Abstract
Traditional models of the human language circuitry encompass three cortical areas, Broca's, Geschwind's and Wernicke's, and their connectivity. In an effort to expand current knowledge, we used diffusion tensor imaging (DTI) to explore subject-specific structural macroscopic connectivity, focusing on Broca’s area. Fascicles were studied using diffusion tensor imaging fiber tracking seeded from volumes placed manually within the white matter. White matter fascicles were co-registered with 3-D renderings of the brain in 12 healthy subjects. We discovered a connectome divisible into three systems --anterior, superior and inferior-- around the insula, more complex than previously thought. The extended Broca’s area involves two new fascicles: the operculo-premotor fascicle comprised of well-organized U-shaped fibers that connect the pars opercularis with the premotor region; and (2) the triangulo-orbitaris system comprised of intermingled U-shaped fibers that connect the pars triangularis with the pars orbitaris. The findings enhance our understanding of human language.
Keywords: language, DTI, fiber tracking, connectome, Broca’s area
Introduction
The seminal works of Broca, Wernicke, and Geschwind (see (Geschwind, 1970)) provided the structural basis of language function, which deeply influenced the understanding of aphasia. Cortical territories were identified and named after the pioneers who described them, i.e., Broca's, Wernicke's, and Geschwind's areas.(Broca, 1861; Geschwind, 1970; Kirshner, 1995).Anatomically, these areas correspond respectively to the inferior frontal gyrus or F3, the superior temporal gyrus or T1 (rather posterior, and a part of the planum temporale), and the inferior parietal cortex (inferior parietal lobule or inferior parietal gyrus) including the supramarginal gyrus. The structural basis of language also encompasses the interconnections among these three areas, as well as the connections between these areas and the deep nuclei (basal ganglia and thalamus). These connections are realized by bundles of nerve fibers known as white matter (WM) fascicles. Although the exact constitution of fascicles remains unknown, they are thought to represent the macroscopic organization of roughly parallel groups of axons (myelinated and unmyelinated) that connect diverse but functionally related regions of the brain.(P J Basser, Mattiello, & LeBihan, 1994; Peter J Basser & Jones, 2002; Mori & Zhang, 2006)
Recent investigations using diffusion tensor imaging (DTI) based tractography (Catani et al., 2005; Frey, Campbell, Pike, & Petrides, 2008; Glasser & Rilling, 2008; Parker et al., 2005; Saur et al., 2008) have led to the description of an extended connectivity of the language circuitry that involves numerous WM connections (Fig 1). However, almost 150 years after Broca first described the relationship between the frontal lobe and a kind of aphasia, the language circuitry in this region remains incompletely characterized. We selected Broca’s area for this investigation, not only because of its key role in language (Keller, Crow, Foundas, Amunts, & Roberts, 2009), but also because of the likely involvement of subcortical WM connectivity in Broca’s aphasia (Dronkers, Plaisant, M. T. Iba-Zizen, & E. A. Cabanis, 2007). We theorized that using subcortical WM analyses we could deliver new insight into its structural organization. The structural organization of the previously uncharted subcortical Broca’s area was probed using DTI, a non-invasive in-vivo imaging method that permits the visualization of WM fascicles by fiber tracking techniques. The overall superficial language circuitry centered on Broca’s area was explored, as well. The connectivity of the language circuitry was described using the informatics concept of the human connectome (Sporns, Tononi, & Kötter, 2005). By using this construct, we aimed to define a single macroscopic connectome for the superficial circuitry of language. We did not explore the deep brain. In summary, we sought to investigate Broca's area in explicit detail with the goal of defining the connectome of the superficial language circuitry, by charting WM fascicles, in healthy subjects.
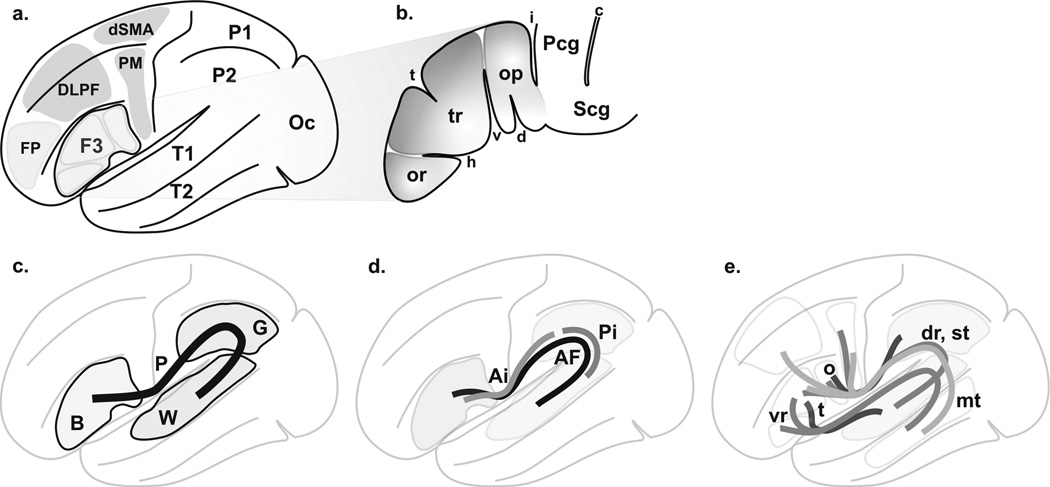
Figure 1. Language circuitry.
A. Overview of cortical language territories: inferior frontal gyrus (F3, or ventrolateral prefrontal cortex, or Broca’s area), superior temporal gyrus (T1), middle temporal gyrus (T2), premotor cortex (PM), dorsal supplementary motor area (dSMA), dorsolateral prefrontal cortex (DLPF) and inferior parietal gyrus (P2, or inferior parietal lobule). The frontopolar region (FP), the superior parietal gyrus (P1, or superior parietal lobule) and the occipital lobe (Oc) are added for further analysis. B. The Broca’s area (adapted from (Geschwind, 1970; Kirshner, 1995; Simmons-Mackie, 1997)): pars opercularis (op), pars triangularis (tr), pars orbitalis (or), horizontal (h) and vertical (v) segments of anterior lateral (or Sylvian) fissure, sulcus triangularis (t) and diagonalis (d). The central region is depicted (precentral gyrus, Pcg, subcentral gyrus, Scg, central sulcus, c and inferior precentral sulcus, i). C. Classical primary perisylvian circuitry (P) from Broca’s area (B) to Wernicke’s area (W); Geschwind’s territory (G) associated with AF, leading to conduction aphasia when lesioned (see(Catani et al., 2005)) is depicted. D. Circuitry according to Catani (Saur et al., 2008). The perisylvian network consists of a direct long pathway (AF) and an indirect pathway with anterior (Ai) and posterior (Pi) segments. E. Other circuitries: ventral (vr) and dorsal (dr, using AF) routes(Frey et al., 2008); opercular (o) and triangular (t) paths(Glasser & Rilling, 2008); superior (st, from operculo-premotor to T1 using AF) and middle (mt; from F3, PM and dorsolateral cortex to T2) temporal gyrus paths(Parker et al., 2005); Parker et al (Parker et al., 2005) results are not depicted because they overlap with the other information.
