Abstract
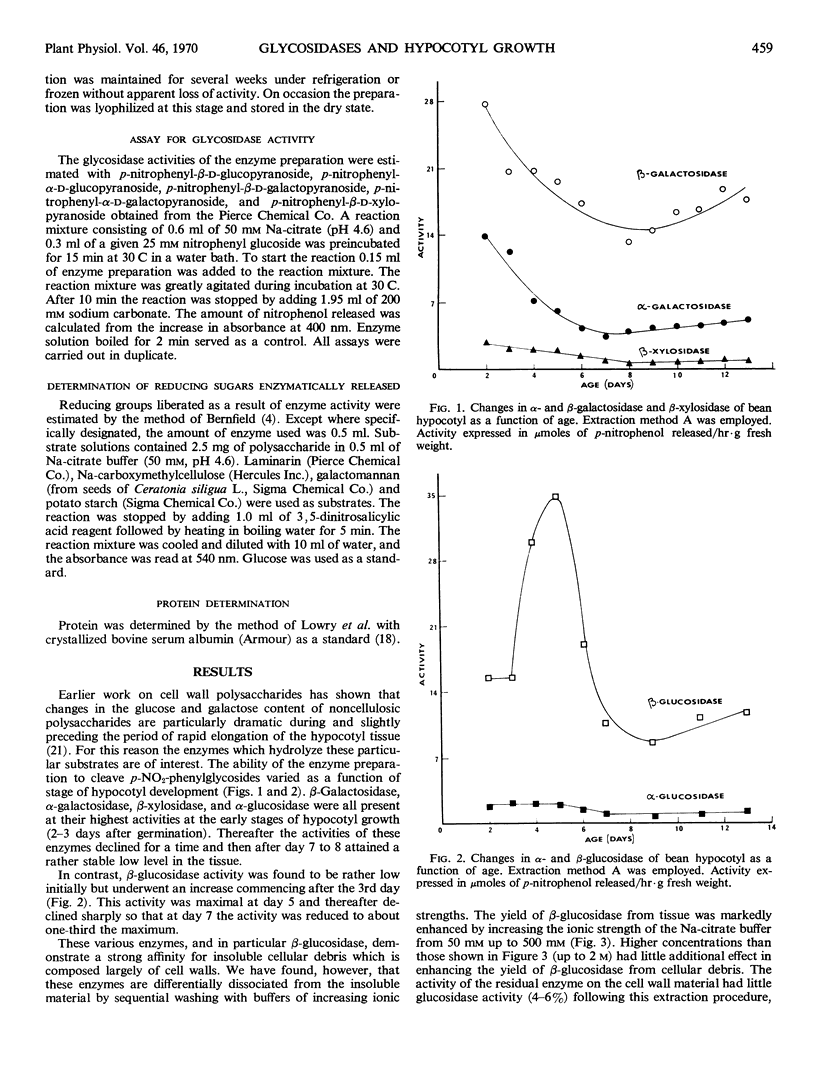
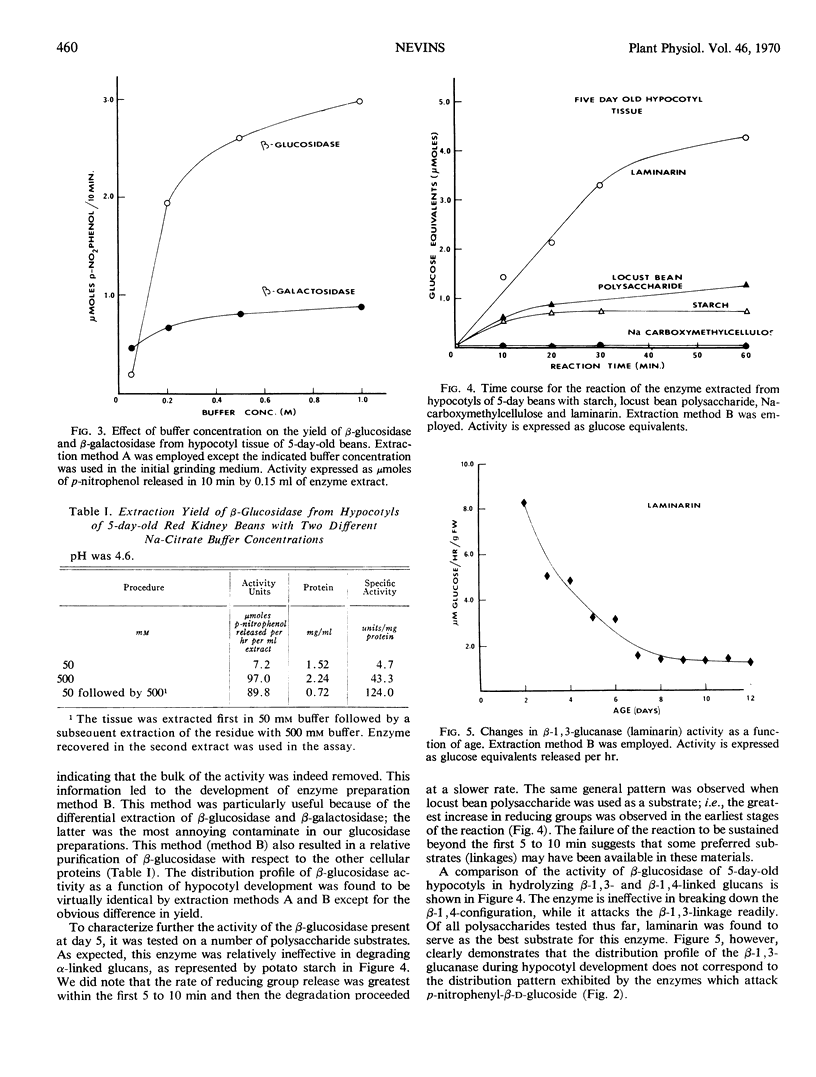
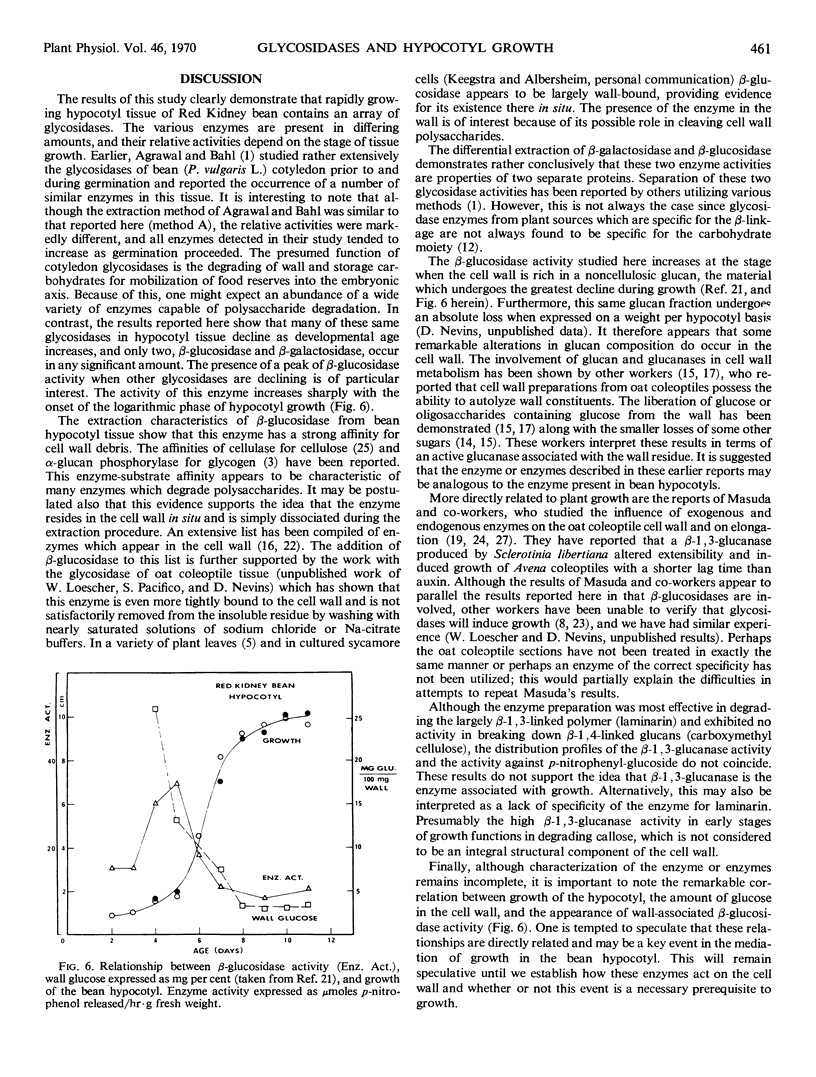
The enzymes β-glucosidase, α-glucosidase, β-galactosidase, α-galactosidase, and β-xylosidase were detected in Phaseolus vulgaris L. var. Red Kidney bean hypocotyl tissue throughout the first 13 days of development with p-nitrophenyl glycosides as substrates. Activities of all enzymes except β-glucosidase declined as a function of increasing tissue age. In contrast, β-glucosidase activity increased rapidly 3 days after imbibition to a maximal activity at 5 days and then subsided to one-third the maximum by day 7. This activity peak immediately preceded the logarithmic phase of hypocotyl growth. This enzyme is strongly associated with cell walls during extraction, suggesting that it is wall-bound in situ. Various polysaccharide substrates were used to evaluate the specificity of this enzyme.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal K. M., Bahl O. P. Glycosidases of Phaseolus vulgaris. II. Isolation and general properties. J Biol Chem. 1968 Jan 10;243(1):103–111. [PubMed] [Google Scholar]
