Abstract
OBJECTIVE
To determine whether the impact of aging on cardiovascular disease (CVD) risk in the general population (as estimated by the Framingham risk score [FRS]) differs in patients with rheumatoid arthritis (RA).
METHODS
A population-based inception cohort of Olmsted County, Minnesota residents aged ≥30 years who fulfilled 1987 ACR criteria for RA in 1988–2008 was assembled and followed until death, migration, or 7-1-2012. Data on CVD events were collected by medical record review. The 10-year FRS for CVD was calculated. Cox models adjusted for FRS were used to examine the influence of age on CVD risk.
RESULTS
The study included 563 patients with RA without prior CVD (mean age: 55 years, 72% women; 69% seropositive [i.e., rheumatoid factor and/or anti-citrullinated protein antibody positive]). During a mean follow-up of 8.2 years, 98 patients developed CVD (74 seropositive and 24 seronegative), but FRS predicted only 59.7 events (35.4 seropositive and 24.3 seronegative). The gap between observed and predicted CVD risk increased exponentially across age, and the age effect on CVD risk in seropositive RA was nearly double its effect in the general population with additional log(age) coefficients of 2.91 for women (p=0.002) and 2.06 for men (p=0.027).
CONCLUSION
Age exerts an exponentially increasing effect on CVD risk in seropositive RA, but no increased effect among seronegative patients. The causes of accelerated aging in patients with seropositive RA deserve further investigation.
Keywords: accelerated aging, rheumatoid arthritis, cardiovascular disease
Introduction
People with rheumatoid arthritis (RA), especially seropositive RA, suffer an excess burden of cardiovascular disease (CVD) (1). We have previously demonstrated that the Framingham risk score for CVD does not accurately predict the overall risk of CVD observed in patients with RA (1). Although inflammation and immune dysregulation are strongly implicated, the precise mechanisms underlying this increased risk of CVD remain elusive. Accelerated aging, due to senescence of multiple systems, represents an attractive biological model that may, in part, explain the excess in CVD and mortality observed in RA (2, 3). Closer examination of the differences in observed and predicted CVD risk according to age may provide additional mechanistic insights. The purpose of this study was to determine whether the impact of aging on CVD risk in the general population (as estimated by the Framingham risk score) differs in patients with RA.
Methods
A population-based inception cohort of Olmsted County, Minnesota residents aged ≥18 years who fulfilled 1987 American College of Rheumatology (ACR) criteria for RA between January 1, 1988 and December 31, 2008 was previously identified and assembled using the resources of the Rochester Epidemiology Project, a population-based medical records linkage system that allows ready access to the complete (in-patient and out-patient) medical records from all community medical providers (4–6). In addition, residents with prevalent RA on January 1, 1995 were identified and assembled using the same procedures. For this analysis, only patients aged ≥30 years were included because CVD events in persons age <30 years are rare (e.g., Framingham risk assessment begins with age 30 years). For each patient, the earliest date of fulfillment of >4 ACR criteria for RA was considered the RA incidence date. For the prevalence patients, physician diagnosis was accepted where the ACR criteria at RA diagnosis were not available (<10% of patients). Data for this analysis was obtained for a random sample of patients in the prevalence cohort. The institutional review boards of the Mayo Clinic and the Olmsted Medical Center approved this study.
Patients in both cohorts were followed through medical record review until death, migration, or July 1, 2012. Medical records were reviewed to ascertain the presence of CVD risk factors (age, systolic and diastolic blood pressure, lipids, smoking status, use of antihypertensive medications, body mass index and diabetes mellitus) at RA incidence and to ascertain the development of CVD (myocardial infarction, CVD death, angina, heart failure, stroke and intermittent claudication) during follow-up.
Diabetes mellitus was defined as at least 2 measurements of fasting plasma glucose ≥126 mg/dl or a 2-hour plasma glucose ≥ 200 mg/dl, physician diagnosis or documented use of insulin and/or oral hypoglycemic agents. For blood pressure, lipids and body mass index, the recorded value closest to RA incidence (or prevalence) date within ± 1 year was used to calculate the risk of CVD. Myocardial infarction was defined according to standardized criteria (7). CVD death was defined using the underlying cause of death obtained from death certificates (International Classification of Diseases version 9, codes 350.0 – 459.9 and version 10, codes I00 – I99). Angina was ascertained by physician diagnosis. Stroke and intermittent claudication were defined using physician diagnosis verified by imaging, cerebrospinal fluid analysis, or autopsy (8). Heart failure was defined using the Framingham criteria (9).
