Abstract
This review provides a comprehensive overview of the established and emerging roles that organelles play in calcium signalling. The function of calcium as a secondary messenger in signal transduction networks is well documented in all eukaryotic organisms, but so far existing reviews have hardly addressed the role of organelles in calcium signalling, except for the nucleus. Therefore, a brief overview on the main calcium stores in plants—the vacuole, the endoplasmic reticulum, and the apoplast—is provided and knowledge on the regulation of calcium concentrations in different cellular compartments is summarized. The main focus of the review will be the calcium handling properties of chloroplasts, mitochondria, and peroxisomes. Recently, it became clear that these organelles not only undergo calcium regulation themselves, but are able to influence the Ca2+ signalling pathways of the cytoplasm and the entire cell. Furthermore, the relevance of recent discoveries in the animal field for the regulation of organellar calcium signals will be discussed and conclusions will be drawn regarding potential homologous mechanisms in plant cells. Finally, a short overview on bacterial calcium signalling is included to provide some ideas on the question where this typically eukaryotic signalling mechanism could have originated from during evolution.
Keywords: Bacteria, calcium flux, calcium signalling, CAS, chloroplast, EF-hand protein, ER, mitochondria, peroxisome
Introduction
Plants react to changing environmental conditions through immediate signal transduction pathways. One integral part of many signal transduction pathways, in both plant and animal cells, is the usage of free calcium ions (Ca2+) as secondary messengers. As Ca2+ forms insoluble precipitates with phosphate, which would interfere with phosphate-based metabolism, cells actively translocate Ca2+ from their cytoplasm to organelles and extracellular compartments. This principle has been conserved throughout evolution from bacteria to eukaryotes. The resulting ~10 000-fold difference between cytoplasmic (~100 nM) and non-cytoplasmic (mM) Ca2+ concentrations enables the generation of calcium signals by fast changes of cytoplasmic Ca2+ levels via membranelocalized Ca2+-permeable channels. A wide variety of signals, including abiotic, biotic, and developmental stimuli, were observed to evoke specific spatiotemporal calcium transients which are further transduced by Ca2+ sensor proteins into a transcriptional and metabolic response. So far in plants, most of the research on Ca2+ signalling has been focused on the transport mechanisms for Ca2+ into and out of the cytoplasm as well as the components involved in decoding of cytoplasmic Ca2+ signals, which has been extensively reviewed (Clapham, 2007; McAinsh and Pittman, 2009;DeFalco et al., 2010; Dodd et al., 2010; Kudla et al., 2010). However, recent advances demonstrate how different organelles are involved in the process of Ca2+ signalling. Therefore, this review starts with a brief summary of the role of the main Ca2+ storage compartments [the vacuole, endoplasmic reticulum (ER) and apoplast] and the main focus is on the emerging role of Ca2+ signalling processes in plastids, mitochondria, and peroxisomes. Finally, considering the endosymbiotic origin of chloroplasts and mitochondria, the review will end with a glimpse at bacterial Ca2+ signalling in order to propose some ideas regarding the evolutionary background of Ca2+ signalling processes in plants.
Main plant calcium stores: the vacuole, the ER, and the apoplast
The vacuole
The main storage compartment of calcium in plants is the central vacuole. Consequently, plants become hypersensitive to Ca2+ if vacuolar uptake is hampered (Cheng et al., 2005). In dicotyledonous plants Ca2+ is preferentially stored in the leaf mesophyll rather than in the epidermal vacuole (Storey and Leigh, 2004; Conn et al., 2011). Estimates of the free vacuolar Ca2+ concentration range from 0.2 mM to 1–5 mM and can reach a maximum of 80 mM of total Ca2+ (free and bound Ca2+ combined, Fig. 1) (Conn and Gilliham, 2010). Most of the Ca2+ is tightly bound to chelating agents, such as malate, citrate, and isocitrate, and, therefore, is not readily available for calcium signalling. Calcium ions might also be transiently bound to proteins, in a fashion comparable with the classical Ca2+-binding storage proteins of the ER (e.g. calreticulin). For example, a radish vacuolar Ca2+-binding protein (RVCaB) was found to improve the calcium storage capacity of the vacuole (Yuasa and Maeshima, 2001), whereas similar proteins in Arabidopsis were only found associated with the plasma membrane and probably do not play a role in calcium storage (Kato et al., 2010). Interestingly, evidence is emerging that calcium is not only stored in the vacuole, but plays an important role in signalling there as well, mainly by influencing the activity of tonoplast (vacuolar membrane) localized ion transporters (Peiter, 2011). Various Ca2+ channels and transporters were identified in the tonoplast (Pottosin and Schonknecht, 2007). In the case of ligand-gated channels, Ca2+ fluxes were measured upon the addition of the signalling molecules inositol 1,4,5-triphosphate (InsP3) and cyclic ADP-ribose (cADPR) (Allen et al., 1995), although this could not be repeated in a more recent study (Pottosin et al., 2009). Voltage-dependent Ca2+ channels and ligand-gated Ca2+ channels release Ca2+ into the cytoplasm and have been characterized mainly during the 1990s through electrophysiological studies (Sanders et al., 2002). Although they have been well characterized for almost 20 years now, no molecular identity has been found for these channels so far, except for TPC1. AtTPC1, which has no other homologues in Arabidopsis, was confirmed to be the slow vacuolar (SV, named after its voltage-gated characteristics) channel of the tonoplast (Hedrich and Neher, 1987; Peiter et al., 2005). Despite being the major vacuolar calcium release channel (Pottosin et al., 2009), debate surrounds the physiological contribution of the SV channel to calcium signalling, since knockout or overexpression of AtTPC1 has no effect on the calcium signals of a whole range of biotic and abiotic stresses (Ranf et al., 2008). Moreover, the robust phenotype of tpc1 mutants—impaired stomatal closure upon extracellular Ca2+ elevation—is not due to impaired Ca2+ homeostasis in guard cells (Peiter et al., 2005; Islam et al., 2010), and evidence for a physiologically relevant release of Ca2+ from the vacuole still needs to be provided (Hedrich and Marten, 2011).
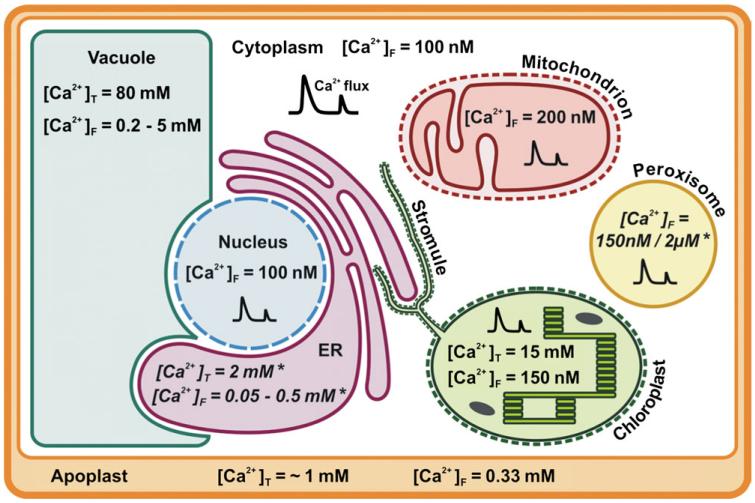
Fig. 1. Summary of organellar Ca2+ concentrations in the plant cell.
Values for reported total ([Ca2+]T) and free resting ([Ca2+]F) Ca2+ concentrations in the different organelles (apoplast, cytoplasm, vacuole, nucleus, ER, chloroplast, mitochondrion, and peroxisome) that were mentioned in the text are depicted here. The values are approximate values and probably vary depending on the tissue or plant species, but nevertheless they provide a general impression of Ca2+ levels across the cell. For ER and peroxisomes, no data on Ca2+ concentration in plants are available. The given values are taken from the animal field and marked with an (*). Calcium fluxes are illustrated by a double peak-shaped symbol ( ).
).
The ER
The calcium storage role of the ER is probably best known from human and animals, where the molecular mechanisms of calcium release from and uptake by the sarcoplasmic endoreticulum (SR; the ER of the muscle cell) during muscle contractions have been described in detail (Rossi and Dirksen, 2006). In animals, the total Ca2+ concentration in the ER is estimated to be 2 mM, while the free Ca2+ concentration varies between 50 μM and 500 μM (Fig. 1) (Coe and Michalak, 2009). In contrast, in plants, few data are available that describe the calcium storage properties of the ER. Research is hampered mainly due to the absence of direct homologues of the well-characterized mammalian InsP3 and ryanodine receptors that are responsible for ER Ca2+ efflux in higher plant genomes. Interestingly though, the genomes of several algae species, including Volvox and Chlamydomonas, do contain these receptor proteins, suggesting they were present in ancestral eukaryotes and were lost by land plants after their divergence from the chlorophyte algae (Wheeler and Brownlee, 2008). Although higher plant genomes lack these canonical receptors, calcium fluxes have been measured from ER membrane preparations upon stimulation with InsP3 and cADPR (Muir and Sanders, 1997; Navazio et al., 2001). Moreover, the signalling molecule nicotinic acid adenine dinucleotide phosphate (NAADP), that was originally described to evoke calcium release from the ER in marine invertebrate species, is active in higher plants as well (Navazio et al., 2000). Voltage-gated Ca2+ fluxes were also measured in ER membrane preparations of the tactile organs of Bryonia dioica (Klusener et al., 1995; Klusener et al., 1997) and in root-tip endomembranes of Lepidium sativum L. (Klusener and Weiler, 1999). However, whether this is a more widespread phenomenon in the ER of higher plants still needs to be established. Calreticulin, a luminal Ca2+-binding protein of the ER, was found to be important for Ca2+ homeostasis in plants, but also serves as a chaperone for protein folding (Christensen et al., 2010). The overexpression of maize calreticulin in tobacco cells lead to increased Ca2+ retention in the ER, and the down-regulation of calreticulin expression in Arabidopsis resulted in enhanced sensitivity of the plants to low Ca2+ (Persson et al., 2001). A comparison of Ca2+-binding proteins between animals and plants suggests that calreticulin is the major Ca2+ storage protein in plants (Nagata et al., 2004). In conclusion, little is known about plant ER Ca2+ storage and release compared with the animal field, which is reflected by the fact that the molecular identity of the plant ER Ca2+ release channel(s) is still unknown to date.
The apoplast
The apoplast is another major plant Ca2+ store. At the same time it acts as the ‘highway’ through which Ca2+ is trafficked to the cells by means of the water transpiration stream. Furthermore, the Ca2+ concentration in the apoplast needs to be tightly regulated as a high apoplastic Ca2+ concentration impairs stomatal movement (Kim et al., 2010) and plant cell wall rigidity depends on Ca2+ for pectate cross-linking (Hepler, 2005). That these two reasons can drastically affect plant growth was recently demonstrated by the analysis of a mutant plant that is deficient in the main vacuolar Ca2+ importers AtCAX1 and AtCAX3 (Conn et al., 2011). The authors reasoned that deficient Ca2+ sequestration in the vacuole led to an increase in free Ca2+ in the apoplast, with the above-mentioned defects as a consequence. Most of the Ca2+ in the apoplast is bound to negatively charged carboxyl groups of pectin and oxalates, and reports on the concentration of Ca2+ vary from 10 μM to 10 mM (Hepler, 2005). Conn and colleagues (2011) estimated the free Ca2+ concentration in the Arabidopsis leaf apoplast to be ~0.33 mM, and the concentration of bound Ca2+ to be 0.5 mM (Fig. 1). In addition to the voltage-gated Ca2+ channels, other ligand-gated ion channels, that can mediate fluxes of Ca2+ into the cytoplasm, include the cyclic nucleotide-gated channels (CNGCs) and glutamate receptor-like channels (GLRs). Both gene families in Arabidopsis contain 20 members and they can carry a diversity of ions, but some members have been implicated in mediating cytosolic increases of Ca2+ (Ali et al., 2006, 2007; Hua et al., 2003; Qi et al., 2006; Kaplan et al., 2007). All CNGCs and GLRs studied so far are targeted to the plasma membrane.
In conclusion, it should be noted that being large calcium stores does not automatically mean that the apoplast and the vacuole are the only relevant organelles for calcium signalling in plants. The vacuole and apopoplast, but also the ER, each contain an equally high potential for unloading Ca2+ into the cytoplasm. Furthermore, the sequestering of such high amounts of Ca2+ plays a role not only in signalling, but also in the general ion homeostasis of the plant. Also the types and amounts of channels that are present at the respective subcellular membranes to generate specific Ca2+ fluxes should be considered. This is exemplified by the SV channel AtTPC1, which is the main vacuolar Ca2+ release channel, but is probably more involved in general ion homeostasis than in signalling to biotic or abiotic stresses. On a related note, calcium release channels from the above-mentioned subcellular calcium stores were found to influence specific Ca2+ fluxes (reviewed in McAinsh and Pittman, 2009). This reflects the heterogeneous nature of calcium signalling in which various organelles can be responsible for calcium uptake and release from the cytosol. Therefore, to obtain a broader, more comprehensive understanding of calcium signalling, the role of other organelles such as chloroplasts and mitochondria should be considered.
Organellar calcium signalling
Chloroplast calcium signalling
Already some decades ago it was found that Ca2+ modulates the metabolic reactions of the chloroplast. Elevated Ca2+ concentrations effectively inhibit the Calvin–Benson cycle enzymes fructose-1,6-bisphophatase and sedoheptulose bisphosphatase, leading to a halt of photosynthetic CO2 fixation (Racker and Schroeder, 1958; Portis and Heldt, 1976; Charles and Halliwell, 1980). The total concentration of Ca2+ in the chloroplast has been estimated at 15 mM or higher (Nobel, 1969; Portis and Heldt, 1976) and increases upon illumination, by the uptake of Ca2+ from the external medium during the daylight (Kreimer et al., 1985; Roh et al., 1998). Because a high amount of free chloroplastic Ca2+ would inhibit photosynthesis and precipitate abundant chloroplastic phosphate, most of the Ca2+ is bound to thylakoid membranes or to stromal proteins (Gross and Hess, 1974; Davis and Gross, 1975; Kreimer et al., 1987). Accordingly, the resting free Ca2+ concentration in the stroma during the day was estimated to be ~150 nM (Fig. 1) (Johnson et al., 1995). Calcium also affects the photosynthetic reactions from the luminal side of the thylakoid. It is an essential cofactor of the oxygen-evolving complex and binds the 8 kDa subunit of the ATP synthase, thereby regulating the photosynthetic proton flow and ATP production (Zakharov et al., 1993; Ifuku et al., 2010). So, the chloroplast has an essential requirement for Ca2+, but needs tight control over its distribution, as indicated in Fig. 2.
