Abstract
Major histocompatibility complex (MHC) class I and II are glycoproteins that can present antigenic peptides at the cell surface for recognition and activation of circulating T lymphocytes. Here, the importance of the modification of protein antigens by glycans on cellular uptake, proteolytic processing, presentation by MHC and subsequent T-cell priming is reviewed. Antigen glycosylation is important for a number of diseases and vaccine design. All of the key proteins involved in antigen recognition and the orchestration of downstream effector functions are glycosylated. The influence of protein glycosylation on immune function and disease is covered.
Most eukaryotic cell surface and secreted proteins are modified by covalently linked glycans, which are essential mediators of biological processes such as protein folding, cell signaling, fertilization and embryogenesis as well as the proliferation of cells and their organization into specific tissues1-9. Overwhelming data support the relevance of glycosylation in pathogen recognition, inflammation, innate immune responses and cancer10-14. The importance of protein glycosylation is also underscored by the developmental abnormalities observed in a growing number of human disorders, known as congenital disorders of glycosylation, caused by defects in the glycosylation machinery15.
During the past decade, it has become evident that glycosylation of protein antigens can greatly influence adaptive immune responses16-19. Antigen glycosylation has been implicated in disease, and several studies have linked immune recognition of glycosylated peptides to autoimmunity20,21. Furthermore, all of the key proteins involved in antigen recognition and the orchestration of downstream effector functions are glycosylated22,23. Changes in glycosylation of these proteins occur during differentiation, immune activation and apoptosis. These alterations have been linked to homeostatic and disease mechanisms including immune-cell trafficking and differentiation, antigen and cytokine receptor activation, autoimmunity and the induction of leukocyte apoptosis. Here, the influence of glycosylation of antigens on cellular uptake, processing, presentation by MHC and subsequent T-cell priming is reviewed. Furthermore, the importance of appropriate glycosylation of proteins involved in immune activation is described.
Protein glycosylation
Almost all of the naturally occurring protein glycosylations can be classified as either N-linked glycosides, in which N-acetylglucosamine is linked to the amide side chain of an asparagine, or as O-glycosides, in which a saccharide is linked to the hydroxyl of serine, threonine or tyrosine (Fig. 1a)24. Usually, O-glycosides are initiated by N-acetylgalactosamine, but O-mannosylation and fucoyslation of these amino acids have also been observed. Recently, several unusual types of glycoproteins have been identified, such as O-glycosides of hydroxylysine and hydroxyproline and the C-glycosides of tryptophan24. Glycosylation was previously considered to occur only in eukaryotes, but through advances in analytical methods and genome sequencing, it is now clear that bacteria can express O- and N-linked glycoproteins25,26. Furthermore, enveloped viruses use the host glycosylation machinery to produce glycoproteins, which may provide opportunities for immune detection or evasion27.
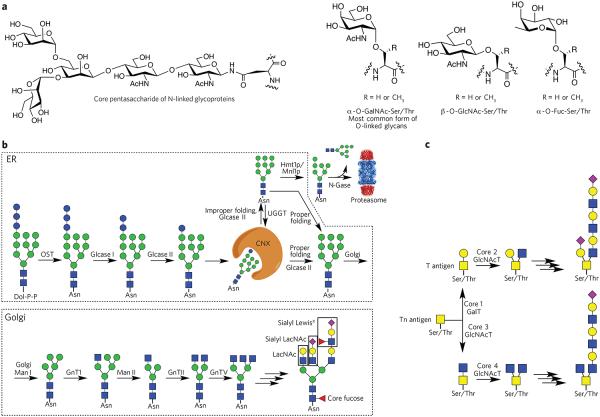
Figure 1. Various classes of glycans and their biosynthesis.
(a) Common types of protein glycosylation: core pentasaccharide common to all N-linked glycans and various types of O-glycosylation. (b) Biosynthesis of N-glycans. The biosynthesis of N-glycans is initiated in the ER by the transfer of a dolichol (Dol-P-P)-linked glycan to an asparagine moiety of an Asn-Xaa-Ser/Thr sequon of a polypeptide. In the ER, protein glycosylation functions in the quality control of protein biosynthesis. Calnexin (CNX) is a chaperone that keeps the unfolded protein in the ER. Improperly folded glycans can encounter the α-mannosidase MNS1, which removes a terminal mannoside, providing a glycoform that will be transported to the cytosol. Here, an N-glycanase (N-Gase) removes the N-linked glycans, and the resulting protein is then imported into the proteasome for proteolysis. Properly folded proteins are transported to the Golgi, where an array of glycosyltransferases diversifies the various antennae of the glycans to give an array of complex structures. The resulting complex-type glycoproteins are transported to the cell surface or secreted. OST, oligosaccharyltransferase; glcase, α-glucosidase; UGGT, UDP–glucose-glycoprotein glucosyltransferase; Hmt1p, HnRNP methyltransferase 1; Mnl1p, mannosidase-like protein 1; N-gase, N-glucosidase; Man, α-mannosidase; GnT, N-acetyl glucosaminyltransferase. (c) Biosynthesis of O-glycans. The biosynthesis of O-glycans that start the structure with an O-GalNAc moiety begins in the Golgi, where various polypeptide polypeptide GalNAc transferases (GlcNAcT) attach a GalNAc residue. The resulting glycoprotein can be converted into various core structures that can be diversified by a range of glycosyltransferases.
The biosynthesis of N-linked oligosaccharides occurs in the endoplasmic reticulum (ER) and Golgi complex. In the ER, a dolichol-linked Glc3Man9GlcNAc2 oligosaccharide precursor is biosynthesized and transferred en bloc to an Asn-Xaa-Ser/Thr sequon on newly synthesized polypeptides through the action of the multisubunit oligosaccharide transferase complex (Fig. 1b)28-31. Subsequent trimming and processing of the transferred oligosaccharide result in a GlcNAcMan3GlcNAc2 structure that is transported to the medial stacks of the Golgi complex, where maturation of the oligosaccharide gives rise to extreme structural diversity32-34. In the early secretory pathway, the glycans have a common role in the promotion of protein folding, quality control and certain sorting events. This is in contrast to their roles in the Golgi complex, where they are modified to perform the functions displayed by the mature glycoproteins.
The biosynthesis of O-glycans occurs in the Golgi apparatus, where the GalNAc moiety of UDP-GalNAc is transferred to the hydroxyl of serine or threonine catalyzed by polypeptide GalNAc transferase (Fig. 1c)35. In contrast to N-glycosylation, a consensus sequence for α-d-GalNAc addition has not been found, although predictive algorithms do exist. Many O-glycans are extended into long biantennary oligosaccharide chains with variable termini that may be similar in structure to those of N-linked glycoproteins. In addition, a highly dynamic type of O-glycosylation at serine and threonine exists in which nuclear and cytoplasmic proteins are modified by a single β-N-acetyl-D-glucosamine (O-GlcNAc) moiety through the action of the cycling enzymes, O-linked GlcNAc transferase and O-GlcNAcase9. Modifications of proteins by O-GlcNAc function as nutrient and stress sensors, and abnormalities in the modification process underlie insulin resistance and glucose toxicity in diabetes, neurodegenerative disorders and dysregulation of tumor suppressors and oncogenic proteins in cancer.
The repertoire of glycan structures biosynthesized by a given cell is largely based on the expression of a subset of more than 200 glycosidases and glycosyl transferases found in the mammalian genome. Glycosyltransferases transfer a monosaccharide from an activated sugar nucleotide to a specific hydroxyl of a growing oligosaccharide chain. The resulting product can then act as an acceptor for another glycosyltransferase, thereby generating an array of glycans36. Glycan biosynthesis is not template-driven like the biosynthesis of polypeptide structures from genome-derived transcripts. The generation of glycan diversity is controlled by factors such as enzyme abundance and specificity, abundance of the respective protein or lipid acceptor substrates, accessibility to glycan modification sites, availability of activated sugar donor substrates and relative levels and locations of other biosynthetic enzymes in the secretory pathway that compete for the same glycan substrates37,38. The total number of distinct glycan structures in the human glycome is not known; however, it has been estimated that approximately 7,000 pentasaccharide sequences exist that represent minimal epitopes for binding to glycan-binding proteins36. Glycans are usually much larger than a pentasaccharide, and thus the total number of structures is probably orders of magnitude larger. Glycosylation can alter the structure and function of proteins by steric influences or by mediating interactions with glycan-binding proteins. Changes in the glycome can occur in response to environmental and genetic stimuli and are often associated with the acquisition of altered cellular phenotypes.
