Abstract
Spinal cord injury (SCI) leads to irreversible neuronal loss and glial scar formation, which ultimately result in persistent neurological dysfunction. Cellular regeneration could be an ideal approach to replenish the lost cells and repair the damage. However, the adult spinal cord has limited ability to produce new neurons. Here we show that resident astrocytes can be converted to doublecortin (DCX)-positive neuroblasts by a single transcription factor, SOX2, in the injured adult spinal cord. Importantly, these induced neuroblasts can mature into synapse-forming neurons in vivo. Neuronal maturation is further promoted by treatment with a histone deacetylase inhibitor, valproic acid (VPA). The results of this study indicate that in situ reprogramming of endogenous astrocytes to neurons might be a potential strategy for cellular regeneration after SCI.
Keywords: spinal cord, astrocytes, SOX2, adult neurogenesis, in vivo reprogramming
INTRODUCTION
Injury to the spinal cord leads to irreversible loss of neurons and the disruption of ascending and descending spinal tracts with consequent impairments of motor and sensory function below the injury1,2. Unlike certain regions in the adult brain where neurogenesis persists3, the spinal cord lacks the ability to produce new neurons in adulthood4–6. A key challenge is how to restore neurons after SCI.
Cell transplantation using stem or differentiated cells has attracted the most attention as a potential therapeutic strategy for SCI7. The establishment of induced pluripotent stem cells (iPSCs)8 from a patient’s somatic cells resolves the ethical controversies and immunological rejection, which are problems associated with cells derived from human fetal tissues. Recent studies also showed that grafted fetal or iPSC-derived neural stem cells (NSCs) can overcome an inhibitory environment to achieve neuronal growth and form a functional relay, thus promoting spinal cord repair9,10. These results clearly demonstrate that neural networks in the adult spinal cord exhibit structural plasticity, which provides a strong rationale for cell-based therapy.
However, transplantation-based cell therapy faces several major hurdles for treating SCI in human patients. First, the time required to prepare cells for autologous transplantation is longer than the therapeutically optimal time window. Extensive studies showed that cells transplanted in the sub-acute phase, but not the chronic phase, could have functional improvement11,12. However, derivation and characterization of iPSCs from patient somatic cells is a very time-consuming process. This is further exacerbated because these pluripotent cells need to be extensively expanded and differentiated in culture before transplantation13. Second, there is potential for tumor formation from residual undifferentiated stem cells in the injured environment14. Third, the transplantation procedure induces secondary injury to the spinal cord. A relatively large quantity of cells is frequently needed for transplantation, which can result in unintended damage to preexisting functional neural circuits, fluid leak, and the formation of cystic tissue at the injection site(s). Continued refinement of cell transplantation is required to overcome these major hurdles.
As an alternative to transplantation, we examined the possibility of reprogramming endogenous non-neuronal cells, such as scar-forming astrocytes, into neurons in the adult spinal cord. This approach is based on recent progress in the reprogramming field. In cell culture, the fates of many somatic cells can be re-specified by the forced expression of a few transcription factors8,15–18. Most importantly, adult pancreatic exocrine cells in vivo can be directly transformed into insulin-secreting β-cells by 3 transcription factors19. Cardiac fibroblasts in injured adult mouse heart could be reprogrammed to cardiomyocytes by 3 or 4 factors20,21. Recent studies also showed the potential of converting brain astrocytes directly to neurons by the forced expression of neurogenic factors22,23. These findings raise the possibility that endogenous non-neuronal cells, such as astrocytes, could be reprogrammed in vivo to neurons in the adult spinal cord.
Astrocytes are broadly distributed throughout the spinal cord24. After SCI, astrocytes proliferate to form a scar that preserves the integrity of surrounding cells. However, the persistence of a glial scar is detrimental to functional recovery of a damaged spinal cord, largely because this scar not only forms a physical barrier but also secretes inhibitors of axonal growth25. Interestingly, astrocytes are amenable to reprogramming in culture17,26–28 and in the adult brain22,23. Our recent studies also showed that brain astrocytes can be converted to induced adult neuroblasts (iANBs)29. Because of regional heterogeneity of astrocytes, however, it is unclear whether the fate of astrocytes in the adult spinal cord can be reprogrammed in vivo.
In this study, we screened for factors that could induce neurogenesis in the adult spinal cord and found that ectopic SOX2 is sufficient to convert endogenous spinal astrocytes to proliferative DCX-positive neuroblasts, which eventually generate mature neurons. Neuronal survival and maturation are further enhanced by treating the mice with VPA. Our study indicates that resident astrocytes in the injured adult spinal cord can be manipulated to produce neurons by defined factors, raising the possibility of using these cells as a source for in situ repair of SCI.
RESULTS
Inducing neurogenesis in the adult spinal cord
We used a lentiviral gene-delivery system to target both proliferating and quiescent cells in the adult mouse spinal cord. Gene expression was regulated by the human glial fibrillary acidic protein (hGFAP) promoter, which is active primarily in astrocytes30. To further examine the cell types targeted by this lentiviral system in the adult spinal cord, 1.5 µl of green fluorescent protein (GFP)-expressing virus (hGFAP-GFP) was injected at two positions 3–5 mm apart at the thoracic level 8 (T8). One week post viral injection (wpi), immunohistochemical analyses of longitudinal sections around the injected regions showed that GFP+ cells were detectable in a broad area, especially in the white matter, reaching a distance of ~3.0 mm from the injection site both rostrally and caudally (Supplementary Fig. 1a). While a few GFP+ cells were stained positive for markers of neurons (NeuN, <0.82%), oligodendrocyte precursors and pericytes (OLIG2, 4.94±3.10% and NG2, 4.36±2.83%; mean±s.d., n=3), the vast majority expressed the astrocyte-specific marker GFAP (95.09±4.15%, mean±s.d., n=3) (Supplementary Fig. 1b–e,i). Markers for mature oligodendrocytes (MBP and PLP) or microglia (IBA1) were not detected in GFP+ cells (Supplementary Fig. 1f–i). These results indicate that spinal astrocytes are the major cell type targeted by lentivirus under the regulation of hGFAP promoter.
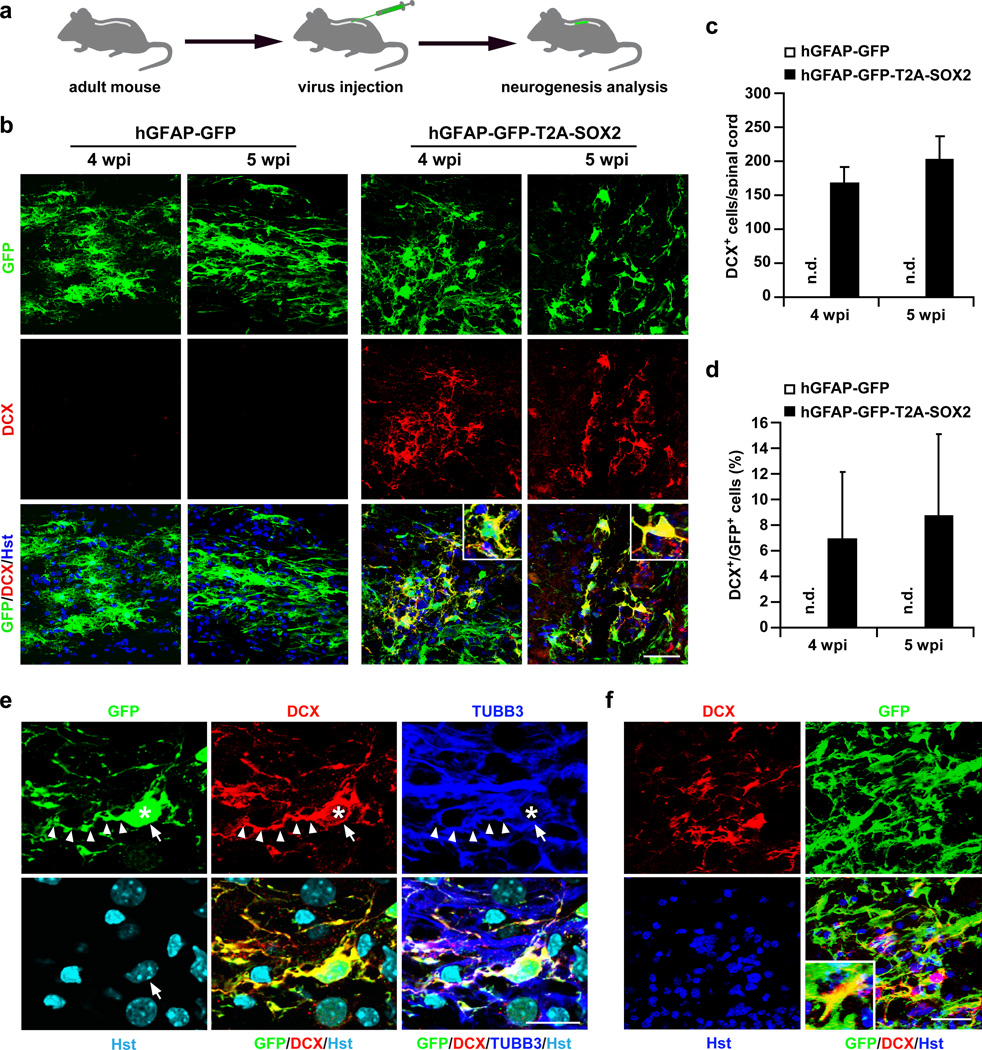
Based on their roles in NSCs and/or neurogenesis, 12 genes (SOX2, PAX6, NKX6.1, NGN2, ASCL1, OLIG2, SOX11, Tlx, OCT4, c-MYC, KLF4, and PTF1a) were chosen as candidates. Lentivirus expressing these candidates under the hGFAP promoter was individually injected into the T8 region of the adult spinal cord and analyzed for their ability to induce adult neurogenesis (Fig. 1a). Neurogenesis was initially examined by staining for the expression of doublecortin (DCX), a microtubule-associated protein that is broadly expressed in neuroblasts and immature neurons during development and in neurogenic regions of the adult brain31,32. DCX expression is mainly associated with adult neurogenesis but not with reactive gliosis or regenerative axonal growth33. Consistent with these results, DCX was not detected in either intact spinal cords or those with hemisection-induced injuries (Supplementary Fig. 2). In sharp contrast, DCX+ cells were identified in spinal cords injected with virus expressing SOX2 but none of the other 11 candidate genes at 4 wpi. This was further confirmed with a virus expressing GFP-T2A-SOX2 so that virus-transduced cells could be identified by the coexpression of GFP (Fig. 1b–d).
