Abstract
A selective KGFR tyrosine kinase inhibitor, N-ethylamino-2-oxo-1,2-dihydro-quinoline-3-carboxamide, was synthesized and its possible inhibitory effects on the development of colon polyps and colorectal tumors was examined in APCMin/+ mice, a mouse model of human intestinal familial adenomatous polyposis. The present study shows for the first time that a dietary administration of a selective KGFR tyrosine kinase inhibitor lacks the overt-toxicities and significantly reduced the growth of small intestinal polyps in both male and female APCMin/+ mice. This inhibition of polyp growth appears to occur at a greater extent in female mice.
Keywords: KFGR, Tyrosine Kinase Inhibitor, Polyps, Chemoprevention, Colon Cancer
Keratinocyte growth factor (KGF, also known as FGF7) is a member of the fibroblast growth factor family that is produced in stromal tissue and stimulates DNA synthesis, proliferation and migration of epithelial cells.1 It is well established that these target epithelial cells contain high affinity keratinocyte growth factor receptor (KGFR, also known as FGFR-2IIIb).2 The biological actions of KGF are involved in normal morphogenesis and tissue repair.3 In addition, over-expression of KGF and/or KGFR is associated with the progression of several types of cancers including colorectal cancers.4–7 KGF/KGFR signaling via the ERK1/2 pathway often occurs early in the process of cancer cell initiation by enhancing cell proliferation and motility resulting in progression to a metastatic phenotype.8 Colorectal cancers are thought to arise due to a series of histopathologic and molecular changes that transform normal colonic epithelial cells into colorectal carcinoma, with an adenomatous polyp as an intermediate step in this process.9 In addition, recent developments in the cellular and molecular pathogenesis of colon cancer provide new insights for developing selective agents with potential chemopreventive properties against colon carcinogenesis. Chemoprevention is likely to play a major role in the management or prevention of colorectal cancer.10, 11 Thus, the present study examines the possible chemoprevention effect of a selective KGFR tyrosine kinase inhibitor, N-ethylamino-2-oxo-1,2-dihydro-quinoline-3-carboxamide (3), on the development of colon polyps and colorectal tumors in an in vivo animal model. In a previous study, compound 3 was shown to reduce KGF-mediated proliferation of MCF-7 human breast cancer cells in vitro.6
The synthesis of 3 was achieved in a three-step synthetic strategy as shown in Figure 1. The experimental details are presented in References and notes.12,13 In the first step, condensation reaction between commercially available Meldrum’s acid and 2-aminobenzaldehyde was carried at 75 °C for 8 hours in water using a previously reported procedure.14 Compound 1 (2-oxo-1,2-dihydro-quinoline-3-carboxylic acid) was obtained in a yield of 57.7%. In the second step, the carboxylic group was converted into carboxyl chloride 2 by refluxing compound 1 in an excess of thionyl chloride for 2 hours. After distilling off the excess thionyl chloride, compound 2 was reacted with an excess of ethylenediamine at room temperature for 15 hours to obtain compound 3. The excess ethylenediamine was removed by distillation under vacuum and the solid was washed several times with diethylether and dichloromethane and dried in vacuum to obtain compound 3 as a white solid in a yield of 66% with a purity of >98%. Compound 3 is soluble in water at a concentration of 2 mg/ml. Compound 3 was characterized by 1H and 13C NMR and HRMS.
Figure 1.
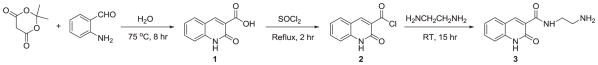
Synthetic scheme for N-ethylamino-2-oxo-1,2-dihydro-quinoline-3-carboxamide (3).
We selected the APCMin/+ mouse model for evaluating the potential chemopreventive properties of compound 3 against colon carcinogenesis because it is one of the most studied animal models of intestinal tumorigenesis and chemoprevention studies.15 It harbors a dominant germline mutation at codon 850 of the mouse homolog of human adenomatous polyposis coli (APC) gene, which is similar to the mutation in patients with familial adenomatous polyposis (FAP). APCMin/+ mice develop multiple adenomas throughout the whole intestinal tract that are primarily small intestinal polyps (tubular adenomas) and fewer colon tumors (adenomas and adenocarcinomas).16 The objective of this study was to determine whether the continuous administration of compound 3 in the diet (150 ppm) can prevent or reduce the incidence and size of colon polyps and intestinal tumors in the APCMin/+ mouse model without causing gross toxicity as determined by a reduction of body weight. The study design is shown in Figure 2. The study was carried out in a similar fashion to the previously reported studies with other drugs in the same mouse model.17, 18 The mice were recruited for the study at the age of 5 weeks. The mice were initially fed a control diet (AIN-76A) for one week and then the control groups were fed with the AIN-76A diet, while the treatment groups were fed the AIN-76A diet containing 150 ppm of compound 3.
Figure 2.