MATERIALS and METHODS
Twelve healthy native English-speaking subjects were included (mean age = 28.8 ± 7.5 years). They were all assessed with the Edinburgh handedness inventory (Oldfield, 1971) and selected to ensure a heterogeneous representation of handedness, blind of the data analysis: five right-handed (score > 40, 1 male); five left-handed (score < −40, 2 male), and two ambidextrous (1 male, score = 0; 1 female, score = 5). All subjects were enrolled after Institutional Review Board (Partners Healthcare, Brigham and Women’s Hospital, Boston, MA, USA) approval and written informed consent was obtained.
Imaging
MRI was performed using a 3-Tesla machine (GE Sigma, General Electronic, Milwaukee, WI, USA). Whole brain T1-weighted axial 3D-SPGR (spoiled gradient recalled) MR images (TR = 7,500 ms, TE = 30 ms, flip angle = 20°, matrix = 512 × 512, 176 slices, voxel size = 0.5 × 0.5 × 1 mm3) were acquired. Single-shot spin-echo echo-planar sequence was used for DTI with diffusion gradients in 31 non-collinear directions (b = 1,000 s/mm2, matrix = 256 × 256, 44 slices to cover the whole brain, voxel size = 1 ×1 × 3 mm3).
Image processing, anatomic registration and 3D visualization
DTI-derived fractional anisotropy (FA) maps, co-registration of data and 3D-volume rendering were achieved using Slicer 3.3 (Surgical Planning Laboratory, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA; www.slicer.org). The coregistration of FA with T1-weighted MR images (anatomical reference) required linear affine registration (translation, rotation and scaling). The accuracy of the anatomic registration was carefully reviewed (visual analysis of merged images; test-retests) using the following landmarks: putamen, pallidum, corpus callosum (whole body and/or major and minor forceps), anterior and posterior limbs of the internal capsule, cerebellar contour, tentorium of the posterior fossa, sylvian region, upper brainstem contour, ventricular system (frontal horns and trigone), the inter hemispheric fissure and main cerebral gyrations. For each individual, we generated 3D surface models for brain renderings from T1-weighted MR.
White matter fascicles
Eight WM fascicles (see glossary) were explored: the fronto-occipital part of the inferior longitudinal fascicle (IFOF), the temporo-occipital fascicle (TOF), the uncinate fascicle (UF), the arcuate fascicle (AF), the inferior fronto-temporo-occipital fascicle (IFTOF), the fronto-parietal branch of the superior longitudinal fascicle (fpSLF), the superficial parieto-temporal system (sPTS), and the transcallosal dorsal supplementary motor area fascicle (TrSMA). New bundles revealed by the exploration of Broca’s area were named according to the topography and the connectivity.
DTI fiber tracking (streamline continuous diffusion tensor algorithm (P J Basser, Pajevic, Pierpaoli, Duda, & Aldroubi, 2000)) was performed in Slicer 3.3, using thresholded linear measurement stopping mode and tracking curvature, respectively > 0.25 and = 0.7 degree/mm. Fiber tracking was seeded from cubes of 2.5-mm edge (namely seeder) placed manually within the WM fascicle (clinical neuroanatomist, J.J.L.) without atlas registration (subject-based approach). The generation of each fascicle was carried out as follows (see Supplementary Material): (i) addition of seeders until generation of the whole macroscopic organization; (ii) real-time, updating of fiber tracking and spatial reorientation, allowing a 3D analysis of fascicles; (iii) iterative test-retests adding seeders until display of unexpected fibers (e.g. fronto-parietal fibers when delineating the inferior fronto-occipital fascicle).
Data analysis
Data collection was carried out in each hemisphere, per subject, each serving as its own anatomical reference. We used the most advanced available tools allowing us to precisely determine the shape and location of WM fascicles and their connections with fMRI clusters, individually and within his/her own 3D anatomic environment. This choice was determined for several reasons: (i) there was no reference 3D atlas or template of human WM fascicles, at least not with enough anatomical details particularly concerning GM connections; (ii) there was no method available to precisely average or normalize WM fascicles.
The overall analysis consisted of exploration and quantification of the WM fascicles. The lateralization between the right (RH) and left (LH) hemispheres was quantified using a laterality index (between + 1 and −1; a positive value means leftward and a negative value rightward: (LH − RH) / (LH + RH)) (Desmond et al., 1995).
We studied the thickness of each fascicle, approximated by the number of fibers that were found (see Supplementary material) across all fascicles. We searched for significant lateralization of fascicle thickness across all subjects and according to handedness (in this latter case, the 2 ambidextrous subjects were excluded because of the small sample size). We tested for rejection of the null hypothesis (no lateralization; setting a type I error to 0.05, adjusted for multiple comparison with Bonferonni correction, using t test after logit transform (natural logarithm, domain adapted to the interval −1 to 1), running in MATLAB® 7.1 (The MathWorks Inc, Natick, MA, USA). For analysis a type I error was set respectively to 0.00555 according to the Bonferonni correction. For the analysis of the AF, we expected left hemisphere lateralization, and used a one-tailed test (†). For the rest we made no specific assumption of lateralization and two-tailed tests were used.
RESULTS
We found, in our 12 subjects, two new fascicles within the subcortical region of Broca’s area, in addition to the 8 known WM fascicles reported by others.(Catani et al., 2005; Frey et al., 2008; Glasser & Rilling, 2008; Parker et al., 2005; Saur et al., 2008). The overall results of the 12 subjects are presented in Figure 2, and Supplementary Tables 1, 2 and 3.
Figure 2. Overall display of the WM fascicles.
Display of the nine WM fascicles, within the right (right column) and the left (left column) hemispheres (lateral views), and of the AF (central column, superior view, right hemisphere on the right): A, right-handed subjects; B, ambidextrous; C, left-handed subjects (Edinburgh handedness inventory score, Ehi: right-handed, Ehi > 40; left-handed, Ehi < −40).
