Abstract
Apnoeic oxygenation is an alternative technique of oxygenation which is recommended in the consecutive oxygen administration with varying flows (2-10 lt/min) through a catheter which is positioned over the keel of the trachea. Apnoeic oxygenation maintains for a significant period of time the oxygenation of blood in breathless conditions. This technique was first applied in 1947 by Draper, Whitehead, and Spencer and it was studied sporadically by other inventors too. However, the international literature shows few studies that have examined closely apnoeic oxygenation and its effects on Hemodynamic image and the respiratory system of the human body. Recently they have begun to arise some studies which deal with the application of this technique in several conditions such as difficult tracheal intubation, ventilation of guinea pigs in campaign conditions where the oxygen supply is limited and calculable, the application of this technique in combination with the use of extracorporeal removal of carbon dioxide (CO2). All the above indicate, the clinical use of this technique.
KEYWORDS : Tracheal apnoeic oxygenation, hemodynamic status, oxygenation indices, hypercapnic acidosis
Introduction
Apnoeic oxygenation is an alternative technique of oxygenation which is recommended in the consecutive oxygen administration with varying flows (2-10 lt/min) through a catheter which is positioned over the keel of the trachea. Apart from this version which consists intratracheal apnoeic oxygenation there is endobronchial apnoeic oxygenation which is done with a probe placed inside one of the two main loops or with two catheters which are positioned in each of the two main loops. Apnoeic oxygenation maintains for a significant period of time the oxygenation of blood in breathless conditions. This effect has its base on the mechanisms by which the gas exchange is done at the level of the alveolar-capillary membrane.
Apnoeic oxygenation was used in 1947 by Draper, Whitehead and Spencer, who studied the oxygenation by diffusion in experimental models with dogs, wanting to focus on changes in alveolar gas and venous pH (1). In their study they applied, consecutive pumping pure oxygen with flows 6-8 lt/min, within a chamber enclosing the head of laboratory animals and had low pressure valve, maintaining the upper airway open with use of artificial airway and causing apnoea with the administration of an overdose from the solution of pentothals 1%. All the laboratory animals survived after oxygenation spread over 45 minutes. The study which was deled with the variation of the veins pH and alveolar PCO2 showed the significant reduction of the first and the increase of the second during the oxygenation diffusion.
In 1959, Frumin, Epstein and Cohen in their study to humans, used the term of “oxygenation diffusion” (2). Their aim was to avoid confusion regarding the mechanism for maintaining oxygenation by this method. The mechanism of diffusion does not transfer oxygen from the outside environment to alveolar film by itself, since the distance from the mouth until the wells are prohibitively large to cover adequately only by the diffusion of oxygen molecules. In their study after denitrification of sick patients they implied apnoeic oxygenation with continued administration of pure oxygen with flows 6-8 lt/min from the respiratory circuit on the edge of the tracheal tube, resulting in more active commuting of oxygen to the alveolar level. They focused on the alterations of pH, SpO2 and PaCO2, adrenaline, plasma noradrenalin, by monitoring AP and ECG. The results of the measurements showed a progressively hypercapnia with reduction of arterial pH to the value 6.72 maintaining saturation of haemoglobin in levels 98-100% for up to and over 30 minutes. The levels of the stress hormones showed an incensement in all the phases of apnoeic oxygenation. However, in this study the conditions of apnoeic oxygenation had not been maintained since as a criterion for granting additional muscle relaxation was considered the beginning of spontaneous breathing by allowing the patient to breathe.
Two years later, 1n 1961, Millar and Morris (3) wanted to study the answer of sympathetic nervous system In experimental models in dogs in which it was applied apnoeic oxygenation for about 60 minutes and they had been undergone adrenalectomy. The researchers found that during apnoeic oxygenation, respiratory acidosis due to summation of CO2 in body causes increased secretion of noradrenalin from outside the adrenal. Apnoeic oxygenation had been done with the connection edge of endotracheal in T-piece with relief valves resulting in a greater sum of CO2 compare to endotracheal pumping at heart level.
In 1973, Fraioli, Scheffer and Steffenson (4) studied the effects of apnoeic oxygenation in two groups of patients. The first group would have been undergone micro laryngoscopy and apnoeic oxygenation would have been applied through nasopharynx airway (flow 6 lt/min). The second group would have been undergone in short-term intervention and apnoeic oxygenation would have been applied trough connection of tracheal tube in spirometer filled with oxygen. The researchers focused on the altercations of PaO2, PaCO2, PAN2, pH, FRC, oxygen uptake, blood pressure and electrocardiogram. They found that the patients who had ratio FRC/weight <50 mL/kg, they presented faster and more largely decrease of PaO2, proposing this value of ratio as criterion of patient selection for the application of apnoeic oxygenation. The faster deterioration of oxygenation was attributed to the fact that in these patients (heavier, similar height) the functional residual capacity (FRC) was smaller; the total amount of nitrogen in the body was larger despite the prior denitrification pnymonon and consequently the he return of nitrogen was greater thus displacing oxygen of the cells. Nevertheless, the patients had acceptable oxygenation for a 15-minute period with lower values of PaO2 at 100 mmHg in patients with the worst oxygenation.
In 1982, Pesenti and collaborators (5) used apnoeic oxygenation in combination with IVF carbon dioxide (CO2) removal in experimental model of preterm sheep with the aim to study the possibility of prevention of hyaline membrane disease. They found that in the group in which it had been fronted in combination with apnoeic oxygenation and IVF dioxide removal (ECCO2R) it had been given time to stabilize the mechanical properties of the respiratory system, with this result to accommodate the subsequent mechanical ventilation with fewer complications compared with the control group which received mechanical ventilation upfront. This has been demonstrated and from the better survival rates in group of apnoeic oxygenation and ECCO2R. Similar is a recent study [2008] by Nielsen and his collaborators (6), in which they used the combination of apnoeic oxygenation and IVF CO2 removal in experimental model of pigs, in which it had been caused acute lung injury by repeated washings of surfactant factor. Here again it had been taken place oxygenation and from the membrane of extracorporeal machine.
In the study of Cook et al. (7) in 1998, apnoeic oxygenation was applied in infants and children in order to research the time in which the method of oxygenation is safe. It had been found that oxygenation was satisfactory during (five minutes) the measurements. They also found in infants that restrictive factor was the progressively decrease of PaO2 despite the upcoming hypercapnia as it happens after application of apnoeic oxygenation in adults.
Moving gas under normal conditions and in conditions of apnoeic oxygenation
The movement of gas from one environment to another is caused by the pressure difference in the two spaces. The difference of pressure can be either hydrostatic pressure so the movement becomes more vivid or partial pressure (percentage of the gas mixture pressure environment due to the presence of this gas), so the movement of gas is macroscopically less pronounced (diffusion). The speed of gas movement depends on either the resistance of airways that the motion of the air mass will find in the case of macroscopic gears (removal) or the by the resistance of the membrane through the gas molecules pass in the case of microscopic motion (diffusion) of gas.
In case of diffusion the molecules of gas, move continuously towards two directions either side of the semi permeable membrane, with rates depending on the partial pressure of the gas in the space being vacated. The net amount of gas diffused is the difference of the number of gas molecules diffused in every direction and is therefore proportional to the difference in partial pressure of the gas on both sides of membrane.
Below it is analyzed the characteristics of the handling for each gas separately.
Oxygen
At the level of the alveolar membrane it takes place the movement of oxygen molecules from the cell to the pulmonary capillary and movement of CO2 from the pulmonary capillaries towards the cell. Both movements happen via the alveolar-capillary membrane.
Oxygen diffusion
The movement of oxygen is made via alveolar membrane from alveolar to pulmonary capillary (diffusion of oxygen from gas phase to liquid phase). This diffusion is preserved from the remained slope partial pressure in the pulmonary capillaries that is created and maintained by the continuous oxygen uptake by reduced haemoglobin (8).
The speed in which is performed this procession depends on the partial pressure difference on either side of membrane, the temperature, surface area spread, distance that oxygen molecule have to spend in order to reach haemoglobin, and lastly molecular weight of oxygen. It should not be forgotten that the path of diffusion of oxygen includes the delay which is due to the connection of oxygen with the molecule of reduced haemoglobin inside the erythrocyte. The bigger is the solubility of gas, there will be more available molecules for diffusion for any pressure difference. The bigger is the surface of diffusion, the bigger the total number of molecules to diffusion will be. On the other hand, the bigger the distance is (membrane thickness) the more time it will take any molecule to diffuse. In addition to that, the greater the speed of molecular motion of the molecule is, which is inversely proportional to the square root of the molecular weight, the greater the rate of diffusion is. Lastly, the diffusion will be quicker when the temperature is greater, because the kinetic energy of the molecules will be greater. Because of stability of temperature in human body, it is not taken into account. The above all can be summarized in below mathematic equation:
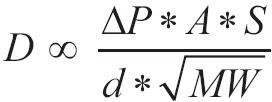 |
[1] |
Where D is the speed of diffusion, ΔΡ is the difference of pressure between the two edges of movement, A is the surface area spread, S is the solubility of gas (oxygen in this case) in water, d is the distance the molecules undergo (membrane thickness) and MW is the molecular weight of gas. In this mathematic formula there are two indicators which describe the characteristics of gas: solubility S and molecular weight MW. The rate 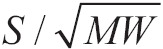 is s proportional to the diffusion coefficient of gas and consequently the speed of diffusion of several molecules for the given difference of pressure and through the same membrane is proportional with the diffusion factor of every gas. If we consider diffusion factor of oxygen to be equal to 1, then the relevant diffusion factors for the gas of aspiratory system are shown in Table 1.
is s proportional to the diffusion coefficient of gas and consequently the speed of diffusion of several molecules for the given difference of pressure and through the same membrane is proportional with the diffusion factor of every gas. If we consider diffusion factor of oxygen to be equal to 1, then the relevant diffusion factors for the gas of aspiratory system are shown in Table 1.
Table 1. Oxygenation equation.
| Factors | Values |
|---|---|
| Oxygen | 1.0 |
| Carbon dioxide | 20.3 |
| Carbon monoxide | 0.81 |
| Nitrogen | 0.53 |
| Helium | 0.95 |
Gas which are diffused in the aspiratory system, are diluted in lipids and through cell membranes. The greater restraint in their diffusion via tissue membranes is the spread of diffusion of water which is included in tissue membranes and in tissues and not the speed of diffusion through membrane of lipids which is very fast.
Pressure gradient responsible for initiating of diffusion
Pulmonary capillary is considered to have a partial part which receives mixed venous blood from pulmonary artery and a final part which includes oxygen fortified with blood, after the procession of diffusion of oxygen has either been completed or has been developed in a great extent. The partial pressure of oxygen in the initial portion of the pulmonary capillaries is that of mixed venous blood PVO2. It can be symbolized as PVCO2 since blood which is contained in pulmonary capillary has vein character.
The partial pressure of oxygen in the final section of pulmonary capillary, after the completion of oxygenation, is increased and approaches partial pressure of oxygen in cell. It can be symbolized as PCO2. The difference of partial pressure ΔP which is responsible for inauguration of diffusion of oxygen via alveolar-capillary membrane, is the difference between partial oxygen pressure in cell PAO2 and partial oxygen pressure in partial part of pulmonary capillary PVCO2. It is given by the following equation:
| [2] |
PAO2 is given by the following equation:
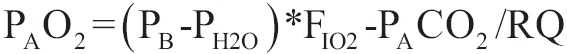 |
[3] |
Where PB is pressure of atmosphere, PH2O is the partial pressure of vapour of water, FIO2 is the partial pressure of CO2 in arterial blood and RQ is the respiratory quotient (rate of VCO2/VO2) which is considered to be equal to 1.
As the process of diffusion of oxygen is passed off and blood flows from initiate part to the final part of pulmonary capillary, the difference of partial pressure ΔP is progressively decreased and under normal circumstances (normal alveolar-capillary membrane thickness, cardiac output calm in normal residence time in the capillaries) is zero in the final part of capillary when blood is fully fortified with oxygen.
Ability of diffusion of oxygen. Integration by Bohr, ante grade completion
There is an indicator which counts the diffusion of oxygen and it is called ability to diffusion. It is given by the following equation:
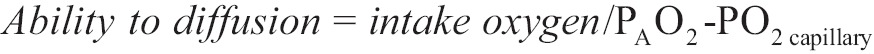 |
[4] |
Alveolar partial pressure of oxygen PAO2 can be calculated from equation [3], while there are severe problems in the estimation of medium capillary partial pressure of oxygen.
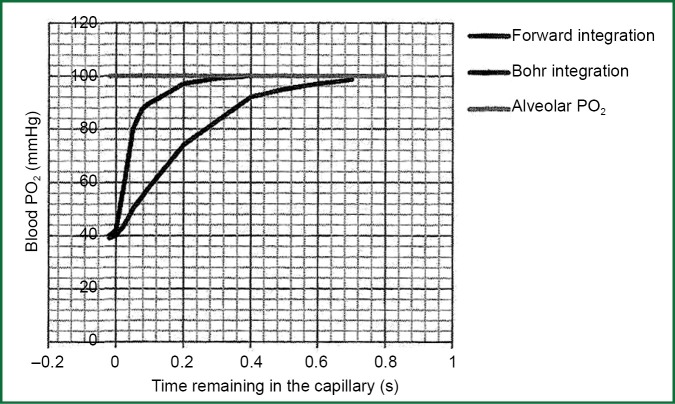
Bohr occupied with the problem of calculation of medium capillary partial pressure of oxygen PO2 capillary and he assumed that the speed of transportation of oxygen from cell air to haemoglobin of erythrocyte is analogue to alveolar-capillary pressure gradient of oxygen at any point along the pulmonary capillary. He also assumed a stabled value for alveolar-capillary difference of partial pressure of oxygen on the final part of pulmonary capillary. Having started from the terminus of the capillary, he tried to construct a chart of alveolar-capillary partial pressure of oxygen according to the residence time of blood in the capillary (Figure 1). This approach was called Bohr integration due to the initiate assumption of final part of capillary (Figure 1).
Figure 1.
Variation of PO2 in pulmonary capillary with two different approaches.
However, it was found that the speed of transportation of oxygen from alveolar air to haemoglobin of erythrocyte is not similar alveolar-capillary pressure gradient of oxygen at any point along the pulmonary capillary and consequently Bohr integration was proved wrong, because the initiate assumption was wrong. The reason is that the speed of transportation of oxygen is slowed because of the chemical connection of oxygen with haemoglobin, a process which is sufficiently slow, so it consists a crucial part of total resistance to oxygen transport. The association rate of oxygen to haemoglobin is not proportional to the partial pressure deviation ΔP for two reasons:
The connection of the fourth oxygen molecules to hemoglobin
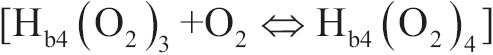 , has greater speed compare to connection of previous three oxygen molecules;
, has greater speed compare to connection of previous three oxygen molecules;As haemoglobin saturation is increased, the number of the reduced haemoglobin molecules is decreased and the speed of reaction attachment is decreased according to the action of the masses.
When the above two factors are combined with, the resistance in diffusion due to chemical connection of oxygen in erythrocyte, is stable until the saturation of haemoglobin up to 80% (PO2 =45 mmHg) and consequently the speed of connection is stable (directly proportional). Thereafter, the speed of connection is rapidly decreased and it is zero when the saturation of haemoglobin is 100%. These new facts of kinetic in connection of oxygen with haemoglobin, consisted the base for Forward integration for the determination of median capillary partial pressure of oxygen. Starting from the arterial edge of pulmonary capillary, partial pressure of oxygen is progressively calculated along the capillary until it is obtained an estimate for the remaining alveolar-capillary oxygen partial pressure deviation in the final part of pulmonary capillary. The ante grade completion proposes much smaller slopes in the final end of the capillary compared to those that were previously thought.
Residence time of blood in the capillaries
The major factor that establishes partial pressure of oxygen in the final part of pulmonary capillary and its capacity of diffusion is the residence time of blood in pulmonary capillaries. As it is shown in Figure 1, if residence time is smaller than 0.25 second, then there will be a significant deviation of oxygen partial pressure between the cell, and the final part of pulmonary capillary. There is no adequate time to become equations of partial pressure of oxygen in the alveolar and capillary. As the deviation of alveolar-capillary oxygen partial pressure is increased, basis of the Eq. [4], the capacity of diffusion of oxygen will be decreased.
Residence time of blood in pulmonary capillaries is equal to the rate of pulmonary blood volume to blood flow via pulmonary circulation (is approximately equal to the cardiac output). In no stress conditions, this time is 0.8 second. However due to these difficult in the calculation of blood in pulmonary circulation, in literature a price range for the residence time in pulmonary capillaries, is displayed and they have been suggested times from 0.1 (9) to 3 seconds (10). Many factors can affect the residence time in capillaries, such as body posture, lung volume, cardiac output, and consequently the normal residence time in capillaries displays a range of normal value rather than one value. Capillaries with brief residence of time, give blood not fully saturated. This fact does not set against during mixing from fully saturated blood derived from capillaries with long residence time.
