Abstract
Toe clipping is used to identify and genotype preweanling mice, but the procedure generates concerns relevant to pain and distress. The few pertinent studies available evaluated mice between postnatal days (PND) 3 and 7, advocate the use of toe clipping in mice PND 7 or younger, and identify handling as the most distressing aspect of the procedure. Because both toe and tail clipping may be necessary in older mice to obtain sufficient DNA for genotyping, we surmised that performing these procedures concurrently to minimize handling would be beneficial. We also examined reflex development until PND 21 and adult behavior at 8 to 10 wk of age in mice toe clipped at PND 7 or 17 and the benefits of using topical vapocoolant anesthesia. C57BL/6J pups at PND 7 and 17 were assigned to 1 of 4 groups: 1) clipping of digit 3 of contralateral fore- and hindpaws; 2) toe clipping after topical vapocoolant anesthesia; 3) unclipped, unsprayed controls; and, 4) unclipped and vapocoolant-sprayed. Compared with unanesthetized pups, those sprayed with vapocoolant vocalized and struggled more when handled and had more bleeding, erythema, and swelling, which persisted for as long as 12 h after toe clipping. Reflex development, anxiety, locomotion, and motor coordination were not different among groups or with regard to the age of toe clipping. No tissue reaction was noted microscopically in paws collected at 10 wk of age. We conclude that the use of vapocoolant cannot be recommended due to its harmful effects and that toe clipping at PND 7 or 17 does not significantly affect the long-term welfare of mice.
Abbreviations: PND, postnatal day; SHIRPA, SmithKline Beecham Pharmaceuticals, Harwell, MRC Mouse Genome Centre and Mammalian Genetics Unit, Imperial College School of Medicine at St Mary's, Royal London Hospital, St Bartholomew's and the Royal London School of Medicine Phenotype Assessment
Toe clipping of juvenile mice constitutes the amputation of individual digits of the forepaw or hindpaw.1,17,18 This technique is used for the purposes of early identification of preweanling mice at ages when other permanent identification techniques are limited and to obtain nucleic acid for genotyping.25,27 Once personnel are trained appropriately, the additional advantages of toe clipping are that it is a fast and easy technique to apply and it provides the ability to assign a unique number when identifying large numbers of mice. The use of toe clipping is a contentious issue because it is presumed by some to cause pain and distress.20,28
A number of UK-based animal welfare organizations, including the British Veterinary Association's Animal Welfare Foundation, the Fund for the Replacement of Animals in Medical Experiments, the Royal Society for the Prevention of Cruelty to Animals and the Universities Federation for Animal Welfare established a Joint Working Group on Refinement to address key issues in the generation of genetically modified mice.28 Their recommendations regarding tissue biopsy indicated that because the removal of any tissue may result in pain, suffering, and distress, care must be taken to select the most appropriate biopsy method under suitable conditions and at the appropriate age. The group suggested that the least invasive method of tissue collection should be used and that the minimal amount of tissue sample collected. Their position on toe clipping was that it should not be used routinely as a method of identification or for the purpose of genotyping because of its assumed potential to cause pain and to affect the animal's ability to grip and groom; that toe clipping should only be used as a last resort in situations where no alternative method of identification or genotyping is possible; and that when used, only a single toe from one hindpaw should be removed under local anesthesia.28 However at the time the group was convened, there were no objective studies published to evaluate methods of toe clipping or its effects.
Subsequently, 2 studies evaluated removal of the distal phalanx for toe clipping in mouse pups at postnatal days (PND) 3 and 7.6,30 These studies concluded that toe clipping at these ages does not appear to have any significant short- or long-term adverse physiologic or behavioral effects on mice;6,30 that it may be preferable to other methods of permanent identification such as ink tattooing and transponder implantation;6 and that the short period of handling, rather than the procedure of toe clipping itself, was the most stressful component of the procedure.30 Stress was assessed by measuring serum corticosterone levels of euthanized clipped, nonclipped and nonhandled pups at PND 7.30
The Guide for the Care and Use of Laboratory Animals,19 referencing these studies, indicates that toe clipping should be reserved for cases where identification is necessary at a young age and when other methods of identification are insufficient or not feasible; that, when used for combined identification and genotyping of neonatal mice, it may be preferable to perform toe clipping prior to PND 7 because it appears to have few adverse effects on behavior or well-being at this age; and that the use of age-appropriate anesthesia and analgesia is recommended. No detailed suggestions for anesthesia or analgesia are provided.19
The Federation of European Laboratory Animal Science Associations established 2 separate working groups that published reports on rodent identification and genotyping methods in early 2013. The Federation's working group on animal identification discourages the use of ‘nonrefined’ toe clipping (defined as the arbitrary removal of the digit) for identification and instead advocates the use of distal phalanx removal. In view of earlier studies,6,30 the working group's recommendation also states that a maximum of one toe per paw should be clipped and that the procedure should take place between PND 5 to 7.9 The working group on genotyping recommends that when both identification and genotyping are required, a single method that achieves both aims should be used.4 This group also recommends the use of distal phalanx biopsy in place of nonrefined toe clipping under the same conditions outlined by the working group on animal identification and that tail biopsy only be used when a combined method of identification and genotyping does not provide a sufficiently large DNA sample for the proposed genotyping assay, such as Southern blot analysis.4
The previous studies conducted on the procedure6,30 did not evaluate toe clipping in mice beyond PND 7. Clipping at such an early age can be impractical due to the small size of the digits. Another limitation of this early time point is that the collection of both toe and tail biopsies is often necessary to obtain sufficient DNA for multiple genotyping assessments by Southern blot analysis. In their intramural program, the NIH currently recommends performing tail biopsy on mice from PND 10.26
Therefore, we sought to measure the developmental and long-term behavioral effects of toe clipping at PND 7 and 17. PND 7 was selected as the lower age limit to test the reproducibility of the previous studies, whereas PND 17 was selected as the upper limit because this age is the maximum at which, in light of previous work,16 our institutions currently allow tail biopsy to be performed without the use of general anesthesia. In addition, because genotyping results would be available by weaning if conducted on or before PND 17, mice of the incorrect genotype could be culled at or before weaning. Our hypothesis was that toe clipping at PND 17 would be no more detrimental to the development and long-term behavior of mice than would be toe clipping at PND 7.
We also evaluated the use of a vapocoolant as a method of pain reduction that might improve animal welfare. Typically, anesthesia of neonatal rodents is achieved by three principal methods: injectable anesthesia, inhalant anesthesia, or hypothermia. Injectable agents, such as ketamine or pentobarbital, are not ideal due to their narrow margin of safety, and their use can result in prolonged recovery.10 The inhalant anesthetic agent isoflurane has been shown to be safe in neonates as young as PND 3.32 However, the practicality of isoflurane anesthesia for this relatively quick procedure is questionable, because this method of anesthesia requires specialized equipment such as a precision vaporizer and the means to scavenge waste gases, and exposure to the gas may be more stressful than the procedure itself. In addition, isoflurane anesthesia does not significantly improve the welfare of mice that undergo tail biopsy between weaning age and PND 31 compared with sham-controls.15 Hypothermic anesthesia is achieved by placing altricial neonates on, but not in direct contact with, ice. The average duration to reach torpor is 15 min, with a 20 to 30 min recovery period when mice are rewarmed in an incubator. Apart from the lengthy duration of recovery given the short duration of toe clipping, this method is associated with the development of ventricular fibrillation, tissue hypoxia, and metabolic acidosis during the rewarming stage.34 We elected to evaluate application of a vapocoolant to provide short-acting topical anesthesia immediately prior to toe clipping. This method is used by some members of the research community when performing tail biopsy and is recommended in the NIH's Intramural Guidelines for the Genotyping of Mice and Rats.26
Materials and Methods
Animals.