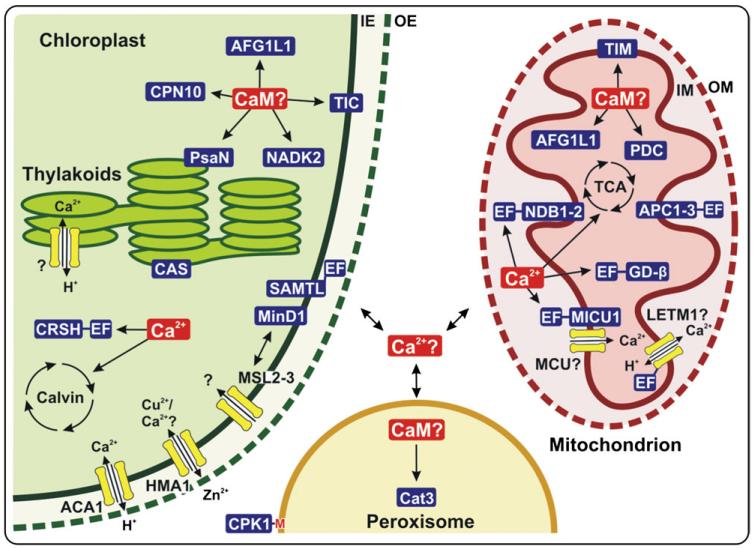
Fig. 2. Overview of proteins involved in calcium signalling in chloroplasts, mitochondria, and peroxisomes of plants.
Calcium and calmodulin (CaM) are highlighted by red boxes. Calcium transporters are depicted in yellow, and calcium/CaM-binding proteins in blue. EF-hand-containing proteins are indicated by the presence of an ‘EF’-box. Proteins of uncertain nature (lack of protein identity or uncertain function) are indicated with a (?). The central ‘Ca2+?’ symbol indicates the possibility of calcium exchange between organelles and the contribution of organelles to the cytoplasmic calcium concentration. Full names for protein abbreviations can be found in the text. Other abbreviations are Calvin (Calvin–Benson cycle), TCA (tricarboxylic acid cycle), IE (inner envelope), OE (outer envelope), IM (inner membrane), OM (outer membrane).
Using chloroplast-targeted Aequorin reporter lines, calcium fluxes were detected in chloroplasts in daily rhythms after the transition from light to dark (Johnson et al., 1995; Sai and Johnson, 2002). This stromal Ca2+ flux after the light–dark transition is proposed to be responsible for inhibiting photosynthetic CO2 fixation during the night and could help to entrain the circadian clock. The characteristics of these Ca2+ fluxes suggest that chloroplasts take up Ca2+ from the cytosol during the light period and store it in the thylakoid membrane or in another as yet unknown store. Upon transition from light to dark the Ca2+ is subsequently released from the store back into the cytosol. Light, via the thylakoid proton gradient, seems to drive Ca2+ uptake into the thylakoid lumen as well, through the activity of a Ca2+/H+ exchanger (Ettinger et al., 1999). However, the inhibition of the photosynthetic electron transport chain, and correspondingly the proton gradient, resulted in a slight increase of stromal Ca2+ during the light period, but did not inhibit the charging of the Ca2+ store that was discharged by lights off (Sai and Johnson, 2002). Hence, the authors proposed the existence of an unknown alternative stromal Ca2+ store.
The molecular nature of the chloroplast Ca2+ store is a long-standing question, and the massive difference between the total and free resting Ca2+ concentration (Fig.1) calls for a strong Ca2+-buffering mechanism in the chloroplast. It has long been known that the net negative charge of the thylakoid surface, which has been referred to as ‘a diffuse electrical layer’ (Barber et al., 1977), is balanced by the positive charge of magnesium ions (Mg2+) (Nakatani et al., 1979; Jennings et al., 1981). Recently, Fristedt and colleagues reported that the negative charge is mainly due to the gross phosphorylation of the photosynthetic complexes and that mutation of the involved kinases, STN7 and STN8 (State Transition 7 and 8), influences the Mg2+-dependent stacking of the thylakoid (Fristedt et al., 2009, 2010). Furthermore, the STN7 and STN8 kinases regulate phosphorylation of the photosynthetic complexes in a light-dependent manner (Depege et al., 2003). Since Ca2+ has highly similar metal properties to Mg2+, it is feasible that phosphorylated protein residues of the thylakoid account for much of the Ca2+-buffering capacity of the chloroplast. Considering Ca2+ transients on light to dark transitions, the link with thylakoid protein phosphorylation seems an interesting route for further investigation. Furthermore, the influence of pH should also be considered, as pH is known to influence the binding capacity of Ca2+ storage proteins (Hidalgo et al., 1996), due to protonation of the acidic residues involved in Ca2+ binding. Particularly in chloroplasts, where dramatic differences in pH are present during light conditions and change in the transition from light to dark, this is expected to impact on calcium signalling significantly.
Active Ca2+ transport has also been measured across the chloroplast inner envelope and could account for the observed Ca2+ uptake of the chloroplast during the light period. A negative inside membrane potential-driven Ca2+ transport (uniport) was measured from inner envelope membrane vesicles of Pisum sativum, confirming previous experiments with intact chloroplasts (Kreimer et al., 1985;Roh et al., 1998). This observation is strengthened by measurements with a calcium ion-selective microelectrode in intact algal cells that recorded a photosynthesis-dependent decrease in cytoplasmic Ca2+ levels (Miller and Sanders, 1987). However, the molecular identity of this channel has not been described. On the other hand, two potential Ca2+ ATPases were identified in the chloroplast envelope. The first is AtACA1 from the autoinhibited Ca2+-ATPases family and is most probably found only in root plastids (Huang et al., 1993). Strangely though, the laboratories of both Huang and Roh could not find Ca2+-ATPase activity at the envelope. Furthermore, since its description in 1993, ACA1 has been found in cauliflower tonoplast and Arabidopsis ER, prompting further study on this Ca2+-ATPase (Malmstrom et al., 1997; Dunkley et al., 2006). The second Ca2+-ATPase might be AtHMA1, a member of the heavy metal P-type ATPases that was shown to have high-affinity Ca2+ transport activity and is specifically inhibited by the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) inhibitor thapsigargin (Moreno et al., 2008). There is dispute about its exact role, because originally it was described to transport Cu2+ into the chloroplast (Seigneurin-Berny et al., 2006) but later on it was shown to function in the tolerance to excess Zn2+, by extruding it from the chloroplast (Kim et al., 2009). ALBINO3 (ALB3), the integral membrane protein and translocase involved in chloroplast biogenesis (Sundberg et al., 1997; Lewis et al., 2010), might function as a Ca2+ transporter at the thylakoid. The pea homologue of ALB3, Pisum-post-floral-specific gene 1 (PPF1), produced significant inward calcium ion currents in Novikoff human hepatoma cells, and the Ca2+ homeostasis of plants with altered expression of PPF1/ALB3 was disrupted in Arabidopsis guard cells, leading to severe growth phenotypes (Wang et al., 2003; Li et al., 2004). In conclusion, although several transporters are hypothesized to aid chloroplast Ca2+ influx, a more comprehensive analysis is needed to link these proteins directly to chloroplast Ca2+ homeostasis. Furthermore, how Ca2+ is exported from the thylakoid lumen to the stroma and subsequently to the cytosol remains largely open. To this end, Roh and colleagues showed that Ca2+ can traverse the inner envelope through a reversal of the membrane potential-driven Ca2+ transporter (Roh et al., 1998), and interesting progress on mitochondrial Ca2+ transport in mammals could shed light on the underlying molecular basis of calcium transport across the chloroplast envelope (see mitochondrial calcium signalling).
From more recent work, it became apparent that calmodulin (CaM) has regulatory roles in chloroplasts. The best example is the import of nuclear-encoded chloroplast proteins via the TOC (translocon at the outer envelope of chloroplasts) and TIC (translocon at the inner envelope of chloroplasts) complexes. Calcium/CaM was found to promote chloroplast import, and this is most likely to be due to the direct interaction of calmodulin with the stromal side of Tic32 (TIC protein of 32 kDa) (Chigri et al., 2005, 2006). Furthermore, in a different study, electrophysiological measurements revealed that the gating properties of the main pore-forming subunit of the TIC complex, Tic110, were affected in a specific manner by Ca2+ (Balsera et al., 2009). Taken together, Ca2+ seems to influence chloroplast protein import at these closely linked sites. Another function of the chloroplast that was found to be modulated by Ca2+ is the chloroplast inner vesicle transport system (Morre et al., 1991). This system is proposed to have a role in thylakoid membrane biogenesis and was found to be disrupted by CaM inhibitors as well as calcium depletion (Westphal et al., 2001, 2003). Chloroplast division might also be regulated in a Ca2+-dependent manner by AtMinD1 (Arabidopsis Minicell D1), which is a part of the chloroplast division machinery, because its ATPase activity depends on Ca2+, rather than Mg2+ as is the case in bacteria (Aldridge and Moller, 2005). Furthermore, two chloroplastic mechanosensitive ion channels MSL2 and 3 [homologues of the bacterial mechanosensitive (MS) channel MscS: MscS-Like 2 and 3] were found to influence chloroplast division and act in concert with the Min proteins (Haswell and Meyerowitz, 2006; Wilson et al., 2011). It would be interesting to see if MSL2 and 3 can directly influence the Ca2+-dependent activity of AtMinD1, either by mediating Ca2+ fluxes or through a depolarization of the chloroplast envelope.
Chloroplast movement in response to fluctuating light conditions is another Ca2+-dependent process shown to occur in different species, such as Lemna trisulca and tobacco (Tlalka and Fricker, 1999; Anielska-Mazur et al., 2009). Chloroplasts move along the actin cytoskeleton in plant cells (Kong and Wada, 2011), and accordingly the use of Ca2+ chelators and CaM inhibitors revealed the stabilizing effect of Ca2+ on actin polymerization and its importance for chloroplast movement. However, chloroplast movement was influenced even in the presence of an intact actin network, thereby evoking a signalling function of Ca2+ in light-induced chloroplast movements.
NAD kinase (NADK) is an intriguing case for chloroplastic calcium-dependent regulations. Through the use of CaM inhibitors, NADK was the first plant enzyme found to be activated by CaM, and the majority of its activity was found to localize to the chloroplast (Jarrett et al., 1982). Jarrett and colleagues purified a CaM-containing fraction from pea chloroplasts to near homogeneity, but did not identify the protein responsible for NADK activation. The initial increase of Ca2+ in the chloroplast upon illumination was proposed to activate NADK via the interaction with CaM. NADK catalyses the light-dependent conversion of NAD to NADP, the final electron acceptor of the photosynthetic electron transport chain. Thereby it provides an important reduction potential for the plant, which becomes obvious in the reduced growth and hypersensitivity to oxidative stress of chloroplastic NADK knock-out plants (Chai et al., 2005; Takahashi et al., 2006). From more recent work, it became apparent that one of the three Arabidopsis NADK isoforms, AtNADK2, is responsible for the CaM-dependent NADK activity in chloroplasts (Turner et al., 2004; Waller et al., 2010). A region of 45 amino acids within the long N-terminal extension of AtNADK2 was found to be sufficient and necessary to bind to CaM. Other examples of chloroplast proteins that were found to interact with CaM are AtPsaN (a subunit of photosystem I), the chaperonin AtCPN10, and AtAFG1L1, an AAA+-ATPase (Yang and Poovaiah, 2000; Reddy et al., 2002; Bussemer et al., 2009). However, the functional relevance of these interactions needs further study. To conclude, although a chloroplastic CaM or CaM-like protein is still unknown, various stromal proteins are able to bind to CaM, leading to a change of their activity. In the same context, a Ca2+-dependent protein kinase activity and cross-talk between Ca2+ signalling and protein phosphorylation is described in two accompanying papers in this issue (Bayer et al., 2012;Stael et al., 2012). Therefore, the identification of CaMs or other Ca2+-binding proteins in chloroplasts is expected greatly to advance the understanding of the physiological relevance of these calcium/CaM-dependent regulations.
So far there are two reports of EF-hand-containing proteins in the chloroplast. The best documented is the Ca2+-activated RelA/SpoT homologous protein (CRSH), that is novel both for being a chloroplastic EF-hand protein and for its alleged function. CRSH contains two EF-hands and a RelA/SpoT enzymatic domain, which is responsible for the calcium-dependent production of a small signalling nucleotide, guanosine 5′-diphosphate 3′-diphosphate (ppGpp) (Masuda et al., 2008). ppGpp signalling was discovered in bacteria as a response to stress conditions, such as nutrient deprivation, in a process called ‘the bacterial stringent response’, and homologous proteins have since been found in various plant species (Tozawa et al., 2007; Masuda et al., 2008). The bacterial RelA and SpoT proteins do not contain EF-hands, which seems to be an exclusive trait of CRSH in higher plants. ppGpp was found mainly in the chloroplast, and the levels changed after different stress and hormonal treatments and upon the transition from light to dark (Takahashi et al., 2004). Similar to its function in transcription and translation in bacteria, ppGpp was found to modulate exclusively the function of the bacterial type plastid-encoded plastid RNA polymerase (PEP), but not the nuclear-encoded plastid RNA polymerase (NEP) (Sato et al., 2009). It seems that the bacterial stringent response has been conserved in chloroplasts from its cyanobacterial origin, but more experimental work is needed to elucidate its physiological function and the interplay with Ca2+ signalling, in plants. The second chloroplastic EF-hand protein is a substrate carrier (AtSAMTL, for SAMT-like) that contains a single EF-hand and belongs to the mitochondrial carrier protein family. It was recently found in a targeted proteomics screen and was confirmed to reside in the chloroplast envelope by yellow fluorescent protein (YFP) fusion analysis (Bayer et al., 2011). Based on a detailed functional prediction, it is proposed to import S-adenosylmethionine (SAM) into the chloroplast in addition to the better described SAM transporter, SAMT (Stael et al., 2011b).