Path of diffusion
Once the oxygen molecules pass the alveolar-capillary membrane, must be diffused through thin layers of plasma and finally through the cell membranes of erythrocytes to enter the red blood cell. Pulmonary capillaries have diameter of 7 μm, which is similar to size of red globules. Only just a small part of membrane of red blood cell is consider as is in close proximity with the capillary endothelial, while the biggest part of red blood cell is consider to be in the central axis of the capillary at an average distance 3.5 μm from the endothelium. Alveolar-capillary membrane thickness is 0.3 μm in the operation sections, where the exchange of gas takes place. This means that the distance that is travelled by molecule of oxygen via alveolar-capillary membrane is ten times smaller from the median distance of membrane of red globule from pulmonary capillary endothelial. The path of diffusion inside the capillary can be longest from the path of alveolar-capillary membrane.
After oxygen molecules inserted into the erythrocyte, must travel intracellular distance up to molecules of haemoglobin in order to be able to connect with rings of hemi. This route of diffusion is governed by the rules which are given by the Eq. [1].
Transportation of air mass from the upper airway to the alveolar. Mechanical insufflations by singlet oxygen catheter
The difference of partial pressure ΔP via alveolar-capillary membrane in the initiate part of pulmonary capillary {Eq. [2]} is preserved constantly due to consecutive return in capillaries of venous blood with low saturation in O2. As a result venous blood absorbs oxygen from alveolar space. The consecutive movement of oxygen out from the alveolar to pulmonary capillary, consists a consecutive removal of oxygen by the alveolar, and it births a power called ΔΡ1 which on conditions of apnea, entice a mass of gas mixture from the adjacent space (alveolar sacks, alveolar ducts, respiratory bronchioles, 12 generations of branching tertiary bronchus, tertiary bronchus, left and right main bronchus, trachea and or pharyngeal). In case of pumping oxygen with stable flow, at some point of tracheobronchial tree (the ideal place to use singleton catheter blow is immediately above the bifurcation of the trachea) is created an additional positive pressure ΔP2.
The difference of pressure ΔP2 is described by the below equation:
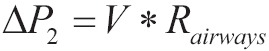 |
[5] |
Where V is flow blowers and Rairways are the resistances of vents. The size of ΔP2 is not stable during the length of the route to the alveolar. ΔP2 at the level of the catheter tip blow is greater in comparison with the lower levels closer to the alveolar, since a part of ΔP2 is always consumed to overwhelm the resistances during the distance of oxygen to alveolar with the stable flow V. ΔP2 has the same direction with ΔP1 so it is added in it. The total pressure gradient ΔPtotal from the one edge of catheter to alveolar side of alveolar-capillary membrane is the sum of ΔP1 and ΔP2 as it is described below:
| [6] |
The speed of transportation air mass to the alveolar is proportional to the resultant force ΔPtotal which is created (pressure gradient from the larger air inlets to the alveolar) and it depends on the rate of the absorption of oxygen from alveolar to pulmonary capillaries, the flow of oxygen blow, the width of the lumen of the catheter blow, and the position of its edge (Figure 2).
Figure 2.
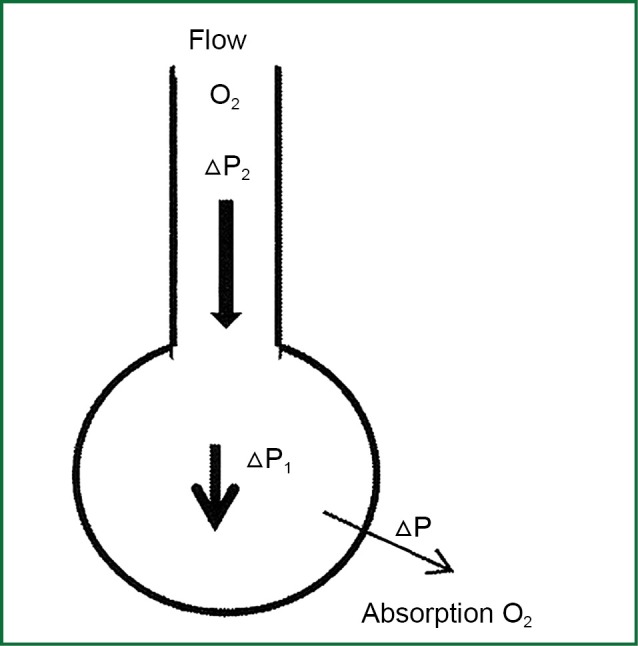
Transportation of air mass from the air vents to the cuvette.
The movement of oxygen mass from the biggest vents to alveolar with the above mechanism, ensures the maintenance of PAO2 in higher values and in this way, the consecutive diffusion of oxygen is maintained through alveolar-capillary membrane to the blood of pulmonary capillaries.
When, singlet oxygen probe blower is used, the point to bifurcation of the trachea has the smallest possible distance from the alveolar level simultaneously from both lungs. For a given oxygen flux measured from the oxygen delivery system, the speed u by which oxygen is popped-out from the catheter tip depends on the width of the lumen that determines the cross-sectional area A and on flow of oxygen:
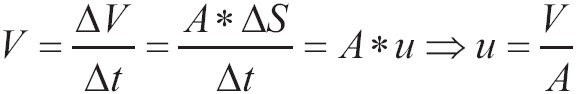 |
[7] |
The thicker is the blow catheter, the bigger is the speed of exit velocity of oxygen from its edge. As a result the pressure gradient from the airways to the cell is greater and the movement of oxygen is more effective (7). As the diameter of catheter shrinks, the resistances of catheter which are displayed on the flow are increased. To maintain stable the flow, it must wield on the upper edge of catheter greater pressure. This difference of pressure is the restraint which is imposed by the characteristics of oxygen system, when flows reach marginally great prices or where the width of the lumen of the catheter approaches marginally small diameter or both.
The role of nitrogen
Everything that has been developed on the operation of gas diffusion of the track of oxygen, applies in the case of dissipated gas is either nitrogen or CO2.
In biota oxygen is consumed by the cells, and CO2 is constantly produced. As consequence, it is not possible the installation of static equilibrium due to the fact that there is constantly alteration of initial partial pressure. On the contrary, dynamic balance is installed, by diffusion of oxygen in the direction of the pressure gradient from alveolar to capillary and diffusion of dioxide in the direction of the pressure gradient from capillary to alveolar.
In case of nitrogen (as for every gas or inhaled anaesthetic which is not metabolized to a significant extent), there is tendency to installation of static equilibrium, in which the partial pressure of the gas in all tissues is equilibrated with the partial pressure of the inhaled mixture. When apnea occurs, instead of progressively decrease of volume of alveolar (and lungs), because of the absorption of oxygen, volume remains stable due to the movement of air gas mixture from the biggest vents, as it was explained above. If gas mixture is the atmosphere air, the containing oxygen will be absorbed, but nitrogen will remain in the alveolar space and it will start to increase its concentration and partial pressure, because nitrogen occupies the position of oxygen which is absorbed by the alveolar blood. Partial pressure of nitrogen in alveolar depends on total pressure in the cuvette (which depends on atmosphere) and on consecration of nitrogen in the gas mixture of alveolar:
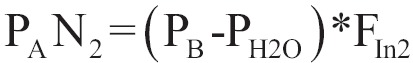 |
[8] |
The result is the decrease of partial pressure of oxygen in alveolar and hypoxia is rapidly installed (two minutes in human).
If air mixture which is close to alveolar space during apnoea is pure oxygen, then the volume of absorbed oxygen in alveolar, is replaced with an equivalent volume of pure oxygen without the addition of nitrogen (at least from the external space) and alveolar PAO2 is decreased in the grade where alveolar PACO2 (3-6 mmHg/min after first minutes), while pure amount of nitrogen in alveolar is stable, because there is no gradually sum of external use. In this case, the time in which hypoxia appears, is quite bigger.
In case of, where before apnoea ventilation has been preceded (automatic or mechanical) with pure oxygen, then in the air mixture of alveolar there is no nitrogen and initial PAO2 is 660 mmHg. It is consider, that in normal adult with normal residual capacity of lungs, inhalation of pure oxygen for four minutes eliminates the major part of alveolar nitrogen, so the concentration of the latter is less than 20%. Since, pure oxygen is granted by blowing into the trachea, hypoxia will be delayed even more, and patients will be oxygenated for 100 minutes, assuming that they have a clear vent and they are connected with source of pure oxygen.
In study of Fraioli, Sheffer et al. (4), an additional mechanism is referred by which nitrogen produces decrease in PAO2. Even when nitrogen is fully purged by alveolar space via hyperventilation with pure oxygen, the circulation of blood returns the dissolved nitrogen in lungs, where this exits from the pulmonary capillaries to the cell, due to the inclination partial pressure of nitrogen from the nitrogen storage of the organization to the leached by nitrogen alveolar. Diffusion of nitrogen decreases partial pressure of total inventories of nitrogen in organization, by the duration of ventilation with pure oxygen, cardiac output, and FRC of the organization.
Carbon dioxide
CO2 had the greater solubility in water compare to oxygen, and besides that its steam are of higher density, it is considered that its capacity for diffusion via hydrated membrane is 20 times bigger than this of oxygen (Table 1). For this reason, in the past it was considered that there would not had been problem with the diffusion of CO2, since patient would had succumbed to hypoxia before hypercapnia was of great importance. All these, ignore the fact that chemical reactions of respiratory molecule are adequately slow, in order to affect the diffusivity of gas. It is considered now, that these are the restraint factor in diffusion of respiratory molecules. CO2 is produced in mitochondria of cells. Partial pressure of CO2 is not similar to all cells. Tissues with low metabolism produce smaller amounts of CO2 per cell in compare to tissues with high metabolism (myocardium). That’s why, venous blood which is removed from various organs does not have the same partial pressure with CO2. From mitochondria with gradually decrease of partial pressure, is transported in cytoplasm, in the interstitium, leaving the regional capillary in venous blood and eventually is diffused through alveolar-capillary film in the alveolar space from which is eliminated by the mechanism of ventilation.
Movement of carbon dioxide in blood
Movement happens with four ways:
As dissolved CO2;
As carbonic acid, that breaks down to CO2 and water with the help of carbonic anhydrase;
As bicarbonate being, that is a major part of CO2 in the blood and is produced by the ionization of carbonic acid;
- With the form of carbonates. Uncharged amino groups R-NH2 of proteins of plasma and the edges of α- and β-chain of haemoglobin are straightway combined with CO2 in order to create carbamate, which is almost completely cleaved:
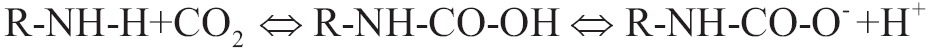
This reaction depends on pH because of the competition of hydrogen and CO2 ions for the connection on uncharged amino groups of proteins. The total amount of CO2 which is transported in this form is very small. Analogue the major part of transport in form of carbamate is done by amino groups of haemoglobin than by the other proteins. Reduced haemoglobin is 3.5 times more effective than ox haemoglobin in this form of transportation of dioxide and this consists a major part of Haldane phenomenon.
Diffusion of oxygen is done in level of alveolar membrane from the liquid phase to the gas phase. The rules that condition the diffusion of CO2 to alveolar are subscribed by the equation [1]. Because of the greater capacity of CO2 to diffusion, the demanding difference of partial pressure of CO2 between pulmonary capillary and alveolar air is less than (6 mmHg) the difference of partial pressure of oxygen between alveolar air and pulmonary capillary (60 mmHg) (11).
The major reactions of the release of chemical connected CO2 is its release from carbamate as it was mentioned above, but mainly the alteration of bicarbonate ions in carbonic acid, followed by dehydration in order to release molecular CO2. Last reaction contains movement of bicarbonate ions via alveolar-capillary membrane and it would have been prohibitively slow if it had not been catalyzed by an enzyme called carbonic anhydrase which is in endothelial of pulmonary capillary and in the surface of red globules:
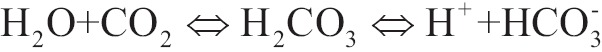 |
[9] |
There are seven isoenzymes of carbonic anhydrase. Two of them are involved in transportation of CO2. In red globules there is isoenzyme II and in endothelial of pulmonary capillary there is isoenzyme IV. There is no act of this enzyme in plasma. This enzyme contains zinc in its composition and the speeds of reactions are so fast, which its kinetic depends on the capacity of neighbouring buffers to offered/abduction ions of H+ to/from enzyme. In case of respite of carbonic anhydrase (e.g., after graduation of 5-20 mg/kg acetazolamide) the gation partial pressure of CO2 is increased between pulmonary capillary and alveolar, revealing the significant decrease in diffusion capacity of CO2 (10). However, because of greatness of enzyme activity, is needed to be ruled at least 98%, in order to display detectable changes in transportation of CO2 and as a consequence hypercapnia.
Effect of breathing in alveolar partial pressure of carbon dioxide
CO2 is diffused in pulmonary capillaries to alveolar, and it increases partial pressure of CO2 in alveolar space PACO2. During inhalation, fresh gas dilute alveolar air mixture, and decrease PACO2 per 0.4 kPa (3 mmHg), and they produce saw tooth form in waveform of PACO2 according to time (Figure 3). As blood leaves pulmonary capillaries has its partial pressure of CO2 close to alveolar partial blood of PACO2. So this alters with the same way as the other one. There is also, regional disparity in which partial pressure of dioxide is related inversely to ratio ventilation/perfusion from different parts of lung (partial part of dioxide is increased when ratio ventilation/perfusion is decreased and vice versa). Partial pressure of dioxide in arterial blood is a median value from blood samples from the total of different parts of lungs.
Figure 3.
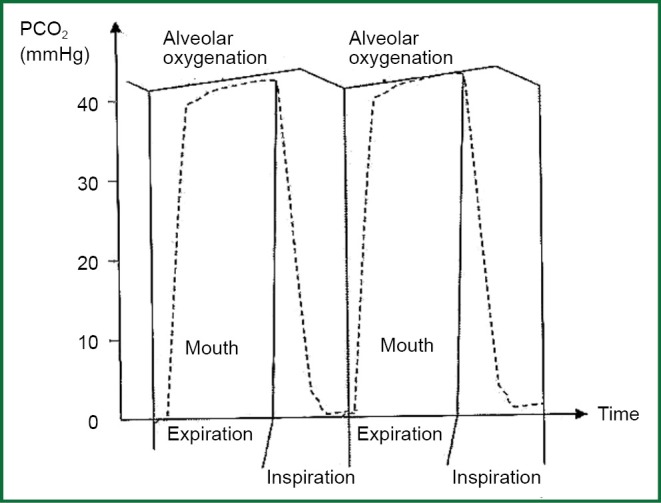
Variation of alveolar and oral PCO2 during respiratory circle.
Alveolar partial pressure of carbon dioxide PACO2
CO2 is added to alveolar air by pulmonary capillaries and it is diverged from cells through respiration. Factors that affect PACO2 are described by the above equation:
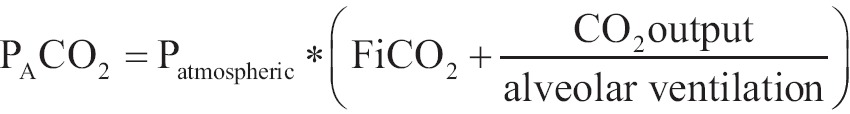 |
[10] |
Small fluctuations of atmospheric pressure in the level of sea cause important altercations of PACO2. At high altitude the hypoxic stimulus leads to hyperventilation and hypocapnia;
Median inhaled concentration of CO2 has additive effect on PACO2. If someone inhales a gas which contains 30 mmHg, then PACO2 will be increased per 30 mmHg above the pressure that it would have had if CO2 had not been inhaled, and other factors including ventilation had been stable;
The way out of CO2 in alveolar space and not its production, determines PACO2. During e steady state of equilibrium in the body, the way out of CO2 is equal to production of CO2. The latter applies to statements that both aeration and cardiac output are altered. In acute hypoventilation a great amount of produced CO2 is travelled to the reserves of dioxide so the way out of CO2 in alveolar space is decreased in low levels. Way out of CO2 in alveolar space is decreased because of the difference of partial pressure of CO2 in alveolar space is abolished due to hypoventilation. Progressively, the alveolar concentration of CO2 is up to new levels, as a result of progressively increases in the amount of dioxide which is heaped in the body and it circulates with blood. This builds a small gradient pressure always from the pulmonary capillaries to alveolar space. Reverse, in curt hypoventilation, there is an increase in the way out of CO2 in alveolar space. A curt decrease of cardiac output decreases the way out of CO2 in alveolar space, and decreases PACO2 until, the concentration of CO2 will be increased in mixed venous blood and the creation of a new pressure gradient of dioxide to alveolar space;
Alveolar ventilation is the volume of air which is expressed by the product of respiratory rate by the difference of reciprocating volume minus the dead space [Minute Ventilation =Respiratory Rate * (Vtidal - V dead)]. It can be fluctuated in a wide range of values, and it is the major factor that determines PACO2;
Influence of deuterium concentration gas. Except from the factors that are described in mathematical Eq. [1-10], alveolar partial pressure of CO2 PACO2 can be affected by pure transport of inert gas along the alveolar-capillary membrane. Fast recruitment of inert gas from alveolar space to pulmonary capillaries increases the concentration (and partial pressure) of CO2 and oxygen in alveolar air. The increase in the concentration is due to the fact that the same amounts of CO2 and oxygen are in smaller volume because a part of initiate of total gas volume which consisted the inert gas which was absorbed. For example this happens during the inception of nitrogen protoxide graduation (N2O) and when big amounts (and volumes) of nitrogen protoxide pass from the cell to the blood, while they are not replenished by the same volumes of nitrogen from the blood to alveolar. Vice versa is done when the graduation of nitrogen protoxide is discontinued, so the vice versa process lead to in transient decrease of partial pressure of CO2 and oxygen in alveolar space (Hypoxia of diffusion).
