Abstract
Natural antibodies produced by B-1a cells are required for immediate protection against infection. The protective capacity of natural antibodies is attributed to germ-line like structure, which includes the relative absence of N-region addition. Previous studies have shown B-1a cell immunoglobulin from aged mice contains abundant N-additions. B-1a cells have been shown to derive from a specific Lineage negative (Lin-)CD45Rlo/-CD19+ progenitor found both in fetal liver and adult bone marrow. Here, we report identification of a fetal liver population characterized phenotypically as Lin-CD45R-CD19-, which gives rise to IgM+IgDloCD45RloCD5+Mac-1+CD19hiCD43+CD23lo B-1a cells upon adoptive transfer to SCID recipients. These B-1a cells derived from the Lin-CD45R-CD19- fetal liver population produce natural antibody that binds pneumococcal antigens, but this immunoglobulin contains substantial N-addition despite initial absence of TdT. Furthermore, we show extensive N-addition is also present in B-1a cells derived from the Lin-CD45Rlo/-CD19+ B-1 progenitor found in the bone marrow. Together these results demonstrate B-1a cell N-addition depends on the type of progenitor and the location of the progenitor during its development. These findings have implications for how regulation of different progenitors from fetal liver and bone marrow may play a role in the age-related increase in N-region addition by B-1a cells in normal animals.
INTRODUCTION
Murine B-1a cells are defined by unique surface marker expression (IgMhiIgDloCD45RloCD5+CD43+CD19hiMAC1+) as well distinct functional characteristics as compared to conventional splenic B-2 cells (1, 2). B-1a cells are found in the peritoneal cavity, spleen, and bone marrow. Functionally, B-1a cells exhibit unique signaling characteristics (2-4), are potent antigen presenting cells (5), and constitutively secrete IgM, which is referred to as natural IgM (6-8). B-1a cells are essential for immediate protection against, and therefore survival from, infection by both bacterial and viral pathogens (9-11). The unique ability of B-1a cells to provide immediate protection against infection is attributed to natural IgM, which is germline-like due to minimal N-region addition with little somatic hypermutation, and includes biased variable heavy chain (VH) gene usage in favor of VH11 and VH12 (1, 12-15). This unique germ-line structure of natural antibody is established during the early development of B-1a cells.
In general, B cell development begins with hematopoietic stem cells (HSC), which are self-renewing pluripotent cells found in fetal liver and adult bone marrow (16). B cell development continues through a series of differentiation steps dictated by expression of transcription factors, cytokines, and cell surface receptors. Proper immunoglobulin rearrangement allows for the B cell to progress through each stage of differentiation culminating in a naïve B cell expressing a B cell receptor (BCR), which is necessary for B cell survival and response to antigen (17). During immunoglobulin gene rearrangement nontemplated (N) nucleotides may be added to joining sites, which increases diversity of the B cell antigen receptor. The process of N-nucleotide addition is mediated by the enzyme terminal deoxynucleotide transferase (TdT) (16-18), which is not expressed in the liver, spleen, or bone marrow during fetal life (19). The limitation of TdT expression until after birth correlates with little to no N-addition observed in fetal derived B cells (12).
Specifically, the B-1 cell population in mice originates mainly from fetal liver precursors and was thought to persist throughout adult life by self-renewal (20-22). Recently, Dorshkind and colleagues identified a B-1 cell specific progenitor with the phenotype, Lineage negative (Lin-)CD45Rlo/-CD19+, found in low numbers in adult bone marrow and abundantly in fetal liver (23). Total Linbone marrow as well as fetal liver progenitors can give rise to B-1a cells upon adoptive transfer (24-26). We and others have shown B-1a cell immunoglobulin from older mice contains more N-addition than B-1a cell immunoglobulin from younger mice (24, 27). Interestingly, an increase in N-addition in TdT transgenic mice produces antibodies less protective against Streptococcus pneumonia (28). This study suggests the increased diversity generated by N-addition can be detrimental for microbial protection. In the course of elucidating the relationship between Lin-CD45Rlo/-CD19+ progenitor cells and immunoglobulin N-addition diversity, we discovered a population of fetal liver cells, characterized as Lin-AA4.1-CD45R-CD19-, that gives rise to B-1a cells containing abundant N-additions. Furthermore, we discovered the Lin-AA4.1+CD45Rlo/-CD19+ B-1 cell progenitor found in the adult bone marrow generates B-1a cells containing abundant N-additions, in keeping with our previous finding that immunoglobulin produced by bone marrow-derived (BMD) B-1a cells differs from that of native B-1a cells by expressing much more N-region addition (24, 25). These results identify a novel B-1a cell progenitor population, and indicate both the progenitor type and progenitor location determine N-region mediated diversity.
MATERIALS AND METHODS
Mice
Male BALB/cByJ and C57BL/6 mice of 6–8 weeks age were obtained from The Jackson Laboratory. CB17-SCID mice of 6-8 weeks of age were obtained from Taconic. Timed pregnant female mice were obtained from either Jackson Laboratory (BALB/c-ByJ) or Taconic (Swiss Webster). TdT knockout mice on the C57BL/6 background were kindly provided by Dr. Ann Feeney (Scripps Research Institute). Mice were cared for and handled in accordance with National Institutes of Health and institutional guidelines.
Adoptive Transfer
Fetal liver was obtained from either BALB/c-ByJ or Swiss Webster timed pregnant females at day 14, 15, or 18 as indicated. Fetal liver cell populations were sort purified using the Influx cell sorter (BD Biosciences), washed twice in 1X PBS, resuspended in 1X PBS, and then injected (i.v.) into recipient CB17-SCID mice at 0.2-1.0×106 cells per mouse in 0.2 ml. Four to five weeks post injection the CB17-SCID recipients were euthanized and examined for B-1a cell development.
Cell Purification and Flow Cytometry
Peritoneal washouts from wild type and fetal liver chimera mice were stained with immunofluorescent antibodies, and then analyzed on a LSRII flow cytometer or Influx cell sorter (BD Biosciences) with gating on live cells by forward side scatter. Images were constructed with FlowJo 6.0 software (Tree Star). The following antibodies were obtained from BD Pharmingen: Biotin-conjugated rat anti-mouse IgMb (clone AF6-78); FITC-conjugated rat anti-mouse IgMa (clone DS-1); PE-conjugated rat anti-mouse CD19 (clone ID3), CD43 (clone S7), Mac-1 (clone M1/70), CD45R/B220 (clone RA3-6B2), CD23 (clone B3B4), PDL2 (clone TY25), CD86 (clone GL1); PE-Cy5-conjugated rat anti-mouse CD5 (clone 53-7.3); Alexa647-conjugated rat anti-mouse CD5 (clone 53-7.3); and PerCP-Cy5.5-conjugated rat anti-mouse CD45R/B220 (clone RA3-6B2). The following antibodies were obtained from Biolegend: PE-Cy7-conjugated rat anti-mouse CD23 (clone B3B4) and Pacific Blue-conjugated rat anti-mouse IgD (clone 11-26c.2a). The following Biotin-conjugated rat anti-mouse antibodies were used to discriminate lineage positive cells: Mac-1 (clone M1/70); TER-119; CD8a (clone 53-6.7); Ly-6C / Ly-6G (clone RB6-8C5); CD5 (clone 53-7.3); and IgM (Southern Biotech).
Gene expression
Gene expression was assayed by Taqman and standard PCR. Briefly, RNA was prepared from B cells using Ultraspec (BiotecX) and chloroform extraction. Following isolation RNA was treated with DNaseI (Ambion) to remove contaminating DNA. cDNA was prepared using avian myeloblastosis virus reverse transcriptase (Bio-Rad). For TdT expression analysis following culture RNA was prepared from 10,000-100,000 cells (Cells-to-Ct kit, Ambion). Gene expression was measured by real-time PCR using Taqman chemistry. Primer and probe sets were obtained from Applied Biosystems for TdT (Mm00493500_m1) and β-Actin, which was used for normalization. Gene expression was also measured by standard PCR. The following primer sets were adapted from (29) and used for standard PCR: Actin (5’ CCTAAGGCCAACCGTGAAAAG; 3’ TCTTCATGGTGCTAGGAGCCA) and TdT (5’ GAAGATGGGAACAACTCGAAGAG; 3’ CAGGTGCTGGAACATTCTGGGAG). Products of amplification were run on a 2% agarose gel.