Antigen presentation and recognition by T cells
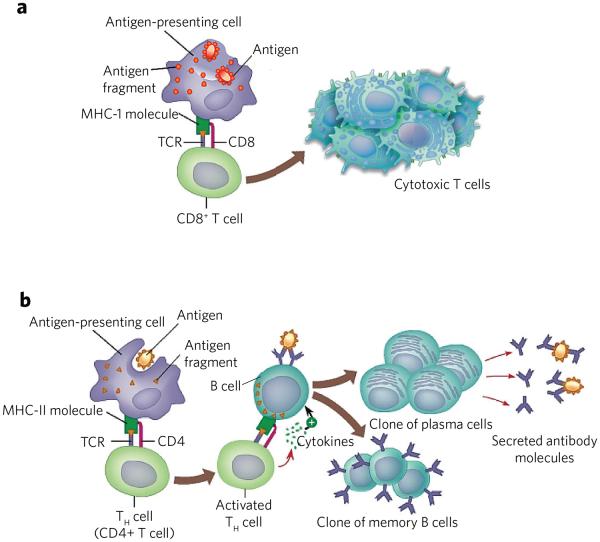
The adaptive immune system, also known as the acquired immune system, relies on the ability to distinguish ‘self’ antigens from pathogen-derived antigens. The MHC is a family of structurally and genetically related glycoproteins that serves a key role in this process by controlling T-cell activation (Fig. 2)39,40. To fulfill their function, MHC proteins must acquire peptide antigen, which is performed by the two main classes of MHC glycoproteins. MHC class I (MHC-I), which is expressed by all nucleated cells, reports on intracellular events such as viral infection, the presence of intracellular bacteria and cellular transformations. In this case, ubiquitinated proteins are degraded in the cytosol by the proteasome followed by further trimming by cytosolic peptidases41. Specific eight- to ten-residue-long peptides are then transported to the lumen of the ER for loading on newly synthesized MHC-I. This complex is transported to the cell surface, where it can interact with an appropriate T-cell receptor (TCR) on the cell surface of a CD8+ T cell. This interaction leads to activation and proliferation of the T cell, leading to the production of mature cytotoxic T cells. These cells can kill infected cells that also present the corresponding peptides on their cell surface in complex with MHC-I.
Figure 2. T-cell activation by endogenous and exogenous antigens.
(a) MHC-I presents peptides derived from endogenous antigens. Antigen is proteolyzed by the immune proteasome and specific peptides loaded to MHC-I. The resulting MHC-I–peptide complex is transported to the cell surface, where it can be recognized by the TCR of CD8+ cells. The resulting CD8+ cells proliferate to give cytotoxic T cells. These cells can kill other cells that present the same antigen in complex with MHC-I. (b) Endocytosis of antigen by antigen-presenting cells followed by proteolysis by lysosomes provides peptides that can be loaded on MHC-II. The resulting complex is transported to the cell surface where it can activate CD4+ cells. The resulting cells can activate B cells that present the same antigenic peptide in complex with MHC-II. The activated B cell differentiates into plasma cells that secrete antibodies and memory B cells.
MHC class II (MHC-II) draws on a different pool of peptides that are generated from exogenous proteins42. The molecular expression of MHC-II molecules is restricted to professional antigen-presenting cells, such as dendritic cells (DCs), macrophages and B cells. In particular, immature DCs reside in tissues such as skin and lungs and in the gastrointestinal tract, where they survey for the presence of pathogens or other foreign entities. These antigens are internalized via endo- or pinocytosis, and, after reaching endosomal or lysosomal compartments, they are processed by resident proteases, and specific 10- to 25-residue-long peptides are loaded onto MHC-II (Fig. 2). Export of the resulting complex to the cell surface allows interactions with TCRs of CD4+ T cells to initiate effector and regulatory functions. In particular, the activated helper T (TH) cells can make a similar interaction with B cells43, thereby instructing them to produce IgG antibodies. A link, termed cross-presentation, exists between the MHC-I and MHC-II pathways, whereby exogenously derived antigens are presented by MHC-I molecules44. Furthermore, peptides derived from endogenous cytosolic proteins can be presented by MHC-II when these proteins are degraded through autophagy or other pathways.
Glycosylation can have a major influence on protein antigen uptake and proteolytic processing, which in turn can affect MHC presentation and subsequent immune responses. Also, it has become apparent that MHC-I and MHC-II can present glycopeptides and that such post-translational modifications can be important for T-cell recognition. The next section highlights examples of the influence of glycosylation on antigen presentation, and its relevance to vaccine design is also discussed.
C-type lectin receptors: immune cell activation and antigen uptake
DCs have a key role in immune surveillance by monitoring the extracellular space for foreign proteins and report by presenting specific peptides in complex with MHC-I and MHC-II to T cells. Dendritic and other myeloid cells express C-type lectin receptors (CLRs) such as DC-SIGN, DEC-205, langerin and dectin-1. This family of proteins contains a highly conserved structural motif (C-type lectin domain) arranged as two protein loops stabilized by two disulfide bridges at the base of each loop. One of these loops is more flexible and generally contains the glycan-binding site45. Ca2+-dependent glycan binding is a common feature of these proteins, giving the name to the family of proteins.
Several CLRs of immune cells preferentially bind microbial glycans, whereas others respond to altered self ligands. Another group of CLRs exhibit binding to microbial as well as to self glycans. Glycan recognition by CLRs can result in cell-signaling events that modulate immune cell responses46,47. For example, several CLRs can recruit the tyrosine kinase Syk, which coordinates many downstream signaling pathways that lead to myeloid cell activation. Other CLRs can recruit phosphatases that negatively regulate signaling through kinase-associated receptors. These CLRs generally modulate myeloid cell activation when they are triggered together with activating receptors such as Toll-like receptors.
CLRs also function as endocytosis receptors, thereby promoting internalization of antigens that leads to their degradation as well as to antigen retrieval and presentation to T cells48. For example, DC-SIGN is a type II transmembrane CLR that contains a carbohydrate recognition domain that can bind glycans containing high-mannose glycans and Lewis-type antigens, which are expressed not only by a wide range of viral, bacterial, fungal and parasitic pathogens but also by host glycoproteins49. Upon ligand binding, DC-SIGN rapidly internalizes and directs its cargo into the lysosomal pathway, which can result in the presentation of specific peptides in complex with MHC-II. Antigen uptake by DC-SIGN can also lead to the priming of CD8+ T cells, and thus an endocytic route should exist that leads to cross-presentation. DC-SIGN forms tetramers that are present in nanodomains on the cell surface of DCs50. This arrangement assures that DC-SIGN exhibits high avidity for multivalent displays of appropriate glycans. It has been proposed that discrimination between host and foreign glycans is based on a density-dependent recognition mechanism51. In particular, pathogens appear to express relevant epitopes in high density, such as in polysaccharides and lipopolysaccharides, resulting in selective cellular uptake.
The multivalent recognition of glycans by DC-SIGN and other glycan-binding proteins of DCs has been exploited for the design of improved vaccines. For example, it has been shown that a dendrimer carrying 32 antigenic peptides and Lewisb epitopes (Fig. 3), the latter being a ligand for DC-SIGN, displays optimal internalization, routing to lysosomal compartments, cytokine response and antigen presentation52,53. In another approach, laminarin, which is a β-glucan ligand of dectin-1, and a synthetic β-mannan derived from Candida albicans54 were individually conjugated to tetanus toxoid to provide a tricomponent conjugate vaccine55. A macrophage cell line expressing dectin-1 readily internalized the conjugate. Furthermore, treatment of immature bone marrow–derived DCs with the tricomponent or control vaccine confirmed that the β-glucan–containing vaccine exerted its enhanced activity by virtue of DC targeting and uptake. Immunization of mice with the vaccine candidate resulted in improved antigenic responses, as manifested by high titers of antibodies recognizing C. albicans β-mannan.