Stocks of carbon dioxide and volatile situation (12)
The amount of CO2 and bicarbonate ions is large, almost Lt, 100 times greater than oxygen. When ventilation is altered irrespective of the metabolic activity, levels of CO2 alter slowly and new balance is installed after 20-30 minutes. Altercations in levels of oxygen occur much faster after changes in the ventilation.
Figure 4 represents a hydrostatic analogue of dioxide removal with three compartments, in which the depth of water represents partial pressure of dioxide PCO2 and volume of water in each compartment represents the volume of CO2 in each compartment. The production of CO2 is represented by the varying flow from the supply tank. The removal of dioxide depends on alveolar ventilation and by observers who watch the level of PCO2 representing chemo receptors. The department of rapid equilibration represents the circulating blood volume, the brain, kidneys and well-blood tissues. The department of Medium-speed balancing represents muscles and mildly-blood tissues while department of slow equilibration represents bones, fat tissue, and tissues with high capacity in CO2. Every compartment has its own time constant and long-time constant of the departments of medium and slow balancing counterforce the altercations in the department of rapid equilibration.
Figure 4.
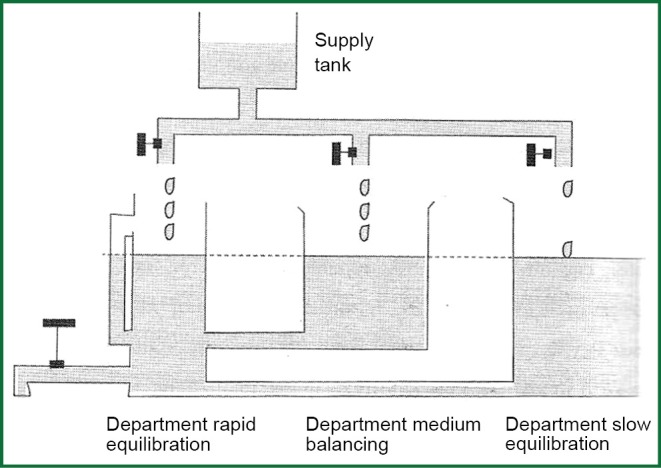
Hydrostatic analogue of the removal of carbon dioxide.
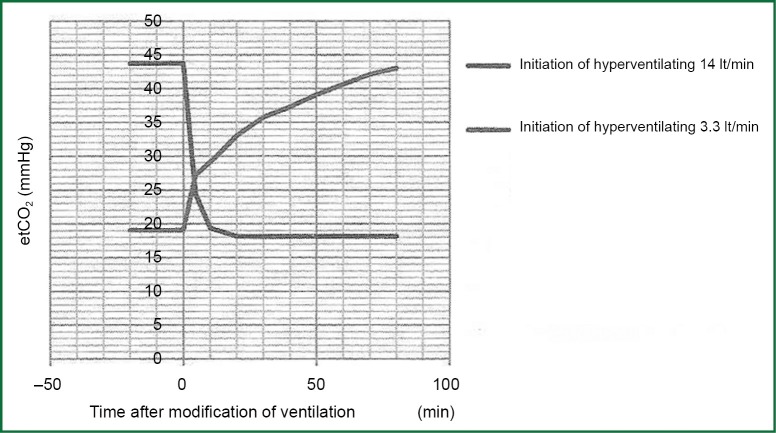
Hyperventilation covers a wide opening of the discharge valve in hydraulic analogue and as result is the exponential decay of levels in three departments. Rapid equilibration apartment lose faster the level of PACO2. The rate of the decrease of PCO2 in each compartment depends on the alveolar ventilation and capacity of every compartment in CO2. Hypoventilation is fundamentally different. The rate of the increase of PCO2 is imposed from the production of CO2 due to metabolism, which is the only factor that increases straightway the amount of CO2 in the body. Consider two different prices of alveolar ventilation, e.g., 3.3 and 14 lt·min-1 and let’s change ventilation sharply from the low price to highest and subsequently from the high price to lower. Graphs that will re-enact the altercation of PCO2 in function of time after the establishment of altercation of ventilation will not have mirror images of each other. The rate of the increase of PCO2 with the inception of ventilation is quite smaller than the rate of decrease of PCO2 with the inception of ventilation, and this is fortunate in cases of chock situations.
When all the produced CO2 by metabolism is retained in the body, the rate of the increase of partial pressure of CO2 in arterial blood (PaCO2) is 3-6 mmHg/min (0.4-0.8 kPa/min). This depends on the rate of production of CO2. In case of hypoventilation (a part of produced CO2 is removed from the body) the rate of the increase of PaCO2 will be smaller than the above. Figure 5 shows the curve of incensement or decrease of end-expiratory partial pressure of CO2 according to time after sharp decrease or increase in alveolar ventilation in anesthetized patients.
Figure 5.
Changes in end expiratory carbon dioxide after sudden changes of alveolar ventilation. During the fall of PCO2 (hyperventilation) the half change comes in three minutes while during the increase in PCO2 (hypoventilation) half change comes in 16 minutes.
Cardiac output and transfer of CO2 (12)
Fluctuations in cardiac output have little effect on partial pressures of CO2 in arterial blood, alveolar, and end-expiratory because of the effectiveness of the breathing control in patient awake. However, with steady alveolar ventilation as it happens in anesthetized patients or during extracorporeal circulation, things are different. In case where cardiac output is zero (cardiac arrest), transportation (that contains CO2) to the lungs is zero. As a result alveolar and end-expiratory is decreased too. Accordingly, the sharp decrease of cardiac output during anaesthesia with controlled ventilation, causes a sharp decrease in end-expiratory PCO2. This was first mentioned in 1957 (13). This is due to dead space because of the increase in number of ventilated but not well-blood alveolar. If low cardiac output is maintained for more than several minutes, partial pressure of CO2 will be increased and end-expiratory PCO2 will be return in normal levels, as blood which oozes pulmonary capillaries releases more CO2 in exhaled air.
Apnoea (14)
When a patient switches from respiration air to apnoea, alveolar gases come to balancing with the gases in mixed venous blood of pulmonary capillaries between few minutes. This balancing will increase alveolar partial pressure of CO2 PACO2 from 40 to 46 mmHg and it will decrease partial pressure of oxygen from 105 to 40 mmHg. These changes in partial pressures correspond to the intake of 230 mL/min of oxygen from mixed venous blood and the assigning of 21 mL/min of CO2. The balancing of CO2 takes place within 10 seconds (15), while oxygen needs one minute, as the latter depends on the ability of cardiac output and arteriovenous dispute partial pressures in level of pulmonary capillaries to absorb 2/3 of oxygen from alveolar air.
The above calculations assume that alveolar air is not exogenously substituted. This depends on patency of the airways and air’s composition (if airways are bramble and deputising of absorbed alveolar air takes place).
Airway obstruction. As it has been described, balance is installed too fast between alveolar PCO2 and PCO2 of mixed venous blood. Thereafter, PCO2 in alveolar space, in arterial blood and in mixed venous blood have similar values with the recirculation of blood. Progressively all of them are increased in a parallel manner and with a rate of increase per 3-6 mmHg/min. More than 90% of metabolic produced CO2 jumps to the reserves of the body. Alveolar partial pressure of oxygen PAO2 follows closely the decrease of PO2 in mixed venous blood within first minute, while after that as recirculation of blood continues, it is further decreased. Volume of lungs is decreased during difference between volume of oxygen which is absorbed by capillaries and in volume of CO2 which is attributed to alveolar. Initially the rate of this decrease of volume of lungs is 230-21=209 mL/min. finally major hypoxia comes within 90 seconds, when the air vents remain excluded and when breathing of atmospheric air has been preceded in the range of FRC.
Bramble air vents and replenishment with ambient air. In this case, the volume of lungs is not decreased because of the exchange of gases (initiate decrease per 209 mL/min) because this volume of air transports from the senior bramble air vents to the level of alveolar-capillary membrane. As the air that replenishes the absorbed volume is atmospheric air [21% O2, 0.04% CO2, 78% N2, 0.95% noble gases (helium, argon, neon, krypton, xenon, radon)] except from oxygen which will be absorbed by pulmonary capillaries, it will give nitrogen, which will be increasingly concentrated in alveolar space. Within two minutes from the inception of apnoea significant hypoxia occurs. It is the moment that the concentration of nitrogen in alveolar space reaches 90% while concentration of CO2 is 8%. The removal of CO2 cannot be happened because there is continuously transport of air mazes from the senior air vents to alveolar, capturing CO2 and preventing its removal either with transport or diffusion.
Bramble air vents and replenishment with pure oxygen. As it has been mentioned, oxygen is continuously absorbed by alveolar space to pulmonary capillaries, but absorbed volume is replenished by graduated oxygen which travels from bramble senior air vents to alveolar. Because graduated gas is 100% oxygen, nitrogen is not added in alveolar gas mixture. As a result alveolar PAO2 is decreased with a rate that PACO2 is increased (3-6 mmHg/min). As a consequence patient is not in hypoxemia for several minutes. If before the installation of apnoea, patient had breathed pure oxygen, so it would not had been residual nitrogen in alveolar air, the initial PAO2 would had been 660 mmHg and the patient would had been able to survived apnoea of 100 min, since air vents would had been bramble and there would had been constantly graduation of 100% oxygen. This is the mechanism of the maintenance of oxygenation during apnoeic oxygenation. With the passage of time produced CO2 which circulates in blood, is progressively increased, by filling storages of the body and simultaneously by increasing its concentration and partial pressure of PACO2 in alveolar air. There have been reported levels and PACO2 up to 140 mmHg during the use of technique of apnoea oxygenation in short periods in air vents interventions (16).
It is believed that, during blowing oxygen in apnoeic oxygenation, with flows greater than the rate of absorption of oxygen in alveolar, a progressive replacement of air mixture inside alveolar is taken place. The rate that the contest of alveolar is renewed depends on the speed of way out of oxygen from the edge of blow catheter, and from the factors that affect the latter. With the partial replacement of alveolar contest, some flushing of dioxide is achieved by alveolar, and a decrease in a small grate of the rate of the increase of PaCO2 and PACO2.
In spite of partial flushing of CO2 by air vents (17,18) during apnoeic oxygenation, a progressive increase of CO2 is observed (less than apnoea) causing hypercapnia and acidosis. A progressive increase of partial pressure of CO2 in alveolar causes decreases in partial pressure of oxygen and as a consequence decreases in the pressure gradient of oxygen to pulmonary capillaries.
Factors that affect diffusion ability (O2, CO2, CO)
The fundamental principles of gases diffusion in lungs show that there are three main mechanisms that affect diffusion ability: changes in potent surface of gas exchange membrane, changes in natural abilities of membrane and changes that are relevant to intake of gases from red globules.
-
Changes in potent surface of gas exchange membrane. The total volume of lungs and consequently the number of available gas cells to exchange, affect diffusion ability. The factors that affect the number of potent alveolar are:
The size of body, as there is a relation between height and lungs’ volume;
Lungs’ volume. Diffusion ability is biggest in peak aspiratory tonnage (19);
Ventilation-perfusion disturbances. The alveoli ventilated but they are not per fused do not participate in gases exchange. In both cases, both the number of active alveolar and the diffusion ability are decreased;
Body posture. Diffusion ability is increased in supine position even though volumes of lungs are decreased. This is due to the increase of pulmonary volume of blood because of increased venous return and to a more uniform distribution of blood (20);
Pathological conditions or surgeries are able to decrease the number of active alveoli. For example, emphysematous decreases diffusion ability, through the disaster of alveolar epithelium and the changes of diffusion ability of monoxide DLCO are relevant to the rate of emphysema changes in lung’s anatomy (21).
Changes in natural abilities of membrane. Chronic heart failure and pulmonary oedema are the only causes that can decrease diffusion of gases via alveolar-capillary membrane. The congestion in pulmonary capillaries increases the distant for the diffusible gases through plasma, while median oedema increases membrane’s thickness. In addition to that, the increased capillary pressure causes damages in endothelial and epithelial cells, leading to the propagation of alveolar cells II and to the fattening of alveolar-capillary membrane (22). Previous study with electron microscope, showed that swollen liquid tends to gather in inactive side of pulmonary capillary, leaving its active side with no fattening. As a result, diffusion of gases in not affected. However, in Chronic heart failure the part of diffusion ability that regards to the properties of membrane is decreased, and the decrease in the ability to diffusion is related to the seriousness of symptoms, while the volume of pulmonary capillaries is increased only in the sever heart failure (22). Besides the negative findings of electronic microscopy it is possible that the severe and prolonged duration of chronic heart failure to cause a form of alveolar-capillary barrier in gases diffusion.
-
Changes that affect gases intake from red globules. The factors that can affect oxygen intake from red globules are:
Haemoglobin concentration. Changing the concentration of haemoglobin, both rate and amount of oxygen intake are changed by the blood which incur in the pulmonary capillaries;
The residence time of blood in pulmonary capillaries. A decrease in residence time causes decrease in diffusion ability. However, the decrease in residence time is done on increased cardiac output (e.g., work out) which increases the amount of oxygen intake and also diffusion ability.
-
Other factors that affect ability to diffusion are:
Age. When age is increased, diffusion ability is decreased in direct proportion (19);
Sex. Women have decreased diffusion ability compare to men same age. This difference is due to the different height and the smaller concentration of haemoglobin in women (23);
Exercise. During work out, the ability to diffusion can be double than at rest. This is due to the increased cardiac output which is decreased in residence time in capillaries (by decreasing the difference in partial pressure of oxygen between initiate and final edge of pulmonary capillaries and by decreasing diffusion). Simultaneously increases both the amount of blood which is taken on by oxygen per unit time and conscripts closed pulmonary capillaries in independent lung’s area. The last two changes increase more the ability to diffusion compare to the decrease of residence time and eventually diffusion ability is increased upon increased cardiac output;
Smoking. The ability to diffusion DLco is decreased in proportion to the number of cigarettes which are smoked per day and in proportion to the number of cigarettes that have been smoked during life (23).
Flow of oxygen through thin air vents (24)
The flow of oxygen via blow catheters but also through thin air vents is described approximately by the law Hagen-Poiseuille which describes the linear flow of fluids through non-operative air ducts. Linear flow is characterized by the flow of liquid in order of concentric parallel cylinders with central axis the longitudinal axis of the pipeline. The speed of liquid in each cylinder is different, rising from zero to a maximum value as cylinders are diverged from the duct wall to the central axis. The increase in liquid’s speed is done by parabolic way, giving the column of liquid a parabolic front:
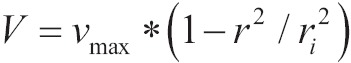 |
[11] |
Where vmax is the maximum speed in central axis, v the speed of liquid in each cylinder and r the distance of cylinder from central axis and ri the radius of the cross-section.
According to this law, the flow via pipe is given by the above equation:
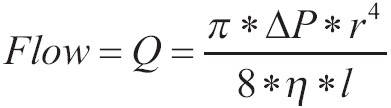 |
[12] |
Where ΔΡ is the difference in edges of pipe, r is the radius of the pipeline, η is the viscosity of liquid and l is the length of the pipeline. The above equation shows that the flow is proportional to the fourth power of the radius of the pipeline, proportional to the ΔΡ and inversely proportional to the viscosity of liquid and the length of pipeline.
There is a number which characterizes each form of flow and it is called number Reynolds. This number characterizes the tendency that certain flow has to go over the linear form to the turbulent form. It is given from the above equation:
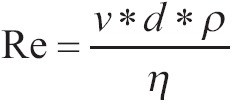 |
[13] |
Where v is the median speed of liquid (cm/second), η the viscosity of liquid (in poise), d the pipe’s diameter (cm) and ρ the density of liquid. When number Reynolds is less than 1,500, then the flow is linear. When it is 1,500-2,000, then linear flow is turbulent, while it overcomes 2,000 then the flow is turbulent.