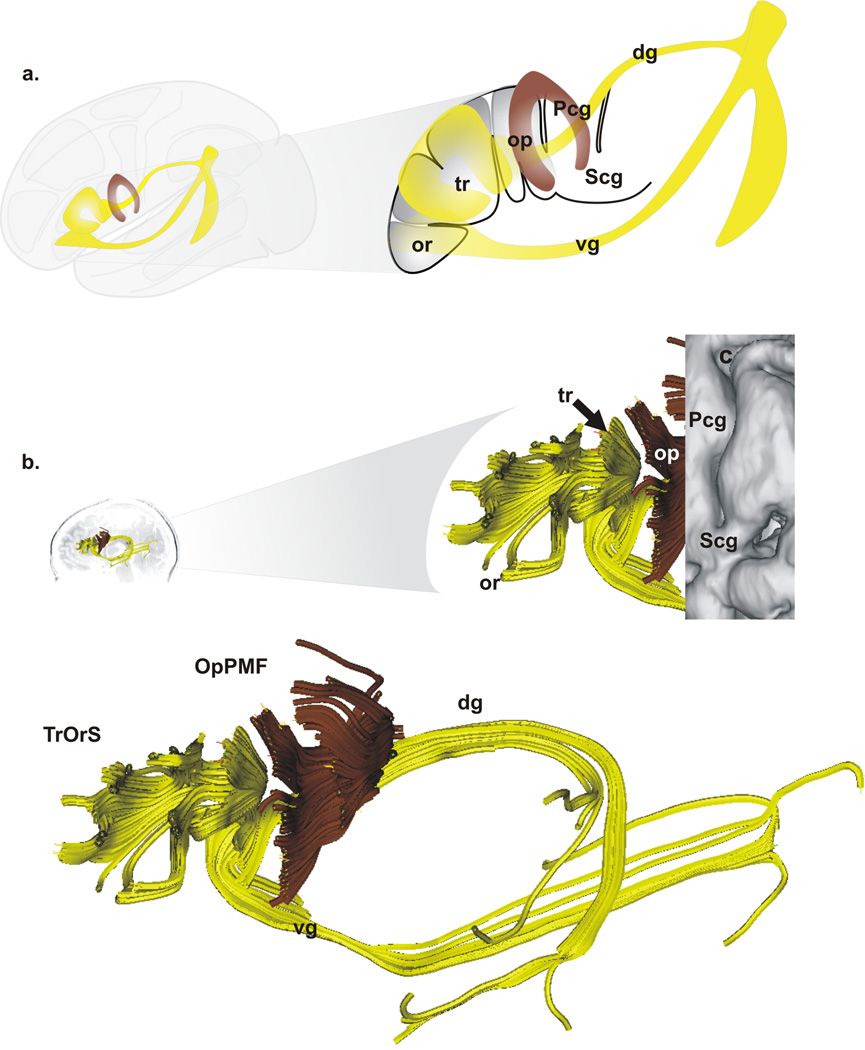
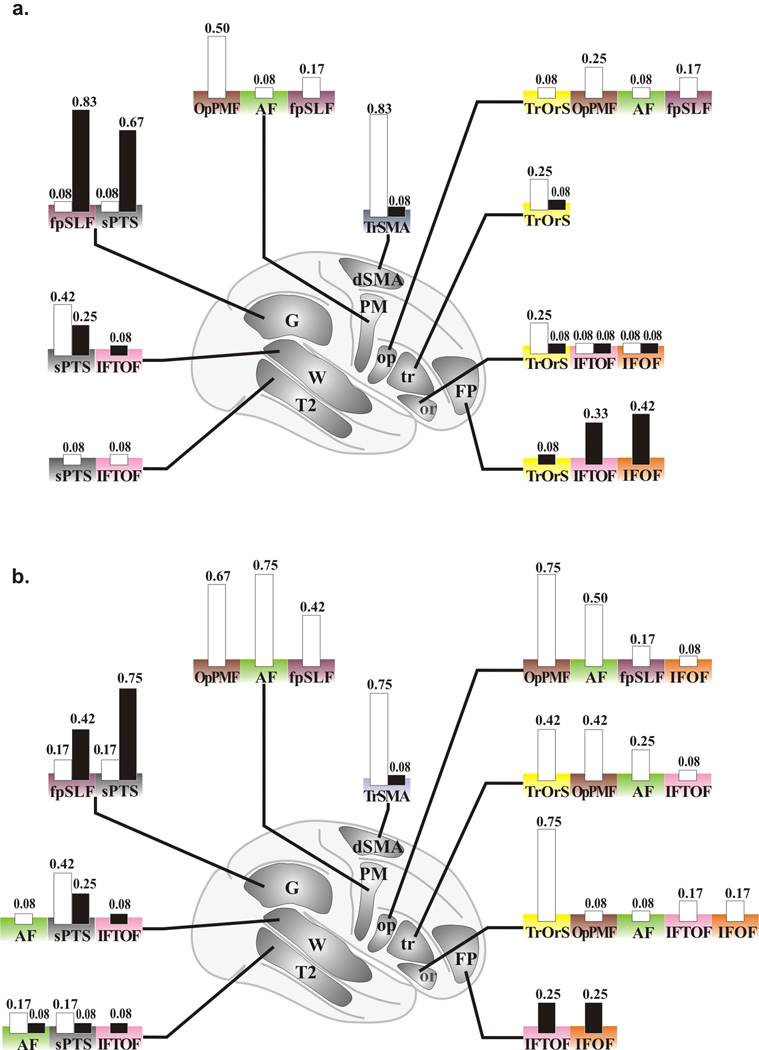
The two previously unreported fascicles of Broca’s area are characterized as follows (Fig. 3): (1) the operculo-premotor fascicle (OpPMF) comprised of well-organized U-shaped fibers that connect the pars opercularis with the premotor region; and (2) the trianguloorbitaris system (TrOrS) comprised of intermingled U-shaped fibers that connect the pars triangularis with the pars orbitaris. Three groups of fiber bundles: frontal, dorsal (placed medially to AF and fpSLF), and ventral (joining the lateral capsule merged with or placed above IFOF and IFTOF) variably emerged from Broca’s area (Fig. 3; Supplementary Table 3). Known fascicles included the fronto-occipital part of the inferior longitudinal fascicle (IFOF), the temporo-occipital fascicle (TOF), the uncinate fascicle (UF), the arcuate fascicle (AF), the inferior fronto-temporo-occipital fascicle (IFTOF), the fronto-parietal branch of the superior longitudinal fascicle (fpSLF), the superficial parieto-temporal system (sPTS), and the transcallosal dorsal supplementary motor area fascicle (TrSMA) (see glossary). The descriptive parameters of these 10 fascicles are reported in Supplementary Table 1.
