Abstract
Functional bowel disorders, including irritable bowel syndrome (IBS), are common disorders that have a significant impact on patients’ quality of life. These disorders present major challenges to healthcare providers, as few effective medical therapies are currently available. Recently, there has been increasing interest in dietary therapies for IBS, particularly a diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs). Since ingestion of FODMAPs increases the delivery of readily fermentable substrates and water to the distal small intestine and colon—which results in luminal distention and gas—the reduction of FODMAPs in a patient’s diet may improve functional gastrointestinal symptoms. This paper will review the pathophysiology of IBS and the role of FODMAPs for the treatment of this condition.
Keywords: Irritable bowel syndrome, FODMAPs, small intestinal bacterial overgrowth, food allergy, food intolerance, hydrogen breath testing
Irritable bowel syndrome (IBS) is a chronic, often disabling, functional disorder characterized by abdominal pain and changes in bowel habits.1 The prevalence of IBS in the US general population varies between 8% and 20% depending on diagnostic criteria and the population that is evaluated.2 Most studies report a higher prevalence of IBS in women than men.3 The average medical expenditure for IBS in the United States is estimated to be $ 1.35 billion in direct costs and $205 million in indirect costs.4 IBS also accounts for almost half of all visits to gastroenterologists.
The pathophysiology of IBS is incompletely understood, and treatment options are limited, partly due to the heterogeneity of the IBS population.5 Nearly two thirds of IBS patients report that their symptoms are related to food.6 The pathogenic mechanism by which food induces IBS symptoms remains unclear, but it includes visceral hypersensitivity, altered motility, abnormal colonic fermentation, and sugar malabsorption, all of which lead to increased gas production and luminal distention.7 The use of elimination diets for the treatment of IBS has yielded conflicting results, although this treatment option has been slightly more successful in IBS patients who have diarrhea.8 However, elimination diets can result in dietary restrictions that can be burdensome to patients and can potentially compromise their nutritional health. In addition, there is a lack of randomized controlled data that show a symptomatic benefit with elimination diets.9
Recently, interest has focused on diets that reduce intake of poorly absorbed, small molecule—sized carbohydrates. These types of carbohydrates are fermented by intestinal bacteria, which produces gas and osmotically active byproducts, causing an increase in fluid in the intestines. The acronym FODMAPs (which stands for fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) was developed to describe these poorly absorbed, short-chain carbohydrates.10 Observational studies have shown that the restriction of FODMAPs in the diet alleviates gastrointestinal symptoms in patients with IBS. Therefore, a low-FODMAP diet represents an opportunity for treatment in these patients. The aim of this paper is to review the pathophysiology of IBS, the current evidence-based literature in this area, and the application of a low-FODMAP diet for treatment of IBS patients. The role of diet in functional bowel disorders such as IBS has become a popular area of interest, given the frequent association of symptoms and foods, as well as the limited availability of effective and safe pharmacologic therapies.
Pathophysiology
Small Intestinal Bacterial Overgrowth in Irritable Bowel Syndrome Patients
Small intestinal bacterial overgrowth (SIBO) is the abnormal growth in the small intestine of bacteria that are normally found only in the colon. The stomach and proximal small bowel (the duodenum and jejunum) normally contain few bacteria (usually <104 colony-forming units per milliliter [CFU/mL]). In contrast, the terminal ileum has significantly more anaerobic bacteria (as high as 109 CFU/mL), and the colon has even more bacteria (as high as 1012 CFU/mL).11 In SIBO patients, the concentration of bacterial flora increases proximally (≥105 CFU/mL).12
Gastric acid and small bowel peristalsis are important mechanisms for the prevention of SIBO. Low gastric pH is an effective antimicrobial agent, as it kills bacteria and suppresses their growth. Likewise, intestinal motility (via the migrating motor complex) has a cleansing effect and prevents excess bacteria from colonizing the small bowel. Conditions that affect these mechanisms—such as scleroderma, hypothyroidism, diabetes, and potentially IBS—can result in SIBO.11 SIBO secondary to impaired motility has different effects than IBS, in which the gut has no structural or functional disruptions.
