Abstract
Objectives:
To study the difference in the prevalence of hypertension and associated risk factors in urban and rural populations and the association of hypertension with various determinants.
Materials and Methods:
A community-based cross-sectional study was conducted in 48 villages and 15 urban wards of Jabalpur District of Central India. Nine hundred and thirty-nine individuals aged 20 years and above (624 from rural areas and 315 from urban areas) were included in the study. The prevalence of hypertension and associated cardiovascular risk factors was assessed in the urban and rural populations. A pretested questionnaire was used to collect data on socio-demographic, behavioral, and dietary factors. Anthropometric measurements of weight, height, waist and hip circumference, and blood pressure measurements were taken using the standard methodology. The glucose oxidase–peroxidase and cholesterol oxidase–cholesterol peroxidase methods were used to measure plasma glucose and serum cholesterol, respectively. Bivariate analysis was followed by multivariate analysis to detect the odds of getting hypertension with various risk factors for the urban and rural populations separately. Hypertension was defined as per Joint National Committee (JNC) - VII criteria.
Results:
The response rate was 97%. Overall prevalence of hypertension was 17%, with 21.4% in the urban population and 14.8% in the rural population. Significantly higher mean values of weight, height, body mass index (BMI), hip circumference (HC), waist circumference (WC), waist hip ratio (WHR), systolic blood pressure (SBP), fasting blood sugar (FBS), and serum cholesterol levels were mapped in the urban population in comparison with the rural population. Multivariate logistic regression analysis identified increasing age, parental history of hypertension, tobacco smoking, tobacco chewing, physical inactivity, high estimated per capita salt consumption, and BMI ≥27.5 kg/m2 as independent predictors for hypertension in the urban population, while in the rural population, increasing age, physical inactivity, central obesity, tobacco chewing and tobacco smoking were independent predictors for hypertension.
Conclusion:
The prevalence of hypertension and other cardiovascular risk factors was high in both urban and rural communities. Therefore, there is a need for comprehensive health promotion programs to encourage lifestyle modification.
Keywords: Blood pressure, cardiovascular risk factors, epidemiology, hypertension, lifestyle, screening
INTRODUCTION
Epidemiological changes have been observed in the prevalence of hypertension (HTN) and associated cardiovascular disease (CVD) risk factors in developing countries.[1,2] HTN is a chronic condition of concern because of its role in the causation of coronary heart disease (CHD), stroke, and other vascular complications. It is the most common CVD disorder which poses a major public health challenge to a population undergoing socioeconomic evolution. It is one of the major risk factors for CVD mortality, accounting for 20-50% of all deaths.[3] The overall burden of blood pressure–related diseases is rapidly rising in countries such as India and China as a consequence of the aging population, increasing urbanization, and an increase in age-specific rates of conditions such as stroke.[4,5]
In India, community surveys have documented that in the last three to six decades, the prevalence of HTN has increased about 30 times among the urban dwellers and about 10 times in the rural communities.[6] The prevalence of HTN differs between urban and rural communities, being higher in urban areas.[7,8]
Data available on the prevalence of CVD risk factors in Madhya Pradesh (MP), a state in Central India, is scarce. A Non-Communicable Diseases (NCD) risk factors survey[9] conducted by the Integrated Disease Surveillance Project (IDSP) in a few states in India documented a high prevalence of HTN in MP. However, at the district level, no data is available on the prevalence of risk factors. Even today, studies that give a comparative picture of CVD risk factors in urban and rural communities in India are rare. In the light of the foregoing, the present study was undertaken to study the prevalence of HTN and its associated factors in urban and rural populations of Central India.
MATERIALS AND METHODS
A community-based comparative cross-sectional study was conducted in the urban and rural populations of Jabalpur district of Central India to assess the prevalence of HTN and associated CVD risk factors in adults aged 20 years and above. Pooled epidemiological studies have documented the average prevalence of HTN in India as 25% in urban areas and 10% in rural areas.[7] Assuming the similar prevalence levels with absolute precision of 5% and 2.5%, sample sizes of 315 and 624 were calculated for urban and rural areas, respectively. The sample size was calculated using the formula n = 4(pq/L2), where P = assumed prevalence of HTN, q = 1 − p, and L = absolute precision.[10] Thus, a total of 939 individuals were included in the study. A total of 48 villages and 15 urban wards of Jabalpur District were selected utilizing population proportional to size (PPS) sampling methodology. In the villages and wards, households and Individuals were selected by simple random sampling. The three components of the study were (a) questionnaire-based survey for behavioral risk factors, (b) anthropometric measurements, and (c) biochemical measurements.
Ethical clearance was obtained from the Institutional Ethics Committee of Netaji Subhash Chandra Bose Medical College, Jabalpur. Informed consent was obtained for the questionnaire-based interview and anthropometric measurements, while written consent was taken for the collection of blood samples. All patients newly diagnosed with HTN, diabetes, and dyslipidemia were referred to the in-house physician for further investigation.
A pretested questionnaire was used to collect data on socio-demographic and behavioral risk factors. Tobacco consumption was assessed using questions on self-reported duration, frequency, and quantity consumed. Individuals were classified as ex-smoker, current smoker, and consumer of smokeless tobacco product. Self-reported alcohol intake data was also collected and subjects were classified as present and past consumer. Data on physical activity, per capita salt estimation, fruit and vegetable intake was collected using a questionnaire-based interview method.