Figure 3. Fascicles of Broca’s area.
A. Schematic representation of the two fascicles identified within Broca’s area: the operculo-premotor fascicle (OpPMF, brown) and the triangulo-orbitaris system (TrOrS, yellow) as well as two groups of variable bundles (the dorsal, dg, and the ventral, vg, groups) linked with the TrOrS: left, overview; right, Broca’s area with the pars opercularis (op), the pars triangularis (tr), the pars orbitalis (or), the central region is depicted with the precentral gyrus (Pcg) and the subcentral gyrus (Pcg). B. Example of OpPMF and TrOrS (left hemisphere) within Broca’s area (overlay of the cortex, top right); bottom, details of the OpPMF and TrOrS fascicles.
The specific connectivity of the AF and fpSLF was respectively: the premotor cortex, the pars opercularis, and the middle temporal gyrus; and the premotor cortex, the pars opercularis, and Geschwind’s area (Supplementary Table 2). Morphologically, the AF was significantly thicker in the left hemisphere; whereas the fpSLF was significantly thicker in the right hemisphere (Fig. 4) regardless of handedness, indicating tangible anatomic differences (see Fig. 2).
Figure 4. Laterality index of the number of fibers of fascicles.
Laterality index of the number of fibers (logit transform on y-axis) of each fascicle (colored bars, bottom; same color-code than in figure 5): the uncinate fascicle (UF, black); the temporo-occipital fascicle (TOF, blue); the inferior fronto-temporo-occipital fascicle (IFTOF, pink); the fronto-occipital part of the inferior longitudinal fascicle (IFOF, orange); the triangulo-orbitaris system (TrOrS, yellow); the operculo-premotor fascicle (OpPMF, brown); the arcuate fascicle (AF, green); the fronto-parietal branch of the superior longitudinal fascicle (fp SLF, purple); the superficial parieto-temporal system (sPTS, grey). The laterality index was calculated from the number of fibers (nf), which approximates the thickness of fascicle, in the left hemisphere, LH, and in the right hemisphere, RH: (LHnf − RHnf) / (LHnf + RHnf); for each of 12 subjects (right handed, n=5, black dash; ambidextrous, n = 2, grey circle; left handed, n= 5, grey dash); p values (italic) were only significant for the AF and the fpSLF (highlighted data, gray boxes; †, one-tailed test)
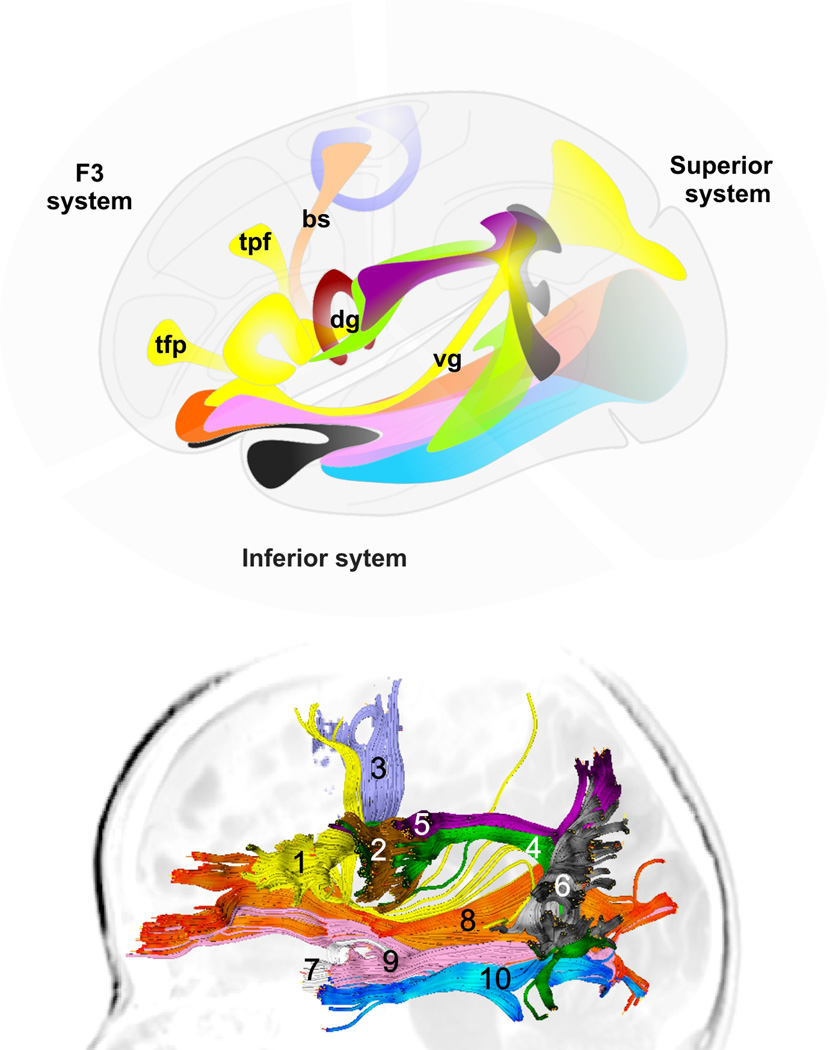
Finally, the 10 fascicles were grouped into three systems to simplify their description: (i) the F3 or anterior system was comprised of the TrOrS, OpPMF, TrSMA, and the variable bundles linked with Broca’s area; (ii) the superior system was comprised of the AF, fpSLF, and sPTS; (iii) the inferior system was comprised of the UF, IFOF, IFTOF, and TOF (Fig. 5).
Figure 5. Fascicles of the superficial language circuitry.
The fascicles are regrouped in three systems: (1) F3 or anterior system, consisting of the triangulo-orbitaris system (yellow, 1), the operculo-premotor fascicle (brown, 2), the transcallosal dorsal supplementary motor area (SMA) fascicle (slate blue, 3), as well as variable bundles regrouped in the frontal (Broca-SMA, bs; TrOrS-Prefrontal, tpf; TrOrS-frontopolar, tfp), dorsal (dg) and ventral (vg) groups; (2) superior system, consisting of the arcuate fascicle (green, 4), the fronto-parietal branch of the superior longitudinal fascicle (purple, 5) and the superficial parieto-temporal system (grey, 6); (3) inferior system, consisting of the uncinate fascicle (white, 7), the fronto-occipital part of the inferior longitudinal fascicle (orange, 8), the inferior fronto-temporo-occipital fascicle (pink, 9) and the temporo-occipital fascicle (blue, 10). Top, schematic representation; bottom, example of one subject, left hemisphere.