Pathophysiologic alterations of apnoeic oxygenation
Common denominator of all effects of apnoeic oxygenation is the developed hypercapnia. Hypercapnia causes changes in acid-base balance, via which the remainder of the effects are practiced in other systems. For as long as oxygenation of blood and tissues is not deranged, apnoeic oxygenation is safe, and the effects of hypercapnia are well tolerated and they are reversed after the reconnection in ventilator with short-term mechanical ventilation.
Effect on acid-base balance (25)
Hypercapnia causes the increase of hydrogen ions in blood and as the consequence decreases pH, causing acidosis. In the context of compensatory mechanisms of body, the buffer which is related to CO2 is consisted by carbonic acid and bicarbonates (H2CO3/HCO3-). The chemical reaction which describes the above buffer is:
| [14] |
Hydrolysis of CO2 is catalyzed by carbonic anhydrase. Because H2CO3 is almost immediately cleaved, it can be replaced by CO2. If all the necessary changes are made for the coefficient decomposition of bicarbonate and if the solubility of CO2 is taken into account (0.03 mmol/L/mmHg in body temperature) the equation Henderson-Hasselbach for bicarbonate takes the following form:
 |
[15] |
A more practical and helpful clinical form of above equation is:
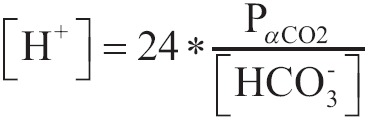 |
[16] |
Where the concentration of [H+] is expressed as nEq/L, partial pressure PaCO2 as mmHg and concentration of [HCO3-] as mEq/L.
With the latten equation we can calculate easy the concentration of [H+] and the corresponding pH according to Table 2.
Table 2. Correlation between pH and [H+].
| pH | [H+] nEq/L |
|---|---|
| 6.80 | 158 |
| 6.90 | 126 |
| 7.00 | 100 |
| 7.10 | 79 |
| 7.20 | 63 |
| 7.30 | 50 |
| 7.40 | 40 |
| 7.50 | 32 |
| 7.60 | 25 |
| 7.70 | 20 |
An acute increase in partial pressure of CO2 in blood causes minimal changes in the concentration of HCO3-. For example an increase in PaCO2 from 40 to 80 mmHg increases the dissolved CO2 from 1.2 to 2.2 mEq/L. In addition to that so is the equilibrium constant of hydrolysis of CO2, that an increase of this size in dissolved CO2 diverts minima the above equation to the right:
It is acceptable that with the acute raise of PaCO2 and with the minimum increase in dissolved CO2, the concentration of [H+] is not altered. According to the Eq. [1-10] it is presented that:
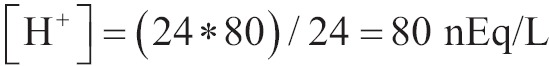 |
And from the Table 1 we find that pH =7.10.
As a consequence [H+] are increased by 40 nEq/L and as HCO3- is produced in rate 1:1 with the H+ according to the Eq. [8] then HCO3- will be raised in 40 nEq/L. the extracellular HCO3- will be increased from 24 to 24.000040 mEq/L. pH is decreased from 7.40 to 7.10 (25).
In acute changes of PaCO2 based on the above reasoning the calculation of the change of pH is feasible.
The value of PaCO2 represents the balance between the production of CO2 with the respiratory movements. During apnoeic oxygenation, the sharp decrease in the removal of CO2 by the lungs due to the abolition of respiratory movements eliminates the deviation of partial pressure of CO2 from pulmonary capillaries to alveolar. As a result the CO2 is not removed from the blood capillary and so the partial pressure of blood is progressively increased. The rate of the increase of CO2 in blood (and in alveolar) depends on many factors, such as body temperature, neuromuscular blockade, changes in cardiac output which are affected by vigilance or anesthesia, pathology primer (fever, sepsis, trauma, tremor, surgery stress), mechanical ventilation etc. The increase in PaCO2 causes acidosis and this is the main mechanism where apnoeic oxygenation effects on several systems of body.
Effect on central nervous system
The effect of apnoeic oxygenation in CNS mediates via the concomitant increase in partial pressure of CO2 in blood. CO2 has at least five different actions in brain:
It is the major regulator of cerebral blood flow;
It affects the pressure of cerebrospinal fluid via changes in cerebral blood flow;
It is the major regulator of intracellular pH, having significant effects on metabolism of cell;
In increased concentrations, has anesthetic properties;
It increases the irritability of certain neurons.
Effect on cerebral blood flow
Hypercapnia causes changes in cerebral blood flow as in consciousness. As PaCO2 is normally increased and cerebral blood flow with rate 1-2 mL. 100 g-1·min-1 per mmHg (26). The curve of the change of cerebral blood flow in conjunction with the changes of PaCO2 has sigmoid form. In lower values of PaCO2 vasoconstriction in cerebral circulation is limited by the vasodilating action of tissue hypoxia, while in higher values of PaCO2 vasodilatation reaches at maximum levels. There is a little documentation that in different areas of CNS in human, there are little fluctuations of the answer in the changes of PaCO2 (27). Hypercapnic acidosis, through a procedure which depends on potassium and calcium channels, causes an increase in the expression of endothelial synthetase of nitric oxide via mediation of prostaglandin PGE2. The final result is the vasodilatation in cerebral circulation and the subsequent increase in blood flow (28).
Effect on consciousness
As PaCO2 is increased in levels 60-75 mmHg in non-anaesthetized body, the feeling of breathlessness is created. As a result the respiratory centre is stimulated and respiratory movements become frequent and deeper.
Higher levels of CO2 have an effect on central functions of CNS. Levels of PaCO2 greater than 90-120 mmHg cause anaesthesia from CO2 (29) while the inhalation of air mixture which contains CO2 in concentration greater than 30% (228 mmHg), causes anaesthesia which is involved in the appearance of spasm (30). It is also reported that in case of provenance of spam because of hyperoxia (e.g., treatment with hyperbaric oxygen in pressure >2 atm) hypercapnia decreases the threshold of occurrence of convulsions. The increase in cerebral blood flow which is caused by hypercapnia, increases the cerebral tissue PO2 in comparison to blood PaO2. The mechanism of anaesthetic effect of CO2 is not due to the physical properties of its molecular but in the changes of intracellular pH, which cause changes in the metabolic processes of cell.
In levels of CO2 greater than 150 mmΗg suppression of respiratory center is taken place and the creation of vicious circle which can lead to death. It is reported that in case of prolonged resuscitation from the anesthesia of an adult who has been undergone in plastic surgery of face and who was ventilated with mask for 4-6 hours with SaO2 >90%. The initiate gas of blood revealed a significant respiratory acidosis with pH 6.60 and PaCO2 375 mmHg. After tracheal intubation, mechanical ventilation and confrontation of respiratory acidosis, patient woke up from comatose without neurological deficit (31).
Effect on the autonomic and endocrine system
The confirmation in significant hypercapnia depends largely on the answer of the automatic nervous system. The effects of hypercapnia on other organic systems are largely explained by the reaction of automatic nervous system.
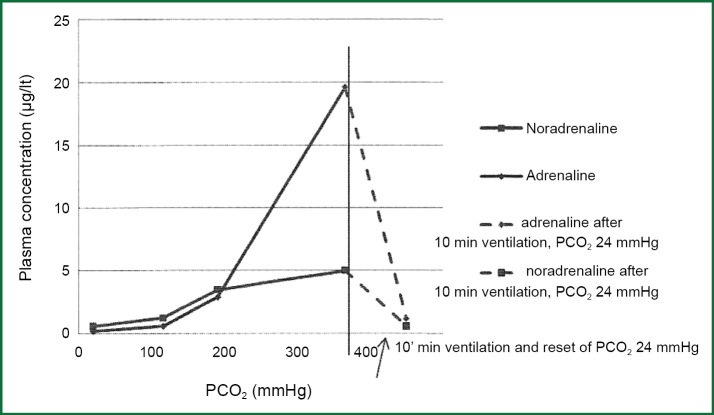
Studies in animals, showed clearly the increase in the concentrations in plasma both adrenaline and noradrenalin after the increase of PaCO2 during apnoeic oxygenation (Figure 6) (32).
Figure 6.
Changes in concentration of catecholamines in dogs’ plasma during the increase in PaCO2 (from 22 to 338 mmHg) at apnoeic oxygenation during one hour. After ventilation 10’ and return of PaCO2 in 24 mmHg, catecholamines decreased in initiate levels.
In moderate increase in CO2 there is an almost analogue and concurrent increase in both adrenaline and noradrenalin, but in higher hypercapnia (PaCO2 >200 mmHg) it is observed a sharp increase of adrenaline. Similar changes of catecholamines have been reported in smaller range of CO2 and in human volunteers who inhaled mixtures of CO2 (33,34).
Effect on cardiovascular system
Effect on cardiomyocytes
Hypercapnia and concurrent acidosis have direct suppressive effects on cardiomyocytes and in smooth muscle vascular. In isolated preparations both contractility and cardiac frequency are decreased probably because of the decrease of pH. These suppressive actions in normal human are hedged by the increase of catecholamine’s which are caused by hypercapnia. In patients who were in mechanical ventilation, the increased partial pressure of CO2 in blood resulted the increase in cardiac output and the decrease of peripheral resistances (34) leading finally to increased blood pressure. In study of Kiely et al., in awake healthy volunteers the examination with no invasive echography Doppler after development of hypercapnia showed similar results (35). During the increase in end-expiratory CO2 at 52 mmHg, cardiac output was increased at 1 lt/min as a consequence of increased cardiac frequency and volume pulse, leading to lightly increase in blood pressure. The measurements of systolic and diastolic function of left ventricular were immutable. This fact advocates in mainly effect of catecholamines in the stimulation of myocardium compare to the direct effects of CO2 on the heart.
Effect on coronary circulation-arrhythmias
According to experimental models in rats, on conditions of hypercapnia, blood flow is increased in coronary circulation. This increase in flow is due to the production of nitric oxide and simultaneously in parallel activation of ATP channels (36).
Hypercapnia and acidosis sensitize myocardium causing arrhythmias from which the most are of no particular importance. However in the study of Kiely et al., healthy volunteers with moderate hypercapnia showed an increase in dispersal change of space QT (35). This finding reflex regional disturbances repolarisation of the ventricles and under other circumstances (e.g., myocardial ischemia) can increase the likelihood of occurrence severe arrhythmias.
Effect on vascular tone
The increased levels of CO2 effect directly on vasomotor centre of brain, which attracts intense sympathetic action causing generalised vasoconstriction in regional, increasing the systemic vascular resistances and blood pressure. As respiratory acidosis becomes more and more intense because of apnoea, the concentration of [H+] and [K+] is increased in blood, effecting directly on the muscular wall of arterioles.
“Pump of K+-H+” correlation to the changes of acid-base balance
It is known that acidosis increases the release of [K+] ions from the cells to the blood causing, hyperkalemia. Vice versa, hypekalemia causes the way out of [H+] ions from the cells, bringing extracellular acidosis. These changes are due to the indirect interactions between [H+] ions and [K+] ions, so the cells seem to have pump exchange of [H+] ions and [K+] ions. It is true, that specialized cells in the stomach and in kidney have a pump which is guided by ATP which exocytose [H+] ions in exchange with [K+] ions. In addition to that, the pump which transfers [K+]/[HCO-3] in some cells, imitates the exchange pump of [H+] - [K+]. However, the fact that have led to the hypothesis of exchange pump [H+] - [K+] reflect indirect interactions in [H+] and [K+] ions. An example of the phenomenal exchange of the [H+] - [K+] ions is the case of hyperkalemia which causes intracellular alkalosis. This phenomenon is not due to the 1:1 exchange of K+ ions with H+ ions but also it is on not due to the clean increase of [K+]extracellular. Vice versa the increased [K+]extracellular depolarize the cell membrane causing the pure intake of HCO3- via electrogenic co transporter Na/HCO3 and as a consequence the increase in intracellular pH—an alkalizing which is due to depolarization of cell membrane.
Extracellular acidosis decreases intracellular pH by inhibiting the transporters which are responsible for the intake of [K+], by decreasing the connection of ions K+ with non-diffusible intracellular anions leading eventually to pure release K+ from cells. In addition to that intracellular acidosis decreases the function of pump Na+/K+ and of pump Na+/K+/Cl- which normally transfer K+ ions inside the cells. The final result is an increase of [K+] in plasma per 0.6 mEq/L for every decrease per 0.10 in blood pH (25). On the other side extracellular alkalosis causes the intake of K+ from the cells leading to hypokalemia. However the mechanism is not known, high levels of HCO3- even in absence of changes in extracellular pH—cause hypokalemia activating the entrance of K+ in cells.
Marked acidosis because of hypercapnia
The mechanism of pump Na+/K+ has the potential to front small deviations of acid-base balance from the normal situation. However in marked acidosis as it happens in intense hypercapnia, the initiate decompensation of the increase of [H+] ions with the increase of extracellular [K+] is followed by furthermore increase in [H+] ions, with final result the concentrations of both ions to be increased intravascularly.
The increased [H+] and [K+] ions inhibit the smooth muscle contraction in the walls of arterioles, bringing vasodilatation and decrease in the systemic vascular resistance.
In case of application of apnoeic oxygenation for extended periods (>30 min) because of the significant vasodilatation which is occurred via the mechanisms which were mentioned before, there is likely to need transient support of circulation system with intense vasopressin agent (such as phenylephrine or noradrenalin) in order to reverse the generalized vasodilatation. The vasodilatation support can begin to the end of apnoeic oxygenation and to be continued and after this, as the patient will be connected to respirator and the severe respiratory acidosis will be restored. In the international literature fewer checked cases of patient with marked acidosis (>200 mmHg) are reported, which are strong enough to substantiate that the full resuscitation after severe hypercapnia in absence of hypoxia, is strong and in fact this tends to be a rule (31,37,38).
Effect on pulmonary circulat/ion
The actions that apnoeic oxygenation has on pulmonary circulation and in pulmonary vascular resistances, are developed in the part of effects on respiratory system. Pulmonary vasoconstrictor reflex plays a significant role which causes different actions in pulmonary resistances, depending on the degree of hypercarbia, hypoxia, the presence of atelectasis, the catecholamine hyper secretion, the intravascular volume and the depth of anesthesia.
Effect on respiratory system
Apnoeic oxygenations effect on respiratory system via the graduation of pure oxygen and the hypercarbia which is developed, and the conforming respiratory acidosis. Central axis of the effect on respiratory system is the hypoxic vasoconstrictor reflux.
Hypoxic vasoconstrictor reflux
The reflux of pulmonary vasoconstriction from hypoxemia is a normal reaction of pulmonary arterioles (which have the greater contribution to pulmonary resistances) in alveolar hypoxia. This reflux regulates the conjugation ventilation/perfusion aiming to the decrease of arteriovenous shunt and to the optimization of the partial pressure of oxygen PaO2 in arterial blood. The heart of the reflux is the smooth muscle cell in pulmonary arterioles and its mechanism is complex. The theory of redox suggests the coordinated action of a redox sensor (the proximal electron transport chain in mitochondria) which produces a chemical ombudsman (free oxygen radicals) which can be diffused and regulates an activate protein [electrically adjustable potassium (Kν) and calcium channels]. Exclusion of sensitive potassium Kν channels to oxygen depolarizes smooth muscle cells of pulmonary arterioles, causing the activation of calcium channels and the following entrance of calcium intracellularly, having as a result the vasoconstriction (39). Acidosis caused by hypoxia and hypercarbia triggers the above procedure, leading to the increase of pulmonary vascular resistances.
The action of hypercarbia to the release of vasoconstrictor reflux is quite weaker compare to the action of alveolar hypoxia (40). The result of the activation of reflux is the increase of pulmonary vascular resistances where in combination with increased cardiac output because of the catecholamine secretion leads to the increase of pulmonary blood pressure. It is believed based on studies in both animals (41) and humans (42) that the responsible factor for the changes of pulmonary vascular bed because of CO2 is pH and not CO2 itself. In study of Lynch et al. the application of hypercapnia in endothelial of isolated pulmonary arteries from rats, caused the contraction of vessels while their stripping to endothelial abolished this reaction in exposure to hypercapnia. Also, the non-specific blockade of isozyme of nitric oxide synthase with solution L-nitro-arginine methyl ester (L-NAME) 10-3 M abolished the vasoconstriction because of hypercapnia. In conclusion the study suggests that hypercapnia causes vasoconstriction in pulmonary circulation, which is related to the function of endothelial through the decreased production of nitric oxide (43).
On the other side, apnoeic oxygenation with the blow of pure oxygen in tracheobronchial tree contributes to the increase of alveolar partial pressure of oxygen, which acts suppressor in the release hypoxic vasoconstrictor reflex. For the activation of the latter, the low alveolar PAO2 is much more significant than the low PVO2 in mixed venous blood (44). The result of the blow of O2 is the decrease in pulmonary resistances. Exceptions are the areas of lung which have atelectasis, so the dominant local conditions emit hypoxic vasoconstrictor reflex by increasing locally the pulmonary vascular contractions.
