Abstract
We studied expression of the Aurora A gene and its clinical significance in a cohort of neuroblastoma patients. In addition, we investigated the antitumor activity of MLN8054, a novel small-molecule inhibitor of Aurora A kinase, on cultured NB cell lines in vitro. Aurora A mRNA expression was assessed by quantitative real-time PCR in tumor tissue specimens from 67 patients at diagnosis and in 9 human neuroblastoma cell lines. Western blot assays for Aurora A protein were done on tumor tissue of 53 patients. The results were correlated with various prognostic factors of neuroblastoma. Aurora A mRNA and protein expression were identified in 9 of 9 neuroblastoma cell lines. Overexpression of Aurora A mRNA in neuroblastoma tumor tissue is associated with high risk (P = 0.019), high-stage (International Neuroblastoma Staging System III and IV) tumors (P = 0.007), unfavorable histology (P = 0.007), MYCN amplification (P = 0.017), disease relapse (P = 0.019), and decreased progression-free survival (P < 0.0001) but not correlated with the age at diagnosis (P = 0.877). Similarly, Aurora A protein expression also significantly correlated with high risk (P = 0.011), high stage (P = 0.0028), unfavorable histology (P = 0.0006), MYCN amplification (P = 0.0029), and disease relapse (P = 0.044). Small interfering RNA–mediated knockdown of the endogenous Aurora A gene causes a proliferation defect and enhances chemosensitivity in human neuroblastoma cell lines. In support of these observations, the Aurora A kinase inhibitor, MLN8054, markedly inhibited growth of cultured neuroblastoma cell lines through an apoptosis-dependent pathway. Overexpression of Aurora A is associated with disease progression in neuroblastoma. Inhibition of this kinase is a promising modality for neuroblastoma treatment.
Introduction
Neuroblastoma is the most common extracranial solid tumor in children and is responsible for 10% to 15% of pediatric cancer deaths (1). Over the past 30 years, much effort has gone into establishing reliable prognostic indicators of high-risk disease. For neuroblastoma, age, stage, and histopathology have consistently correlated well with outcomes. Chromosomal number, or ploidy, and amplification of the MYCN oncogene have also proven to be important and are commonly used to stratify patient risk (2–4). Despite recent advances in cancer therapy, the prognosis of patients with high-risk neuroblastoma is still very poor. Currently, much effort is focused on developing novel therapeutic strategies and improving the prognosis of higher-stage neuroblastoma patients. To fulfill this purpose, discovery and characterization of new molecular targets involved in neuroblastoma progression is urgently required.
The Aurora A gene (serine/threonine kinase 15, also known as STK15, BT AK, and Aurora 2), encoding a centrosome-associated kinase, is amplified and overexpressed in multiple human adult tumor cell types (5–11) and is involved in the induction of centrosome duplication-distribution abnormalities and aneuploidy in mammalian cells (5). Recent studies have shown that Aurora A is overexpressed and/or amplified in breast cancers (26%; refs. 5, 6), hepatocellular carcinoma (61%; refs. 7, 8), laryngeal squamous cell carcinoma (68%; refs. 9–11), and ovarian cancer (67%; ref. 12) as well as in neuroblastoma cell lines (5). The specific knockdown of Aurora A strongly suppresses in vitro cell growth and in vivo tumorigenicity and enhances the taxane or docetaxel chemosensitivity of human cancer cells (13, 14), substantially suppresses cell migration in human esophageal squamous cell carcinoma cells (15), and suppresses cell cycle progression in HeLa cells (16). These findings imply that Aurora A is a critical oncogene. Its overexpression in multiple tumor types shows that Aurora A may be a potential therapeutic target.
The role of Aurora A in neuroblastoma progression has not been well characterized. In the present study, we have examined the expression of Aurora A mRNA and protein both in a set of 67 neuroblastoma primary tumor tissue samples and 9 tumor cell lines and analyzed the clinicopathologic features of Aurora A expression in neuroblastoma patients. In addition, the cytotoxic activity and the mechanism of action of the Aurora A inhibitor MLN8054 was analyzed in a series of neuroblastoma cell lines. We hypothesize that Aurora A could be an important prognostic factor and a new therapeutic target in human neuroblastoma.
Materials and Methods
Patients and Tumor Tissue Collection
The neuroblastoma patients participating in this study were recruited from the Texas Children's Cancer Center at Texas Children's Hospital from 1995 to 2006. All of the procedures were approved by the Baylor College of Medicine Institutional Review Board. Tumor samples were obtained from 67 neuroblastoma patients with International Neuroblastoma Staging System (INSS) stage I to IV or IVS disease in this study. Fresh tumor tissues were collected from patients with pathologically and clinically confirmed neuroblastoma. A portion of tumor specimens were kept in −80°C and sectioned for total RNA and protein extraction. Clinical information was obtained by chart review.
Cell Culture and Compound Treatment
Human neuroblastoma tumor cell lines (IMR-32, SK-NSH, SH-SY5Y, SK-N-AS, SH-EP, and LAN-1), breast normal epithelial cell line MCF-10A, and human breast cancer cell line MCF-7 were obtained from the American Type Culture Collection, and the neuroblastoma cell line JF was kindly provided by Dr. M. Brenner (Baylor College of Medicine). NB19 and SMS-KCN were kindly provided by Dr. A. Davidoff (St. Jude's Children's Hospital). SH-SY5Y-Luc cells were kindly provided by Dr. Eugene S. Kim (Baylor College of Medicine). Briefly, cell lines were maintained in MEM (IMR-32, SK-N-SH, NB19, SMS-KCN, and LAN-1), RPMI 1640 (JF, SH-EP, and SH-SY5Y-Luc) and DMEM (SK-N-AS, MCF-10A, and MCF-7). All media were supplemented with 10% heat-inactivated FCS, 2 mmol/L glutamine, 100 units/mL penicillin, and 100 µg/mL streptomycin (all from Invitrogen). MLN8054 (Millennium Pharmaceuticals) was diluted in distilled water and added to the cell culture medium at a final concentration of 0.1 to 50 µmol/L. Doxorubicin (Sigma-Aldrich) was diluted in HBSS and added to the cell culture medium at a final concentration of 0.1 to 1 µmol/L.