DISCUSSION
We report herein two new WM fascicles in Broca's area using targeted DTI fiber tracking analysis. The fascicles were found reproducibly in all 12 subjects, regardless of handedness. The two new fascicles within the subcortical region of Broca’s area are the operculo-premotor fascicle (OpPMF) and the triangulo-orbitaris system (TrOrS). Thereby we expand recent reports, which, while confirming known morphologic structures also present in nonhumans, failed to describe any new structures that could be unique to humans (Davis et al., 2008; Keller et al., 2009; Tsapkini, Vivas, & Triarhou, 2008).
This study permits us to add to the 8 previously documented WM fascicles and to report a new integrated functional connectome, for Broca's area in particular, and a new organization for the superficial cortical language circuitry consisting of 3 clearly defined systems of WM fiber connections, namely, the anterior (or F3), the superior, and the inferior (Fig 5). The anterior system is composed of the TrOrS, the OpPMF, and the trans-SMA, which connects the right and left dorsal supplementary motor areas (TrSMA). A number of additional smaller and inconsistent (across individuals) bundles were found, but were not amenable to this type of analysis. The superior system is composed of the AF, the fpSLF, and the superficial parieto-temporal system (sPTS). The inferior system is composed of the uncinate fascicle (UF), the inferior fronto-occipital fascicle, the inferior fronto-temporooccipital fascicle, and the temporo-occipital fascicle. The description of these three systems of connections, although a simplification of the full complexity seen in the language network (see(Angela D Friederici, 2009; Lee et al., 2009; Nowak, Komarova, & Niyogi, 2002; Pulvermüller, 2005; Rizzolatti & Craighero, 2004; Sandrini & Rusconi, 2009) for a review), provides a complete and consistent model of the experimental data currently available through neuroimaging studies (compare Fig 1 with Figs 5). The new connectome is consistent with physiologic data (Catani et al., 2005; Frey et al., 2008; Glasser & Rilling, 2008; Parker et al., 2005; Saur et al., 2008). It is also consistent with non-physiologic data using direct electrical stimulation of cortical and subcortical regions (Boatman et al., 2000; Duffau et al., 2002, 2005; Mandonnet, Nouet, Gatignol, Capelle, & Duffau, 2007; G. Ojemann, J. Ojemann, Lettich, & Berger, 1989; Quigg & Fountain, 1999) although the underlying biophysical mechanism is still partially understood (P J Basser & Roth, 2000; Manola, Holsheimer, Veltink, & Buitenweg, 2007). As a consequence, the new connectome could be used to interpret language function and aphasia.
Fiber tracing of WM fascicles
There is an important body of literature regarding the efficacy of using DTI to trace WM fascicles, (see for example, P J Basser, Mattiello, & LeBihan, 1994; Peter J Basser & Jones, 2002; Mori & Zhang, 2006), revealing the macroscopic organization of myelinated axons regrouped in bundles, similar to the dissections of hardened brains (Dejerine, 1901; Klingler, 1935). Modern, in-vivo, 3D rendering of fascicles dramatically facilitates the analysis of WM fascicles and it has been demonstrated that DTI fiber tracking can reveal known connectivity between gray matter territories, in particular within Broca’s area (Anwander, Tittgemeyer, von Cramon, A D Friederici, & Knösche, 2007). Cutting-edge tractography techniques, such as the real-time tracing tool used in this study, permit precise 3D exploration up to the confined region of Broca, at least of well-organized anisotropic bundles of fibers (P J Basser et al., 2000). However, there remain several limitations (see for an overview (Mori & Zhang, 2006)), particularly with regard to the exploration of small fascicles like those in our study that emerged from Broca’s area. Their small size prevents any detailed analysis using current imaging resolutions. Thus different techniques will likely be necessary in the future to analyze the complexity of the human language circuitry. Partial biologic and anatomic knowledge of human brain fascicles added with still on-going research on mathematical models used for the display of fascicles preclude any firm and definitive conclusion about the detailed biologic organization hidden behind DTI fiber tracking technique. This challenge fosters the research of new methodologies (Leow et al., 2009; Raj, Hess, & Mukherjee, 2011). Our subject-based reasoning, however, gathered new insights ignored by other seeding techniques for DTI tracing; i.e., manually drawing within cortical territories (Frey et al., 2008), placement of 4-mm radius spheres in major cortical BOLD clusters of voxels (Saur et al., 2008), manual drawing on DTI maps according to the topography of fascicles (Catani et al., 2007, 2005; Glasser & Rilling, 2008), and constrained multifiber streamline tracing between two cortical territories (Parker et al., 2005).
The extended Broca’s area
The extended notion of Broca’s area includes cortico-gyral, i.e., Broca’s area stricto sensu, and subcortical, i.e., the OpPMF and TrOrS, components. This extended Broca’s area is situated in the lateral and inferior region of the frontal lobe where the anterior system is developed. The lateral and inferior region of the frontal lobe is divisible into two subregions.
The posterior subregion includes the premotor cortex and the pars opercularis, connected by the OpPMF. The well-organized nature of the U-shaped fibers that constitute the OpPMF could indicate that there is a somatotopic connection between the pars opercularis and the premotor region involved with oro-facial motor control.
The anterior subregion includes the pars triangularis (Tr) and the pars orbitaris (Or), connected by the TrOrS. The pars triangularis and pars orbitaris are less well understood in humans. However, they are part of the prefrontal lateral (dorsal and ventral) cortex, which participates in behavioral controls. The less organized, intermingled U-shaped fibers characteristic of the TrOrS could reflect a more complex spatial organization, more consistent with a behavior-related function than somatotopy. Hence, this fascicle supports the notion of a behavior-related region, which has a specific superficial connectivity.
The posterior subregion uses the superior system of fascicles, (over the insula) to connect the temporal region and Geschwind’s area. The anterior subregion uses the inferior system of fascicles (through the inferior part of the lateral capsule) to connect to the temporal pole.