The effect of apnoeic oxygenation in pulmonary circulation is the result of the two opposite mechanisms; each of them depending on the dominant conditions has variable gravity. Furthermore, the change of the pulmonary resistances depends on the situation of intravascular volume and the depth of anesthesia. The flows of pumping, the position of the tip of the catheter blow, anatomic factors, the place of patient, and any factors that increase intra-abdominal pressure will determine the extent of lung atelectasis and so the extent of activation of hypoxic vasoconstrictor reflex according to with what has been mentioned.
Effect of hypercapnia on respiratory drive
It is known that the increased partial pressure of CO2 in arterial blood is a stimulating factor that increases the minute ventilation of patient. Ventilation is essentially a function of pH in cerebrospinal fluid. The curve of the changes in the ventilation in relation to PaCO2 is affected by the presence of hypoxia or hyperoxia and the presence of metabolic acidosis or not. In any case apnoeic oxygenation is applied on conditions of general anesthesia and under generalized muscle relaxation so the changes of PaCO2 send incentive to the CNS for the change of ventilation but the latter do not change because it is controlled by the reactions of general anesthetics and muscle relaxants to the CNS and neuromuscular junction.
Effect on kidney
The renal blood flow and the rate of glomerular filtration are at least affected by the small changes of PaCO2. However, in high values of PaCO2 vasoconstriction occurs to arterioles. This vasoconstriction leads to the decrease of the rate of the urine production and in extreme situations it can reach until the installation of complete anuria. Kidney cells are very sensitive on hypoxia conditions with the danger of necrosis when it occurs for more than ten minutes. If during apnoeic oxygenation, oxygenation of kidney cells is not deranged these do not incur any danger of disruption of their functionality.
Heart-lung interactions
Interactions of the mechanism of ventilation/circulation
During mechanical ventilation, the fluctuations of the pressures in air vents are transmitted in vascular of pulmonary circulation, in heart, and in central venous. The size of the transmitted pressure on heart and on circulation depends on distensibility of lungs and chest wall. During the lungs’ development a part of the applied positive pressure is consumed as transmural pressure, while the remainder part is transmitted to the contents of thoracic cavity and chest. The above equation is valid:
| [17] |
Where PMV is the pressure inside the air vents, during mechanical ventilation, Ctotal is the total distensibility of the chest wall-lungs system, Cπν is the distensibility of lungs, Cθτ the distensibility of the chest wall and ΔV is the change of the volume which is common both for the lungs and the chest wall (Figure 7).
Figure 7.
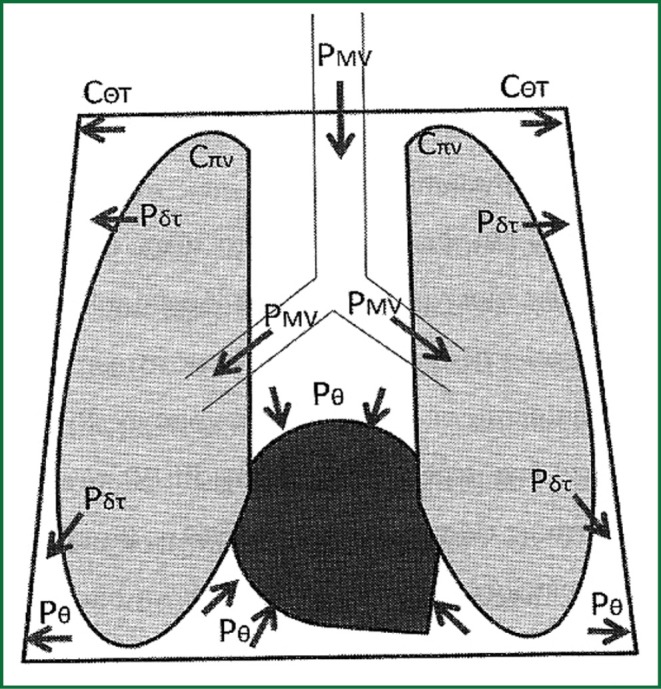
Distribution of positive pressure from the mechanical ventilation of the lungs within the thoracic cavity. Implementation of intrathoracic pressure on the heart and vascular formations. PΘ =Pintrathoracic, Pδτ = Ptrasnmular, PMV = Pmechanical ventilation, CΘΤ = compliance of chest wall, Cπν = lungs’ compliance.
According to the above mentioned, lungs with small distensibility (e.g., in ARDS) results in less transmission of positive pressure from ventilation to the chest (and the pulmonary vascular), while the chest wall with small compliance (e.g., in obesity) results in greater transmission of positive pressure on chest and its vascular. The latter happens because the pressure which compresses intrathoracic organs is the pressure which is demanded to deployed chest wall. In opposite situations, lungs with greater compliance (emphysematous) will transfer more the positive pressures in chest, while the chest wall will great compliance (muscle relaxation) will transfer less the positive pressures in chest and its vascular. In other words lungs “consume” a part of the positive pressures before they transferred inside the chest, while the thoracic wall which hems the lungs and the big vascular formations inside of it expands the remainder of the positive pressures which was finally transferred inside the chest from the lungs.
Effect of mechanical insufflations on central pressures
During mechanical inflation (inspiratory phase) the positive pressures which are intrathoracic transferred increase the pulmonary blood pressure (PAPs, PAPd) the pressure of wedge in pulmonary capillaries (PCWP), the pressures in cardiac chambers and the central venous pressure (CVP).
During inspiratory phase, and especially towards the end of this, intrathoracic increase of the pressure in lifted and as a consequence the transferred pressure is lifted to the central pressures, and their records approaches closely as possible the real values of central pressures. Exceptionally in case of application PEEP, central pressures will be increased in a part of the PEEP price over the real value at the end of the inspiratory phase. How great the additional increase of the pressure will be, depends on the lungs compliance, which determines which part of PEEP will be consumed for the deployment of lungs and how much the remainder of the PEEP part which eventually will be transferred in intrathoracic cavity and will increase the central pressures, will be.
Effect of mechanical inflation on preload
Regarding the preload and after load, the effect of the increase of intrathoracic pressure is different upon the right heart rather than upon left heart. More specifically, the increase in intrathoracic pressure during inspiratory phase of mechanical inflation decreases the preload of left ventricle, because the increase in pressure of right atrium (CVP) decreases the venous return and obstructs the right ventricular filling. The transmural pressure of right ventricular (but also the left) is given by the following equation:
| [18] |
As intrathoracic pressure is increased during inspiratory phase of mechanical inflation, for given pressure inside the ventricular, the transmural pressure of the cavity (right heart) is increased. This becomes, although the recorded pressure of right atrium or CVP is increased. The transmural pressure of a cavity during expansion determines its preload, the end-diastolic volume, and the length of muscle fibers (45,46). The decrease of the transmural pressure of right ventricle in inspiratory phase of mechanical inflation represents the decreased filling of right ventricular.
On the contrary, in this phase of mechanical ventilation, the preload of left heart is increased, because the blood which is contained in pulmonary circulation crushed to the left atrium as lungs distend, increasing venous return to the left heart. The increase in the recorded pressures of the left ventricular (PCWP, Pinside the ventricle) is greater than the increase in the intrathoracic pressure because of mechanical insufflation. This is due to the increase in PCWP is attributed to the direct effect of increased intrathoracic pressure in left heart, and to the increased venous return from the pulmonary circulation. The final result is the increase in the transmural pressure of left ventricular and the increase in its preload. The right and left ventricular are characterized by different pressure-volume curves and as a result same change of volume implies different changes in their corresponding pressure filling so reliable predictions can not be made for the preload of the left ventricle only by the value of CVP.
These changes in the preload of the right and left ventricle are affected by many factors. First and foremost, the changes in intrathoracic pressure during mechanical inflation are depended by the retrograde volume, lung compliance, and chest wall compliance. Great ventilation volumes, compliant lungs, and unyielding chest wall cause great increase in intrathoracic pressure, affecting mostly the changes in preload. The situation of the intravascular volume will determine the rate in which the changes of intrathoracic pressure will affect heart filling. For example, in hypovolemia, inside the pulmonary circulation will be less blood volume, so during pulmonary inflation less blood volume will be transposed to the left heart while the decrease in venous return to right heart will be even greater.
The mechanical inflation of positive pressure, supplants a discrepancy between the fillings and ejections of both ventricles with the left heart to increase the stroke volume and the right heart to decrease it. During the expiry of the inflation and the outset of the expiration phase, things are reversed. Intrathoracic pressure is zero (if PEEP is not applied), venous return is increased and also the filling of right ventricle and as a result right ventricle increases its stroke volume. On the contrary, the filling of heart ventricle is decreased leading to the decrease of its stroke volume. So, the appliance of mechanical ventilation with positive pressures causes circular changes in intrathoracic pressure, which are perceived as changes in preload and stroke volume of left and right ventricle.
Effect of mechanical inflation on heart’s after load
The transmural pressure across the wall of the ventricle (right or left) during contraction, determines the after load of ventricle. The changes in after load of right ventricle during inflation with positive pressure do not affect its version (47) which is due to the fact that both right ventricle and the whole pulmonary circulation incur same changes during mechanical ventilation. However, the same change in intrathoracic pressures during mechanical inflation decreases the after load of left ventricle, enhancing its version. Left ventricle and thoracic aorta incur the same increases in intrathoracic pressures but compare to the right heart, the biggest part of the systemic circulation is out of the chest and it does not incur the increase in the intrathoracic pressures. As intrathoracic pressure is increased during mechanical inflation, this increase in pressure is transferred to the left heart and thoracic aorta, increasing their pressure above the pressures of the vascular which are out of the ribcage. As a consequence the force which is demanded to the promotion of stroke volume of left heart is decreased, by decreasing the after load of the latter. Alternatively another manner of visa effect is transmural pressure of left ventricle. The mechanical inflation of positive pressure increases intrathoracic pressure and according to the equation [18] decreases the transmural pressure, decreasing its after load. Both theories lead to the conclusion that after load of left ventricle is decreased during the increase in intrathoracic pressure which accompanies the mechanical inflation of positive pressure.
Fluctuation of systemic blood pressure
When systemic blood pressure is watched by system of direct measurement it is likely that periodic changes in systemic blood pressure will be observed. These changes are in concurrency with respiratory circle of mechanical ventilation. Positive pressure during inflation decreases stroke volume of right ventricle, via the decrease of its preload, while at the same time stoke volume is increased via the decrease in its preload, and the decrease in after load of left ventricle. Systemic blood pressure is increased during the outset of inflation of positive pressure. few beats later near at the end of expiration phase, the decreased stroke volume of right ventricle will be in left ventricle, decreasing its filling and consequently its stroke volume, fact that leads to the decrease of systemic blood pressure.
Systolic variation of systemic blood pressure is the difference between the maximum and minimum systolic blood pressure during respiratory circle (47). Using the end of expiration phase as the report period (baseline plateau) for the pressure’s measurements, the total variation of blood pressure can be diverged into a premature expiration increase of pressure which symbolized as Δup and in a ultimate reduction which is symbolized as Δdown (47). Δup waves reflect the inspiratory strengthening of stoke volume of left ventricle while Δdown waves reflect the decrease in venous return in right heart which is conceived in waveform of systemic blood pressure. Normally, patients who are mechanically ventilated do not appear Δup and Δdown waved bigger than 5 mmHg.
When there is hypovolemia then the total variation of blood pressure will be greater than 15 mmHg, with Δdown waves to present bigger deviation from blood pressure during the end of exhale (baseline plateau) rather than Δup waves.
On the other side, Δup waves can give information for any reliance of the function of left heart from the after load. When with the outset of the inflation of positive pressure, a big increased in Δup waves is shown, this indicated how important the decrease of after load of left ventricle is for the enhancement of the extrusion of its stroke volume, fact that indicates the deficiency in left ventricle.
Disconnection from the ventilator, entry of inflation
The entry of apnoeic oxygenation implies the disconnection of patient from the mechanical ventilation. From a situation where intermittently intrathoracic pressures are increased (mechanical ventilation) switching to another state in which intrathoracic pressures do not appear variations, only a small steady increase due to constant pumping of oxygen.
With the disconnection from the ventilator in muscle relaxation and the entry of apnoeic oxygenation all the periodic changes which were developed in the previous paragraph stop. The periodic fluctuations in central pressures are disappeared, which presents a stable small (frequent negligible) increase which is corresponds to small increase in pressures of air vents of possible hyperinflation from the constant blowing of oxygen. The size of hyperinflation and the concomitant increase in the pressure of air vents depends on several factors, such as the size of the blow catheter, the spot of the tracheobronchial tree and its segmentation in this spot, the flows of inflation, and the compliance of respiratory system.
The removal of periodic fluctuations abolishes the respective fluctuations of venous return to the right atrium. Venous return is directly increased and subsequently is maintained stable leading to the direct increase in CVP. The filling of right ventricle is directly increased and its stroke volume, increasing the amount of blood which is instigate to the pulmonary circulation. As a consequence the venous return is increased (a few beats later from the disconnection) to the left heart, it’s filling and eventually its stroke volume. After the installation of stable intrathoracic pressures, the variations of filling and stroke volume are disappeared both right and left heart.
Since normal periodic deviation is stoke volume of left ventricle is not observed, the variations in systemic blood pressured are not observed (Δup waves, Δdown waves) during respiratory circle. In case where left heart is not sick, systemic blood pressure after an initiate small increase immediately after the disconnection from the mechanical ventilation, because of directly venous return, will be maintained stable in its new increases levels.
In case of deficiency of left ventricle, the increase in after load (increase in the transmural pressure) involved the disconnection from the ventilator causes the decrease in stoke volume and systemic blood pressure. As left heart is poor and ceases the inotropic effects of mechanical inflation of positive pressure above the left ventricle, a part from the blood volume which returns from the pulmonary circulation is not promoted to the systemic circulation and it is summed up to pulmonary circulation causing pulmonary congestion or pulmonary edema in severe situations.
Because of the sustained pumping of oxygen during apnoeic oxygenation the lungs’ hyperinflation is possible. In case that inflation of lungs is such is such as to significant positives pressures be transferred to the intrathoracic (especially in lungs with great compliance), venous return to the right ventricular will be inhibited (increased intrathoracic pressures) and the stroke volume of right ventricular will be decreased. Finally, the filling and stroke volume of left ventricular will be decreased leading to hypotension syndrome. The tension of this phenomenon varies depending on the size of positive pressures which are transferred in intrathoracic space, and in severe situations is called extracardiac intrathoracic tamponade. The latter case is this where while pumping of oxygen is continued the remainder direction of escape of gases is extremely small and inadequate for the free gas venting outside the lungs.
Clinical applications of apnoeic oxygenation
Taking into account that apnoeic oxygenation can maintain a satisfactory level of oxygenation of body for short but substantial time; the method can be used in conditions of impossible intubation and impossible ventilation (jet ventilation is a version of apnoeic oxygenation). It consists a way of buying time, in order to ensure a more efficient and permanent way of ventilation or until the influence of muscle relaxation to be brought and automatic ventilation to be started.
In study of Lee et al. (48) the application of apnoeic oxygenation via nasopharyngeal air vent during tracheal intubation enhanced the oxygenation of patients and delayed hemoglobin desaturation for three minutes at least. In study of Taha et al. (49) the application of nasopharyngeal oxygen pumping during laryngoscopy after pre-oxygenation with the technique of four deep breaths, maintained the saturation of arterial blood at 100% for six minutes.
Apnoeic oxygenation with transtracheal oxygen inflation is used in test of apnea during the procedure of diagnosis of brain death (50). The effective oxygenation is ensured and possibility of hypoxia is removed for a significant time (residence time ten minutes) in order to allow time to detect respiratory movement due to increased hypercapnia on arterial blood. According to the guidelines of USA, partial pressure of CO2 is suggested to rise in 60 or 20 mmHg upon the basic measurement before the reflex spontaneous breathing be marked as absent (51,52). Other ventilation methods intervene more strongly in handling of CO2 and impose some degree of respiratory movement, abolishing the worth of procedure. First, the required increase of PaCO2 in order to spontaneous breathing to be caused may be considerably higher of 20 mmHg upon the basic measurement; secondly the increased levels of CO2 can act repressive in Central Nervous System.