Study design for the evaluation of chemoprevention of intestinal tumorigenesis by compound 3. Animals: APCMin/+ male and female mice (~8–10/group). Experimental diets: a) AIN-76A diet and b) AIN-76A diet + Compound 3 (150 ppm)
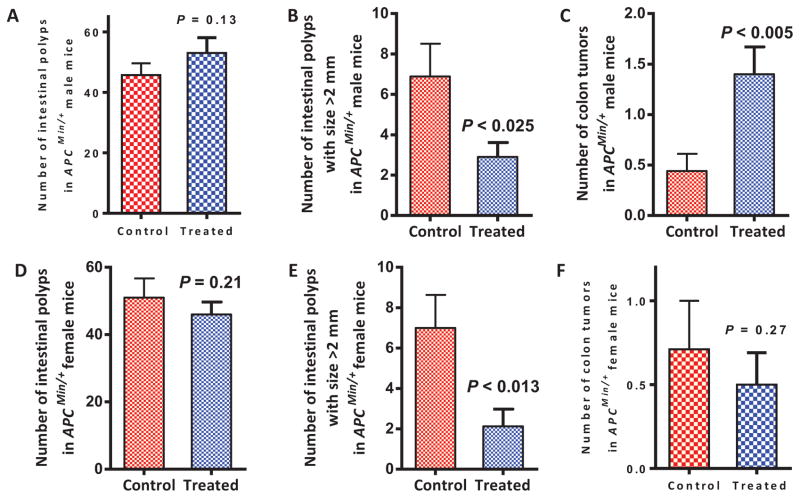
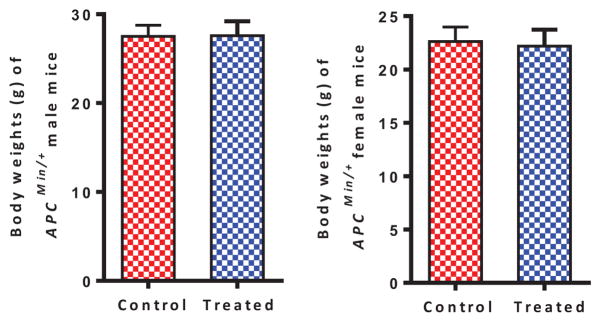
Figure 3 summarizes the chemopreventive effect of compound 3 on tumor multiplicity in the small intestine. Male and female APCMin/+ mice fed the control diet developed a total of 45.8 ± 3.9 and 51.0 ± 5.7 (mean ± SEM) intestinal polyps, respectively (Figure 3A and 3C). Whereas male and female APCMin/+ mice fed with the compound 3 containing diet developed a total of 53.0 ± 5.1 and 45.3 ± 3.7 intestinal polyps, respectively (Figure 3B and 3D). These results indicate that compound 3 did not prevent small intestinal polyp formation. However, the number of large-sized polyps (>2 mm) was significantly reduced with compound 3 treatment in both male (6.9 ± 1.6 verses 2.9 ± 0.7 control verses treatment groups) and female mice (7.0 ± 1.6 verses 2.1 ± 0.9 control verses treatment groups) as shown in Figure 3B and 3E. The effect is more profound in female mice (Figure 3E). As shown in Figure 3C and 3F, compound 3 did not inhibit colon tumor formation in either the male or the female mice. However, the body weights of both control and compound 3 treated mice were essentially unchanged at the end of study, indicating that compound 3 did not cause any gross toxicity at the dosage level used in this study (Figure 4). In addition, no evidence of gross toxicity was visually observed in the major organs when the animals were dissected at the conclusion of the study.
Figure 3.
Effects of compound 3 on small intestinal polyp and colon tumor incidence in APCMin/+ male and female mice. Date represents mean ± SEM (~8–10/group). P values (P < 0.05, statistically significant) were derived using ANOVA Tukey post test.
Figure 4.
Changes in body weight over time in APCMin/+ mice. Date represents mean ± SEM (~8–10/group). P values (P < 0.05, statistically significant) were derived using ANOVA Tukey post test.
In summary, the present study demonstrates for the first time that dietary administration of a selective KGFR tyrosine kinase inhibitor may stabilize the growth of small intestinal polyps in both male and female APCMin/+ mice. Also compound 3 appear to cause a reduction of tumor development to a greater extent in the female mice when compared to the male response.