Reports on a chloroplast-localized protein involved in ‘calcium sensing’ (AtCAS) evoke the idea that chloroplasts may modulate cytoplasmic Ca2+ signalling. AtCAS was first reported as a plasma membrane-localized Ca2+-sensing receptor, important for inducing stomatal closure provoked by elevation of the extracellular Ca2+ concentration ([Ca2+]ext)—a hallmark of stomatal movement (Han et al., 2003). The protein was found to bind Ca2+ with a low affinity and high capacity, and down-regulation of its expression impaired the production of [Ca2+]ext-induced cytoplasmic Ca2+ oscillations. However, subsequent reports identified AtCAS to be targeted to the thylakoid membrane (Friso et al., 2004; Nomura et al., 2008; Vainonen et al., 2008; Weinl et al., 2008). Nevertheless, knock-out of AtCAS was still confirmed, by different labs, to impair [Ca2+]ext-induced stomatal closure, whereas overexpression of CAS promoted stomatal closure in the absence of externally applied Ca2+ (Nomura et al., 2008; Weinl et al., 2008). Disruption of AtCAS in Arabidopsis retarded plant growth, and AtCAS was found to be increasingly phosphorylated by the state transition kinase AtSTN8 under increasing light intensities (Vainonen et al., 2008). Similarly, CAS is required for photoacclimation in the process of non-photochemical quenching (NPQ) in Chlamydomonas where cas-knockdown lines displayed a high-light-sensitive phenotype (Petroutsos et al., 2011). Stomata of cas Arabidopsis plants displayed normal closure after the application of externally imposed cytoplasmic Ca2+ oscillations, indicating that the guard cells are still responsive to Ca2+ signals but most probably have a defect in the generation of Ca2+ fluxes (Weinl et al., 2008). This suggests that the chloroplast can sense and influence cytoplasmic Ca2+ levels; however, the molecular mechanisms behind these processes still await discovery.
Mitochondrial calcium signalling
In contrast to the animal and human field, where mitochondrial calcium uptake and release are well studied and known to play important cellular roles, relatively few data are available on calcium signalling in plant mitochondria. It is now well established that mitochondria in animals function as transient calcium stores that can produce Ca2+ microdomains through a close interaction with the ER, thereby modulating Ca2+ signatures (Clapham, 2007; Laude and Simpson, 2009). On the other hand, the elevation of Ca2+ inside the mitochondria positively affects ATP production by up-regulating the major limiting enzymes of the citric acid cycle (similar to the up-regulation of the Calvin cycle in chloroplasts) (McCormack et al., 1990). The overaccumulation of Ca2+ in the mitochondria is linked to the induction of apoptosis by opening of the mitochondrial permeability transition pore (mPTP) and the subsequent release of mitochondrial apoptosis markers, such as cytochrome c (Giacomello et al., 2007; Szabadkai and Duchen, 2008). This process apparently occurs in plants as well (Arpagaus et al., 2002; Tiwari et al., 2002; Virolainen et al., 2002).
In plants, the resting free Ca2+ concentration in the mitochondria was estimated to be ~200 nM (Logan and Knight, 2003), with most of the mitochondrial Ca2+ probably being bound in the form of a ready-releasable amorphous phosphate precipitate (Fig. 1) (Chalmers and Nicholls, 2003; Starkov, 2010). Ca2+ fluxes in Arabidopsis mitochondria have been observed upon various stimulations (Logan and Knight, 2003). Whereas most mitochondrial Ca2+ fluxes were similar, H2O2 and touch stimulation produced a signal that was different from the concurrently occurring cytosolic Ca2+ fluxes. This indicates that mitochondria are not just passive calcium sinks, but are able to regulate their own Ca2+ fluxes as depicted in Fig. 2.
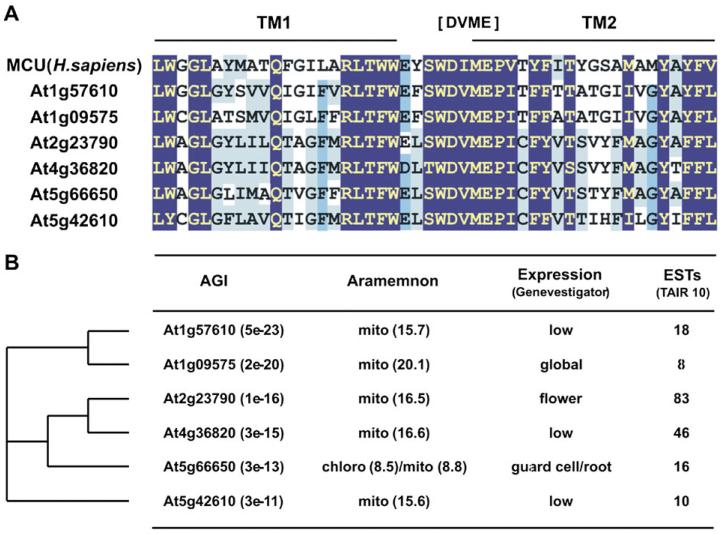
Surprisingly little information about Ca2+ transporters of plant mitochondria exists, compared with the knowledge on this topic in the animal and human field (Collins and Meyer, 2010; Starkov, 2010). Animal mitochondria take up high or low concentrations of Ca2+ differently, and various Ca2+ channels have recently been described (Hajnoczky and Csordas, 2010; Hoppe, 2010). The mitochondrial calcium uniporter (MCU) imports Ca2+ from ‘microdomains’ with highly elevated cytosolic Ca2+ concentrations. MCU has been extensively studied and its properties are well delineated: (i) electrogenic transport that is driven by a negative inside membrane potential; (ii) sensitivity to ruthenium red; (iii) low affinity for Ca2+; and (iv) regulation by Ca2+. After almost 50 years, the molecular nature of the uniporter has been discovered in animals in a series of recent studies (Perocchi et al., 2010; Baughman et al., 2011; De Stefani et al., 2011). In an integrative genomics and proteomics search, Perocchi and colleagues found an EF-hand-containing protein, which is targeted to the mitochondrial inner membrane, to induce a Ca2+-dependent calcium influx into the mitochondria. They called it MICU1, for mitochondrial Ca2+ uptake 1, and reasoned that it is most probably not the uniporter itself, but the sensor for Ca2+ that regulates the MCU. In two independent follow-up experiments, based on the phylogenetic profile, protein distribution, and characteristics of MICU1, a 40 kDa protein was identified as the actual Ca2+ pore and therefore it was called MCU. The MCU protein has two transmembrane helices, connected by a conserved loop containing the amino acids DIME, and most probably oligomerizes in the mitochondrial inner membrane. Intriguingly, the Arabidopsis genome contains six uncharacterized genes with a rather low homology to the human MCU, which share the pore-forming domain with two transmembrane helices connected by a conserved DVME motive (Fig. 3A). These potential Arabidopsis MCU isoforms are highly predicted to localize to the mitochondria, except for At5g66650, which is equally predicted to target to the chloroplast according to Aramemnon (Schwacke et al., 2003) (Fig. 3B). A quick survey of the expression profiles by GENEVESTIGATOR (Zimmermann et al., 2004) suggests a differential tissue expression of the various isoforms, for example to the flower, root, or guard cell. The possibility that an MCU isoform is located in the chloroplast is supported by the notion that the light-dependent Ca2+ influx in chloroplasts, reported by Kreimer et al. (1985), is inhibited by ruthenium red and that the overall characteristics are similar to the mitochondrial Ca2+ uniport. Moreover, the potential chloroplastic localization and preferential guard cell expression of At5g66650 are interesting in the light of stomatal movement, since chloroplasts have been implicated in ‘sensing’ extracellular calcium transients through CAS protein and the modulation of stomatal closure.
Fig. 3. Conserved regulators of mitochondrial Ca2+ import between humans and plants.
(A) Alignment of the transmembrane domain of the human mitochondrial calcium uniporter (MCU, gi24308400) with its six homologues from Arabidopsis thaliana displays the two transmembrane helices (TM1 and TM2) connected by a highly conserved loop domain containing the amino acids DIME in human and DVME in Arabidopsis. (B) Overview of targeting and expression of the MCU homologues. The cladogram displays the homology relationship between the Arabidopsis proteins. The blast e-value for each protein relative to the human MCU is written in parentheses after the Arabidopsis gene identifier (AGI). Protein localization was predicted by Aramemnon (Schwacke et al., 2003) and the meta-score is indicated in parentheses. Organ-related RNA expression profiles were examined with the help of the GENEVESTIGATOR browser (Zimmermann et al., 2004) and the presence of expressed sequence tags (ESTs) in the TAIR 10 database (www.arabidopsis.org).
In addition to the low-affinity MCU system, mammalian mitochondria take up Ca2+ at lower cytoplasmic concentrations via the high-affinity Ca2+/H+ antiporter, LETM1 (Jiang et al., 2009). Interestingly, the closest Arabidopsis homologue of LETM1, the mitochondrial high-affinity Ca2+/H+ antiporter, contains two EF-hands and was recently found to localize to the mitochondria by green fluorescent protein (GFP) fusion analysis (Van Aken et al., 2009). In this study the authors attempted to delineate the mitochondrial stress response by searching for mitochondrial proteins that show the greatest expression variation upon 16 selected stress treatments. The fact that the AtLETM1-like protein was included in this set further prompts the study of this protein for its Ca2+/H+ activity and the involvement of mitochondrial calcium signalling upon stress conditions in Arabidopsis. In mammals, calcium efflux from mitochondria can occur through a reversal of LETM1 or through the Na+/Ca2+ exchanger NCLX, that was recently found to be targeted to the mitochondria (Palty et al., 2010). To conclude, the presence of homologues in the Arabidopsis genome of all the above-mentioned proteins raises the interesting possibility of translating the mammalian findings directly into plants.
Calcium/CaM was reported to promote mitochondrial protein import in a manner similar to how it occurs in chloroplasts (Kuhn et al., 2009). Furthermore, it was found to be a plant-specific trait, because yeast mitochondria were not susceptible to the CaM inhibitors and calcium ionophores used in the study. Pyruvate dehydrogenase activity was also found to decline with the use of CaM inhibitors (Miernyk et al., 1987). The pyruvate dehydrogenase complex (PDC) assists in the conversion of pyruvate into acetyl-CoA and, therefore, an important connection between glycolysis and the citric acid cycle might be regulated by Ca2+ in plants. The finding of a CaM-binding AAA+-ATPase, AtAFG1L1, with dual localization to chloroplasts and mitochondria further suggests the presence of CaM in the plant mitochondria (Bussemer et al., 2009). So far, the only report on CaM in the mitochondria came from Biro and colleagues, who found an oat CaM in the intermembrane space that was lost upon removal of the outer mitochondrial membrane (Biro et al., 1984).
The glutamate dehydrogenase β subunit (GD-β) localizes to the mitochondrial matrix, contains a single EF-hand, and its activity was reported to be stimulated by the addition of Ca2+ (Turano et al., 1997). Two isoforms of the type II NAD(P)H:quinone oxidoreductases of Arabidopsis, AtNDB1 and AtNDB2 were found to be externally attached to the inner membrane of mitochondria and to contain functional EF-hands (Geisler et al., 2007).
Ample evidence for the influence of mitochondrial Ca2+ on cytoplasmic Ca2+ fluxes exists in animals (Laude and Simpson, 2009), and these observations probably hold true for plants, given that plant mitochondria exhibit a similar handling of Ca2+ (Silva et al., 1992). Maize mitochondria have been shown to release Ca2+ upon hypoxia, and their internal Ca2+ concentration changes rapidly and in a reversible manner when returning back to normoxia (Subbaiah et al., 1994, 1998). The recently described mitochondrial carrier proteins APC1, 2, and 3 (short for ATP-phosphate carrier1, 2, and 3) could be immediate targets of these mitochondrial Ca2+ fluxes (Stael et al., 2011b). APC1–APC3 each contain four EF-hands and are better known from yeast and mammals to import ATP into mitochondria in a Ca2+-dependent manner (Chen, 2004; Traba et al., 2008). Mitochondrial ATP import might be a first line of defence against hypoxia, in order to maintain the mitochondrial membrane potential and cell viability by a reversal of the FoF1 H+-ATP synthase. Other evidence for mitochondrial Ca2+ efflux comes from the measurement of Ca2+ changes in the root hair in response to the disruption of actin polymerization by latrunculin B (Lat-B) and Jasplakinolide (Jas) (Wang et al., 2010). Root hair cells produce a tip-focused Ca2+ gradient, with Ca2+ oscillation lagging behind growth oscillation, in a similar fashion to growing pollen tubes (Cardenas et al., 2008; Monshausen et al., 2008). Modulation of actin polymerization caused a release of Ca2+ from the mitochondria, probably via the mPTP, and a concurrent increase in cytosolic Ca2+ concentrations. Furthermore, Wang and colleagues showed that the concentration of free Ca2+ in mitochondria displays a gradient from a high concentration at the growing tip (~500 nM) to low at the base (~200 nM). Together with the observation that mitochondria shuttle up and down the actin filaments through the root hair (Zheng et al., 2009), mitochondria may play a role in the removal or buffering of the Ca2+ fluxes in the root hair apex, as was previously hypothesized (Hetherington and Brownlee, 2004).
Peroxisomal calcium signalling
Information on calcium signalling in the peroxisome is scarce. It was only recently that the occurrence of calcium fluxes in the peroxisome was recognized in mammals (Raychaudhury et al., 2006; Drago et al., 2008; Lasorsa et al., 2008). Two different resting free Ca2+ concentrations have been reported from these experiments, namely 150 nM (Drago et al., 2008) and 2 μM (Lasorsa et al., 2008). A similar study, based on the expression of a peroxisomal-targeted chameleon probe (a Ca2+ reporter protein construct), showed that plant peroxisomes undergo Ca2+ fluxes as well (Costa et al., 2010). Furthermore, the peroxisomal Ca2+ increase was found to enhance the detoxification of the reactive oxygen species (ROS) H2O2 in vivo through the activity of the catalase isoform 3 (AtCAT3), as was proposed earlier. In 2002, Yang and Poovaiah demonstrated the in vitro stimulation of AtCAT3 activity by the calcium-dependent binding of CaM, and provided evidence for the presence of CaM in peroxisomes. However, they did not find its molecular identity (Yang and Poovaiah, 2002). A member of the Arabidopsis calcium-dependent protein kinase family, AtCPK1, was found to bind to the external surface of peroxisomes (Dammann et al., 2003; Coca and San Segundo, 2010) and lipid bodies, which is most likely to be due to an N-terminal myristoylation signal, which determines the localization of many calcium-dependent protein kinases (CDPKs) and other kinases (Lu and Hrabak, 2002; Benetka et al., 2008; Stael et al., 2011a). AtCPK1 was shown to mediate pathogen resistance (Coca and San Segundo, 2010). Given the few reports, the already presented interplay between calcium and ROS prompts the further study of the molecular nature of calcium handling and signalling in and around peroxisomes.