Apnoeic oxygenation can be used as a method of oxygenation in surgeries and radiological cases in which the absence of movement is demanded in the lungs, the ribcage, diaphragm and in the ventricular area for a short time. Such surgeries are done in Cardiovascular Surgery and Thoracic Surgery and Surgery of the trachea, bronchus and larynx. Especially in the surgeries of trachea, because pump is incised, the distance that respiratory gases must cover to escape from the atmosphere is smaller and as a consequence the flushing and removal of CO2 is easier. It is referred the successful resection of the handle of the sternum (53) with the application of apnoeic oxygenation in patient with ankylosing spondylitis who have been undergone fracture and spondylolisthesis on the sixth of seventh cervical vertebra, causing massive swelling of the tissues between A4-A7 vertebrae, resulting in severe dyspnea and dysphagia. Apnoeic oxygenation with flows 10-15 lt/min and with blow catheter which was supernatants introduced at the keel of the trachea, increased in maximum the report in the surgical field and the rectification of the damage was done without stopping to ventilate the patient.
In coronary artery surgery, it has been proved that the use of mammary artery as an implant prevails over vein grafts for long-term severity. In case those mammary arteries are used, central anastomoses are not over the aorta and this decreases the danger of posting the plaque from the inside of the aorta and its plunger in cerebral circulation. But in surgical field of the production of mammary arteries is possible that the cognate lung is inserted during inspiratory phase, inhibiting the procedure of production and maybe by increasing the danger of invitation of traumatic injuries in mammary artery, especially when PEEP is applied. One strategy is the ventilation of lungs with smaller volumes, greater respiratory frequency, and small PEEP or ZEEP. However, in emphysematous patients with lungs that have pathological increased compliance, even this ventilation can inflate lungs in such degree that they annoy the production of mammary arteries. A second alternative strategy of ventilation of one lung (the opposite side) which requires the installation of endotracheal channel something that is difficult to be done at the start of surgery. A third alternative strategy of ventilation is apnoeic oxygenation which offers an ideal field for the production of mammary artery. In the study of Watson et al. (54), the use of apnoeic oxygenation for 20-30 minutes offered enhanced surgery filed without respiratory movements, graduating oxygen with flows 45 L/min. The oxygenation of blood was satisfied (in all patients except from one PaO2 was greater than 200 mmHg) while PaCO2 was not increased more than 60-65 mmHg, as a result of the sizable flows which caused the flushing of CO2 from the lungs.
In Thorax it is common the ventilation of one lung (of the depended) in order the suffered lung to be isolated by the pulmonary movements which is about to be operated and the cognate side. The same need for ventilation of one lung is accrued in selected cardiac surgeries, where the access to the heart becomes with lateral thoracotomy, so the cognate lung must deduct in order to ensure the required space of accesses. Prerequisite for this type of mechanical ventilation is the placement of tracheal channel. When ventilation of depended lung starts, independent lung is excluded, it does not have respiratory movements and it is opened to the atmosphere to be compacted. These actions result in sharp and big increase of intrapulmonary shut (20-30%), because blood which posed to vascular of independent lung in not oxygenated. The big increase of the disturbances of ventilation-perfusion cause the appearance of hypoxemia, which often is resistant to the appliance of PEEP in depended lung, because latter exacerbates the shunt, as it can increase the diversion of blood to the depended-unvented lung. The appliance of apnoeic oxygenation to the dependent lung can enhance the conditions of ventilation and hypoxemia, because it allows both the oxygenation of blood which incurs in independent lung, and the positive pressures which are finally applied (according to the flows of pumping, the size of catheter and the lungs compliance) decrease the shunt via diversion of a part of blood flow to circulation of vented lung so they give the opportunity to PEEP to be applied in dependent lung. If in a relief valve, additional PEEP is applied in independent-unvented lung (with the lesser of its partial unfolding) the benefit from the oxygenation is greater, because shunt is decreased more to the independent-unvented lung.
In all cases of apnoeic oxygenation, the edge of blow catheter must be close the respiratory membrane. When it is about oxygenation of both lungs, this spot is upon the belly pan. When it is about oxygenation of left lung, this point is above the bifurcation of the left main bronchus. When it is about oxygenation of right lung, because the bronchus of the upper lobe explants almost immediately after the bifurcation of the trachea in two bronchi, the optimum spot for apnoeic oxygenation is through the right main bronchus after its outgrowth from the trachea.
There are thoughts about the possible use of oxygen inflation only during inspiratory phase of mechanical ventilation in patients with acute respiratory distress syndrome (ARDS) in whom mechanical ventilation is suggested with small volumes focusing on the smallest possible increase in intrapulmonary pressures. The protected ventilation of small volumes results the decreased removal of CO2 and the increase in its partial pressure in arterial blood. Blow oxygen during inspiratory phase in level above the bell pan helps in the easier flushing of CO2, via the flushing of air content in the supernatant keel airway and the avoid recycling at the next inhalation. Final goal is the decreased of intrapulmonary pressures which are demanded for standard oxygenation and flushing of CO2 to avoiding hypercapnia. However, up to clarify the issue of safety and feasibility of this method, as the functional details of system which will do the synchronizing of blow to the expiration phase; the possible use of it must be done with skepticism (55).
Adverse reactions from the application of apnoeic oxygenation
The application of apnoeic oxygenation as every medical action can cause adverse effects, which are not frequent and they do not usually have clinical significance.
Lungs’ hyperinflation
The sustained graduation of oxygen endotracheal or endobronchial carries the risk of lungs’ hyperinflation. The endobronchial graduation causes greater possibility for hyperinflation (56). This happens because with the sustained graduation of oxygen, the intrapulmonary pressures during respiratory circle, which is contrary to a small part in the expiration (56). The size of hyperinflation of one or both lungs depends on their compliance, flows of blow, size of the space which leaving between the walls of the external air vents (trachea, bronchus or endotracheal tube), blow catheter, and on the spot in which the edge of the blow catheter is. If the latter is above the belly pan then both lungs are contended equally, but if it is endobronchial, stretching is greater in the array rather than the corresponding lung.
The size of space which is leaving between the walls of the bronchial or trachea and blow catheter, determines the rate of removal of oxygen which has already been blown and CO2 which is in alveolar. The greater the remaining space is, the easier is the removal of gases (greater flow of removal) and so the smaller is the increase in the intrapulmonary pressures and as a consequence it’s stretching or the lungs. According to the principle of the maintenance of mass, as much quantity of air masses entering the unit time proportional amounts of air masses must exit the lungs at equilibrium subtracting of course the volumes absorbed by the alveolar membrane into the blood. The latter is smaller compare to the total flows which are graduated and can be overlooked in this syllogism. It is necessary the diatom of blow catheter to be smaller or equal to the diatom of remainder space which is given for the gases escape. The relationship of diatoms can be altered into ratio of air inlet diameter (D) to the diatom of catheter blow (d). It is obvious that endotracheal tube is composed airway escape of blow gases and not the trachea, which has bigger diatom from endotracheal tube. And for this reason it is more accurate to take into account the diameter of the tracheal tube as the diameter of the airway escape. This applies to the case that the catheter tip is located blow into the trachea or in the tracheal tube. If the edge is inside of some bronchus which has diameter smaller than trachea and tracheal tube, then as diameter of the airway escape must be taken as the diameter of the loop which clinically is difficult to measure.
Let’s consider that trachea, bronchus or tracheal tube have a loop as blow catheter as is shown in Figure 8.
Figure 8.
Relation of cross section of trachea and probe blow. The remainder space has A-α cross section.
Suppose that D is the diameter of tracheal tube, trachea or bronchus and d the outer diameter of blow catheter. The relevant sections are A=π*(D/2)2 and a=π*(d/2)2. To be the diameter of blow catheter α smaller or equal to the diameter of remainder space A-α which is given for the gases escape, the following relation to the diameters of the two intangibles must be in force:
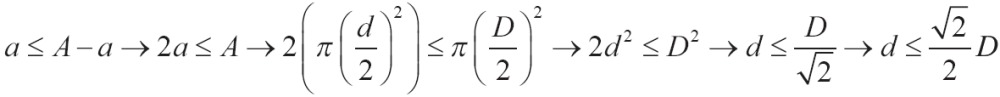 |
It must be in force:
| [19] |
Or else:
| [20] |
In vitro study of Olguner et al. (57) the importance of diameter of blow catheter, the position of the edge inside the tracheal tube and the flows of oxygen blow was looked into for the creation of barotraumas. The model on which changes of pressures were measured during oxygen inflation, was consisted by reserve windbag connected in series with a pressure transducer and tracheal tube through which the blow of oxygen was done.
Endotracheal tubes were tested of three different inside diameters (7, 7.5, 8 mm), catheters blow of four different external diameters (2.66, 3.33, 4, 4.66 mm) with the edge in three locations within the tracheal tube (1/3, and ultimate 1/3 of tracheal tube) and three different flows of blow (6, 8 and 10 lt/min). There were in total 108 measurements. It was seemed that the airway pressure is significantly increased when the flows of inflation are increased more than 10 lt/min compare to flows of 6 L/min. Finally, the different positions of the catheter tip though it gave greater airway pressure in distal position, it did not give any statistical significance.
Based on [5-1] and [5-2] equation and when it is known the internal diameter of the tracheal tube which has been inserted in trachea of patient, it is easy to calculate the diameter of blow catheter which must be inserted in tracheal tube. It is clear that the presence of secretions anywhere in the common course of tracheal tube-trachea and catheter narrows the remainder space, affects the flows of escape and affects the rate of hyperinflation (56). Every time the remaining space on escape has smaller cross section from catheter blow (D/d <1.41414), as it was developed above, there will be the tendency to hyperinflation of lungs, something that in boundary conditions (very big catheter, big flows) can lead to barotraumas or pneumothorax. In case of apnoeic oxygenation application in cardiac the rate of hyperinflation of lungs is directly estimated with simple review of the surgical field into the open rib cage.
Subcutaneous emphysema-pneumothorax
If during the application of apnoeic oxygenation, the placement of the blow catheter is done blindfold either with traumatically way in retropharyngeal or trachea because of the big flows which are used and the positive pressures which are developed, it is possible to be caused subcutaneous emphysema through traumatic dissolution of continuity the tissue. This is typically small size and asymptomatic, it is quick to perceive, requires monitoring and usually subsides within a few hours after discontinuation of blow.
In references they have been reported some of the incidents of apnoeic oxygenation during tests of brain death, in which pneumonothrorax was developed under extension. The possible mechanism of creation of pneumonothorax in the study of Burns and Russell (58) was the blow catheter’s insertion inside the endotracheal tube which was deeply placed and injury of tracheal wall after repeated attempts of bronchial suction. In study of Bar-Joseph et al. as well as Marks and Zisfein as mechanism of the creation of pneumothorax the massive air trapping was speculated beyond the edge of blow catheter which was occluded the air vent (59,60).
The placement of blow catheter to such a depth that does not protrude from the edge of endotracheal tube and the previous bronchoscopic checking of the correct position of the edge of endotracheal tube, decrease the risk of injury of trachea and remove the possibility of subcutaneous emphysema and pneumothorax, with all the consequent effects on the cardiovascular system.
Atelectasis
In contrast to the hyperinflation of the lungs is the development of atelectasis during apnoeic oxygenation under general anesthesia. Lungs’ hyperinflation function deterrent to some extent in the creation of atelectasis during apnoeic oxygenation. However, the protective action is not extended in the total of pulmonary alveolar in certain areas can be created atelectasis. The mechanisms which are responsible for are three:
Occlusion of small air vents;
Compression of the dependent part of the diaphragm from pressure by intra-abdominal organs;
Absorption of oxygen from alveolar air.
Atelectasis because of occlusion of small air vents
Occlusion of small air vents is caused by the decrease in FRC as a result of general anesthesia. In human FRC during general anesthesia can be decreased up to 16-20%. Possible causes for the decrease in FRC are the changes in shape of rib cage [decrease per 200 mL in human (61,62)], the diaphragm’s position (63) and perhaps the changes in intrathoracic blood volume. The decrease in FRC decreases the volume of lungs and it can decrease it below the levels of capacity closure. In such case some of the small air vents will be closed, and as a consequence an increase of normal shunt and the investigation of alveolar-arterial partial pressure of oxygen. Occlusion takes place in dependent areas of lung, where small air vents and alveolar have smaller diameter compare to independent area of lung which are in the upper levels of the lungs. With the outset of apnoeic oxygenation, respiratory movements are stopped and by extension lungs’ volume remain stable with no variations. As lungs’ volume is decreased in lower levels from those of tonnage occlusion, then atelectasis will be created by occlusion of part of the small air vents, leading to disturbances of ventilation/perfusion, as it was above mentioned.
Atelectasis from compression
At the supine position and in full consciousness vaulted muscle of diaphragm maintains satisfaction tone (64) during the end of expiration which is effective to the prevention of diaphragm’s movement to the cephalic direction due to compressive forces exerted by the weight of intra-abdominal organs. General anesthesia tend to disappear the end-expiratory tone of diaphragm allowing its cephalic movement, mainly dependent areas and less or even no its non-dependent areas (62,65). In the study of Reber et al. it was shown that the graduation of relaxant agents during general anesthesia causes substantial cephalic movements of the diaphragm in relation to the sick who had not been undergone muscle relaxation (63). The changes in the position of diaphragm with the changes in respiratory muscle function and the curvature of the spine that genetic anesthesia causes, changes the shape of the rib cage. All these changes act cumulative and lead to the broadcasting high intra-abdominal pressure to the diaphragm compressing mainly the dependent lung areas which are overlaid above the diaphragm causing atelectasis.
The application of apnoeic oxygenation can not reverse fully the above changes which are caused supine position, general anesthesia and muscle relaxation, at least in the dependent lungs’ areas, even if lung’s hyperinflation had been installed.
Atelectasis by absorption
Atelectasis from absorption is caused when an air vent is partially or completely excluded and the gas content within the cell which leads to the obstructed airway is absorbed by mixed venous blood. The absorption of gas content without partial or complete airway obstruction does not lead to atelectasis. The use of gas mixtures with high concentrations of oxygen increase the likelihood of atelectasis from absorption something which is supported by sustained increases references for several stages of general anesthesia. It is noted that the concentration of inhaled mixture at oxygen and not the partial pressure of oxygen, correlates to the creation of atelectasis from absorption.
During anesthesia critical value of FiO2 is 0.6. In the study of Edmark et al. the comparison of inhaled FiO2 1.0, 0.8, 0.6 showed sections of atelectasis areas CT scan after anesthesia induction of 5.6%, 1.3%, and 0.2% (66). The same increased possibility to the creation of atelectasis by absorption presents the use of high inhaled concentrations of FiO2 both in maintenance of anesthesia after manipulation of development (67) and in extubation (68).
If before apnoeic oxygenation ventilation is preceded with pure oxygen for an appropriate time, all the nitrogen is removed from the alveolar and stocks of alveolar oxygen are enhanced, thereby adequate oxygenation levels are maintained for longer. The absence of nitrogen from the alveolar can contribute to the development of atelectasis areas from complete absorption of the oxygen content in the alveoli. Basic conditions as it was above mentioned is a partial or complete blockage of relevant air vent, something which can be done when FRC is decreased in lower levels on the occlusions’ capacity. The higher the inspired oxygen concentration during mechanical ventilation is, the more likely the development of atelectasis from absorption is.
The application of PEEP with specific relief valve during apnoeic oxygenation can prevent the development of atelectasis because of the combination of denitrification and partial or complete airway obstruction. Unfortunately, the application of high values of PEEP, more effective in preventing development of atelectasis, causes hyperinflation of lungs, fact that is no desirable in cases where apnoeic oxygenation is used, when it is intended the respiratory movements to be overcome.
Attenuation of acetic vasopressin reflex
It is known that acetic vasopressin reflex in pulmonary circulation is an instrument which aims to overthrow the perfusion in satisfactorily ventilated areas and to decrease it from the alveolar which are sub ventilated, tending to the decrease in ventilation-perfusion disturbances. Stimulus which releases vasoconstriction in the pulmonary circulation is the low alveolar partial pressure of oxygen (<60 mmHg) and not the partial pressure of oxygen in mixed venous blood (44). On the contrary in the presence of increased alveolar partial pressure of oxygen acetic vasopressin reflex is inhibited, with the result that vasoconstriction do not occur in these areas. The application of apnoeic oxygenation gives pure oxygen is no-vented areas, increases the alveolar partial pressure of oxygen and inhibits acetic vasopressin reflex in these areas, fact that according to the extent it may affect the total pressure in the pulmonary circulation. However, if the suspension of reflex extends to lungs’ adjacent areas of atelectasis, then the blood flow from non-vented areas will be increased, increasing shunt, leading to a likely deterioration of hypoxia.
Potential toxicity of oxygen to tissues
A concern arising from the application of apnoeic oxygenation with the graduation of pure oxygen is the possibility of toxic effects of oxygen in the lungs and in the body, through the production of free radicals. The graduation of 100% oxygen, causes a big increase in alveolar PAO2 and in arterial PAO2 but PvO2 in mixed venous blood and the smaller tissue PO2 are not increased significantly (Table 3). In order to be significantly increased the partial pressure of O2 in tissue level; hyperbaric ventilation is demanded in pressure 3 atm. Apnoeic oxygenation is applied in conditions of atmospheric pressure, so tissue partial pressure of oxygen can not be increased in such levels to cause toxicity in other organs apart from lungs. It is also mentioned that body’s defense mechanisms against free radicals function effectively until levels of tissue PO2 of 450 mmHg.