C57BL/6J mice purchased from the Jackson Laboratory (Bar Harbor, ME) or obtained from an in-house breeding colony were used to generate the pups used in this study. Mice were individually identified by lightly grasping the animal by the tail, which then was marked with between 1 and 5 dashes by using a nontoxic, fine point permanent marker (Sharpie, Newell Rubbermaid, Oak Brook, IL) and remarking as needed throughout the study period, to avoid using invasive methods of identification.
Mice were free of mouse hepatitis virus, mouse rotavirus, lymphocytic choriomeningitis virus, ectromelia virus, mouse parvovirus, minute virus of mice, pneumonia virus of mice, reovirus type 3, Sendai virus, Theiler mouse encephalomyelitis virus, mouse adenovirus, K virus, polyoma virus, mouse cytomegalovirus, mouse thymic virus, Haantan virus, lactic dehydrogenase elevating virus, cilia-associated respiratory bacillus, Mycoplasma pulmonis, as well as ectoparasites including Myobia spp., Myocoptes spp., and Radfordia spp. and endoparasites including Syphacia spp. and Aspiculuris spp.
All mice were group-housed (2 to 3 male mice or 3 to 5 female mice per cage) in solid-bottom, polysulfone, individually ventilated cages (Thoren Caging Systems, Hazelton, PA) on autoclaved aspen-chip bedding (PWI Industries Canada, Quebec, Canada) and were provided γ-irradiated feed (LabDiet 5058, PMI, St Louis, MO) and acidified water (pH, 2.5 to 2.8) ad libitum. Cages were changed weekly in a vertical flow, mass air-displacement unit (AllerGard NU612-400, Nuaire, Plymouth, MN). Animal-handling forceps were dipped and disinfected in chlorine dioxide (1:18:1 dilution; Clidox-S, Pharmacal Research Laboratories, Naugatuck, CT) between cages. The holding room was ventilated with 95% filtered outside air at 15 air changes per hour, temperature was maintained at 72 ± 2 °F (21.5 ± 1 °C), relative humidity was between 30% and 70%, and a 12:12-h light:dark cycle was used. Animals were maintained in accordance with the Guide for the Care and Use of Laboratory Animals19 in an AAALAC-accredited facility. All procedures outlined in the study were approved by the Memorial Sloan–Kettering Cancer Center's IACUC.
Preliminary study.
C57BL/6J pups (n = 12) were acquired from an in-house breeding colony to conduct a preliminary study examining toe ossification and innervation at multiple preweaning time points. Toes were clipped from the third digit of the right forepaw and left hindpaw from euthanized pups at PND 7, 10, 12, 14, 17, and 21 (n = 2 at each time point). Both the amputated digit and the corresponding paw were fixed in 10% neutral buffered formalin, decalcified in a decalcifying solution (Surgipath Decalcifier I, Leica Biosystems, Richmond, IL), processed, and embedded in paraffin. Tissue sections (thickness, 4 µm) were stained with hematoxylin and eosin. These sections were analyzed to evaluate the anatomic site at which the digit was amputated by using the toe clipping method chosen for the experimental study. Transverse sections of the digits were immunohistochemically stained with antiS100 antibody to characterize digit innervation by age. The primary antibody (product code Z0628, Dako North America, Carpinteria, CA) was diluted 1:10,000, and the sections were incubated overnight at 4 °C; the remaining steps were performed according to vendor's recommendations. Negative controls were prepared for each toe by using an irrelevant immunoglobulin from the same species and subtype as a substitute for the primary antibody.
To evaluate the level of phalanx ossification at various time points, whole-mount staining of the left forepaw and right hindpaw was performed. The paws were stored in 100% ethanol until transported to the laboratory, where they were submerged in hot water and then skinned. The skinned paws were stored and delipidated in acetone for 48 h. The paws were double-stained for 24 h and incubated at 37°C in Alcian blue–Alizarin red S staining solution comprising 2 mL 0.14% Alcian blue solution (Sigma–Aldrich, St Louis, MO; 0.14 g Alcian Blue 8 GX certified in 100 mL 70% ethanol), 1 mL 0.12% Alizarin red S solution (Sigma–Aldrich; 0.12 g Alizarin red S certified in 100 mL 95% ethanol), 8 mL glacial acetic acid, and 50 mL 70% ethanol. Stained tissues were rinsed in distilled water and cleared in 1% potassium hydroxide for 8 h at room temperature. The tissues then underwent 8 hourly sequential clearings in 20% glycerol solution (1 part glycerol, 4 parts 1% potassium hydroxide), 50% glycerol solution (1 part glycerol, 1 part 1% potassium hydroxide), 80% glycerol solution (4 parts glycerol, 1 part 1% potassium hydroxide), and finally storage in 100% glycerol. The tissues were examined by using a dissecting microscope and analyzed for stage of ossification by noting the presence of bone and cartilage, as stained by Alizarin red S and Alcian blue, respectively.
Experimental design.
Timed matings were conducted by using monogamous pairs (n = 24); 2 cohorts of pups, aged PND 7 (n = 54) and PND 17 (n = 58), were generated and randomly assigned to 1 of 4 experimental groups.
Group 1 (n = 12 at PND 7; n = 15 at PND 17).
These mice underwent toe clipping after manual restraint by grasping of the skin overlying the dorsal cervicothoracic region of the pup without prior administration of a vapocoolant.
Group 2 (n = 12 at PND 7; n = 14 at PND 17).
Mice in group 2 underwent toe clipping under manual restraint (as described) and topical administration of a vapocoolant (Spray and Stretch [95% 1,1,1,3,3-pentafluoropropane; 5% 1,1,1,2-tetrafluoroethane], Gebauer Company, Cleveland, OH). The paw to be clipped was sprayed for approximately 4 s until the skin blanched, as described in the manufacturer's instructions for topical anesthesia. The pup's limb was restrained and extended, while the spray, which is dispensed in a fine stream, was directed at the paw, away from the rest of the body.
Group 3 (untreated controls; n = 16 at PND 7; n = 15 at PND 17).
Group 3 mice were manually restrained (as described), and toe clipping was simulated by lightly touching the paw with the tip of a pair of scissors.
Group 4 (vapocoolant controls; n = 14 at PND 7; n = 14 at PND 17).
Mice in this group were manually restrained (as described) and received vapocoolant; toe clipping was simulated by lightly touching the paw with the tip of a pair of scissors.
Toe clipping.
An experienced technician completed the toe-clipping procedure by using newly purchased microsurgery scissors (no. 14090-09, Fine Science Tools, Foster City, CA), which had been steam sterilized prior to initial use for each age cohort and wiped with 70% ethanol between each animal. Two toes were clipped per animal according to the institution's IACUC-recommended toe-clipping scheme (Figure 1). Digit 3 of the right forepaw and left hindpaw (corresponding to numbers 3 and 70 according to the toe clipping scheme) were clipped at the level of the distal end of the 1st phalange. These toes were selected because they are the longest on each respective paw and presumed to exert the most downward pressure during ambulation. We speculated that their removal would have the greatest potential effect on the animal's gait and grasping ability. Gauze swabs were applied to the clipped toes to achieve hemostasis, the pups were returned to their home cage, and one author (LP) monitored the animals for 5 min after the procedure to record immediate reactions, including absence of suckling behavior and evidence of maternal rejection.