Nuclear calcium signalling
Nuclear Ca2+ in plants has recently been reviewed comprehensively (Mazars et al., 2009, 2011) and will therefore be discussed only briefly. Calcium signals in the nucleus enable the cell to react to environmental changes by alteration of gene expression in animals and plants (Ikura et al., 2002; M.C. Kim et al., 2009; Galon et al., 2010;Reddy et al., 2011). This may sound straightforward; however, only recently were direct target genes of Ca2+-dependent gene expression reported in Arabidopsis (Kaplan et al., 2006). The main problem is to distinguish them from non-Ca2+-dependent gene expression mediated via different nuclear signalling pathways in response to stress treatment (Finkler et al., 2007; Wurzinger et al., 2011). Various studies revealed that many of the Ca2+-regulated genes contained abscisic acid-responsive element (ABRE)-related cis-elements and were already implicated in the abiotic stress response before (Hirayama and Shinozaki, 2010). Ca2+ can influence transcription through Ca2+-binding transcription factors, CaM-binding transcription activators [AtCAMTAs, six members in Arabidopsis (Bouche et al., 2002)], or phosphorylation of transcription factors by CDPKs, of which quite a number are present in the nucleus (Dammann et al., 2003;Choi et al., 2005; Zhu et al., 2007; Boudsocq et al., 2010;Mehlmer et al., 2010).
Various stimuli such as abiotic or biotic stresses as well as symbiotic signals elicit nuclear Ca2+ fluxes (Pauly et al., 2000;Lecourieux et al., 2005; Oldroyd and Downie, 2006; Sieberer et al., 2009). A lot of the research on nuclear Ca2+ signalling has been focused on the question whether nuclei can generate Ca2+ fluxes autonomously from the cytosol. The nuclear resting free Ca2+ concentration was measured at ~100 nM (Brini et al., 1993; Mazars et al., 2009), which is similar to the cytosolic value (Fig. 1). The fact that the nucleoplasm and cytosol are connected by relatively large pores in the nuclear envelope might suggest that nuclear Ca2+ fluxes are the result of passive influx from the cytosol. However, the delays that have been measured between cytosolic and nuclear Ca2+ fluxes implicate the opposite. Furthermore, when tobacco cells were treated with a biologically active derivate of jasmonate (jasmonate-isoleucine), nuclei were able to generate Ca2+ fluxes without any measurable cytosolic Ca2+ responses (Walter et al., 2007). Experiments with isolated nuclei further emphasized the autonomous nature of plant nuclei from the extranuclear environment with regards to Ca2+ signalling (Xiong et al., 2004, 2008).
If the nucleus is able to produce its own Ca2+ fluxes, than the nuclear envelope is most likely to serve as the responsible Ca2+ store. Analysis of its protein components has been hampered by the difficult extraction of intact nuclei and the contamination by ER membranes (Matzke et al., 2010). Nonetheless, several Ca2+ channels and transporters have been found at the inner and outer membrane of the nuclear envelope, and it is hypothesized that the nuclear pores can act as Ca2+-selective channels. Two interesting examples of nuclear ion channels are Castor and Polux. Initially they had been reported to localize to chloroplasts (Imaizumi-Anraku et al., 2005), but more recent work showed that they are cation channels in the nuclear envelope (Charpentier et al., 2008). Mutation of Castor and Polux abolishes perinuclear Ca2+ spiking and, concurrently, root symbioses with arbuscular mycorrhizal fungi and rhizobial bacteria in legume species, such as Lotus japonicas, or non-legume species, such as rice (Chen et al., 2009). Root symbiosis in legumes is probably the best example to demonstrate the importance of nuclear calcium signalling and has been extensively reviewed elsewhere (Oldroyd and Downie, 2006; Murray, 2011).
Bacterial calcium signalling
The observation that Ca2+ signalling is present in organelles of endosymbiotic origin leads to the question of whether some basic mechanisms of generation or decoding of Ca2+ signals can also be found in bacteria. Ca2+ signalling is generally considered to be a typical eukaryotic invention. However, bacteria also need to exclude Ca2+ actively from the cytosol in order to operate their phosphate-based metabolism. Similar to eukaryotes, the resulting unequal distribution of free Ca2+ between the cytosol and the extracellular space would provide the basis to use Ca2+ as a signalling molecule. Furthermore, the discovery of various Ca2+ transporters, Ca2+-binding proteins, and Ca2+-dependent processes all argue in favour of some calcium signalling taking place in bacteria.
It has been shown that the intracellular free Ca2+ concentration in bacteria is actively maintained in the range of 100-300 nM (Knight et al., 1991; Herbaud et al., 1998;Jones et al., 1999; Torrecilla et al., 2000). Like in eukaryotes, distinct transient changes in the cytoplasmic Ca2+ concentration were also observed in bacteria (Dominguez, 2004) for different stimuli such as heat, cold (Torrecilla et al., 2000), salt, osmotic stress (Torrecilla et al., 2001), and oxidative stress (Herbaud et al., 1998), or in response to nitrogen deprivation (Torrecilla et al., 2004; Zhao et al., 2005; Shi et al., 2006; Leganes et al., 2009).
An early bioinformatic investigation revealed the presence of Na+, K+, and Ca2+ selective voltage-gated ion channels, Ca2+ cation antiporters, and P-type Ca2+-ATPases in 18 bacterial genomes based on sequence homology to eukaryotic counterparts (Paulsen et al., 2000). Since then, experimental evidence for Ca2+ transporters in bacteria has accumulated (Raeymaekers et al., 2002;Fujisawa et al., 2009; Faxen et al., 2011). In the cyanobacteria, Synechococcus elongatus PCC 7942, the PacL gene product was identified as a P-type Ca2+-ATPase (Berkelman et al., 1994), and in Synechocystis sp. PCC 6803 the mechanosensitive ion channel MscL was reported to be responsible for major Ca2+ effluxes observed after membrane depolarization and high temperature (Nazarenko et al., 2003). Finally, Ca2+/H+ antiporters, homologous to the CAX gene family in Arabidopsis, have also been identified in cyanobacterial genomes (Waditee et al., 2004). The CAX protein from Synechocystis sp. PCC 6803 was shown to be biochemically functional, localized at the plasma membrane, and required for salt tolerance, as well as for adaptation to an alkaline milieu in this strain. In contrast, molecular identities of Ca2+-specific influx systems in bacteria are largely missing so far.
If intracellular Ca2+-mediated signalling should take place in bacteria like in eukaryotic cells, then calcium sensors in the form of Ca2+-binding proteins must exist. During the last decade various successful attempts have been made to identify EF-hand-containing proteins in bacterial genomes (Michiels et al., 2002; Zhou et al., 2006;Rigden et al., 2011). Globally, it can be said that EF-hand-containing proteins are found in most bacterial genomes and that they are evenly distributed throughout families and genera. In addition, new calcium-binding motifs are predicted and found in bacterial proteins, such as the Dx[DN]xDG motif, which is part of the EF-hand motif. This further increases the number of potential Ca2+-binding proteins in bacteria (Morgan et al., 2006; Hu et al., 2011;Rigden et al., 2011). For example, the calmodulin-related protein CasA from the symbiotic bacterium Rhizobium etli is secreted during infection and colonization of its plant host (Xi et al., 2000).
How calcium signalling can impact on bacterial physiology is best illustrated by the effect of a Ca2+-binding protein on nitrogen fixation. CcbP, a non-EF hand-containing protein from Nostoc (Anabaena) sp. PCC7120, has been demonstrated to be an intracellular Ca2+-binding protein which is involved in heterocyst differentiation upon limitation of combined nitrogen (Zhao et al., 2005; Hu et al., 2011). Several genera of cyanobacteria grow heterocysts, which are specialized cells capable of fixing N2, and they make up a considerable part of the global nitrogen cycle. At the initial stage of differentiation, CcbP becomes degraded by hetR, a serine protease specifically expressed at that stage of development, resulting in a rise of intracellular free [Ca2+] and subsequently heterocyst formation (Shi et al., 2006). Furthermore, alterations of the nitrogen depletion-induced Ca2+ transient led to an arrest of heterocyst differentiation (Torrecilla et al., 2004).
Except for the Ca2+ transporters, none of the already described bacterial Ca2+-binding proteins in the literature has clear homologues in higher plant species. The apparent lack of conservation of calcium signalling components is most probably due to the rather different physiology of bacteria and higher plants, whereas the active extrusion of Ca2+ by transporters from the cytosol seems to be conserved and universal. Moreover, the eukaryotic cell acquired more complexity in terms of compartmentalization than a bacterial cell, and consequently needs a signal transduction system, such as calcium signalling, to orchestrate all processes in those different subcellular compartments. Interestingly, highly conserved bacterial processes governed by proteins such as MinD and the RelA/SpoT homologue CRSH (Givens et al., 2004; Aldridge and Moller, 2005) might have acquired Ca2+ regulation to be able to function coordinately in the cellular environment of higher plants. Table 1 lists 19 conserved proteins between Arabidopsis thaliana and Nostoc (Anabaena) sp. PCC7120, containing the Ca2+-binding Dx[DN]xDG motif. It is interesting that most of these proteins localize, or are predicted to localize to different compartments other than to the plastid. Nevertheless, together with two other proteins, CML41 is predicted to localize to the plastid, which may suggest that the so far ‘missing’ plastidal CaM might be a CaM-like protein.
Table 1. Arabidopsis proteins of putative cyanobacterial origin containing the Ca2+ binding Dx[DN]xDG motif.
| AGI code | Annotation | Aramemnon predictiona |
MS/MSb | ||
|---|---|---|---|---|---|
| Plastids | Mitochondria | Secretory pathway | |||
| At3g12290 | Amino acid dehydrogenase family protein | 1.2 | 0 | 0 | PM, plastid |
| At3g09090 | DEX1 defective in exine formation | 0 | 3.3 | 28.2 | PM |
| At2g34020 | Calcium-binding EF-hand family protein | 5 | 6.2 | 16 | |
| At3g50770 | CML41 calmodulin-like 41 | 11.4 | 2.3 | 0 | |
| At4g10060 | β-Glucosidase GBA2 type family protein | 0 | 0 | 0 | |
| At1g33700 | β-Glucosidase GBA2 type family protein | 0 | 0 | 0 | PM |
| At5g49900 | β-Glucosidase GBA2 type family protein | 0 | 0 | 0.8 | Vacuole |
| At1g74960 | KAS2 fatty acid biosynthesis 1 | 27.2 | 0 | 7.5 | Plastid |
| At2g04540 | mtKAS1 β-ketoacyl synthase | 2.2 | 19 | 0.5 | |
| At1g62810 | Copper amine oxidase family protein | 0.4 | 0 | 30.6 | |
| At1g31690 | Copper amine oxidase family protein | 3.3 | 0 | 27.7 | Peroxisomes |
| At3g43670 | Copper amine oxidase family protein | 0.1 | 0 | 35.2 | |
| At1g31670 | Copper amine oxidase family protein | 0 | 0 | 29.1 | |
| At4g12290 | Copper amine oxidase family protein | 2.2 | 0 | 24.3 | PM |
| At4g14940 | AO1 amine oxidase 1 | 0 | 0 | 28.9 | Extracellular |
| At3g48560 | CSR1 Chlorsulphuron/imidazolinone resistant 1 | 30.6 | 0.6 | 0 | Plastid |
| At5g04130 | GYRB2 DNA gyrase B2 | 3.6 | 18.9 | 2.6 | Mitochondrion |
| At3g10270 | GYRB1 DNA gyrase B1 | 0 | 0 | 0 | Mitochondrion |
| At3g23890 | TOPII topoisomerase II | 4 | 3.2 | 0 | |
Aramemnon score refers to the likelihood of localization for a protein predicted with Aramemnon (Schwacke et al., 2003).
MS/MS indicates evidence for the localization of the protein in a cellular compartment based on proteomics studies summarized in the SUBA database (Heazlewood et al., 2007).
PM, plasma membrane.
Conclusions and future perspectives
A central question in Ca2+ signalling is how such a simple ion can mount an appropriate and specific physiological response, when almost every stress or developmental process elicits calcium fluxes in the cell. While the answer to the specificity question is manifold (Sanders et al., 2002), it can partly be explained by the compartmentalized nature of calcium signalling. In other words, the possibility to mobilize Ca2+ from various subcellular stores, including chloroplasts and mitochondria, combined with its rapid release and uptake, implies that the effect of a Ca2+ flux can be highly localized to microdomains in the cytosol (Laude and Simpson, 2009). Furthermore, through the physical separation of organelles from the cytosol, Ca2+-dependent regulation inside organelles, such as chloroplasts, mitochondria, peroxisomes, and nuclei, can be exerted in a highly localized and specific manner.
The question arises then: what is the physiological relevance of the organelles to cellular Ca2+ signalling? At least in chloroplasts, the impact of impaired organellar Ca2+ handling on plant physiology has already been demonstrated by CAS and PPF1. The mutation of, respectively, a putative chloroplastic Ca2+ sensor and a transporter led to impaired stomatal movement and impaired plant growth. To provide further insight into the physiological relevance of organellar Ca2+ signalling, it will be crucial to identify the proteins that are responsible for calcium transport (channels and transporters), storage, and decoding of the Ca2+ signals that have been observed. Likewise, more should be known on the stimuli that provoke Ca2+ signals in mitochondria and peroxisomes, and especially in chloroplasts, where until now only light and dark were found to induce Ca2+ fluxes. For future research, the combination of the molecular players and the elicitors of calcium signalling in organelles together with newly generated detection systems for measuring organellar Ca2+ concentrations in planta (Krebs et al., 2012; Mehlmer et al., 2012) should provide fruitful grounds for further discoveries.
Interplay between organelles can greatly affect Ca2+ signalling. In human and animal cells, the intimacy of ER and mitochondria was found to be beneficial for movement of Ca2+ between the two stores, and this was further strengthened by the identification of the molecular bridge between them (Kornmann et al., 2009; de Brito and Scorrano, 2010). Similarly in plants, Ca2+ might be exchanged between chloroplasts, mitochondria, and peroxisomes, while they are required to be closely connected for photorespiration and the exchange of metabolites (Majeran et al., 2010; Gowik et al., 2011). Moreover, Ca2+ exchange between chloroplasts and the ER cannot be excluded. The co-localization of the ER with stromules—stroma-filled tubules that extend from plastids (Fig. 1)—considerably increases the interactive surface between the plastid and the ER (Schattat et al., 2011a, b). The observation that sugars and abiotic stresses induce stromule formation (Gray et al., 2012; Schattat and Klosgen, 2011) further supports the idea that Ca2+ signals could be modulated by the interplay between organelles.