Quantitative Real-time PCR
Total RNA was extracted from tumor cell lines and tumor tissues using Trizol reagent (Invitrogen), and the purity of RNA was determined by measuring the absorbance at 260/280 nm (A260/A280) in a spectrophotometer. The following primer pairs were used: Aurora A mRNA (forward 5′-TGGAATATGCACCACTTGGA-3′ and reverse 5′-GGCATTTGCCAATTCTGTTA-3′; the product size is 101 bp) and glyceraldehyde-3-phosphate dehydrogenase mRNA (forward 5′-CCACATCGCTCAGACACCAT-3′ and reverse 5′-GGCAACAATATCCACTTTACCAGAGT-3′; the product size is 113 bp). Glyceraldehyde-3-phosphate dehydrogenase was used as an internal control. One hundred nanograms of RNA were used as the template for a one-step real-time PCR (RT-PCR). QuantiTect SYBR Green (Qiagen) RT-PCR mixes were used for RT-PCR. For each unknown test sample, the amount of Aurora A and endogenous reference glyceraldehyde-3-phosphate dehydrogenase was determined from the respective standard curve. Subtracting the glyceraldehyde-3-phosphate dehydrogenase level from the Aurora A level resulted in a normalized Aurora A value (Table 1). For the cell cultures, the MCF-10A mammary epithelial cell line was considered the normal threshold value, whereas for the neuroblastoma tissue samples the average normalized Aurora A level of seven ganglioneuroma samples established the threshold below which the value was considered background (17).
Table 1.
Clinicopathologic characteristics of the patients with neuroblastoma and Aurora A mRNA and protein expression
| Patient characteristics | mRNA | Protein | ||||
|---|---|---|---|---|---|---|
| n | X ± SD | P | n | X ± SD | P | |
| Age at diagnosis (y) | ||||||
| ≤1 | 28 | 3.10 ± 2.64 | 0.877 | 25 | 1.37 ± 1.67 | 0.29 |
| >1 | 39 | 2.98 ± 3.30 | 28 | 1.82 ± 1.37 | ||
| INSS stage | ||||||
| I + II + IVS | 31 | 1.98 ± 1.34 | 0.007 | 26 | 1.28 ± 1.24 | 0.0028 |
| III + IV | 36 | 3.94 ± 3.72 | 27 | 2.85 ± 2.23 | ||
| MYCN status | ||||||
| Nonamplified | 49 | 2.64 ± 2.66 | 0.017 | 36 | 1.30 ± 1.04 | 0.0029 |
| Amplified (>10 copies) | 14 | 4.84 ± 3.90 | 11 | 2.50 ± 1.31 | ||
| Histology | ||||||
| Favorable | 28 | 2.42 ± 2.00 | 0.007 | 23 | 1.03 ± 0.87 | 0.0006 |
| Unfavorable | 19 | 4.89 ± 3.94 | 14 | 2.26 ± 1.11 | ||
| Disease risk | ||||||
| Low + intermediate | 42 | 2.31 ± 1.80 | 0.019 | 35 | 1.22 ± 0.94 | 0.011 |
| High | 22 | 4.74 ± 4.18 | 16 | 2.11 ± 1.47 | ||
| Relapse | ||||||
| Yes | 19 | 4.59 ± 4.41 | 0.019 | 14 | 2.42 ± 2.07 | 0.044 |
| No | 43 | 2.63 ± 2.00 | 35 | 1.45 ± 1.17 | ||
NOTE: n, number of neuroblastoma tumor tissues tested; X, mean mRNA and protein expression levels between selected groups and determined by RT-PCR and Western blotting assay.
Western Blotting Assay
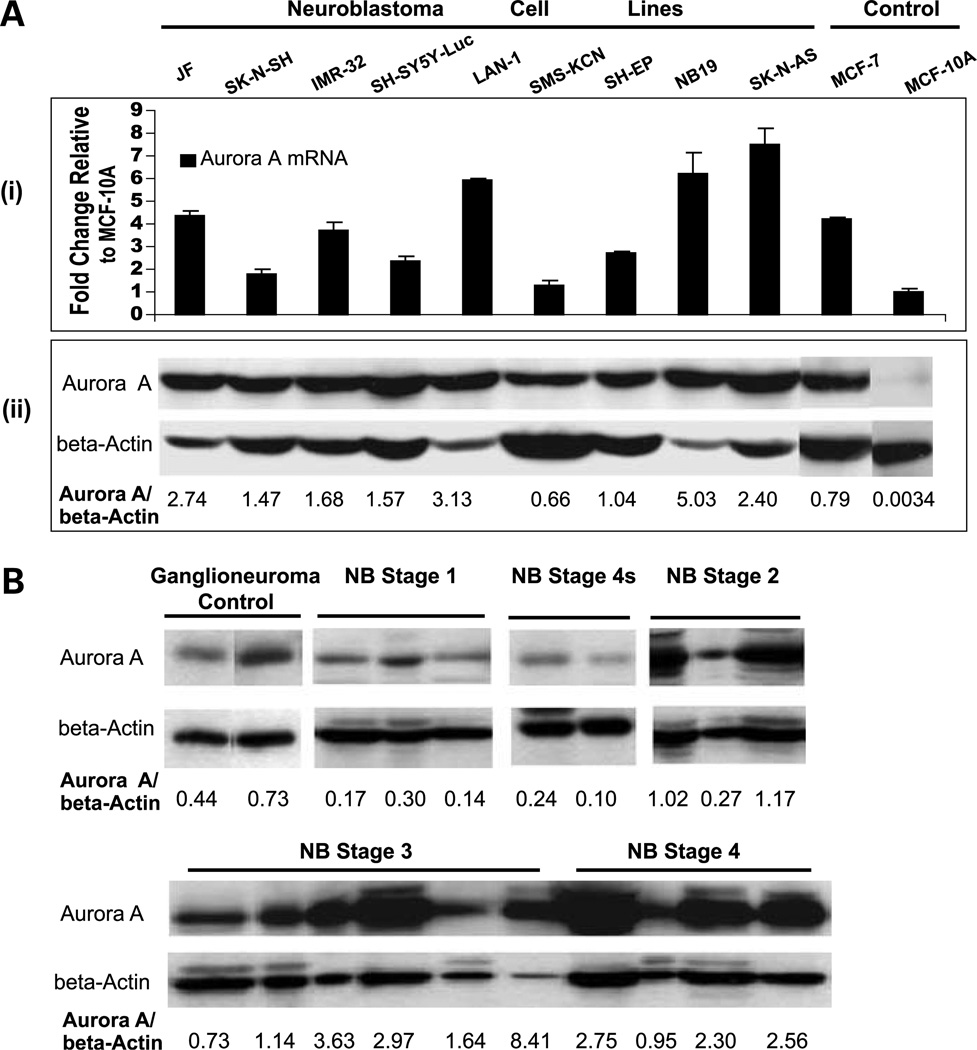
For Aurora A protein extraction, neuroblastoma cells were lysed in prewarmed (95°C) cell lysate buffer [2% SDS/300 mmol/LTris-HCl (pH 6.8)/10% glycerol]; frozen tissue samples were sectioned into small pieces and proteins were extracted by using Trizol reagent. For poly(ADP-ribose) polymerase (PARP) protein cleavage assay, radioimmuno-precipitation assay buffer was used as described (18). Equal amounts of protein (30 µg) were separated by SDS-PAGE on 10% gels, transferred to polyvinylidene difluoride membrane (Amersham Pharmacia), and incubated with antibodies specific for Aurora A (1:1,000 dilution), PARP (1:1,000 dilution), or the secondary antibodies horseradish peroxidase–linked anti-rabbit immunoglobulin (1:1,000 dilution) and horseradish peroxidase–linked anti-mouse immunoglobulin (1:1,000 dilution; all from Cell Signaling Technology) as well as antibodies specific for β-actin (A2228; 1:5,000 dilution; Sigma-Aldrich). Electrochemiluminescence