SIBO can cause a wide range of symptoms, including those consistent with IBS. Commonly, patients with SIBO experience nausea, abdominal cramping, bloating, flatus, and diarrhea. Patients with more severe disease can experience malabsorption due to the inflammatory effects of bacteria on small bowel mucosa. For example, macrocytic anemia can result from vitamin B12 deficiency, while hypocalcemia can result from vitamin D deficiency.13 Children with SIBO are susceptible to more severe disease; they may develop malnutrition and/or steatorrhea and may have difficulty maintaining their weight and growth.14
SIBO can be diagnosed via several methods.15 The gold standard for diagnosis is a jejunal aspirate with at least 105 CFU/mL of bacteria. Several endoscopic techniques can be used to sample the contents of the small bowel. Classically, the jejunum is intubated under fluoroscopic guidance, but this method has fallen out of practice due to its invasive nature and the possibility for contamination of the aspirate by Gram-positive organisms in the oropharyngeal flora. The reproducibility of the culture technique has also been shown to be suboptimal (<38% vs 92% for breath testing). In addition, the criteria commonly used to diagnose SIBO (≥105 CFU/mL of bacteria)—which were proposed by Reid and colleagues—have not been validated.16 A systematic review by Khoshini and associates found that there was no adequately validated diagnostic test for SIBO.12 The researchers also suggested that there was a lack of evidence to justify the use of culture as the gold standard test for SIBO.12
Given these limitations, noninvasive and less expensive tests such as breath testing are more commonly used for diagnosing SIBO. Breath testing is based on the premise that bacteria are the sole producers of intestinal hydrogen, some of which is exhaled. Therefore, testing can measure the amount of hydrogen gas that is produced when a fixed dose of a substrate (ie, a carbohydrate) is encountered by bacteria in the bowel. The most commonly used substrates are glucose and lactulose. Glucose is absorbed in the first 3 ft of the small intestine; therefore, it is only capable of detecting SIBO in the proximal small bowel. An increase of at least 12 parts per million at 120 minutes after ingestion is generally considered to be a positive test result for SIBO.11 In contrast, lactulose is a nonabsorbable carbohydrate that is eventually fermented by colonic bacteria. The diagnosis of SIBO via the lactulose breath hydrogen test (LBHT) is based on the following criteria: The first peak is caused by the production of gas due to bacterial overgrowth in the small bowel, and the second peak results from the action of colonic bacteria on lactulose.17 The LBHT has a higher specificity compared to the glucose hydrogen breath test (~86% vs ~80%, respectively), but the former has lower sensitivity and accuracy.18
Many, but not all, studies have shown that patients with IBS have abnormal LBHT results, which suggests that SIBO may be involved in the pathogenesis of IBS. This association was first reported by Pimentel and coworkers in 2003.19 In this study of 111 IBS patients, approximately 84% had an abnormal LBHT result compared to only 20% of healthy subjects (n=15). Patients who received neomycin had a 35% improvement in symptoms compared to a 11.4% improvement in patients who received placebo. Importantly, normalization of LBHT results was associated with the use of neomycin in patients who reported improvement in symptoms (n=8). This study also suggested that excessive breath methane on a LBHT was associated with constipation-predominant IBS.19
In 2009, Ford and colleagues conducted a systematic review and meta-analysis of 12 studies (a total of 1,921 subjects) that met the following inclusion criteria: case series or case-control design; adults with a presumed diagnosis of IBS; participants not specially selected; tests for SIBO given to all patients with their results recorded; and more than 90 subjects in each study.20 The authors concluded that the likelihood of having a positive test result for SIBO was increased 3—5-fold in IBS patients compared to healthy controls, although this finding was not statistically significant. In addition, this finding was independent of the type of test used.20 Although IBS patients appear to have an increased rate of positive breath test results, the accuracy and interpretation of breath testing in these patients are not entirely clear. Therefore, the exact role of SIBO in the pathophysiology of IBS remains controversial.
Food Allergy and Intolerance
True food allergy caused by immunoglobulin (Ig) E— mediated type 1 hypersensitivity is rare in adults, occurring in only 1-2% of the adult population. Although food allergy symptoms may include pruritus, erythema, urticaria, angioedema, eczema, and rhinitis, symptoms may be limited to the gastrointestinal tract and consist of nausea, vomiting, bloating, pain, diarrhea, and edema of the lips and tongue. Most true food allergies occur in children, particularly infants. Over 90% of food allergies are caused by eggs, peanuts, milk, soy, nuts, shellfish, fish, or wheat. Food hypersensitivity is suggestive of an underlying allergy or atopy to specific components in food products. The innate and adaptive immune systems of the gut act as active barriers to foreign antigens. Therefore, maintaining intestinal permeability is crucial for preventing the development of food allergies.7 There is little evidence to suggest that the classical IgE-mediated type 1 hypersensitivity reaction plays a role in the pathogenesis of IBS. In addition, an IgE response to dietary antigens may be localized to bowel mucosa and, therefore, may not correlate with serum antibody levels.
