Abstract
Background
Cardiac myxoma is a benign neoplasm that represents the most prevalent primary tumor of the heart. If not treated with the right surgical technique recurrence occurs. Aim of our study is to present our surgical approach and the histology of the tumors resected.
Methods
All patients, except for one, underwent extracorporeal circulation and mild hypothermia, right atrial or both atrial incision and excision of the fossa ovalis, followed by prosthetic patch suturing. All specimens were submitted for microscopic evaluation (haematoxylin-eosin). We contacted personally each patient and asked them to complete a standardized questionnaire, concerning their peri-operative characteristics.
Results
Six cases were “active” myxomas, 3 were “mildly active” and 3 were “inactive”. “Normal differentiation” was seen in 6, “medium” in 1 and “poor” in 5 cases. In our series there were no recurrences recorded during the follow-up period.
Conclusions
The ideal approach, according to our experience is right atrial or both atrial incision as described by Shumacker and King, with excision of the fossa ovalis and the surrounding tissues and closure with a pericardial patch. Such a technique provides an excellent long-term survival in these patients.
KEYWORDS : Surgery of myxomas, atrial myxoma, cardiac surgery
Introduction
Cardiac myxoma represents the most common type of primary cardiac tumor. Typically it is regarded as a benign neoplasm (1) and although it can occur in almost any age, usually patient ages range from 30-60 years, with a female predilection. Clinically myxomas may present with a variety of symptoms such as obstructive cardiac, embolic and constitutional.
Obstructive cardiac signs include dyspnea, thoracic pain, cough, dizziness and heart failure due to tumor prolapse into the mitral orifice (2) mimicking heart failure symptoms (3). Embolic manifestations can occur in any organ (4) and usually present with peripheral or pulmonary emboli or stroke. Constitutional symptoms include arthralgia, myalgia, fever, rash, weight loss, cachexia, fatigue, Reynaud’s phenomenon and they are believed to be related with IL-6 production by tumor cells (5).
Cardiac myxoma most commonly occurs in the left atrium, originating from the interatrial septum near or on the fossa ovalis. However, it can arise in any of the heart chambers. Most myxomas are single and sporadic although familiar cases are also well known. (Carney syndrome represents the majority of familiar cases).
The diagnosis is usually based on transthoracic and transesophageal echocardiography (better approach to the tumor’s location and characteristics). Surgical resection under cardiopulmonary bypass and cardiac arrest is the commonest treatment, regardless of tumor size. There have been many reports of postoperative local recurrence and of distant metastases with aneurysmal changes of vessel walls and independent growth (6-10).
The aim of our study is to present our surgical technique that is characterized by total removal of heart myxoma without heart block and no recurrences during a follow-up period of 13 years. Moreover we attempted to associate the histopathologic findings and the clinical presentation of myxomas.
Patients and methods
In the Cardiothoracic Surgery Department of our University Hospital we treated 13 cardiac myxomas (Table 1). The patients’ age was between 20-76 years. More particularly the study group included 9 women (69.23% of the whole population, range, 20-76 years) and 4 men (30.76% of the whole population, range, 43-71 years).
Table 1. Patients’ characteristics diagnosed with heart myxoma during the 13 years period.
| No | Age | Sex | Clinical presentation | Postoperative complications | Follow-up (months) | Follow-up findings |
|---|---|---|---|---|---|---|
| 1 | 76 | F | Stroke | nil | 154 | Neurologic residue |
| 2 | 63 | F | Dyspnea | nil | 135 | nil |
| 3 | 48 | M | Peripheral embolism | nil | 114 | nil |
| 4 | 71 | M | Random finding | AF | 113 | nil |
| 5 | 72 | F | Syncope | AF | 65 | nil |
| 6 | 70 | M | Stroke | AF | 14 | Death from accident |
| 7 | 57 | F | Random finding | nil | 43 | nil |
| 8 | 71 | F | Random finding | nil | 41 | nil |
| 9 | 73 | F | Dyspnea | nil | 38 | nil |
| 10 | 43 | M | Stroke | nil | 23 | nil |
| 11 | 28 | F | Fever, rash, fatigue | nil | 19 | nil |
| 12 | 72 | F | Stroke | Inoperable | 62 | nil |
| 13 | 20 | F | Stroke | nil | 3 | nil |
F, female; M, male; AF, atrial fibrillation.