detection (GE Healthcare) with documentation on X-ray films was used for the detection of protein, the lysate from NB cell line SK-N-AS was used as an internal control, and the same exposure time (40 s) was set up to standardize the analysis. The net intensity of the immunoreactive bands was quantified by scanning densitometry (Scan Molecular Dynamics; GE Healthcare) using Imaging Analysis software (Image-Quant Tool version 5.2). Specific protein level was expressed as the ratio of the net intensity of the protein band to β-actin band of the same sample [Fig. 1A (ii) and B; Table 1].
Figure 1.
A, Aurora A expression in human neuroblastoma cell lines determined by quantitative RT-PCR (i) and Western blot analysis (ii). Western blots were probed with anti-Aurora A antibody and reprobed with anti-β-actin antibody to show equal protein loading. Breast tumor cell line MCF-7 and normal breast epithelial cell line MCF-10A were used as positive and negative controls for Aurora A expression, respectively. B, analysis of Aurora A expression in neuroblastoma and ganglioneuroma tumor tissue samples by Western blot. Representative neuroblastoma tumor samples are shown, grouped by INSS stage.
Establishment of Stable Short Hairpin RNA Aurora A Knockdown Neuroblastoma Cell Lines
Aurora A short hairpin RNA expression was achieved by cloning the following ligated sequences into the pSuper-PURO retrovirus expression vectors per manufacturer's protocol (Oligoengine). The RNA interference target sequences used were as follows: pSuper-Scrambled control (Sh-Control), CGTCTTTTCGGACTTAGAGAG; pSuper-No.1-sh Aurora A (Sh-Aurora A #1), AACGTGTTCTCGTGACTCAGC; and pSuper-No.2-sh Aurora A (Sh-Aurora A #2), AAATGCCCTGTCTTACTGTCA (13). For retroviral transduction, we used a previously published protocol (19). Stable cell lines were established after 10 days of puromycin (2 µg/mL) selection.
Proliferation and Soft-Agar Assays
Cells were plated in 96-well flat-bottomed plates at a concentration of 1 × 103 or 1 × 104 per well. Using the Cell Counting Kit-8 (Dojindo Molecular Technologies), cells were stained at the indicated time point. After a 3 h incubation period, absorbance was measured at 450 nm using a standard plate reader.
For the soft-agar assays, a layer of 0.5% agarose/culture medium was plated and allowed to solidify. Cells (1 × 104 or 5 × 103) were suspended in 0.3% agarose/culture medium and plated on top of the 0.5% layer. Culture medium (500 µL) was added on top of the agarose to prevent drying of the soft agar. Wells were photographed after 14 days of growth and colonies (>30 cells) were counted. All experiments were carried out in triplicate with the mean ± SD reported.
Flow Cytometry for Detection of Apoptotic Cells and Cell Cycle Analysis
Neuroblastoma cells were cultured in 6-cm dishes (2 × 105 per dish) for 24 h and then treated for 48 h with MLN8054 at concentrations between 0 and 50 µmol/L. Subsequently, floating cells in the medium and adherent cells were collected. Using an Annexin V-FITC Apoptosis Detection kit (BD Biosciences), cells were stained with Annexin V-FITC and propidium iodide according to the manufacturer's instructions. Untreated cells were used as the negative control for double staining. Cells were analyzed immediately after staining by using a FACScan flow cytometer and the Cell-Quest software. For each measurement, >10,000 events were counted. A flow cytometric analysis of DNA content was done to assess the cell cycle phase distribution (20). DNA content was evaluated using a FACScan flow cytometer (Becton Dickinson) and CellQuest software (Becton Dickinson). The experiment was repeated three times.