Figure 1. Induction of DCX+ cells in the adult mouse spinal cord.
(a) Experimental scheme. (b) DCX+ cells are detected in animals injected with lentivirus expressing SOX2 but not control GFP at 4 or 5 weeks post viral injection (wpi). Nuclei were counterstained with Hoechst 33342 (Hst) (c–d) Quantification of SOX2-induced DCX+ cells around the virus-injected regions in adult spinal cords (mean+s.d.; n=3 mice per group; n.d., not detected). (e) Confocal images of a representative DCX+ cell (indicated by arrows). SOX2-induced DCX+ cells are traced by the co-expressed GFP, indicating an origin of virus-infected cells. They are also co-labeled by TUBB3 and have bipolar or multipolar processes. Asterisks and arrowheads indicate cell bodies and neuronal processes, respectively. (f) Representative images of DCX+ cells induced in the spinal cord of aged mice (>12 months). Scales: 50 µm (b, f) and 20 µm (e).
SOX2-induced DCX+ cells were mainly identified surrounding the virus-injected region and showed typical immature neuronal morphology with bipolar or multipolar processes (Fig. 1b,e). They co-expressed betaIII-tubulin (TUBB3, also known as TUJ1), a pan-neuronal marker, and were labeled by GFP, showing an origin of virus-transduced cells (Fig. 1e). The induction efficiency of DCX+ cells was estimated at 6–8% of GFP+ cells surrounding the core injection sites at 4 or 5 wpi (Fig. 1d). Interestingly, ectopic SOX2 also resulted in the production of DCX+ cells in aged mice (>12 month, Fig. 1f). Together, these data suggest that neurogenesis can be induced by a single transcription factor, SOX2, in the adult spinal cord, similar to observations made in the adult striatum29.
Inducing neurogenesis in spinal cords with severe injuries
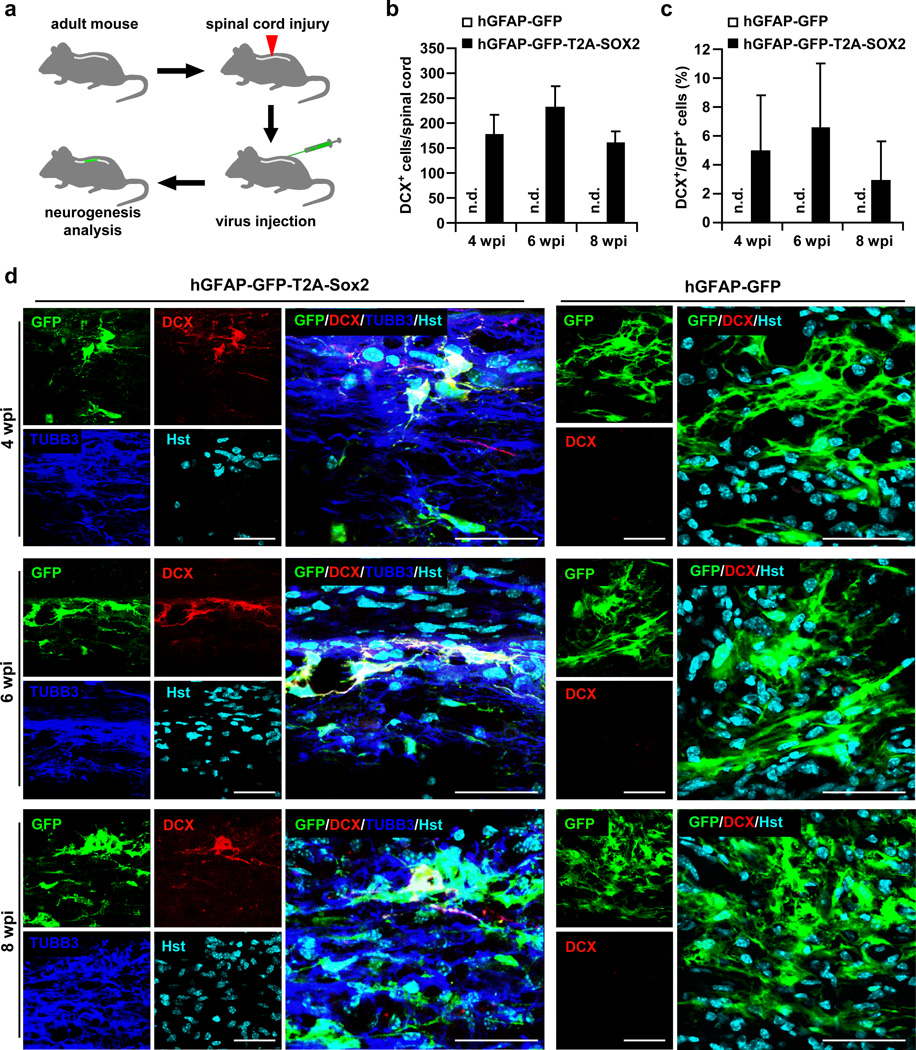
Severe traumatic injury to the adult spinal cord causes massive cell death, inflammation, and gliosis1,25,34, which result in a pathological microenvironment drastically different from that of the needle injection-induced stab wound injury. To examine whether neurogenesis could also be induced under this clinically-relevant pathological condition, we injected lentivirus into the parenchyma of severely injured spinal cord immediately after hemisection at the T8 level (Fig. 2a). The two injection sites were 1.5 mm away from the injury core on each side (Supplementary Fig. 3a). Histological analyses were performed on spinal cord sections spanning the lesion site. Similar to what was observed in the intact spinal cord, the control virus hGFAP-GFP could efficiently transduce cells surrounding the injection sites with a majority expressing the astrocyte marker GFAP (95.21±3.95%, mean±s.d., n=3; Supplementary Fig. 3b,i). Only a small percentage of GFP+ cells expressed markers for neurons, oligodendrocyte precursors or pericytes (NeuN+, <0.91%; OLIG2, 4.73±2.94%; NG2, 3.73±2.74%; mean±s.d., n=3) (Supplementary Fig. 3c–e,i). None expressed the markers for mature oligodendrocytes MBP and PLP or the microglia marker IBA1 (Supplementary Fig. 3f–i). These results are very similar to those in spinal cords without the hemisection (Supplementary Fig. 1), suggesting that severe injury does not change the cell types targeted by lentivirus.
Figure 2. Inducing neurogenesis in the adult spinal cord after severe injury.
(a) Experimental scheme. Lentivirus was injected into the spinal cord immediately after hemisection at the T8 level. (b) Quantification of SOX2-induced DCX+ cells around the virus-injected regions in injured spinal cords (mean+s.d.; n=4 mice per group; n.d., not detected). (c) Percentage of GFP+ cells expressing DCX around the virus-injected regions in injured spinal cords (mean+s.d.; n=4 mice per group; n.d., not detected). (d) Representative images of DCX+ cells induced by virus expressing SOX2 but not the GFP control at 4, 6, or 8 wpi. Scales: 40 µm.
No DCX+ cells were detected in the injured spinal cords injected with the control virus hGFAP-GFP at 4, 6 or 8 wpi (Fig. 2b–d). In stark contrast, these cells were specifically induced by the injection of hGFAP-GFP-T2A-SOX2 virus (Fig. 2b–d). All the induced DCX+ cells also expressed GFP indicating an origin from virus-transduced cells (Fig. 2d). An estimation of 3–6% of GFP+ cells surrounding the core viral injection sites were reprogrammed by SOX2 to become DCX+ cells between 4–8 wpi (Fig. 2c). These DCX+ cells also stained positive for the neuronal marker TUBB3 (Fig. 2d). Together, these data indicate that neurogenesis can be induced by SOX2 in an injured environment of the adult spinal cord.