The data on bacterial calcium signalling clearly indicate that the prerequisites for eukaryotic Ca2+ signalling, an actively maintained Ca2+ gradient across membranes and Ca2+ sensor proteins, are already present in bacteria. The even distribution and frequent occurrence of Ca2+ transporters and EF-hand-containing proteins throughout genomes of bacterial families (Paulsen et al., 2000; Zhou et al., 2006) further indicate a prokaryotic origin of these core elements in eukaryotic Ca2+ signalling. For this, it is reasonable to search for conserved Ca2+ signalling processes between bacteria, eukaryotes, and their endosymbionts, mitochondria and plastids, too.
Acknowledgements
Work in the author’s lab has been funded by the Austrian GEN-AU program in the ERA-PG project CROPP (FFG Project No. 818514), the Austrian Science Foundation (FWF) (P19825-B12), and by the EU in the Marie-Curie ITN COSI (ITN 2008 GA 215-174).
Abbreviations
- ABA
abscisic acid
- CAS
calcium sensing
- CDPK
calcium-dependent protein kinase
- PM
plasma membrane
References
- Aldridge C, Moller SG. The plastid division protein AtMinD1 is a Ca2+-ATPase stimulated by AtMinE1. Journal of Biological Chemistry. 2005;280:31673–31678. doi: 10.1074/jbc.M505126200. [DOI] [PubMed] [Google Scholar]
- Ali R, Ma W, Lemtiri-Chlieh F, Tsaltas D, Leng Q, von Bodman S, Berkowitz GA. Death don’t have no mercy and neither does calcium: Arabidopsis CYCLIC NUCLEOTIDE GATED CHANNEL2 and innate immunity. The Plant Cell. 2007;19:1081–1095. doi: 10.1105/tpc.106.045096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali R, Zielinski RE, Berkowitz GA. Expression of plant cyclic nucleotide-gated cation channels in yeast. Journal of Experimental Botany. 2006;57:125–138. doi: 10.1093/jxb/erj012. [DOI] [PubMed] [Google Scholar]
- Allen GJ, Muir SR, Sanders D. Release of Ca2+ from individual plant vacuoles by both InsP3 and cyclic ADP-ribose. Science. 1995;268:735–737. doi: 10.1126/science.7732384. [DOI] [PubMed] [Google Scholar]
- Anielska-Mazur A, Bernas T, Gabrys H. In vivo reorganization of the actin cytoskeleton in leaves of Nicotiana tabacum L. transformed with plastin–GFP. Correlation with light-activated hloroplast responses. BMC Plant Biology. 2009;9:64. doi: 10.1186/1471-2229-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpagaus S, Rawyler A, Braendle R. Occurrence and characteristics of the mitochondrial permeability transition in plants. Journal of Biological Chemistry. 2002;277:1780–1787. doi: 10.1074/jbc.M109416200. [DOI] [PubMed] [Google Scholar]
- Balsera M, Goetze TA, Kovacs-Bogdan E, Schurmann P, Wagner R, Buchanan BB, Soll J, Bolter B. Characterization of Tic110, a channel-forming protein at the inner envelope membrane of chloroplasts, unveils a response to Ca2+ and a stromal regulatory disulfide bridge. Journal of Biological Chemistry. 2009;284:2603–2616. doi: 10.1074/jbc.M807134200. [DOI] [PubMed] [Google Scholar]
- Barber J, Mills J, Love A. Electrical diffuse layers and their influence on photosynthetic processes. FEBS Letters. 1977;74:174–181. doi: 10.1016/0014-5793(77)80841-7. [DOI] [PubMed] [Google Scholar]
- Baughman JM, Perocchi F, Girgis HS, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer RG, Stael S, Csaszar E, Teige M. Mining the soluble chloroplast proteome by affinity chromatography. Proteomics. 2011;11:1287–1299. doi: 10.1002/pmic.201000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer RG, Stael S, Rocha AG, Mair A, Vothknecht UC, Teige M. Chloroplast localised protein kinases: a step forward towards a complete inventory. Journal of Experimental Botany. 2012;63:1713–1723. doi: 10.1093/jxb/err377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetka W, Mehlmer N, Maurer-Stroh S, Sammer M, Koranda M, Neumuller R, Betschinger J, Knoblich JA, Teige M, Eisenhaber F. Experimental testing of predicted myristoylation targets involved in asymmetric cell division and calcium-dependent signalling. Cell Cycle. 2008;7:3709–3719. doi: 10.4161/cc.7.23.7176. [DOI] [PubMed] [Google Scholar]
- Berkelman T, Garret-Engele P, Hoffman NE. The pacL gene of Synechococcus sp. strain PCC 7942 encodes a Ca2+-transporting ATPase. Journal of Bacteriology. 1994;176:4430–4436. doi: 10.1128/jb.176.14.4430-4436.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro RL, Daye S, Serlin BS, Terry ME, Datta N, Sopory SK, Roux SJ. Characterization of oat calmodulin and radioimmunoassay of its subcellular distribution. Plant Physiology. 1984;75:382–386. doi: 10.1104/pp.75.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouche N, Scharlat A, Snedden W, Bouchez D, Fromm H. A novel family of calmodulin-binding transcription activators in multicellular organisms. Journal of Biological Chemistry. 2002;277:21851–21861. doi: 10.1074/jbc.M200268200. [DOI] [PubMed] [Google Scholar]
- Boudsocq M, Willmann MR, McCormack M, Lee H, Shan L, He P, Bush J, Cheng SH, Sheen J. Differential innate immune signalling via Ca2+ sensor protein kinases. Nature. 2010;464:418–422. doi: 10.1038/nature08794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brini M, Murgia M, Pasti L, Picard D, Pozzan T, Rizzuto R. Nuclear Ca2+ concentration measured with specifically targeted recombinant aequorin. EMBO Journal. 1993;12:4813–4819. doi: 10.1002/j.1460-2075.1993.tb06170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussemer J, Chigri F, Vothknecht UC. Arabidopsis ATPase family gene 1-like protein 1 is a calmodulin-binding AAA+-ATPase with a dual localization in chloroplasts and mitochondria. FEBS Journal. 2009;276:3870–3880. doi: 10.1111/j.1742-4658.2009.07102.x. [DOI] [PubMed] [Google Scholar]
- Cardenas L, Lovy-Wheeler A, Kunkel JG, Hepler PK. Pollen tube growth oscillations and intracellular calcium levels are reversibly modulated by actin polymerization. Plant Physiology. 2008;146:1611–1621. doi: 10.1104/pp.107.113035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai MF, Chen QJ, An R, Chen YM, Chen J, Wang XC. NADK2, an Arabidopsis chloroplastic NAD kinase, plays a vital role in both chlorophyll synthesis and chloroplast protection. Plant Molecular Biology. 2005;59:553–564. doi: 10.1007/s11103-005-6802-y. [DOI] [PubMed] [Google Scholar]
- Chalmers S, Nicholls DG. The relationship between free and total calcium concentrations in the matrix of liver and brain itochondria. Journal of Biological Chemistry. 2003;278:19062–19070. doi: 10.1074/jbc.M212661200. [DOI] [PubMed] [Google Scholar]
- Charles SA, Halliwell B. Action of calcium ions on spinach (Spinacia oleracea) chloroplast fructose bisphosphatase and other nzymes of the Calvin cycle. Biochemical Journal. 1980;188:775–779. doi: 10.1042/bj1880775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier M, Bredemeier R, Wanner G, Takeda N, Schleiff E, Parniske M. Lotus japonicus CASTOR and POLLUX are ion channels essential for perinuclear calcium spiking in legume root ndosymbiosis. The Plant Cell. 2008;20:3467–3479. doi: 10.1105/tpc.108.063255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Fan C, Gao M, Zhu H. Antiquity and function of CASTOR and POLLUX, the twin ion channel-encoding genes key to the evolution of root symbioses in plants. Plant Physiology. 2009;149:306–317. doi: 10.1104/pp.108.131540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XJ. Sal1p, a calcium-dependent carrier protein that suppresses an essential cellular function associated with the Aac2 isoform of ADP/ATP translocase in Saccharomyces cerevisiae. Genetics. 2004;167:607–617. doi: 10.1534/genetics.103.023655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng NH, Pittman JK, Shigaki T, Lachmansingh J, LeClere S, Lahner B, Salt DE, Hirschi KD. Functional association of Arabidopsis CAX1 and CAX3 is required for normal growth and ion omeostasis. Plant Physiology. 2005;138:2048–2060. doi: 10.1104/pp.105.061218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chigri F, Hormann F, Stamp A, Stammers DK, Bolter B, Soll J, Vothknecht UC. Calcium regulation of chloroplast protein translocation is mediated by calmodulin binding to Tic32. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:16051–16056. doi: 10.1073/pnas.0607150103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chigri F, Soll J, Vothknecht UC. Calcium regulation of chloroplast protein import. The Plant Journal. 2005;42:821–831. doi: 10.1111/j.1365-313X.2005.02414.x. [DOI] [PubMed] [Google Scholar]
- Choi HI, Park HJ, Park JH, Kim S, Im MY, Seo HH, Kim YW, Hwang I, Kim SY. Arabidopsis calcium-dependent protein kinase AtCPK32 interacts with ABF4, a transcriptional regulator of abscisic acid-responsive gene expression, and modulates its activity. Plant Physiology. 2005;139:1750–1761. doi: 10.1104/pp.105.069757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen A, Svensson K, Thelin L, et al. Higher plant calreticulins have acquired specialized functions in Arabidopsis. PLoS One. 2010;5:e11342. doi: 10.1371/journal.pone.0011342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Coca M, San Segundo B. AtCPK1 calcium-dependent protein kinase mediates pathogen resistance in Arabidopsis. The Plant Journal. 2010;63:526–540. doi: 10.1111/j.1365-313X.2010.04255.x. [DOI] [PubMed] [Google Scholar]
- Coe H, Michalak M. Calcium binding chaperones of the endoplasmic reticulum. General Physiology and Biophysics. 2009;28:F96–F103. Spec No Focus. [PubMed] [Google Scholar]
- Collins S, Meyer T. Cell biology: a sensor for calcium uptake. Nature. 2010;467:283. doi: 10.1038/467283a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn S, Gilliham M. Comparative physiology of elemental istributions in plants. Annals of Botany. 2010;105:1081–1102. doi: 10.1093/aob/mcq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn SJ, Gilliham M, Athman A, et al. Cell-specific vacuolar calcium storage mediated by CAX1 regulates apoplastic calcium concentration, gas exchange, and plant productivity in Arabidopsis. The Plant Cell. 2011;23:240–257. doi: 10.1105/tpc.109.072769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Drago I, Behera S, Zottini M, Pizzo P, Schroeder JI, Pozzan T, Lo Schiavo F. H2O2 in plant peroxisomes: an in vivo analysis uncovers a Ca2+-dependent scavenging system. The Plant Journal. 2010;62:760–772. doi: 10.1111/j.1365-313X.2010.04190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammann C, Ichida A, Hong B, Romanowsky SM, Hrabak EM, Harmon AC, Pickard BG, Harper JF. Subcellular targeting of nine calcium-dependent protein kinase isoforms from Arabidopsis. Plant Physiology. 2003;132:1840–1848. doi: 10.1104/pp.103.020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DJ, Gross EL. Protein–protein interactions of light-harvesting pigment protein from spinach chloroplasts. I. Ca2+-binding nd its relation to protein association. Biochimica et Biophysica Acta. 1975;387:557–567. doi: 10.1016/0005-2728(75)90093-6. [DOI] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L. An intimate liaison: spatial rganization of the endoplasmic reticulum-mitochondria relationship. EMBO Journal. 2010;29:2715–2723. doi: 10.1038/emboj.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFalco TA, Bender KW, Snedden WA. Breaking the code: Ca2+ sensors in plant signalling. Biochemical Journal. 2010;425:27–40. doi: 10.1042/BJ20091147. [DOI] [PubMed] [Google Scholar]
- Depege N, Bellafiore S, Rochaix JD. Role of chloroplast rotein kinase Stt7 in LHCII phosphorylation and state transition in Chlamydomonas. Science. 2003;299:1572–1575. doi: 10.1126/science.1081397. [DOI] [PubMed] [Google Scholar]
- Dodd AN, Kudla J, Sanders D. The language of calcium signaling. Annual Review of Plant Biology. 2010;61:593–620. doi: 10.1146/annurev-arplant-070109-104628. [DOI] [PubMed] [Google Scholar]
- Dominguez DC. Calcium signalling in bacteria. Molecular Microbiology. 2004;54:291–297. doi: 10.1111/j.1365-2958.2004.04276.x. [DOI] [PubMed] [Google Scholar]
- Drago I, Giacomello M, Pizzo P, Pozzan T. Calcium dynamics in the peroxisomal lumen of living cells. Journal of Biological Chemistry. 2008;283:14384–14390. doi: 10.1074/jbc.M800600200. [DOI] [PubMed] [Google Scholar]
- Dunkley TP, Hester S, Shadforth IP, et al. Mapping the Arabidopsis organelle proteome. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6518–6523. doi: 10.1073/pnas.0506958103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger WF, Clear AM, Fanning KJ, Peck ML. Identification of a Ca2+/H+ antiport in the plant chloroplast thylakoid membrane. Plant Physiology. 1999;119:1379–1386. doi: 10.1104/pp.119.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faxen K, Andersen JL, Gourdon P, Fedosova N, Morth JP, Nissen P, Moller JV. Characterization of a Listeria monocytogenes Ca2+ pump: a SERCA-type ATPase with only one Ca2+-binding site. Journal of Biological Chemistry. 2011;286:1609–1617. doi: 10.1074/jbc.M110.176784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkler A, Kaplan B, Fromm H. Ca-responsive cis-elements in plants. Plant Signaling Behavior. 2007;2:17–19. doi: 10.4161/psb.2.1.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friso G, Giacomelli L, Ytterberg AJ, Peltier JB, Rudella A, Sun Q, Wijk KJ. In-depth analysis of the thylakoid membrane proteome of Arabidopsis thaliana chloroplasts: new proteins, new functions, and a plastid proteome database. The Plant Cell. 2004;16:478–499. doi: 10.1105/tpc.017814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fristedt R, Granath P, Vener AV. A protein phosphorylation threshold for functional stacking of plant photosynthetic membranes. PLoS One. 2010;5:e10963. doi: 10.1371/journal.pone.0010963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fristedt R, Willig A, Granath P, Crevecoeur M, Rochaix JD, Vener AV. Phosphorylation of photosystem II controls functional macroscopic folding of photosynthetic membranes in Arabidopsis. The Plant Cell. 2009;21:3950–3964. doi: 10.1105/tpc.109.069435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa M, Wada Y, Tsuchiya T, Ito M. Characterization of Bacillus subtilis YfkE (ChaA): a calcium-specific Ca2+/H+ antiporter of the CaCA family. Archives of Microbiology. 2009;191:649–657. doi: 10.1007/s00203-009-0494-7. [DOI] [PubMed] [Google Scholar]