Single Cell Sequencing and Analysis
Peritoneal washout cells and splenocytes were obtained from 8-14 week old wild-type BALB/c-ByJ mice, wild-type C57BL/6 mice, TdT-/- C57BL/6 mice, or chimeras 4-5 weeks post transplant and were stained with fluorescence labeled antibodies to IgMa, IgMb, CD45R, CD5, and CD23. B cell populations (native peritoneal CD5+ B1 cells: IgMa+/CD45Rlo/CD5+; adoptive transfer-derived peritoneal CD5+ B1 cells: IgMa+/CD45Rlo/CD5+; native splenic B2 cells: CD23+/CD45Rhi/CD5-) were then purified using an Influx cell sorter (BD Biosciences). Post-sort re-analysis of B cell populations showed them to be ≥98% pure. Native peritoneal CD5+ B-1, adoptive transfer-derived peritoneal CD5+ B-1, and native splenic B2 cells were sorted onto a 48-well AmpliGrid slide (Advalytix). Reverse transcription and PCR (Qiagen OneStep RT-PCR) were carried out as described previously (24). The products were purified then sequenced (Biotic Solutions) using the MsVHE primer. Sequences were then analyzed using an online sequence analysis tool for VDJ sequences (IMGT, the international ImMunoGeneTics information system). Each of the sequences analyzed and reported in this manuscript, from all populations, has a unique V, D, and J segment along with a unique CDR3. Sequences with identical V, D, and J segments as well as identical CDR3 regions were eliminated from consideration according to the criteria of Kantor et al (29). As stated in previously published work, since it cannot be determined whether these sequences containing identical V, D, J, and CDR3 regions result from a single clonal expansion, or from analysis of multiple cells with identical rearrangements, we removed these sequences from the analysis presented herein (30). Nevertheless, upon analysis including these sequences containing identical V, D, J, and CDR3 regions the results (significant differences) and conclusions of N-region addition remain the same.
In vitro Cell Culture
Fetal liver was obtained from Swiss Webster timed pregnant females at day 18. Fetal liver cell populations were sort purified using the Influx cell sorter (BD Biosciences), washed once in complete medium (RPMI containing 10% FBS, penicillin/streptomycin, L-glutamine, and β-mercaptoethanol), resuspended in complete medium, then plated for one day with 100 ng/ml stem cell factor (SCF), 100 ng/ml Flt3-L, and 50 ng/ml TPO. After the 24-hour culture the cells were collected for analysis by flow cytometry. Alternatively, sorted cells were washed once in complete medium, resuspended in complete medium, then plated in the bottom of a 6-well 0.45-μm transwell plate at 10,000 cells per well with one of the two cytokine cocktails as indicated: 1) 20 ng/ml SCF, 10 ng/ml Flt3-L, 10 ng/ml TSLP, 20 ng/ml IL-3, and 20 ng/ml IL-6 (-IL-7); or, 2) 20 ng/ml SCF, 10 ng/ml Flt3-L, 10 ng/ml TSLP, and 10 ng/ml IL-7 (+IL-7). The upper chambers of the transwell plates were seeded with OP9 cells (ATCC) the day before culture in alpha-MEM without ribonucleosides and deoxyribonucleosides containing 2 mM L-glutamine, 1.5 g/L sodium bicarbonate, and 20% FBS. The cells were cultured for 3 or 9 days and then collected for analysis by cell counting and flow cytometry.
ELISA
Serum was collected from individual naïve chimera mice four to five weeks post transplantation or from 3-month old BALB/c-ByJ mice at the time of euthanasia. The serum was analyzed for total IgM by ELISA according to the manufacturer’s instructions (Bethyl Laboratories). IgM specific PC and PPS3 serum levels were measured by coating 96-well plates with PC-BSA (Biosearch Technologies) or PPS3 (American Type Culture Collection) at 5 μg/ml in 1X PBS. All plates were washed with TBS Tween (0.05%) and blocked with 1% BSA for 1 hour at room temperature. Bound IgM was measured using HRP-conjugated goat anti-mouse IgM (Bethyl Labs). IgM standards were included on each plate and PC / PPS3 specific antibody levels were interpreted as volume equivalent of IgM, as described in (31).
RESULTS
Peritoneal IgMa+CD45RloCD5+ B-1 cells develop from Lin-CD45R-CD19- fetal liver cells
Fetal liver cells were collected at day 14, 15, or 18 of embryonic life from either BALB/c-ByJ or Swiss Webster mice. The B-1 cell specific progenitor described by Dorshkind and colleagues, Lin-CD45Rlo/-CD19+, was sort purified and adoptively transferred by intravenous inoculation into adult CB17-SCID mice. At the same time we identified among Lin- fetal liver cells a CD45R-CD19- population that was transferred to separate CB17-SCID recipients. Four to five weeks post transfer, recipient mice were euthanized at which time peritoneal washouts and spleens were collected for analysis. To identify donor-derived cells in the CB17-SCID recipients, allotypic differences at the Igh locus between the donor (IgMa) and recipient (IgMb) strains were utilized. We found the Lin-CD45R-CD19- fetal liver cell population reconstituted IgMa+CD45RloCD5+ and IgMa+CD45RloCD5- B-1 cell populations in the peritoneal cavity (Figure 1), with resultant B-1 cells amounting to about 40% of peritoneal cells, which is compared to native B-1a cells from un-manipulated 3 month old mice and B-1a cells derived from Lin-CD45Rlo/-CD19+ fetal liver cells wherein B-1a cells constituted 41% and 53% of peritoneal cells respectively (Figure 1). In contrast, neither fetal liver cell population reconstituted peritoneal B-2 cells, nor splenic B or T cells, to any significant extent. Total fetal liver cells (non-lineage depleted) reconstituted a significant proportion of splenic and peritoneal T cells as previously reported (data not shown) (32). The total number of each population in fetal liver was assessed at 15, 16, and 18 days of gestation. We found the number of Lin-CD45R-CD19- cells exceeded the number of Lin-CD45Rlo/-CD19+ cells at each time point (Supplemental Figure 1). These results demonstrate there is a population of fetal liver cells defined as Lin-CD45R-CD19-, which can preferentially repopulate the peritoneal cavity with IgMa+CD45RloCD5+ lymphocytes.
Figure 1. Lin-CD45R-CD19- fetal liver cells reconstitute peritoneal IgMa+CD45RloCD5+ cells.
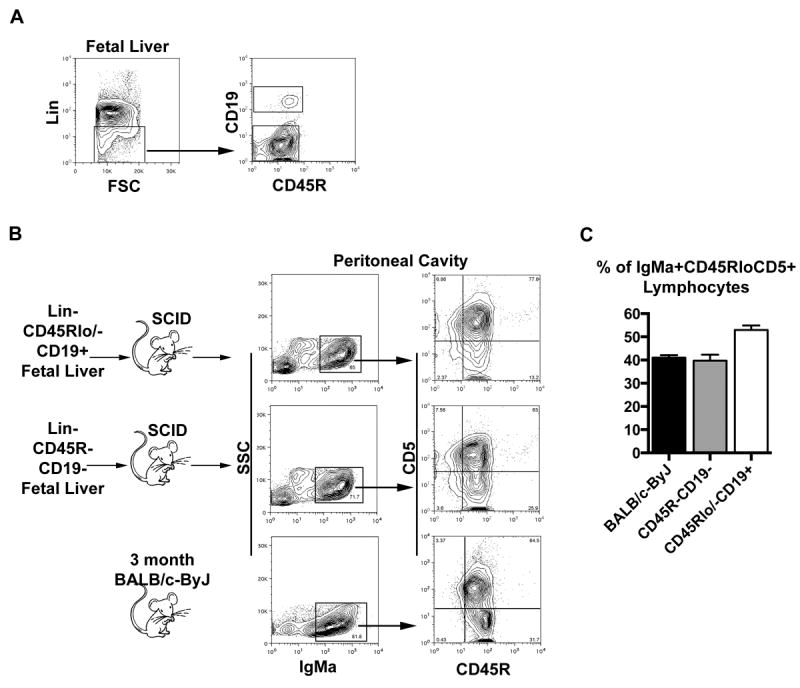
Fetal liver cells collected from day 14, 15, or 18 embryos were stained with lineage markers, CD19, and CD45R. Lin-CD45R-CD19- and Lin-CD45Rlo/-CD19+ cells were sorted for transplantation. (A) A representative sorting scheme is displayed for day 15 fetal liver. (B) CB17 SCID mice were injected intravenously with 1-2 × 105 Lin-CD45R-CD19- or 1-2 × 105 Lin-CD45Rlo/-CD19+ fetal liver cells. Four to five weeks post transplantation the mice were euthanized and evaluated for reconstitution of lymphocytes in the spleen and peritoneal cavities. Results for a wild type 3-month old BALB/c-ByJ mouse are shown for comparison. (C) The percent of live lymphocytes in the peritoneal cavities of recipient SCID mice which are IgMa+ (fetal liver derived) CD45RloCD5+ (B-1a cells), as compared to the fraction of B-1a cells found in the peritoneal cavities of wild type 3-month old BALB/c-ByJ mice is displayed. The data shown are an average of six independent experiments with 3-5 mice per group in each experiment. Embryos were obtained from timed pregnant BALB/c-ByJ mice for three experiments (Jackson Labs) and Swiss Webster (Taconic) mice for three experiments.