SOX2-induced neurogenesis originates from spinal astrocytes
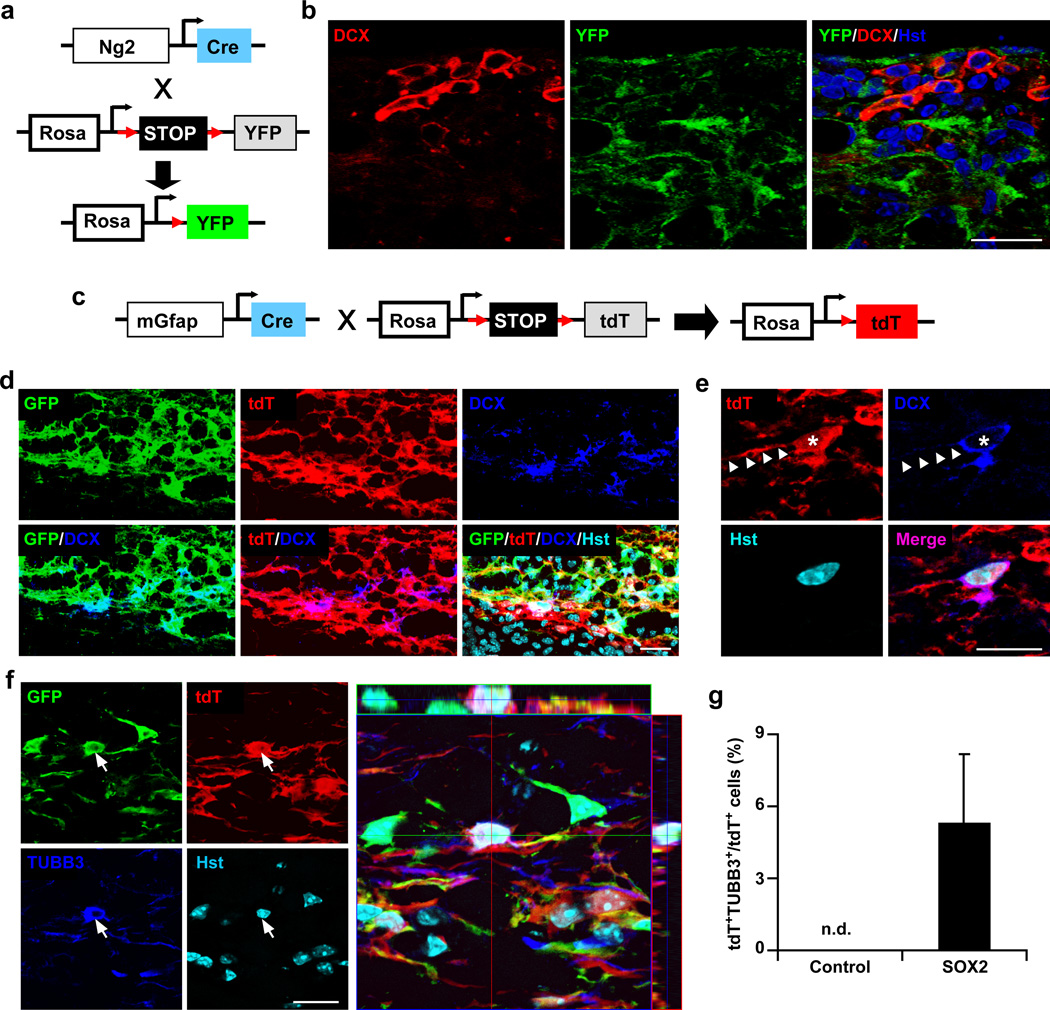
The cellular source for SOX2-induced DCX+ cells was determined by genetic lineage tracing. Gene expression under the hGFAP promoter was not detected in mature oligodendrocytes or microglia (Supplementary Fig. 1,3), thus excluding them as a possible origin for SOX2-induced DCX+ cells. Although less than 5% of NG2+ cells were targeted by the hGFAP promoter (Supplementary Fig. 1,3), these cells were specifically examined as they are cycling precursors for oligodendrocytes, exhibit plasticity after injury in the adult central nervous system35, and are amenable to fate reprogramming in culture15. NG2+ cells and their derivatives were traced using Ng2-Cre BAC transgenic mice36 and the reporter Rosa-YFP (Fig. 3a). A respective 71% and 64% of YFP+ cells expressed NG2 and OLIG2 in the adult spinal cord (Supplementary Fig. 4a,b,d). Markers for astrocytes (GFAP), microglia (IBA1), or neurons (TUBB3) were not detected in YFP-traced cells (Supplementary Fig. 4c,d). Transgenic mice were then injected with hGFAP-SOX2 lentivirus and examined at 5 wpi. Immunohistochemistry showed that DCX+ cells were induced around the virus-injected regions but none co-labeled with YFP (Fig. 3b). This result indicates that DCX+ cells induced by SOX2 under the hGFAP promoter do not originate from NG2+ cells in the adult spinal cord.
Figure 3. SOX2-induced new neurons originate from spinal astrocytes.
(a–b) SOX2-induced DCX+ cells do not come from NG2+ cells. Ng2-Cre;Rosa-YFP mice (a) were injected with SOX2-expressing or empty lentivirus and were analyzed at 5 wpi. Confocal images are shown in panel (b). (c–g) SOX2-induced new neurons originate from astrocytes. (c) mGfap-Cre;Rosa-tdT mice were injected with virus expressing either GFP-T2A-SOX2 or GFP (as a control) and were analyzed at 5 wpi. (d) Representative confocal images showing that SOX2-induced DCX+ cells originate from lentivirus-infected astrocytes (indicated by GFP+tdT+). (e) Higher magnification views of a tdT-labeled DCX+ cell. Asterisks and arrowheads indicate cell bodies and processes, respectively. (f–g) SOX2-induced TUBB3+ neurons are derived from virus-infected astrocytes (indicated by GFP+tdT+). Traced neurons were quantified in virus-injected regions (mean+s.d.; n=5 mice per group; n.d., not detected). Scales: 20 µm (b,d-f).
In contrast, astrocytes are the most likely cellular origin for the induced neurons since they are the predominant cell type targeted by lentivirus under the hGFAP promoter (Supplementary Fig. 1,3). This hypothesis was examined by genetic lineage tracing using the transgenic mGfap-Cre line 77.6, which was reported to exclusively trace astrocytes in the forebrain23. We crossed this line to Rosa-tdTomato (tdT) reporter37 and examined the identity of the labeled cells in the adult spinal cord (Fig. 3c, Supplementary Fig. 5). Based on GFAP expression, 71.32 ± 6.75% of astrocytes were labeled by the reporter tdT. Among tdT+ cells, the vast majority expressed the astrocyte markers GFAP (97.36±3.24%, mean±s.d., n=3) and glutamine synthetase (GS, 95.14±3.86%, mean±s.d., n=3), while fewer than 5% were positive for NG2 or OLIG2 (Supplementary Fig. 5a–d,f). Markers for microglia (IBA1+) or neurons (NeuN+, MAP2+, and TUBB3+) were not expressed in traced cells (Supplementary Fig. 5e,f). Adult mGfap-Cre;Rosa-tdT mice were then injected with lentivirus expressing either GFP (as a control) or GFP-T2A-SOX2 and examined at 5 wpi. Immunohistochemistry showed that ectopic SOX2 induced DCX expression in tdT+ cells (Fig. 3d). Importantly, a fraction of the traced cells were also labeled by the neuronal marker TUBB3 (~5%) in the core spinal regions injected with SOX2 virus (Fig. 3f,g). In stark contrast, these neuronal markers were not detectable in tdT-traced cells in spinal cords injected with the control virus (Fig. 3g), although GFP expression showed a clear infection of these cells (Supplementary Fig. 6). These results indicate that SOX2-induced new neurons come from resident astrocytes.
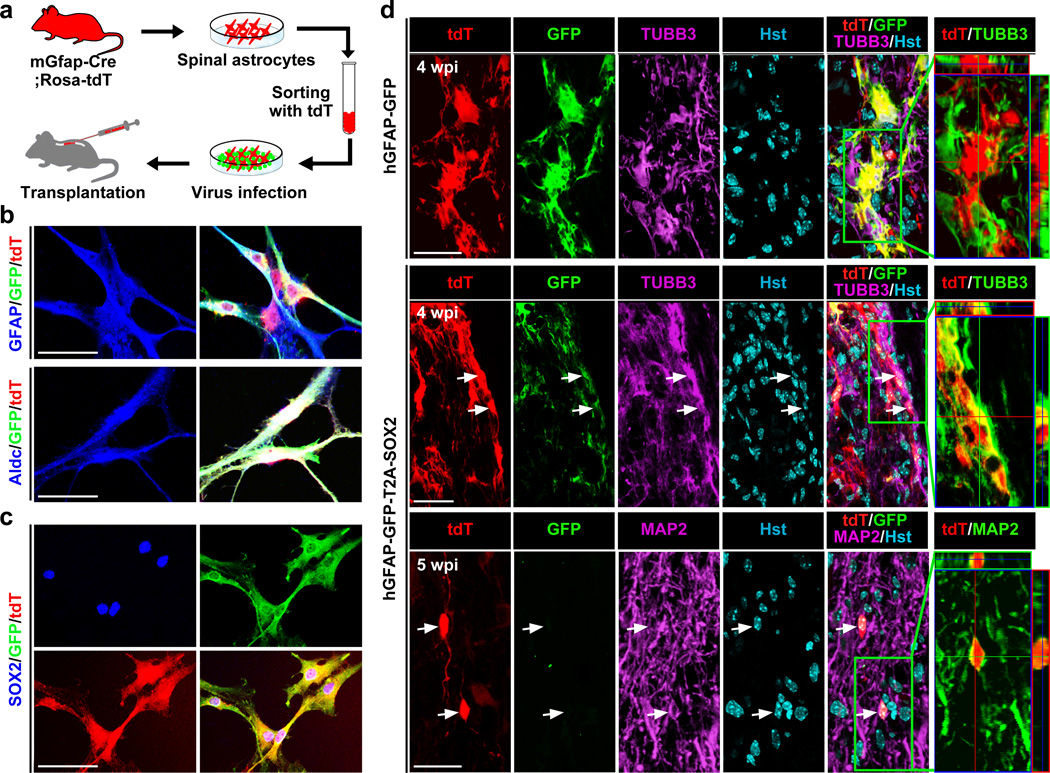
Cell transplantation assays were performed to further confirm these results. Spinal astrocytes were isolated from neonatal mGfap-Cre;Rosa-tdT mice, cultured for 7–10 days in serum-containing medium, and subsequently passaged once. tdT+ astrocytes were then purified by fluorescence-activated cell sorting and infected with either hGFAP-GFP or hGFAP-GFP-T2A-SOX2 lentivirus (Fig. 4a). The astrocytic identity and expression of ectopic GFP and SOX2 were confirmed by immunocytochemistry (Fig. 4b,c). Three days post viral infection, these cells were transplanted into the spinal cords of adult immunodeficient NOD scid gamma (NSG) mice. When examined at 4 wpi, none of the control GFP virus-infected tdT+ cells stained positive for the neuronal marker TUBB3 (Fig. 4d). In contrast, TUBB3 expression was clearly detectable in tdT+ cells that were infected with the hGFAP-GFP-T2A-SOX2 virus prior to transplantation (Fig. 4d). GFP expression in the latter group was also downregulated, reflecting much reduced activities of the hGFAP promoter in converted cells. By 5 wpi, some of the tdT+ cells in the SOX2 group started to express MAP2, a marker for mature neurons. These results demonstrate that the ectopic expression of SOX2 can convert transplanted spinal astrocytes to neurons in the adult spinal cord.