Statistical Analysis
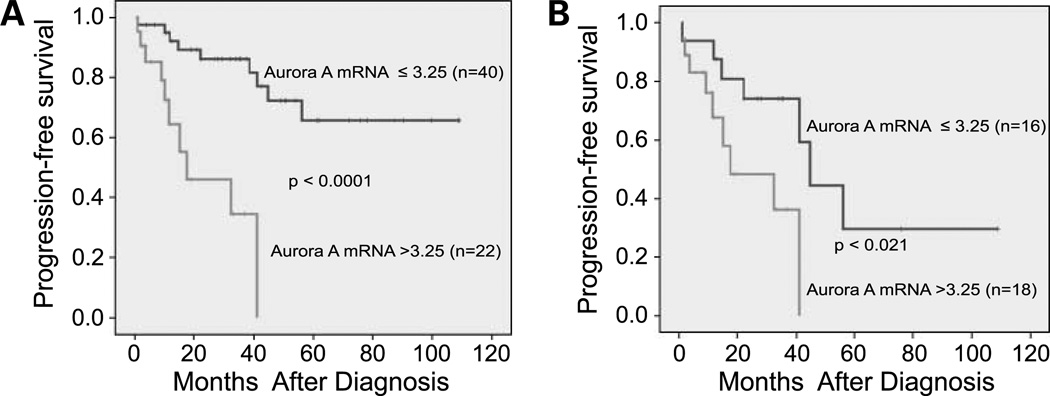
We computed basic descriptive statistics for age at diagnosis, gender, histology, extent of surgery, radiation therapy, chemotherapy, and level of mRNA and protein expression for Aurora A. Two-sample t test or ANOVA was used to compare the mean mRNA and protein expression levels (X value) between selected groups (Table 1). Kaplan-Meier procedure was used to estimate the progression-free survival (21). The cutoff to discriminate low versus high Aurora A mRNA expression (3.25) was selected because it separated the population into two distinct survival groups (Fig. 2; Supplementary Table S1). Survival time was calculated in 62 patients for whom the date of diagnosis, date of progression or relapse, death, or date of last follow-up were available. Cox proportional hazard analysis was used for multivariable analyses adjusting for age at diagnosis, gender, risk group (low-intermediate versus high), stage (stages I, II, and IVS versus stages III and IV), MYCN amplification status, and histology. All statistical tests were two-sided and P < 0.05 was considered significant. For all of the analyses, we used SPSS (version 15.0) software package.
Figure 2.
Aurora A mRNA expression level is significantly associated with decreased progression-free survival in patients with neuroblastoma. A, effect of Aurora A mRNA expression on progression-free survival in all patients in this study (P < 0.0001). B, effect of Aurora A mRNA expression on progression-free survival in INSS stage III and IV patients (P < 0.021).
Results
Aurora A Is Overexpressed in Human Neuroblastoma Cell Lines
To address the possibility of whether Aurora A could serve as a therapeutic target for neuroblastoma, we measured the protein and mRNA levels of Aurora A in nine cultured neuroblastoma cell lines. Because neuroblastic (N) type and substrate adherent (S) type cells are two distinct cell types in neuroblastoma tumors and tumor cell cultures, we tested the Aurora A expression in both four N-type cell lines (JF, SH-SY5Y-Luc, IMR-32, and Lan-1) and four S-type cell lines (SMS-KCN, SH-EP, NB19, and SK-N-AS) as well as one mixed N/S-type cell line (SK-N-SH; Table 2). Total RNA and protein were harvested at the same time from each cell line. As shown in Fig. 1A (i), with the exception of SMS-KCN, the neuroblastoma cell lines had higher levels of Aurora A mRNA ranging from 1.7- to 7.5-fold higher than the control level. Also, compared with the human mammary epithelial cell line MCF-10A (negative control), all nine neuroblastoma cell lines had high levels of Aurora A protein expression [Fig. 1A (ii)]. The Aurora A mRNA level correlated well with protein expression in the majority of tested neuroblastoma cell lines (Fig. 1A).
Table 2.
Biological characteristics and sensitivity to MLN8054 in human neuroblastoma cell lines
| Cell line | Origin | MYCN | p53 status | Cell type | IC50 of MLN8054 (µmol/L) |
|---|---|---|---|---|---|
| SK-N-SH | NB tumor | SC | WT | N + S | 0.46 ± 0.01 (2) |
| SH-SY5Y-Luc | NB tumor | SC | WT | N | 0.24 ± 0.02 (4) |
| IMR-32 | NB tumor | A | WT | N | 0.30 ± 0.08 (2) |
| LAN-1 | NB tumor | A | Null | N | 0.67 ± 0.04 (4) |
| JF | NB tumor | A | N/D | N | 0.26 ± 0.06 (3) |
| SH-EP | NB tumor | SC | WT | S | 0.92 (1) |
| NB19 | NB tumor | A | WT | S | 0.50 (1) |
| SMS-KCN | NB tumor | A | N/D | S | 2.77 ± 0.18 (2) |
| SK-N-AS | NB tumor | SC | mt | S | 4.11 ± 1.03 (2) |
| MCF-10A | Breast normal epithelial | WT | 12.92 (1) | ||
| MCF-7 | Breast tumor | WT | 0.44 (1) |
NOTE: Numbers represent average ± SD IC50 derived from a Cell Counting Kit-8 proliferation assay. Values in parentheses represent the number of experiments completed for each cell line.
Abbreviations: NB, neuroblastoma; SC, single copy; A, amplified; WT, wild-type; mt, mutated; N/D, not described; N, neuroblastic; S, substrate adherent.
High Aurora A Expression Is Associated with Poor Clinical Outcome in Human Neuroblastoma
Aurora A mRNA expression was measured in tumor tissue specimens from 67 patients using quantitative RT-PCR. Quantification of the kinase protein was done in a subset of 53 specimens for whom sufficient protein was available. Among the 67 patients, the mean patient age was 18 months (range, 1–75 months), 14 of 63 (22%) patients were MYCN-amplified, and 22 of 64 (34%) patients were considered high risk according to the Children's Oncology Group neuroblastoma risk classification system (22). The distribution among the INSS stages was stage I, 18%; stage II, 21%; stage III, 15%; stage IV, 39%; and stage IVS, 7%. Patient characteristics and the relative Aurora A expression levels are shown in Table 1.
Aurora A mRNA expression in neuroblastoma tissue samples was compared with the mean of seven ganglioneuroma specimens, with the ratio expressed as fold change. The latter neoplasm was chosen because it is considered a benign cognate of malignant neuroblastoma (23). As shown in Table 1, expression level of Aurora A mRNA is associated with high INSS stage (stage III or IV; P = 0.007), MYCN amplification (P = 0.017), unfavorable histology (P = 0.007), high Children's Oncology Group risk group (P = 0.019), and disease relapse (P = 0.019) but did not correlate with the age at diagnosis (P = 0.877). As shown in Fig. 1B and Table 1, Aurora A protein expression also significantly correlated with high INSS stage (stage III or IV; P = 0.0028), MYCN amplification (P = 0.0029), unfavorable histology (P = 0.0006), high Children's Oncology Group risk classification (P = 0.011), and disease relapse (P = 0.044). The kinase protein level did not correlate with age at diagnosis (P = 0.29).