Figure 1.
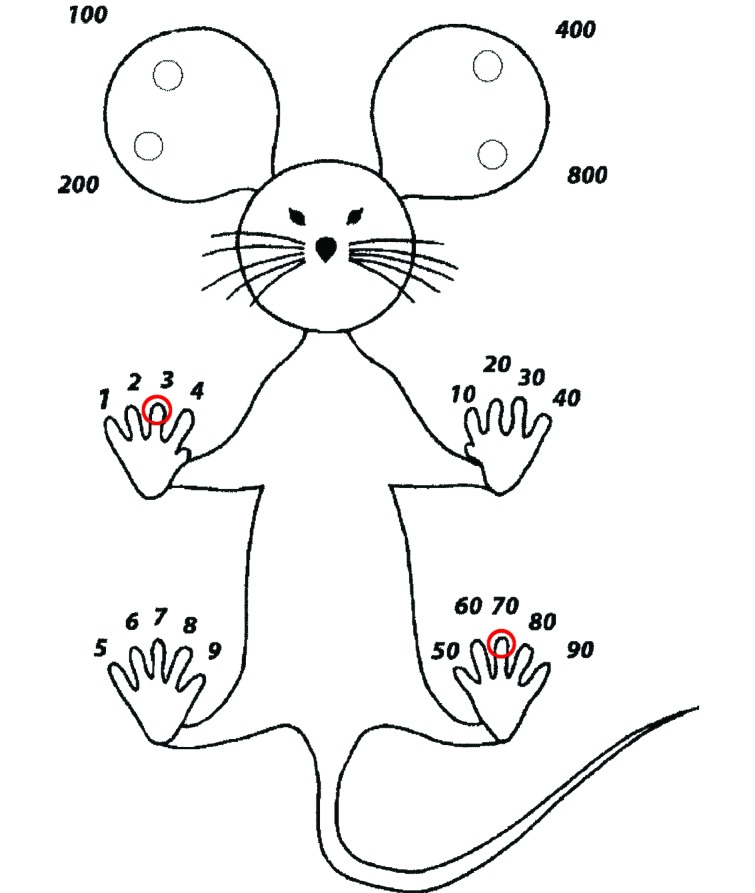
Memorial Sloan–Kettering Cancer Center's toe-clipping scheme. The third digit of the right forepaw (no. 3; circled in red) and left hindpaw (no. 70; circled in red) were clipped from each mouse in groups 1 and 2 at PND 7 or PND 17.
Condition assessment.
Each mouse's overall condition was assessed subjectively at 1, 3, 5, 8 and 12 h after toe clipping. At each time point, pups were monitored for signs of hunching and reluctance to bear weight on the affected feet; in addition, the clipped or sprayed paws were examined for signs of swelling or erythema. Furthermore, the mice were monitored for suckling behavior and evidence of maternal rejection at each time point. Body weight was measured by using a digital scale (model KD-160, Tanita, Arlington Heights, IL) once each week from PND 6 (to obtain baseline values prior to clipping on day 7) until 10 wk of age.
Developmental assessment.
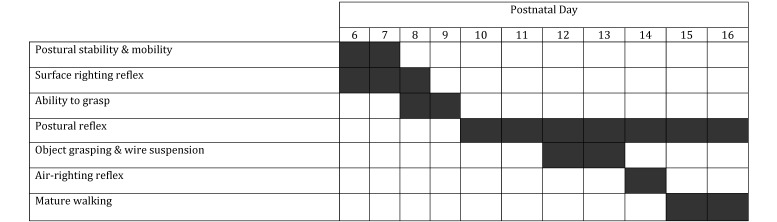
Beginning at PND 6 and continuing until PND 21, mice were evaluated daily for neurologic reflex development (Figure 2). Each neurologic reflex was assessed within the time periods described and was recorded as either absent (score, 0) or present (score, 1). Once a pup successfully achieved a score of 1, it no longer was tested for that reflex. The time frames were selected based on the age at which a mature response was expected to have developed.23
Figure 2.
Developmental assessment. The pups were assessed for each of the listed reflexes within the time frames designated by the shaded boxes.
Postural stability and mobility was evaluated once each day on PND 6 and 7 by placing the pup with its head faced downward on a grid tilted at a 45° angle. A mature response was achieved when the pup was able to rotate itself to face upward and climb up the grid within 30 s. The surface righting reflex was tested once each day between days PND 6 and 8 by placing the pup in a supine position and allowing 30 s for the animal to flip to ventral recumbency. The ability to grasp was tested on PND 8 and 9 by prodding the forepaw with a thin wire. A mature reaction was defined as immediate grasping on stimulation of the paw. The postural reflex was tested once every 1 to 2 d between PND 10 and 16 by placing a pup in an empty cage and gently shaking the cage to make the pup's stance unsteady. This test differs from that of postural stability, in that the postural reflex test measures the ability of a pup to maintain its position by outstretching all 4 limbs. Object grasping and wire suspension were tested once daily on days PND 12 and 13 by touching the pup's forepaws to a wire (diameter, 3 mm). The pup was expected to hold on to the wire and maneuver its body to grasp the wire bar with all 4 paws. Mature walking without dragging of the belly along the floor of the cage was observed once daily on PND 15 and 16. The air-righting reflex, defined as the pup's ability to rotate its body midflight when dropped, back first, onto a soft surface from a height of 20 cm and land on all 4 paws, was evaluated at PND 14.
Behavioral assessment.
To familiarize the mice with the handler, they were handled daily from 7 wk of age, beginning 1 wk prior to behavioral testing, by a single author (LP). Mice were lifted by the tail and placed on the back of the handler's hand, to avoid excessive restraint. Behavioral assays were conducted between 0800 and 1300, and mice were allowed to acclimate to the testing room for 30 min before testing commenced. All of the equipment was thoroughly wiped down with 40% ethanol before each testing session and between animals.
The open-field test was used to assess locomotor ability and exploratory behavior once at 8 wk of age. Each mouse was placed in the open arena (45 cm × 45 cm; wall height, 20 cm) and its activity recorded for 5 min by using a video tracking system (Ethovision 3.1, Noldus Technology, Attleborough, MA). The total distance traveled, rearing frequency, and percentage of time spent in the center were recorded.
The elevated plus-maze test was used to measure long-term anxiety-like behavior once at 8 wk of age, after the open-field test. The maze consists of an elevated platform (height, 39 cm) with 4 arms (30 cm × 5 cm) arranged in the shape of a cross. Two arms are open (without walls), and the remaining 2 arms are enclosed by 15-cm walls on both sides and at the end. Each respective set of arms faces the other. The mouse was placed in the center of the maze and monitored for 5 min. The frequency of entries into both the open and closed arms and the percentage of time spent in the open arms of the maze were recorded.