Figure 4. In vivo conversion of engrafted astrocytes to neurons.
(a) Experimental scheme. Spinal astrocytes cultured from mGfap-Cre;Rosa-tdT mice were purified based on tdT expression. Three days post viral infection, cells were transplanted into the spinal cord of NSG mouse. (b) Immunocytochemistry showing the expression of GFAP and Aldolase c (Aldc), markers for astrocytes, in purified tdT+ cells. (c) Immunocytochemistry confirming SOX2 expression in spinal astrocytes infected with the hGFAP-GFP-T2A-SOX2 virus. (d) Confocal images showing astrocyte-derived neurons in the spinal cord transplanted with the SOX2 but not control virus-infected astrocytes. Orthogonal views of cells in the boxed regions are shown in the right panels. Astrocyte-derived neurons (tdT+TUBB3+ or tdT+MAP2+) are indicated by arrows. Scales: 60 µm (b,c) and 30 µm (d).
Cell proliferation during induced neurogenesis
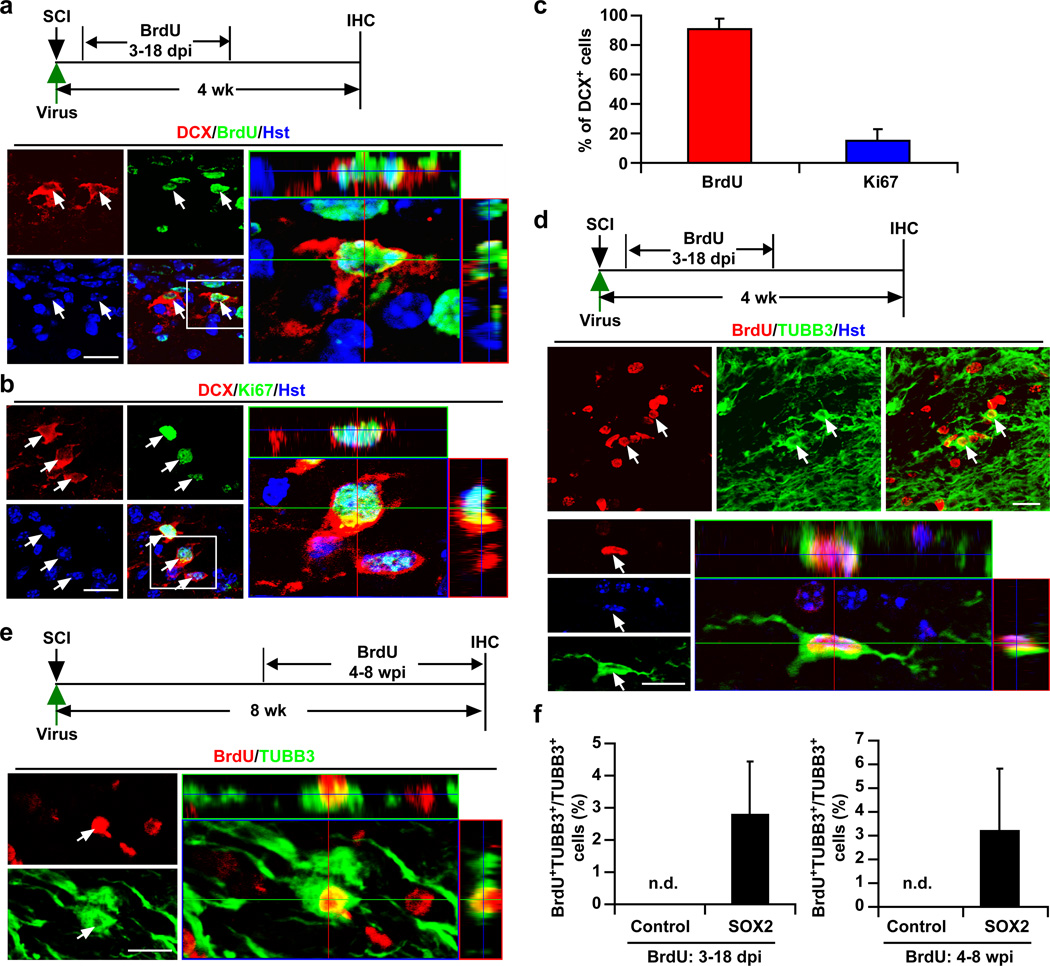
SOX2-induced neurons could be converted from astrocytes by a direct lineage switch without the addition of new cells. We tested this hypothesis by examining cell proliferation during the process of SOX2-induced neurogenesis. Proliferating cells in the injured adult spinal cord were continually labeled by intraperitoneal injection of BrdU (100 mg/kg, twice a day) from 3 to 18 days post viral injection. When examined at 4 wpi, around 90% of induced DCX+ cells were clearly labeled by BrdU indicating that they passed through a proliferative stage (Fig. 5a,c). Interestingly, nearly 17% of DCX+ cells also expressed the cell proliferation marker Ki67, indicating that these cells were still in a cycling state (Fig. 5b–c). Using TUBB3 as an additional marker for neurons, we found that approximately 3% of neurons surrounding the core viral injection sites could be labeled by BrdU (Fig. 5d,f). Administration of BrdU during 4–8 wpi also led to a similar number of TUBB3+ cells being labeled (Fig. 5e–f). These data collectively demonstrate that adult neurogenesis induced by the ectopic expression of SOX2 is a continuous process, which passes through a proliferation phase.
Figure 5. Cell proliferation during SOX2-induced neurogenesis in the adult spinal cord.
(a) Incorporation of BrdU in SOX2-induced DCX+ cells. An orthogonal view of BrdU+DCX+ cells in the boxed region is shown in the right panel (dpi, days post viral injection; wk, weeks; IHC, immunohistochemistry). (b) Ki67-staining showing that SOX2-induced DCX+ cells are proliferative at 5 wpi. An orthogonal view of Ki67+DCX+ cells in the boxed region is shown in the right panel. (c) Quantification of BrdU or Ki67-labeled DCX+ cells (mean+s.d.; n=3 mice per group). (d–e) Newly generated neurons indicated by BrdU-traced TUBB3+ cells in the adult spinal cord. Orthogonal views of BrdU+TUBB3+ cell are also shown. (f) Quantification of SOX2-induced new neurons (indicated by BrdU+TUBB3+) surrounding the virus-injected regions in adult spinal cords (mean+s.d.; n=3 mice per group; n.d., not detected). Scales: 20 µm (a,b,d,e).
SOX2 induces neuroblasts in the adult spinal cord
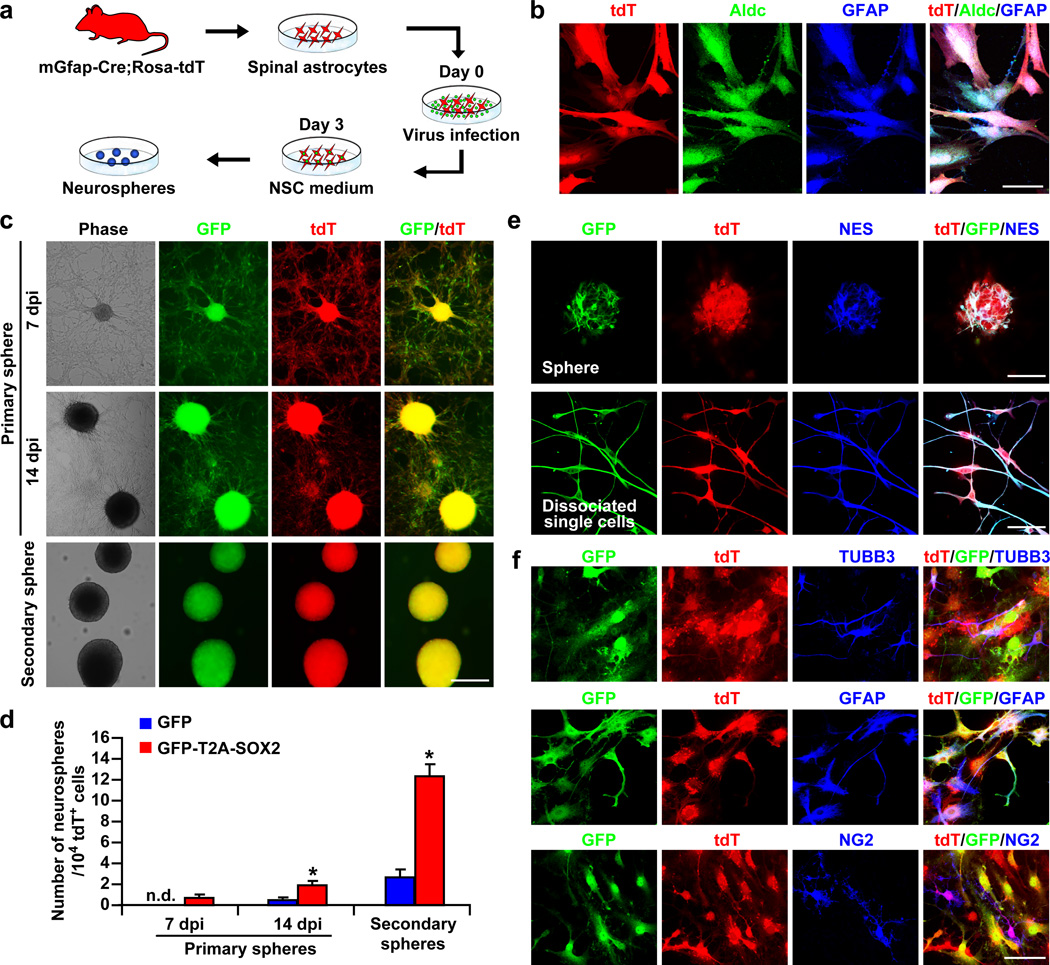
Ectopic expression of SOX2 alone was able to convert fibroblasts or human cortical astrocytes to proliferative and multipotent NSCs28,38, raising the possibility that spinal astrocytes could be similarly reprogrammed by SOX2. Spinal astrocytes were isolated from early postnatal mGfap-Cre;Rosa-tdT mice and infected with lentivirus expressing either GFP-T2A-SOX2 or the control GFP alone (Fig. 6a,b). Three days later, the fetal bovine serum-containing medium for astrocytes was switched to complete synthetic medium for NSCs. Neurospheres, which indicate the presence of self-renewable NSCs, were observed as early as 7 days post viral infection (dpi) in cultures with ectopic SOX2 but not the control GFP (Fig. 6c,d). These spheres were clearly labeled by tdT and GFP indicating an origin for SOX2 virus-infected astrocytes. Consistent with neural stem-like cells being present in postnatal spinal cords39, neurospheres were also detectable in the GFP group by 14 dpi. However, their number was significantly lower than that in the SOX2 group (Fig. 6d). Interestingly, ectopic SOX2 further dramatically enhanced the generation of secondary neurospheres from the primary ones (Fig. 6c,d). These spheres and the single cells dissociated from them were labeled by tdT, GFP, and the NSC marker nestin (NES; Fig. 6e). When cultured under differentiation conditions for 6 days, neurosphere-derived single cells could become TUBB3+ neurons, GFAP+ astrocytes, and NG2+ oligodendrocyte precursors (Fig. 6f). All of these cells were traced by the marker tdT indicating an origin from astrocytes. Together, these data show that SOX2 can promote the generation of multipotent NSCs from spinal astrocytes in culture consistent with results from cultured fibroblasts and cortical astrocytes42, 43.