Weight was measured in the upright position to the nearest 0.1 kg using pre-calibrated electronic weighing scale. Height was measured without shoes to the nearest 0.1 cm using calibrated stadiometer. Body mass index (BMI) was calculated by dividing the observed weight by height squared (kg/m2). Waist circumference (WC) was measured to the nearest 0.1 cm at the narrowest point between the lower end of the rib cage and the iliac crest. Hip circumference (HC) was measured to the nearest 0.1 cm at the greatest horizontal circumference below the iliac crest, at the level of greater trochanter. Blood pressure (BP) was measured from the right arm after the subject had been sitting for at least 5 min using digital automatic blood pressure apparatus (Omron HEM-7080, Omron Corporation, Kyoto, Japan). Three readings were taken 10 min apart, and the final reading was the average of the two closest readings.
Five milliliters of blood was collected from antecubital vein in two test tubes 10 h after overnight fasting. Blood sample for plasma glucose was collected in the test tube containing heparin sodium fluoride. Plasma glucose and total cholesterol were measured using an auto-analyzer. The glucose oxidase–peroxidase method and cholesterol oxidase–cholesterol peroxidase method were used to measure plasma glucose and serum cholesterol, respectively, in the Central Laboratory of Pathology Department, Netaji Subhash Chandra Bose Medical College and Hospital.
Definitions utilized for the various variables are as follows:
Socioeconomic status (SES): Defined as per modified Prasad's classification[11]
Current smoker: A person who has smoked at least 100 cigarettes in his lifetime and has continued to smoke every day or some days in the last 30 days
Ex-smoker or former smoker: Defined as a person who has smoked more than a 100 cigarettes in his lifetime and who has not smoked for the last 12 months[12]
Tobacco chewing: Defined as a person who has consumed smokeless tobacco once a day or nearly every day in any form for the last 12 months
Alcohol use: Present consumer was defined as a person who has consumed alcohol everyday or some days in the last 30 days. Past consumer was defined as a person who used to consume alcohol but stopped taking alcohol 12 months ago
Estimated per capita salt consumption was quantified by 24-h dietary recall method for three consecutive days and an average was calculated[13]
Type of diet was categorized as vegetarian and nonvegetarian
Fruit and vegetable intake was classified according to the number of servings per week.[14]
Physical activity: Physical activity levels were classified into four categories: Sedentary = sedentary work (sitting jobs, working at a desk, computer) and at home (watching television, reading, or sitting) with no exercise (no leisure time physical activity); mild = mild activity at work (standing jobs or jobs involving walking a short distance) and at home (cooking and cleaning house) with no exercise; moderate = moderate activity at work (agricultural work, walking long distances, climbing more than 20 stairs in a day) and at home (gardening) with non-vigorous exercise (walking, yoga during leisure time) at least three times a week; and strenuous = strenuous activity at work (job involving manual labor) and at home (lifting heavy weights) with vigorous exercise (weight lifting, jogging, running as leisure time activity) at least three times a week
BMI classification: Subjects were classified using the World Health Organization (WHO) classification and the classification recently recommended for Asians.[15]
Central obesity was defined as waist–hip ratio (WHR) >0.85 for females and >0.90 for males[16]
WC was divided into category I [WC <80 cm (female) and WC <90 cm (male)], category II [WC = 80-89 cm (female) and WC = 90-99 cm (male)], and category III [WC >90 cm (female) and WC >100 cm (male)]
HTN: Defined as systolic blood pressure (SBP) ≥140 mmHg or diastolic blood pressure (DBP) ≥90 mmHg as per the WHO criteria or having a history of previously known disease. Pre-HTN was defined as SBP between 120 and 139 mmHg or DBP between 80 and 89 mmHg[17]
Type 2 diabetes mellitus: Diagnosed either by history of previously known disease or fasting plasma glucose ≥126 mg/dl, and impaired fasting glucose (pre-diabetes) was defined as fasting plasma glucose between 100 and 125 mg/dl[18]
Hypercholesterolemia: Defined as total fasting cholesterol level ≥200 mg/dl according to USA - Adult Treatment Panel (ATP) III guidelines[19]
Data collection was done by trained field investigators and data of completed forms was entered on the same day in order to maintain the quality. All the instruments were calibrated each day before the field work. After data collection, relevant statistical analysis was done using SPSS 17.0, and various statistical tests, e.g. Pearson's Chi-square test and t-test, were used for bivariate analysis. Multiple logistic regression (MLR) was used for all variables which were found significant during bivariate analysis.
RESULTS
Of the total of 939 individuals invited to participate, 28 refused to give their consent for participation in the study. Hence, the remaining 911 (309 from urban areas and 602 from rural areas) subjects were included in the final survey. The overall response rate was 97.02% (98.1% in urban areas and 96.5% in rural areas). Also, 47.6% of the subjects in the urban areas were females and males made up 54.8% of the subjects in the rural areas. The mean age of male and female subjects from urban areas was 38.64 ± 13.53 and 38.75 ± 14.47 years, respectively, and in rural areas, it was 39.64 ± 14.04 and 38.85 ± 14.04 years, respectively.
A comparative socio-demographic profile of individuals in the urban and rural populations is shown in Table 1. A significant difference was seen in the socio-demographic features such as caste, religion, educational status, occupation, family type, total number of family members, and SES in urban and rural communities. There was a significant difference between the socio-demographic profiles of individuals from the urban and rural communities.
Table 1.