All rheumatoid factor (RF), anti-citrullinated protein antibody (ACPA), erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) values performed during usual clinical care were collected. Positivity was defined using the relevant cutoffs used for clinical decision making at the time each test was performed. Patients with a positive test for RF or ACPA will be referred to as seropositive, and those who were never positive for either test will be referred to as seronegative.
Statistical Methods
Descriptive statistics (means, proportions, etc.) were used to summarize the characteristics of patients with RA. Chi-square and rank sum tests were used to examine differences in CV risk factors between patients with seropositive and seronegative RA. Patients with CVD outcomes prior to RA incidence were excluded from the analysis.
The 10-year general Framingham risk score (FRS) for CVD and the office-based 10-year FRS, which does not include laboratory values, were calculated according to published algorithms (10). For patients without available lipid measures, the office-based FRS was used.
Poisson methods were used to compare the observed and predicted number of events. For this analysis, the FRS probabilities of CVD events were converted to numbers of predicted CVD events, which were adjusted proportionately for the actual length of follow-up for each patient if it differed from 10 years (with a maximum follow-up of 12 years per patient to parallel the Framingham study). Proportional adjustment of the expected risk among patients with less than 10 years of follow-up assumes that the 10 years risk is equally accrued throughout the follow-up period (e.g., 1/10 of the 10 year risk occurs during each of the 10 years of follow-up). Sensitivity analyses were performed to examine whether assumptions of greater risk in the beginning or the end of the 10 year period would change the results.
Cox models for each sex were used to examine the influence of age on CVD risk in patients with RA after adjusting for the FRS alone and with additional adjustment for the other traditional CVD risk factors. For plotting, estimated 10 year CVD rates were directly adjusted for the distribution of CVD risk factors observed in the RA cohort. The adjusted CVD rates were plotted against age for women and men. Similarly, the FRS estimates and projections for various multipliers and age effects were directly adjusted for the distribution of CVD risk factors observed in the RA cohort.
Results
The incidence cohort included 563 patients of at least 30 years of age without prior CVD at the time of RA incidence (mean age: 55 years, 72% women; 69% seropositive). Characteristics and cardiovascular risk factors for seropositive and seronegative patients are shown in Table 1. Patients with seropositive RA were younger, were more likely to have abnormal ESR at RA incidence, and were somewhat more likely to be current smokers than patients with seronegative RA.
Table 1.
Characteristics of 563 patients with incident rheumatoid arthritis in 1980–2008
| Characteristic | Seronegative (N=172) | Seropositive (N=391) | p value |
|---|---|---|---|
| Age, years | 56.8 (±14.3) | 54.0 (±13.4) | 0.030 |
| Female sex | 124 (72%) | 279 (71%) | 0.86 |
| Abnormal erythrocyte sedimentation rate | 37 (22%) | 131 (34%) | 0.004 |
| Total Cholesterol, mg/dL | 197.9 (±36.3) | 194.0 (±35.3) | 0.18 |
| N | 248 | 102 | |
| Low Density Lipoprotein, mg/dL | 114.7 (±32.4) | 114.9 (±31.2) | 0.90 |
| N | 145 | 320 | |
| High Density Lipoprotein, mg/dL | 56.6 (±18.0) | 53.4 (±15.8) | 0.10 |
| N | 230 | 94 | |