How does this extended description of Broca's area affect our understanding and interpretation of aphasia and language? Since this is the first report of these subcortical fascicles, we cannot say with certainty. These answers await further corroboration by others. We can only speculate. On the basis of the connectivity of the OpPMF, a lesion specific to this fascicle might lead to a pure "naked" Broca's aphasia (an isolated loss of word production), or semantic errors (De Carli et al., 2007; Glasser & Rilling, 2008). Another term for this fascicle might be the "premotor connection", because the OpPMF makes a strong, well organized, and direct connection between the pars opercularis and the premotor cortex. The TrOrS makes the link between the pars triangularis and the pars orbitaris, both involved in behavioral controls. The discovery of an actual link (TrOrS) between these two gyri supports the hypothesis of a more behavioral-related region which has a specific superficial connectivity. On the basis of its connectivity, a lesion of the TrOrS would lead to a behavioral apraxia linked with language. In other words, disruption of the TrOrS might lead to a loss of behavioral automatisms like those used in mirror actions (Rizzolatti & Craighero, 2004) or loss of ability to make gestures related to words (Pulvermüller, 2005).
Structural hemisphere asymmetry
The relationship between handedness and language is well accepted based on abundant literature and pioneering evidence following brain injury. Irrespective of handedness, we confirmed the presence of strong leftward lateralization in the AF (Catani et al., 2007; Vernooij et al., 2007), thereby reinforcing other reports of leftward brain asymmetry (Keller et al., 2009; Parker et al., 2005). Interestingly, the size of the AF and the sPTS appear to be balanced, with AF predominating within the left hemisphere and sPTS within the right. We still do not have an explanation for this asymmetry, but the asymmetric distribution of several subfunctionalities (De Carli et al., 2007; Fink et al., 2009; Glasser & Rilling, 2008) of language is one rationale
In conclusion we have reported a new superficial connectome of language circuitry in the human brain that is characterized by: (i) a specific structural organization of an extended Broca’s area; (ii) divisibility into three systems--anterior, superior and inferior-- around the insula; (iii) an asymmetric (right versus left hemisphere) organization. These findings correlate with previously reported data and provide new insight into the structural organization of language in adults. The two new fascicles within the subcortical region of Broca’s area, the operculo-premotor fascicle (OpPMF) and the triangulo-orbitaris system (TrOrS), are composed of short connection fibers that belong to two categories of WM fibers, U-fibers between adjacent gyri and neighborhood association fibers (Dejerine, 1901; J. D. Schmahmann, Smith, Eichler, & Filley, 2008). These two new fascicles could be specific to human, as they are not described explicitly in monkeys (Petrides & D. N. Pandya, 2009).
These data have the potential to lead to new advanced solutions for the treatment of aphasia and closely related syndromes (i.e., apraxia, alexia, and dysarthria) as well as new basic research applications. In medical prospect, individual connectome of language circuitry could be explored to understand syndromes and possibly to propose therapeutic solutions fitting with the residual functional connectivity. From a more forward thinking perspective, deeper understanding of local WM connectivity also paves the way for precise positioning of future brain-computer interfaces (BCI). Current BCI (also termed direct neural interfaces) are configured to interface directly with neurons (Hatsopoulos & Donoghue, 2009). However, interfacing with WM fascicles, or WM gates, may also prove to be a promising alternative. Hence, knowledge of the superficial functional connectome would enable one to hypothesize solutions for rewiring the language circuitry after lesion, infarct or injury, up to the extended Broca’s area, thereby alleviating or reducing the symptoms of aphasia. Whatever configuration the future WM technologies assume, it will require a deeper understanding of brain biology, in terms of functionality, plasticity and topography, from macro- to meso- to micro- scopic scales.
Supplementary Material
Acknowledgments
We thank Wentao Wu, Isaiah Norton, Wendy Plesniak, and Nicole Aucoin for their technical support. We thank Ann Adams for her editorial advice. We are grateful to the community and major sponsors and contributors to Slicer (http://www.slicer.org/pages/Acknowledgments) who permitted us to use the most recent technologies of real-time fiber tracking. This work is part of the National Alliance for Medical Image Computing (NA-MIC), funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant U54 EB005149. Information on the National Centers for Biomedical Computing can be obtained from http://nihroadmap.nih.gov/bioinformatics. This work was also supported in part by NIH grants U41-RR019703, P41-RR13218, and P01 CA067165 and by the Inserm (Institut national de la santé et de la recherche médicale).
Glossary
The sometimes-confusing terminology of fascicles is revisited for further analysis. Some of the fascicles have already been defined (Dejerine, 1901; Riley, 1953): the fronto-occipital part of the inferior longitudinal fascicle or the inferior fronto-occipital fascicle (IFOF), the temporo-occipital fascicle (TOF), the uncinate fascicle (UF), and the arcuate fascicle (AF). The joined and merged fibers of IFOF and TOF were renamed the inferior fronto-temporo-occipital fascicle (IFTOF). Catani’s anterior and posterior segments of the indirect Broca-Wernicke pathway (Catani, Jones, & ffytche, 2005) were renamed, respectively, the fronto-parietal branch of the superior longitudinal fascicle (fpSLF) and the superficial parietotemporal system (sPTS). The dorsal supplementary motor area (SMA), connected with its contralateral symmetric region through a component of the callosal system, was renamed the transcallosal SMA fascicle (TrSMA).
- Superior longitudinal fascicle (SLF)
The SLF connects the frontal lobe with the parietal and occipital lobes. It is located superficially and laterally to the superior corona radiata. It has also been called the superior fronto-occipital fascicle or fascicle occipito-frontal of Forel (Dejerine, 1901; Riley, 1953). Along its superficial path, different bundles of fibers merge with the SLF. We named these bundles "branches," for example, the long fronto-parietal branch and several short U-shaped branches. One of the strongest bundles is the fronto-parietal branch, which corresponds to the anterior segment of the indirect Broca-Wernicke pathway defined by Catani et al (Catani et al., 2005). This branch might partially correspond to the superior longitudinal fascicle III of the monkey anatomy (Makris et al., 2005; J. Schmahmann & D. Pandya, 2006).
- Inferior longitudinal fascicle (ILF)
The ILF connects the inferior or basal frontal lobe with the temporo-occipito-parietal region. It is located within the lateral capsule between the lenticular nucleus and the insula (anteriorly it is placed inferiorly). It has also been called the inferior fronto-occipital fascicle (Dejerine, 1901; Riley, 1953). The fronto-occipital fibers constitute the inferior part of this fascicle; they were specifically explored in this study, regrouped, and named the inferior fronto-occipital fascicle (IFOF).
- The uncinate fascicle (UF)
The UF connects the latero-basal frontal lobe with the anterior temporal lobe. It is located just beneath the IFOF within the anterior region of the lateral capsule.