An abridged version of the SHIRPA screening protocol29 was used once, at 8 wk of age, to test various behavioral and phenotypic parameters in the adult mice. Briefly, each mouse was placed in an open-bottomed transparent plastic cylindrical viewing jar (diameter, 14 cm; height, 18 cm) on top of a sanded acrylic sheet, and the following was recorded: body position, tremor, palpebral closure, lacrimation, and defecation. The mouse then was transferred to a clear acrylic arena (45 cm × 45 cm × 20 cm), the floor of which was divided into 25 squares (9 × 9 cm each), by holding the jar and sheet 20 cm above the arena floor and sliding the sheet from beneath the mouse so that it dropped into the arena. The immediate reaction of the mouse was observed as hypoactive (mouse immobile for more than 2 s), normal (mouse transiently immobile for 2 s or less before exploring the arena), or hyperactive (movement immediately on landing). The other recorded observations included: locomotor activity (the number of squares entered with all 4 feet in 30 s), gait (abnormal compared with normal appearance), pelvic elevation (no dragging of the hindquarters along the floor), tail elevation (normal, extended horizontally; abnormal, dragging on ground or extended vertically, also referred to as the Straub response), startle response (evaluated by making a sudden beeping noise by using a stopwatch alarm over the mouse's head), and touch escape (evaluated by approaching the mouse from the front with a bent index finger and then stroking the back of the mouse's neck). The mouse then was removed from the arena by grasping of the animal by the tail, and the following observations were made: whether the mouse struggled when held by its tail, whether it curled its trunk forward when held by the tail, and whether it grasped at the air while held by the tail. In addition, the pinna reflex was assessed by gently probing inside the external ear canal by using a fine cotton probe and noting whether the mouse retracted its ears; the corneal reflex was tested by lightly touching the cornea with the cotton probe and observing the blink response. The mouse was then placed in a clear plastic tube (diameter, 3.5 cm; length, 20 cm) and the tube rolled slowly until the mouse was upside down; the righting reflex was observed. The mouse was subsequently placed on a grid and allowed to clasp the wire surface. The grid was then inverted and held over a soft surface while the mouse grasped the grid. The latency to fall on to a soft padded surface was measured. Throughout this panel of evaluations, biting attempts and vocalization were recorded.
The balance beam test was used to assess motor coordination and balance by measuring foot slips and latency to fall from a cylindrical beam (diameter, 2.5 cm; length, 110 cm; divided into eleven 10-cm segments) that was elevated above a soft, padded bench top and clamped between 2 support stands. The beam was constructed from a plastic broomstick covered in textured gaffer tape to ensure the mice were able to walk easily on a nonslippery surface. Beginning at 9 wk of age, the mice underwent 3 d of daily testing (data were collected during the initial training day). The mice were timed as they traversed the beam (maximum, 60 s) in 3 consecutive trials per day.
The rotarod test was used to further assess motor coordination and balance in the mice, beginning at 10 wk of age. The mice were trained to walk on an accelerated rotarod apparatus (Economex, Columbus Instruments, Columbus, OH) starting at an initial speed of 4 rpm, accelerating at a rate of 0.2 rpm/s. Mice underwent 3 daily trials with 15-min rest periods between trials for 3 consecutive days. The maximal speed achieved was calculated by recording the latency to fall from the rotarod.
Postmortem analyses.
The mice were euthanized at the end of the study (after completion of the rotarod test) by exposure to carbon dioxide gas, and the paws of all euthanized animals were harvested for histologic processing. The individual digits of the right forepaw and left hindpaw from the animals in groups 1 and 2 (toe clipping without and with vapocoolant anesthesia, respectively) were removed and placed in separate cassettes. The paws of animals from groups 3 and 4 were kept whole. All tissues were fixed in 10% neutral buffered formalin and decalcified in decalcifying solution (Surgipath Decalcifier I, Leica Biosystems). The tissues were paraffin-embedded and sectioned (thickness, 4 µm) for processing and were stained with hematoxylin and eosin. The tissues of the amputated digits were analyzed for a healing response and signs of regeneration.
Statistical methods.
The data were analyzed by using SAS (version 9.2, SAS Institute, Cary, NC). Differences between groups (group 1 compared with group 2; group 1 compared with group 3; and group 2 compared with group 4) for continuous variables were assessed by using the Wilcoxon rank-sum test. In addition, within-group comparisons were made by age group (PND 7 compared with PND 17). The P values were adjusted for multiple comparisons by using the method of Benjamini and Hochberg;3 P values less than or equal to 0.05 were considered significant.
Results
Preliminary study.
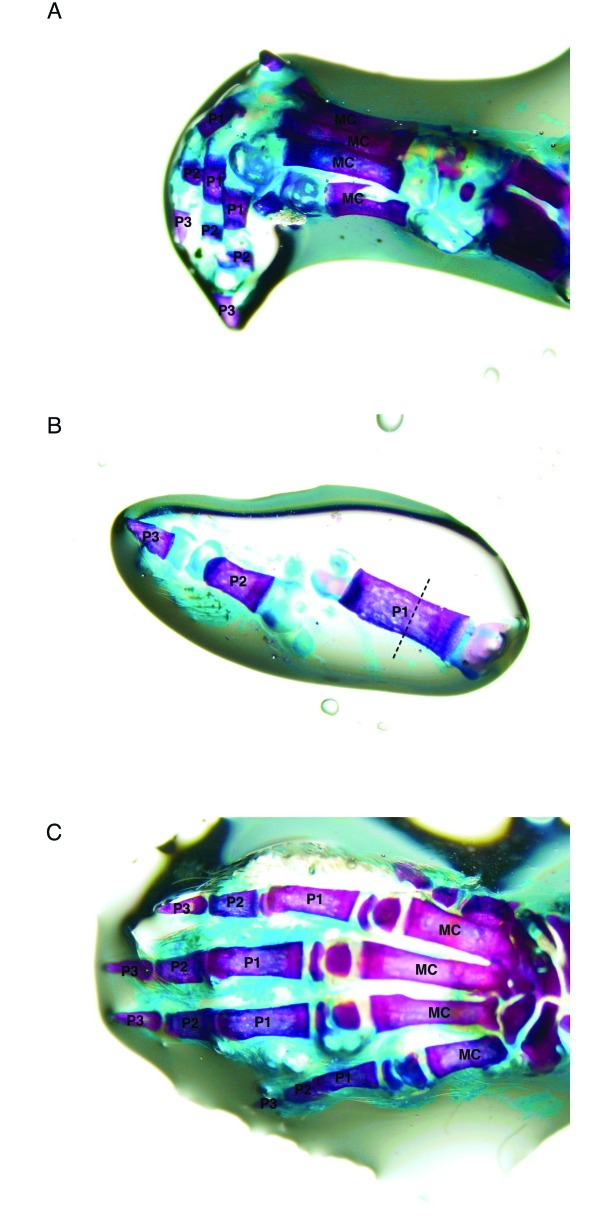
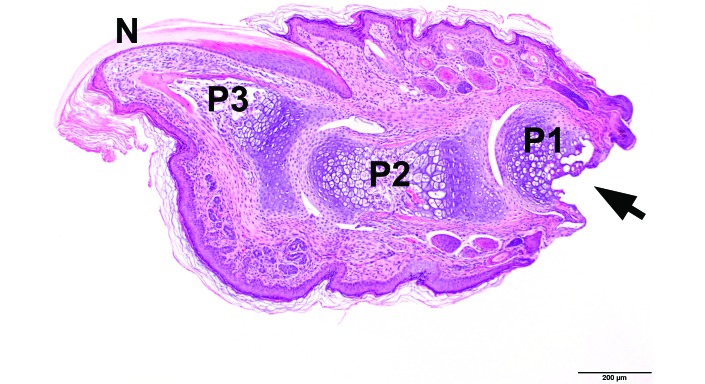
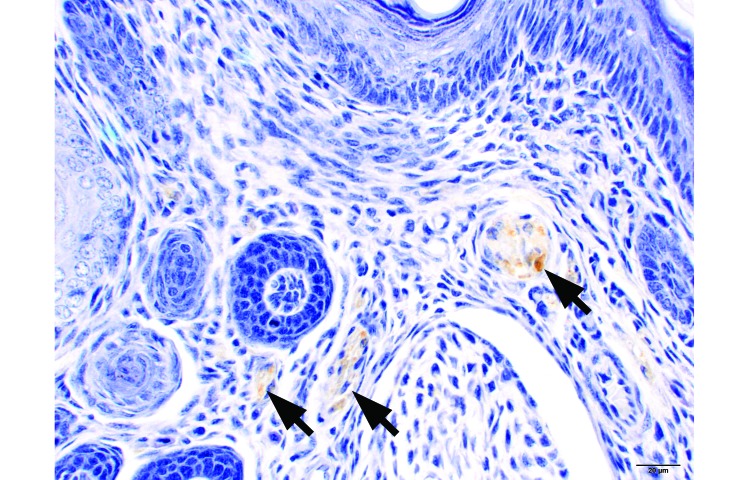
At PND 7, the distal ends of the individual phalanges were not completely ossified, but a large proportion of the central shaft of the proximal, intermediate, and distal phalanges was ossified (Figure 3A and B). By PND 10 (Figure 3 C), the cartilage present at PND 7 appeared to have completed ossification. The level of amputation was consistently the ossified midsection of the proximal phalanx (Figure 4). Innervation of the toe was present at PND 7 (Figure 5) and was consistent among age groups.