Peritoneal B lymphocytes derived from Lin-CD45R-CD19- fetal liver cells phenotype as B-1a cells
Beyond IgM, CD45R and CD5, peritoneal B-1a cells express additional cell surface markers in a distinctive pattern consisting of IgDloCD19hiMac-1+CD43+CD23lo, in contrast to B-2 cells that are IgDhiCD19loMac-1-CD43-CD23hi (1, 2, 33). To verify the identity of fetal liver Lin-CD45R-CD19- progeny, peritoneal IgMa+CD45RloCD5+ cells were immunofluorescently stained and analyzed. We found IgMa+CD45RloCD5+cells derived from both Lin-CD45R-CD19- and Lin-CD45Rlow/-CD19+ adoptively transferred populations differed from native splenic B-2 cells by expressing less IgD and more CD19 (Figure 2A), which is comparable to native B-1a cells. Similarly, we demonstrated like native B-1a cells, a much higher proportion of Lin-CD45R-CD19- and Lin-CD45Rlo/-CD19+ derived IgMa+CD45RloCD5+ cells expressed Mac-1 and CD43, and a much lower proportion expressed CD23, as compared to native splenic B-2 cells (Figure 2B). In sum, IgMa+CD45RloCD5+ B cells derived from Lin-CD45R-CD19- fetal liver cells express cell surface markers in a pattern that is characteristic of native peritoneal B-1a cells and is also observed in IgMa+CD45RloCD5+ B cells derived from fetal Lin-CD45Rlo/-CD19+ B-1 cell progenitors. These results indicate peritoneal IgMa+CD45RloCD5+ cells derived from Lin-CD45R-CD19- fetal liver cells phenotype as authentic B-1a cells.
Figure 2. Peritoneal B cells derived from Lin-CD45R-CD19- fetal liver cells phenotype as B-1a cells.
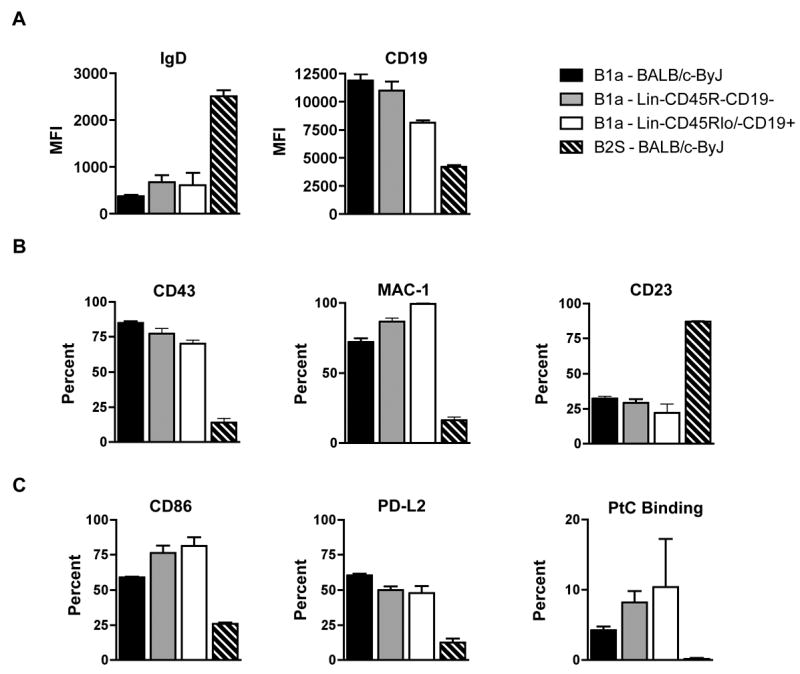
Peritoneal IgMa+CD45RloCD5+ cells derived from Lin-CD45R-CD19- fetal liver cells were analyzed for surface markers typically found on peritoneal B-1a cells. These cells were compared to peritoneal B-1a and splenic B-2 cells from 3-month old BALB/c-ByJ mice in addition to peritoneal IgMa+CD45RloCD5+ cells derived from Lin-CD45Rlo/-CD19+ fetal liver cells. (A) MFI values of IgD and CD19 are shown. (B) Percent of peritoneal IgMa+CD45RloCD5+ cells or splenic IgMa+CD45RhighCD5- cells expressing CD43, Mac-1 (CD11b), or CD23 are shown. (C) Percent of peritoneal IgMa+CD45RloCD5+ cells or splenic IgMa+CD45RhighCD5- cells expressing CD86, PD-L2, or PtC liposome binding are shown. The data shown are an average of six independent experiments with 3-5 mice per group in each experiment.
Other phenotypic characteristics have been described for peritoneal B-1a cells, which include surface expression of CD86 (5) and PD-L2 (34), and binding of phosphatidylcholine (PtC) (35). As with native B-1a cells, we found many more B-1a cells derived from both Lin-CD45R-CD19- and Lin-CD45Rlo/-CD19+ fetal liver cells displayed CD86 and PD-L2 as compared to splenic B-2 cells (Figure 2C). Furthermore, we found both fetal liver-derived and native B-1a cell populations bound PtC liposomes whereas splenic B-2 cells did not (Figure 2C). These results on CD86, PD-L2, and PtC binding provide additional evidence that IgMa+CD45RloCD5+ B cells derived from Lin-CD45R-CD19- fetal liver cells are B-1a cells, and indicate they are repertoire skewed toward PtC as are native B-1a cells. From this point on we refer to B-1a cells derived from Lin-CD45R-CD19- fetal liver cells as B-1aFN (for fetal liver CD19 negative progenitor derived), B-1a cells derived from Lin-CD45Rlo/-CD19+ fetal liver cells as B-1aFP (for fetal liver CD19 positive progenitor derived), and B-1a cells derived from Lin-CD45Rlo/-CD19+ bone marrow cells as B-1aBP (for bone marrow CD19 positive progenitor derived).
B-1aFN and B-1aFP cells produce PC and PPS3-specific IgM
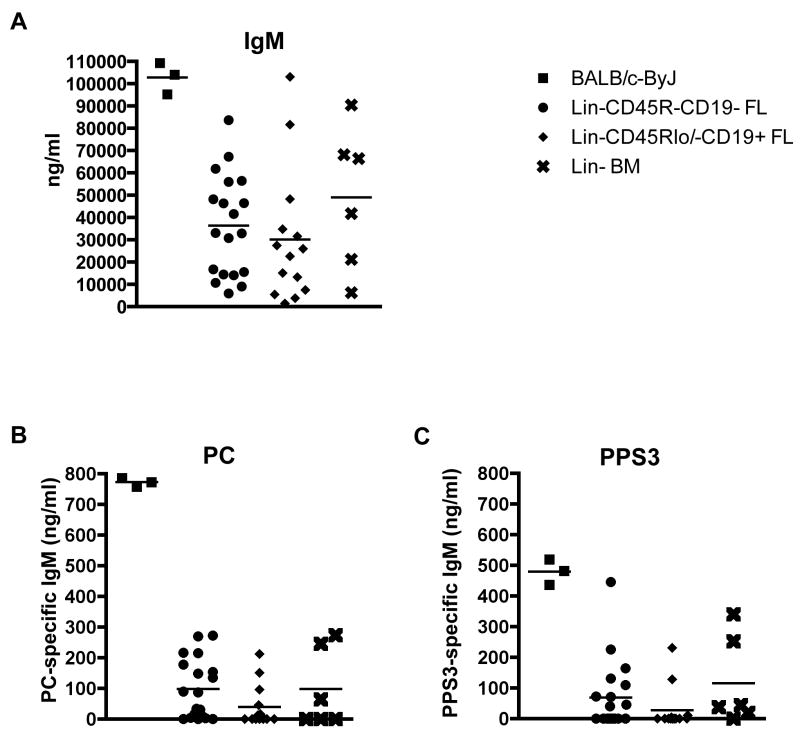
B-1a cells produce natural IgM, which is essential for immediate protection against bacterial and viral infections (9-11). In particular, anti-phosphorylcholine (PC) antibody derived from B-1a cells has been well characterized and shown to be protective against Streptococcus pneumoniae infection (9, 36). The importance of anti-PC and anti-pneumococcal capsular polysaccharide 3 (PPS3) germline-like natural antibody produced by B-1a cells was also shown in mice lacking B-1a cells, as they were unable to survive acute infection with S. pneumoniae (9). Serum obtained from individual SCID mice transplanted with either Lin-CD45R-CD19- fetal liver cells, Lin-CD45Rlo/-CD19+ fetal liver cells, or total Lin- bone marrow cells was screened by ELISA for total IgM (Figure 3A), anti-PC specific IgM (Figure 3B), or PPS3 (Figure 3C) specific IgM. These results show mice transplanted with Lin-CD45R-CD19- and Lin-CD45Rlo/-CD19+ fetal liver cells contain serum with slightly lower levels of total IgM as compared to mice transplanted with total Lin- bone marrow. All three populations produced much less IgM after the 4-week transfer than that present in 3-month old adult BALB/c-ByJ mice. Lin-CD45R-CD19- fetal liver progenitors gave rise to cells producing similar levels of PC-specific and PPS3-specific IgM as compared to mice transplanted with bone marrow progenitors. Lin-CD45Rlo/-CD19+ fetal liver progenitors gave rise to cells producing lower levels of PC-specific and PPS3-specific IgM as compared to mice transplanted with bone marrow progenitors. Despite small disparities no statistically significant differences were observed in the amount of total IgM, PC-specific, or PPS3-specific IgM between mice transplanted with either fetal liver population or Lin- bone marrow. Regardless, these results demonstrate both fetal liver B-1a progenitors are capable of producing total IgM, PC-specific, and PPS3-specific serum IgM, which are hallmarks of B-1a cell natural antibody.