- Galon Y, Finkler A, Fromm H. Calcium-regulated transcription in plants. Molecular Plant. 2010;3:653–669. doi: 10.1093/mp/ssq019. [DOI] [PubMed] [Google Scholar]
- Geisler DA, Broselid C, Hederstedt L, Rasmusson AG. Ca2+-binding and Ca2+-independent respiratory NADH and NADPH dehydrogenases of Arabidopsis thaliana. Journal of Biological Chemistry. 2007;282:28455–28464. doi: 10.1074/jbc.M704674200. [DOI] [PubMed] [Google Scholar]
- Giacomello M, Drago I, Pizzo P, Pozzan T. Mitochondrial Ca2+ as a key regulator of cell life and death. Cell Death and Differentiation. 2007;14:1267–1274. doi: 10.1038/sj.cdd.4402147. [DOI] [PubMed] [Google Scholar]
- Givens RM, Lin MH, Taylor DJ, Mechold U, Berry JO, Hernandez VJ. Inducible expression, enzymatic activity, and origin of higher plant homologues of bacterial RelA/SpoT stress proteins in Nicotiana tabacum. Journal of Biological Chemistry. 2004;279:7495–7504. doi: 10.1074/jbc.M311573200. [DOI] [PubMed] [Google Scholar]
- Gowik U, Brautigam A, Weber KL, Weber AP, Westhoff P. Evolution of c4 photosynthesis in the genus flaveria: how many and which genes does it take to make c4? The Plant Cell. 2011;23:2087–2105. doi: 10.1105/tpc.111.086264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JC, Hansen MR, Shaw DJ, Graham K, Dale R, Smallman P, Natesan SK, Newell CA. Plastid stromules are induced by stress treatments acting through abscisic acid. The Plant Journal. 2012;69:387–398. doi: 10.1111/j.1365-313X.2011.04800.x. [DOI] [PubMed] [Google Scholar]
- Gross EL, Hess SC. Correlation between calcium ion binding to chloroplast membranes and divalent cation-induced structural changes and changes in chlorophyll a fluorescence. Biochimica et Biophysica Acta. 1974;339:334–346. doi: 10.1016/0005-2736(74)90160-6. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G, Csordas G. Calcium signalling: fishing out molecules of mitochondrial calcium transport. Current Biology. 2010;20:R888–R891. doi: 10.1016/j.cub.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Tang R, Anderson LK, Woerner TE, Pei ZM. A cell surface receptor mediates extracellular Ca2+ sensing in guard cells. Nature. 2003;425:196–200. doi: 10.1038/nature01932. [DOI] [PubMed] [Google Scholar]
- Haswell ES, Meyerowitz EM. MscS-like proteins control plastid size and shape in Arabidopsis thaliana. Current Biology. 2006;16:1–11. doi: 10.1016/j.cub.2005.11.044. [DOI] [PubMed] [Google Scholar]
- Heazlewood JL, Verboom RE, Tonti-Filippini J, Small I, Millar AH. SUBA: the Arabidopsis Subcellular Database. Nucleic Acids Research. 2007;35:D213–D218. doi: 10.1093/nar/gkl863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R, Marten I. TPC1-SV channels gain shape. Molecular Plant. 2011;4:428–441. doi: 10.1093/mp/ssr017. [DOI] [PubMed] [Google Scholar]
- Hedrich R, Neher E. Cytoplasmic calcium regulates voltage-dependent ion channels in plant vacuoles. Nature. 1987;329:833–836. [Google Scholar]
- Hepler PK. Calcium: a central regulator of plant growth and development. The Plant Cell. 2005;17:2142–2155. doi: 10.1105/tpc.105.032508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbaud ML, Guiseppi A, Denizot F, Haiech J, Kilhoffer MC. Calcium signalling in Bacillus subtilis. Biochimica et Biophysica Acta. 1998;1448:212–226. doi: 10.1016/s0167-4889(98)00145-1. [DOI] [PubMed] [Google Scholar]
- Hetherington AM, Brownlee C. The generation of Ca2+ signals in plants. Annual Review of Plant Biology. 2004;55:401–427. doi: 10.1146/annurev.arplant.55.031903.141624. [DOI] [PubMed] [Google Scholar]
- Hidalgo C, Donoso P, Rodriguez PH. Protons induce calsequestrin conformational changes. Biophysics Journal. 1996;71:2130–2137. doi: 10.1016/S0006-3495(96)79413-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama T, Shinozaki K. Research on plant abiotic stress responses in the post-genome era: past, present and future. The Plant Journal. 2010;61:1041–1052. doi: 10.1111/j.1365-313X.2010.04124.x. [DOI] [PubMed] [Google Scholar]
- Hoppe UC. Mitochondrial calcium channels. FEBS Letters. 2010;584:1975–1981. doi: 10.1016/j.febslet.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Hu Y, Zhang X, Shi Y, Zhou Y, Zhang W, Su XD, Xia B, Zhao J, Jin C. Structures of Anabaena calcium-binding protein CcbP: insights into Ca2+ signaling during heterocyst differentiation. Journal of Biological Chemistry. 2011;286:12381–12388. doi: 10.1074/jbc.M110.201186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua BG, Mercier RW, Leng Q, Berkowitz GA. Plants do it differently. A new basis for potassium/sodium selectivity in the pore of an ion channel. Plant Physiology. 2003;132:1353–1361. doi: 10.1104/pp.103.020560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Berkelman T, Franklin AE, Hoffman NE. Characterization of a gene encoding a Ca2+-ATPase-like protein in the plastid envelope. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:10066–10070. doi: 10.1073/pnas.90.21.10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifuku K, Ishihara S, Sato F. Molecular functions of oxygen-evolving complex family proteins in photosynthetic electron flow. Journal of Integrative Plant Biology. 2010;52:723–734. doi: 10.1111/j.1744-7909.2010.00976.x. [DOI] [PubMed] [Google Scholar]
- Ikura M, Osawa M, Ames JB. The role of calcium-binding proteins in the control of transcription: structure to function. Bioessays. 2002;24:625–636. doi: 10.1002/bies.10105. [DOI] [PubMed] [Google Scholar]
- Imaizumi-Anraku H, Takeda N, Charpentier M, et al. Plastid proteins crucial for symbiotic fungal and bacterial entry into plant roots. Nature. 2005;433:527–531. doi: 10.1038/nature03237. [DOI] [PubMed] [Google Scholar]
- Islam MM, Munemasa S, Hossain MA, Nakamura Y, Mori IC, Murata Y. Roles of AtTPC1, vacuolar two pore channel 1, in Arabidopsis stomatal closure. Plant and Cell Physiology. 2010;51:302–311. doi: 10.1093/pcp/pcq001. [DOI] [PubMed] [Google Scholar]
- Jarrett HW, Brown CJ, Black CC, Cormier MJ. Evidence that calmodulin is in the chloroplast of peas and serves a regulatory role in photosynthesis. Journal of Biological Chemistry. 1982;257:13795–13804. [PubMed] [Google Scholar]
- Jennings RC, Gerola PD, Garlaschi FM, Forti G. Effects of trypsin and cations on chloroplast membranes. Plant Physiology. 1981;67:212–215. doi: 10.1104/pp.67.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Zhao L, Clapham DE. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science. 2009;326:144–147. doi: 10.1126/science.1175145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CH, Knight MR, Kondo T, Masson P, Sedbrook J, Haley A, Trewavas A. Circadian oscillations of cytosolic and chloroplastic free calcium in plants. Science. 1995;269:1863–1865. doi: 10.1126/science.7569925. [DOI] [PubMed] [Google Scholar]
- Jones HE, Holland IB, Baker HL, Campbell AK. Slow changes in cytosolic free Ca2+ in Escherichia coli highlight two putative influx mechanisms in response to changes in extracellular calcium. Cell Calcium. 1999;25:265–274. doi: 10.1054/ceca.1999.0028. [DOI] [PubMed] [Google Scholar]
- Kaplan B, Davydov O, Knight H, Galon Y, Knight MR, Fluhr R, Fromm H. Rapid transcriptome changes induced by cytosolic Ca2+ transients reveal ABRE-related sequences as Ca2+-responsive cis elements in Arabidopsis. The Plant Cell. 2006;18:2733–2748. doi: 10.1105/tpc.106.042713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan B, Sherman T, Fromm H. Cyclic nucleotide-gated channels in plants. FEBS Letters. 2007;581:2237–2246. doi: 10.1016/j.febslet.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Kato M, Nagasaki-Takeuchi N, Ide Y, Maeshima M. An Arabidopsis hydrophilic Ca2+-binding protein with a PEVK-rich domain, PCaP2, is associated with the plasma membrane and interacts with calmodulin and phosphatidylinositol phosphates. Plant and Cell Physiology. 2010;51:366–379. doi: 10.1093/pcp/pcq003. [DOI] [PubMed] [Google Scholar]
- Kim MC, Chung WS, Yun DJ, Cho MJ. Calcium and calmodulin-mediated regulation of gene expression in plants. Molecular Plant. 2009;2:13–21. doi: 10.1093/mp/ssn091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Bohmer M, Hu H, Nishimura N, Schroeder JI. Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annual Review of Plant Biology. 2010;61:561–591. doi: 10.1146/annurev-arplant-042809-112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YY, Choi H, Segami S, Cho HT, Martinoia E, Maeshima M, Lee Y. AtHMA1 contributes to the detoxification of excess Zn(II) in Arabidopsis. The Plant Journal. 2009;58:737–753. doi: 10.1111/j.1365-313X.2009.03818.x. [DOI] [PubMed] [Google Scholar]
- Klusener B, Boheim G, Liss H, Engelberth J, Weiler EW. Gadolinium-sensitive, voltage-dependent calcium release channels in the endoplasmic reticulum of a higher plant mechanoreceptor organ. EMBO Journal. 1995;14:2708–2714. doi: 10.1002/j.1460-2075.1995.tb07271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusener B, Boheim G, Weiler EW. Modulation of the ER Ca2+ channel BCC1 from tendrils of Bryonia dioica by divalent cations, protons and H2O2. FEBS Letters. 1997;407:230–234. doi: 10.1016/s0014-5793(97)00364-5. [DOI] [PubMed] [Google Scholar]
- Klusener B, Weiler EW. A calcium-selective channel from root-tip endomembranes of garden cress. Plant Physiology. 1999;119:1399–1406. doi: 10.1104/pp.119.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight MR, Campbell AK, Smith SM, Trewavas AJ. Recombinant aequorin as a probe for cytosolic free Ca2+ in Escherichia coli. FEBS Letters. 1991;282:405–408. doi: 10.1016/0014-5793(91)80524-7. [DOI] [PubMed] [Google Scholar]
- Kong SG, Wada M. New insights into dynamic actin-based chloroplast photorelocation movement. Molecular Plant. 2011;4:771–781. doi: 10.1093/mp/ssr061. [DOI] [PubMed] [Google Scholar]
- Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, Walter P. An ER–mitochondria tethering complex revealed by a synthetic biology screen. Science. 2009;325:477–481. doi: 10.1126/science.1175088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs M, Held K, Binder A, Hashimoto K, Den Herder G, Parniske M, Kudla J, Schumacher K. FRET-based genetically encoded sensors allow high-resolution live cell imaging of Ca2+ dynamics. The Plant Journal. 2012;69:181–192. doi: 10.1111/j.1365-313X.2011.04780.x. [DOI] [PubMed] [Google Scholar]
- Kreimer G, Melkonian M, Holtum JAM, Latzko E. Characterization of calcium fluxes across the envelope of intact spinach chloroplasts. Planta. 1985;166:515–523. doi: 10.1007/BF00391276. [DOI] [PubMed] [Google Scholar]
- Kreimer G, Surek B, Woodrow IE, Latzko E. Calcium binding by spinach stromal proteins. Planta. 1987;171:259–265. doi: 10.1007/BF00391103. [DOI] [PubMed] [Google Scholar]
- Kudla J, Batistic O, Hashimoto K. Calcium signals: the lead currency of plant information processing. The Plant Cell. 2010;22:541–563. doi: 10.1105/tpc.109.072686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S, Bussemer J, Chigri F, Vothknecht UC. Calcium depletion and calmodulin inhibition affect the import of nuclear-encoded proteins into plant mitochondria. The Plant Journal. 2009;58:694–705. doi: 10.1111/j.1365-313X.2009.03810.x. [DOI] [PubMed] [Google Scholar]
- Lasorsa FM, Pinton P, Palmieri L, Scarcia P, Rottensteiner H, Rizzuto R, Palmieri F. Peroxisomes as novel players in cell calcium homeostasis. Journal of Biological Chemistry. 2008;283:15300–15308. doi: 10.1074/jbc.M800648200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laude AJ, Simpson AW. Compartmentalized signalling: Ca2+ compartments, microdomains and the many facets of Ca2+ signalling. FEBS Journal. 2009;276:1800–1816. doi: 10.1111/j.1742-4658.2009.06927.x. [DOI] [PubMed] [Google Scholar]
- Lecourieux D, Lamotte O, Bourque S, Wendehenne D, Mazars C, Ranjeva R, Pugin A. Proteinaceous and oligosaccharidic elicitors induce different calcium signatures in the nucleus of tobacco cells. Cell Calcium. 2005;38:527–538. doi: 10.1016/j.ceca.2005.06.036. [DOI] [PubMed] [Google Scholar]
- Leganes F, Forchhammer K, Fernandez-Pinas F. Role of calcium in acclimation of the cyanobacterium Synechococcus elongatus PCC 7942 to nitrogen starvation. Microbiology. 2009;155:25–34. doi: 10.1099/mic.0.022251-0. [DOI] [PubMed] [Google Scholar]