| Triglycerides, mg/dL | 125.8 (±65.2) | 129.2 (±74.0) | 0.94 |
| N | 154 | 338 | |
| Systolic blood pressure, mmHg | 133.2 (±18.6) | 130.1 (±18.2) | 0.08 |
| Diastolic blood pressure, mmHg | 76.3 (±9.9) | 77.1 (±10.8) | 0.54 |
| Anti-hypertensive use | 51 (30%) | 88 (22%) | 0.07 |
| Current smoker | 23 (13%) | 78 (20%) | 0.06 |
| Diabetes mellitus | 21 (12%) | 29 (7%) | 0.07 |
| Body Mass Index, kg/m2 | 28.4 (5.8) | 28.5 (6.5) | 0.96 |
| Framingham risk score, % | 14.1 (±14.6) | 11.4 (±11.1) | 0.07 |
Values in the table are mean (±SD) for continuous characteristics and N (%) for discrete characteristics
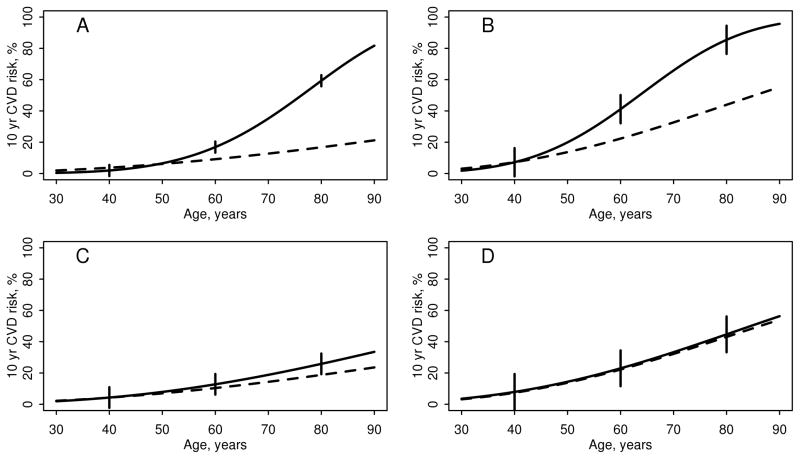
The patients were followed for a mean of 8.2 years (maximum: 12 years), during which 98 developed CVD (74 seropositive and 24 seronegative). The FRS predicted only 59.7 events in these patients (35.4 seropositive and 24.3 seronegative). Figure 1 shows the 10 year risk of CVD for each sex according to age, among seropositive and seronegative patients with RA along with the FRS-predicted 10 year risk of CVD. The observed and predicted risk of CVD agreed closely for all ages among patients with seronegative RA, but the observed risk of CVD in patients with seropositive RA was much greater than the predicted risk of CVD from the FRS at older ages.
Figure 1.
The influence of age on the observed and predicted 10 year risk of cardiovascular disease in patients with rheumatoid arthritis by seropositivity and sex (panel A: seropositive women; panel B: seropositive men; panel C: seronegative women; panel D: seronegative men). The solid line is the observed risk, the dashed line is predicted risk from the Framingham risk score. The error bars are 95% confidence intervals for the observed risk. Among patients with seropositive RA, additional log(age) coefficients of 2.91 for women (p=0.002) and 2.06 for men (p=0.027) were found. Among patients with seronegative RA, the additional log(age) coefficients of 1.34 for women (p=0.30) and 0.30 for men (p=0.85) were not statistically significant.
Among patients with seropositive RA, the gap between the observed and predicted risk of CVD widened with increasing age, which demonstrated an accelerated aging effect. Cox modeling also revealed a significantly greater effect of age on the risk of developing CVD after adjusting for the FRS. In the FRS, the coefficients for log(age) were 2.33 for women and 3.06 for men. Among patients with seropositive RA, additional age coefficients (or multipliers) of 2.91 for women (p=0.002) and 2.06 for men (p=0.027) were found. Hence, the effect of age on CVD risk in the seropositive women was more than double its effect in the general population, and for seropositive men, it was nearly double. Among patients with seronegative RA, the additional log(age) coefficients of 1.34 for women (p=0.30) and 0.30 for men (p=0.85) were not statistically significant.
The impact of this additional aging effect was negligible for patients younger than 50 years, when CVD risk in the general population is generally low. However, the impact was observed to increase exponentially as CVD risk rose with age. For example, patients over 65 years with seropositive RA had similar CVD risk as persons in the general population older than 90 years. Because the FRS was originally built using only patients between the ages of 30 and 74 years, we conducted subgroup analyses of the 360 seropositive patients who were 30–74 years old. The findings of accelerated aging on risk of CVD were essentially unchanged in this subgroup. Models adjusted for the other traditional CVD risk factors also did not explain this age effect.