Table 3. Oxygen levels in normal adult in various recommendations and pressures of inhaled mixture.
| Air 1 atm | O2 1 atm | O2 1 atm | O2 1 atm | |
|---|---|---|---|---|
| PO2 of inhaled mixture (mmHg) | 150 | 713 | 1,425 | 2,138 |
| PaO2 | 98 | 600 | 1,313 | 2,025 |
| CaO2 | 19.3 | 21.3 | 23.4 | 25.5 |
| Arteriovenous oxygen content difference Ca-vO2 (mL/dL) |
5 | 5 | 5 | 5 |
| CvO2 | 14.3 | 16.3 | 18.4 | 20.5 |
| PvO2 | 39 | 48 | 68 | 360 |
Lungs are the first organ which comes into contact with a high partial pressure of oxygen during ventilation with pure oxygen and therefore it is more exposed to oxygen toxicity. The latter seems to correlated more with partial pressure of oxygen in inhaled mixture and not with its % concentration in it (69). Different species have different resistances during ventilation with pure oxygen. Most species of mice will not survive ventilation with pure oxygen for more than three days, monkeys generally survive for two weeks and the man seems to be even more resistant.
Oxygen toxicity to the lungs begins to give the first symptoms after prolonged ventilation with 100% oxygen in pressure 1 atm for more than 24 hours and these are recommended in addiction of tracheobronchial tree chest pain and cough. Further exposure leads to structural changes in the lungs, setting the stage for the development of acute liver injury (ALI). The application of apnoeic oxygenation is done in shorter time frames (a few minutes to one hour) and on the basis of the above it is completely safe as to the possibility of harm to the lungs through the toxicity from their exposure to pure oxygen.
Assessment of respiratory system under general anesthesia during apnoeic oxygenation
During apnoeic oxygenation, lugs are disconnected from the respirator, resulting in air vents’ pressure is may not significantly increased, and certainly not with the typical way that they are installed on mechanical insufflations. Increasing air vents’ pressure depends on many factors, among which are the flows blows, the position of the catheter tip blow, the diameter of the probe and the corresponding diameter of the external air inlet (air vent that surrounds the probe), respiratory system compliance. In any case the increase in pressure of air vents is gradually installed and it acquires a plateau during the blow. Only after reconnecting the test animal from ventilator it was possible to monitor the airway pressure and the finally exhaled CO2. Therefore, the evaluation of the respiratory system was limited to the assessment of oxygenation of experimental animals which is a criterion for the effectiveness of apnoeic oxygenation and encourages the continuation of its application to the experimental animals.
In our study oxygenation is tested by the method of pulse oximetry, by periodically taking samples and analyzing arterial and mixed venous blood and with the periodic import of data for taking respiratory profiles based on proper software. There was a thought about the use of paratrend device for blood gas analysis in real time, but the idea was abandoned because the cost was prohibitive.
Pulse oximetry is an indispensable monitor of oxygenation according to ASA. The principle of its operation is based on spectrophotometric absorbance of light of specific frequency of blood components in order to calculate the hemoglobin saturation (70). The application of probe was in the ear of experimental animal, fact that is responsible for the non-good transmission of biological signal, as probe was used in clinical practice in finger of patients. However, this is more likely in the case that blood pressure will be reduced and, the irrigation of the respective tissue.
The sampling of arterial and mixed venous blood was going on every ten minutes giving a picture of oxygenation of animals. The parameters which were measured were PaO2, PACO2, pH, HCO3, BE and SBE for the corresponding blood sample.
Every ten minutes the appropriate data were entered in the software of monitor continuous cardiac output to yield the values of parameters of respiratory profile. The latter includes the oxygen content in arterial blood CaO2, in mixed venous blood CvO2 and in the blood of pulmonary capillaries CcO2, the arteriovenous oxygen difference C(a-v)O2, the transportable oxygen in arterial blood DO2, the consumption of oxygen by the body VO2, posting the fraction of oxygen from the arterial blood ERO2 and impurities of shunt of oxygenated blood from the open pulmonary capillaries to non-oxygenated blood. The equations describing the above parameters are listed below:
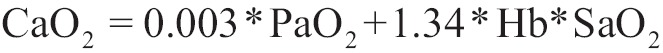 |
[21] |
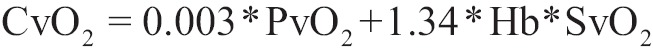 |
[22] |
| [23] |
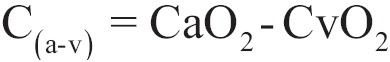 |
[24] |
The units of measure for the above parameters are in mL O2 /dL of blood.
| [25] |
| [26] |
The measurement unit of the transportable oxygen in arterial blood and oxygen consumption is mL O2/min.
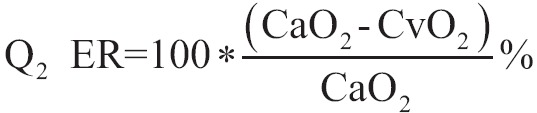 |
[27] |
 |
[28] |
O2ER and shunt are pure numbers and they do not have unit of measurement.
Assessment of circulatory system under general anesthesia during apnoeic oxygenation
Assessment of circulatory system under general anesthesia during apnoeic oxygenation follows the principles of hemodynamic regarding to the measured pressures. Heart rate is the key parameter that measures the remaining hemodynamic parameters, which are named by the temporal relation with the phases of the cardiac cycle [e.g., systolic or diastolic blood pressure, the adequacy (a, c, v, x, y) at the waveform of CVP or pulmonary artery, which are determined by time position in relation to the cardiac cycle and the adequacy of the electro cardio watchtower etc.].
Heart rate is measured in real time by the method of electro cardio watchtower all the remaining parameters are marked as systolic or diastolic.
Blood pressure is measured by indirect mean via the catheterization of the femoral artery of the animal. The intra-arterial catheter is connected in series with order of cannula filled with saline solution which conveys non-pressurized way the pressure changes of the catheter tip to the transducer, which ascribes them at its output as conversion of electric power. The last are noticed by the system processor monitor, which displayed them as waveforms on screen of monitor within a predetermined price range, displaying simultaneously after completion digital indication. The provision of measurement of direct blood pressure is the same which is used to the measure of other hemodynamic pressures (CVP, pulmonary artery pressure, pressure of the pulmonary capillary wedge etc.) and constitutes the cornerstone of intravascular hemodynamic monitoring. Because of the interposer connection of endovascular catheter with the transducer there is a delay between the actual variation of intravascular pressure and finally alleged waveform in the screen of monitor. The greater the length provision is the greater the delay in the appearance of the waveform is, compare to the adequacy of electrocardiogram, which is considered to have almost zero underdeveloped relative to the creation time of biological signal. The further behind appears in the waveform wedge pressure in the pulmonary capillaries because in this case the length of arrangement is added and the length of the column of blood in the pulmonary vascular bed which is excluded by balloon inflation of Swan-Ganz catheter.
Blood pressure is distinguished in systolic and diastolic and median blood pressure (SAPs, DAPd, SAPm). The latter is derived size has biological significance but is used to calculate other derived hemodynamic parameters and it is given by the following equation:
 |
[29] |
SAP is the result of extrusion of blood (stroke volume) from the heart in each cardiac cycle to the arterial tree. Systolic pressure is the section of waveform of blood pressure by the time the aortic valve remains open. After the closure of the aortic valve the phase of diastolic blood pressure begins. The systolic phase is contemporary with the complex QRS of electrocardiogram, while diastolic phase starts after the end of QRS, include the adequacy T and covers the period until the next QRS of subsequent cardiac contractile. The size of the area which is enclosed below the waveform of blood pressure is correlated with stroke volume. The greater the surface area is in each cardiac cycle theories the greater is the stroke volume. The particular characteristics of the waveform such as the speed of ascent and descent of the wave (which is exported by each slope of the tangent to the waveform) allow for reliable conclusions about the after load of left heart (e.g., resistance to extrusion through the aortic valve) and peripheral vascular resistance (in vasodilatation there is a rapid decline of the wave during the diastolic phase) (71).
The cautery central venous strain as the internal jugular vein, the subclavian or femoral vein enables the establishment of an importer for the promotion of Swan Ganz catheter, and allows measuring CVP and the recovery of the waveform of CVP in real time. The latter requires appropriate provisions of connective cannulas and pressure transducer, just as in the case of blood pressure. CVP is indicative of left arterial pressure and in general of the situation of intravascular volume, while its waveform presents five adequacies (a, c, v, x, y) which are correlated to different moments of cardiac circle based on electrocardiogram and provide substantial information about the function of tricuspid valve, right ventricle’s compliance and stunt, the presence and the tension of right atrium’s contraction etc. CVP is directly affected by the pressures which endues and transmits in right heart (pressure pericardium, mesaflia pressure, pulmonary pressures secondary to mechanical or automatic ventilation, pleural pressure) and for this reason its changes should be analyzed and interpreted in the light of these factors (72).
The importation and use of respiratory artery catheter allows measurement and real-time monitoring beyond CVP, the pressure of pulmonary artery and distension of the balloon on its edge, the pressure on pulmonary capillary wedge. The pulmonary artery pressure is divided into systolic and diastolic in full correspondence with the systemic blood pressure based on timing with electrocardiogram. Producer size is the mean pulmonary artery pressure that is given in full correspondence with the equation [29,30]:
 |
[30] |
The latter has no biological significance but is used to calculate other derived hemodynamic parameters. From the waveforms of pulmonary artery pressure can be drawn on the right ventricular after load (gradient of the wave) and the pulmonary vascular resistances (slope and descent speed of the waveform during the diastolic phase).
The pressure on the wedge of pulmonary capillaries PWCP is the best approach of pressure of left ventricular filling which is used in clinical practice. The most reliable measurements of diastolic filling pressure of the left ventricle is done by catheterization of the left ventricle in the catheterization laboratory, but is far from routine clinical practice. PWCP allows better hemodynamic approach of patients, particularly when these displayed hemodynamic instability (45,73-75). Also the variations of its waveforms enable early recognition phenomena before they have significant effects on hemodynamic state of the patient. The correlation of wedge pressure of pulmonary capillaries (the a-wave) with the end-diastolic pressure of the left ventricle and then the preload must be handled with care because it can lead to false conclusions: pressure depends on both filling volume of a cavity, and its compilation. Therefore, without taking into account the conditions which limit compilation of left ventricle (reducing acids of compilation in ischemia or heart attack and chronic decrease of its compilation at hypertrophy of this) and assessment is reduced only in PWCP, then we can lead to inconsistent in the conclusion of increased filling from a premium value of PWCP (76) while in fact there is sub-filling of the less compliant concavity.
As CVP waveform, so PWCP waveform presents the same adequacies (a, c, v, x, y) in temporal association with the adequacies of the electrocardiogram slightly delayed due to interstitial property blood column from the point of the wedge of the catheter’s edge to the arterial side of the pulmonary vasculature until the corresponding point in the venous limb them. Also the greater length of Swan-Ganz catheter compare to the tube length which carries the waveform of CVP adds an additional little delay on the waveform of PWCP (76).
By adding thermistor at the edge of Swan-Ganz catheter the calculation of cardiac output is permitted by thermo dilution method. Using appropriate monitor and software is able to measure cardiac output in real time with seconds delay minutes for the measured parameters and export the results (77-79).
The change in cardiac output in function of time is illustrated in the monitor’s screen while there is the possibility of printing. With the Swan-Ganz catheter CCO cardiac output and saturation of hemoglobin of mixed venous blood are counted on an ongoing time (SVO2). The latest indication is more sensitive and changes more rapidly than cardiac output.
By measuring cardiac output and the set values to various parameters (height, weight, hemoglobin levels), the software monitor exports results for derivatives size, very useful for the assessment of hemodynamic picture of the patient, and his oxygenation.
Cardiac output is the size which can lead to comparison patients of similar physical characteristics (height, weight). To smooth this difficult to compare between patients with different physical characteristics, the cardiac index CI is used, which is given by the above equation:
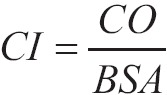 |
[31] |
Where BSA is Body Surface Area, it is provided by special normogrammata and it is counted in m2. Cardiac output CO is counted in lt/min, so the unit of measurement of CI is lt (min·m2).
The stroke volume is the amount of blood forward at every cardiac contractile to the aorta. The sum of the volumes of each pulse in one minute is the cardiac output. Assuming the rhythmic pulse (which implies similar filling of the left heart with blood in each cardiac cycle and the residence of steady equation and stroke volume) and dividing the measured with the Swan-Ganz catheter an average is produced which is the stroke volume:
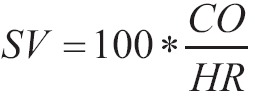 |
[32] |
The unit of measurement is mL/beat.
Where HR is cardiac output (beats/min). If instead of cardiac output we use the cardiac index CI, then the stroke volume will arise:
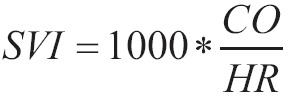 |
[33] |
With unit of measurement mL/(beat*m2).
Systemic vascular conditions are a derivative size which characterizes the resistance to flow of blood from the root of the aorta to the confluence of the vena cava into the right atrium. They are given by the equation [34]:
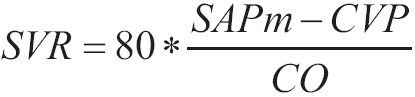 |
[34] |
The unit of measurement is dyn·second/cm5.
Correspondingly with the systemic vascular resistance, pulmonary vascular resistance PVR characterizes the resistance in blood flow from the root of the pulmonary artery to the confluence of the pulmonary veins to the left atrium. PVR are given from the equation [35] and PVRI from the equation [36]:
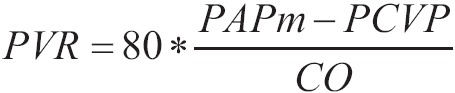 |
[35] |
With unit of measurement dyn*second/cm5.
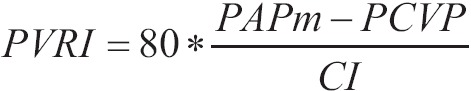 |
[36] |
With unit of measurement dyn*second*m2/cm5.
SVR and SVRI describe the resistance in flow along the arterial and venous limb of the systemic circulation, which are connected in series. The concept of the series connection contains the sum of the individual components that have the corresponding lower sections of series connection, yielding the total resistance of the entire route. PVR, and PVRI describe the resistance in blood flow from the pulmonary arteries to the confluence of the pulmonary veins. Bothe categories of sizes do not regard the difficulty that blood can meet during its movement inside the cavities of right and left heart. This gap is covered by four other derivatives sizes, RVSW, RVSWI, LVSW, LVSWI, which do not describe these resistances via the cardiac cavities, but the work which is paid by every heart in each heartbeat for ejection of blood through cavities in the correspondingly large arterial strain.