Overexpression of Aurora A mRNA Is Significantly Associated with Decreased Progression-Free Survival in Patients with Neuroblastoma
For the tumors assessed in this study, 19 of 62 (30.65%) patients had progression of their disease and 9 of 62 (14.52%) patients died. Three-year progression-free survival rate ± SE was 72 ± 6.5% with a median follow-up period of 33 months (range, 0.7–122 months) in the survivors. Two Kaplan-Meier progression-free survival analysis curves with stratification by Aurora A mRNA expression are shown in Fig. 2. The cut point for Aurora A mRNA (3.25) was chosen because it separated the population into two distinct survival groups. In the entire population of patients, survival was significantly worse in those with Aurora A expression >3.25 (P < 0.0001). In the subgroup of patients with stage III and IV tumors, Aurora A expression continued to have a significant association with decreased progression-free survival (P < 0.021). In multivariable analysis, patients with high Aurora A expression (>3.25) had a 4.8 (95% confidence interval, 1.6–14.4) times higher risk for progression independent of the risk group (Supplementary Table S1).
Knocking Down the Aurora A Gene Causes a Proliferation Defect and Enhances Chemosensitivity in Human Neuroblastoma Cell Lines
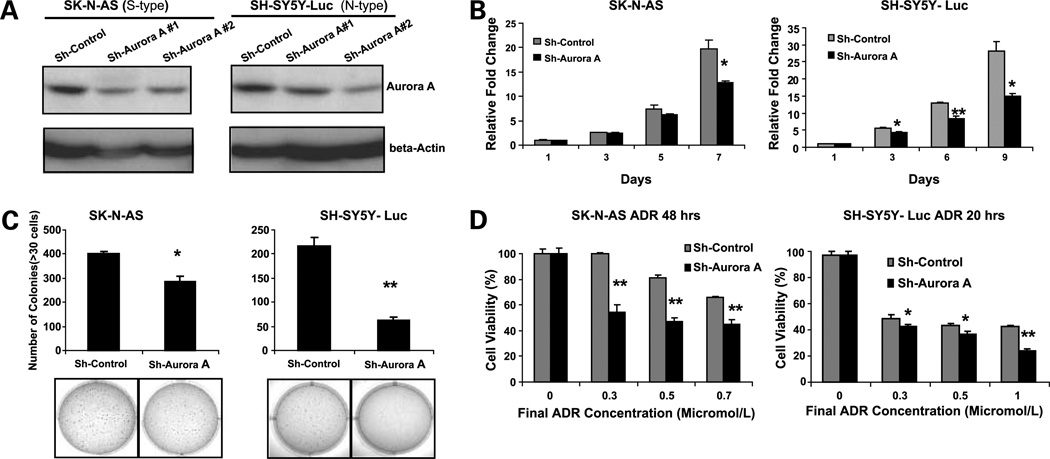
Given the observed correlation between Aurora A expression and clinical outcome in our cohort of neuroblastoma patients, we proposed that inhibition of this kinase might inhibit growth of neuroblastoma tumor cells. The siRNA method was employed to deplete the expression of Aurora A in cultured neuroblastoma cell lines, SH-SY5Y-Luc (N-type) and SK-N-AS (S-type). As shown in Fig. 3A, transduction with siRNA targeting Aurora A caused a 50% to 60% of decrease in Aurora A protein that was not observed with the siRNA scramble control. The effect of Aurora A siRNA on the in vitro proliferation and colony formation is shown in Fig. 3B and C, respectively. Compared with the scramble control transduced cells, the Aurora A siRNA group had much less growth and formed fewer colonies in soft agar. These results indicate the RNA interference–mediated specific knockdown of Aurora A induced significant inhibition of neuroblastoma cell growth in vitro. In addition, we noted an increase in the relative G2-M population in the Aurora A knockdown cells compared with the scramble controls in these two cell lines (Supplementary Fig. S1).
Figure 3.
Inhibition of Aurora A expression causes a proliferation defect and enhances chemosensitivity in both S-type and N-type human neuroblastoma cell lines. Aurora A expression in S-type SK-N-AS and N-type SH-SY5Y-Luc cells stably transduced with pSR-Sh-scramble control and pSR-Sh-Aurora A (#1 and #2 sequence). A, cells were analyzed by Western blot after 10 d of puromycin (2 µg/mL) selection. B, inhibition of Aurora A expression causes a proliferation defect. The SK-N-AS and SH-SY5Y-Luc Sh-Control and Sh-Aurora A #2 cell lines were plated in 96-well plates at 1 × 103 cells per well. The Cell Counting Kit-8 tetrazolium salt–based proliferation assay was used to quantify cellular proliferation relative to day 1 absorbance measured at 450 nm. These experiments were done in triplicate and reported as mean ± SD. C, SK-N-AS and SH-SY5Y-Luc Sh-Control and Sh-Aurora A #2 stably transduced cell lines were plated in 0.3% agarose/DMEM on top of a 0.5% agarose/DMEM layer. After 14 d of growth, colonies were stained with MTT (bottom) and colonies >30 cells were counted (top). A similar result was observed using Sh-Aurora A #1 cells (data not shown). D, inhibition of Aurora A expression increases chemosensitivity. SK-N-AS and SH-SY5Y-Luc Sh-Control and Sh-Aurora A #2 cell lines were plated in 96-well plates at 1 × 104 cells per well. After 24 h of growth, all of the cell lines were treated with the indicated micromolar concentration of doxorubicin for the indicated amount of time. Cell viability was determined with the Cell Counting Kit-8 cell viability assay relative to the 0 µmol/L group. All experiments were done in triplicate and statistical significance was determined by Student's t test where P < 0.05 was statistically significant. *, P < 0.05; **, P < 0.001 compared with the Sh-Control group.