Figure 3. Multivalent presentation of DC-SIGN ligands for enhanced cellular uptake of antigen.
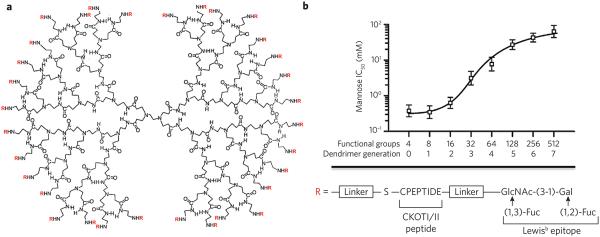
(a) Glycopeptide dendrimers were synthesized by attaching a glycopeptide to the primary amines of PAMAM dendrimers by functionalization with a maleimide-containing linker followed by reaction with the thiol of the C-terminal cysteine of the CKOTI/II peptide, which was further extended by a Lewisb epitope. The structure of a third-generation dendrimer is shown. (b) Cellular binding of glycopeptide dendrimers. The glycopeptide dendrimers were labeled with a fluorophore and incubated with mutant DC-SIGN–expressing cells in the presence of increasing concentrations of mannose as a binding inhibitor. The resulting half-maximum inhibitory concentration (IC50) values are plotted against the number of functional groups attached to the dendrimer. Reprinted with permission from reference 52.
Glycosylation and antigen processing
Protein glycosylation can influence proteolytic processing of protein antigens by sterically blocking the action of proteases56. For example, the gp120 subunit, which is an HIV envelope glycoprotein involved in host cell binding, is a heavily glycosylated protein expressing ~25 N-linked glycans per protein molecule. Several studies57,58 have shown that N-glycans on gp120 can facilitate viral escape from the host immune system by constraining proteolytic processing of the protein antigen required for antigen presentation and cytotoxic T-cell priming. The N-glycans can also block access of neutralizing antibodies to critical epitopes.
An elegant study by Hanisch and co-workers59 has shown that O-glycosylation can influence proteolytic processing by the immunoproteasome. They performed an in vitro study in which a range of synthetic glycopeptides derived from the mucin MUC1 were exposed to immune proteasomes, and then the products were analyzed by HPLC and MS. It was found that O-glycosylated MUC1 glycopeptides that carried GalNAc or Gal-GalNAc moieties could be proteolytically processed. However, the efficacy and site specificity of proteolysis was greatly influenced by the length of the glycopeptide, abundance and position of glycosylation sites, glycosylation type and amino acid sequence. These findings have important implications for vaccine design. For example, glycosylation of amino acids near proteolytic cleavage sites can block proteolysis, which in turn prevents the formation of peptides or glycopeptides for presentation to MHC1 or MHCII.
Glycopeptides and antigen presentation and T-cell priming
Several studies have shown that N- and O-linked glycosides can survive lysosomal degradation and that the formed glycopeptides can be presented in complex with MHC-II18,21,60. O-linked glycans can also remain intact during processing by the immune proteasomes, and resulting glycopeptides can be complexed by MHC-I18,21,60. N-glycosylated peptides have not been found in complex with MHC-I, and, although controversial, it has been proposed that N-glycans need to be removed by a cytosolic peptide-N-glycanase before proteins can enter the cylinder-shaped proteasome60,61. N-glycosylation can, however, influence MHC-I peptide presentation because removal of N-glycans results in the conversion of glycosylated asparagine to aspartic acid and thus leads to the presentation of an epitope with a different amino acid62.
During the past decade, it has become clear that a T-cell repertoire exists that specifically can recognize glycopeptides complexed with MHC-I and MHC-II63. In an early study, MHC-II–restricted T-cell hybridomas were raised against a synthetic glycopeptide derived from mouse hemoglobin that was known to strongly bind MHC-II E(k)64. The specificity of the T-cell hybridomas for the glycan moiety was investigated by examining their responses toward a panel of glycopeptides carrying different glycans. It was found that the hybridomas displayed high specificity for the α-GalNAc moiety, and only a few of the T-cell clones showed weak cross-reactivity with glycopeptides having a different glycan moiety, even though some of these were structurally very similar to α-GalNAc. Subsequently, MHC-II–associated glycopeptide epitopes were implicated in inflammatory autoimmune diseases such as rheumatoid arthritis and lupus erythematosus20.
Rheumatoid arthritis, which is an autoimmune disease that causes chronic inflammation of peripheral joints and subsequent destruction of cartilage and bone, has been genetically linked to the MHC-II proteins DR1 and DR4. There is evidence to support that these MHC-II molecules can present glycopeptides derived from type II collagen (CII) to circulating CD4+ TH cells, leading to the initiation of autoimmune responses. CII is a cartilage-specific homotrimeric fibrillar protein that contains a large number of lysine residues that are often hydroxylated and subsequently glycosylated with a β-D-galactopyranosyl or an α-D-glucopyranosyl-(1→2)-β-D-galactopyranosyl moiety. By employing a panel of synthetic glycopeptides, it was found that most T-cell hybridomas obtained in collagen-induced arthritis specifically recognize a β-D-galactopyranoside moiety located at Hyl264 of the peptide sequence CII256–270 (refs. 65,66). Further studies showed that the axial hydroxyl group of the galactoside was critical for T-cell activation. Vaccination with a collagen type II glycopeptide in complex with MHC-II prevented the development of collagen-induced arthritis in mice and improved chronic relapsing disease67.
In several studies, peptides have been employed that are known binders of MHC class I and modified with relatively short oligosaccharides18,56. Examination of binding to MHC-I and induction of the T-cell immune response showed that glycans are tolerated only at amino acid positions that are not critical for anchoring the peptide to MHC-I. In many cases, immunizations of mice with glycosylated peptides, known to bind MHC-I, activated T cells that required the peptide and glycan for recognition. Support for direct recognition of glycans by TCRs of CD8+ cells came from crystal structures of MHC-I (H-2Db) complexed with glycopeptides derived from an immunodominant epitope of the Sendai virus nucleoprotein68. The peptide carried a GlcNAcβ1-O-Ser moiety at the fourth or fifth amino acid. The crystal structure of the complex showed that the glycans of the glycopeptides were solvent exposed and available for direct binding to TCRs. Modeling of the ternary complex of MHC, glycopeptide and TCR indicated that the monosaccharide residue can be accommodated by the TCR. The models also suggested the possible molecular basis for the observed cross-reactivity pattern of the cytotoxic T lymphocyte (CTL) clones, which appeared to be influenced by the length of the CDR3 loop and the nature of the immunizing ligand.
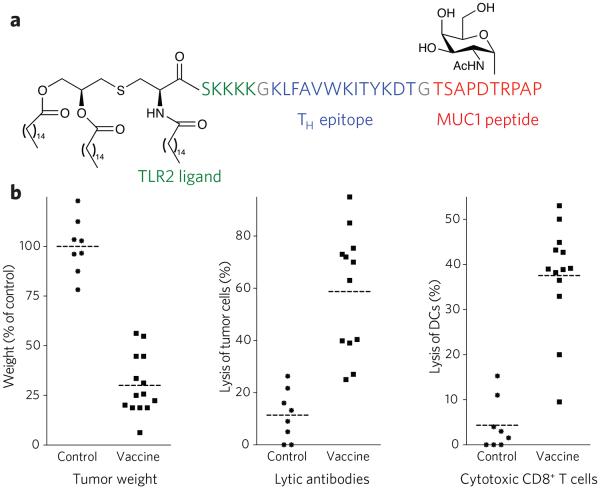
Appropriate glycosylation of MHC-I epitopes is relevant for the design of cancer vaccines56. In this respect, the mucin MUC1 is often aberrantly glycosylated by epithelial cancer cells manifested by truncated O-linked saccharides such as Tn (αGalNAc-Thr), STn (αNeu5Ac-(2,6)-αGalNAc-Thr) and Thomsen-Friedenreich antigen (βGal-(1,3)-αGalNAc-Thr)69. CTLs isolated from patients with breast carcinoma can recognize epitopes derived from the MUC1 tandem repeat peptide70. It has been proposed that tumor cells present epitopes derived from the core domain of MUC1 in their truncated glycosylation state in complex with MHC-I molecules, leading to natural MHC-restricted recognition of ‘hypoglycosylated’ epitopes59,71-73. Early efforts to develop MUC1-based cancer vaccines focused on the use of unglycosylated MUC1 tandem repeat peptides of different lengths, conjugated to different carriers and/or administered with an adjuvant74,75. In general, these strategies have failed to elicit effective immune responses to MUC1-expressing cancer cells, probably owing to the conformational disparities between nonglycosylated vaccine sequences and tumor-expressed, aberrantly glycosylated MUC1 (refs. 76-78). Immunizations with densely glycosylated MUC1 peptides have also been ineffective owing to impaired susceptibility to antigen processing. We have identified the minimum requirements to consistently induce CTLs and cytotoxic antibodies specific for the tumor form of MUC1, resulting in a therapeutic response in a mouse model of mammary cancer. The vaccine is composed of the immunoadjuvant Pam3CysSK4, a peptide TH epitope and an aberrantly glycosylated MUC1 peptide (Fig. 4)79. The vaccine elicited cytotoxic T cells that recognized both glycosylated and unglycosylated peptides, whereas a similar nonglycosylated vaccine gave cytotoxic T cells that recognized only nonglycosylated peptide. Antibodies elicited by the glycosylated tripartite vaccine were significantly more lytic compared to the unglycosylated control. As a result, immunization with the glycosylated tripartite vaccine was superior in tumor prevention. Other efforts have to develop MUC1-based glycopeptide vaccines been reported80.