We reviewed these 13 cases and collected data concerning: (I) the clinical manifestations of cardiac myxoma; (II) the serologic alterations; (III) the patients’ medical history; (IV) their treatment; (V) post-operative course; (VI) the detailed histopathologic features of each case and (VII) the follow-up findings. Follow-up was achieved by contacting each patient personally and completing a standardized questionnaire.
In particular, they were asked if they had a regular follow-up and what the findings were, if they had any disturbances in their everyday life or any changes towards a healthier lifestyle and if they had been hospitalized again after their heart surgery.
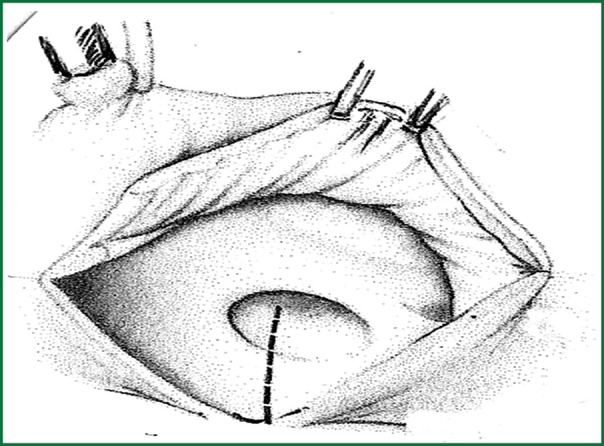
Surgical resection with bicaval extracorporeal circulation and mild hypothermia was performed in all cases, except for one patient to whom surgery was refused due to high risk of cerebral bleeding from systemic heparin administration during the extracorporeal circulation (11). The approach was achieved with right atrial incision and excision of the fossa ovalis, followed by prosthetic patch suturing. In addition to this in cases where the tumor was occupying the majority of the left atrium another approach was selected as described by Shumacker and King (12). The incision starts from the right pulmonary vein continues vertically to the interatrial septum and free wall of the right atrium (Figures 1,2,3). With this method the left atrium opens wide (Figure 4) and it is impossible to escape a remnant of the tumor.
Figure 1.
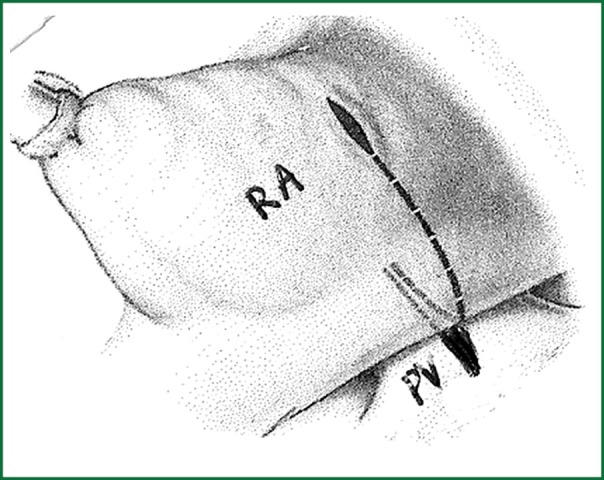
Incision line from pulmonary vein to the free wall of atrium.
Figure 2.
Incision line to the interatrial septum.
Figure 3.
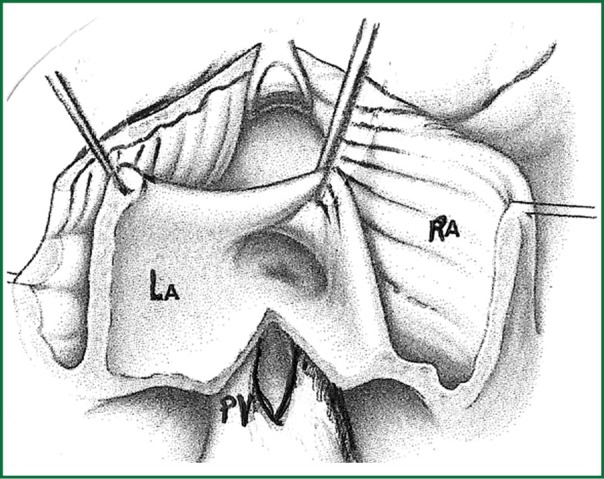
Final view of the approach to both atria.
Figure 4.