Figure 6. Induction of NSCs from spinal astrocytes by SOX2 in culture.
(a) Experimental scheme. (b) Immunocytochemistry confirming the astrocyte identity of cultured tdT+ cells. (c) Representative micrographs of neruospheres from spinal astrocytes infected with the hGFAP-GFP-T2A-SOX2 lentivirus (dpi, days post infection). (d) Frequency of neurosphere-forming cells from spinal astrocytes infected with the indicated lentivirus. Secondary spheres were quantified at 7 days after plating (mean+s.d.; n=5; *p < 0.01 by Student’s t-test). (e) Nestin (NES) expression in SOX2-induced neurospheres. (f) Multiple lineage differentiation of SOX2-induced secondary neurospheres. Immunocytochemistry was performed 6 days after plating dissociated single cells. Scales: 50 µm (b), 200µm (c), 60 µm (e), and 40 µm (f).
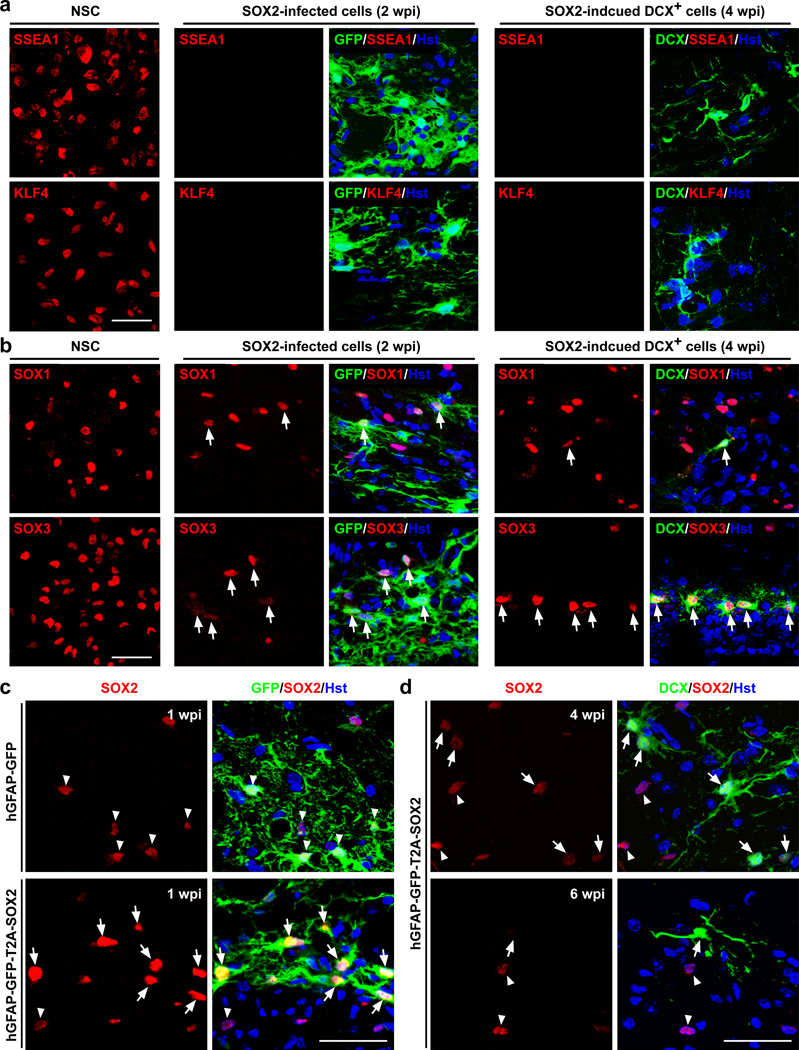
We then examined the SOX2-induced neurogenesis in the adult spinal cord using immunohistochemistry. A time course analysis showed that DCX+ cells were not readily detectable until 4 wpi suggesting that the induced neurogenesis was a slow process. SSEA1 (also known as LeX or CD15) and KLF4, markers for multipotent stem cells, were not expressed in virus-infected astrocytes or the induced DCX+ cells in the adult spinal cord at 1, 2, 3 or 4 wpi (Fig. 7a). Interestingly, SOX1 and SOX3, members of the SOXB1 subfamily of SOX transcription factors and key players in neural progenitors, were expressed in a subset of SOX2 virus-infected astrocytes, although these two factors were also distributed in some of the non-infected cells (Fig. 7b). The expression of SOX1 and SOX3 persisted into the DCX+ stage, consistent with their reported expression in neuroblasts in the subventricular zone of the adult mouse brain40,41.
Figure 7. Expression of SOXB1 factors during the reprogramming process.
(a) Markers for multipotent stem cells are not detectable in the spinal cord injected with the hGFAP-GFP-T2A-SOX2 virus. The expression in neural stem cells (NSCs) was served as a control. (b–d) The SOXB1 transcription factors are expressed during the reprogramming process. (b) Expression of SOX1 and SOX3 in SOX2 virus-infected cells and induced neuroblasts (indicated by arrows). Cultured NSCs served as controls. (c) Robust expression of SOX2 in the spinal cord injected with the hGFAP-GFP-T2A-SOX2 virus (indicated by arrows). Arrowheads indicate endogenous SOX2 expression. (d) Downregulation of SOX2 in converted neuroblasts (indicated by arrows). Arrowheads indicate endogenous SOX2 expression. Scales: 40 µm (a–d).
Although endogenous SOX2 is broadly expressed in astrocytes in the adult spinal cord42, immunostaining showed that cells infected with the hGFAP-GFP-T2A-SOX2 virus had a significantly higher level of total SOX2 expression when compared to the surrounding non-infected cells (Fig. 7c). In induced DCX+ cells, SOX2 expression was downregulated to a level similar to their neighboring non-DCX+ cells at 4 wpi and became almost non-detectable at 6 wpi (Fig. 7d). We hypothesized that such dynamic expression of SOX2 that was delivered by the hGFAP promoter was critical for induced neurogenesis. This hypothesis was tested by controlling SOX2 expression through the constitutively active CMV early enhancer/chicken beta actin (CAG) promoter. Robust SOX2 expression was observed in virus-infected cells; however, DCX+ cells were not detected at 4 wpi (Supplementary Fig. 7). These results suggest that the initial higher level of SOX2 expression and its subsequent downregulation was indeed necessary for the reprogramming process.
In summary, these results reveal that the ectopic expression of SOX2 reprograms resident astrocytes to proliferative DCX+ neuroblasts in the adult spinal cord contrary to in vitro cell culture conditions that enable SOX2 to convert astrocytes to multipotent stem cells (Fig. 6). This highlights the importance of the cellular milieu on the reprogramming process.