Figure 3. Lin-CD45R-CD19- fetal liver derived B-1a cells produce natural IgM antibody that binds pneumococcal antigens.
Serum was obtained from CB17 SCID mice transplanted with Lin-CD45R-CD19- fetal liver cells, Lin-CD45Rlo/-CD19+ fetal liver cells, or total Lin- bone marrow cells four to five weeks post transfer. The serum was evaluated for (A) total IgM, (B) PC-specific IgM, and (C) PPS3-specific IgM levels by ELISA. As a reference, serum from 3-month old BALB/c-ByJ mice was also evaluated at the same time. The results represent an average of serum samples taken from individual mice, which demonstrated reconstitution by flow cytometry analysis. The number of individual serum samples analyzed per group is as follows: 19 Lin-CD45R-CD19- fetal liver cell chimera mice, 14 Lin-CD45Rlo/-CD19+ fetal liver cell chimera mice, and 6 total Lin- bone marrow chimera mice.
B-1aFN and B-1aBP cells produce immunoglobulin with substantial N-region addition unlike B-1aFP cells
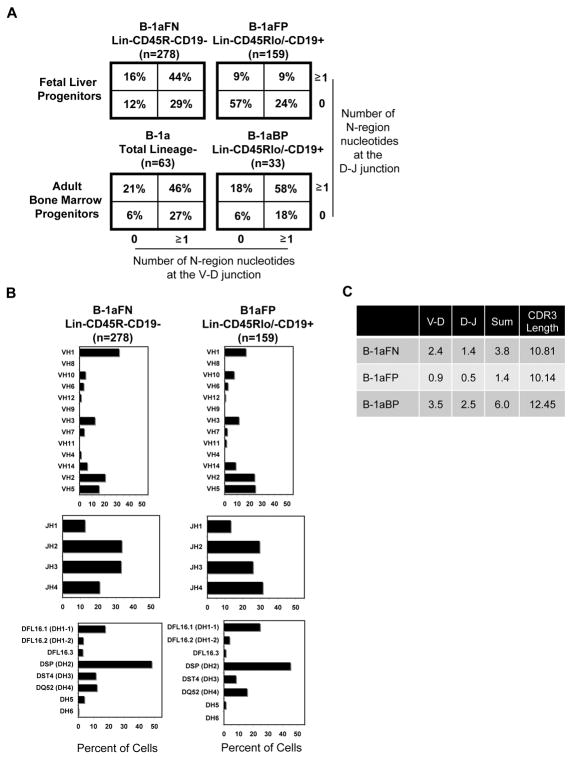
Native peritoneal B-1a cells contain few N-region additions at both the V-D and D-J junctions (55% of sequences with no N-additions), as well as differential VH, DH, and JH gene usage, in comparison to splenic B-2 cells (24, 30). To examine the immunoglobulin heavy chain structure of fetal liver Lin-CD45R-CD19- B-1a progeny, fetal liver Lin-CD45Rlo/-CD19+ B-1a progeny, and bone marrow Lin-CD45Rlo/-CD19+ B-1a progeny, B-1a cells from SCID recipients were single cell sorted 4 weeks following adoptive transfer. Immunoglobulin heavy chains were then amplified with promiscuous primers, sequenced, and analyzed (30). All sequences are provided as supplemental Figure 4. As expected, B-1aFP cells produced immunoglobulin containing very few N-region additions, with 57% of 159 analyzed heavy chains characterized by zero nontemplated sequences at both the V-D and D-J junctions (Figure 4A). Surprisingly, however, we found B-1aFN and B-1aBP cells expressed immunoglobulin containing abundant N-region additions. Only 12% of the 278 heavy chains analyzed from B-1aFN and 6% of the 33 heavy chains analyzed from B-1aBP cells contained zero N-additions at both junctions, which are both significantly different from B-1aFP sequences by chi-square analysis (p<0.0001). This level of N-addition is similar to that which characterizes B-2 cells, and B-1 cells produced by adoptive transfer of Lin- adult bone marrow (24, 25). Further, we found N-addition lengths were significantly longer in B-1aFN and B-1aBP cells as compared to B-1aFP cells at the V-D junction (p<0.0001), the D-J junction (p<0.0001), and for the sum of the two junctions (p<0.0001). These results are summarized in Figure 4C. Thus, Lin-CD45R-CD19- fetal liver cells and Lin- CD45Rlo/-CD19+ bone marrow cells function differently from Lin-CD45Rlo/-CD19+ fetal liver cells in giving rise to different kinds of B-1a cells; progeny of the former two add non-templated nucleotides at heavy chain V-D and D-J junctions, whereas progeny of the latter largely do not.
Figure 4. Immunoglobulin heavy chain sequence analysis of Lin-CD45R-CD19- fetal liver and Lin-CD45Rlo/-CD19+ bone marrow derived B-1a cells show abundant N-addition.
(A) N-region addition analysis at the D-J and V-D junctions in B-1a cells derived from Lin-CD45R-CD19- and Lin-CD45Rlo/-CD19+ fetal liver cells, Lin-CD45Rlo/-CD19+ bone marrow cells, and total Lin- bone marrow is shown. (B) V, D, and J gene segment usage in B-1a cells derived from Lin-CD45R-CD19- or Lin-CD45Rlo/-CD19+ fetal liver cells is displayed. (C) Average number of N-additions at the V-D, D-J, or sum of the two junctions is shown along with CDR3 length. Results are based on 3-5 independent experiments with sequences combined from each independent experiment.
CDR3 length was also evaluated in B-1aFN, B-1aFP, and B-1aBP cells (Figure 4C). We found B-1aFN cells expressed immunoglobulin with significantly longer average CDR3 length (10.81 ± 2.49) as compared to B-1aFP cells (10.14 ± 2.44) (p=0.0073). B-1aBP cells expressed immunoglobulin with significantly longer average CDR3 length (12.45 ± 2.57) as compared to B-1aFP cells (10.14 ± 2.44) (p<0.0001) and as compared to B-1aFN cells (10.81 ± 2.49) (p=0.0006). These results are consistent with the increased N-addition present in B-1aFN and B-1aBP cells. In sum, Lin-CD45R-CD19- fetal liver cells give rise to B-1aFN cells and Lin-CD45Rlo/-CD19+ bone marrow cells give rise to B-1aBP cells, both of which differ dramatically from B-1aFP cells in N-addition, which is reflected in average CDR3 length.
VH, DH, and JH gene usage was also analyzed in B-1a cell sequences derived from the fetal liver progenitor populations and unlike N-addition these aspects of immunoglobulin structure differed little. Overall we found VH gene usage was similar between B-1aFN and B-1aFP cells (Figure 4B) with the exception of some differences in VH1 (J558 family) and VH5 (7183 family) gene usage. B-1aFN cells used VH1 more frequently (32%) than B-1aFP cells (17%) (p=0.0008). Conversely, B-1aFP cells used VH5 more frequently (25%) than B-1aFN cells (16%) (p=0.02). Although JH gene usage was similar between B-1aFN and B-1aFP cells, B-1aFN cells utilized JH4 less frequently (21%) than B-1aFN cells (31%) (p=0.0136) (Figure 4B). No significant differences in DH usage were observed between B-1aFN and B-1aFP cells.