- The temporo-occipital fascicle (TOF)
The TOF connects the temporal lobe with the occipital lobe. It has also been called the fasciculus longitudinalis inferior (Dejerine, 1901; Riley, 1953). It is located superficially and laterally within the temporo-occipital region. The inferior longitudinalis fascicle of monkeys (J. Schmahmann & D. Pandya, 2006) broadly includes the fibers of the human TOF as well as temporo-parietal fibers. The latter may correspond to the human superficial parieto-temporal system (see below).
- The extreme capsule system
This terminology is used in monkey anatomy (J. Schmahmann & D. Pandya, 2006) and it corresponds to the system of fibers that lie between the claustrum and the insula, which link the frontal and the temporal lobe. The extreme capsule system partially corresponds to humans, to the IFL and the UF, as well as joined fibers between the IFOF and the TOF, attributed either to the former or latter, particularly in pioneering works (see (Dejerine, 1901; Riley, 1953)). In this study, these joined fibers have been incorporated into the inferior fronto-temporo-occipital fascicle (IFTOF). IFTOF fibers may partially correspond to fibers of the middle longitudinal fascicle described in monkeys (J. Schmahmann & D. Pandya, 2006).
- The arcuate fascicle (AF)
The AF connects the frontal pre-central region with the temporal region. It is superficial, lying inferior to the SLF, and makes a turn around the posterior region of the insula from whence it derives its typical curved shape and name. It has also been called the arcuate fascicle of Burdach, the frontal superior fascicle or the fasciculus longitudinalis superior (Dejerine, 1901; Riley, 1953). Catani et al (Catani et al., 2005) associated the posterior segment of the indirect Broca-Wernicke pathway with the AF. We prefer to separate this posterior segment and have named it the superficial parieto-temporal system (sPTS). The sPTS consists of long and short arched fibers that connect several cortical areas of the inferior parietal gyrus (or inferior parietal lobule) and the superior and middle temporal gyrus.
- The inferior frontal gyrus
This fascicle is comprised of three parts: the pars opercularis (op), the pars triangularis (tr) and the pars orbitaris (or). It is also named the 3rd frontal gyrus (F3). The frontal operculum includes the pars triangularis and the pars opercularis (Duvernoy, E.-A. Cabanis, M.-T. Iba-Zizen, Tamraz, & Guyot, 1992). The horizontal and vertical segments of the anterior lateral (or Sylvian) fissure separate, respectively, the pars orbitaris and the pars triangularis, and the pars triangularis and the pars opercularis. Two fissures that represent variable anatomy, the sulcus triangularis and the sulcus diagonalis, subdivide respectively, the pars triangularis and the pars opercularis.
References
- Anwander A, Tittgemeyer M, von Cramon DY, Friederici AD, Knösche TR. Connectivity-Based Parcellation of Broca’s Area. Cerebral Cortex (New York, N.Y.:1991) 2007;17(4):816–825. doi: 10.1093/cercor/bhk034. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophysical Journal. 1994;66(1):259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2000;44(4):625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Roth BJ. New currents in electrical stimulation of excitable tissues. Annual Review of Biomedical Engineering. 2000;2:377–397. doi: 10.1146/annurev.bioeng.2.1.377. [DOI] [PubMed] [Google Scholar]
- Basser, Peter J, Jones DK. Diffusion-tensor MRI: theory, experimental design and data analysis - a technical review. NMR in Biomedicine. 2002;15(7–8):456–467. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- Boatman D, Gordon B, Hart J, Selnes O, Miglioretti D, Lenz F. Transcortical sensory aphasia: revisited and revised. Brain: A Journal of Neurology. 2000;123(Pt 8):1634–1642. doi: 10.1093/brain/123.8.1634. [DOI] [PubMed] [Google Scholar]
- Broca P. Perte de la parole, ramollissement chronique et destruction partielle du lobe antérieur gauche du cerveau. Bulletins de la Société d’Anthroplogie de Paris. 1861:235–238. [Google Scholar]
- Catani M, Allin MPG, Husain M, Pugliese L, Mesulam MM, Murray RM, Jones DK. Symmetries in human brain language pathways correlate with verbal recall. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(43) doi: 10.1073/pnas.0702116104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Annals of Neurology. 2005;57(1):8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Davis C, Kleinman JT, Newhart M, Gingis, Leila, Pawlak M, Hillis AE. Speech and language functions that require a functioning Broca’s area. Brain and Language. 2008;105(1):50–58. doi: 10.1016/j.bandl.2008.01.012. [DOI] [PubMed] [Google Scholar]
- De Carli D, Garreffa G, Colonnese C, Giulietti G, Labruna L, Briselli E, Ken S, et al. Identification of activated regions during a language task. Magnetic Resonance Imaging. 2007;25(6):933–938. doi: 10.1016/j.mri.2007.03.031. [DOI] [PubMed] [Google Scholar]
- Dejerine J. Anatomie des centres nerveux (Tomes 1 and 2) (Rueff et Cie.) Paris: 1901. [Google Scholar]
- Desmond JE, Sum JM, Wagner AD, Demb JB, Shear PK, Glover GH, Gabrieli JD, et al. Functional MRI measurement of language lateralization in Wada-tested patients. Brain: A Journal of Neurology. 1995;118(Pt 6):1411–1419. doi: 10.1093/brain/118.6.1411. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Plaisant O, Iba-Zizen MT, Cabanis EA. Paul Broca’s historic cases: high resolution MR imaging of the brains of Leborgne and Lelong. Brain: A Journal of Neurology. 2007;130(Pt 5):1432–1441. doi: 10.1093/brain/awm042. [DOI] [PubMed] [Google Scholar]
- Duffau H, Capelle L, Sichez N, Denvil D, Lopes M, Sichez J-P, Bitar A, et al. Intraoperative mapping of the subcortical language pathways using direct stimulations. An anatomo-functional study. Brain: A Journal of Neurology. 2002;125(Pt 1):199–214. doi: 10.1093/brain/awf016. [DOI] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Mandonnet E, Peruzzi P, Tzourio-Mazoyer N, Capelle L. New insights into the anatomo-functional connectivity of the semantic system: a study using cortico-subcortical electrostimulations. Brain: A Journal of Neurology. 2005;128(Pt 4):797–810. doi: 10.1093/brain/awh423. [DOI] [PubMed] [Google Scholar]
- Duvernoy H, Cabanis E-A, Iba-Zizen M-T, Tamraz J, Guyot J. Le cerveau humain: Surfaces, coupes sériées tridimentionelles et IRM (Springer-Verlag.) Paris Berlin Heidelberg New York Londres Tokyo Hong Kong Bracelone Budapest: 1992. [Google Scholar]
