization of the PYRIN domain of ASC. Biochemistry. 2005;44:575–583. doi: 10.1021/bi048374i. [DOI] [PubMed] [Google Scholar]
- 17.Poyet JL, Srinivasula SM, Tnani M, Razmara M, Fernandes-Alnemri T, Alnemri ES. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J Biol Chem. 2001;276:28309–28313. doi: 10.1074/jbc.C100250200. [DOI] [PubMed] [Google Scholar]
- 18.Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shyu YJ, Liu H, Deng X, Hu CD. Identification of new fluorescent protein fragments for bimolecular fluorescence complementation analysis under physiological conditions. Biotechniques. 2006;40:61–66. doi: 10.2144/000112036. [DOI] [PubMed] [Google Scholar]
- 20.Sali A, Potterton L, Yuan F, van Vlijmen H, Karplus M. Evaluation of comparative protein modeling by MODELLER. Proteins. 1995;23:318–326. doi: 10.1002/prot.340230306. [DOI] [PubMed] [Google Scholar]
- 21.Fernandes-Alnemri T, Alnemri ES. Assembly, purification, and assay of the activity of the ASC pyroptosome. Methods Enzymol. 2008;442:251–270. doi: 10.1016/S0076-6879(08)01413-4. [DOI] [PubMed] [Google Scholar]
- 22.de Alba E. Structure and interdomain dynamics of apoptosis-associated speck-like protein containing a CARD (ASC). J Biol Chem. 2009;284:32932–32941. doi: 10.1074/jbc.M109.024273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Humke EW, Shriver SK, Starovasnik MA, Fairbrother WJ, Dixit VM. ICEBERG: a novel inhibitor of interleukin-1beta generation. Cell. 2000;103:99–111. doi: 10.1016/s0092-8674(00)00108-2. [DOI] [PubMed] [Google Scholar]
- 24.Kersse K, Lamkanfi M, Bertrand MJ, Vanden Berghe T, Vandenabeele P. Interaction patches of procaspase-1 caspase recruitment domains (CARDs) are differently involved in procaspase-1 activation and receptor-interacting protein 2 (RIP2)-dependent nuclear factor kappaB signaling. J Biol Chem. 2011;286:35874–35882. doi: 10.1074/jbc.M111.242321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 26.Martinon F, Agostini L, Meylan E, Tschopp J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol. 2004;14:1929–1934. doi: 10.1016/j.cub.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 27.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 28.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, Superti-Furga G. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 30.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koonin EV, Aravind L. The NACHT family - a new group of predicted NTPases implicated in apoptosis and MHC transcription activation. Trends Biochem Sci. 2000;25:223–224. doi: 10.1016/s0968-0004(00)01577-2. [DOI] [PubMed] [Google Scholar]
- 32.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, Grant EP, Nunez G. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 33.Amer A, Franchi L, Kanneganti TD, Body-Malapel M, Ozoren N, Brady G, Meshinchi S, Jagirdar R, Gewirtz A, Akira S, Nunez G. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem. 2006;281:35217–35223. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.