TdT expression is limited to progenitors found in the bone marrow
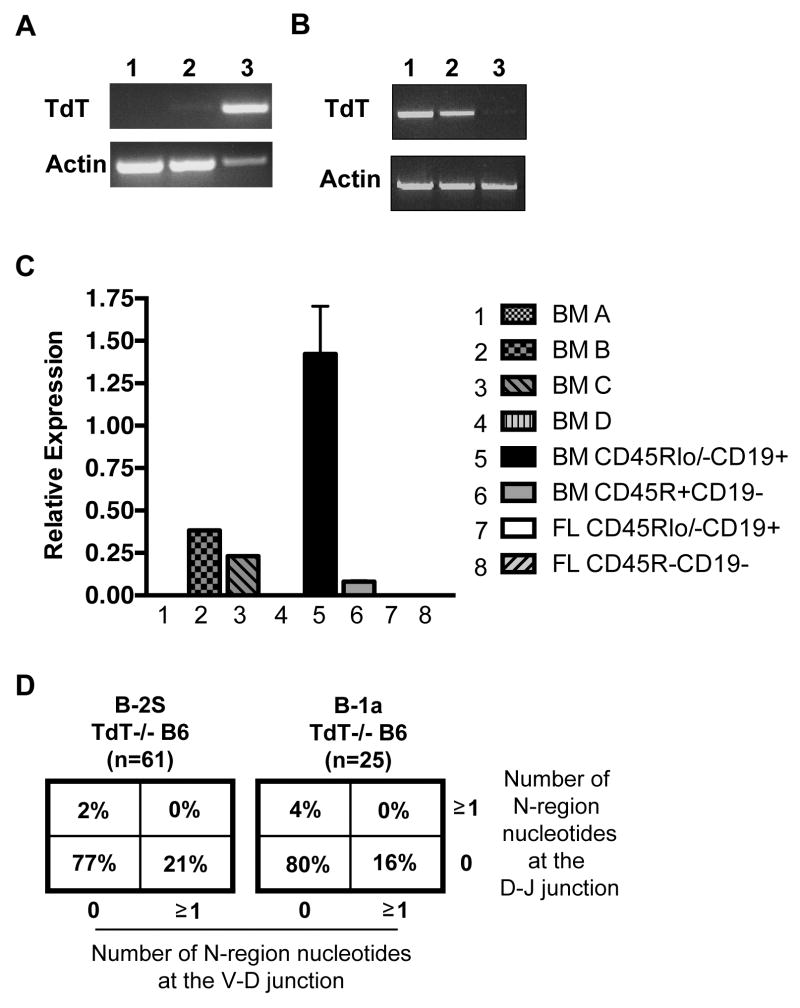
The process of N-nucleotide addition is mediated by the enzyme terminal deoxynucleotidyl transferase, TdT (18). Since B-1a cells derived from Lin-CD45R-CD19- fetal liver cells produced immunoglobulin containing levels of N-addition indistinguishable from splenic B-2 cells, B-1a progenitors were examined for TdT. Lin-CD45R-CD19- and Lin-CD45Rlo/-CD19+ cells from day 15 or day 18 fetal liver cells and Lin-CD45Rlo/-CD19+ bone marrow cells were sort purified after which TdT transcripts were evaluated by PCR. As expected, we found bone marrow Lin-CD45R+CD19- and bone marrow Lin-CD45Rlo/-CD19+ progenitors expessed TdT transcripts, in keeping with the level of N-addition found in the immunoglobulin of their B-2 cell or B-1a cell progeny respectively (Figure 5B). In direct contrast, we found fetal liver Lin-CD45R-CD19- progenitors did not express TdT, despite the fact that their B-1aFN progeny produced immunoglobulin with substantial, B-2-like levels of N-addition (Figure 5A and B). The absence of TdT gene expression in fetal liver Lin-CD45R-CD19- progenitors was further confirmed by real-time PCR. As a control for positive and negative TdT expression, Hardy fractions A-D were sort purified from bone marrow cells. As previously reported, we found TdT is expressed in fractions B (Pro-B) and C (late Pro-B) from the bone marrow but there is little to no expression in fractions A (Pre-Pro-B) and D (small Pre-B) (29). As shown in Figure 5C, Lin-CD45Rlo/-CD19+ and Lin-CD45R+CD19- progenitors from bone marrow express TdT, but Lin-CD45Rlo/-CD19+ and Lin-CD45R-CD19- cells from fetal liver do not express TdT. Serial dilution studies indicate that real time PCR detects TdT transcripts from as few as 250 Lin-CD45Rlo/-CD19+ bone marrow progenitors, a population that is TdT positive as a whole. This number represents 0.1% the number of adoptively transferred cells, but could be less, because not all Lin-CD45Rlo/-CD19+ bone marrow progenitors may express TdT so the detection limit may be fewer than 250 TdT+ cells. Together these results demonstrate both Lin-CD45Rlo/-CD19+ and Lin-CD45R+CD19- progenitors found in the bone marrow express TdT, whereas all progenitors found in the fetal liver do not express TdT, which might be expected from the generally acknowledged absence of TdT during fetal development.
Figure 5. The Lin-CD45R-CD19- fetal liver progenitor population does not express TdT.
(A) cDNA was obtained from sort purified populations: 1) Lin-CD45R-CD19- fetal liver cells, 2) Lin-CD45Rlo/-CD19+ fetal liver cells, and 3) Lin-CD45Rlo/-CD19+ bone marrow cells, and assessed for TdT expression by PCR. (B) cDNA was obtained from sort purified bone marrow cell populations: 1) Lin-CD45Rlo/-CD19+, 2) Lin-CD45R+CD19-, or 3) total bone marrow cells, and assessed for TdT expression by PCR. (C) TdT expression in sort purified bone marrow and fetal liver populations was analyzed using Taqman PCR. The results shown are an average of 5 independent experiments. (D) N-region addition analysis at the D-J and V-D junctions in B-1a and B-2 cells obtained from CD57/B6 TdT-/- mice is shown.
Despite the lack of TdT expression found in the Lin-CD45R-CD19- fetal liver progenitors, extensive N-addition was observed in the B-1aFN cells with 88% of sequences containing N-additions at one or both junctions (Figure 4A). These results call into question whether a TdT independent mechanism of N-addition exists. Therefore, we evaluated B-1a cell immunoglobulin obtained from TdT knockout (TdT-/-) mice for N-insertions. Results shown in Figure 5D demonstrate B-1a cells express immunoglobulin with N-additions in the absence of TdT, albeit significantly fewer N-additions than found in comparable B-1a cells from WT mice. These results suggest a TdT-independent mechanism could be partially at work after transfer of Lin-CD45R-CD19- fetal liver cells into adult SCID mice to produce B-1aFN cells with extensive N-additions (Figure 4A).
Lin-CD45R-CD19- fetal liver cell characteristics
AA4.1 is expressed early during B cell development (37). Furthermore, it has been shown that c-Kit along with AA4.1 marks early lymphoid progenitors in the mouse (38). The Lin-CD45Rlo/-CD19+ B-1 cell progenitor reported by Dorshkind and colleagues expresses AA4.1 in both fetal liver and bone marrow (23). To address similarities and differences between the CD19- and CD19+ fetal liver progenitor cells, AA4.1 and c-Kit surface expression was examined on fetal liver Lin-CD45R-CD19- and Lin-CD45Rlo/-CD19+ cells by immunofluorescent staining. In contrast to Lin-CD45Rlo/-CD19+ fetal liver cells that are AA4.1 positive (99%), we found Lin-CD45R-CD19- fetal liver cells are largely negative for AA4.1 expression, with only 4.4% positive (Figure 6). However, c-Kit was partially and similarly expressed on Lin-CD45R-CD19- and Lin-CD45Rlo/-CD19+ fetal liver cells at 68% and 63% respectively (Figure 6A). These results clearly differentiate AA4.1- Lin-CD45R-CD19- from AA4.1+ Lin-CD45Rlow/-CD19+ fetal liver progenitors.
Figure 6. Lin-CD45R-CD19- fetal liver cells express little to no AA4.1 and are responsive to IL-7.
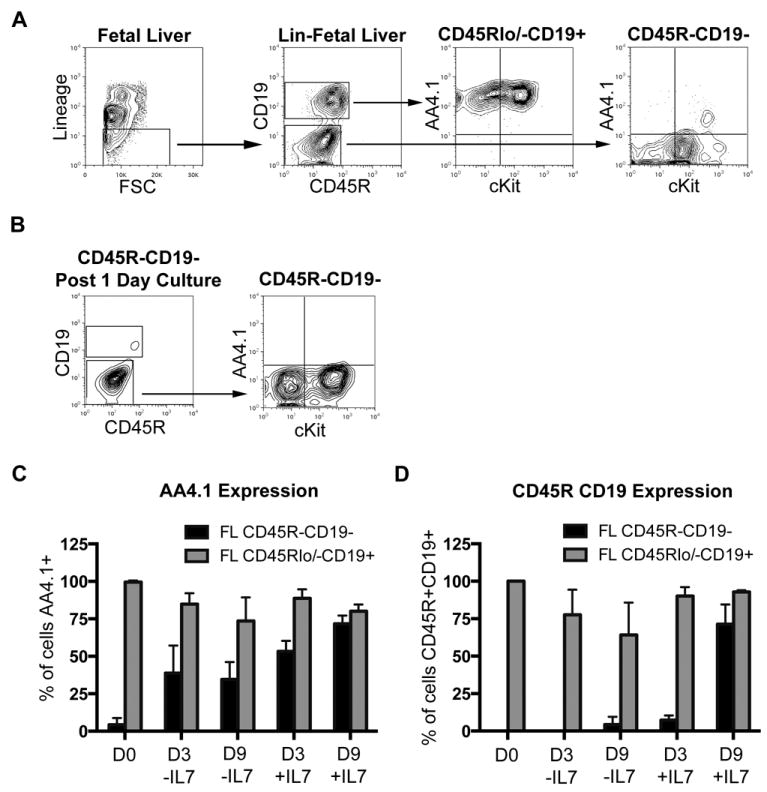
(A) Lin-CD45R-CD19- and Lin-CD45Rlo/-CD19+ fetal liver cells were assessed for AA4.1 and cKit expression at day 18 by immunofluorescent staining. (B) Lin-CD45R-CD19- fetal liver cells were sorted from day 18 embryos. The cells were placed in culture overnight with SCF, TPO, and Flt-3L. Expression of AA4.1 and cKit was determined following the 24-hour culture. The results shown are representative of 3 independent experiments. (C, D) Lin-CD45R-CD19- (black bars) and Lin-CD45Rlo/-CD19+ (grey bars) fetal liver cells were sorted from day 18 embryos. The cells were placed in culture with a cytokine mix containing IL-7 (SCF, Flt3-L, TSLP, IL-7) or lacking IL-7 (SCF, Flt3-L, TSLP, IL-6, IL-3). After 3 and 9 days expression of AA4.1 (C) or CD45R and CD19 (D) expression was determined. The results shown are an average of 3 independent experiments.