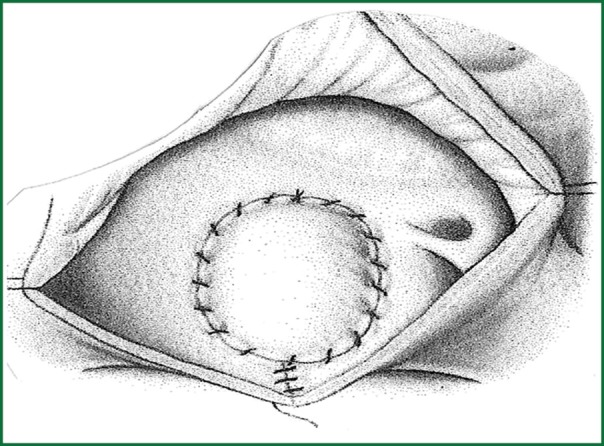
Final view of the repaired atria.
Histopathologic analysis (haematoxylin-eosin) was possible in all cases. For detailed microscopic analysis modification of a previously published sub typing was employed (5). Briefly, myxomas were differentiated as “active”, with increased cellularity of myxoma cells, “mildly active”, with areas of variable cellularity and “inactive”, with low cellularity. Furthermore, based on the architecture of the lesions, “normal differentiation “was considered when rudimentary or well-developed vessels were surrounded by cells and then by matrix, “poor differentiation” when cells were singly dispersed, or in rows within matrix and “medium differentiation” for cases with intermediate features between the two.
Results
The predominant clinical manifestations (Table 2) were embolic (stroke and peripheral embolism) in 46.15% of the patients, followed by cardiovascular (dyspnea, cough, dizziness, chest pain) in 23.07% and constitutional (fever, rash and fatigue) in 7.69%. In three cases (23.07% of the patients) the myxoma was diagnosed accidentally, during regular echocardiographic control. Only four patients presented laboratory abnormalities. In all four of them elevated E.S.R >20 mm/hr was found and anemia was observed in two of them.
Table 2. Patients’ histology divided to 2 groups of clinical presentation.
| No | Clinical presentation | Laboratory tests (+) | Tumor surface | Histologic classification | Architecture of lesions |
|---|---|---|---|---|---|
| 1 | Stroke | E.S.R | Smooth | Active | Poor differentiation |
| 3 | Peripheral embolism | nil | Villous | Mildly active | Medium differentiation |
| 5 | Syncope | nil | Smooth | Mildly active | Poor differentiation |
| 6 | Stroke | nil | Villous | Active | Normal differentiation |
| 10 | Stroke | nil | Smooth | Active | Poor differentiation |
| 12 | Stroke | nil | Inoperable | Inoperable | Inoperable |
| 13 | Stroke | E.S.R, anemia | Smooth | Active | Poor differentiation |
| 2 | Dyspnea | E.S.R | Smooth | Mildly active | Normal differentiation |
| 4 | Random finding | nil | Smooth,ossification | Inactive | Poor differentiation |
| 7 | Random finding | nil | Smooth | Active | Normal differentiation |
| 8 | Random finding | nil | Smooth | Inactive | Normal differentiation |
| 9 | Dyspnea | nil | Smooth | Inactive | Normal differentiation |
| 11 | Fever, rash, fatigue | E.S.R, anemia | Smooth | Active | Normal differentiation |
E.S.R, erythrocyte sedimentation rate.
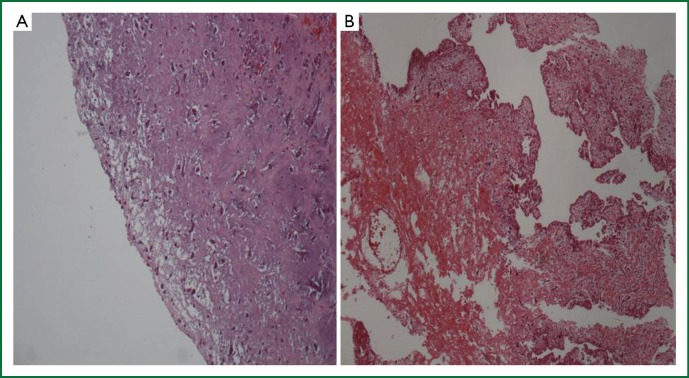
All tumors were located in the left atrium, attached to the atrial septum (fossa ovalis). No tumors were located in the right atrium or the ventricles. The size of the tumors ranged between 2 cm × 2 cm and 4.2 cm × 5.9 cm. Two of them were attached to the interatrial septum and were petiolate. All specimens were submitted in total for microscopic evaluation. The surface of the tumor was evaluated in all tumors (Table 2). It was covered by a single layer of cuboital, endothelial-like cells and was smooth in 10 cases (#1, #2, #4, #5, #7, #8, #9, #10, #11, #13) and villous in 2 cases (#3, #6) (Figure 5). Ossification was observed in one case (#4) and calcification in none.