Area wise socio-demographic profile of study subjects
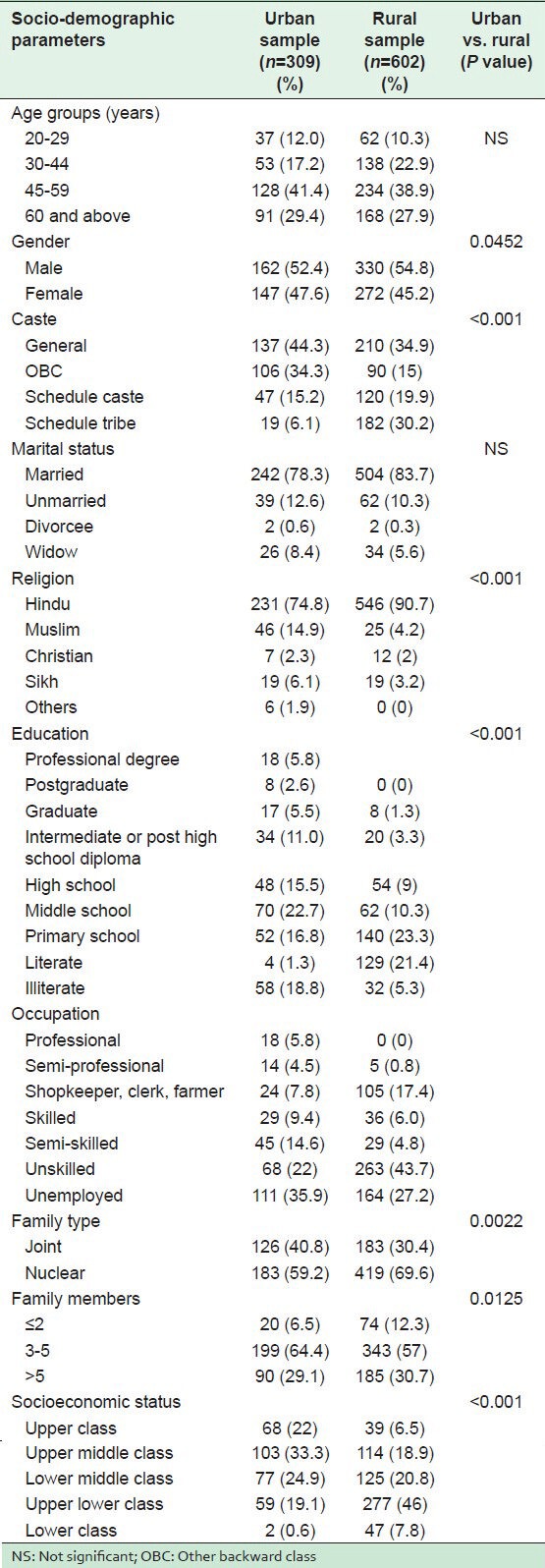
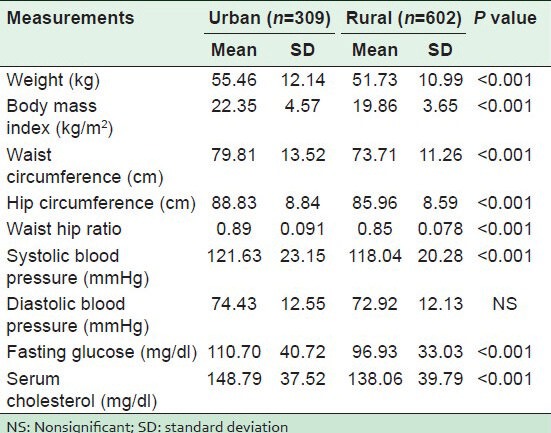
Table 2 shows the distribution of CVD risk factors in both the communities. In the urban population, a significantly higher proportion of HTN, parental history of HTN, nonvegetarian meals ≥1 per week, physical inactivity, category III of WC, high WHR (central obesity), BMI ≥23 kg/m2, diabetes, and hypercholesterolemia were observed. However, in the rural population, there was a significantly high proportion of low fruit intake (≤1 per week), the estimated per capita salt consumption of >10 g/day, and tobacco smoking. There was an insignificant difference in the proportion of vegetable intake, alcohol and smokeless tobacco consumption. As shown in Table 3, significantly higher mean values of weight, BMI, WC, HC, WHR, SBP, fasting blood sugar (FBS), and serum cholesterol were observed in urban dwellers than in the rural subjects. An insignificantly higher mean value of DBP was recorded in the urban subjects.
Table 2.
Area wise prevalence of hypertension and other cardiovascular risk factors
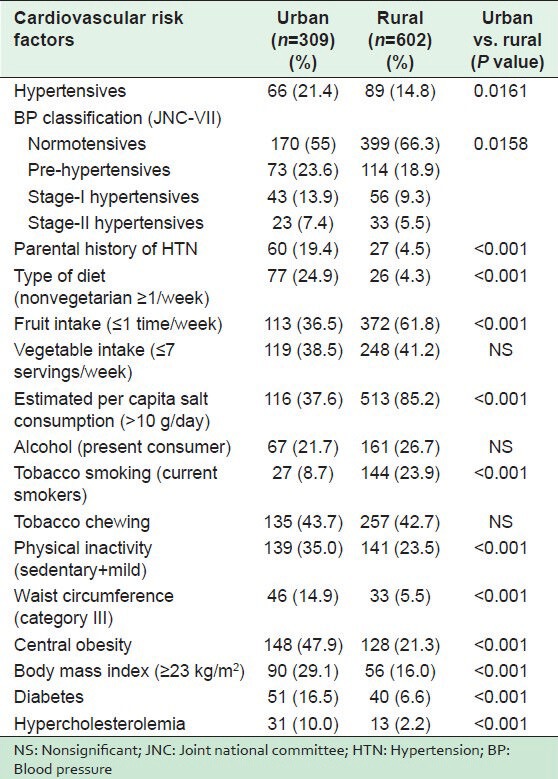
Table 3.
Mean and standard deviation for various cardiovascular disease risk factors in the study population as per area of residence
Tables 4 and 5 show the results of bivariate analysis, while Table 6 shows the results of MLR to detect the association of HTN with socio-demographic profiles and other CVD risk factors in urban and rural areas.
Table 4.