To determine whether the accelerated aging effect is simply an influence of age at onset of RA on CVD risk or if it can be extrapolated to prevalent RA, the analysis was repeated using a prevalence cohort. The prevalence cohort included 238 patients of ≥30 years of age without prior CVD at the prevalence date (mean age: 58 years; 73% female; 66% seropositive). During a mean follow-up of 9.0 years, 70 patients developed CVD (42 seropositive and 28 seronegative). Among the seropositive patients, additional age coefficients of 2.32 for women (p=0.11) and 4.63 for men (p=0.002) were found. The coefficient for women was similar to our findings in the incidence cohort, and the coefficient for men with prevalent RA was larger than in the incidence cohort. In addition, there was no association between CVD risk and disease duration (linear p=0.49; non-linear p=0.90). These findings support an accelerated aging effect that is not limited to age at onset of RA.
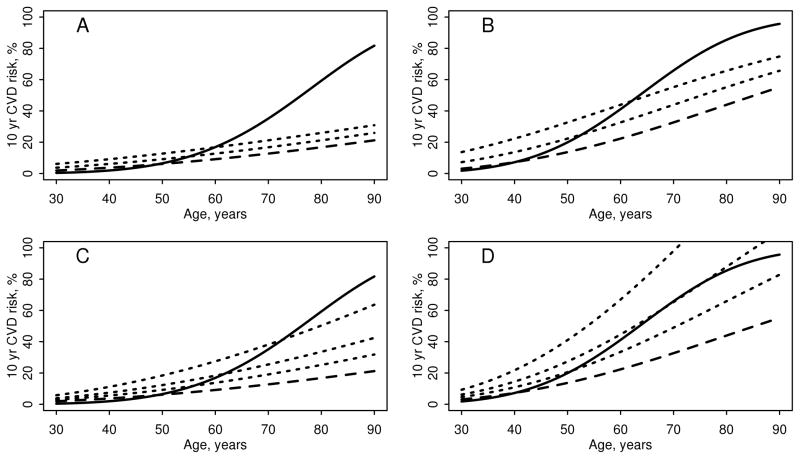
To demonstrate the accelerated influence of age on risk of CVD, the effect of adding a constant value to age was also examined. Panels A and B in Figure 2 demonstrated that adding an additional 10 or 20 years to the age of each patient with RA when computing the FRS did not accurately predict the observed risk of CVD among patients with seropositive RA. Clearly, the observed CVD risk outpaced this adjustment at older ages. Panels C and D in Figure 2 demonstrated that applying a multiplier of 1.5, 2 or 3 to the FRS increased the FRS more steeply across the age range than the constant age increases in Panels A and B of Figure 2. However, the observed age effect on CVD risk could not be accurately predicted using any of these multipliers.
Figure 2.
The influence of age on the observed and predicted 10 year risk of cardiovascular disease in patients with seropositive rheumatoid arthritis by sex. The solid line is the observed risk, the dashed line is predicted risk from the Framingham risk score. Dotted lines in panels A and B add 10 and 20 years to age when computing the Framingham risk score (Panel A: women; Panel B: men). Dotted lines in panels C and D depict the Framingham risk score with multipliers of 1.5, 2 and 3 (Panel C: women; Panel D: men).
Sensitivity analyses examining the impact of assuming equal risk across the 10 year follow-up when defining predicted CVD risk for patients with <10 years of follow-up revealed no significant changes in these results (data not shown).
Discussion
Patients with seropositive RA experienced an exponentially increasing effect of age on CVD risk. In contrast, the impact of age on CVD risk among seronegative patients was similar to the general population. In addition, the impact of age on CVD risk cannot be easily captured by adding a constant to patient’s age when computing the FRS or by applying a multiplier to the FRS.
These findings support prior reports of accelerated aging in patients with RA. We have previously demonstrated that accelerated aging may play a role in the increased mortality of patients with seropositive RA (3). At a cellular level, the hypothesis of accelerated aging is supported by a considerable body of literature on accelerated immune system aging, or immunosenescence, in RA (2). While this phenomenon was formerly thought to result from chronic inflammatory activity, recent findings revealed that telomerase deficiency and overall DNA instability occur in patients with RA and lead to apoptosis of T cells, which imposes proliferative pressure resulting in premature aging of the immune system (11). In addition, excessive telomere shortening was found in patients with HLA-DRB1*04 alleles, which are strongly associated with seropositivity and with disease severity in RA (12). Furthermore, telomere shortening has been shown to precede the development of CVD (13). Thus, immunosenescence may be a common antecedent to both RA and CVD.