Figure 4. Properties of the three-component cancer vaccine.
(a) Chemical structure of synthetic vaccine. (b) Left, glycosylated multi-component vaccine reduces MMT tumor burden in MUC1.Tg mice. MUC1.Tg mice were immunized with empty liposomes (EL) as control or with liposomes containing the vaccine. Three biweekly immunizations were given before a tumor challenge with MUC1-expressing MMT tumor cells followed by one boost one week after. Middle, induction of antibody-dependent cell-mediated cytotoxicity (ADCC). Right, induction of CD8+ cytolytic T cells in MUC1.Tg mice. Reprinted with permission from reference 79. Each data point represents an individual mouse, and the horizontal lines indicate the mean for the group of mice.
Glycosylation of protein antigen is expected to be relevant for the development of many types of vaccines. A major stumbling block in the design of such vaccines is a lack of technology to determine protein glycosylation sites81. In general, the discovery of glycosylation sites has been performed on isolated proteins. A recently developed method is beginning to address the deficiencies of analyzing complex mixtures of glycoproteins82. In this approach, zinc finger nuclease–based gene targeting was applied to mutate the gene for the chaperone Cosmc, which is essential for translocation of the glycosyl transferase C1GalT1 to the Golgi apparatus. This enzyme is the only one that extends core-1–type O-glycans (Fig. 1c), and, as a result, only simple GalNAc-O-Ser/Thr or NeuAc(2-6)GalNAc-O-Ser/Thr glycoforms will be biosynthesized. The identity of proteins and the localization of glycosylation sites were determined by proteolysis of glycoproteins and treatment with a sialidase, followed by the isolation of O-GalNAc–modified peptides using nanoflow lectin chromatography combined with MS detection by electron transfer dissociation fragmentation. This approach led to the identification of well over 100 O-glycoproteins with more than 350 O-glycan sites.
T-cell priming and glycoconjugate vaccine development
It has long been thought that T cells can only recognize peptides presented in complex with MHC-II that are modified by relatively simple glycans. Recent studies have, however, shown that a CD4+ T-cell population exists, designated as Tcarbs, which can recognize polysaccharides83. It appears that priming of such T cells is relevant for eliciting optimal immune responses against glycoconjugates vaccines.
In general, bacterial polysaccharides cannot activate T cells and therefore elicit only IgM antibodies and do not induce memory responses. Conjugation of a polysaccharide to a foreign carrier protein such as tetanus toxoid, CRM197 or KLH will provide a source of peptides that can be presented in the context of MHC-II, thereby overcoming the T cell–independent properties of polysaccharides84. Therefore, glycoconjugate vaccines can readily induce a class switch to the superior IgG antibodies and immunological memory (Fig. 2). These vaccines are among the safest and most efficacious vaccines developed to date and are widely used for the prevention of life-threatening bacterial infections such as meningitis and pneumonia.
Until recently, little was known about the fate of the polysaccharide conjugated to a carrier protein during antigen processing and presentation by APCs. Studies employing fluorescently labeled pneumococcal polysaccharides conjugated to CRM197 labeled with another dye indicated that the polysaccharide component of the glycoconjugate can enter endosomes, travel with peptides to surface of an APC and co-localize with MHC-II85. Further studies by Kasper and co-workers83 have demonstrated that endolysosomal processing by APCs of group B streptococcal type III polysaccharides coupled to a carrier protein results in peptides modified by the polysaccharide. MHC-II can present these glycopeptides, and the resulting complex can activate CD4+ T cells. Specifically, it was shown that the glycoconjugates can stimulate carbohydrate-specific CD4+ T-cell clones to produce interleukins 2 and 4, which are cytokines that provide T-cell help to antibody-producing B cells. A glycoconjugate vaccine was designed to maximize the presentation of carbohydrate-specific T-cell epitopes, which was 50–100 times more potent and substantially more protective in a neonatal mouse model of group B Streptococcus infection than a vaccine constructed by a conventional conjugation method.
Glycosylation and immune cell receptor function
All of the key proteins involved in antigen recognition and the orchestration of effector functions including MHC class I and II are glycosylated86. A diverse range of glycan-binding proteins, such as galectins (sialic acid–binding Ig-like lectins, also known as Siglecs) and CLRs, can interact with these glycoproteins, thereby modulating cell-cell interactions and cell signaling events48. It is becoming increasingly apparent that the regulation of glycan structures, the expression of glycan-binding proteins by immune cells and their complex interplay is crucial to proper functioning of immune system events, including antigen presentation and T-cell activation. A detailed understanding of these interactions and their downstream effector functions is providing unique opportunities for the design of peptide or glycopeptide-specific immunotherapies for the treatment of diseases such as allergy and autoimmunity.
The best-studied involvement of cell-surface glycans in modulating immune responses involves selectins, which are surface-localized CLRs that mediate leukocyte rolling, ultimately resulting in extravasation into inflamed or infected tissues87. Thus, the regulation of glycan structures on endothelial and leukocyte surfaces during an inflammatory response is a key factor in the recruitment and homing of immune cells.
Galectins are an ancient family of proteins that preferentially bind N-acetyllactosamine sequences (Galβ(1,4)GlcNAc) of O- and N-linked glycoproteins88,89. They typically act as cell surface scaffolding proteins to organize specific cell surface glycoproteins into lattices. The resulting galectin-glycoprotein lattices can modulate intracellular signaling events and regulate cellular processes such as apoptosis, proliferation and migration90. Galectins have been implicated in a wide range of immune responses and can provide positive as well as negative regulatory signals. For example, the binding of galectin-1 to developing thymocytes influences the strength of TCR signaling, which is an important determinant for proper recognition of antigen by a T cell91. Galectin-1 can also induce apoptosis of specific thymocyte subsets and activated T cells92. As expected, galectin-1 knockout mice exhibit aberrant thymocyte selection, resulting in alteration in the T-cell repertoire and the responses of the mature T cell.