Unadjusted odds ratios of getting hypertensive for various socio-demographic determinants (bivariate analysis)
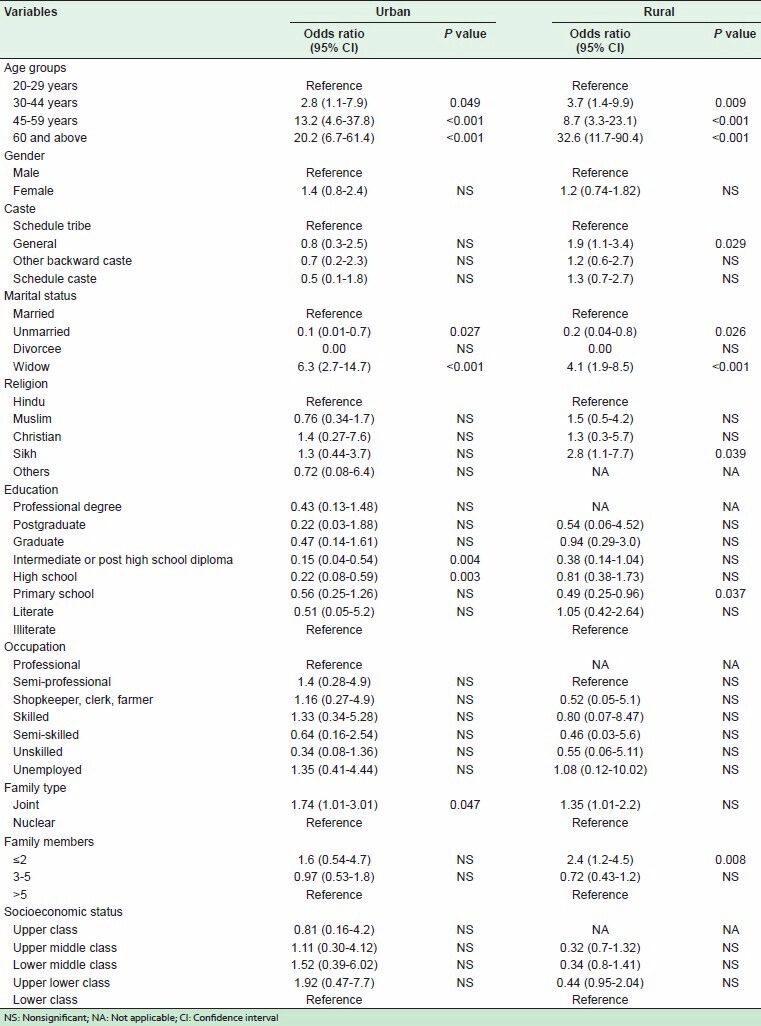
Table 5.
Unadjusted odds ratios of getting hypertensive for various risk factors (bivariate analysis)
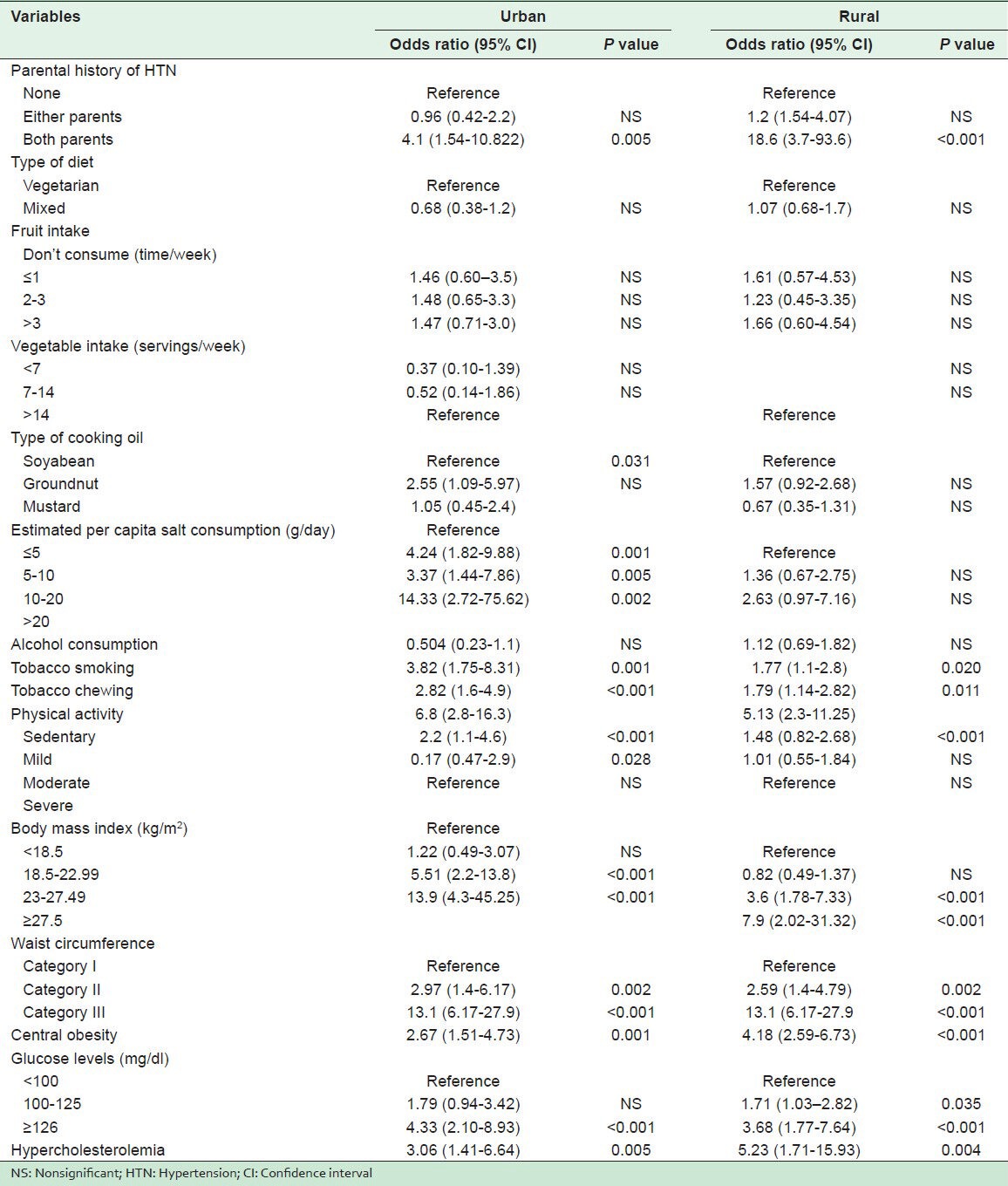
Table 6.