Doxorubicin, also known as Adriamycin, is an antibiotic of the anthracycline group that has been used for the clinical treatment of neuroblastoma for >30 years. We examined the effect of Aurora A siRNA on growth of doxorubicin-treated neuroblastoma cells. As shown in Fig. 3D, there is a significant enhancement of the cytotoxic effect of doxorubicin in the Aurora A siRNA-transduced cells that is not observed in the scramble control transduced cells.
Aurora A Inhibitor MLN8054 Inhibits the Growth of Neuroblastoma Cell Lines
Aurora kinases have been proposed as a promising target for cancer therapy (24–26). MLN8054 is a novel small-molecule Aurora A inhibitor discovered and developed by scientists at Millennium Pharmaceuticals for the treatment of human cancers (27). The Aurora A inhibitor MLN8054 was tested in vitro against a panel of neuroblastoma cell lines as well as the normal and cancer-derived breast cell lines, MCF-10 A and MCF-7. MLN8054 exhibited dose-dependent cytotoxicity against in both S-type and N-type cell cultures (Fig. 4A) during 96 h of exposure. Based on the IC50, the compound had less activity against the normal breast cell line, MCF-10A, compared with the cancer cell lines (Table 2). Among the neuroblastoma cell lines tested, SH-SY5Y-Luc, JF, IMR-32, and LAN-1 (N-type) were generally more sensitive than the SK-N-AS, SH-EP, SMS-KCN, and NB19 (S-type) cell lines, although the difference in mean IC50 values between the two groups did not achieve statistical significance.
Figure 4.
A, growth-inhibitory effect of Aurora A inhibitor MLN8054 on cultured NB cell lines in vitro. N-type and S-type NB cells were plated in 96-well flat-bottomed plates at a concentration of 5 × 103 or 1 × 104 per well and NB cells were treated with increasing concentrations of MLN8054 for 96 h. Cytotoxic activity was determined by the Cell Counting Kit-8 assay. B, effect of the Aurora A inhibitor MLN8054 on apoptosis and cell cycle distribution in two representative NB cell lines, SK-N-AS and SH-SY5Y-Luc. Cells were preincubated in the absence or presence of different concentrations of MLN8054 for 48 h and stained for Annexin V and propidium iodide [PI; (i)], stained with propidium iodide and analyzed for relative DNA content (ii), and harvested and submitted to immunoblotting of full-length (116 kDa) and cleaved (89 kDa) PARP (iii).
Aurora A Inhibitor MLN8054 Triggers Apoptosis in NB Cell Lines
To determine the mechanism of action of the Aurora A inhibitor MLN8054 in neuroblastoma, Annexin V/propidium iodide staining, cell cycle analysis, and PARP cleavage immunoblotting were done after incubating two representative neuroblastoma cell lines SK-N-AS (S-type) and SH-SY5Y-Luc (N-type) for 48 h with MLN8054. As shown in Fig. 4B (i), apoptosis (Annexin V–positive population) was induced by MLN8054 in a dose-dependent fashion. Drug treatment resulted in a decreased population of cells in the G0-G1 phases and increased numbers of cells in the G2-M phases of the cell cycle in both SK-N-AS and SH-SY5Y-Luc cell lines [Fig. 4B (ii)]. PARP, a 116-kDa nuclear PARP, is involved in DNA repair predominantly in response to environmental stress (28). PARP is important for cells to maintain their viability: cleavage of PARP facilitates cellular disassembly and serves as a marker of cells undergoing apoptosis (29). As shown in Fig. 4B (iii), the control cells treated with DMSO alone did not manifest appreciable cleavage of the 116 kDa PARP, whereas the cells treated with MLN8054 had significant cleavage of PARP after 48 h incubation. Between the neuroblastoma cell lines tested, SH-SY5Y-Luc is more sensitive than SK-N-AS to MLN8054, resulting in a higher Annexin V–positive cell population and a high percentage of PARP cleavage at lower concentrations of MLN8054. These results suggest that the Aurora A inhibitor MLN8054 inhibits the proliferation of neuroblastoma cells by an apoptotic pathway and is associated with mitotic delay.
Discussion
In the current study, we describe the first systematic survey of Aurora A expression in human neuroblastoma. We have shown that Aurora A, a centrosome-associated oncogene, is overexpressed in human neuroblastoma primary tumor tissue and cell lines at both mRNA and protein levels. Interestingly, overexpression of Aurora A is associated with clinically aggressive neuroblastoma and correlates with tumor stage, relapse risk, Children's Oncology Group risk group, and histology. In multivariate analysis, patients with high Aurora A expression had a significantly increased risk of disease progression that was independent of the Children's Oncology Group risk group. Also, we found that small interfering RNA (siRNA)–mediated knockdown of the endogenous Aurora A gene causes a proliferation defect and enhances chemosensitivity in human neuroblastoma cell lines. In support with these results, we report significant in vitro antitumor activity of the Aurora A inhibitor, MLN8054, against neuroblastoma cell lines.
Although MYCN amplification is an important marker to predict poor patient outcome in neuroblastoma, this genetic aberration only occurs in ~20% of primary neuroblastomas (30). Approximately 65% of patients with metastatic disease have tumors that lack amplification of the MYCN proto-oncogene. Thus, identification of other oncogenes involved in neuroblastoma progression is needed. Our results provide strong evidence that overexpression of Aurora A could be critical in determining the malignant behavior of neuroblastoma tumor cells. Furthermore, Cox proportional hazard analysis shows that Aurora A is an independent marker to predict poor patient outcome in neuroblastoma. A recent report has identified Aurora A as a protein that is required for growth of MYCN-amplified neuroblastoma cells (31). We found that high Aurora A mRNA and protein expression levels correlate with MYCN amplification in neuroblastoma tumor tissue samples.
Aurora kinases represent an important therapeutic target for small-molecule inhibitors (5–11). MLN8054 (Millennium Pharmaceuticals) was the first selective small-molecule inhibitor of Aurora A to enter clinical trials in adult patients with advanced cancers (32). Because pediatric cancers are extremely rare, it is difficulty for pharmaceutical corporations to commit the necessary resources to development of drugs specific for these malignancies. Therefore, we chose to investigate the anti-neuroblastoma activity of MLN8054 because it has been vetted in appropriate preclinical trials with common adult malignancies.