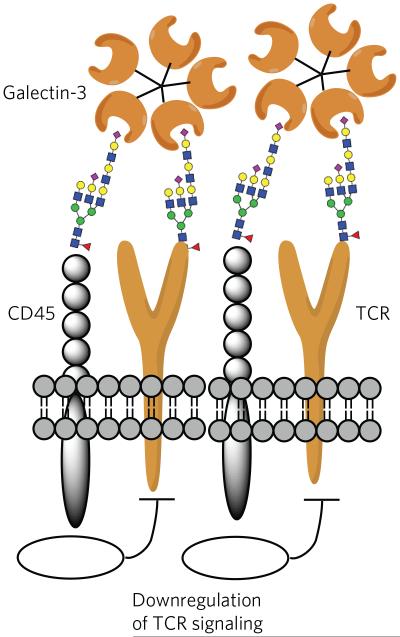
Galectin-3, which is absent in resting CD4+ and CD8+ T cells but inducible by various stimuli, such as TCR ligation, can also induce suppressive responses93. In this respect, each subunit of αβTCRs contains multiple acceptor sites for N-linked glycans. It is thought that the N-glycans do not directly influence MHC interactions but influence the threshold of immune activation. For example, a deficiency in the β(1,6)-N-acetylglucosaminyltransferase V (Mgat5) alters the branching pattern of N-glycans, which in turn leads to a lower activation threshold of T cells in vitro and increases the incidence of autoimmunity in vivo94. These effects are a result of a lack of galectin-binding sites on αβTCR, and therefore no protein complexes can be formed with CD45, which, if appropriately glycosylated, can also be recruited by galectin-3 (ref. 95). The cytoplasmic tail of CD45 contains phosphatase activity that maintains Lck, which is a critical signaling molecule for initiating the signaling cascade, leading to T-cell activation from a dephosphorylated and inactive state (Fig. 5). Thus, galectin-3 is responsible for holding CD45 and the TCR signaling complex in close proximity via their glycans, thereby preventing low-avidity T-cell activation. Galectin-1 also binds CD45 but organizes it differently on the cell surface. The differential effects of galectin-1 and galectin-3 on CD45 binding may be due to their structural differences; the former is a rigid homodimer, whereas the latter forms pentamers upon glycoprotein binding through its flexible N-terminal domain96. Although galectins often act as suppressors of inflammation and T-cell responses, some members can induce activation signals; for example, binding of galectin-8 to T cells promotes T-cell proliferation, possibly through unique interactions with CD45 (ref. 97).
Figure 5. Galectins can reorganize cell surface receptors by binding their glycans.
Galectin-3 can organize the TCR and CD45 of T cells into lattices by binding their glycans. In this way, galectin-3 is proposed to block direct interactions of TCRs. Furthermore, the phosphatase activity of CD45 causes downregulation of T-cell signaling.
With the exception of resting T cells, most human and mouse immune cells express at least one Siglec12. Some are expressed in a cell type–restricted manner, whereas others are found on multiple cell types. Furthermore, each Siglec has a unique specificity for a sialylated glycan, supporting the notion that each protein mediates a distinct or partially overlapping function. CD22 and most CD33-related Siglecs have one or more cytosolic immune-receptor tyrosine-based inhibitory motifs that can modulate cell signaling events.
CD22, which recognizes α(2,6)-linked sialylated glycans, is a B-cell co-receptor that can attenuate BCR signaling98. Such signals are critical for the prevention of autoimmunity. For example, CD22-deficient mice exhibit hyperimmune responses in vitro and in vivo99. In contrast, ST6Gal1–deficient mice, which cannot make CD22 ligands, exhibit hypoimmune responses100. Immune activation to foreign antigens leads to the crosslinking of BCR and exclusion of CD22 from the BCR signalosome. This leads to robust cell signaling, culminating in robust downstream positive signals for B-cell activation. In contrast, self antigens that carry a CD22 ligand recruit CD22 to the signalosome, which provides negative cell signals that lead to the suppression of B-cell activation. Thus, the latter condition has been proposed to induce tolerance.
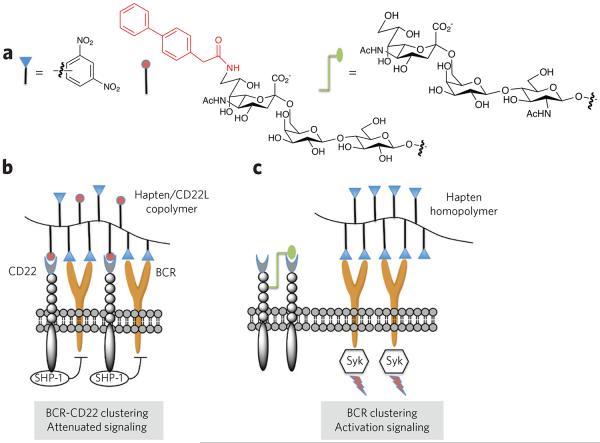
The proposed model for the CD22-mediated attenuation of B-cell responses was probed by a series of glycopolymers that displayed (i) a high-affinity ligand for CD22, (ii) the 2,4-dinitrophenyl hapten that can engage with BCR of a particular B-cell line and (iii) a combination of CD22 ligands and dinitrophenyl haptens (Fig. 6)101. As expected, the CD22 ligand homopolymer did not elicit a change in intracellular Ca2+, consistent with its inability to bind the BCR. Importantly, the copolymer that can recruit CD22 to the BCR complex exhibited a suppressed Ca2+ influx compared to the dinitrophenyl homopolymer. These data support a model in which coclustering of CD22 and the BCR results in signal attenuation. A subsequent in vivo study highlighted the role of trans interactions with CD22 for attenuating B-cell function102. In this experiment, mice were treated with polyacrylamide functionalized with a nitrophenyl hapten and a high-affinity CD22 ligand. The glycopolymers were nonimmunogenic and promoted long-lived tolerance, preventing B-cell responses when the mice were challenged with the hapten. No tolerogenic responses were observed when CD22-deficient mice were treated with the polymers. On the basis of these findings, it is to be expected that glycopolymers containing an immunodominant peptide or glycopeptide epitope that drives autoimmune responses and a CD22 ligand can serve as therapeutics that inhibit autoimmune responses.
Figure 6. Modulation of B-cell activation by glycosylation.
(a) Polymers were designed that contain dinitrophenyl as a hapten, a carbohydrate ligand for CD22 (CD22L) or both. A biphenyl moiety at C6 of the sialoside of the CD22L greatly increases the affinity of binding. (b) The copolymer bearing the hapten and CD22 ligand induces clustering of the B-cell receptor (BCR) and CD22, which results in a suppression of B-cell signaling and tolerance. In this case, the immune-receptor tyrosine-based inhibitory motifs of CD22 recruit the inhibitory phosphatases SHP-1 and SHIP, which suppress BCR signaling through unknown mechanisms. It is thought that trans interactions with 2,6-linked sialosides of self cells induce tolerance through a similar mechanism. (c) A polymer containing only hapten mimics a foreign entity that can cluster BCRs, resulting in cell signaling. In this case, CD22 is largely excluded from the BCR signaling complex. CD22 is a glycoprotein, and it has been shown that it can bind with its own glycans forming homomultimeric protein complexes110.
Conclusions and perspectives
Protein glycosylation has been implicated in many aspects of adaptive immune activation. It can greatly influence uptake and proteolytic processing of antigens. Glycosylated peptides can be presented by MHC-I and MHC-II, and the resulting complex can be recognized by T cells, leading to glycan-dependent TH cell and cytotoxic T-cell responses. Appropriate glycosylation of antigens is being exploited for the development of more efficacious prophylactic as well as therapeutic vaccines79,83.
Most of the receptors involved in adaptive immune activation are glycosylated. The interaction of these glycoproteins with glycan-binding proteins, such as C-type lectins, galectins and Siglecs, can regulate many aspects of immune cell activation. Glycopolymers have been designed that can interfere with these processes, leading to tolerance or immune activation101,102.
A considerable expansion in glycomics capabilities103-105 will be required to fully exploit protein glycosylation for the development of the next generation of immune therapies. The design of appropriately glycosylated antigens requires knowledge of glycosylation patterns of proteins in complex mixtures, which is difficult to obtain by current analytic methodologies. Although MS has been employed to study the glycome of immune cells106, it has not been possible to establish the glycosylation status of individual proteins of immune cells. Glycan microarray technology is beginning to provide important information about the ligands of glycan-binding proteins107,108. However, these arrays contain only a fraction of the naturally occurring glycans, and new synthetic methodologies109 are required that make full cellular glycomes. The future capability to determine the biologically relevant glycoforms of immune receptors combined with an ability to selectively modulate cellular glycomes are expected to provide exciting opportunities to control adaptive immune responses.