Figure 5.
Surface of the excised myxomas, covered by a single layer of flattened endothelial-like cells. (A) Smooth surface, (haematoxylin-eosin ×100); (B) Villous surface with multiple, friable villous extensions, (haematoxylin-eosin ×100).
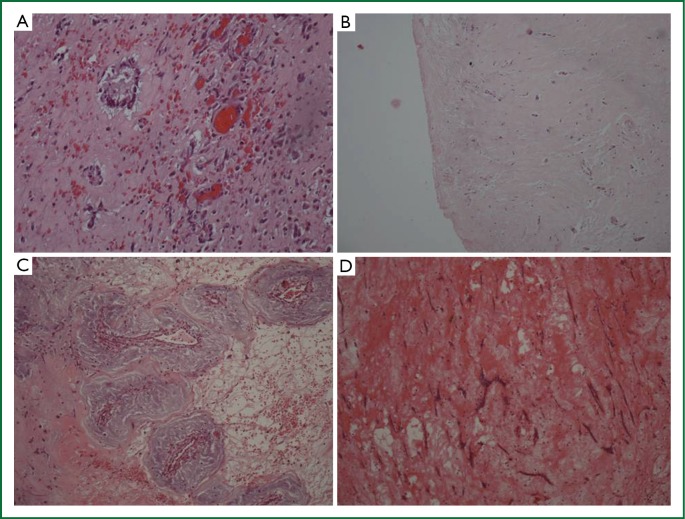
Six cases (46.15%) were “active” myxomas, 3 were “mildly active” (23.07%) and 3 were “inactive” (23.07%). “Normal differentiation” was seen in 6 cases (46.15%), “medium” in 1 (7.69%) and “poor differentiation” in 5 (38.46%) (Figure 6). The myxoma case with “medium” differentiation was also “mildly” active. Three out of 6 “active” myxomas (50%) showed “normal differentiation”, while three were “poorly differentiated”.
Figure 6.
Histologic characteristics of myxomas. (A) Active myxoma with normal differentiation. Increased cellularity of myxoma cells, which form rudimentary vascular structures, (Haematoxylin-eosin ×200); (B) Mildly active myxoma with medium differentiation—Variable cellularity of myxoma cells that form vascular structures, (Haematoxylin-eosin ×200); (C) Inactive myxoma with normal differentiation—Low cellularity of myxoma cells, which form rudimentary vascular structures, (Haematoxylin-eosin ×200); (D) Inactive myxoma with poor differentiation—Low cellularity of myxoma cells, dispersed or lepodic, which form short chains, (Haematoxylin-eosin ×200).
One third of the mildly active myxomas were normal differentiated, one third medium differentiated and one third poorly differentiated. Two thirds of the “inactive” myxomas were “normally differentiated”, and one “poorly”.
All patients, apart from one, underwent surgery. The only postoperative complication was atrial fibrillation in 3 patients (20%), which was treated with Amiodarone.
Follow-up was between 3-154 months. In our series there was only one death during the follow-up period, which was not related with the patient’s medical history of heart myxoma. All cases recovered fully except for one patient who had suffered preoperatively a stroke (as the initial symptom of heart myxoma) and has a neurological residue after the excision of the heart tumor (case #1 has difficulties in the movement of the right arm and in speech). No recurrences were recorded.
Discussion
Myxomas represent the most common primary cardiac tumors. They can appear in almost all age groups but most commonly between the third and the sixth decades of life with a female predilection. In our series the patients’ mean age was 58.76 years and 69.23% of the whole group was women. Myxomas can originate from any of the heart chambers, most commonly from the left atrium. Right atrial myxomas (13) and heart valve myxomas (14) are rare but still there have been reported such cases.
It appears that the clinical features of these tumors are not pathognomonic for the diagnosis of myxoma. Actually in a few cases there were no symptoms at all and the tumors were discovered incidentally during regular echocardiographic examination. The most common symptoms though are obstructive, embolic and constitutional. This classic triad of symptoms depends on the tumor’s location, size, mobility and its surface. Embolism (mainly affects the central nervous system) was the most frequent clinical manifestation in our series, occurring in 46.15% of the patients in our study. Cerebral emboli may lead to numerous aneurysms, as in one patient (11) in our study, to whom surgery was refused, but his long-term outcome was finally good without having any disturbances or ever being hospitalized again (case #12). Obstructive symptoms (cardiovascular) occurred in 23.07% of patients in our series with manifestations such as dyspnea, cough, chest pain and dizziness and they are related with mechanical interference with the mitral valve. Finally, constitutional signs were observed only in one patient in our series who presented fever and rash. There are cases though in literature where cardiac myxoma was presented only with dermatologic lesions (purpuric macules on palms and soles) (15).