Although skin-prick testing can be helpful for identifying systemic responses to food antigens, the utility of this test in IBS patients is dubious.21 In a study of 88 patients with gastrointestinal symptoms that were thought to be caused by a food allergy, only 15 patients had reproducible symptoms in a double-blind, placebo-controlled (DBPC) trial, and none of the patients had a positive skin-prick test result or a positive radioallergo-sorbent test score for the food that reproduced the symptoms.22 In a study of 81 patients with IBS symptoms that were thought to be caused by a food allergy, 48 patients had a positive skin-prick test result. However, there was little consistency between the food that reportedly caused the adverse reaction and the food that produced a positive skin-prick test result.23
Bischoff and colleagues utilized the colonoscopic allergen provocation (COLAP) test to examine patients’ responses to food antigens that were injected into the submucosa of the colon.24 In this study, 70 patients with chronic abdominal symptoms and suspected food allergies underwent COLAP testing. A positive COLAP test result was found in 77% of individuals with chronic abdominal pain, of whom 74% had a suspected diagnosis of IBS. Biopsies from the response site revealed an increased number of mast cells and eosinophils. Once the suspected foods were eliminated from the patients’ diets, 83% reported improvement in their symptoms. These patients had normal skin-prick test results and normal serum levels of IgE antibodies to common food antigens.24 Although the COLAP test appears to be promising, further studies are clearly needed to corroborate these findings.
IgG antibodies—specifically subclass 4, which usually provides a delayed response following exposure to an antigen—have also been implicated in food hypersensitivity.25 Although food hypersensitivity may be associated with IBS, the current data on this issue are limited and, therefore, difficult to apply in the clinical setting.
Most elimination diets remove the foods that are most commonly associated with adverse reactions in IBS patients, as well as any foods thought to provoke symptoms, for at least 14 days. Patients who respond to the elimination diet are then gradually reintroduced to individual foods to determine whether symptoms recur. The resolution of symptoms suggests, but does not confirm, a causal relationship between the food and IBS. A DBPC food challenge is needed to establish an association. However, DBPC food challenges are rarely performed in clinical practice. The response rate to elimination diets in IBS patients ranges from 15% to 71%.8 IBS patients with diarrhea-predominant symptoms have the greatest number of adverse food reactions and the highest response rates to elimination diets. However, all studies performed to date have had major limitations in their trial designs, including patient selection, the appropriateness and duration of elimination diets, and the methods of food challenge. Therefore, no definitive conclusions can be made regarding the effectiveness of elimination diets for treating IBS patients.
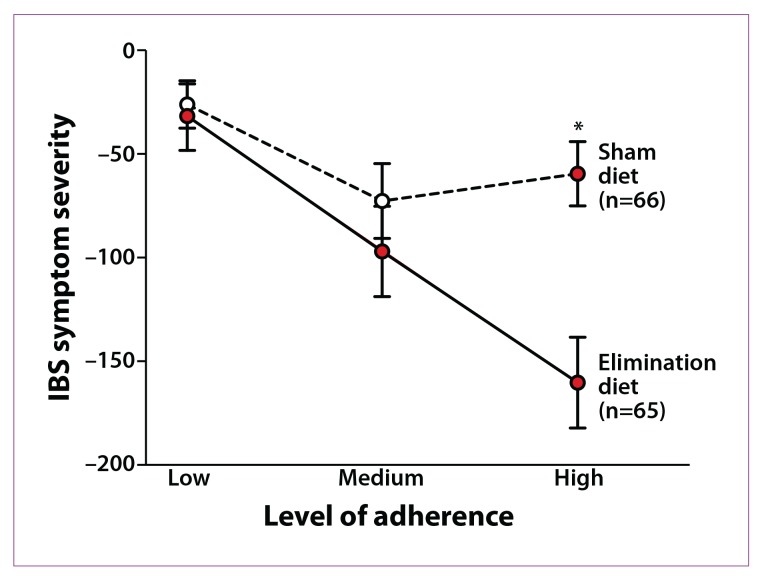
An interesting study evaluated the efficacy of an elimination diet based on the presence of IgG antibodies to food. In this study, IgG antibody levels to 29 food antigens were measured. IBS patients were randomized to either an elimination diet based on their true sensitivity results (ie, elevated IgG antibody levels to the food antigen) or a “sham” diet (in which the same number of foods were excluded but not the foods to which the patients had antibodies). After 12 weeks, the elimination diet resulted in a 10% greater reduction in symptom score than the sham diet, and this finding increased to 26% in fully compliant patients (Figure 1).26 However, the elimination and sham diets were not properly matched, which was a limitation of the study.
Figure 1.
Mean change in irritable bowel syndrome (IBS) symptom severity scores at 12 weeks according to degree of adherence to an elimination diet. The difference between the treatment and control groups with high adherence to the elimination diet is 101 units (95% confidence interval, 54-147).
*P<.001.