- Lewis NE, Marty NJ, Kathir KM, Rajalingam D, Kight AD, Daily A, Kumar TK, Henry RL, Goforth RL. A dynamic cpSRP43-Albino3 interaction mediates translocase regulation of chloroplast signal recognition particle (cpSRP)-targeting components. Journal of Biological Chemistry. 2010;285:34220–34230. doi: 10.1074/jbc.M110.160093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang DY, Li Q, Xu YJ, Cui KM, Zhu YX. PPF1 inhibits programmed cell death in apical meristems of both G2 pea and transgenic Arabidopsis plants possibly by delaying cytosolic Ca2+ elevation. Cell Calcium. 2004;35:71–77. doi: 10.1016/j.ceca.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Logan DC, Knight MR. Mitochondrial and cytosolic calcium dynamics are differentially regulated in plants. Plant Physiology. 2003;133:21–24. doi: 10.1104/pp.103.026047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SX, Hrabak EM. An Arabidopsis calcium-dependent protein kinase is associated with the endoplasmic reticulum. Plant Physiology. 2002;128:1008–1021. doi: 10.1104/pp.010770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeran W, Friso G, Ponnala L, et al. Structural and metabolic transitions of C4 leaf development and differentiation defined by microscopy and quantitative proteomics in maize. The Plant Cell. 2010;22:3509–3542. doi: 10.1105/tpc.110.079764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmstrom S, Askerlund P, Palmgren MG. A calmodulin-stimulated Ca2+-ATPase from plant vacuolar membranes with a putative regulatory domain at its N-terminus. FEBS Letters. 1997;400:324–328. doi: 10.1016/s0014-5793(96)01448-2. [DOI] [PubMed] [Google Scholar]
- Masuda S, Mizusawa K, Narisawa T, Tozawa Y, Ohta H, Takamiya K. The bacterial stringent response, conserved in chloroplasts, controls plant fertilization. Plant and Cell Physiology. 2008;49:135–141. doi: 10.1093/pcp/pcm177. [DOI] [PubMed] [Google Scholar]
- Matzke AJ, Weiger TM, Matzke M. Ion channels at the nucleus: electrophysiology meets the genome. Molecular Plant. 2010;3:642–652. doi: 10.1093/mp/ssq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazars C, Bourque S, Mithofer A, Pugin A, Ranjeva R. Calcium homeostasis in plant cell nuclei. New Phytologist. 2009;181:261–274. doi: 10.1111/j.1469-8137.2008.02680.x. [DOI] [PubMed] [Google Scholar]
- Mazars C, Briere C, Bourque S, Thuleau P. Nuclear calcium signaling: an emerging topic in plants. Biochimie. 2011;93:2068–2074. doi: 10.1016/j.biochi.2011.05.039. [DOI] [PubMed] [Google Scholar]
- McAinsh MR, Pittman JK. Shaping the calcium signature. New Phytologist. 2009;181:275–294. doi: 10.1111/j.1469-8137.2008.02682.x. [DOI] [PubMed] [Google Scholar]
- McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiological Reviewa. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- Mehlmer N, Parvin N, Hurst CH, Knight MR, Teige M, Vothknecht UC. A toolset of aequorin expression vectors for in planta studies of subcellular calcium concentrations in Arabidopsis thaliana. Journal of Experimental Botany. 2012;63:1751–1761. doi: 10.1093/jxb/err406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlmer N, Wurzinger B, Stael S, Hofmann-Rodrigues D, Csaszar E, Pfister B, Bayer R, Teige M. The Ca2+-dependent protein kinase CPK3 is required for MAPK-independent salt-stress acclimation in Arabidopsis. The Plant Journal. 2010;63:484–498. doi: 10.1111/j.1365-313X.2010.04257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels J, Xi C, Verhaert J, Vanderleyden J. The functions of Ca2+ in bacteria: a role for EF-hand proteins? Trends in Microbiology. 2002;10:87–93. doi: 10.1016/s0966-842x(01)02284-3. [DOI] [PubMed] [Google Scholar]
- Miernyk JA, Fang TK, Randall DD. Calmodulin antagonists inhibit the mitochondrial pyruvate dehydrogenase complex. Journal of Biological Chemistry. 1987;262:15338–15340. [PubMed] [Google Scholar]
- Miller AJ, Sanders D. Depletion of cytosolic free calcium induced by photosynthesis. Nature. 1987;326:397–400. [Google Scholar]
- Monshausen GB, Messerli MA, Gilroy S. Imaging of the Yellow Cameleon 3.6 indicator reveals that elevations in cytosolic Ca2+ follow oscillating increases in growth in root hairs of Arabidopsis. Plant Physiology. 2008;147:1690–1698. doi: 10.1104/pp.108.123638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno I, Norambuena L, Maturana D, Toro M, Vergara C, Orellana A, Zurita-Silva A, Ordenes VR. AtHMA1 is a thapsigargin-sensitive Ca2+/heavy metal pump. Journal of Biological Chemistry. 2008;283:9633–9641. doi: 10.1074/jbc.M800736200. [DOI] [PubMed] [Google Scholar]
- Morgan RO, Martin-Almedina S, Garcia M, Jhoncon-Kooyip J, Fernandez MP. Deciphering function and mechanism of calcium-binding proteins from their evolutionary imprints. Biochimica et Biophysica Acta. 2006;1763:1238–1249. doi: 10.1016/j.bbamcr.2006.09.028. [DOI] [PubMed] [Google Scholar]
- Morre DJ, Sellden G, Sundqvist C, Sandelius AS. Stromal low temperature compartment derived from the inner membrane of the chloroplast envelope. Plant Physiology. 1991;97:1558–1564. doi: 10.1104/pp.97.4.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir SR, Sanders D. Inositol 1,4,5-trisphosphate-sensitive Ca2+ release across nonvacuolar membranes in cauliflower. Plant Physiology. 1997;114:1511–1521. doi: 10.1104/pp.114.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JD. Invasion by invitation: rhizobial infection in legumes. Molecular Plant-Microbe Interactions. 2011;24:631–639. doi: 10.1094/MPMI-08-10-0181. [DOI] [PubMed] [Google Scholar]
- Nagata T, Iizumi S, Satoh K, et al. Comparative analysis of plant and animal calcium signal transduction element using plant full-length cDNA data. Molecular Biology and Evolution. 2004;21:1855–1870. doi: 10.1093/molbev/msh197. [DOI] [PubMed] [Google Scholar]
- Nakatani HY, Barber J, Minski MJ. The influence of the thylakoid membrane surface properties on the distribution of ions in chloroplasts. Biochimica et Biophysica Acta. 1979;545:24–35. doi: 10.1016/0005-2728(79)90110-5. [DOI] [PubMed] [Google Scholar]
- Navazio L, Bewell MA, Siddiqua A, Dickinson GD, Galione A, Sanders D. Calcium release from the endoplasmic reticulum of higher plants elicited by the NADP metabolite nicotinic acid adenine dinucleotide phosphate. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8693–8698. doi: 10.1073/pnas.140217897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navazio L, Mariani P, Sanders D. Mobilization of Ca2+ by cyclic ADP-ribose from the endoplasmic reticulum of cauliflower florets. Plant Physiology. 2001;125:2129–2138. doi: 10.1104/pp.125.4.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarenko LV, Andreev IM, Lyukevich AA, Pisareva TV, Los DA. Calcium release from Synechocystis cells induced by depolarization of the plasma membrane: MscL as an outward Ca2+ channel. Microbiology. 2003;149:1147–1153. doi: 10.1099/mic.0.26074-0. [DOI] [PubMed] [Google Scholar]
- Nobel PS. Light-induced changes in the ionic content of chloroplasts in. Pisum sativum. Biochimica et Biophysica Acta. 1969;172:134–143. doi: 10.1016/0005-2728(69)90098-x. [DOI] [PubMed] [Google Scholar]
- Nomura H, Komori T, Kobori M, Nakahira Y, Shiina T. Evidence for chloroplast control of external Ca2+-induced cytosolic Ca2+ transients and stomatal closure. The Plant Journal. 2008;53:988–998. doi: 10.1111/j.1365-313X.2007.03390.x. [DOI] [PubMed] [Google Scholar]
- Oldroyd GE, Downie JA. Nuclear calcium changes at the core of symbiosis signalling. Current Opinion in Plant Biology. 2006;9:351–357. doi: 10.1016/j.pbi.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Palty R, Silverman WF, Hershfinkel M, et al. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:436–441. doi: 10.1073/pnas.0908099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen IT, Nguyen L, Sliwinski MK, Rabus R, Saier MH., Jr. Microbial genome analyses: comparative transport capabilities in eighteen prokaryotes. Journal of Molecular Biology. 2000;301:75–100. doi: 10.1006/jmbi.2000.3961. [DOI] [PubMed] [Google Scholar]
- Pauly N, Knight MR, Thuleau P, van der Luit AH, Moreau M, Trewavas AJ, Ranjeva R, Mazars C. Control of free calcium in plant cell nuclei. Nature. 2000;405:754–755. doi: 10.1038/35015671. [DOI] [PubMed] [Google Scholar]
- Peiter E. The plant vacuole: emitter and receiver of calcium signals. Cell Calcium. 2011;50:120–128. doi: 10.1016/j.ceca.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Peiter E, Maathuis FJ, Mills LN, Knight H, Pelloux J, Hetherington AM, Sanders D. The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature. 2005;434:404–408. doi: 10.1038/nature03381. [DOI] [PubMed] [Google Scholar]
- Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, Mootha VK. MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature. 2010;467:291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson S, Wyatt SE, Love J, Thompson WF, Robertson D, Boss WF. The Ca2+ status of the endoplasmic reticulum is altered by induction of calreticulin expression in transgenic plants. Plant Physiology. 2001;126:1092–1104. doi: 10.1104/pp.126.3.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroutsos D, Busch A, Janssen I, Trompelt K, Bergner SV, Weinl S, Holtkamp M, Karst U, Kudla J, Hippler M. The chloroplast calcium sensor CAS is required for photoacclimation in Chlamydomonas reinhardtii. The Plant Cell. 2011;23:2950–2963. doi: 10.1105/tpc.111.087973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis AR, Jr, Heldt HW. Light-dependent changes of the Mg2+ concentration in the stroma in relation to the Mg2+ dependency of CO2 fixation in intact chloroplasts. Biochimica et Biophysica Acta. 1976;449:434–436. doi: 10.1016/0005-2728(76)90154-7. [DOI] [PubMed] [Google Scholar]
- Pottosin II, Schonknecht G. Vacuolar calcium channels. Journal of Experimental Botany. 2007;58:1559–1569. doi: 10.1093/jxb/erm035. [DOI] [PubMed] [Google Scholar]
- Pottosin I, Wherrett T, Shabala S. SV channels dominate the vacuolar Ca2+ release during intracellular signaling. FEBS Letters. 2009;583:921–926. doi: 10.1016/j.febslet.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Qi Z, Stephens NR, Spalding EP. Calcium entry mediated by GLR3.3, an Arabidopsis glutamate receptor with a broad agonist profile. Plant Physiology. 2006;142:963–971. doi: 10.1104/pp.106.088989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racker E, Schroeder EA. The reductive pentose phosphate cycle. II. Specific C-1 phosphatases for fructose 1,6-diphosphate and sedoheptulose 1,7-diphosphate. Archives of Biochemistry and Biophysics. 1958;74:326–344. doi: 10.1016/0003-9861(58)90004-3. [DOI] [PubMed] [Google Scholar]
- Raeymaekers L, Wuytack E, Willems I, Michiels CW, Wuytack F. Expression of a P-type Ca2+-transport ATPase in Bacillus subtilis during sporulation. Cell Calcium. 2002;32:93. doi: 10.1016/s0143-4160(02)00125-2. [DOI] [PubMed] [Google Scholar]
- Ranf S, Wunnenberg P, Lee J, Becker D, Dunkel M, Hedrich R, Scheel D, Dietrich P. Loss of the vacuolar cation channel, AtTPC1, does not impair Ca2+ signals induced by abiotic and biotic stresses. The Plant Journal. 2008;53:287–299. doi: 10.1111/j.1365-313X.2007.03342.x. [DOI] [PubMed] [Google Scholar]
- Raychaudhury B, Gupta S, Banerjee S, Datta SC. Peroxisome is a reservoir of intracellular calcium. Biochimica et Biophysica Acta. 2006;1760:989–992. doi: 10.1016/j.bbagen.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Reddy AS, Ali GS, Celesnik H, Day IS. Coping with stresses: roles of calcium- and calcium/calmodulin-regulated gene expression. The Plant Cell. 2011;23:2010–2032. doi: 10.1105/tpc.111.084988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy VS, Ali GS, Reddy AS. Genes encoding calmodulin-binding proteins in the Arabidopsis genome. Journal of Biological Chemistry. 2002;277:9840–9852. doi: 10.1074/jbc.M111626200. [DOI] [PubMed] [Google Scholar]
- Rigden DJ, Woodhead DD, Wong PW, Galperin MY. New structural and functional contexts of the Dx[DN]xDG linear motif: insights into evolution of calcium-binding proteins. PLoS One. 2011;6:e21507. doi: 10.1371/journal.pone.0021507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh MH, Shingles R, Cleveland MJ, McCarty RE. Direct measurement of calcium transport across chloroplast inner-envelope vesicles. Plant Physiology. 1998;118:1447–1454. doi: 10.1104/pp.118.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi AE, Dirksen RT. Sarcoplasmic reticulum: the dynamic calcium governor of muscle. Muscle and Nerve. 2006;33:715–731. doi: 10.1002/mus.20512. [DOI] [PubMed] [Google Scholar]
- Sai J, Johnson CH. Dark-stimulated calcium ion fluxes in the chloroplast stroma and cytosol. The Plant Cell. 2002;14:1279–1291. doi: 10.1105/tpc.000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D, Pelloux J, Brownlee C, Harper JF. Calcium at the crossroads of signaling. The Plant Cell. 2002;14(Suppl):S401–S417. doi: 10.1105/tpc.002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Takahashi K, Ochiai Y, Hosaka T, Ochi K, Nabeta K. Bacterial alarmone, guanosine 5′-diphosphate 3′-diphosphate (ppGpp), predominantly binds the beta′ subunit of plastid-encoded plastid RNA polymerase in chloroplasts. Chembiochem. 2009;10:1227–1233. doi: 10.1002/cbic.200800737. [DOI] [PubMed] [Google Scholar]