- Fink M, Wadsak W, Savli M, Stein P, Moser U, Hahn A, Mien L-K, et al. Lateralization of the serotonin-1A receptor distribution in language areas revealed by PET. NeuroImage. 2009;45(2):598–605. doi: 10.1016/j.neuroimage.2008.11.033. [DOI] [PubMed] [Google Scholar]
- Frey S, Campbell JSW, Pike GB, Petrides M. Dissociating the human language pathways with high angular resolution diffusion fiber tractography. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2008;28(45):11435–11444. doi: 10.1523/JNEUROSCI.2388-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici, Angela D. Pathways to language: fiber tracts in the human brain. Trends in Cognitive Sciences. 2009;13(4):175–181. doi: 10.1016/j.tics.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Geschwind N. The organization of language and the brain. Science (New York, N.Y.) 1970;170(961):940–944. doi: 10.1126/science.170.3961.940. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Rilling JK. DTI tractography of the human brain’s language pathways. Cerebral Cortex (New York, N.Y.:1991) 2008;18(11):2471–2482. doi: 10.1093/cercor/bhn011. [DOI] [PubMed] [Google Scholar]
- Hatsopoulos NG, Donoghue JP. The science of neural interface systems. Annual Review of Neuroscience. 2009;32:249–266. doi: 10.1146/annurev.neuro.051508.135241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SS, Crow T, Foundas A, Amunts K, Roberts N. Broca’s area: nomenclature, anatomy, typology and asymmetry. Brain and Language. 2009;109(1):29–48. doi: 10.1016/j.bandl.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Kirshner H. Handbook of neurological speech and language disorders. New York Basel Honk Kong: H.S. Kirshner. Dekker Inc.; 1995. Classical aphasia syndromes; pp. 57–89. H.S. Kirshner. Dekker Inc. [Google Scholar]
- Klingler J. Erleichterung des makroskopischen praeparation des gehirns durch den gefrierprozess. Schweiz Arch Neurol Psychiatr. 1935;36:247–256. [Google Scholar]
- Lee HW, Shin JS, Webber WRS, Crone NE, Gingis L, Lesser RP. Reorganisation of cortical motor and language distribution in human brain. Journal of Neurology, Neurosurgery, and Psychiatry. 2009;80(3):285–290. doi: 10.1136/jnnp.2008.156067. [DOI] [PubMed] [Google Scholar]
- Leow AD, Zhu S, Zhan L, McMahon K, de Zubicaray GI, Meredith M, Wright MJ, et al. The tensor distribution function. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2009;61(1):205–214. doi: 10.1002/mrm.21852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Pandya DN. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cerebral Cortex (New York, N.Y.:1991) 2005;15(6):854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Mandonnet E, Nouet A, Gatignol P, Capelle L, Duffau H. Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain: A Journal of Neurology. 2007;130(Pt 3):623–629. doi: 10.1093/brain/awl361. [DOI] [PubMed] [Google Scholar]
- Manola L, Holsheimer J, Veltink P, Buitenweg JR. Anodal vs cathodal stimulation of motor cortex: a modeling study. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology. 2007;118(2):464–474. doi: 10.1016/j.clinph.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51(5):527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Nowak MA, Komarova NL, Niyogi P. Computational and evolutionary aspects of language. Nature. 2002;417(6889):611–617. doi: 10.1038/nature00771. [DOI] [PubMed] [Google Scholar]
- Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. Journal of Neurosurgery. 1989;71(3):316–326. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- Parker GJM, Luzzi S, Alexander DC, Wheeler-Kingshott CAM, Ciccarelli O, Lambon Ralph MA. Lateralization of ventral and dorsal auditory-language pathways in the human brain. NeuroImage. 2005;24(3):656–666. doi: 10.1016/j.neuroimage.2004.08.047. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Distinct parietal and temporal pathways to the homologues of Broca’s area in the monkey. PLoS Biology. 2009;7(8):e1000170. doi: 10.1371/journal.pbio.1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvermüller F. Brain mechanisms linking language and action. Nature Reviews. Neuroscience. 2005;6(7):576–582. doi: 10.1038/nrn1706. [DOI] [PubMed] [Google Scholar]
- Quigg M, Fountain NB. Conduction aphasia elicited by stimulation of the left posterior superior temporal gyrus. Journal of Neurology, Neurosurgery, and Psychiatry. 1999;66(3):393–396. doi: 10.1136/jnnp.66.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A, Hess C, Mukherjee P. Spatial HARDI: improved visualization of complex white matter architecture with Bayesian spatial regularization. NeuroImage. 2011;54(1):396–409. doi: 10.1016/j.neuroimage.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley H. An atlas of the basal ganglia, brain stem and spinal cord. Baltimore: Williams & Wilkins; 1953. [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annual Review of Neuroscience. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Sandrini M, Rusconi E. A brain for numbers. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2009;45(7):796–803. doi: 10.1016/j.cortex.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kümmerer D, Kellmeyer P, Vry M-S, Umarova R, et al. Ventral and dorsal pathways for language. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(46):18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann J, Pandya D. Fiber pathways of the brain. New York: Oxford University Press, Inc.; 2006. [Google Scholar]
- Schmahmann JD, Smith EE, Eichler FS, Filley CM. Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Annals of the New York Academy of Sciences. 2008;1142:266–309. doi: 10.1196/annals.1444.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons-Mackie N. Aphasia and related neurologenic language disorders (Thieme. Stuttgard New York: LaPointe LL; 1997. Conduction aphasia; pp. 63–90. [Google Scholar]
- Sporns O, Tononi G, Kötter R. The Human Connectome: A Structural Description of the Human Brain. PLoS Comput Biol. 2005;1(4):e42. doi: 10.1371/journal.pcbi.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapkini K, Vivas AB, Triarhou LC. « Does Broca »s area exist?’ Christofredo Jakob’s 1906 response to Pierre Marie’s holistic stance. Brain and Language. 2008;105(3):211–219. doi: 10.1016/j.bandl.2007.07.124. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, Smits M, Wielopolski PA, Houston GC, Krestin GP, van der Lugt A. Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right- and left-handed healthy subjects: a combined fMRI and DTI study. NeuroImage. 2007;35(3):1064–1076. doi: 10.1016/j.neuroimage.2006.12.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.