Reproduced from Atkinson W, Sheldon TA, Shaath N, Whorwell PJ.26
Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols
Definition
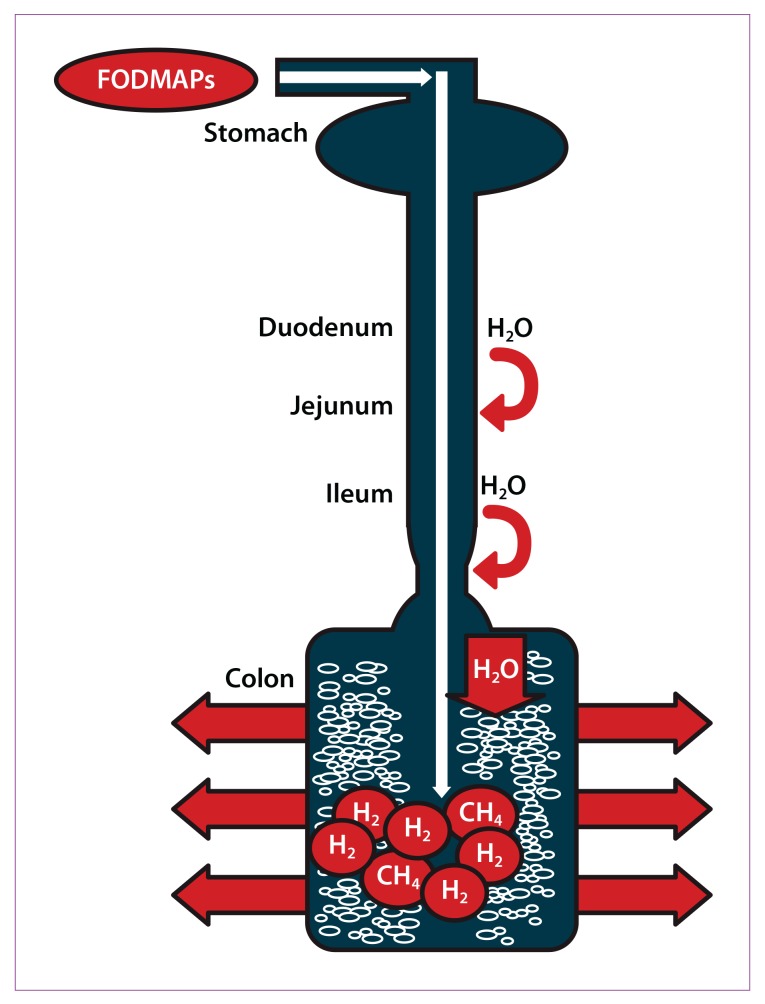
The acronym FODMAPs was created to describe poorly absorbed, short-chain carbohydrates that can lead to excessive fluid and gas accumulation, resulting in bloating, abdominal pain, and distention (Figure 2). FODMAPs are found in a wide variety of foods, including those containing lactose, fructose in excess of glucose, fructans, galacto-oligosaccharides, and polyols (sorbitol, mannitol, xylitol, and maltitol). All FODMAPs have poor absorption and rapid fermentation, and they are comprised of small, osmotically active molecules. FODMAPs are poorly absorbed for a number of reasons, including the absence of luminal enzymes capable of hydrolyzing the glycosidic bonds contained in carbohydrates, the absence or low activity of brush border enzymes (eg, lactase), or the presence of low-capacity epithelial transporters (fructose, glucose transporter 2 [GLUT-2], and glucose transporter 5 [GLUT-5]). Fructose, which is an important FODMAP in the Western diet, is absorbed across villous epithelium through low-capacity, carrier-mediated diffusion involving GLUT-5. The absorption of free fructose is markedly enhanced in the presence of glucose via GLUT-2. Therefore, if fructose is present in excess of glucose, the risk of fructose malabsorption is increased. In addition, some molecules, such as polyols, are too large for simple diffusion. The fermentation rate is determined by the chain length of the carbohydrate.27
Figure 2.
Ingested fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) are poorly absorbed in the small intestine. Their small molecular size results in an osmotic effect, drawing water (H20) through to the large intestine. FODMAPs are then fermented by colonic microflora, producing hydrogen (H2) and/or methane gas (CH4). The increase in fluid and gas leads to diarrhea, bloating, flatulence, abdominal pain, and distension.
Reproduced from Barrett JS, Gearry RB, Muir JG, et al.10
For example, oligosaccharides are rapidly fermented, compared to polysaccharides. Fermentation results in the production of carbon dioxide, hydrogen, and/or methane gas. Finally, small, osmotically active molecules draw more water and other liquid into the small bowel. Given these properties, a diet low in FODMAPs has become a potential therapy for IBS patients.