Odds ratios of getting hypertensive for various risk factors (multivariate logistic regression)
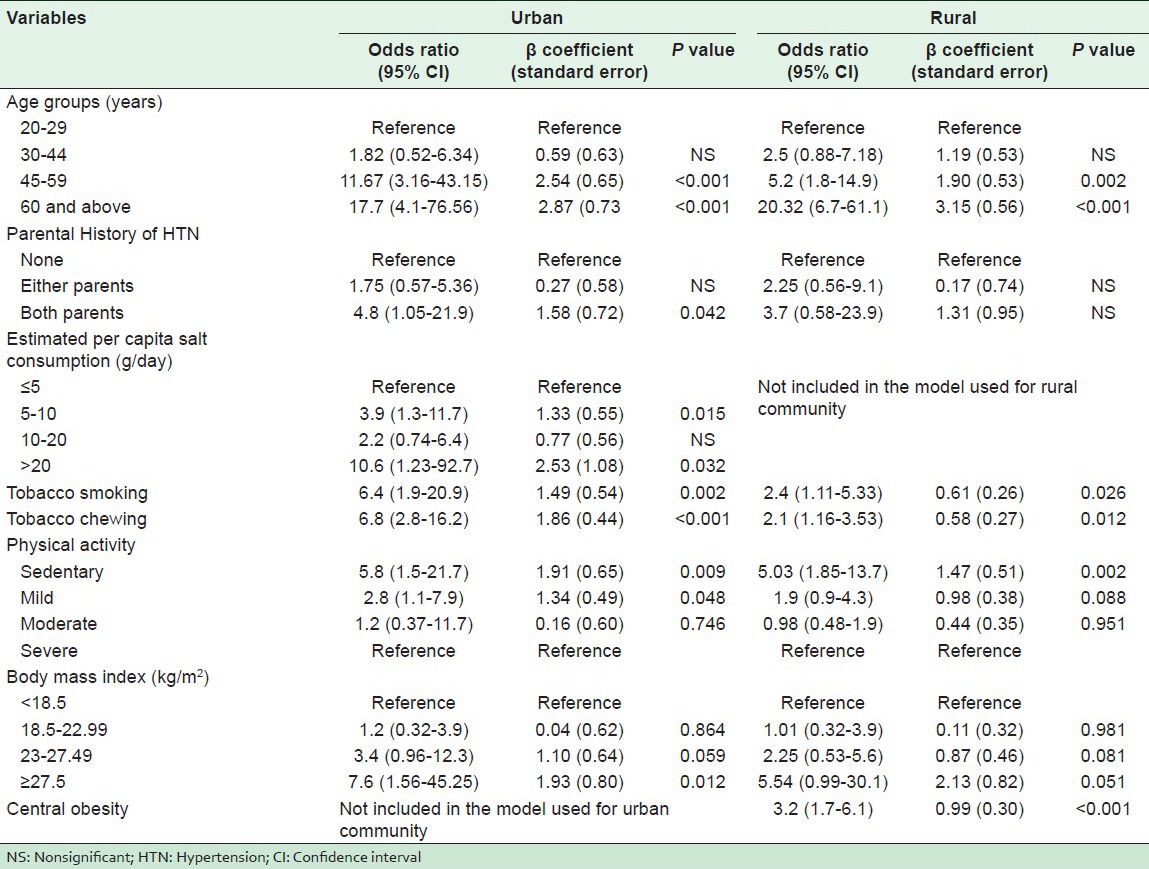
All the covariates of bivariate analysis have been incorporated in the multivariate forward step-wise conditional logistic regression analysis using the inclusion criterion of P value 0.05 or less.
In the urban population, with the help of bivariate analysis, increasing age, marital status (unmarried and widowed), educational status (middle school, high school, intermediate, and post high school diploma), joint family, history of HTN in both parents, use of groundnut oil in cooking, high estimated per capita salt consumption, tobacco smoking, tobacco chewing, physical inactivity (sedentary and mild), being overweight (>23 kg/m2), central obesity, high WC, diabetes, and hypercholesterolemia were found to be significantly associated with HTN. However, analysis by multivariate logistic regression showed increasing age, parental history of HTN in both parents, high estimated per capita salt consumption, tobacco smoking, tobacco chewing, sedentary and mild physical activity, and BMI ≥27.5 kg/m2 to be significantly associated with HTN.
In the rural subjects, bivariate analysis showed increasing age, caste (general), religion (Sikh), marital status (unmarried and widowed), educational status (primary school and middle school), family members ≤2, history of HTN in both parents, tobacco smoking, tobacco chewing, physical inactivity (sedentary), overweight (>23 kg/m2), central obesity, high WC, diabetes, pre-diabetes, and hypercholesterolemia to be significantly associated with HTN, and multivariate logistic regression identified increasing age, tobacco smoking, tobacco chewing, sedentary physical activity, and central obesity to be significantly associated with HTN.