The mechanism underlying the observed accelerated aging effect and the potential impact of other time-related factors, such as disease duration or cumulative disease burden are unknown. However, we found no association between disease duration and risk of CVD in our prevalence cohort.. It is also unlikely that disease burden could completely explain our findings, because patients across the spectrum of age could have high disease burden. Further research is needed to better elucidate these complex interactions.
Strengths of our study include its population-based cohort of patients who meet objective criteria for RA, and the comprehensive review of all inpatient and outpatient medical records allowing complete ascertainment of clinically identified CVD events. Potential study limitations include its modest sample size, particularly in men, which may influence the ability to accurately assess the accelerated aging effect. Also the population of Olmsted County, Minnesota is predominately white, which may limit the generalizability of these results to other populations. In addition, lipid measures were not available for some patients, so the office-based FRS was used. This may slightly diminish the differences between observed and predicted risks, since the office-based FRS was slightly higher than the general FRS in patients for whom both could be assessed (1).
In conclusion, age exerts an exponentially increasing effect on CVD risk in patients with seropositive RA, supporting prior reports of accelerated aging in this disease. In contrast, the impact of age on CVD risk among seronegative patients was similar to the general population. The causes of accelerated aging in patients with seropositive RA deserve further investigation.
Acknowledgments
Funding: This work was supported by a grant from Pfizer, Inc., by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01AR46849, and by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
References
- 1.Crowson CS, Matteson EL, Roger VL, Therneau TM, Gabriel SE. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol. 2012;110(3):420–4. doi: 10.1016/j.amjcard.2012.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hohensinner PJ, Goronzy JJ, Weyand CM. Telomere dysfunction, autoimmunity and aging. Aging Dis. 2011;2(6):524–37. [PMC free article] [PubMed] [Google Scholar]
- 3.Crowson CS, Liang KP, Therneau TM, Kremers HM, Gabriel SE. Could accelerated aging explain the excess mortality in patients with seropositive rheumatoid arthritis? Arthritis Rheum. 2010;62(2):378–82. doi: 10.1002/art.27194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maradit Kremers H, Crowson CS, Gabriel SE. Rochester Epidemiology Project: a unique resource for research in the rheumatic diseases. Rheum Dis Clin North Am. 2004;30(4):819–34. doi: 10.1016/j.rdc.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 6.Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the Incidence of Rheumatoid Arthritis Rising? Results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 2010;62(6):1576–82. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mascioli SR, Jacobs DR, Jr, Kottke TE. Diagnostic criteria for hospitalized acute myocardial infarction: the Minnesota experience. International Journal of Epidemiology. 1989;18(1):76–83. doi: 10.1093/ije/18.1.76. [DOI] [PubMed] [Google Scholar]
- 8.Bacani AK, Gabriel SE, Crowson CS, Heit JA, Matteson EL. Noncardiac vascular disease in rheumatoid arthritis: Increase in venous thromboembolic events? Arthritis Rheum. 2012;64(1):53–61. doi: 10.1002/art.33322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22(4 Suppl A):6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 10.D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 11.Weyand CM, Fujii H, Shao L, Goronzy JJ. Rejuvenating the immune system in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5(10):583–8. doi: 10.1038/nrrheum.2009.180. [DOI] [PubMed] [Google Scholar]
- 12.Schonland SO, Lopez C, Widmann T, Zimmer J, Bryl E, Goronzy JJ, et al. Premature telomeric loss in rheumatoid arthritis is genetically determined and involves both myeloid and lymphoid cell lineages. Proc Natl Acad Sci USA. 2003;100(23):13471–6. doi: 10.1073/pnas.2233561100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samani NJ, van der Harst P. Biological ageing and cardiovascular disease. Heart. 2008;94(5):537–9. doi: 10.1136/hrt.2007.136010. [DOI] [PubMed] [Google Scholar]