LVSWI and RVSWI are referred to the work of every heart in each heartbeat reducible to the body surface. The major values of RVSW and RVSWI, LVSW, LVSWI show that there are big resistances in transcardially promote of blood (e.g., in aortic or pulmonary valve) or a big amount of energy is lost during the extrusion (e.g., in deficiency of mitral or tricuspid valve). On the contrary, low values show that corresponding heart is not able because of pathological reasons to produce enough project to promote adequate cardiac output through its cavity (e.g., dilated cardiomyopathy). These are the equations which describe the above mentioned:
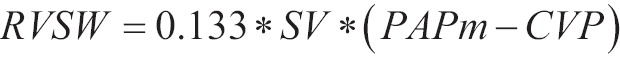 |
[37] |
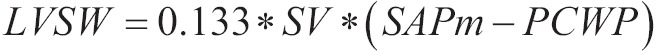 |
[38] |
The unit of measurements in SI system is g*m2/(beat/second2)
Also,
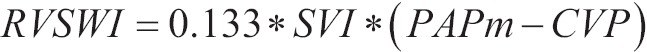 |
[39] |
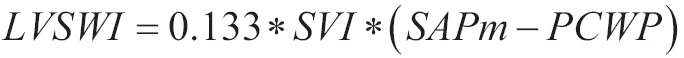 |
[40] |
The unit of measurements in SI system is g/(beat/second2).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Draper WB, Whitehead RW, Spencer JN. Studies on diffusion respiration: alveolar gases and venous blood pH of dogs during diffusion respiration. Anesthesiology 1947;8:524-33 [DOI] [PubMed] [Google Scholar]
- 2.Frumin MJ, Epstein RM, Cohen G. Apneic oxygenation in man. Anesthesiology 1959;20:789-98 [DOI] [PubMed] [Google Scholar]
- 3.Millar RA, Morris ME. Apneic oxygenation in adrenalectomized dogs. Anesthesiology 1961;22:433-9 [DOI] [PubMed] [Google Scholar]
- 4.Fraioli RL, Sheffer LA, Steffenson JL. Pulmonary and cardiovascular effects of apneic oxygenation in man. Anesthesiology 1973;39:588-96 [DOI] [PubMed] [Google Scholar]
- 5.Pesenti A, Kolobow T, Buckhold DK, et al. Prevention of hyaline membrane disease in premature lambs by apneic oxygenation and extracorporeal carbon dioxide removal. Intensive Care Med 1982;8:11-7 [DOI] [PubMed] [Google Scholar]
- 6.Nielsen ND, Kjaergaard B, Koefoed-Nielsen J, et al. Apneic oxygenation combined with extracorporeal arteriovenous carbon dioxide removal provides sufficient gas exchange in experimental lung injury. ASAIO J 2008;54:401-5 [DOI] [PubMed] [Google Scholar]
- 7.Cook TM, Wolf AR, Henderson AJ. Changes in blood-gas tensions during apnoeic oxygenation in paediatric patients. Br J Anaesth 1998;81:338-42 [DOI] [PubMed] [Google Scholar]
- 8.Smyth E, Egan TD. Apneic oxygenation associated with patient-controlled analgesia. J Clin Anesth 1998;10:499-501 [DOI] [PubMed] [Google Scholar]
- 9.McHardy GJ. Diffusing capacity and pulmonary gas exchange. Br J Dis Chest 1972;66:1-20 [PubMed] [Google Scholar]
- 10.Cain SM, Otis AB. Carbon dioxide transport in anesthetized dogs during inhibition of carbonic anhydrase. J Appl Physiol 1961;16:1023-8 [DOI] [PubMed] [Google Scholar]
- 11.Kanthakumar P, Oommen V.A simple model to demonstrate perfusion and diffusion limitation of gases. Adv Physiol Educ 2012;36:352-5 [DOI] [PubMed] [Google Scholar]
- 12.Vance JW. Stores of carbon dioxide in emphysema. Dis Chest 1966;49:147-54 [DOI] [PubMed] [Google Scholar]
- 13.Leigh MD, Jenkins LC, Belton MK, et al. Continuous alveolar carbon dioxide analysis as a monitor of pulmonary blood flow. Anesthesiology 1957;18:878-82 [DOI] [PubMed] [Google Scholar]
- 14.Rigg CD, Cruickshank S. Carbon dioxide during and after the apnoea test--an illustration of the Haldane effect. Anaesthesia 2001;56:377. [DOI] [PubMed] [Google Scholar]
- 15.Stock MC, Downs JB, McDonald JS, et al. The carbon dioxide rate of rise in awake apneic humans. J Clin Anesth 1988;1:96-103 [DOI] [PubMed] [Google Scholar]
- 16.Payne JP. Apnoeic oxygenation in anaesthetised man. Acta Anaesthesiol Scand 1962;6:129-42 [DOI] [PubMed] [Google Scholar]
- 17.Benditt J, Pollock M, Roa J, et al. Transtracheal delivery of gas decreases the oxygen cost of breathing. Am Rev Respir Dis 1993;147:1207-10 [DOI] [PubMed] [Google Scholar]
- 18.Nahum A, Burke WC, Ravenscraft SA, et al. Lung mechanics and gas exchange during pressure-control ventilation in dogs. Augmentation of CO2 elimination by an intratracheal catheter. Am Rev Respir Dis 1992;146:965-73 [DOI] [PubMed] [Google Scholar]
- 19.Stam H, Hrachovina V, Stijnen T, et al. Diffusing capacity dependent on lung volume and age in normal subjects. J Appl Physiol (1985) 1994;76:2356-63 [DOI] [PubMed] [Google Scholar]
- 20.Cotes JE, Chinn DJ, Quanjer PH, et al. Standardization of the measurement of transfer factor (diffusing capacity). Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993;16:41-52 [PubMed] [Google Scholar]
- 21.Cotton DJ, Soparkar GR, Grahan BL. Diffusing capacity in the clinical assessment of chronic airflow limitation. Med Clin North Am 1996;80:549-64 [DOI] [PubMed] [Google Scholar]
- 22.Puri S, Baker BL, Dutka DP, et al. Reduced alveolar-capillary membrane diffusing capacity in chronic heart failure. Its pathophysiological relevance and relationship to exercise performance. Circulation 1995;91:2769-74 [DOI] [PubMed] [Google Scholar]
- 23.Neas LM, Schwartz J. The determinants of pulmonary diffusing capacity in a national sample of U.S. adults. Am J Respir Crit Care Med 1996;153:656-64 [DOI] [PubMed] [Google Scholar]
- 24.Farag A.Use of the Hagen-Poiseuille law: a new mathematical approach for the integration and evaluation of anorectal physiological testing in patients with faecal incontinence and pelvic dyschezia and in normal controls. Eur Surg Res 1998;30:279-89 [DOI] [PubMed] [Google Scholar]
- 25.Morgan HG. Acid-base balance in blood. Br J Anaesth 1969;41:196-212 [DOI] [PubMed] [Google Scholar]
- 26.Brian JE., Jr Carbon dioxide and the cerebral circulation. Anesthesiology 1998;88:1365-86 [DOI] [PubMed] [Google Scholar]
- 27.Wilkinson IM, Browne DR. The influence of anaesthesia and of arterial hypocapnia on regional blood flow in the normal human cerebral hemisphere. Br J Anaesth 1970;42:472-82 [DOI] [PubMed] [Google Scholar]
- 28.Najarian T, Marrache AM, Dumont I, et al. Prolonged hypercapnia-evoked cerebral hyperemia via K(+) channel- and prostaglandin E(2)-dependent endothelial nitric oxide synthase induction. Circ Res 2000;87:1149-56 [DOI] [PubMed] [Google Scholar]
- 29.Refsum HE. Relationship between state of consciousness and arterial hypoxaemia and hypercapnia in patients with pulmonary insufficiency, breathing air. Clin Sci 1963;25:361-7 [PubMed] [Google Scholar]
- 30.Leake CD. A vasopressor local anesthetic. Science 1937;85:242-3 [DOI] [PubMed] [Google Scholar]
- 31.Potkin RT, Swenson ER. Resuscitation from severe acute hypercapnia. Determinants of tolerance and survival. Chest 1992;102:1742-5 [DOI] [PubMed] [Google Scholar]
- 32.Millar RA. Plasma adrenaline and noradrenaline during diffusion respiration. J Physiol 1960;150:79-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sechzer PH, Egbert LD, Linde HW, et al. Effect of carbon dioxide inhalation on arterial pressure, ECG and plasma catecholamines and 17-OH corticosteroids in normal man. J Appl Physiol 1960;15:454-8 [DOI] [PubMed] [Google Scholar]
- 34.Cullen DJ, Eger EI., 2nd Cardiovascular effects of carbon dioxide in man. Anesthesiology 1974;41:345-9 [DOI] [PubMed] [Google Scholar]
- 35.Kiely DG, Cargill RI, Lipworth BJ. Effects of hypercapnia on hemodynamic, inotropic, lusitropic, and electrophysiologic indices in humans. Chest 1996;109:1215-21 [DOI] [PubMed] [Google Scholar]
- 36.Heintz A, Damm M, Brand M, et al. Coronary flow regulation in mouse heart during hypercapnic acidosis: role of NO and its compensation during eNOS impairment. Cardiovasc Res 2008;77:188-96 [DOI] [PubMed] [Google Scholar]
- 37.Goldstein B, Shannon DC, Todres ID. Supercarbia in children: clinical course and outcome. Crit Care Med 1990;18:166-8 [DOI] [PubMed] [Google Scholar]
- 38.Slinger P, Blundell PE, Metcalf IR. Management of massive grain aspiration. Anesthesiology 1997;87:993-5 [DOI] [PubMed] [Google Scholar]
- 39.Moudgil R, Michelakis ED, Archer SL. Hypoxic pulmonary vasoconstriction. J Appl Physiol (1985) 2005;98:390-403 [DOI] [PubMed] [Google Scholar]
- 40.Barer GR, Howard P, McCurrie JR. The effect of carbon dioxide and changes in blood pH on pulmonary vascular resistance in cats. Clin Sci 1967;32:361-76 [PubMed] [Google Scholar]
- 41.Domino KB, Swenson ER, Hlastala MP. Hypocapnia-induced ventilation/perfusion mismatch: a direct CO2 or pH-mediated effect? Am J Respir Crit Care Med 1995;152:1534-9 [DOI] [PubMed] [Google Scholar]
- 42.Loeppky JA, Scotto P, Riedel CE, et al. Effects of acid-base status on acute hypoxic pulmonary vasoconstriction and gas exchange. J Appl Physiol (1985) 1992;72:1787-97 [DOI] [PubMed] [Google Scholar]
- 43.Lynch F, Sweeney M, O'Regan RG, et al. Hypercapnia-induced contraction in isolated pulmonary arteries is endothelium-dependent. Respir Physiol 2000;121:65-74 [DOI] [PubMed] [Google Scholar]
- 44.Marshall C, Marshall B.Site and sensitivity for stimulation of hypoxic pulmonary vasoconstriction. J Appl Physiol Respir Environ Exerc Physiol 1983;55:711-6 [DOI] [PubMed] [Google Scholar]
- 45.O’Quin R, Marini JJ. Pulmonary artery occlusion pressure: clinical physiology, measurement, and interpretation. Am Rev Respir Dis 1983;128:319-26 [DOI] [PubMed] [Google Scholar]
- 46.Tuman KJ, Carroll GC, Ivankovich AD. Pitfalls in interpretation of pulmonary artery catheter data. J Cardiothorac Anesth 1989;3:625-41 [DOI] [PubMed] [Google Scholar]
- 47.Frazier SK. Cardiovascular effects of mechanical ventilation and weaning. Nurs Clin North Am 2008;43:1-15; v. [DOI] [PubMed] [Google Scholar]
- 48.Lee SC. Improvement of gas exchange by apneic oxygenation with nasal prong during fiberoptic intubation in fully relaxed patients. J Korean Med Sci 1998;13:582-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taha SK, Siddik-Sayyid SM, El-Khatib MF, et al. Nasopharyngeal oxygen insufflation following pre-oxygenation using the four deep breath technique. Anaesthesia 2006;61:427-30 [DOI] [PubMed] [Google Scholar]
- 50.Belsh JM, Blatt R, Schiffman PL. Apnea testing in brain death. Arch Intern Med 1986;146:2385-8 [PubMed] [Google Scholar]
- 51.Wijdicks EF. The diagnosis of brain death. N Engl J Med 2001;344:1215-21 [DOI] [PubMed] [Google Scholar]
- 52.Nakagawa TA, Ashwal S, Mathur M, et al. Guidelines for the determination of brain death in infants and children: an update of the 1987 task force recommendations-executive summary. Ann Neurol 2012;71:573-85 [DOI] [PubMed] [Google Scholar]
- 53.Go T, Altmayer M, Richter M, et al. Decompressing manubriectomy under apneic oxygenation to release the median thoracic outlet compartment in Bechterew disease. J Thorac Cardiovasc Surg 2003;126:867-9 [DOI] [PubMed] [Google Scholar]
- 54.Watson RJ, Szarko R, Mackenzie CF, et al. Continuous endobronchial insufflation during internal mammary artery harvest. Anesth Analg 1992;75:219-25 [DOI] [PubMed] [Google Scholar]
- 55.Marini JJ. Phasic expiratory tracheal gas insufflation: short but sweet. Crit Care Med 1998;26:825-6 [DOI] [PubMed] [Google Scholar]
- 56.Pinsky MR, Delgado E, Hete B. The effect of tracheal gas insufflation on gas exchange efficiency. Anesth Analg 2006;103:1213-8 [DOI] [PubMed] [Google Scholar]
- 57.Olguner C, Koca U, Akan M, et al. The safe limits of mechanical factors in the apnea testing for the diagnosis of brain death. Tohoku J Exp Med 2007;211:115-20 [DOI] [PubMed] [Google Scholar]
- 58.Burns JD, Russell JA. Tension pneumothorax complicating apnea testing during brain death evaluation. J Clin Neurosci 2008;15:580-2 [DOI] [PubMed] [Google Scholar]
- 59.Bar-Joseph G, Bar-Lavie Y, Zonis Z.Tension pneumothorax during apnea testing for the determination of brain death. Anesthesiology 1998;89:1250-1 [DOI] [PubMed] [Google Scholar]
- 60.Marks SJ, Zisfein J. Apneic oxygenation in apnea tests for brain death. A controlled trial. Arch Neurol 1990;47:1066-8 [DOI] [PubMed] [Google Scholar]
- 61.Gunnarsson L, Tokics L, Gustavsson H, et al. Influence of age on atelectasis formation and gas exchange impairment during general anaesthesia. Br J Anaesth 1991;66:423-32 [DOI] [PubMed] [Google Scholar]
- 62.Hedenstierna G, Strandberg A, Brismar B, et al. Functional residual capacity, thoracoabdominal dimensions, and central blood volume during general anesthesia with muscle paralysis and mechanical ventilation. Anesthesiology 1985;62:247-54 [DOI] [PubMed] [Google Scholar]
- 63.Reber A, Nylund U, Hedenstierna G.Position and shape of the diaphragm: implications for atelectasis formation. Anaesthesia 1998;53:1054-61 [DOI] [PubMed] [Google Scholar]
- 64.Muller N, Volgyesi G, Becker L, et al. Diaphragmatic muscle tone. J Appl Physiol Respir Environ Exerc Physiol 1979;47:279-84 [DOI] [PubMed] [Google Scholar]
- 65.Froese AB, Bryan AC. Effects of anesthesia and paralysis on diaphragmatic mechanics in man. Anesthesiology 1974;41:242-55 [DOI] [PubMed] [Google Scholar]
- 66.Edmark L, Kostova-Aherdan K, Enlund M, et al. Optimal oxygen concentration during induction of general anesthesia. Anesthesiology 2003;98:28-33 [DOI] [PubMed] [Google Scholar]
- 67.Rothen HU, Sporre B, Engberg G, et al. Influence of gas composition on recurrence of atelectasis after a reexpansion maneuver during general anesthesia. Anesthesiology 1995;82:832-42 [DOI] [PubMed] [Google Scholar]
- 68.Benoît Z, Wicky S, Fischer JF, et al. The effect of increased FIO(2) before tracheal extubation on postoperative atelectasis. Anesth Analg 2002;95:1777-81, table of contents. [DOI] [PubMed] [Google Scholar]
- 69.Mach WJ, Thimmesch AR, Pierce JT, et al. Consequences of hyperoxia and the toxicity of oxygen in the lung. Nurs Res Pract 2011;2011:260482. [DOI] [PMC free article] [PubMed]
- 70.Kelleher JF. Pulse oximetry. J Clin Monit 1989;5:37-62 [DOI] [PubMed] [Google Scholar]
- 71.Ochagavia A, Baigorri F, Mesquida J, et al. Hemodynamic monitoring in the critically patient. Recomendations of the Cardiological Intensive Care and CPR Working Group of the Spanish Society of Intensive Care and Coronary Units. Med Intensiva. 2013 doi: 10.1016/j.medin.2013.10.006. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 72.Boghossian E, Clement R, Redpath M, et al. Respiratory, circulatory, and neurological responses to hanging: a review of animal models. J Forensic Sci 2010;55:1272-7 [DOI] [PubMed] [Google Scholar]
- 73.Hellems HK, Haynes FW, Dexter L. Pulmonary capillary pressure in man. J Appl Physiol 1949;2:24-9 [DOI] [PubMed] [Google Scholar]
- 74.Calvin JE, Driedger AA, Sibbald WJ. Does the pulmonary capillary wedge pressure predict left ventricular preload in critically ill patients? Crit Care Med 1981;9:437-43 [DOI] [PubMed] [Google Scholar]
- 75.Raper R, Sibbald WJ. Misled by the wedge? The Swan-Ganz catheter and left ventricular preload. Chest 1986;89:427-34 [DOI] [PubMed] [Google Scholar]
- 76.Finkelhor RS, Scrocco JD, Madmani M, et al. Discordant doppler right heart catheterization pulmonary artery systolic pressures: importance of pulmonary capillary wedge pressure. Echocardiography 2014;31:279-84 [DOI] [PubMed] [Google Scholar]
- 77.Boldt J, Menges T, Wollbruck M, et al. Is continuous cardiac output measurement using thermodilution reliable in the critically ill patient? Crit Care Med 1994;22:1913-8 [PubMed] [Google Scholar]
- 78.Haller M, Zollner C, Briegel J, et al. Evaluation of a new continuous thermodilution cardiac output monitor in critically ill patients: a prospective criterion standard study. Crit Care Med 1995;23:860-6 [DOI] [PubMed] [Google Scholar]
- 79.Mihaljevic T, von Segesser LK, Tonz M, et al. Continuous versus bolus thermodilution cardiac output measurements--a comparative study. Crit Care Med 1995;23:944-9 [DOI] [PubMed] [Google Scholar]