Acknowledgments
The authors receive research support by the National Cancer Institute (R01CA88986) and the National Institute of General Medical Sciences (R01GM061761 and R01GM090269) of the US National Institutes of Health. We thank Z.S. Chinoy for assistance with figure preparation.
Footnotes
Competing financial interests
The authors declare competing financial interests: details accompany the online version of the paper.
Additional information
Reprints and permissions information is available online at http://www.nature.com/reprints/index.html. Correspondence and requests for materials should be addressed to G.-J.B.
references
- 1.Varki A. Biological roles of oligosaccharides—all of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth J. Protein N-glycosylation along the secretory pathway: relationship to organelle topography and function, protein quality control, and cell interactions. Chem. Rev. 2002;102:285–303. doi: 10.1021/cr000423j. [DOI] [PubMed] [Google Scholar]
- 3.Kleene R, Schachner M. Glycans and neural cell interactions. Nat. Rev. Neurosci. 2004;5:195–208. doi: 10.1038/nrn1349. [DOI] [PubMed] [Google Scholar]
- 4.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 5.Prescher JA, Bertozzi CR. Chemical technologies for probing glycans. Cell. 2006;126:851–854. doi: 10.1016/j.cell.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Lanctot PM, Gage FH, Varki AP. The glycans of stem cells. Curr. Opin. Chem. Biol. 2007;11:373–380. doi: 10.1016/j.cbpa.2007.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor ME, Drickamer K. Paradigms for glycan-binding receptors in cell adhesion. Curr. Opin. Cell Biol. 2007;19:572–577. doi: 10.1016/j.ceb.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Hart GW, Copeland RJ. Glycomics hits the big time. Cell. 2010;143:672–676. doi: 10.1016/j.cell.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu. Rev. Biochem. 2011;80:825–858. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brockhausen I. Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions. EMBO Rep. 2006;7:599–604. doi: 10.1038/sj.embor.7400705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown JR, Crawford BE, Esko JD. Glycan antagonists and inhibitors: a fount for drug discovery. Crit. Rev. Biochem. Mol. Biol. 2007;42:481–515. doi: 10.1080/10409230701751611. [DOI] [PubMed] [Google Scholar]
- 12.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 13.Varki A. Glycan-based interactions involving vertebrate sialic-acid–recognizing proteins. Nature. 2007;446:1023–1029. doi: 10.1038/nature05816. [DOI] [PubMed] [Google Scholar]
- 14.van Kooyk Y, Rabinovich GA. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat. Immunol. 2008;9:593–601. doi: 10.1038/ni.f.203. [DOI] [PubMed] [Google Scholar]
- 15.Freeze HH. Congenital disorders of glycosylation: CDG-I, CDG-II, and beyond. Curr. Mol. Med. 2007;7:389–396. doi: 10.2174/156652407780831548. [DOI] [PubMed] [Google Scholar]
- 16.Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA. Glycosylation and the immune system. Science. 2001;291:2370–2376. doi: 10.1126/science.291.5512.2370. [DOI] [PubMed] [Google Scholar]
- 17.Baum LG, Crocker PR. Glycoimmunology: ignore at your peril! Immunol. Rev. 2009;230:5–8. doi: 10.1111/j.1600-065X.2009.00800.x. [DOI] [PubMed] [Google Scholar]
- 18.Petersen J, Purcell AW, Rossjohn J. Post-translationally modified T cell epitopes: immune recognition and immunotherapy. J. Mol. Med. 2009;87:1045–1051. doi: 10.1007/s00109-009-0526-4. [DOI] [PubMed] [Google Scholar]
- 19.Avci FY, Li X, Tsuji M, Kasper DL. Carbohydrates and T cells: a sweet twosome. Semin. Immunol. 2013;25:146–151. doi: 10.1016/j.smim.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderton SM. Post-translational modifications of self antigens: implications for autoimmunity. Curr. Opin. Immunol. 2004;16:753–758. doi: 10.1016/j.coi.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Purcell AW, van Driel IR, Gleeson PA. Impact of glycans on T-cell tolerance to glycosylated self-antigens. Immunol. Cell Biol. 2008;86:574–579. doi: 10.1038/icb.2008.48. [DOI] [PubMed] [Google Scholar]
- 22.Marth JD, Grewal PK. Mammalian glycosylation in immunity. Nat. Rev. Immunol. 2008;8:874–887. doi: 10.1038/nri2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan SO, Cobb BA. Host glycans and antigen presentation. Microbes Infect. 2012;14:894–903. doi: 10.1016/j.micinf.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buskas T, Ingale S, Boons GJ. Glycopeptides as versatile tools for glycobiology. Glycobiology. 2006;16:113R–136R. doi: 10.1093/glycob/cwj125. [DOI] [PubMed] [Google Scholar]
- 25.Calo D, Kaminski L, Eichler J. Protein glycosylation in Archaea: sweet and extreme. Glycobiology. 2010;20:1065–1076. doi: 10.1093/glycob/cwq055. [DOI] [PubMed] [Google Scholar]
- 26.Nothaft H, Szymanski CM. Protein glycosylation in bacteria: sweeter than ever. Nat. Rev. Microbiol. 2010;8:765–778. doi: 10.1038/nrmicro2383. [DOI] [PubMed] [Google Scholar]
- 27.Vigerust DJ, Shepherd VL. Virus glycosylation: role in virulence and immune interactions. Trends Microbiol. 2007;15:211–218. doi: 10.1016/j.tim.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lizak C, Gerber S, Numao S, Aebi M, Locher KP. X-ray structure of a bacterial oligosaccharyltransferase. Nature. 2011;474:350–355. doi: 10.1038/nature10151. [DOI] [PubMed] [Google Scholar]
- 29.Schwarz F, Aebi M. Mechanisms and principles of N-linked protein glycosylation. Curr. Opin. Struct. Biol. 2011;21:576–582. doi: 10.1016/j.sbi.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Taniguchi N, Korekane H. Branched N-glycans and their implications for cell adhesion, signaling and clinical applications for cancer biomarkers and in therapeutics. BMB Rep. 2011;44:772–781. doi: 10.5483/bmbrep.2011.44.12.772. [DOI] [PubMed] [Google Scholar]
- 31.Moremen KW, Tiemeyer M, Nairn AV. Vertebrate protein glycosylation: diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 2012;13:448–462. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291:2364–2369. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- 33.Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 2003;4:181–191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- 34.Helenius A, Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 2004;73:1019–1049. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- 35.Bennett EP, et al. Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology. 2012;22:736–756. doi: 10.1093/glycob/cwr182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cummings RD. The repertoire of glycan determinants in the human glycome. Mol. Biosyst. 2009;5:1087–1104. doi: 10.1039/b907931a. [DOI] [PubMed] [Google Scholar]
- 37.Nairn AV, et al. Regulation of glycan structures in animal tissues: transcript profiling of glycan-related genes. J. Biol. Chem. 2008;283:17298–17313. doi: 10.1074/jbc.M801964200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nairn AV, Moremen KW. In: Handbook of Glycomics. Cummings R, Pierce JM, editors. Academic Press; Burlington, MA: 2009. pp. 95–136. [Google Scholar]
- 39.Vyas JM, van der Veen AG, Ploegh HL. The known unknowns of antigen processing and presentation. Nat. Rev. Immunol. 2008;8:607–618. doi: 10.1038/nri2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neefjes J, Jongsma ML, Paul P, Bakke O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat. Rev. Immunol. 2011;11:823–836. doi: 10.1038/nri3084. [DOI] [PubMed] [Google Scholar]