Examination of the Low-FODMAP Diet
To date, few studies have evaluated the efficacy of a low-FODMAP diet for treating IBS and other gastrointestinal symptoms. Before the use of low-FODMAP diets, early observational studies examined the role of sugar malabsorption in patients with functional gut symptoms. For example, a study by Fernandez-Banares and associates evaluated 36 patients with functional abdominal bloating and gas-related symptoms via hydrogen breath tests to assess for lactose and/or fructose-plus-sorbitol malabsorption.28 Of the 26 patients (72.2%) who had evidence of sugar malabsorption, 17 patients (65%) had symptoms of sugar intolerance during the 3-hour breath testing period. Eighty-one percent of patients experienced clinical improvement after 1 month on a lactose-free diet and/or a fructose-plus-sorbitol—free diet; at 12 months, 67% of patients experienced clinical improvement, with complete improvement in 50% of patients and partial improvement in 16.7% of patients.28 Although this study lacked a control group, it supports the potential role of carbohydrate malabsorption in at least a subset of patients with functional bowel disorders.
A more recent randomized, placebo-controlled trial by Shepherd and coworkers sought to determine whether the efficacy of a low-FODMAP diet was primarily due to a reduction in fructose or to a reduction in poorly absorbed, short-chain carbohydrates in general.29 This study was a DBPC, randomized, quadruple-arm, rechallenge trial. Twenty-five patients with IBS (based on Rome II criteria) had evidence of fructose malabsorption on fructose breath tests and experienced improvement in symptoms while on a low-FODMAP diet. Patients were recruited from a hospital-based dietetic practice that served the community. Patients were randomly challenged by graded-dose introduction of fructose and fructans (alone or in combination) or glucose (ie, placebo), which were administered as drinks with meals for a maximum test period of 2 weeks (with at least a 10-day washout period between test periods). Patients continued the low-FODMAP diet during the washout period and were not permitted to move to the next phase until their symptoms had returned to baseline levels. Inadequate symptom control was reported by 70% of patients who received fructose, 77% of patients who received fructans, and 79% of patients who received a combination of fructose and fructans, compared to 14% of patients who received glucose. All IBS symptoms were significantly greater in patients who received fructose, fructans, or a combination of the 2 foods, compared to glucose. There was also a dose-dependent response, with worsening of IBS symptoms as the dose of fructose, fructans, or the combination increased.29 This study provided strong evidence that FODMAPs can induce symptoms in at least a subset of IBS patients (ie, patients who have fructose malabsorption and respond to a low-FODMAP diet).
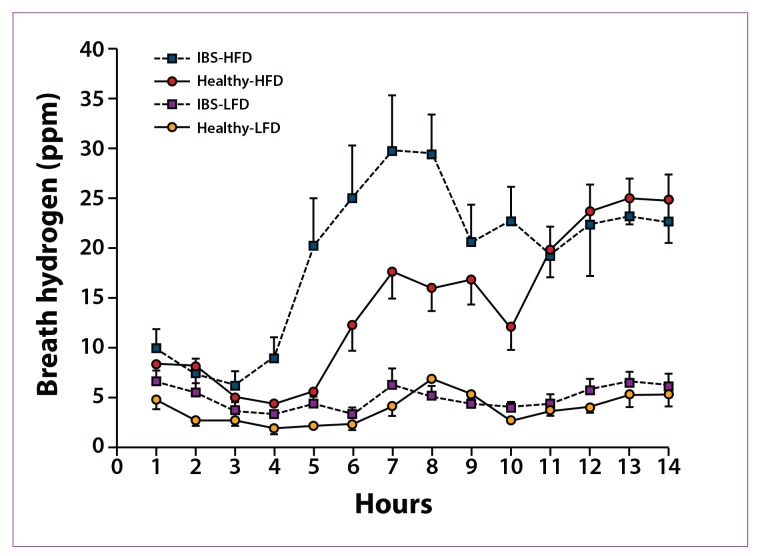
Ong and colleagues examined the effects of a low-FODMAP diet on hydrogen gas production in IBS patients.30 In this study, 15 healthy controls and 15 IBS patients (based on Rome III criteria) underwent breath testing while on a low-FODMAP diet (9 g of FODMAPs) or a high-FODMAP diet (50 g of FODMAPs) for 2 days. Following a 7-day washout period, patients were crossed over to the other diet. IBS patients on a high-FODMAP diet produced a significantly greater amount of hydrogen gas compared to IBS patients on a low-FODMAP diet (Figure 3). In addition, compared to healthy controls, IBS patients produced more hydrogen gas, regardless of their diet. Interestingly, significantly less hydrogen gas was produced in healthy controls on a high-FODMAP diet compared to those on a low-FODMAP diet (P=.043); in contrast, patients with IBS had no change in methane production with either diet. IBS symptoms significantly worsened with a high-FODMAP diet. A composite IBS symptom score that included the most commonly reported IBS symptoms was significantly higher for IBS patients on a high-FODMAP diet (P=.002).30 This study showed that the ingestion of FODMAPs leads to prolonged intestinal hydrogen production in healthy controls as well as in IBS patients in whom gastrointestinal and systemic symptoms were induced.