Acknowledgments
We acknowledge funding from the NIH grant NCI-R01-CA094962 and Kerley-Cade Chair Endowment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Rubin JS, Osada H, Finch PW, Taylor WG, Rudikoff S, Aaronson SA. Proc Natl Acad Sci U S A. 1989;86:802. doi: 10.1073/pnas.86.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bottaro DP, Rubin JS, Ron D, Finch PW, Florio C, Aaronson SA. J Biol Chem. 1990;265:12767. [PubMed] [Google Scholar]
- 3.Finch PW, Cunha GR, Rubin JS, Wong J, Ron D. Developmental dynamics: an official publication of the American Association of Anatomists. 1995;203:223. doi: 10.1002/aja.1002030210. [DOI] [PubMed] [Google Scholar]
- 4.Yoshino M, Ishiwata T, Watanabe M, Komine O, Shibuya T, Tokunaga A, Naito Z. Oncol Rep. 2005;13:247. [PubMed] [Google Scholar]
- 5.Otte JM, Schmitz F, Banasiewicz T, Drews M, Folsch UR, Herzig KH. Eur J Clin Invest. 2000;30:222. doi: 10.1046/j.1365-2362.2000.00617.x. [DOI] [PubMed] [Google Scholar]
- 6.Mehta M, Kesinger JW, Zang XP, Lerner ML, Brackett DJ, Brueggemeier RW, Li PK, Pento JT. Anticancer Res. 2010;30:4883. [PubMed] [Google Scholar]
- 7.Zang XP, Lerner M, Brackett D, Pento JT. Anticancer Res. 2009;29:3417. [PubMed] [Google Scholar]
- 8.Pento JT. Drug Future. 2009;34:915. [Google Scholar]
- 9.Cho KR, Vogelstein B. Cancer. 1992;70:1727. doi: 10.1002/1097-0142(19920915)70:4+<1727::aid-cncr2820701613>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 10.Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM. Cell. 1996;87:803. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 11.Kelloff GJ. Adv Cancer Res. 2000;78:199. doi: 10.1016/s0065-230x(08)61026-x. [DOI] [PubMed] [Google Scholar]
- 12.2-Oxo-1,2-dihydro-quinoline-3-carboxylic acid (1). 2-Aminobenzaldehyde (1.0 g, 8.26 mmol) and Meldrum’s acid (1.19 g, 8.26 mmol) were combined in H2O (20 ml). The reaction mixture was stirred at 75 °C for 8 hr. The progress of the reaction was monitored by TLC. After completion of reaction, the reaction mixture was cooled to room temperature, the precipitate was filtered on sintered funnel and dried at suction to obtain the 2-Oxo-1,2-dihydro-quinoline-3-carboxylic acid as a solid (0.9 g, 57.7 %). 1H NMR (DMSO-d6, 300 MHz): δ (ppm) = 7.35 – 7.43 (m, 1H), 7.45 – 7.52 (m, 1H), 7.72 – 7.78 (m, 1H), 8.02 (d, J = 8.2 Hz, 1H), 8.96 (s, 1H).
- 13.N-ethylamino-2-oxo-1,2-dihydro-quinoline-3-carboxamide (3). To a sample of 1 (0.9 g, 4.76 mmol) was added freshly distilled thionyl chloride (10 ml) at room temperature. The reaction mixture was heated under reflux for 1 hr. The excess thionyl chloride was removed by distillation and co distilled with dichloromethane (2 × 10 ml) to obtain 2 in a quantitative yield. This compound was used immediately for next step without further purification. To execess of ethylene diamine (10 ml) was added a solution of 2 (0.83 g, 4 mmol) in 10 ml of acetone to at 0 °C. The reaction mixture was stirred at room temperature for overnight. The ethylene diamine was evaporated on rotary evaporator and residue was washed several times with ether and dichloromethane and dried to obtain the L-27 compound as a solid(0.61 g, 66%). 1H NMR (DMSO-d6, 300 MHz): δ (ppm) = 2.96 – 3.02 (m, 2H), 3.55 – 3.63 (m, 2H) 7.26 – 7. 32 (m, 1H), 7.42 – 7.47 (m, 1H), 7.62 – 7.70 (m, 1H), 7.94 (d, J = 7.6 Hz, 1H), 8.84 (s, 1H), 9.85 – 9.92 (m, 1H). 13C NMR (DMSO-d6, 75 MHz): δ (ppm) = 36.75, 37.30, 115.20, 118.40, 121.43, 122.68, 129.61, 132.61, 139.38, 143.73, 161.62, 163.60. Electrospray MS calcd. m/z for C12H13N3O2: 231.1; found: 232.1 ([M+H]+)
- 14.Renslo AR, Wells JM, Wolan D, Zorn J. [Google Scholar]
- 15.Rosenberg DW, Giardina C, Tanaka T. Carcinogenesis. 2009;30:183. doi: 10.1093/carcin/bgn267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA, Dove WF. Science. 1992;256:668. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 17.Swamy MV, Patlolla JM, Steele VE, Kopelovich L, Reddy BS, Rao CV. Cancer Res. 2006;66:7370. doi: 10.1158/0008-5472.CAN-05-4619. [DOI] [PubMed] [Google Scholar]
- 18.Mohammed A, Janakiram NB, Li Q, Choi CI, Zhang Y, Steele VE, Rao CV. Cancer prevention research. 2011;4:2015. doi: 10.1158/1940-6207.CAPR-11-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]