DISCUSSION
It was found in the study that HTN and almost all risk factors except for alcohol consumption, tobacco chewing, and vegetable intake were more prevalent in the urban population.
The overall prevalence of HTN in the present study was 17.1% with 21.4% in urban and 14.8% in rural residents, while a study conducted at Moradabad District, India by Singh et al.[20] found the prevalence of HTN as 24% and 17% in urban and rural areas, respectively. IDSP-NCD risk factor survey[9] had shown 24% prevalence of HTN in MP, with a prevalence of 27% in urban areas and 23% in rural areas. A study carried out in 2005 in different parts of India revealed a dramatic rise in the prevalence of HTN in both urban and rural communities.[6]
The prevalence of normotensive, pre-HTN, stage-I and stage-II HTN was found in the present study to be 62.5%, 20.5%, 10.9%, and 6.1%, respectively, while the IDSP-NCD risk factor survey 2007-2008[9] reported the prevalence as 32.6% for normotensive, 46.2% for pre-hypertensive, 16.1% for stage-I, and 5% for stage-II HTN in MP. The significantly higher proportion of HTN among urban residents that was found in the present study is also supported by other studies.[7,8,21,22]
Similarly, high prevalence of physical inactivity, abdominal obesity, high WHR, BMI, diabetes, and hypercholesterolemia has been observed in urban populations compared to rural populations in many other studies.[8,9,20,21,22,23,24,25,26,27]
Also, we found that a higher number of people consumed alcohol in the rural areas (26.7%) as compared to the number in urban areas (21.7%). While contrary results were reported by Ebrahim et al.,[21] the study by Kumar et al. showed no dissimilarity.[8] Significantly higher prevalence of tobacco smoking was found in rural subjects (24.9%) as compared to urban subjects (9.7%) in the present study, similar to that reported by other studies.[8,21,28] There was not much difference in the prevalence of tobacco chewing in urban (43.7%) and rural (42.7%) areas, though a study in North India[28] had shown a significantly higher prevalence in urban compared to rural communities (12% vs. 6.8%).
Significantly higher mean values of weight, BMI, WC, HC, WHR, SBP, FBS, and serum cholesterol were seen in the urban community than in the rural subjects. Mean values of DBP was insignificantly higher among urban subjects. Similar results were obtained in many studies except that they also reported significantly higher values of DBP in the urban subjects.[21,26,27,29,30]
Compared to other studies, the present study also found that the prevalence of HTN was significantly associated with increasing age,[9,22,23,24,31,32,33] caste,[23] religion,[23] family type,[22] marital status, and education, while an insignificant association with gender, occupation,[9] and SES[22] was also observed. In the IDSP-NCD risk factor survey in 2007-2008,[9] increased prevalence of HTN was seen with advancing age, while no significant urban and rural variation was observed with education and occupation. In the rural survey reported by National Nutrition Monitoring Bureau (NNMB) 2006,[23] a significant association was observed with religion, caste, per capita income, and family size. Badaruddoza et al.[33] found significant differences in terms of age, religion, current job status, occupation, and SES in the urban population of Punjab when compared to the rural population. Vimala et al.[34] reported an insignificant association of educational standard with HTN. In a study carried out in the urban and rural areas of North Indian districts, Midha et al.[22] found age and family type to be significantly associated with the prevalence of isolated systolic HTN while an insignificant association was observed with the area of residence, religion, marital status, and socioeconomic class.
Similar to other studies, parental history of HTN was found to be significantly associated with high BP.[35,36,37] Rodger et al.[38] and Vimala et al.[34] found high consumption of salt to be significantly associated with HTN, as was found in the present study. Tobacco consumption either by smoking or chewing was found to be significantly associated with HTN. This was also revealed in the rural survey report by NNMB 2006[23] and two other studies.[38,39] Alcohol consumption was not found to be significantly associated with high BP.
HTN was also seen to be significantly associated with physical inactivity, as seen in other studies.[23–24,36,38] Similar to the findings of the present study, Gupta et al.[40] and other studies[23–24,32] found overweight and central obesity to be significantly associated with HTN. Biochemical markers such as increased fasting glucose levels and serum cholesterol levels were also observed to be significantly associated with high BP, as reported in other studies.[20,34,41]
Multivariate logistic regression analysis identified increasing age, parental history of HTN, tobacco smoking, tobacco chewing, physical inactivity, high estimated per capita salt consumption, and BMI ≥27.5 kg/m2 as the risk factors for HTN in the urban population, while increasing age, physical inactivity, high WHR, tobacco chewing, and tobacco smoking were the risk factors for HTN in the rural population. Yadav et al.[42] reported increasing age, BMI, WHR, and high FBS as the risk factors significantly associated with HTN by MLR analysis in an affluent North Indian population. Thankappan et al.[7] found higher odds of HTN with advancing age, alcohol use, and low physical activity, while lower odds were observed with tobacco use and female gender in a community-based study at Kerala. Subburam et al.[36] found that in bivariate analysis, many of the independent variables correlated with HTN, but in multivariate analysis, using multiple logistic regression only, BMI, family history, and age remained significantly associated. Similarly, Manimunda et al.[32] reported that age and BMI were significantly associated using MLR analysis in the Nicobar tribe. In a study conducted by Todkar et al.[35] in the rural population of Maharashtra, multiple logistic regression analysis identified increasing age, sex, BMI, high estimated per capita salt consumption, smoking, and high FBS to be the risk factors significantly associated with HTN. Midha et al.[22] found in multivariate logistic regression that age, gender, physical activity, and WC were significantly associated with HTN in the rural areas of North Indian districts.