Figure 3.
Profiles of breath hydrogen production over 14 hours of each dietary period in healthy subjects and patients with irritable bowel syndrome (IBS) on high-FODMAP (fermentable oligosaccharide, disaccharide, monosaccharide, and polyol) diets (HFD) and low-FODMAP diets (LFD). Total breath hydrogen was significantly greater in patients on the HFD in both groups (P<.0001). Patients with IBS produced significantly more breath hydrogen over the 14-hour period than healthy controls.
ppm=parts per million.
Reproduced from Ong DK, Mitchell SB, Barrett JS, et al.30
Finally, Staudacher and colleagues randomized 82 consecutive IBS patients from a dietary clinic to either a standard diet or a low-FODMAP diet for 9 months.31 Patients on a low-FODMAP diet reported greater symptomatic improvement—with significant improvement in bloating, abdominal pain, and flatulence—compared to patients on a standard diet. Seventy-six percent of patients on a low-FODMAP diet reported satisfaction with their symptom response compared to 54% of patients on a standard diet. However, there were several limitations to this study. Because patients were seen by different dieticians, differences in the communication and style of dietary education may have affected patient adherence. In addition, although symptom response was recorded prospectively, a questionnaire was completed at the time of the consultation between the patient and the dietician, which may have resulted in bias.31
Taken together, these studies suggest that a low-FODMAP diet may result in significant symptom improvement in at least a subset of IBS patients; however, additional controlled clinical trials are warranted.
Clinical Application of the Low-FODMAP Diet
Despite limited data, implementation of a low-FODMAP diet should be considered in patients with IBS, particularly those in whom food is a trigger for symptoms. Implementation of a low-FODMAP diet is best done with the help of a dietician during a one-to-one consultation so that the dietician can understand the patient’s symptoms; this process also ensures that any diet modifications are individualized and still provide a balanced diet. A diet history should be taken to determine the composition of FODMAPs in an individuals diet. Education can then be tailored appropriately rather than focusing on FODMAPs that may never be consumed. Lists of food substitutions can help patients understand what they can and cannot eat (Table 1).32 For good symptom control, individuals should restrict their total FODMAP load for 6-8 weeks. After this time, the diet may be modified to be less restrictive based on symptom response.
Table 1.
Foods High in Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols (FODMAPs) and Suitable Alternatives
| FODMAP | Foods high in FODMAPs | Suitable alternatives low in FODMAPs |
|---|---|---|
| Excess fructose | Fruits: apple, clingstone peach, mango, nashi pear, pear, sugar snap pea, tinned fruit in natural juice, watermelon | Fruits: banana, blueberry, cantaloupe, carambola, durian, grape, grapefruit, honeydew melon, kiwi, lemon, lime, orange, passion fruit, pawpaw, raspberry, strawberry, tangelo |
| Honey sweeteners: fructose, high-fructose corn syrup | Honey substitutes: golden syrup, maple syrup | |
| Large total fructose dose: concentrated fruit sources, large servings of fruit, dried fruit, fruit juice | Sweeteners: any sweeteners except polyols | |
| Lactose | Milk: regular and low-fat cow, goat, and sheep milk; ice cream | Milk: lactose-free milk, rice milk
Ice cream substitutes: gelato, sorbet |
| Yogurts: regular and low-fat yogurts | Yogurts: lactose-free yogurts | |
| Cheeses: soft and fresh cheeses | Cheeses: hard cheeses | |
| Oligosaccharides (fructans and/or galactans) | Vegetables: artichoke, asparagus, beetroot, broccoli, Brussels sprout, cabbage, fennel, garlic, leek, okra, onion, pea, shallot | Vegetables: bamboo shoot, bok choy, capsicum, carrot, celery, chives, choko, choy sum, corn, eggplant, green bean, lettuce, parsnip, pumpkin, silverbeet, spring onion (green part only) |
| Cereals: rye and wheat cereals when eaten in large amounts (eg, biscuit, bread, couscous, cracker, pasta) | Onion/garlic substitutes: garlic-infused oil | |
| Legumes: baked bean, chickpea, lentil, red kidney bean | Cereals: gluten-free and spelt bread/cereal products | |