Figure 3.
Whole-mount bone preparations. The purple-stained tissue comprises bone, and the blue-staining tissue comprises cartilage. (A) Lateral view of PND 7 left forepaw. (B) PND 7 fifth (most lateral) digit of a hindpaw. The dotted line indicates the approximate site of amputation. (C) Dorsal view of PND 10 left forepaw. MC, metacarpal; P1, proximal phalanx; P2, intermediate phalanx; P3, terminal phalanx. Alizarin red S–Alcian blue double stain.
Figure 4.
PND 7 forepaw, digit, longitudinal section. Clipped (arrow) at the level of mid P1. N, nail; P1, proximal phalanx; P2, intermediate phalanx; P3, terminal phalanx. Hematoxylin and eosin stain; bar, 200 µm.
Figure 5.
PND 7 hindpaw, digit 3, transverse section. Nerves (arrows) are labeled with S100 (brown staining). Immunohistochemistry for S100, hematoxylin counterstain; bar, 20 µm.
Toe clipping.
Due to their small size, clipping toes at PND 7 was technically challenging and more time-consuming than at PND 17. Regardless of the cohort (PND 7 and 17), vapocoolant complicated the procedure, because it caused the toes to adhere to one another and made it more difficult to isolate individual toes for clipping. The difficulty in clipping toes within these groups prolonged the period of animal restraint by approximately 2 min.
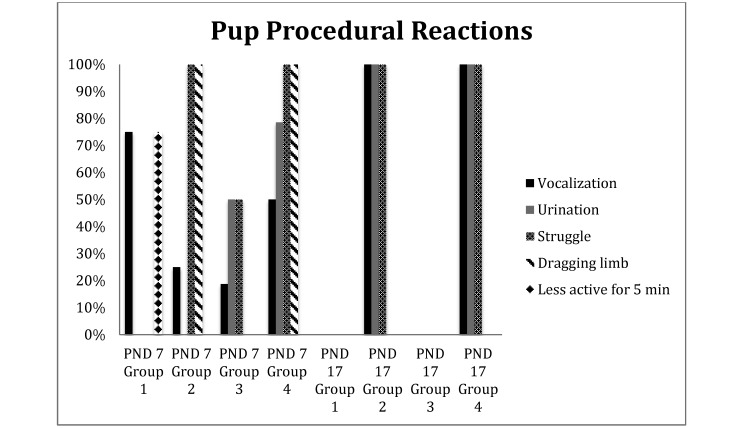
At PND 7, 9 of the 12 pups that underwent toe clipping without vapocoolant (that is, group 1) vocalized briefly (less than 3 s) on toe clipping and showed decreased activity for 5 min after the procedure. This behavior was in contrast to that of the 15 PND 17 pups in group 1, which did not demonstrate any noticeable reaction to toe amputation. All 12 PND 7 pups in group 2 (toe clipping with vapocoolant) and the 14 pups in group 4 (vapocoolant only) struggled throughout the procedure and dragged the sprayed limb for as long as 5 min after return to their cages. Two of the 12 group 2 pups at PND 7 vocalized briefly (less than 3 s) when being sprayed with vapocoolant, and one other pup from this group vocalized on toe clipping. At PND 7, 7 of the 14 group 4 pups, which were sprayed with vapocoolant without having their toes clipped, vocalized briefly (less than 3 s), and 11 urinated when sprayed with the topical anesthetic. At PND 17, all 14 pups in group 2 and all 14 pups in group 4 vocalized briefly (less than 3 s) and urinated upon being sprayed with vapocoolant and withdrew their limbs, regardless of whether toe clipping followed vapocoolant application. On PND 7, 8 of the 16 pups in group 3 (untreated controls) urinated and struggled on restraint, with 3 of the 16 vocalizing; in contrast, none of the 15 group 3 pups vocalized, urinated, or struggled (Figure 6).
Figure 6.
Percentage of pups within each group that displayed the indicated reactions during the toe-clipping procedure and within 5 min afterward.
Regardless of age, toe clipping without prior vapocoolant administration resulted in minimal bleeding and the formation of a clot within seconds. Although they bled minimally on clipping, vapocoolant-sprayed groups that had their toes amputated experienced profuse bleeding approximately 5 min after amputation. These mice required application of gauze to the clipped toes to ensure hemostasis.
Condition assessment.
No sign of maternal rejection or absence of suckling was noticed in any of the groups at any age after manipulation of pups.
In group 1 mice that underwent toe clipping on PND 7, erythema and swelling of the clipped toes was noted in 7 of the 12 pups at 1 h after clipping and lasted for as long as 5 h in 4 pups. No swelling was observed past 1 h in group 1 PND 17 pups. Of the 12 group 2 pups at PND 7, 9 had red, swollen paws for as long as 12 h when vapocoolant anesthesia preceded toe clipping. This same effect was noted in the older (PND 17) group 2 cohort. None of the group 4 PND 7 pups, which had been sprayed with vapocoolant but not clipped, displayed swelling of the paws past 1 h after amputation, whereas 5 of the 14 PND 17 pups from this group displayed swelling and erythema of the paw for as long as 5 h.