Though studies conducted in the last few years suggest rising trends of CVD risk factors in rural areas, the picture in states like MP, with the lowest level of urbanization is dissimilar. Reddy et al.[43] indicated the growing vulnerability of lower socioeconomic groups to cardiovascular risk factors, mentioning reversal of social gradient (the presence of a graded inverse relationship of SES with CVD risk factors). The disparate results from diverse locations in India indicate that the different regions of India are in different stages of epidemiological development.
CONCLUSIONS
This study documents a high burden of HTN and other CVD risk factors in Central India. Despite the higher prevalence of almost all factors in urban areas, rural areas are also not far behind. This suggests that there should be public health remedial measures to address growing trends of CVD risk factors in both communities through Information, Education, and Communication (IEC) or Behavior Change Communication (BCC) activities about weight reduction, restriction of tobacco smoking and chewing, increase in physical activity, salt restriction, proper screening of BP, blood sugar and serum cholesterol levels, as well as increase in the intake of fruits and vegetables. Utilization of this information, especially by stakeholders and policy makers in the regional health sector, can avert a problematic health situation. In a milieu of a health system with limited resources such as those obtained in India, a better approach might be to focus jointly on population risk factor modification together with screening and evidence-based treatment.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Nissien A, Bothig S, Grenroth H, Lopez AD. Hypertension in developing countries. World Health Stat Q. 1988;41:141–54. [PubMed] [Google Scholar]
- 2.Reddy KS. Hypertension control in developing countries: Generic issues. J Hum Hypertens. 1996;10:S33–8. [PubMed] [Google Scholar]
- 3.Park K. Park's Textbook of Preventive and Social Medicine. 18th ed. Jabalpur, India: M/s Banarasidas Bhanot Publishers; 2005. Epidemiology of chronic non-communicable diseases and conditions; p. 293. [Google Scholar]
- 4.Singh RB, Suh IL, Singh VP, Chaithiraphan S, Laothavorn P, Sy RG, et al. Hypertension and stroke in Asia: Prevalence, control and strategies in developing countries for prevention. J Hum Hypertens. 2000;14:749–63. doi: 10.1038/sj.jhh.1001057. [DOI] [PubMed] [Google Scholar]
- 5.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: Part I: General considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–53. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 6.Gupta R. Meta-analysis of prevalence of hypertension in India. Indian Heart J. 1997;49:43–8. [PubMed] [Google Scholar]
- 7.Thankappan KR, Shah B, Mathur P, Sarma PS, Srinivas G, Mini GK, et al. Risk factor profile for chronic diseases: Results of a community based study in Kerala, India. Indian J Med Res. 2010;131:53–63. [PubMed] [Google Scholar]
- 8.Kumar R, Singh MC, Singh MC, Ahlawat SK, Thakur JS, Srivastava A, et al. Urbanization and coronary heart disease: A study of urban-rural differences in northern India. Indian Heart J. 2006;58:126–30. [PubMed] [Google Scholar]
- 9.Indian Council of Medical Research. IDSP Non Communicable Disease Risk Factors Survey Phase-I Report 2007-08. [Last accessed on 2011 Aug 06]. Available from: http://icmr.nic.in/final/IDSP-NCD%20Reports/Summary.pdf .
- 10.Rao NSN, Murthy Ns. 1st ed. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd; 2008. Applied Statistics in Health Sciences; pp. 105–7. [Google Scholar]
- 11.Kumar P. Modified Prasad classification. Indian J Community Med. 1993;19:21–61. [Google Scholar]
- 12.Atlanta: US Department of Health and Human Services, CDC, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. [Last accessed on 2013 Mar 31]. US Department of Health and Human Services: The health consequences of smoking: A report of the surgeon general. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20669512 . [Google Scholar]
- 13.Thimmayamma BV. Hyderabad: National Institute of Nutrition Press; 1987. A handbook of schedules and guidelines in socio-economic and diet surveys. National Institute of Nutrition, Indian Council of Medical Research; pp. 18–23. [Google Scholar]
- 14.Hall JN, Moore S, Harper SB, Lynch JW. Global variability in fruit and vegetable consumption. Am J Prev Med. 2009;36:402–9. doi: 10.1016/j.amepre.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 15.WHO Expert Consultation. Appropriate BMI for Asian populations and its implications for policy intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 16.Sydney: Health Communications; 2000. [Last accessed on 2013 Mar 31]. WHO/IASO/IOTF. The Asia – Pacific prospective. Redifining obesity and its treatmeant. Available from: http://www.wpro.who.int/nutrition/documents/docs/Redefiningobesity.pdf . [Google Scholar]
- 17.Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 18.American Diabetic Association. Diagnosis and classification of DM. Diabetes Care. 2004;27:S5–10. [Google Scholar]
- 19.US Department of Health and Human Services, National Institute of Health Publication No. 89-2925,1989; [Last accessed on 2013 Mar 31]. The Expert Panel, Report of the expert panel on detection, evaluation and treatment of high blood cholesterol in adults. Available from: http://www.nhlbi.nih.gov/guidelines/cholesterol/atp3full.pdf . [Google Scholar]
- 20.Singh RB, Bajaj S, Niaz MA, Rastogi SS, Moshiri M. Prevalence of type 2 DM and risk of hypertension and CAD in rural and urban population with low rates of obesity. Int J Cardiol. 1998;66:65–72. doi: 10.1016/s0167-5273(98)00141-7. [DOI] [PubMed] [Google Scholar]
- 21.Ebrahim S, Kinra S, Bowen L, Andersen E, Ben-Shlomo Y, Lyngdoh T, et al. The Effect of Rural-to-Urban Migration on Obesity and Diabetes in India: A Cross-Sectional Study. PLoS Med. 2010;7:e1000268. doi: 10.1371/journal.pmed.1000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Midha T, Idris MZ, Saran RK, Srivastav AK, Singh SK. Prevalence and determinants of hypertension in the urban and rural population of a north Indian district. East Afr J Public Health. 2009;6:268–73. [PubMed] [Google Scholar]
- 23.Indian Council of Medical Research. National Nutritional Monitoring Bureau Report Nov 2006. [Last accessed on 2011 Aug 14]. Available from: http://www.nnmbindia.org/NNMBReport06Nov20.pdf .