| Fruits: custard apple, persimmon, rambutan, watermelon, white peach | Fruit: tomato | |
| Polyols | Fruits: apple, apricot, avocado, cherry, longon, lychee, nashi pear, nectarine, peach, pear, plum, prune, watermelon | Fruits: banana, blueberry, cantaloupe, carambola, durian, grape, grapefruit, honeydew melon, kiwi, lemon, lime, orange, passion fruit, pawpaw, raspberry |
| Vegetables: cauliflower, mushroom, snow pea | ||
| Sweeteners: isomalt, maltitol, mannitol, sorbitol, xylitol, and other sweeteners ending in “-ol” | Sweeteners: glucose, sugar (sucrose), other artificial sweeteners not ending in “-ol” |
However, there are also several limitations to low-FODMAP diets. Most foods do not list their FODMAP content. In addition, the cutoff levels for FODMAP content are not clearly defined. When this diet was first examined, cutoff values were proposed based on foods that patients identified to be triggers for their symptoms. Foods and beverages with the following amounts of FODMAPs were considered to have risk for inducing symptoms: more than 0.5 g of fructose in excess of glucose per 100 g, more than 3 g of fructose in an average serving quantity regardless of glucose amount, and more than 0.2 g of fructans per serving.27 Although a low-FODMAP diet has been shown to be helpful in patients with IBS or other functional bowel disorders, further randomized controlled trials should be conducted.
Summary
A low-FODMAP diet appears to be effective for treatment of at least a subset of patients with IBS. FODMAPs likely induce symptoms in IBS patients due to luminal distention and visceral hypersensitivity. Whenever possible, implementation of a low-FODMAP diet should be done with the help of an experienced dietician. More research is needed to determine which patients can benefit from a low-FODMAP diet and to quantify the FODMAP content of various foods, which will help patients follow this diet effectively.
References
- 1.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 2.Hungin AP, Chang L, Locke GR, Dennis EH, Barghout V. Irritable bowel syndrome in the United States: prevalence, symptom patterns, and impact. Aliment Pharmacol Ther. 2005;21:1365–1375. doi: 10.1111/j.1365-2036.2005.02463.x. [DOI] [PubMed] [Google Scholar]
- 3.Adeyemo MA, Spiegel BM, Chang L. Meta-analysis: do irritable bowel syndrome symptoms vary between men and women? Aliment Pharmacol Ther. 2010;32:738–755. doi: 10.1111/j.1365-2036.2010.04409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaman A, Bucur MC, Kuo B. Therapeutic advances in functional gastrointestinal disease: irritable bowel syndrome. Therap Adv Gastroenterol. 2009;2:169–181. doi: 10.1177/1756283X08103656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayer EA. Clinical practice. Irritable bowel syndrome. N Engl J Med. 2008;358:1692–1699. doi: 10.1056/NEJMcp0801447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simren M, Mansson A, Langkilde AM, et al. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion. 2001;63:108–115. doi: 10.1159/000051878. [DOI] [PubMed] [Google Scholar]
- 7.Camilleri M, McKinzie S, Busciglio I, et al. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:772–781. doi: 10.1016/j.cgh.2008.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niec AM, Frankum B, Talley NJ. Are adverse food reactions linked to irritable bowel syndrome? Am J Gastroenterol. 1998;93:2184–2190. doi: 10.1111/j.1572-0241.1998.00531.x. [DOI] [PubMed] [Google Scholar]
- 9.Gibson PR. Food intolerance in functional bowel disorders. J Gastroenterol Hepatol. 2011;26(suppl 3):128–131. doi: 10.1111/j.1440-1746.2011.06650.x. [DOI] [PubMed] [Google Scholar]
- 10.Barrett JS, Gearry RB, Muir JG, et al. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment Pharmacol Ther. 2010;31:874–882. doi: 10.1111/j.1365-2036.2010.04237.x. [DOI] [PubMed] [Google Scholar]
- 11.Quigley EM, Abu-Shanab A. Small intestinal bacterial overgrowth. Infect Dis Clin North Am. 2010;24:943–959. doi: 10.1016/j.idc.2010.07.007. viii-ix. [DOI] [PubMed] [Google Scholar]
- 12.Khoshini R, Dai SC, Lezcano S, Pimentel M. A systematic review of diagnostic tests for small intestinal bacterial overgrowth. Dig Dis Sci. 2008;53:1443–1454. doi: 10.1007/s10620-007-0065-1. [DOI] [PubMed] [Google Scholar]