- Barber A. A., Orrell S. A., Jr, Bueding E. Association of enzymes with rat liver glycogen isolated by rate-zonal centrifugation. J Biol Chem. 1967 Sep 25;242(18):4040–4044. [PubMed] [Google Scholar]
- Cleland R. Auxin and wall extensibility: reversibility of auxin-induced wall-loosening process. Science. 1968 Apr 12;160(3824):192–194. doi: 10.1126/science.160.3824.192. [DOI] [PubMed] [Google Scholar]
- HEYWORTH R., WALKER P. G. Almond-emulsin beta-D-glucosidase and beta-D-galactosidase. Biochem J. 1962 May;83:331–335. doi: 10.1042/bj0830331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyn A. N. Dextranase activity in coleoptiles of Avena. Science. 1970 Feb 6;167(3919):874–875. doi: 10.1126/science.167.3919.874. [DOI] [PubMed] [Google Scholar]
- Heyn A. N. Glucanase activity in coleoptiles of Avena. Arch Biochem Biophys. 1969 Jul;132(2):442–449. doi: 10.1016/0003-9861(69)90387-7. [DOI] [PubMed] [Google Scholar]
- Katz M., Ordin L. A cell wall polysaccharide-hydrolyzing enzyme system in Avena sativa L. coleoptiles. Biochim Biophys Acta. 1967 Jun 13;141(1):126–134. doi: 10.1016/0304-4165(67)90251-6. [DOI] [PubMed] [Google Scholar]
- Katz M., Ordin L. Metabolic turnover in cell wall constituents of Avena sativa L. coleoptile sections. Biochim Biophys Acta. 1967 Jun 13;141(1):118–125. doi: 10.1016/0304-4165(67)90250-4. [DOI] [PubMed] [Google Scholar]
- Kivilaan A., Beaman T. C., Bandurski R. S. Enzymatic activities associated with cell wall preparations from corn coleoptiles. Plant Physiol. 1961 Sep;36(5):605–610. doi: 10.1104/pp.36.5.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee S. H., Kivilaan A., Bandurski R. S. In vitro autolysis of plant cell walls. Plant Physiol. 1967 Jul;42(7):968–972. doi: 10.1104/pp.42.7.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins D. J., English P. D., Albersheim P. Changes in cell wall polysaccharides associated with growth. Plant Physiol. 1968 Jun;43(6):914–922. doi: 10.1104/pp.43.6.914. [DOI] [PMC free article] [PubMed] [Google Scholar]