- 24.Kaur P, Rao TV, Sankarassubbaiyan S, Narayanan AM, Ezhil R, Rao SR, et al. Prevalence and distribution of cardiovascular risk factors in an urban industrial population in South India: A cross sectional study. J Assoc Physicians India. 2007;55:771–6. [PubMed] [Google Scholar]
- 25.Nongkynrih B, Acharya A, Ramakrishnan L, Ritvik, Anand K, Shah B. Profile of biochemical risk factors for non communicable diseases in urban, rural and periurban Haryana. India. J Assoc Physicians India. 2008;56:165–70. [PubMed] [Google Scholar]
- 26.Bhongir AV, Nemani S, Reddy PS. Rural-Urban transition of risk factors for CAD in college students of Hyderabad and near-by rural area-a pilot study. J Assoc Physicians India. 2011;59:222–6. [PubMed] [Google Scholar]
- 27.Allender S, Lacey B, Webster P, Rayner M, Deepa M, Scarborough P, et al. Level of urbanization and non-communicable disease risk factors in Tamil Nadu, India. Bull World Health. 2010;88:297–304. doi: 10.2471/BLT.09.065847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta V, Yadav K, Anand K. Patterns of tobacco use across rural, urban and urban slum population in a North Indian Community. Indian J Community Med. 2010;35:245–51. doi: 10.4103/0970-0218.66877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Das M, Pal S, Ghosh A. Rural -urban differences of cardiovascular risk factors in adult Asian Indians. Am J Hum Biol. 2008;20:440–5. doi: 10.1002/ajhb.20757. [DOI] [PubMed] [Google Scholar]
- 30.Pandey RM, Gupta R, Misra A, Misra P, Singh V, Agraval A, et al. Determinants of urban-rural differences in cardiovascular risk factors in middle-aged women in India: A cross-sectional study. Int J Cardiol. 2013;163:157–62. doi: 10.1016/j.ijcard.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Whelton PK. Epidemiology of hypertension. Lancet. 1994;344:101–6. doi: 10.1016/s0140-6736(94)91285-8. [DOI] [PubMed] [Google Scholar]
- 32.Manimunda SP, Sugunan AP, Benegal V, Balakrishna N, Rao MV, Pesala KS. Hypertension among Nicobarese Tribe. Ind J Med Res. 2011;133:287–293. [PMC free article] [PubMed] [Google Scholar]
- 33.Badaruddoza, Kumar R. Cardiovascular Risk factor and familial aggregation of BP with respect to anthropometric variables in a SC population in Punjab, a north Indian state. Anthropol Anz. 2009;67:111–9. doi: 10.1127/0003-5548/2009/0014. [DOI] [PubMed] [Google Scholar]
- 34.Vimala A, Ranji SA, Jyosana MT, Chandran V, Mathews SR, Pappachan JM. The prevalence, risk factors and awareness of hypertension in an urban population of Kerala [South India] Saudi J Kidney Dis Transpl. 2009;20:685–9. [PubMed] [Google Scholar]
- 35.Todkar SS, Gujarathi VV, Tapare VS. Period prevalence and sociodemographic factors of hypertension in rural Maharashtra: A cross sectional Study. Indian J Community Med. 2009;34:183–7. doi: 10.4103/0970-0218.55269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Subburam R, Sankarapandian M, Gopinath DR, Selvarajan SK, Kabilan L. Prevalence of hypertension and correlates among adults of 45-60 yrs in a rural area of Tamil Nadu. Indian J Public Health. 2009;53:37–40. [PubMed] [Google Scholar]
- 37.Divan V, Chauhan V, Panchal S, Bansal RK. Prevalence of hypertension amongst workers of a fertilizer company in Surat district. Natl J Community Med. 2010;1:153–5. [Google Scholar]
- 38.Rodger RA, Lawes CM, Gaziano T, Vos T. The growing burden of risk from high BP, Cholesterol and body weight. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P, editors. Disease Control Priorities in Developing World. Oxford: Oxford University Press; 2006. pp. 851–68. [Google Scholar]
- 39.Pandey A, Patni N, Sarangi S, Singh M, Sharma K, Vellimana AK, et al. Association of exclusive smokeless tobacco consumption with hypertension in an adult male rural population of India. Tob Induc Dis. 2009;5:15. doi: 10.1186/1617-9625-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta R, Gupta VP. Hypertension epidemiology in India. Curr Sci. 2009;97:349–55. [Google Scholar]
- 41.Bhasin SK, Dwivedi S, Dehghani A, Sharma R. Conventional risk factors among newly diagnosed CHD patients in Delhi. World J Cardiol. 2011;3:210–6. doi: 10.4330/wjc.v3.i6.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yadav S, Boddula R, Genitta G, Bhatia V, Bansal B, Kongara S, et al. Prevalence and risk factors of pre-hypertension and hypertension in an affluent North Indian population. Indian J Med Res. 2008;128:712–20. [PubMed] [Google Scholar]
- 43.Reddy KS, Prabhakaran D, Jeemon P, Thankappan KR, Joshi P, Chaturvedi V, et al. Educational status and cardiovascular risk profile in Indians. Proc Natl Acad Sci. 2007;104:16263–8. doi: 10.1073/pnas.0700933104. [DOI] [PMC free article] [PubMed] [Google Scholar]