- 13.Gasbarrini A, Lauritano EC, Gabrielli M, et al. Small intestinal bacterial overgrowth: diagnosis and treatment. Dig Dis. 2007;25:237–240. doi: 10.1159/000103892. [DOI] [PubMed] [Google Scholar]
- 14.DiBaise JK. Nutritional consequences of small intestinal bacterial overgrowth. Practical Gastroenterology. 2008;32:15–28. [Google Scholar]
- 15.Lin HC. Small intestinal bacterial overgrowth: a framework for understanding irritable bowel syndrome. JAMA. 2004;292:852–858. doi: 10.1001/jama.292.7.852. [DOI] [PubMed] [Google Scholar]
- 16.Reid MC, Lachs MS, Feinstein AR. Use of methodological standards in diagnostic test research. Getting better but still not good. JAMA. 1995;274:645–651. [PubMed] [Google Scholar]
- 17.Simren M, Stotzer PO. Use and abuse of hydrogen breath tests. Gut. 2006;55:297–303. doi: 10.1136/gut.2005.075127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghoshal UC, Ghoshal U, Das K, Misra A. Utility of hydrogen breath tests in diagnosis of small intestinal bacterial overgrowth in malabsorption syndrome and its relationship with oro-cecal transit time. Indian J Gastroenterol. 2006;25:6–10. [PubMed] [Google Scholar]
- 19.Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98:412–419. doi: 10.1111/j.1572-0241.2003.07234.x. [DOI] [PubMed] [Google Scholar]
- 20.Ford AC, Spiegel BM, Talley NJ, Moayyedi P. Small intestinal bacterial overgrowth in irritable bowel syndrome: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2009;7:1279–1286. doi: 10.1016/j.cgh.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 21.Philpott H, Gibson P, Thien F. Irritable bowel syndrome—an inflammatory disease involving mast cells. Asia Pac Allergy. 2011;1:36–42. doi: 10.5415/apallergy.2011.1.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bengtsson U, Nilsson-Balknas U, Hanson LA, Ahlstedt S. Double-blind, placebo-controlled food reactions do not correlate to IgE allergy in the diagnosis of staple food-related gastrointestinal symptoms. Gut. 1996;39:130–135. doi: 10.1136/gut.39.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dainese R, Galliani EA, De Lazzari F, Di Leo V, Naccarato R. Discrepancies between reported food intolerance and sensitization test findings in irritable bowel syndrome patients. Am J Gastroenterol. 1999;94:1892–1897. doi: 10.1111/j.1572-0241.1999.01226.x. [DOI] [PubMed] [Google Scholar]
- 24.Bischoff SC, Mayer J, Wedemeyer J, et al. Colonoscopic allergen provocation (COLAP): a new diagnostic approach for gastrointestinal food allergy. Gut. 1997;40:745–753. doi: 10.1136/gut.40.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zar S, Benson MJ, Kumar D. Food-specific serum IgG4 and IgE titers to common food antigens in irritable bowel syndrome. Am J Gastroenterol. 2005;100 doi: 10.1111/j.1572-0241.2005.41348.x. 1550-1557-26. [DOI] [PubMed] [Google Scholar]
- 26.Atkinson W, Sheldon TA, Shaath N, Whorwell PJ. Food elimination based on IgG antibodies in irritable bowel syndrome: a randomised controlled trial. Gut. 2004;53:1459–1464. doi: 10.1136/gut.2003.037697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibson PR, Shepherd SJ. Evidence-based dietary management of functional gastrointestinal symptoms: the FODMAP approach. J Gastroenterol Hepatol. 2010;25:252–258. doi: 10.1111/j.1440-1746.2009.06149.x. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez-Banares F, Rosinach M, Esteve M, Forne M, Espinos JC, Maria Viver J. Sugar malabsorption in functional abdominal bloating: a pilot study on the long-term effect of dietary treatment. Clin Nutr. 2006;25:824–831. doi: 10.1016/j.clnu.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Shepherd SJ, Parker FC, Muir JG, Gibson PR. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin Gastroenterol Hepatol. 2008;6:765–771. doi: 10.1016/j.cgh.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 30.Ong DK, Mitchell SB, Barrett JS, et al. Manipulation of dietary short-chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25:1366–1373. doi: 10.1111/j.1440-1746.2010.06370.x. [DOI] [PubMed] [Google Scholar]
- 31.Staudacher HM, Whelan K, Irving PM, Lomer MC. Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. J Hum Nutr Diet. 2011;24:487–495. doi: 10.1111/j.1365-277X.2011.01162.x. [DOI] [PubMed] [Google Scholar]
- 32.Gibson PR, Barrett JS. Clinical ramifications of malabsorption of fructose and other short-chain carbohydrates. Practical Gastroenterology. 2007;31:51–65. [Google Scholar]