- Schattat M, Barton K, Baudisch B, Klosgen RB, Mathur J. Plastid stromule branching coincides with contiguous endoplasmic reticulum dynamics. Plant Physiology. 2011a;155:1667–1677. doi: 10.1104/pp.110.170480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattat M, Barton K, Mathur J. Correlated behavior implicates stromules in increasing the interactive surface between plastids and ER tubules. Plant Signal Behav. 2011b;6:715–718. doi: 10.4161/psb.6.5.15085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattat MH, Klosgen RB. Induction of stromule formation by extracellular sucrose and glucose in epidermal leaf tissue of Arabidopsis thaliana. BMC Plant Biology. 2011;11:115. doi: 10.1186/1471-2229-11-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwacke R, Schneider A, van der Graaff E, Fischer K, Catoni E, Desimone M, Frommer WB, Flugge UI, Kunze R. ARAMEMNON, a novel database for Arabidopsis integral membrane proteins. Plant Physiology. 2003;131:16–26. doi: 10.1104/pp.011577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigneurin-Berny D, Gravot A, Auroy P, et al. HMA1, a new Cu-ATPase of the chloroplast envelope, is essential for growth under adverse light conditions. Journal of Biological Chemistry. 2006;281:2882–2892. doi: 10.1074/jbc.M508333200. [DOI] [PubMed] [Google Scholar]
- Shi Y, Zhao W, Zhang W, Ye Z, Zhao J. Regulation of intracellular free calcium concentration during heterocyst differentiation by HetR and NtcA in Anabaena sp. PCC 7120. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11334–11339. doi: 10.1073/pnas.0602839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieberer BJ, Chabaud M, Timmers AC, Monin A, Fournier J, Barker DG. A nuclear-targeted cameleon demonstrates intranuclear Ca2+ spiking in Medicago truncatula root hairs in response to rhizobial nodulation factors. Plant Physiology. 2009;151:1197–1206. doi: 10.1104/pp.109.142851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MA, Carnieri EG, Vercesi AE. Calcium transport by corn mitochondria: evaluation of the role of phosphate. Plant Physiology. 1992;98:452–457. doi: 10.1104/pp.98.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stael S, Bayer RG, Mehlmer N, Teige M. Protein N-acylation overrides differing targeting signals. FEBS Letters. 2011a;585:517–522. doi: 10.1016/j.febslet.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stael S, Rocha AG, Robinson AJ, Kmiecik P, Vothknecht UC, Teige M. Arabidopsis calcium-binding mitochondrial carrier proteins as potential facilitators of mitochondrial ATP-import and plastid SAM-import. FEBS Letters. 2011b;585:3935–3940. doi: 10.1016/j.febslet.2011.10.039. [DOI] [PubMed] [Google Scholar]
- Stael S, Rocha AG, Wimberger T, Anrather D, Vothknecht UC, Teige M. Crosstalk between calcium signalling and protein phosphorylation at the thylakoid. Journal of Experimental Botany. 2012;63:1725–1733. doi: 10.1093/jxb/err403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkov AA. The molecular identity of the mitochondrial Ca2+ sequestration system. FEBS Journal. 2010;277:3652–3663. doi: 10.1111/j.1742-4658.2010.07756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey R, Leigh RA. Processes modulating calcium distribution in citrus leaves. An investigation using x-ray microanalysis with strontium as a tracer. Plant Physiology. 2004;136:3838–3848. doi: 10.1104/pp.104.045674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaiah CC, Bush DS, Sachs MM. Elevation of cytosolic calcium precedes anoxic gene expression in maize suspension-cultured cells. The Plant Cell. 1994;6:1747–1762. doi: 10.1105/tpc.6.12.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaiah CC, Bush DS, Sachs MM. Mitochondrial contribution to the anoxic Ca2+ signal in maize suspension-cultured cells. Plant Physiology. 1998;118:759–771. doi: 10.1104/pp.118.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg E, Slagter JG, Fridborg I, Cleary SP, Robinson C, Coupland G. ALBINO3, an Arabidopsis nuclear gene essential for chloroplast differentiation, encodes a chloroplast protein that shows homology to proteins present in bacterial membranes and yeast mitochondria. The Plant Cell. 1997;9:717–730. doi: 10.1105/tpc.9.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadkai G, Duchen MR. Mitochondria: the hub of cellular Ca2+ signaling. Physiology (Bethesda) 2008;23:84–94. doi: 10.1152/physiol.00046.2007. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Watanabe A, Tanaka A, Hashida SN, Kawai-Yamada M, Sonoike K, Uchimiya H. Chloroplast NAD kinase is essential for energy transduction through the xanthophyll cycle in photosynthesis. Plant and Cell Physiology. 2006;47:1678–1682. doi: 10.1093/pcp/pcl029. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Kasai K, Ochi K. Identification of the bacterial alarmone guanosine 5′-diphosphate 3′-diphosphate (ppGpp) in plants. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4320–4324. doi: 10.1073/pnas.0308555101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari BS, Belenghi B, Levine A. Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiology. 2002;128:1271–1281. doi: 10.1104/pp.010999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlalka M, Fricker M. The role of calcium in blue-light-dependent chloroplast movement in Lemna trisulca L. The Plant Journal. 1999;20:461–473. doi: 10.1046/j.1365-313x.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Torrecilla I, Leganes F, Bonilla I, Fernandez-Pinas F. Use of recombinant aequorin to study calcium homeostasis and monitor calcium transients in response to heat and cold shock in cyanobacteria. Plant Physiology. 2000;123:161–176. doi: 10.1104/pp.123.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrecilla I, Leganes F, Bonilla I, Fernandez-Pinas F. A calcium signal is involved in heterocyst differentiation in the cyanobacterium Anabaena sp. PCC7120. Microbiology. 2004;150:3731–3739. doi: 10.1099/mic.0.27403-0. [DOI] [PubMed] [Google Scholar]
- Torrecilla I, Leganés F, Bonilla I, Fernández-Piñas F. Calcium transients in response to salinity and osmotic stress in the nitrogen-fixing cyanobacterium Anabaena sp. PCC7120 expressing cytosolic apoaequorin. Plant, Cell and Environment. 2001;24:641–648. [Google Scholar]
- Tozawa Y, Nozawa A, Kanno T, Narisawa T, Masuda S, Kasai K, Nanamiya H. Calcium-activated (p)ppGpp synthetase in chloroplasts of land plants. Journal of Biological Chemistry. 2007;282:35536–35545. doi: 10.1074/jbc.M703820200. [DOI] [PubMed] [Google Scholar]
- Traba J, Froschauer EM, Wiesenberger G, Satrustegui J, Del Arco A. Yeast mitochondria import ATP through the calcium-dependent ATP-Mg/Pi carrier Sal1p, and are ATP consumers during aerobic growth in glucose. Molecular Microbiology. 2008;69:570–585. doi: 10.1111/j.1365-2958.2008.06300.x. [DOI] [PubMed] [Google Scholar]
- Turano FJ, Thakkar SS, Fang T, Weisemann JM. Characterization and expression of NAD(H)-dependent glutamate dehydrogenase genes in Arabidopsis. Plant Physiology. 1997;113:1329–1341. doi: 10.1104/pp.113.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner WL, Waller JC, Vanderbeld B, Snedden WA. Cloning and characterization of two NAD kinases from Arabidopsis. Identification of a calmodulin binding isoform. Plant Physiology. 2004;135:1243–1255. doi: 10.1104/pp.104.040428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainonen JP, Sakuragi Y, Stael S, et al. Light regulation of CaS, a novel phosphoprotein in the thylakoid membrane of Arabidopsis thaliana. FEBS Journal. 2008;275:1767–1777. doi: 10.1111/j.1742-4658.2008.06335.x. [DOI] [PubMed] [Google Scholar]
- Van Aken O, Zhang B, Carrie C, Uggalla V, Paynter E, Giraud E, Whelan J. Defining the mitochondrial stress response in Arabidopsis thaliana. Molecular Plant. 2009;2:1310–1324. doi: 10.1093/mp/ssp053. [DOI] [PubMed] [Google Scholar]
- Virolainen E, Blokhina O, Fagerstedt K. Ca2+-induced high amplitude swelling and cytochrome c release from wheat (Triticum aestivum L.) mitochondria under anoxic stress. Annals of Botany. 2002;90:509–516. doi: 10.1093/aob/mcf221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waditee R, Hossain GS, Tanaka Y, Nakamura T, Shikata M, Takano J, Takabe T, Takabe T. Isolation and functional characterization of Ca2+/H+ antiporters from cyanobacteria. Journal of Biological Chemistry. 2004;279:4330–4338. doi: 10.1074/jbc.M310282200. [DOI] [PubMed] [Google Scholar]
- Waller JC, Dhanoa PK, Schumann U, Mullen RT, Snedden WA. Subcellular and tissue localization of NAD kinases from Arabidopsis: compartmentalization of de novo NADP biosynthesis. Planta. 2010;231:305–317. doi: 10.1007/s00425-009-1047-7. [DOI] [PubMed] [Google Scholar]
- Walter A, Mazars C, Maitrejean M, Hopke J, Ranjeva R, Boland W, Mithofer A. Structural requirements of jasmonates and synthetic analogues as inducers of Ca2+ signals in the nucleus and the cytosol of plant cells. Angewandte Chemie International Edition in English. 2007;46:4783–4785. doi: 10.1002/anie.200604989. [DOI] [PubMed] [Google Scholar]
- Wang D, Xu Y, Li Q, Hao X, Cui K, Sun F, Zhu Y. Transgenic expression of a putative calcium transporter affects the time of Arabidopsis flowering. The Plant Journal. 2003;33:285–292. doi: 10.1046/j.1365-313x.2003.01627.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhu Y, Ling Y, Zhang H, Liu P, Baluska F, Samaj J, Lin J, Wang Q. Disruption of actin filaments induces mitochondrial Ca2+ release to the cytoplasm and [Ca2+]c changes in Arabidopsis root hairs. BMC Plant Biology. 2010;10:53. doi: 10.1186/1471-2229-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinl S, Held K, Schlucking K, Steinhorst L, Kuhlgert S, Hippler M, Kudla J. A plastid protein crucial for Ca2+-regulated stomatal responses. New Phytologist. 2008;179:675–686. doi: 10.1111/j.1469-8137.2008.02492.x. [DOI] [PubMed] [Google Scholar]
- Westphal S, Soll J, Vothknecht UC. A vesicle transport system inside chloroplasts. FEBS Letters. 2001;506:257–261. doi: 10.1016/s0014-5793(01)02931-3. [DOI] [PubMed] [Google Scholar]
- Westphal S, Soll J, Vothknecht UC. Evolution of chloroplast vesicle transport. Plant and Cell Physiology. 2003;44:217–222. doi: 10.1093/pcp/pcg023. [DOI] [PubMed] [Google Scholar]
- Wheeler GL, Brownlee C. Ca2+ signalling in plants and green algae—changing channels. Trends in Plant Science. 2008;13:506–514. doi: 10.1016/j.tplants.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Jensen GS, Haswell ES. Two mechanosensitive channel homologs influence division ring placement in arabidopsis chloroplasts. The Plant Cell. 2011;23:2939–2949. doi: 10.1105/tpc.111.088112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurzinger B, Mair A, Pfister B, Teige M. Cross-talk of calcium-dependent protein kinase and MAP kinase signaling. Plant Signaling Behavior. 2011;6:8–12. doi: 10.4161/psb.6.1.14012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi C, Schoeters E, Vanderleyden J, Michiels J. Symbiosis-specific expression of Rhizobium etli casA encoding a secreted calmodulin-related protein. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11114–11119. doi: 10.1073/pnas.210181097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong TC, Coursol S, Grat S, Ranjeva R, Mazars C. Sphingolipid metabolites selectively elicit increases in nuclear calcium concentration in cell suspension cultures and in isolated nuclei of tobacco. Cell Calcium. 2008;43:29–37. doi: 10.1016/j.ceca.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Xiong TC, Jauneau A, Ranjeva R, Mazars C. Isolated plant nuclei as mechanical and thermal sensors involved in calcium signalling. The Plant Journal. 2004;40:12–21. doi: 10.1111/j.1365-313X.2004.02184.x. [DOI] [PubMed] [Google Scholar]
- Yang T, Poovaiah BW. Arabidopsis chloroplast chaperonin 10 is a calmodulin-binding protein. Biochemical and Biophysical Research Communications. 2000;275:601–607. doi: 10.1006/bbrc.2000.3335. [DOI] [PubMed] [Google Scholar]
- Yang T, Poovaiah BW. Hydrogen peroxide homeostasis: activation of plant catalase by calcium/calmodulin. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:4097–4102. doi: 10.1073/pnas.052564899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa K, Maeshima M. Organ specificity of a vacuolar Ca2+-binding protein RVCaB in radish and its expression under Ca2+-deficient conditions. Plant Molecular Biology. 2001;47:633–640. doi: 10.1023/a:1012355205991. [DOI] [PubMed] [Google Scholar]
- Zakharov SD, Ewy RG, Dilley RA. Subunit III of the chloroplast ATP-synthase can form a Ca(2+)-binding site on the lumenal side of the thylakoid membrane. FEBS Letters. 1993;336:95–99. doi: 10.1016/0014-5793(93)81617-9. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Shi Y, Zhao W, Huang X, Wang D, Brown N, Brand J, Zhao J. CcbP, a calcium-binding protein from Anabaena sp. PCC 7120, provides evidence that calcium ions regulate heterocyst differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5744–5748. doi: 10.1073/pnas.0501782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Beck M, Muller J, et al. Actin turnover is required for myosin-dependent mitochondrial movements in Arabidopsis root hairs. PLoS One. 2009;4:e5961. doi: 10.1371/journal.pone.0005961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Yang W, Kirberger M, Lee HW, Ayalasomayajula G, Yang JJ. Prediction of EF-hand calcium-binding proteins and analysis of bacterial EF-hand proteins. Proteins. 2006;65:643–655. doi: 10.1002/prot.21139. [DOI] [PubMed] [Google Scholar]
- Zhu SY, Yu XC, Wang XJ, et al. Two calcium-dependent protein kinases, CPK4 and CPK11, regulate abscisic acid signal transduction in Arabidopsis. The Plant Cell. 2007;19:3019–3036. doi: 10.1105/tpc.107.050666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W. GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiology. 2004;136:2621–2632. doi: 10.1104/pp.104.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]