Induced neuroblasts mature into synapse-forming interneurons
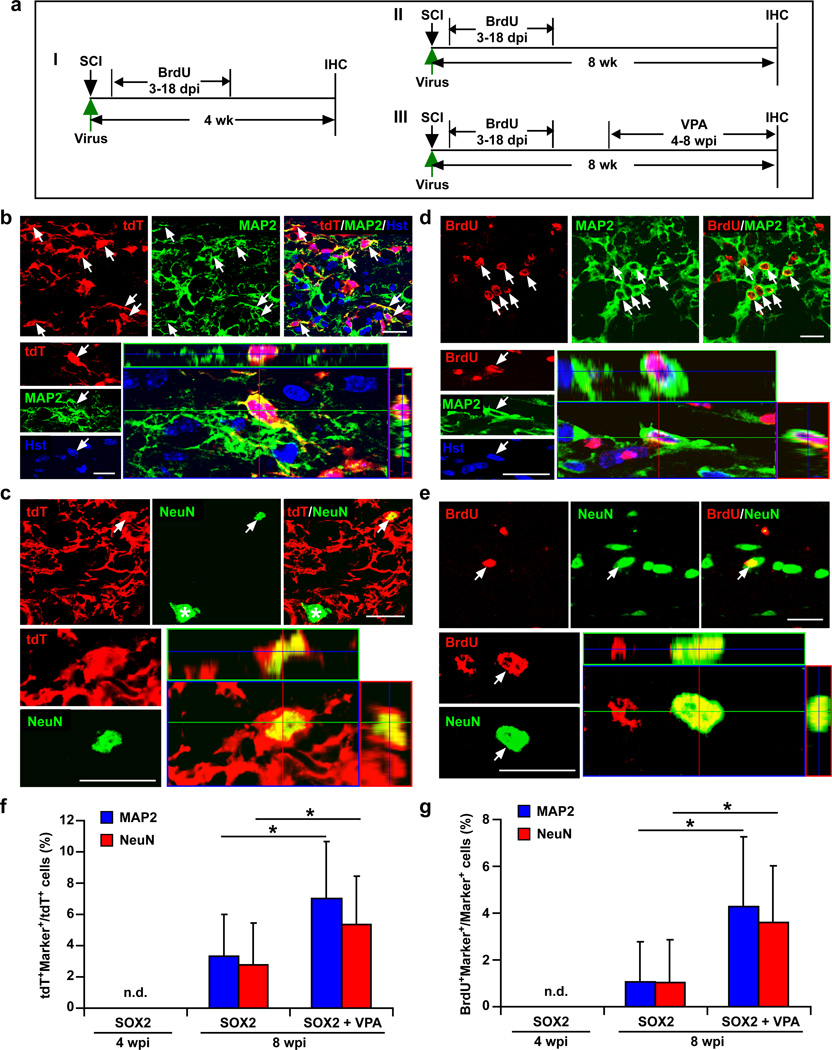
As DCX is restricted to neuroblasts and immature neurons and TUBB3 is also broadly expressed in both immature and mature neurons, the expression of these two markers during SOX2-induced neurogenesis does not indicate that these new neurons can become mature. In comparison, MAP2 and NeuN are markers for mature neurons. These markers were undetected in both BrdU- and tdT-traced cells in mGfap-Cre;Rosa-tdT mice that were injected with SOX2-expressing virus at 4 wpi (Fig. 8a,f–g). When examined at 8 wpi, however, approximately 3% of tdT+ cells were labeled by either MAP2 or NeuN around the virus-injected region (Fig. 8b,c,f). Correspondingly, an estimated 1% of either MAP2- or NeuN-positive cells within the injected region also incorporated BrdU (Fig. 8d,e,g), indicating that they were mature neurons reprogrammed through cell division. Valproic acid (VPA) is a histone deacetylase inhibitor that enhances cellular reprogramming43 and promotes normal neurogenesis and maturation of iANBs in the adult brain29,44. Here, we examined the effect of VPA on SOX2-induced neurogenesis in the adult spinal cord. Interestingly, 4 weeks of intraperitoneal injection of VPA (100 mg/kg, twice daily) resulted in nearly a 2-fold increase of tdT+ cells being labeled by either MAP2 or NeuN in mGfap-Cre;Rosa-tdT mice (Fig. 8f). These neurons could survive up to 30 wpi, the longest time examined (Supplementary Fig. 8). Quantification of BrdU-labeled cells also showed a 3-fold increase of newly generated MAP2+ or NeuN+ mature neurons (Fig. 8g). Together, these data indicate that SOX2-induced neurons become mature by 8 wpi and can be further enhanced by treatment with VPA.
Figure 8. Maturation of SOX2-induced new neurons in the injured adult spinal cord.
(a) Experimental scheme. mGfap-Cre;Rosa-tdT mice were injected with hGFAP-SOX2 or a control virus immediately after injury and analyzed by IHC at 4 (I) or 8 (II, III) wpi (dpi, days post viral injection; VPA, valproic acid). (b–c) Expression of the mature neuronal markers MAP2 (b) or NeuN (c) in tdT+ cells in SOX2 virus-injected spinal cords at 8 wpi. tdT-traced MAP2+ or NeuN+ cells were not detectable in control virus-injected spinal cords. Orthogonal views of cells with expression of the indicated markers are also shown. Compared to endogenous spinal motoneurons (indicated by an asterisk in c), the SOX2-induced neurons are interneuron-like with a smaller soma (indicated by an arrow in c). (d–e) SOX2-induced mature neurons pass through a proliferative stage. Mice were treated with BrdU at 3–18 dpi and analyzed at 8 wpi. Orthogonal views of BrdU-traced mature neurons are also shown. (f–g) Quantification of SOX2-induced mature neurons in the injured adult spinal cord (mean+s.d.; n=5 mice per group; *p<0.01 by Student’s t-test). Scales: 20 µm (b–e).
We analyzed the cellular identity of the reprogrammed neurons in mGfap-Cre;Rosa-tdT mice that were injected with hGFAP-SOX2 virus and treated with VPA for 4 weeks beginning at 4 wpi (Fig. 9a). These mice were also treated with BrdU from 3 to 18 dpi to label newly generated cells (Fig. 9a). Immunohistochemistry showed that none of the tdT+ cells expressed choline acetyltransferase (ChAT), a marker for cholinergic motor neurons (Supplementary Fig. 9a). In contrast, the astrocyte-derived mature neurons (indicated by the expression of tdT and MAP2) were positive for GABA or vGLUT1, markers for inhibitory or excitatory neurons, respectively (Fig. 9b and Supplementary Fig. 9b–c). Importantly, co-staining with GABA and BrdU confirmed that these cells were indeed newly reprogrammed (Fig. 9c). The GABAergic neuronal identity was further demonstrated by staining with an antibody against glutamate decarboxylase (GAD65) (Fig. 9d). Interestingly, some tdT-traced cells were co-labeled by synapsin-1 (SYN1), a marker for presynaptic terminals, in spinal cords injected with SOX2 virus but not with a control virus (Fig. 9e). Confocal analyses under higher magnifications showed that dense bouton-like terminals co-stained with SYN1 and tdT were juxtaposed to the soma and axon of ChAT+ cells (Fig. 9f–g), indicating the formation of synapses between SOX2-induced new neurons and local motor neurons. Together, these results showed that ectopic SOX2 could convert local astrocytes into synapse-forming GABAergic interneurons in the adult spinal cord.
Figure 9. SOX2-induced neurons resemble GABAergic interneurons in the adult spinal cord.
(a) Experimental scheme to examine SOX2-induced mature neurons. (b–c) Ectopic SOX2 converts astrocytes (indicated by tdT) to GABAergic interneurons (indicated by GABA and GAD65 expression). Co-expression of the indicated markers is also shown by an orthogonal view. These cells were not detectable in spinal cords injected with a control virus. (d) BrdU-labeling indicates that SOX2-induced GABA+ cells are newly generated. Co-expression of the indicated markers is shown by an orthogonal view. (e) Expression of synapsin-I (SYN1) in tdT+ cells in spinal cords injected with SOX2 but not a control virus. An orthogonal view is to show co-localization of the indicated markers. (f–g) Confocal images showing synapse-formation between SOX2-induced neurons (labeled by tdT) and the soma (f) or axon (g) of endogenous cholinergic motoneurons (labeled by ChAT). Synapses are indicated by SYN1-expression. Scales: 20 µm (b–g).
DISCUSSION
Supporting the most recent results that the fate of non-neuronal cells in the adult brain could be reprogrammed in vivo22,23,29,45, we provide further evidence showing that the single transcription factor SOX2 is also sufficient to convert endogenous differentiated astrocytes into neuroblasts and mature neurons in the adult spinal cord with or without severe injury. The SOX2-induced neurogenesis passes through a proliferation phase and can give rise to synapse-forming interneurons.
Postnatal spinal cords retain cells resembling NSCs that can self-renew and generate neurons, astrocytes, and oligodendrocytes when cultured in vitro39,46. Although new neurons were reported in the adult spinal cord under physiological or pathological conditions47,48; the other studies rather showed that the adult spinal cord is not a neurogenic region and failed to identify new neurons under either the injured or uninjured state4,6,49,50. We are also unable to detect any newly generated neurons in the adult spinal cord non-injected or injected with lentivirus expressing the control GFP or other 11 transcription factors under the hGFAP promoter. Notwithstanding these discrepancies, spontaneous neurogenesis in the adult spinal cord is extremely limited, if there is any, and requires strategies to overcome this limitation. Through a combination of growth factor treatment and ectopic expression of the transcription factor NGN2, neurogenesis can be stimulated from endogenous proliferative neural progenitor cells in the adult spinal cord50. As an alternative strategy, our current study reveals that the fate of resident astrocytes can be reprogrammed to become neuroblasts and mature neurons in the adult spinal cord. NGN2-mediated differentiation of neural progenitors is a fast process with neurons being detectable as early as 3 days post injection, although a majority of the induced neurons cannot survive beyond 56 days even in the presence of exogenous BDNF50. In sharp contrast, our time course analyses reveal that SOX2-mediated in vivo reprogramming is a slow process with neuroblasts and mature neurons not readily detectable until 4 wpi and 8 wpi, respectively. Differentiated astrocytes might be more refractory to fate reprogramming as compared to neural progenitors50 and thus potentially contribute to the slow reprogramming observed in our study. It is remarkable that SOX2-induced mature neurons from astrocytes were still detectable at 210 dpi when the mice were treated with VPA.
Astrocytes are abundantly and ubiquitously distributed throughout the central nervous system. In response to injury, they are activated, proliferate, and contribute to the formation of a glial scar24,25,51. These reactive astrocytes play a critical role in sealing the lesion site in the early phase of neural damage but constitute a mechanical and biochemical obstacle to axonal regeneration at later stages52. When isolated and cultured in vitro, activated astrocytes can form neurospheres that are able to generate neurons, oligodendrocytes, and astrocytes51,53. Glutamatergic and GABAergic neurons can be respectively induced from reactive astrocytes by the forced expression of NGN2 and DLX2 in culture27. Despite these observations, astrocyte-derived neurons are not detectable around injured regions of the brain or spinal cord4,6,49–51,54. Nonetheless, these data indicate that endogenous astrocytes might be ideal targets for in vivo lineage reprogramming. Supporting this hypothesis, our in vivo screens for reprogramming factors and genetic lineage tracings showed that ectopic SOX2 uniquely converts resident astrocytes to DCX+ neuroblasts and MAP2+ mature neurons in the adult spinal cord. NG2+ cells are also amenable to fate reprogramming15,22; however, SOX2 under the hGFAP promoter is rarely expressed in these cells, potentially accounting for our failure to detect induced neuroblasts from these cells in the adult spinal cord.