- 41.Basler M, Kirk CJ, Groettrup M. The immunoproteasome in antigen processing and other immunological functions. Curr. Opin. Immunol. 2013;25:74–80. doi: 10.1016/j.coi.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Garstka MA, Neefjes J. How to target MHC class II into the MIIC compartment. Mol. Immunol. 2013;55:162–165. doi: 10.1016/j.molimm.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 43.Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat. Rev. Immunol. 2009;9:15–27. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- 44.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu. Rev. Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 45.Taylor ME, Drickamer K. Structure-function analysis of C-type animal lectins. Methods Enzymol. 2003;363:3–16. doi: 10.1016/S0076-6879(03)01039-5. [DOI] [PubMed] [Google Scholar]
- 46.Sancho D, Reis e Sousa C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu. Rev. Immunol. 2012;30:491–529. doi: 10.1146/annurev-immunol-031210-101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plato A, Willment JA, Brown GD. C-type lectin-like receptors of the dectin-1 cluster: ligands and signaling pathways. Int. Rev. Immunol. 2013;32:134–156. doi: 10.3109/08830185.2013.777065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Osorio F, Reis e Sousa C. Myeloid C-type lectin receptors in pathogen recognition and host defense. Immunity. 2011;34:651–664. doi: 10.1016/j.immuni.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 49.Švajger U, Anderluh M, Jeras M, Obermajer N. C-type lectin DC-SIGN: an adhesion, signalling and antigen-uptake molecule that guides dendritic cells in immunity. Cell. Signal. 2010;22:1397–1405. doi: 10.1016/j.cellsig.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitchell DA, Fadden AJ, Drickamer K. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. Subunit organization and binding to multivalent ligands. J. Biol. Chem. 2001;276:28939–28945. doi: 10.1074/jbc.M104565200. [DOI] [PubMed] [Google Scholar]
- 51.Dam TK, Brewer CF. Lectins as pattern recognition molecules: the effects of epitope density in innate immunity. Glycobiology. 2010;20:270–279. doi: 10.1093/glycob/cwp186. [DOI] [PubMed] [Google Scholar]
- 52.García-Vallejo JJ, et al. Multivalent glycopeptide dendrimers for the targeted delivery of antigens to dendritic cells. Mol. Immunol. 2013;53:387–397. doi: 10.1016/j.molimm.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 53.van Kooyk Y, Unger WW, Fehres CM, Kalay H, Garcia-Vallejo JJ. Glycan-based DC-SIGN targeting vaccines to enhance antigen cross-presentation. Mol. Immunol. 2013;55:143–145. doi: 10.1016/j.molimm.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 54.Johnson MA, Bundle DR. Designing a new antifungal glycoconjugate vaccine. Chem. Soc. Rev. 2013;42:4327–4344. doi: 10.1039/c2cs35382b. [DOI] [PubMed] [Google Scholar]
- 55.Lipinski T, et al. Enhanced immunogenicity of a tricomponent mannan tetanus toxoid conjugate vaccine targeted to dendritic cells via Dectin-1 by incorporating β-glucan. J. Immunol. 2013;190:4116–4128. doi: 10.4049/jimmunol.1202937. [DOI] [PubMed] [Google Scholar]
- 56.Hanisch FG, Ninkovic T. Immunology of O-glycosylated proteins: approaches to the design of a MUC1 glycopeptide-based tumor vaccine. Curr. Protein Pept. Sci. 2006;7:307–315. doi: 10.2174/138920306778018034. [DOI] [PubMed] [Google Scholar]
- 57.Doe B, Steimer KS, Walker CM. Induction of HIV-1 envelope (gp120)-specific cytotoxic T lymphocyte responses in mice by recombinant CHO cell-derived gp120 is enhanced by enzymatic removal of N-linked glycans. Eur. J. Immunol. 1994;24:2369–2376. doi: 10.1002/eji.1830241017. [DOI] [PubMed] [Google Scholar]
- 58.Li H, et al. Proximal glycans outside of the epitopes regulate the presentation of HIV-1 envelope gp120 helper epitopes. J. Immunol. 2009;182:6369–6378. doi: 10.4049/jimmunol.0804287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ninkovic T, Hanisch FG. O-glycosylated human MUC1 repeats are processed in vitro by immunoproteasomes. J. Immunol. 2007;179:2380–2388. doi: 10.4049/jimmunol.179.4.2380. [DOI] [PubMed] [Google Scholar]
- 60.Werdelin O, Meldal M, Jensen T. Processing of glycans on glycoprotein and glycopeptide antigens in antigen-presenting cells. Proc. Natl. Acad. Sci. USA. 2002;99:9611–9613. doi: 10.1073/pnas.152345899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kario E, Tirosh B, Ploegh HL, Navon A. N-linked glycosylation does not impair proteasomal degradation but affects class I major histocompatibility complex presentation. J. Biol. Chem. 2008;283:244–254. doi: 10.1074/jbc.M706237200. [DOI] [PubMed] [Google Scholar]
- 62.Altrich-VanLith ML, et al. Processing of a class I-restricted epitope from tyrosinase requires peptide N-glycanase and the cooperative action of endoplasmic reticulum aminopeptidase 1 and cytosolic proteases. J. Immunol. 2006;177:5440–5450. doi: 10.4049/jimmunol.177.8.5440. [DOI] [PubMed] [Google Scholar]
- 63.Dengjel J, Stevanovic S. Naturally presented MHC ligands carrying glycans. Transfus. Med. Hemother. 2006;33:38–44. [Google Scholar]
- 64.Jensen T, et al. Carbohydrate and peptide specificity of MHC class II-restricted T cell hybridomas raised against an O-glycosylated self peptide. J. Immunol. 1997;158:3769–3778. [PubMed] [Google Scholar]
- 65.Bäcklund J, et al. Predominant selection of T cells specific for the glycosylated collagen type II epitope (263–270) in humanized transgenic mice and in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA. 2002;99:9960–9965. doi: 10.1073/pnas.132254199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andersson IE, et al. Design of glycopeptides used to investigate class II MHC binding and T-cell responses associated with autoimmune arthritis. PLoS ONE. 2011;6:e17881. doi: 10.1371/journal.pone.0017881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dzhambazov B, et al. Therapeutic vaccination of active arthritis with a glycosylated collagen type II peptide in complex with MHC class II molecules. J. Immunol. 2006;176:1525–1533. doi: 10.4049/jimmunol.176.3.1525. [DOI] [PubMed] [Google Scholar]
- 68.Apostolopoulos V, et al. A glycopeptide in complex with MHC class I uses the GalNAc residue as an anchor. Proc. Natl. Acad. Sci. USA. 2003;100:15029–15034. doi: 10.1073/pnas.2432220100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tarp MA, Clausen H. Mucin-type O-glycosylation and its potential use in drug and vaccine development. Biochim. Biophys. Acta. 2008;1780:546–563. doi: 10.1016/j.bbagen.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 70.Roulois D, Gregoire M, Fonteneau JF. MUC1-specific cytotoxic T lymphocytes in cancer therapy: induction and challenge. Biomed. Res. Int. 2012 doi: 10.1155/2013/871936. doi:10.1155/2013/871936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haurum JS, et al. Presentation of cytosolic glycosylated peptides by human class I major histocompatibility complex molecules in vivo. J. Exp. Med. 1999;190:145–150. doi: 10.1084/jem.190.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vlad AM, et al. Complex carbohydrates are not removed during processing of glycoproteins by dendritic cells: processing of tumor antigen MUC1 glycopeptides for presentation to major histocompatibility complex class II-restricted T cells. J. Exp. Med. 2002;196:1435–1446. doi: 10.1084/jem.20020493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stepensky D, Tzehoval E, Vadai E, Eisenbach L. O-glycosylated versus non-glycosylated MUC1-derived peptides as potential targets for cytotoxic immunotherapy of carcinoma. Clin. Exp. Immunol. 2006;143:139–149. doi: 10.1111/j.1365-2249.2005.02965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beatson RE, Taylor-Papadimitriou J, Burchell JM. MUC1 immunotherapy. Immunotherapy. 2010;2:305–327. doi: 10.2217/imt.10.17. [DOI] [PubMed] [Google Scholar]
- 75.Kimura T, Finn OJ. MUC1 immunotherapy is here to stay. Expert Opin. Biol. Ther. 2013;13:35–49. doi: 10.1517/14712598.2012.725719. [DOI] [PubMed] [Google Scholar]