To determine whether Lin-CD45R-CD19- fetal liver cells are preprogrammed to differentiate into Lin-CD45Rlo/-CD19+ progenitors, day 18 Lin-CD45R-CD19- fetal liver cells were placed in culture with or without a cytokine cocktail of SCF, TPO, and Flt-3L. After 24 hours, the cells were assessed for expression of CD19, CD45R, AA4.1 and c-Kit expression by immunofluorescent staining. We found Lin-CD45R-CD19- fetal liver cells did not acquire CD45R, CD19, or AA4.1 during culture in medium alone or with SCF, TPO, and Flt-3L (Figure 6B). Further, c-Kit expression on Lin-CD45R-CD19- fetal liver cells did not change in the absence or presence of cytokine stimulation. These data demonstrate Lin-CD45R-CD19- fetal liver cells are not pre-progammed to become AA4.1+Lin-CD45Rlo/-CD19+ cells within a 24 hour period.
It has been shown bone marrow B-1 cell progenitors are responsive to TSLP in an in vitro culture system (23) and are sensitive to IL-7 signaling in the bone marrow (26). To further investigate whether the Lin-CD45R-CD19- fetal liver cells can acquire AA4.1, CD45R, and/or CD19 we cultured these cells using two previously described in vitro culture systems either with or without IL-7. For cultures with IL-7 we used the cytokine cocktail reported by Esplin et al, which included SCF, Flt-3L, TSLP, and IL-7 (26). For cultures without IL-7 we used the cytokine cocktail reported by Montecino-Rodriguez et al, which included SCF, Flt-3L, TSLP, IL-3, and IL-6 (23). Cultures were harvested at day 3 and day 9. Results shown in Figure 6C demonstrate Lin-CD45R-CD19- fetal liver cells start to gain AA4.1 in the absence of IL-7 but gain more AA4.1 expression in the presence of IL-7 by day 9 of culture. However, Lin-CD45R-CD19- fetal liver cells only acquire significant levels of CD45R and CD19 expression in the presence of IL-7 at day 9 (Figure 6D). These results demonstrate Lin-CD45R-CD19- fetal liver cells are able to progress forward in development by acquisition of AA4.1 in the presence of TSLP with or without IL-7 but only in the presence of TSLP and IL-7 do they acquire CD45R and CD19 expression.
We evaluated whether isolated Lin-CD45R-CD19- fetal liver cells up-regulate TdT in vitro in the presence or absence of IL-7, using the culture conditions described in Figure 6C and 6D. The Lin-CD45R-CD19- fetal liver cells did not express TdT in the absence or presence of IL-7 after 3 or 9 days of culture (no detectable TdT mRNA expression by realtime PCR analysis). Lin-CD45Rlo/-CD19+ bone marrow cells were used as a positive control for TdT expression (Supplemental Figure 3).
We also assessed Lin-CD45R-CD19- fetal liver cells for the presence of the hematopoietic stem cell-enriched population referred to as LSK (Lin-Sca-1+c-Kit+) (26). Results demonstrate an average of 1.8% (± 0.57 SEM) of Lin-CD45R-CD19- fetal liver cells express Sca-1 and c-Kit (Supplemental Figure 2A and 2B). The results show a small number of HSC are present within the Lin-CD45R-CD19- fetal liver population. Previously reported data suggests B-1a cell specific progenitors present in the fetal environment arise independently of HSCs (39).
DISCUSSION
N-addition plays a key role in antigen receptor diversity and antibody effector fitness. Anti-phosphorylcholine (PC) antibodies derived from B-1a cells have been shown to be protective against Streptococcus pneumoniae infection (9, 36), and the prototypical B-1a anti-PC antibody, T15, has no N-addition (36, 40). Conversely, TdT transgenic mice vaccinated with heat killed S. pneumoniae generated an anti-PC response, but these anti-PC antibodies were not protective against S. pneumoniae infection (28). These findings highlight the importance of antibody structure in terms of N-addition in the protection afforded by natural antibody.
Herein we identify two progenitor populations, distinguished by phenotype and location, that give rise to B-1a cells expressing immunoglobulin characterized by abundant N-region addition. This differs from the accepted paradigm that B-1a cells produce protective germline-like immunoglobulin with few N-additions (1, 41). The first progenitor population described herein is a population of fetal liver cells, defined as Lin-CD45R-CD19-, which repopulates the peritoneal cavity with authentic, phenotypic IgM+IgDloCD45RloCD5+Mac-1+CD19hiCD43+CD23lo B-1a cells. Furthermore, these B-1a cells derived from Lin-CD45R-CD19- fetal liver cells, B-1aFN cells, exhibit additional characteristics typical of B-1a cells which include, surface expression of CD86 and PD-L2, PtC binding, as well as production of natural antibodies against PC and PPS3. Yet, we show here that the immunoglobulin produced by B-1aFN cells contains abundant N-region addition, which distinguishes B-1aFN cells functionally from native B-1a cells. The N++ immunoglobulin produced by B-1aFN cells further distinguishes them from B-1aFP cells, which are derived from Lin-CD45Rlo/-CD19+ fetal liver progenitor cells described by Dorshkind and colleagues and produce immunoglobulin with little N-addition. Thus, Lin-CD45R-CD19- fetal liver cells represent the first fetal liver cell population shown to be capable of giving rise to B-1a cells with abundant N-addition and hence enhanced diversity.
The second progenitor population described herein is the aforementioned Lin-CD45Rlo/-CD19+ progenitor as it develops in the adult bone marrow rather than the fetal liver. Bone marrow Lin-CD45Rlo/-CD19+ progenitor cells give rise to authentic B-1a cells and these B-1aBP cells produce immunoglobulin with abundant N-addition. This is in direct contrast to the phenotypically identical Lin-CD45Rlo/-CD19+ progenitor cells obtained from fetal liver, which give rise to B-1a cells expressing immunoglobulin with minimal N-addition. Thus, Lin-CD45R-CD19- fetal liver cells, and Lin-CD45Rlo/-CD19+ bone marrow cells, each give rise to N++ B-1a cells and either of these progenitor populations, or both, may contribute to the age-related increase in B-1a cell antibody N-addition documented elsewhere (24, 27). Moreover, our results highlight two variables that determine enhanced, N-addition-mediated B-1a diversity, namely the nature of the B-1a progenitor population (in particular, CD45R-CD19- vs CD45Rlo/-CD19+) and the location in which the B-1a progenitor develops (in particular, fetal liver vs adult bone marrow).
Since B-1aFN and B-1aBP cells displayed immunoglobulin with high level N-addition we evaluated fetal liver and bone marrow progenitors for TdT expression. We found no expression of TdT in Lin-CD45R-CD19- or Lin-CD45Rlo/-CD19+ fetal liver cells, whereas TdT was expressed in Lin-CD45Rlo/-CD19+ bone marrow progenitors. The importance of progenitor expression of TdT for B-1a Naddition is emphasized by our finding that unlike TdT-negative Lin-CD45Rlo/-CD19+ fetal liver cells, TdT-positive Lin-CD45Rlo/-CD19+ bone marrow cells give rise to B-1aBP progeny expressing immunoglobulin with abundant, B-2 cell-like levels of N-addition (Figure 4A). These results are consistent with the generally accepted notion that TdT is not expressed in hematopoietic tissue during fetal life but is abundantly expressed in adult animals (19, 29, 40). Nevertheless, these results raise the question of how Lin-CD45R-CD19- cells function to generate B-1aFN progeny characterized by N-replete immunoglobulin in the absence of TdT. It may be that another route to N-addition beyond TdT exists, or that Lin-CD45R-CD19- progenitors acquire TdT upon adoptive transfer to SCID recipients. It is unlikely to result from repertoire skewing produced by selective pressure because both Lin-CD45R-CD19- and Lin-CD45Rlo/-CD19+ progenitors experienced the same SCID environment upon adoptive transfer.