Reprogramming of somatic cells into neurons represents a novel approach towards understanding and treating neural injury and degeneration. The reprogramming process can be initiated by a few cell fate-determining transcription factors in culture55. SOX2, a high-mobility group DNA-binding domain transcription factor56, is essential for specification and/or maintenance of progenitor identity57–59. It also plays a key role in reprogramming somatic cells to pluripotent stem cells or NSCs in culture8,38,60–63. After screening pools of transcriptional regulators, we previously showed that SOX2 is alone sufficient to convert resident astrocytes to neuroblasts in the adult brain29. Nevertheless, it is not clear whether astrocytes in different regions of the central nervous system can be similarly reprogrammed, as they exhibit regional diversity64. It might not be coincident, though, that our additional screens also identified SOX2 as a key reprogramming factor in the adult spinal cord. Interestingly, SOX2 is endogenously expressed in spinal astrocytes42; yet, no new neurons are detected in the spinal cord injected with the control virus or virus expressing many other transcription factors in our study. This suggests that a threshold of SOX2 expression is required to induce cell fate change. SOX2 expression under the hGFAP promoter is dynamic with a much higher level in reactive astrocytes and a subsequent downregulation in converted neuroblasts. The initial robust SOX2 expression may induce a “plastic” state in infected astrocytes and cues in the local environment may help push them towards a neuroblast phenotype in vivo. A future detailed analysis is required to tease out the underlying molecular mechanism.
Injury to spinal cords frequently disrupts the long ascending and descending spinal tracts and leads to functional impairments and long-term disability. There is currently no effective treatment for SCI. Reconstructing the neural circuits to mediate transmission of electrical impulses through the lesion site has been a major research focus65. Indeed, transplanted neurons are able to reinstate the disrupted neural circuitry in a mouse model of SCI66. Our present study demonstrates that SOX2-induced adult neurogenesis can generate mature neurons with features of GABAergic interneurons in injured VPA-treated spinal cords. The new neurons are capable of forming synapses with pre-existing ChAT+ motor neurons, suggesting potential integration into the local neural network of the injured spinal cord. Future work will examine whether induced neurons can form intraspinal relays that are able to mediate communication between segments above and below the lesion site.
In summary, the results of this study raise a promising potential for treating SCI through cellular regeneration by in situ conversion of patients’ endogenous glial cells to neurons. This strategy might face fewer obstacles to clinical applications than other approaches since exogenous cells and transplantation are not required. However, the current reprogramming efficiency and the number of converted neurons are low. Further studies are necessary to enhance the reprogramming process and to define a precise strategy to generate subtype-specific neurons that are required for functional recovery after SCI.
METHODS
Animals
Wild type male C57/BL6J and the immunodeficient NOD scid gamma (NSG) mice were purchased from the Jackson Laboratories. The following transgenic mice have been previously described and were obtained from the Jackson Laboratories: mGfap-Cre line 77.667, Ng2-Cre36, Rosa-tdTomato (tdT)37, and Rosa-YFP68. Adult male and female mice at 2–3 months of age were used unless otherwise stated. All mice were housed under a 12 h light/dark cycle and had ad libitum access to food and water in the UT Southwestern animal facility. No statistical method was used to predetermine sample size. The experiments were not randomized and the experimenters were not blinded to the allocation of animals during experiments and outcome assessment. All experimental procedures and protocols were approved by the Institutional Animal Care and Use Committee at UT Southwestern.
SCI model
Surgeries were conducted under deep anesthesia induced by a cocktail of ketamine and xylazine69. Briefly, a laminectomy was performed to expose the dorsal surface of the T7–9 segment. A hemisection was then introduced at the right side of the spinal cord by cutting twice at the same place using a fine corneal blade. After surgery, animals were returned to their home cages and received manual bladder expression twice daily until reflexive bladder control returned.
Lentivirus preparation and stereotactic injection
Lentivirus was used to deliver candidate factors into the adult spinal cords. Lentiviral vector hGFAP-GFP was generated by sub-cloning the synthetic hGFAP promoter30 into CS-CDF-CG-PRE vector with EcoRI and AgeI sites. Candidate genes from human or mouse sources were then amplified by PCR and sub-cloned into the hGFAP-GFP vector by replacing the GFP gene at AgeI and XhoI sites. The third generation, replication-deficient lentivirus was generated in HEK293T cells by transient transfections with lentiviral vectors and the packaging plasmids (pMDL, VSV-G, and pREV). Lentivirus was collected, precipitated with polyethylene glycol (PEG) 8000, and concentrated by centrifugation. Viral titers were determined by measuring the coexpressed GFP or antibody staining after infection of Cos-7 cells70. Using a Hamilton syringe and a 33 gauge, 45 degree-beveled needle (Hamilton, Reno, NV), 1.5 µl of lentivirus (0.5–2 × 109 colony-forming unit/ml) was manually injected into the spinal cord parenchyma at each of the two locations 3 mm apart at the T8 level. For animals with injured spinal cords, 1.5 µl of virus was immediately injected after hemisection at each of the two locations (1.5 mm proximal and distal to the lesion site). Virus was slowly injected within 1 min, followed by holding the needle at the injection site for 3 min and another minute to slowly withdraw the injection needle.
BrdU labeling
Proliferating cells in the spinal cord were labeled by intraperitoneal injection of 5-bromo-2-deoxyuridine (BrdU, Sigma) at a dose of 100 mg/kg body weight, twice daily for the indicated duration. BrdU incorporation was detected by fluorescent staining using an anti-BrdU antibody (rat, 1:500, Accurate Chemical).
Immunohistochemistry
Mice were killed by CO2 overdose and fixed by intracardial perfusion with 4% (w/v) paraformaldehyde in phosphate-buffered saline (PBS). Spinal cords were surgically dissected out, post-fixed overnight and cryoprotected with 30% sucrose at 4°C for 48 hr. Transverse sections or 1.5 cm longitudinal sections of spinal cords spanning the injection/injury sites were sectioned on a cryostat set at 14 µm thickness. The primary antibodies listed in Supplementary Table 1 were used for immunohistochemical analyses. Alexa Fluor 488-, 594-, or 647-conjugated corresponding secondary antibodies from Jackson ImmunoResearch were used for indirect fluorescence. Nuclei were counterstained with Hoechst 33342 (Hst). Images were captured and examined by an Olympus fluorescence microscope or a Zeiss LSM 510 confocal microscope. A Cell Counter software plugin in the ImageJ program was used for cell counting.
Primary astrocyte culture
Primary astrocytes were isolated from the spinal cord of postnatal day 2 to day 4 mGfap-Cre;Rosa-tdT mouse. After removal of the meninges, the spinal cord was dissociated into a single-cell suspension through trypsinization and mechanical disruption. Cells were pelleted for 5 min at 1,000 rpm, re-suspended and plated in DMEM/F-12 (1:1) medium containing 15% fetal bovine serum (Life Technologies). Culture medium was replaced 24 hr later and every 3 days thereafter. When cultures became confluent, loosely attached microglia and oligodendrocyte precursor cells were removed from the cell monolayer by shaking vigorously. Cells were then passaged after dissociation with trypsin/EDTA.
Neurosphere formation assays
Spinal astrocytes from the mGfap-Cre;Rosa-tdT mice were purified through fluorescence activated cell sorting based on tdT expression. These astrocytes were then infected with the hGFAP-GFP-T2A-SOX2 or hGFAP-GFP lentivirus. Three days later, the culture was switched to NSC medium, which consists of DMEM:F12:Neurobasal (2:2:1), 0.8% N2, 0.4% B27 (Life Technologies), 20 ng/ml FGF2, and 20 ng/ml EGF (Peprotech). Primary neurospheres were collected at day 14 and serially passaged under the same culture condition. To induce differentiation, secondary neurospheres were dissociated into single cells, which were then seeded onto Matrigel-coated glass coverslips in 24-well plate at a density of 2 × 104 cells per well. Cells were further cultured for 6 days in the NSC medium without FGF2 and EGF. Immunocytochemistry was performed on 4% paraformaldehyde-fixed cells using the indicated primary antibodies.
Cell transplantation
Purified tdT+ spinal astrocytes were infected with the hGFAP-GFP-T2A-SOX2 or hGFAP-GFP lentivirus. Three days later, these cells (1 × 105/1.5 µl) were stereotactically injected into the spinal cord of 2–3 month old NSG mice at T7–9 segment using a pulled glass micropipette with an inner diameter of 40 µm. Animals were killed 4 or 5 weeks after transplantation. A 1.5 cm length of the spinal cord centered at the injection site was separated and subjected to immunohistochemical analyses.
Statistical analysis
The quantitative data were expressed as mean±s.d. from three to five mice. Statistical analysis was performed using unpaired Student’s t tests. Differences were considered significant at P< 0.05.
Supplementary Material
Acknowledgements
We thank members of the Zhang laboratory for discussions and reagents and Derek Smith for critical comments on the manuscript. C.L.Z. is a W. W. Caruth, Jr. Scholar in Biomedical Research. This work was supported by the American Heart Association (09SDG2260602), the Welch Foundation Award (I-1724), the Ellison Medical Foundation Award (AG-NS-0753-11), and NIH Grants (1DP2OD006484 and R01NS070981; to C.L.Z.).
Footnotes
Author Contributions. Z.S. and C.L.Z. conceived and designed the experiments. Z.S. performed the experiments. W.N. and M.L.L provided critical reagents and scientific input. Y.Z. maintained the mouse colonies. Z.S. and C.L.Z. analyzed data and prepared the manuscript.
Competing Financial Interests: The authors declare no competing financial interests.