- 76.Coltart DM, et al. Principles of mucin architecture: structural studies on synthetic glycopeptides bearing clustered mono-, di-, tri-, and hexasaccharide glycodomains. J. Am. Chem. Soc. 2002;124:9833–9844. doi: 10.1021/ja020208f. [DOI] [PubMed] [Google Scholar]
- 77.Karsten U, Serttas N, Paulsen H, Danielczyk A, Goletz S. Binding patterns of DTR-specific antibodies reveal a glycosylation-conditioned tumor-specific epitope of the epithelial mucin (MUC-1) Glycobiology. 2004;14:681–692. doi: 10.1093/glycob/cwh090. [DOI] [PubMed] [Google Scholar]
- 78.Dziadek S, Griesinger C, Kunz H, Reinscheid UM. Synthesis and structural model of an a(2,6)-sialyl-t glycosylated MUC1 eicosapeptide under physiological conditions. Chemistry. 2006;12:4981–4993. doi: 10.1002/chem.200600144. [DOI] [PubMed] [Google Scholar]
- 79.Lakshminarayanan V, et al. Immune recognition of tumor-associated mucin MUC1 is achieved by a fully synthetic aberrantly glycosylated MUC1 tripartite vaccine. Proc. Natl. Acad. Sci. USA. 2012;109:261–266. doi: 10.1073/pnas.1115166109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gaidzik N, Westerlind U, Kunz H. The development of synthetic antitumour vaccines from mucin glycopeptide antigens. Chem. Soc. Rev. 2013;42:4421–4442. doi: 10.1039/c3cs35470a. [DOI] [PubMed] [Google Scholar]
- 81.Wells L, Hart GW. Glycomics: building upon proteomics to advance glycosciences. Mol. Cell. Proteomics. 2013;12:833–835. doi: 10.1074/mcp.E113.027904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Steentoft C, et al. Mining the O-glycoproteome using zinc-finger nucleaseglycoengineered SimpleCell lines. Nat. Methods. 2011;8:977–982. doi: 10.1038/nmeth.1731. [DOI] [PubMed] [Google Scholar]
- 83.Avci FY, Li X, Tsuji M, Kasper DL. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat. Med. 2011;17:1602–1609. doi: 10.1038/nm.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Costantino P, Rappuoli R, Berti F. The design of semi-synthetic and synthetic glycoconjugate vaccines. Expert Opin. Drug Discov. 2011;6:1045–1066. doi: 10.1517/17460441.2011.609554. [DOI] [PubMed] [Google Scholar]
- 85.Lai Z, Schreiber JR. Antigen processing of glycoconjugate vaccines; the polysaccharide portion of the pneumococcal CRM(197) conjugate vaccine co-localizes with MHC II on the antigen processing cell surface. Vaccine. 2009;27:3137–3144. doi: 10.1016/j.vaccine.2009.03.064. [DOI] [PubMed] [Google Scholar]
- 86.Johnson JL, Jones MB, Ryan SO, Cobb BA. The regulatory power of glycans and their binding partners in immunity. Trends Immunol. 2013;34:290–298. doi: 10.1016/j.it.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barthel SR, Gavino JD, Descheny L, Dimitroff CJ. Targeting selectins and selectin ligands in inflammation and cancer. Expert Opin. Ther. Targets. 2007;11:1473–1491. doi: 10.1517/14728222.11.11.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu FT. Regulatory roles of galectins in the immune response. Int. Arch. Allergy Immunol. 2005;136:385–400. doi: 10.1159/000084545. [DOI] [PubMed] [Google Scholar]
- 89.Vasta GR. Roles of galectins in infection. Nat. Rev. Microbiol. 2009;7:424–438. doi: 10.1038/nrmicro2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Garner OB, Baum LG. Galectin-glycan lattices regulate cell-surface glycoprotein organization and signalling. Biochem. Soc. Trans. 2008;36:1472–1477. doi: 10.1042/BST0361472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu SD, et al. Endogenous galectin-1 enforces class I-restricted TCR functional fate decisions in thymocytes. Blood. 2008;112:120–130. doi: 10.1182/blood-2007-09-114181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Perillo NL, Pace KE, Seilhamer JJ, Baum LG. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378:736–739. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- 93.Hsu DK, Chen HY, Liu FT. Galectin-3 regulates T-cell functions. Immunol. Rev. 2009;230:114–127. doi: 10.1111/j.1600-065X.2009.00798.x. [DOI] [PubMed] [Google Scholar]
- 94.Demetriou M, Granovsky M, Quaggin S, Dennis JW. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature. 2001;409:733–739. doi: 10.1038/35055582. [DOI] [PubMed] [Google Scholar]
- 95.Chen IJ, Chen HL, Demetriou M. Lateral compartmentalization of T cell receptor versus CD45 by galectin-N-glycan binding and microfilaments coordinate basal and activation signaling. J. Biol. Chem. 2007;282:35361–35372. doi: 10.1074/jbc.M706923200. [DOI] [PubMed] [Google Scholar]
- 96.Clark MC, Baum LG. T cells modulate glycans on CD43 and CD45 during development and activation, signal regulation, and survival. Ann. NY Acad. Sci. 2012;1253:58–67. doi: 10.1111/j.1749-6632.2011.06304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tribulatti MV, Cattaneo V, Hellman U, Mucci J, Campetella O. Galectin-8 provides costimulatory and proliferative signals to T lymphocytes. J. Leukoc. Biol. 2009;86:371–380. doi: 10.1189/jlb.0908529. [DOI] [PubMed] [Google Scholar]
- 98.Poe JC, Tedder TF. CD22 and Siglec-G in B cell function and tolerance. Trends Immunol. 2012;33:413–420. doi: 10.1016/j.it.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Collins BE, Smith BA, Bengtson P, Paulson JC. Ablation of CD22 in ligand-deficient mice restores B cell receptor signaling. Nat. Immunol. 2006;7:199–206. doi: 10.1038/ni1283. [DOI] [PubMed] [Google Scholar]
- 100.Hennet T, Chui D, Paulson JC, Marth JD. Immune regulation by the ST6Gal sialyltransferase. Proc. Natl. Acad. Sci. USA. 1998;95:4504–4509. doi: 10.1073/pnas.95.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Courtney AH, Puffer EB, Pontrello JK, Yang ZQ, Kiessling LL. Sialylated multivalent antigens engage CD22 in trans and inhibit B cell activation. Proc. Natl. Acad. Sci. USA. 2009;106:2500–2505. doi: 10.1073/pnas.0807207106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Duong BH, et al. Decoration of T-independent antigen with ligands for CD22 and Siglec-G can suppress immunity and induce B cell tolerance in vivo. J. Exp. Med. 2010;207:173–187. doi: 10.1084/jem.20091873. [DOI] [PMC free article] [PubMed] [Google Scholar]; erratum. 2010;207:445. [Google Scholar]
- 103.Pilobello KT, Mahal LK. Deciphering the glycocode: the complexity and analytical challenge of glycomics. Curr. Opin. Chem. Biol. 2007;11:300–305. doi: 10.1016/j.cbpa.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 104.Vanderschaeghe D, Festjens N, Delanghe J, Callewaert N. Glycome profiling using modern glycomics technology: technical aspects and applications. Biol. Chem. 2010;391:149–161. doi: 10.1515/bc.2010.031. [DOI] [PubMed] [Google Scholar]
- 105.Kolarich D, Lepenies B, Seeberger PH. Glycomics, glycoproteomics and the immune system. Curr. Opin. Chem. Biol. 2012;16:214–220. doi: 10.1016/j.cbpa.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 106.Haslam SM, et al. Characterizing the glycome of the mammalian immune system. Immunol. Cell Biol. 2008;86:564–573. doi: 10.1038/icb.2008.54. [DOI] [PubMed] [Google Scholar]
- 107.Rillahan CD, Paulson JC. Glycan microarrays for decoding the glycome. Annu. Rev. Biochem. 2011;80:797–823. doi: 10.1146/annurev-biochem-061809-152236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smith DF, Cummings RD. Application of microarrays for deciphering the structure and function of the human glycome. Mol. Cell. Proteomics. 2013;12:902–912. doi: 10.1074/mcp.R112.027110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang Z, et al. A general strategy for the chemoenzymatic synthesis of asymmetrically branched N-glycans. Science. 2013;341:379–383. doi: 10.1126/science.1236231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Han S, Collins BE, Bengtson P, Paulson JC. Homomultimeric complexes of CD22 in B cells revealed by protein-glycan cross-linking. Nat. Chem. Biol. 2005;1:93–97. doi: 10.1038/nchembio713. [DOI] [PubMed] [Google Scholar]