Diagnosis was initially achieved by TTE, which was then complemented by TOE. Both diagnostic methods and especially TOE are excellent for characterizing the tumor’s appearance, location and dimensions. Most myxomas measure a few centimeters, however there have been reported cases of huge atrial myxomas (16,17).
According to histopathologic features the tumor’s surface is either villous and friable or smooth. It appears that the presence of a villous surface is associated with cerebral and peripheral embolism (cases #3 and #6) whereas smooth surfaced tumors can appear either as a random finding (cases #4, #7, #8) or with cardiovascular manifestations (cases #2, #5, #9) or with embolic manifestations (cases #1, #10, #13). No immediate correlation was detected, between the histologic differentiation-architecture of lesions and the clinical manifestations. There could be though some type of correlation with the recurrence rate, but sine no recurrences were recorded in our study, no such relation could be estimated.
Urgent surgical resection with median sternotomy and the patient placed on bicaval cardiopulmonary bypass under mild hypothermia and cardioplegic cardiac arrest is the treatment of choice. Such an operation is safe and curative with a low peri-operative risk. Resection of the tumor and the area of endocardium that is attached plus a margin of safety (18) should be complete and it is necessary to prevent recurrence, since incomplete resection (19), along with peri-operative manipulations causing detachment of tumor pieces, are definitely risk factors for post-operative recurrence.
According to literature recurrence of sporadic myxoma develops in about 3% of tumors (20,21). It may occur several months after the first operation or even years later. Ricardo Oliveira et al. (21) presented a study where among the 19 patients, to whom follow-up was achieved, in a mean follow-up period of 5.2±3.7 years, there were 2 recurrences (10.5% recurrence rate).
Sheng Wen-Bo et al. (19) had 5 recurrences (4.67% recurrence rate) in their series, G. Samanidis et al. (18) presented a study where during a mean follow-up period of 3.1±3.2 years there were two recurrences. Finally in Laurent Pinede et al. series (5), in a median follow-up period of 3 years there were 6 recurrences recorded (5% recurrence rate). Malignant heart tumors are sarcomas and they relapse but occur preferentially in the right side of the heart. An exception to this rule is leiomyosarcoma (22), a rare form of primary cardiac sarcoma that occurs predominantly in the left atrium, as does cardiac myxoma.
In our series follow-up was up to 13 years. All patients who underwent surgical excision of heart myxoma were reached personally and follow-up was achieved in all of them. No recurrences were recorded during the follow-up period (0% recurrence rate). We consider that the total excision of the interatrial septum is strongly related with no recurrences. Recurrence involves two main risks: the risk of a re-operation which is more difficult and causes more suffering to the patient and off course the risk of the clinical manifestations of the tumor itself. The surgeon should therefore not hesitate to remove totally the tumor.
According to our experience the ideal approach is right atriotomy with excision of the fossa ovalis and the surrounding tissues and closure with a pericardial patch. In addition to this in cases where the tumor is occupying the majority of the left atrium we suggest another approach as described for congenital surgery by Shumacker and King (12). The incision starts from the right pulmonary vein continues vertically to the interatrial septum and the free wall of the right atrium. With this method the left atrium opens wide and it is impossible to escape a remnant of the tumor. The worst approach is through the left atrium, because this technique does not allow us to see properly the base and the petiole of the myxoma (there could be remaining tissues). Surgical excision of the tumor provides an excellent long-term survival in most patients, compared to other primary cardiac tumors (20).