MLN8054 induces apoptosis in human colon cancer cell lines and inhibits tumor growth in xenograft models (19). Prior work has shown that Aurora A inhibition kills tumor cells through the development of deleterious aneuploidy (33). We observe that MLN8054 also inhibits the growth of neuroblastoma tumor cell lines by an apoptotic mechanism. In our studies, MLN 8054 increases the percentage of cells in the G2 and M phases of the cell cycle, consistent with induction of mitotic delay. We found no direct correlation between the Aurora A expression level and the cytotoxic effect of MLN8054 compound on NB cell lines (Fig. 1A; Table 2).
N-type and S-type cells are two distinct cell types in neuroblastoma tumors and tumor cell cultures. Compared with S-type cells, N-type cells are more tumorigenic in immunodeficient mice and express higher levels of the N-myc and the antiapoptotic protein Bcl-2 (34). The mechanism of doxorubicin-induced death has been reported to be different in these two cell types. Cell death is caspase independent in N-type cells, whereas in S-type cells it does depend on caspase-8 but does not involve death receptors (35). We found high Aurora A expression in both N-type and S-type neuroblastoma cells. In our limited sample, we have observed a somewhat increased sensitivity to MLN8054 in N-type versus S-type cells, although this difference did not achieve statistical significance. This differential effect does not correlate with Aurora A mRNA or protein levels or MYCN amplification status. It is interesting to note that p53 status appears to be a determinant of sensitivity to Aurora A inhibition: the two cell lines with null or mutant p53 status, LAN-1 and SK-N-AS, each had the highest MLN8054 IC50 value among the N-type and S-type groups, respectively (Fig. 1A; Table 2). This observation is consistent with a mechanism in which Aurora A and p53 have opposite effects on apoptotic signaling pathways in these tumor cells. In addition, we noted that diminution of Aurora A activity, by either siRNA knockdown or MLN8054 inhibition, results in an increase in sensitivity to doxorubicin, suggesting a potential synergy between Aurora A inhibitors and standard chemotherapy agents.
In summary, our studies show that Aurora A may play an important role in tumorigenesis and progression of neuroblastoma. In addition, our work strongly suggests that MLN8054 may be an effective therapeutic agent for treatment of neuroblastoma. Further in vivo studies will provide a better assessment of the potential therapeutic utility of Aurora kinase inhibitors in this pediatric tumor.
Supplementary Material
Acknowledgments
We thank Millennium Pharmaceuticals for supplying the Aurora A inhibitor MLN8054 and Dr. Subrata Sen (M. D. Anderson Cancer Center) for providing anti-Aurora A antibody, Dr. Akira Horii (Tohoku University School of Medicine) for providing the pSR-sh AURKA plasmid, and Dr. Susan Blaney (Baylor College of Medicine) and Dr. Jeffrey A. Ecsedy (Millennium Pharmaceuticals) for their critical review of this manuscript.
Grant support: Children's Oncology Group Translational Research Award (J.G. Nuchtern) and National Cancer Institute grant 1R21CA106513-01A2 and Hope Street Kids Foundation (J. Yang).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Note: Supplementary material for this article is available at Molecular Cancer Therapeutics Online (http://mct.aacrjournals.org/).
References
- 1.Castleberry RP. Neuroblastoma. Eur J Cancer. 1997;33:1430–1437. doi: 10.1016/s0959-8049(97)00308-0. [DOI] [PubMed] [Google Scholar]
- 2.Look AT, Hayes FA, Shuster JJ, et al. Clinical relevance of tumor cell ploidy and N-myc gene amplification in childhood neuroblastoma: a Pediatric Oncology Group study. J Clin Oncol. 1991;9:581–591. doi: 10.1200/JCO.1991.9.4.581. [DOI] [PubMed] [Google Scholar]
- 3.Kaneko Y, Knudson AG. Mechanism and relevance of ploidy in neuroblastoma. Genes Chromosomes Cancer. 2000;29:89–95. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1021>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 4.Brodeur GM, Seeger RC, Schwab M, et al. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984;224:1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- 5.Zhou H, Kuang J, Zhong L, et al. Tumour amplified kinase AURORA A/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet. 1998;20:189–193. doi: 10.1038/2496. [DOI] [PubMed] [Google Scholar]
- 6.Poyce ME, Xia W, Sahin AA, et al. AURORA A/Aurora-A expression in primary breast tumors is correlated with nuclear grade but not with prognosis. Cancer. 2004;100:12–19. doi: 10.1002/cncr.11879. [DOI] [PubMed] [Google Scholar]
- 7.Zhao JM, Li FC, Xu XY, Fu BY. Deletion of p15 and pl6 genes and overexpression of AURORA A gene in primary hepatocellular carcinoma. Zhonghua Gan Zang Bing Za Zhi. 2005;13:202–204. [in Chinese]. [PubMed] [Google Scholar]
- 8.Jeng YM, Peng SY, Lin CY, Hsu HC. Overexpression and amplification of Aurora-A in hepatocellular carcinoma. Clin Cancer Res. 2004;10:2065–2071. doi: 10.1158/1078-0432.ccr-1057-03. [DOI] [PubMed] [Google Scholar]
- 9.Li YH, Li FC, Wang X, et al. Study on AURORA A gene abnormality and centrosomal amplification in laryngeal carcinoma. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2004;21:240–244. [in Chinese]. [PubMed] [Google Scholar]
- 10.Li FC, Li YH, Zhao X, et al. Deletion of p15 and p16 genes and overexpression of AURORA A gene in human laryngeal squamous cell carcinoma. Zhonghua Yi Xue Za Zhi. 2003;83:316–319. [in Chinese]. [PubMed] [Google Scholar]