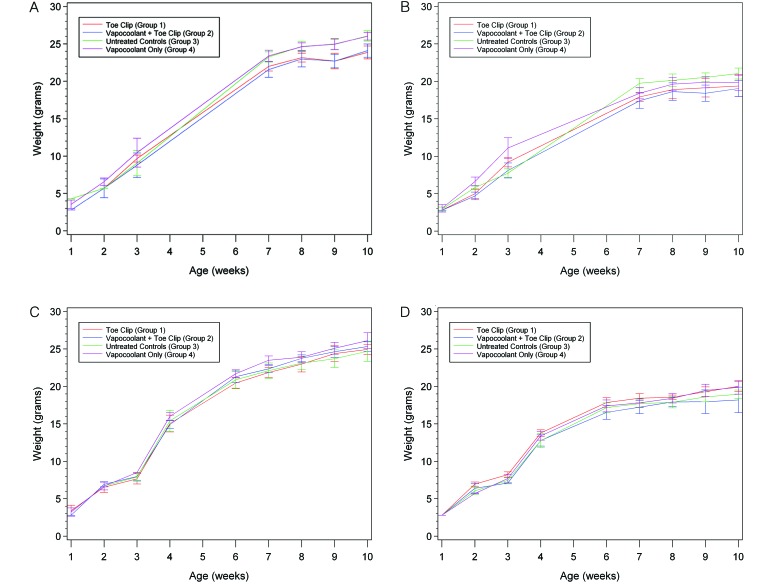
No differences in the pattern of body weight gain from birth to 10 wk of age were observed (Figure 7 A through D).
Figure 7.
Body weights (g) from PND 6 to 10 wk of age plotted by experimental group. (A) Male mice in PND 7 cohort. (B) Female mice in PND 7 cohort. (C) Male mice in PND 17 cohort. (D) Female mice in PND 17 cohort. Data are presented as mean ± 2 SE.
Developmental assessment.
All animals displayed normal development of reflexes within the established normal time frames.
Behavioral assessment.
Open-field test.
No significant differences in the average percentage of time spent in the center of the arena, the mean distance traveled in centimeters, and the average rearing frequency were observed between treatment groups or between cohorts in the open-field test (data not shown).
Elevated plus-maze test:
No significant differences in the overall percentage of time spent in the open arms and the ratio of entries into open compared with closed arms were noted between treatment groups within cohorts of the same age (data not shown). However, group 3 (untreated control) PND 17 pups spent a higher (P = 0.04) percentage of time in the open arms than did group 3 PND 7 pups (mean, 33.4% compared with 21.3%). We attributed this difference to individual animal variability rather than as a result of any experimental manipulations. No other measurements were found to be statistically different between experimental groups or age cohorts.
SHIRPA:
All of the mice appeared healthy and alert without any obvious physical abnormalities or impediments. All animals behaved normally within the arena, not displaying differences between transfer arousal responses nor any obvious gait abnormalities. The vast majority of mice appeared to have normal startle responses and touch escape behavior, and all presented with normal pinna and corneal reflexes. None of the mice attempted to bite the handler, nor did any vocalize throughout the assay. All animals displayed normal postural reflexes when suspended by the tail. All animals were able to right themselves when placed in a tube that had been rotated such that they were positioned in dorsal recumbency.
No significant differences between animals of any group were detected for the grip test (data not shown).
Beam test:
No significant differences between groups were detected for any of the parameters measured in the beam test, including latency to fall, number of foot slips, and segments traversed on the beam (data not shown).
Rotarod test:
No significant differences were detected between any groups for performance on the rotarod, as measured by the maximal speed achieved (data not shown).
Postmortem analyses.
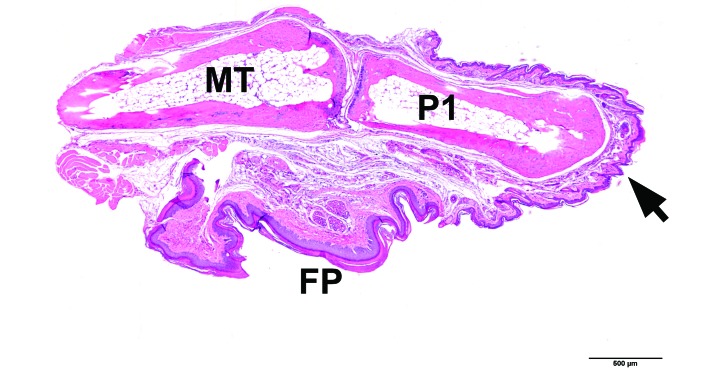
Histologic analysis of the remaining stumps of amputated digits did not reveal any abnormalities in healing responses (Figure 8).
Figure 8.
Postmortem analysis of healed stump from the third digit (hindpaw) of a 10-wk-old mouse that was toe-clipped on PND 17 without vapocoolant anesthesia (group 1). Metatarsus and digit, longitudinal section. Distal extremity (arrow) at the level of midP1. No abnormalities are observed. FP, foot pad; MT, metatarsal bone; P1, proximal phalanx. Hematoxylin and eosin stain; bar, 500 µm.
Discussion
We confirmed the findings of our contemporaries in Europe in that there appear to be no significant long-term differences between mice toe clipped at PND 7 and naïve control animals.6,30 We also observed the same results when mice had their toes clipped at PND 17. We similarly observed inter-animal variability in response to toe clipping alone at PND 7, with just over half of the pups vocalizing upon amputation, whereas the remaining animals displayed no reaction.30 We did not observe any immediate behavioral response to toe clipping in the PND 17 cohort unless the topical anesthetic was used. Of note, group 1 mice in the PND 17 cohort showed little behavioral reaction and no obvious swelling of the clipped paws within 12 h after the procedure. As observed in prior studies,6,30 none of the mice we studied displayed maternal rejection or unwillingness to suckle; the pups continued to develop normally after toe clipping.
Our conclusions were similar to those of the European researchers in terms of welfare and lack of adverse impacts on the mice despite our use of a different toe clipping technique, in which we amputated at the level of the midsection of the proximal phalanx. This technique is discouraged by the FELASA Working Group on animal identification and is referred to as the nonrefined method of toe clipping.9 The FELASA report advocates the method of distal phalanx removal, defined as cutting the digit at the distal end of the second phalanx, which is equivalent to 2 to 3 mm in the 7-d-old pup.9 The FELASA Working Group explains that application of this method to pups at PND 3 is too difficult due to the small size of the toes and that from PND 12 onward, the pups are too active to accurately perform distal phalanx removal, potentially leading to regrowth of the toe due to incomplete removal of the nail bed.9
In our experience, toe clipping was challenging to perform in pups as young as PND 7 due to the small size of their digits and therefore actually prolonged the period of restraint. We question the ease of performing distal phalanx biopsy and how one would ascertain the accuracy of the personnel charged with performing toe clipping in this manner in neonatal animals in the day-to-day laboratory setting.
As reported by others, stress in the mice appeared to result from handling rather than the toe amputation.6,30 We observed that handling alone was enough to elicit a stress-like response (vocalization) from many of the PND 7 pups. This finding correlated with those of earlier studies, which demonstrated that capture and restraint were the most stressful intervention for mice subject to tissue biopsy techniques other than toe clipping, increasing heart rate and body temperature for as long as 1 h after manipulation.7
Our evaluation of vapocoolant as a method of local anesthesia yielded unexpected results: the change in tissue temperature appeared to be distressful. The mice may experience a similar sensation to that of humans, in whom pain is associated with the initial vasoconstriction response resulting from exposure to extremely cold temperatures.8 Not only did vapocoolant seem to adversely affect the psychologic state of the pups, it also elicited tissue swelling and increased blood loss after toe clipping. The profuse bleeding noted in the toe clipped animals after the administration of vapocoolant was likely a result of cold-induced vasodilation.8 This condition is assumed to be the body's defense against thermal injury and develops 5 to 10 min after cold exposure.8 One group of investigators previously reported that accidental inhalation of topical vapocoolant by mice can result in respiratory distress and therefore recommended taking appropriate precautions, such as directing the spray away from the mouse's covered head.21 We did not encounter any respiratory difficulties in any of the pups after our application method.