Conclusions
Heart myxoma is the most common primary cardiac tumor which is usually located in the left atrium. Operative treatment is urgently required. The best approach is through the right atrium with excision of the fossa ovalis plus a margin of safety, followed by sewing a prosthetic patch. In addition to in cases where the tumor is occupying the majority of the left atrium in order to avoid the possibility of recurrence we suggest another approach as described by Shumacker and King. Regarding histopathologic features the tumor’s surface is either villous and friable or smooth. It appears that the presence of a villous surface is associated with cerebral and peripheral embolism whereas smooth surfaced tumors can appear either as a random finding or with cardiovascular manifestations.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Guiraudon C. Cardiac tumors. In: Crawford M, Di Marco J, Paulus W. Cardiology. 2nd edition. Elsevier (publisher) Ltd, 2004:1508. [Google Scholar]
- 2.Yuan SM. Mitral valve myxoma: clinical features, current diagnostic approaches, and surgical management. Cardiol J 2012;19:105-9 [DOI] [PubMed] [Google Scholar]
- 3.Ojji DB, Mamven MH, Omonua O, et al. Left atrial myxoma mimicking mitral stenosis. Clin Med Insights Case Rep 2012;5:111-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogel B, Thomas D, Mereles D, et al. Systemic Embolization and Myocardial Infarction due to Clinically Unrecognized Left Atrial Myxoma. Case Rep Med 2011;2011:159024. [DOI] [PMC free article] [PubMed]
- 5.Pinede L, Duhaut P, Loire R.Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine (Baltimore) 2001;80:159-72 [DOI] [PubMed] [Google Scholar]
- 6.Dang CR, Hurley EJ. Contra lateral recurrent myxoma of the heart. Ann Thorac Surg 1976;21:59-62 [DOI] [PubMed] [Google Scholar]
- 7.Kimbrell OC, Jr, Kaasa LJ. Primary intraluminal aortic myxoma with involvement of several vertebrae. JAMA 1973;226:459-60 [PubMed] [Google Scholar]
- 8.Pastakia B.Malignant atrial myxoma presenting as intracranial mass. Chest 1979;75:531-2 [DOI] [PubMed] [Google Scholar]
- 9.Read RC, White HJ, Murphy ML, et al. The malignant potentiality of left atrial myxoma. J Thorac Cardiovasc Surg 1974;68:857-68 [PubMed] [Google Scholar]
- 10.Seo IS, Warner TF, Colyer RA, et al. Metastasizing atrial myxoma. Am J Surg Pathol 1980;4:391-9 [DOI] [PubMed] [Google Scholar]
- 11.Baikoussis NG, Siminelakis SN, Kotsanti A, et al. Multiple cerebral mycotic aneurysms due to left atrial myxoma: Are there any pitfalls for the cardiac surgeon? Hellenic J Cardiol 2011;52:466-8 [PubMed] [Google Scholar]
- 12.Shumacker HB, Jr, King H. A modified procedure for complete repair of total anomalous pulmonary venous drainage. Surg Gyn Obstet 1961;112:763-5 [Google Scholar]
- 13.Jung J, Hong YS, Lee CJ, et al. Successful surgical treatment of a right atrial myxoma complicated by pulmonary embolism. Korean J Thorac Cardiovasc Surg 2013;46:63-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernández AL, Vega M, El-Diasty MM, et al. Myxoma of the aortic valve. Interact CardioVasc Thorac Surg 2012;15:560-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee HJ, Park JY, Kim YS, et al. Cardiac myxoma diagnosed by signs of purpuric macules on both palms and soles. Ann Dermatol 2012;24:337-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strecker T, Agaimy A.Giant left atrial myxoma causing drop attacks by prolapsing into the mitral valve. Int J Clin Exp Pathol 2012;5:996-9 [PMC free article] [PubMed] [Google Scholar]
- 17.Avakian SD, Takada JY, Mansur Ade P. Giant obstructive left atrial myxoma resembling mitral valve stenosis. Clinics (Sao Paulo) 2012;67:853-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samanidis G, Perreas K, Kalogris P, et al. Surgical treatment of primary intracardiac myxoma: 19 years of experience. Interact Cardiovasc Thorac Surg 2011;13:597-600 [DOI] [PubMed] [Google Scholar]
- 19.Sheng WB, Luo BE, Liu Y, et al. Risk factors for postoperative recurrence of cardiac myxoma and the clinical managements: a report of 5 cases in one center and review of literature. Chin Med J (Engl) 2012;125:2914-8 [PubMed] [Google Scholar]
- 20.Elbardissi AW, Dearani JA, Daly RC, et al. Survival after resection of primary cardiac tumors: a 48-year experience. Circulation 2008;118:S7-15 [DOI] [PubMed] [Google Scholar]
- 21.Oliveira R, Branco L, Galrinho A, et al. Cardiac myxoma: a 13-year experience in echocardiographic diagnosis. Rev Port Cardiol 2010;29:1087-100 [PubMed] [Google Scholar]
- 22.Morin JE, Rahal DP, Hüttner I. Myxoid leiomyosarcoma of the left atrium: a rare malignancy of the heart and its comparison with atrial myxoma. Can J Cardiol 2001;17:331-6 [PubMed] [Google Scholar]