To address the possibility of a TdT-independent mechanism of N-addition, we evaluated B-1a cell immunoglobulin from TdT-/- mice. We found a limited number of N-additions in native B-1a cells, in the absence of TdT (Figure 5C). These results are the first to examine TdT-/- B-1a cells, but are congruent with previously published data showing about 3% of the VH5 sequences analyzed from splenic B cells of TdT knockout mice display N-additions (42). As suggested by Gilfillan et al, it is probable in the absence of TdT N-additions are inserted during non-homologous recombination at the time of the joining process, or before the joining process, by DNA polymerase misincorporation of nucleotides (42). Therefore, it is plausible a TdT-independent mechanism could be at work after transfer of Lin-CD45R-CD19- fetal liver cells into adult SCID mice to produce B-1a cells with N-additions.
However, the data presented here supports at most only a minor role for a TdT-independent mechanism for N-addition in the immunoglobulin of B-1aFN cells. The N-additions seen in B-1aFN cells are much more extensive (average N-length at both junctions = 3.8) than those seen in B-1a cells from TdT-/- mice (average N-length at both junctions = 0.6). Thus, a TdT-independent mechanism of N-addition may contribute to N-addition in antibodies produced by B-1a cells derived from TdT-negative progenitors, but is unlikely to be fully responsible.
Interestingly, induction of TdT expression by IL-7 has been demonstrated in bone marrow pro-B cells (43). IL-7-/- knockout studies have shown mouse fetal B cell development is not dependent upon IL-7, whereas B cell development in the adult is IL-7 dependent (44). These previous reports suggest B cell development in the fetal liver environment takes place in the absence of IL-7. In context with the data presented here showing TdT-negative progenitors transferred into adult SCID mice produce B-1a cells expressing immunoglobulin with abundant N-additions, it is reasonable to hypothesize that upon transfer into the adult environment fetal progenitors are exposed to IL-7 (along with other factors), which up-regulates TdT expression. The absence of TdT expression in Lin-CD45R-CD19- fetal liver cells cultured with IL-7 does not rule out induction of TdT in vivo after transfer to SCID mice because in vitro conditions cannot completely reproduce the natural bone marrow environment. Together our results along with previous findings suggest the numerous N-additions observed in B-1a cells derived from TdT-negative Lin-CD45R-CD19- fetal liver cells are likely the result of TdT up-regulation in the adult environment in combination with minimal contribution of TdT-independent N-addition. In this scheme, the key functional difference between Lin-CD45R-CD19- and Lin-CD45Rlo/-CD19+ progenitor populations lies in responsiveness to the adult bone marrow milieu in terms of TdT up-regulation.
Peritoneal B-1a cells from young (3 month old) mice express immunoglobulin with little N-addition, similar to the level of N-addition found in B-1aFP progeny of Lin-CD45Rlo/-CD19+ fetal liver progenitors, whereas native B-1a cells do not begin to express immunoglobulin with substantial N-addition until well into adulthood. Several groups have shown total lineage negative adult bone marrow can give rise to B-1a cells, inferring that B-1a cells are generated de novo throughout adulthood (24-26). Notably, hematopoietic stem cells, and common lymphoid progenitors, largely fail to give rise to B-1a cells beyond the second week of life (45-47). These studies imply mainly bone marrow cells other than HSC and CLP produce N++ B-1a cells in the adult. This focuses attention on the behavior of the Lin-AA4.1-CD45R-CD19- and Lin-AA4.1+CD45Rlo/-CD19+ progenitor populations, which could provide a source of non-HSC B-1a progenitors in the adult.
Lin-CD45R-CD19- cells may correspond to an earlier stage of development than Lin-CD45Rlo/-CD19+ cells because the former lack AA4.1 and acquire CD45R and CD19 during in vitro culture with IL-7. We have shown AA4.1-Lin-CD45R-CD19- fetal liver cells lack TdT but presumably acquire it in the adult bone marrow, where resident AA4.1+Lin-CD45Rlo/-CD19+ cells express TdT constitutively (although the phenotypically identical AA4.1+Lin-CD45Rlo/-CD19+ cells developing in the fetal liver appear refractory to TdT induction). Thus, there are at least 2 ways in which the AA4.1-Lin-CD45R-CD19- progenitor cells described here may give rise to N++ B-1a cells in vivo. (1) AA4.1-Lin-CD45R-CD19- progenitor cells may differentiate into AA4.1+Lin-CD45Rlo/-CD19+ progenitor cells that give rise to N- B-1a cells early in life but migrate to the bone marrow in adulthood where, having acquired TdT, they generate N++ B-1a cells. (2) AA4.1-Lin-CD45R-CD19- progenitor cells are quiescent early in life but migrate to the bone marrow in adulthood where they acquire TdT and generate N++ B-1a cells, or they give rise to TdT+ AA4.1+Lin-CD45Rlo/-CD19+ progenitors that generate N++ B-1a cells. At present it is not possible to differentiate between these mechanisms. We hypothesize option (2) is the more likely scenario because results presented here demonstrate a) AA4.1-Lin-CD45R-CD19- progenitor cells do not acquire CD19 or AA4.1 without IL-7 in vitro (Figure 6C and 6D), and b) AA4.1+Lin-CD45Rlo/-CD19+ fetal liver cells do not give rise to N++ B-1a cells upon transfer to the adult (Figure 4). These results suggest bone marrow migration of fetal liver AA4.1+Lin-CD45Rlo/-CD19+ cells cannot account for generation of N++ B-1a cells.
In sum, our data suggests Lin-CD45R-CD19- fetal liver cells, which grow out B-1aFN cells in SCID mice, add to the adult pool of B-1a cells present in older animals. Their relationship to Lin-CD45Rlo/-CD19+ progenitors in adult bone marrow, which also generate B-1a cells with abundant N-additions, remains uncertain.
Supplementary Material
Acknowledgments
We kindly thank Dr. Stephen H. Clarke for providing PtC liposomes and Dr. Ann Feeney for providing TdT deficient mice.
Abbreviations
- B-1aBP
B-1a cells derived from Lin-CD45Rlo/-CD19+ adult bone marrow cells
- B-1aFN
B-1a cells derived from Lin-CD45R-CD19- fetal liver cells
- B-1aFP
B-1a cells derived from Lin-CD45Rlo/-CD19+ fetal liver cells
- Lin-
Lineage negative cells
Footnotes
This work was supported by PHS grant AI29690 awarded by the National Institutes of Health.
References
- 1.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 2.Rothstein TL. Cutting edge commentary: two B-1 or not to be one. J Immunol. 2002;168:4257–4261. doi: 10.4049/jimmunol.168.9.4257. [DOI] [PubMed] [Google Scholar]
- 3.Wong SC, Chew WK, Tan JE, Melendez AJ, Francis F, Lam KP. Peritoneal CD5+ B-1 cells have signaling properties similar to tolerant B cells. J Biol Chem. 2002;277:30707–30715. doi: 10.1074/jbc.M202460200. [DOI] [PubMed] [Google Scholar]
- 4.Holodick NE, Tumang JR, Rothstein TL. Continual signaling is responsible for constitutive ERK phosphorylation in B-1a cells. Mol Immunol. 2009;46:3029–3036. doi: 10.1016/j.molimm.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong X, Gao W, Degauque N, Bai C, Lu Y, Kenny J, Oukka M, Strom TB, Rothstein TL. Reciprocal generation of Th1/Th17 and T(reg) cells by B1 and B2 B cells. Eur J Immunol. 2007;37:2400–2404. doi: 10.1002/eji.200737296. [DOI] [PubMed] [Google Scholar]
- 6.Sidman CL, Shultz LD, Hardy RR, Hayakawa K, Herzenberg LA. Production of immunoglobulin isotypes by Ly-1+ B cells in viable motheaten and normal mice. Science. 1986;232:1423–1425. doi: 10.1126/science.3487115. [DOI] [PubMed] [Google Scholar]
- 7.Tumang JR, Frances R, Yeo SG, Rothstein TL. Spontaneously Ig-secreting B-1 cells violate the accepted paradigm for expression of differentiation-associated transcription factors. J Immunol. 2005;174:3173–3177. doi: 10.4049/jimmunol.174.6.3173. [DOI] [PubMed] [Google Scholar]
- 8.Forster I, Rajewsky K. Expansion and functional activity of Ly-1+ B cells upon transfer of peritoneal cells into allotype-congenic, newborn mice. Eur J Immunol. 1987;17:521–528. doi: 10.1002/eji.1830170414. [DOI] [PubMed] [Google Scholar]
- 9.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med. 2000;192:271–280. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alugupalli KR, Gerstein RM, Chen J, Szomolanyi-Tsuda E, Woodland RT, Leong JM. The resolution of relapsing fever borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J Immunol. 2003;170:3819–3827. doi: 10.4049/jimmunol.170.7.3819. [DOI] [PubMed] [Google Scholar]