- 11.Yang SB, Zhou XB, Zhu HX, et al. Amplification and overexpression of Aurora-A in esophageal squamous cell carcinoma. Oncol Rep. 2007;17:1083–1088. [PubMed] [Google Scholar]
- 12.Hu W, Kavanagh JJ, Deaver M, et al. Frequent overexpression of AU-RORA A/Aurora-A/BTAK and chromosomal instability in tumorigenic cell cultures derived from human ovarian cancer. Oncol Res. 2005;15:49–57. doi: 10.3727/096504005775082101. [DOI] [PubMed] [Google Scholar]
- 13.Hata T, Furukawa T, Sunamura M, et al. RNA interference targeting aurora kinase a suppresses tumor growth and enhances the taxane chemosensitivity in human pancreatic cancer cells. Cancer Res. 2005;65:2899–2905. doi: 10.1158/0008-5472.CAN-04-3981. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka E, Hashimoto Y, Ito T, et al. The suppression of aurora-A/STK15/BTAK expression enhances chemosensitivity to docetaxel in human esophageal squamous cell carcinoma. Clin Cancer Res. 2007;13:1331–1340. doi: 10.1158/1078-0432.CCR-06-1192. [DOI] [PubMed] [Google Scholar]
- 15.Tong T, Zhong Y, Kong J, et al. Overexpression of Aurora-A contributes to malignant development of human esophageal squamous cell carcinoma. Clin Cancer Res. 2004;10:7304–7310. doi: 10.1158/1078-0432.CCR-04-0806. [DOI] [PubMed] [Google Scholar]
- 16.Du J, Hannon GJ. Suppression of p160ROCK bypasses cell cycle arrest after Aurora-A/AURORA A depletion. Proc Natl Acad Sci U S A. 2004;101:8975–8980. doi: 10.1073/pnas.0308484101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dosch J, Repp R, Rascher W, Christiansen H. Diagnostic and scientific applications of TaqMan real-time PCR in neuroblastomas. Expert Rev Mol Diagn. 2001;1:233–238. doi: 10.1586/14737159.1.2.233. [DOI] [PubMed] [Google Scholar]
- 18.Kapasi AA, Singhal PC. Aging splenocyte and thymocyte apoptosis is associated with enhanced expression of p53, bax, caspase-3. Mol Cell Biol Res Commun. 1999;1:78–81. doi: 10.1006/mcbr.1999.0106. [DOI] [PubMed] [Google Scholar]
- 19.Yu Y, Ge NL, Xie M, et al. Phosphorylation of Thr-178 and Thr-184 in the TAK1 T-loop is required for interleukin (IL)-1-mediated optimal NFκB and AP-1 activation as well as IL-6 gene expression. J Biol Chem. 2008;36:22497–22505. doi: 10.1074/jbc.M802825200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nunez R. DNA measurement and cell cycle analysis by flow cytometry. Curr Issues Mol Biol. 2001;3:67–70. [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 22.Shimada H, Ambros IM, Dehner LP, et al. The International Neuroblastoma Pathology Classification (the Shimada system) Cancer. 1999;86:364–372. [PubMed] [Google Scholar]
- 23.Lonergan GJ, Schwab CM, Suarez ES, Carlson CL. Neuroblastoma, ganglioneuroblastoma, and ganglioneuroma: radiologic-pathologic correlation. Radiographics. 2002;22:911–934. doi: 10.1148/radiographics.22.4.g02jl15911. [DOI] [PubMed] [Google Scholar]
- 24.Bolanos-Garcia VM. Aurora kinases. Int J Biochem Cell Biol. 2005;37:1572–1577. doi: 10.1016/j.biocel.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 25.Doggrell SA. Dawn of Aurora kinase inhibitors as anticancer drugs. Expert Opin Investig Drugs. 2004;13:1199–1201. doi: 10.1517/13543784.13.9.1199. [DOI] [PubMed] [Google Scholar]
- 26.Harrington EA, Bebbington D, Moore J, et al. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat Med. 2004;10:262–267. doi: 10.1038/nm1003. [DOI] [PubMed] [Google Scholar]
- 27.Manfredi MG, Ecsedy JA, Meetze KA, et al. Antitumor activity of MLN8054, an orally active small-molecule inhibitor of Aurora A kinase. Proc Natl Acad Sci U S A. 2007;104:4106–4111. doi: 10.1073/pnas.0608798104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satoh MS, Lindahl T. Role of poly (ADP-ribose) formation in DNA repair. Nature. 1992;356:356–358. doi: 10.1038/356356a0. [DOI] [PubMed] [Google Scholar]
- 29.Oliver FJ, de la Rubia G, Rolli V, Ruia-Ruiz MC, de Murcia G, Murcia JM. Importance of poly (ADP-ribose) polymerase and its cleavage in apoptosis. Lesson from an uncleavable mutant. J Biol Chem. 1998;273:33533–33539. doi: 10.1074/jbc.273.50.33533. [DOI] [PubMed] [Google Scholar]
- 30.Seeger RC, Brodeur GM, Sather H, et al. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985;313:1111–1116. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- 31.Otto T, Horn S, Brockmann M, et al. Stabilization of N-Myc is a critical function of Aurora A in human neuroblastoma. Cancer Cell. 2009;15:67–78. doi: 10.1016/j.ccr.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 32.LeRoy PJ, Hunter JJ, Hoar KM, et al. Localization of human TACC3 to mitotic spindles is mediated by phosphorylation on Ser558 by Aurora A: a novel pharmacodynamic method for measuring Aurora A activity. Cancer Res. 2007;67:5362–5370. doi: 10.1158/0008-5472.CAN-07-0122. [DOI] [PubMed] [Google Scholar]
- 33.Hoar K, Chakravarty A, Rabino C, et al. MLN8054, a small-molecule inhibitor of Aurora A, causes spindle pole and chromosome congression defects leading to aneuploidy. Mol Cell Biol. 2007;27:4513–4525. doi: 10.1128/MCB.02364-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piacentini M, Piredda L, Starace DT, et al. Differential growth of N- and S-type human neuroblastoma cells xenografted into scid mice correlation with apoptosis. J Pathol. 1996;180:415. doi: 10.1002/(SICI)1096-9896(199612)180:4<415::AID-PATH684>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 35.Hopkins-Donaldson S, Yan P, Bourloud KB, et al. Doxorubicin-induced death in neuroblastoma does not involve death receptors in S-type cells and is caspase-independent in N-type cells. Oncogene. 2002;21:6132–6137. doi: 10.1038/sj.onc.1205879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.