REFERENCES
- 1.Bradbury EJ, McMahon SB. Spinal cord repair strategies: why do they work? Nat Rev Neurosci. 2006;7:644–653. doi: 10.1038/nrn1964. [DOI] [PubMed] [Google Scholar]
- 2.Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- 3.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 4.Horky LL, Galimi F, Gage FH, Horner PJ. Fate of endogenous stem/progenitor cells following spinal cord injury. The Journal of comparative neurology. 2006;498:525–538. doi: 10.1002/cne.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang H, et al. Endogenous neurogenesis replaces oligodendrocytes and astrocytes after primate spinal cord injury. J Neurosci. 2006;26:2157–2166. doi: 10.1523/JNEUROSCI.4070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horner PJ, et al. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snyder EY, Teng YD. Stem cells and spinal cord repair. N Engl J Med. 2012;366:1940–1942. doi: 10.1056/NEJMcibr1200138. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Ogawa Y, et al. Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J Neurosci Res. 2002;69:925–933. doi: 10.1002/jnr.10341. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi Y, et al. Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PloS one. 2012;7:e52787. doi: 10.1371/journal.pone.0052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishimura S, et al. Time-dependent changes in the microenvironment of injured spinal cord affects the therapeutic potential of neural stem cell transplantation for spinal cord injury. Mol Brain. 2013;6:3. doi: 10.1186/1756-6606-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsui T, Akamatsu W, Nakamura M, Okano H. Regeneration of the damaged central nervous system through reprogramming technology: Basic concepts and potential application for cell replacement therapy. Exp Neurol. 2012 doi: 10.1016/j.expneurol.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Okano H, et al. Steps toward safe cell therapy using induced pluripotent stem cells. Circ Res. 2013;112:523–533. doi: 10.1161/CIRCRESAHA.111.256149. [DOI] [PubMed] [Google Scholar]
- 14.Knoepfler PS. Deconstructing stem cell tumorigenicity: a roadmap to safe regenerative medicine. Stem cells. 2009;27:1050–1056. doi: 10.1002/stem.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karow M, et al. Reprogramming of pericyte-derived cells of the adult human brain into induced neuronal cells. Cell stem cell. 2012;11:471–476. doi: 10.1016/j.stem.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Vierbuchen T, et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinrich C, et al. Generation of subtype-specific neurons from postnatal astroglia of the mouse cerebral cortex. Nat Protoc. 2011;6:214–228. doi: 10.1038/nprot.2010.188. [DOI] [PubMed] [Google Scholar]
- 18.Liu ML, et al. Small molecules enable neurogenin 2 to efficiently convert human fibroblasts into cholinergic neurons. Nat Commun. 2013;4:2183. doi: 10.1038/ncomms3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song K, et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian L, et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo Z, et al. In Vivo Direct Reprogramming of Reactive Glial Cells into Functional Neurons after Brain Injury and in an Alzheimer’s Disease Model. Cell Stem Cell. 2013 doi: 10.1016/j.stem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torper O, et al. Generation of induced neurons via direct conversion in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:7038–7043. doi: 10.1073/pnas.1303829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heins N, et al. Glial cells generate neurons: the role of the transcription factor Pax6. Nat Neurosci. 2002;5:308–315. doi: 10.1038/nn828. [DOI] [PubMed] [Google Scholar]
- 27.Heinrich C, et al. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol. 2010;8:e1000373. doi: 10.1371/journal.pbio.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corti S, et al. Direct reprogramming of human astrocytes into neural stem cells and neurons. Experimental cell research. 2012;318:1528–1541. doi: 10.1016/j.yexcr.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niu W, et al. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat Cell Biol. 2013;15:1164–1175. doi: 10.1038/ncb2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee Y, Messing A, Su M, Brenner M. GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia. 2008;56:481–493. doi: 10.1002/glia.20622. [DOI] [PubMed] [Google Scholar]
- 31.Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- 32.Brown JP, et al. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- 33.Couillard-Despres S, et al. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- 34.Fleming JC, et al. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- 35.Richardson WD, Young KM, Tripathi RB, McKenzie I. NG2-glia as multipotent neural stem cells: fact or fantasy? Neuron. 2011;70:661–673. doi: 10.1016/j.neuron.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- 37.Luche H, Weber O, Nageswara Rao T, Blum C, Fehling HJ. Faithful activation of an extra-bright red fluorescent protein in “knock-in” Cre-reporter mice ideally suited for lineage tracing studies. Eur J Immunol. 2007;37:43–53. doi: 10.1002/eji.200636745. [DOI] [PubMed] [Google Scholar]
- 38.Ring KL, et al. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell. 2012;11:100–109. doi: 10.1016/j.stem.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss S, et al. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996;16:7599–7609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Venere M, et al. Sox1 marks an activated neural stem/progenitor cell in the hippocampus. Development. 2012;139:3938–3949. doi: 10.1242/dev.081133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang TW, et al. Sox3 expression identifies neural progenitors in persistent neonatal and adult mouse forebrain germinative zones. The Journal of comparative neurology. 2006;497:88–100. doi: 10.1002/cne.20984. [DOI] [PubMed] [Google Scholar]
- 42.Guo F, et al. Macroglial plasticity and the origins of reactive astroglia in experimental autoimmune encephalomyelitis. J Neurosci. 2011;31:11914–11928. doi: 10.1523/JNEUROSCI.1759-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huangfu D, et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nature biotechnology. 2008;26:795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hao Y, et al. Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J Neurosci. 2004;24:6590–6599. doi: 10.1523/JNEUROSCI.5747-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grande A, et al. Environmental impact on direct neuronal reprogramming in vivo in the adult brain. Nat Commun. 2013;4:2373. doi: 10.1038/ncomms3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kehl LJ, Fairbanks CA, Laughlin TM, Wilcox GL. Neurogenesis in postnatal rat spinal cord: a study in primary culture. Science. 1997;276:586–589. doi: 10.1126/science.276.5312.586. [DOI] [PubMed] [Google Scholar]
- 47.Shechter R, Ziv Y, Schwartz M. New GABAergic interneurons supported by myelin-specific T cells are formed in intact adult spinal cord. Stem Cells. 2007;25:2277–2282. doi: 10.1634/stemcells.2006-0705. [DOI] [PubMed] [Google Scholar]
- 48.Ke Y, et al. Early response of endogenous adult neural progenitor cells to acute spinal cord injury in mice. Stem Cells. 2006;24:1011–1019. doi: 10.1634/stemcells.2005-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johansson CB, et al. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- 50.Ohori Y, et al. Growth factor treatment and genetic manipulation stimulate neurogenesis and oligodendrogenesis by endogenous neural progenitors in the injured adult spinal cord. J Neurosci. 2006;26:11948–11960. doi: 10.1523/JNEUROSCI.3127-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buffo A, et al. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc Natl Acad Sci U S A. 2008;105:3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okada S, et al. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med. 2006;12:829–834. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- 53.Nakagomi T, et al. Isolation and characterization of neural stem/progenitor cells from post-stroke cerebral cortex in mice. Eur J Neurosci. 2009;29:1842–1852. doi: 10.1111/j.1460-9568.2009.06732.x. [DOI] [PubMed] [Google Scholar]
- 54.Buffo A, et al. Expression pattern of the transcription factor Olig2 in response to brain injuries: implications for neuronal repair. Proc Natl Acad Sci U S A. 2005;102:18183–18188. doi: 10.1073/pnas.0506535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang N, Ng YH, Pang ZP, Sudhof TC, Wernig M. Induced neuronal cells: how to make and define a neuron. Cell Stem Cell. 2011;9:517–525. doi: 10.1016/j.stem.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chew LJ, Gallo V. The Yin and Yang of Sox proteins: Activation and repression in development and disease. J Neurosci Res. 2009;87:3277–3287. doi: 10.1002/jnr.22128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bylund M, Andersson E, Novitch BG, Muhr J. Vertebrate neurogenesis is counteracted by Sox1–3 activity. Nat Neurosci. 2003;6:1162–1168. doi: 10.1038/nn1131. [DOI] [PubMed] [Google Scholar]
- 58.Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- 59.Episkopou V. SOX2 functions in adult neural stem cells. Trends Neurosci. 2005;28:219–221. doi: 10.1016/j.tins.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 60.Han DW, et al. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell. 2012;10:465–472. doi: 10.1016/j.stem.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 61.Lujan E, Chanda S, Ahlenius H, Sudhof TC, Wernig M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc Natl Acad Sci U S A. 2012;109:2527–2532. doi: 10.1073/pnas.1121003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim J, et al. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc Natl Acad Sci U S A. 2011;108:7838–7843. doi: 10.1073/pnas.1103113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thier M, et al. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell. 2012;10:473–479. doi: 10.1016/j.stem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 64.Tsai HH, et al. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science. 2012;337:358–362. doi: 10.1126/science.1222381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ben-Hur T. Reconstructing neural circuits using transplanted neural stem cells in the injured spinal cord. J Clin Invest. 2010;120:3096–3098. doi: 10.1172/JCI43575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abematsu M, et al. Neurons derived from transplanted neural stem cells restore disrupted neuronal circuitry in a mouse model of spinal cord injury. J Clin Invest. 2010;120:3255–3266. doi: 10.1172/JCI42957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gregorian C, et al. Pten deletion in adult neural stem/progenitor cells enhances constitutive neurogenesis. J Neurosci. 2009;29:1874–1886. doi: 10.1523/JNEUROSCI.3095-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Su Z, et al. Triptolide promotes spinal cord repair by inhibiting astrogliosis and inflammation. Glia. 2010;58:901–915. doi: 10.1002/glia.20972. [DOI] [PubMed] [Google Scholar]
- 70.Niu W, Zou Y, Shen C, Zhang CL. Activation of postnatal neural stem cells requires nuclear receptor TLX. J Neurosci. 2011;31:13816–13828. doi: 10.1523/JNEUROSCI.1038-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.