- 12.Feeney AJ. Lack of N regions in fetal and neonatal mouse immunoglobulin V-D-J junctional sequences. J Exp Med. 1990;172:1377–1390. doi: 10.1084/jem.172.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pennell CA, Arnold LW, Haughton G, Clarke SH. Restricted Ig variable region gene expression among Ly-1+ B cell lymphomas. J Immunol. 1988;141:2788–2796. [PubMed] [Google Scholar]
- 14.Hardy RR, Carmack CE, Shinton SA, Riblet RJ, Hayakawa K. A single VH gene is utilized predominantly in anti-BrMRBC hybridomas derived from purified Ly-1 B cells. Definition of the VH11 family. J Immunol. 1989;142:3643–3651. [PubMed] [Google Scholar]
- 15.Wang H, Clarke SH. Positive selection focuses the VH12 B-cell repertoire towards a single B1 specificity with survival function. Immunol Rev. 2004;197:51–59. doi: 10.1111/j.0105-2896.2004.0098.x. [DOI] [PubMed] [Google Scholar]
- 16.Spangrude GJ, Smith L, Uchida N, Ikuta K, Heimfeld S, Friedman J, Weissman IL. Mouse hematopoietic stem cells. Blood. 1991;78:1395–1402. [PubMed] [Google Scholar]
- 17.Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 18.Desiderio SV, Yancopoulos GD, Paskind M, Thomas E, Boss MA, Landau N, Alt FW, Baltimore D. Insertion of N regions into heavy-chain genes is correlated with expression of terminal deoxytransferase in B cells. Nature. 1984;311:752–755. doi: 10.1038/311752a0. [DOI] [PubMed] [Google Scholar]
- 19.Gregoire KE, Goldschneider I, Barton RW, Bollum FJ. Ontogeny of terminal deoxynucleotidyl transferase-positive cells in lymphohemopoietic tissues of rat and mouse. J Immunol. 1979;123:1347–1352. [PubMed] [Google Scholar]
- 20.Hayakawa K, Hardy RR, Stall AM, Herzenberg LA. Immunoglobulin-bearing B cells reconstitute and maintain the murine Ly-1 B cell lineage. Eur J Immunol. 1986;16:1313–1316. doi: 10.1002/eji.1830161021. [DOI] [PubMed] [Google Scholar]
- 21.Lalor PA, Herzenberg LA, Adams S, Stall AM. Feedback regulation of murine Ly-1 B cell development. Eur J Immunol. 1989;19:507–513. doi: 10.1002/eji.1830190315. [DOI] [PubMed] [Google Scholar]
- 22.Kantor AB. V-gene usage and N-region insertions in B-1a, B-1b and conventional B cells. Semin Immunol. 1996;8:29–35. doi: 10.1006/smim.1996.0005. [DOI] [PubMed] [Google Scholar]
- 23.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 24.Holodick NE, Repetny K, Zhong X, Rothstein TL. Adult BM generates CD5+ B1 cells containing abundant N-region additions. Eur J Immunol. 2009;39:2383–2394. doi: 10.1002/eji.200838920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duber S, Hafner M, Krey M, Lienenklaus S, Roy B, Hobeika E, Reth M, Buch T, Waisman A, Kretschmer K, Weiss S. Induction of B-cell development in adult mice reveals the ability of bone marrow to produce B-1a cells. Blood. 2009;114:4960–4967. doi: 10.1182/blood-2009-04-218156. [DOI] [PubMed] [Google Scholar]
- 26.Esplin BL, Welner RS, Zhang Q, Borghesi LA, Kincade PW. A differentiation pathway for B1 cells in adult bone marrow. Proc Natl Acad Sci U S A. 2009;106:5773–5778. doi: 10.1073/pnas.0811632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu H, Forster I, Rajewsky K. Sequence homologies, N sequence insertion and JH gene utilization in VHDJH joining: implications for the joining mechanism and the ontogenetic timing of Ly1 B cell and B-CLL progenitor generation. EMBO J. 1990;9:2133–2140. doi: 10.1002/j.1460-2075.1990.tb07382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benedict CL, Kearney JF. Increased junctional diversity in fetal B cells results in a loss of protective anti-phosphorylcholine antibodies in adult mice. Immunity. 1999;10:607–617. doi: 10.1016/s1074-7613(00)80060-6. [DOI] [PubMed] [Google Scholar]
- 29.Li YS, Hayakawa K, Hardy RR. The regulated expression of B lineage associated genes during B cell differentiation in bone marrow and fetal liver. J Exp Med. 1993;178:951–960. doi: 10.1084/jem.178.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kantor AB, Merrill CE, Herzenberg LA, Hillson JL. An unbiased analysis of V(H)-D-J(H) sequences from B-1a, B-1b, and conventional B cells. J Immunol. 1997;158:1175–1186. [PubMed] [Google Scholar]
- 31.Shriner AK, Liu H, Sun G, Guimond M, Alugupalli KR. IL-7-dependent B lymphocytes are essential for the anti-polysaccharide response and protective immunity to Streptococcus pneumoniae. J Immunol. 185:525–531. doi: 10.4049/jimmunol.0902841. [DOI] [PubMed] [Google Scholar]
- 32.Kantor AB, Stall AM, Adams S, Herzenberg LA. Differential development of progenitor activity for three B-cell lineages. Proc Natl Acad Sci U S A. 1992;89:3320–3324. doi: 10.1073/pnas.89.8.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato S, Ono N, Steeber DA, Pisetsky DS, Tedder TF. CD19 regulates B lymphocyte signaling thresholds critical for the development of B-1 lineage cells and autoimmunity. J Immunol. 1996;157:4371–4378. [PubMed] [Google Scholar]
- 34.Zhong X, Tumang JR, Gao W, Bai C, Rothstein TL. PD-L2 expression extends beyond dendritic cells/macrophages to B1 cells enriched for V(H)11/V(H)12 and phosphatidylcholine binding. Eur J Immunol. 2007;37:2405–2410. doi: 10.1002/eji.200737461. [DOI] [PubMed] [Google Scholar]
- 35.Mercolino TJ, Locke AL, Afshari A, Sasser D, Travis WW, Arnold LW, Haughton G. Restricted immunoglobulin variable region gene usage by normal Ly-1 (CD5+) B cells that recognize phosphatidyl choline. J Exp Med. 1989;169:1869–1877. doi: 10.1084/jem.169.6.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Briles DE, Forman C, Hudak S, Claflin JL. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J Exp Med. 1982;156:1177–1185. doi: 10.1084/jem.156.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li YS, Wasserman R, Hayakawa K, Hardy RR. Identification of the earliest B lineage stage in mouse bone marrow. Immunity. 1996;5:527–535. doi: 10.1016/s1074-7613(00)80268-x. [DOI] [PubMed] [Google Scholar]
- 38.Yamane T, Hosen N, Yamazaki H, Weissman IL. Expression of AA4.1 marks lymphohematopoietic progenitors in early mouse development. Proc Natl Acad Sci U S A. 2009;106:8953–8958. doi: 10.1073/pnas.0904090106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshimoto M, Montecino-Rodriguez E, Ferkowicz MJ, Porayette P, Shelley WC, Conway SJ, Dorshkind K, Yoder MC. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc Natl Acad Sci U S A. 2011;108:1468–1473. doi: 10.1073/pnas.1015841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benedict CL, Gilfillan S, Thai TH, Kearney JF. Terminal deoxynucleotidyl transferase and repertoire development. Immunol Rev. 2000;175:150–157. [PubMed] [Google Scholar]
- 41.Tung JW, Herzenberg LA. Unraveling B-1 progenitors. Current opinion in immunology. 2007;19:150–155. doi: 10.1016/j.coi.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 42.Gilfillan S, Dierich A, Lemeur M, Benoist C, Mathis D. Mice lacking TdT: mature animals with an immature lymphocyte repertoire. Science. 1993;261:1175–1178. doi: 10.1126/science.8356452. [DOI] [PubMed] [Google Scholar]
- 43.Wei C, Zeff R, Goldschneider I. Murine pro-B cells require IL-7 and its receptor complex to up-regulate IL-7R alpha, terminal deoxynucleotidyltransferase, and c mu expression. J Immunol. 2000;164:1961–1970. doi: 10.4049/jimmunol.164.4.1961. [DOI] [PubMed] [Google Scholar]
- 44.Carvalho TL, Mota-Santos T, Cumano A, Demengeot J, Vieira P. Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7(-/)- mice. J Exp Med. 2001;194:1141–1150. doi: 10.1084/jem.194.8.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kikuchi K, Kondo M. Developmental switch of mouse hematopoietic stem cells from fetal to adult type occurs in bone marrow after birth. Proc Natl Acad Sci U S A. 2006;103:17852–17857. doi: 10.1073/pnas.0603368103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghosn EE, Yamamoto R, Hamanaka S, Yang Y, Herzenberg LA, Nakauchi H, Herzenberg LA. Distinct B-cell lineage commitment distinguishes adult bone marrow hematopoietic stem cells. Proc Natl Acad Sci U S A. 2012;109:5394–5398. doi: 10.1073/pnas.1121632109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barber CL, Montecino-Rodriguez E, Dorshkind K. Reduced production of B-1-specified common lymphoid progenitors results in diminished potential of adult marrow to generate B-1 cells. Proc Natl Acad Sci U S A. 2011;108:13700–13704. doi: 10.1073/pnas.1107172108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.