Other investigators have proposed that toe clipping should take place “between PND 5 and 7, when…ossification is not yet complete.”6,14 However, we observed, in the C57BL6/J strain (other authors14 examined the development of neonatal digits in outbred CD1 mice), that a substantial amount of bone is already present at PND 7. This finding challenges the accuracy of this statement, at least for the commonly used C57BL6/J strain, and calls into question whether the presence or lack of ossification is substantially relevant to the age at which toe clipping should be performed. This point is especially germane, because the PND 17 mice responded as well or better than those clipped at PND 7. In addition, we noted innervation in the toes of mice as young as PND 7, thereby suggesting the potential for nociception at this age.
Although we did not observe any long-term adverse effects on mice subject to toe clipping according to the assessment methods we used, we were limited in our ability to objectively assess the acute pain response to the procedure, and no previous study evaluates this response. Identifying pain and distress in mice, especially neonates, is extremely difficult. Measuring changes in corticosterone levels requires euthanasia of the animals and in the postnatal period, for example, is problematic due to the reduced responsiveness of the pituitary–adrenal axis.31 Neonates have very low basal corticosterone levels and are generally unable to elicit a corticosterone response to stressors.31 Another limitation is the inability to use telemetry in neonates due to size constraints. Additional research is needed to evaluate the acute pain responses to toe clipping. Assessing ultrasonic vocalization may be useful to measure immediate pain or distress,5 although a 2008 study33 did not find ultrasonic measurements to be a reliable method of assessing acute pain in mice. The same study concluded that although the presence of audible vocalizations may be an indicator of pain, their absence does not necessarily reflect its absence. Measurement of acute phase proteins such as serum amyloid A as a measure of acute injury and the associated inflammatory response may be useful for pain determination.5
Studies in neonatal rats have demonstrated that despite the anatomic presence of descending inhibitory pathways in the dorsolateral funiculus, these pathways don't become functional until PND 10 to PND 12.13 This pathway is important, given that it blocks the afferent nociceptive or C-fiber signals from reaching the brain. One might infer from this finding that, compared with older animals, neonatal rats experience more intense pain from a nociceptor, because the sensation is not mitigated by the inhibitory pathways present in more mature animals. Similar studies have not been conducted in neonatal mice, but it would not be surprising to find this developmental phenomenon in this species.
Studies in human newborns have shown that neonates were more sensitive to painful stimuli such as circumcision and heel lancing than were infants of 3 to 12 mo old. The proposed mechanism is that the slower conduction speed of the neonatal nervous system is off set by the smaller interneuron distances traveled by the impulse.12 Conversely, one should not over-interpret pain responses observed in neonates. Although neonates may be able to sense nociceptive stimuli, the neonatal nervous system is not yet mature enough to organize a directed response to stimulus. Therefore neonates may display a more exaggerated response than would their adult counterparts.2
Another area of future investigation is the effect of toe clipping on the representation of the paws and individual digits in the mouse's somatosensory cortex. Such information would indicate whether differences between the representation of the forepaws and hindpaws, or even between digits, on a rodent somatotopic map translate to differing reliance of the animal on particular digits in manipulating objects or ambulation. The removal of digits in rats younger than PND 5 apparently does not alter the forepaw barrel subfield.11 However, remapping occurs in older animals in which the areas of the forepaw barrel subfield representing the remaining adjacent digits expand to include the area once representative of the removed digit, such that the remaining phalanges excite the neurons previously stimulated by the missing toe.22 This phenomenon has also been documented in higher vertebrate species such as nonhuman primates and is likely to occur in mice.24
Toe clipping is a controversial practice due to perceived animal welfare concerns. The technique is used in many institutions to identify genetically modified mice at early ages. Our current study aimed to determine whether toe clipping mice as old as PND 17 is more problematic than when then the procedure is performed at PND 7. Based on observational, developmental, and behavioral assessments, we conclude that toe clipping of mice at PND 17 is an acceptable method when other less painful methods are not practical. Because toe clipping at PND 7 or PND 17 does not significantly impact the long-term welfare of mice, we suggest that the currently accepted age at which toe clipping is performed should be extended to PND 17, bearing in mind that toe biopsies from younger animals may yield larger amounts of DNA for genotyping.16,27 However, the potential for subtle changes in the nervous system exist and may be problematic when mice are used in specific studies.
Given that restraint and handling have consistently been identified as stressful for neonatal mice,6,30 we suggest that when both permanent identification and Southern blot analysis are needed, toe clipping and toe biopsy should be performed simultaneously between PND 10 to PND 17, in harmony with both the NIH's guidelines26 and the recommendations of other colleagues regarding tail biopsy.16 We also evaluated the potential advantages of using a vapocoolant to provide local anesthesia to reduce pain and distress that may be associated with the procedure. We cannot recommend the use of vapocoolant during toe clipping, because the adverse effects markedly outweigh any potential benefit.
Acknowledgments
We acknowledge and thank the following people: Yuri Igarashi for her technical assistance in performing the toe clipping procedures; Willie Mark and Peter Romanienko for their helpful discussions and advice regarding genotyping of juvenile mice; Cliona Stack, Ceyhan Elipenahli, and Shari Jainuddin for their patience and training in how to perform behavioral assays; the members of the Laboratory of Comparative Pathology—particularly Nancy Pinard, Maria Jiao, Kim McBride, and Jacqueline Candelier; Jennifer Herlihy, Rona Lester, Leslie Diaz, and Kimberly Such for their technical assistance; and Odessa Giardino for her interesting and useful insights.
References
- 1.American Association for Laboratory Animal Science 2009. Chapter 10: Husbandry, p 74–75. ALAT manual. Memphis (TN): American Association for Laboratory Animal Science [Google Scholar]
- 2.Beggs S, Fitzgerald M. 2007. Development of peripheral and spinal nociceptive systems, p 11–24. In: Anand KJS, Stevens BJ, McGrath PJ, editors. Pain in neonates and infants; New York (NY): Elsevier [Google Scholar]
- 3.Benjamini Y, Hochberg Y. 1995. Controlling the false-discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc, B 57:289–300 [Google Scholar]
- 4.Bonaparte D, Cinelli P, Douni E, Herault Y, Maas A, Pakarinen P, Poutanen M, Lafuente MS, Scavizzi F. 2013. FELASA guidelines for the refinement of methods for genotyping genetically modified rodents: a report of the Federation of European Laboratory Animal Science Associations Working Group. Lab Anim 47:134–145 [DOI] [PubMed] [Google Scholar]
- 5.Branchi I, Santucci D, Alleva E. 2001. Ultrasonic vocalisation emitted by infant rodents: a tool for assessment of neurobehavioural development. Behav Brain Res 125:49–56 [DOI] [PubMed] [Google Scholar]
- 6.Castelhano-Carlos MJ, Sousa N, Ohl F, Baumans V. 2010. Identification methods in newborn C57BL/6 mice: a developmental and behavioural evaluation. Lab Anim 44:88–103 [DOI] [PubMed] [Google Scholar]
- 7.Cinelli P, Rettich A, Seifert B, Burki K, Arras M. 2007. Comparative analysis and physiological impact of different tissue biopsy methodologies used for the genotyping of laboratory mice. Lab Anim 41:174–184 [DOI] [PubMed] [Google Scholar]
- 8.Daanen HA. 2003. Finger cold-induced vasodilation: a review. Eur J Appl Physiol 89:411–426 [DOI] [PubMed] [Google Scholar]
- 9.Dahlborn K, Bugnon P, Nevalainen T, Raspa M, Verbost P, Spangenberg E. 2013. Report of the Federation of European Laboratory Animal Science Associations Working Group on Animal identification. Lab Anim 47:2–11 [DOI] [PubMed] [Google Scholar]
- 10.Danneman PJ, Mandrell TD. 1997. Evaluation of 5 agents/methods for anesthesia of neonatal rats. Lab Anim Sci 47:386–395 [PubMed] [Google Scholar]
- 11.Dawson DR, Killackey HP. 1987. The organization and mutability of the forepaw and hindpaw representations in the somatosensory cortex of the neonatal rat. J Comp Neurol 256:246–256 [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald M, Anand KJS. 1993. Developmental neuroanatomy and neurophysiology of pain, p 11–31. In: Schechter NL, Berde C, Yaster M, editors. Pain in infants, children, and adolescents. Baltimore (MD): Williams and Wilkins [Google Scholar]
- 13.Fitzgerald M, Koltzenburg M. 1986. The functional development of descending inhibitory pathways in the dorsolateral funiculus of the newborn rat spinal cord. Brain Res 389:261–270 [DOI] [PubMed] [Google Scholar]
- 14.Han M, Yang X, Lee J, Allan CH, Muneoka K. 2008. Development and regeneration of the neonatal digit tip in mice. Dev Biol 315:125–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hankenson FC, Braden-Weiss GC, Blendy JA. 2011. Behavioral and activity assessment of laboratory mice (Mus musculus) after tail biopsy under isoflurane anesthesia. J Am Assoc Lab Anim Sci 50:686–694 [PMC free article] [PubMed] [Google Scholar]
- 16.Hankenson FC, Garzel LM, Fischer DD, Nolan B, Hankenson KD. 2008. Evaluation of tail biopsy collection in laboratory mice (Mus musculus): vertebral ossification, DNA quantity, and acute behavioral responses. J Am Assoc Lab Anim Sci 47:10–18 [PMC free article] [PubMed] [Google Scholar]
- 17.Hayward AM, Lemke LB, Bridgeford EC, Theve EJ, Jackson CN, Cunliffe-Beamer TL, Marini RP. 2007. Biomethodology and surgical techniques, p 437–488. In: Fox JG, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith A, editors. The mouse in biomedical research: normative biology, husbandry and models. San Diego (CA): Elsevier Academic Press [Google Scholar]
- 18.Hedrich HJ, Mossmann H, Nicklas W. 2004. Housing and maintenance, p 395–408. In: Hedrich HJ, Bullock GR, editors. The laboratory mouse. Amsterdam (The Netherlands): Elsevier Academic Press. [Google Scholar]
- 19.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press [Google Scholar]
- 20.Karas A, Silverman J. 2007. Pain and distress, p 275–276. In: Silverman J, Suckow MA, Murthy S, editors. The IACUC handbook. Boca Raton (FL): CRC Press. [Google Scholar]
- 21.Matthias N, Robinson MA, Crook R, Lockworth CR, Goodwin BS., Jr 2013. Local cryoanalgesia is effective for tail-tip biopsy in mice. J Am Assoc Lab Anim Sci 52:171–175 [PMC free article] [PubMed] [Google Scholar]
- 22.McCandlish CA, Li CX, Waters RS, Howard EM. 1996. Digit removal leads to discrepancies between the structural and functional organization of the forepaw barrel subfield in layer IV of rat primary somatosensory cortex. Exp Brain Res 108:417–426 [DOI] [PubMed] [Google Scholar]
- 23.Mertens C, Rulicke T. 2000. [A comprehensive form for the standardized characterization of transgenic rodents: genotype, phenotype, welfare assessment, recommendations for refinement.] ALTEX 17:15–21[Article in German]. [PubMed] [Google Scholar]
- 24.Merzenich MM, Nelson RJ, Stryker MP, Cynader MS, Schoppmann A, Zook JM. 1984. Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol 224:591–605 [DOI] [PubMed] [Google Scholar]
- 25.Nadon NL, Draeger K. 1996. Genomic DNA analysis from mouse toe lysates. Transgenic Res 5:209–211 [DOI] [PubMed] [Google Scholar]
- 26.National Institutes of Health. [Internet] 2002. Guidelines for the genotyping of mice and rats. [Cited July 29, 2011]. Available at: http://oacu.od.nih.gov/ARAC/documents/Rodent_Genotyping.pdf
- 27.Pinkert CA. 2003. Transgenic animal technology: alternatives in genotyping and phenotyping. Comp Med 53:126–139 [PubMed] [Google Scholar]
- 28.Robinson VMD, Anderson D, Carver JFA, Francis RJ, Hubrecht R, Jenkins E, Mathers KE, Raymond R, Rosewell I, Wallace J, Wells DJ, BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement 2003. Refinement and reduction in production of genetically modified mice. Lab Anim 37:S1–S49 [PubMed] [Google Scholar]
- 29.Rogers DC, Peters J, Martin JE, Ball S, Nicholson SJ, Witherden AS, Hafezparast M, Latcham J, Robinson TL, Quilter CA, Fisher EM. 2001. SHIRPA, a protocol for behavioral assessment: validation for longitudinal study of neurological dysfunction in mice. Neurosci Lett 306:89–92 [DOI] [PubMed] [Google Scholar]
- 30.Schaefer DC, Asner IN, Seifert B, Burki K, Cinelli P. 2010. Analysis of physiological and behavioural parameters in mice after toe clipping as newborns. Lab Anim 44:7–13 [DOI] [PubMed] [Google Scholar]
- 31.Schmidt MV, Enthoven L, van der Mark M, Levine S, de Kloet ER, Oitzl MS. 2003. The postnatal development of the hypothalamic–pituitary–adrenal axis in the mouse. Int J Dev Neurosci 21:125–132 [DOI] [PubMed] [Google Scholar]
- 32.Wiesmann F, Ruff J, Hiller KH, Rommel E, Haase A, Neubauer S. 2000. Developmental changes of cardiac function and mass assessed with MRI in neonatal, juvenile, and adult mice. Am J Physiol Heart Circ Physiol 278:H652–H657 [DOI] [PubMed] [Google Scholar]
- 33.Williams WO, Riskin DK, Mott AK. 2008. Ultrasonic sound as an indicator of acute pain in laboratory mice. J Am Assoc Lab Anim Sci 47:8–10 [PMC free article] [PubMed] [Google Scholar]
- 34.Wixson SK, Smiler KL. 1997. Anesthesia and analgesia in rodents, p 190–191. In: Kohn D, Wixson SK, White WJ, Benson GJ, editors. Anesthesia and analgesia in laboratory animals. Burlington (MA): Elsevier. [Google Scholar]