Abstract
Whether social isolation of adult rats under standard laboratory conditions produces significant long-term alterations in behavior and physiology is unclear. In the present study, male Sprague–Dawley rats were singly or paired-housed for 10 wk. During this period, they were tested for acquisition, extinction, and reacquisition of heroin (0.3 mg/kg)-conditioned place preference. Fecal corticoid metabolite levels were analyzed several times throughout the period of housing, and food consumption and body weight were monitored. During place conditioning, heroin induced a significant increase in locomotor activity in both singly and pair housed rats, and the resulting place preference was similar in both groups. However, singly housed rats showed increased motor reactivity to heroin on reconditioning after extinction and displayed significant reacquisition of conditioned place preference, compared with pair-housed animals. Over the 10-wk period of the study, there were no differences in body weight or food consumption between groups. Mild significant increases in relative adrenal gland weight and decreases in relative brain weight were noted in singly housed animals compared with those paired. Significant decreases in nocturnal fecal corticoid metabolite output were noted in both groups, with loss of circadian variation in fecal corticoid levels over the course of the study. These data suggest that male Sprague–Dawley rats, irrespective of single or pair housing, develop reduced hypothalamic–pituitary–adrenal axis activity over time under standard laboratory housing conditions. Single housing can enhance both this effect and sensitivity to the stimulatory and rewarding actions of heroin after withdrawal.
Abbreviations: CPP, conditioned place preference; HPA, hypothalamic–pituitary–adrenal axis; CORT, fecal corticoid metabolites
The importance of the preweaning social environment on animal behavior, physiology, and brain plasticity has been well described (reviewed in references 6 and 30). Maternal care of mammalian young is accepted as a critical life event that determines key aspects of postweaning behavior, response to stressors, and hippocampal development.1,20,41 In addition, social housing of preweaned rats alters behavioral and motivational responses to various drugs of abuse, including opiates.5,42,52 Recent studies have shown that rats socially isolated from weaning display enhanced self-administration of opiates and drug-seeking behaviors and that social housing reduces these effects,31 although the underlying mechanism for this response is unknown. Few studies have been conducted to ascertain whether social housing affects other responses to these same drugs of abuse in mature animals, which is a relevant question because commercial vendors typically rear rats in social groups prior to distribution, whereas purchased adult animals may be individually housed on arrival at a facility because of experimental constraints or to minimize extrinsic experimental variability.
Rearing rats in a physically and socially enriched environment can affect reactivity to stressors but the effects are stock and strain dependent. In Sprague–Dawley and Long Evans rats, for example, combined social and environmental enrichment is reported to result in faster systemic responses to stressors, indicated by more rapid increases in plasma corticosterone levels and by a more rapid return to baseline levels after termination of the stressor, as compared with the responses in singly housed animals of the same strain maintained in nonenriched cages.22 Single housing of rats is reported to increase baseline anxiety, exploration, and locomotion, compared with those in group-housed rats, but the effect is highly stock- and strain-dependent.15,19,41,44,49 Neither male nor female Sprague–Dawley rats display more locomotion when singly housed.50 However, locomotor activity is an important parameter for evaluating the response to pharmacologic agents, such as heroin, which may differ between singly and pair-housed animals.
The current study evaluated the effect of social environment on fecal corticosterone and on the rewarding properties of heroin in adult rats by using conditioned place preference (CPP). To maximize the likelihood of detecting significant differences between groups, we used 2 approaches. First the dose of heroin that we used for CPP was relatively low (that is, 0.3 mg/kg). In adult rats, this dose has only mild stimulatory properties, and it induces a significant CPP, which is significantly smaller than the CPP produced by doses equal to or greater than 1 mg/kg.25 Second, considering that spontaneous recovery after extinction and rate of reacquisition partially reflect strength of original conditioning,32,48 we evaluated whether single compared with pair housing was associated with differences in spontaneous recovery and magnitude of reacquisition of CPP induced by reexposure to the same dose of heroin after extinction or withdrawal.
Because of the potential effect of social isolation on the hypothalamic–pituitary–adrenal (HPA) axis function, and because HPA axis activity in turn modulates responses to opioids,12 we evaluated alterations in glucocorticoid output at various intervals during the duration of the study. In the current study, basal HPA axis activity was assessed by monitoring fecal corticoid metabolite (CORT) levels. In mammals, glucocorticoids are released in pulsatile bursts throughout the day, with increased release during the time of the day at which the species is most active.9,26 Rats show a 2- to 3-fold variation in nocturnal:diurnal levels of CORT.43,53 When 3H-corticosterone is injected into rats, approximately 80% of metabolites are collected in the feces, and the remaining 20% are excreted in the urine.2 Levels of CORT reflect systemic arousal that occurred 6 to 10 h prior to collection, and feces can be collected during light and dark periods to estimate plasma corticosterone levels.9 Furthermore, paired adrenal gland to body weight ratios were collected postmortem during this study to determine whether single housing resulted in gross physical changes suggestive of chronically altered HPA function.35 The null hypothesis was that single housing of male Sprague–Dawley rats would have no effect on our measures of HPA function or on development, recovery, or strength of CPP after low-dose heroin exposure.
Methods and Materials
Animals.
Studies were conducted on adult, male Sprague–Dawley rats, obtained at 5 wk of age from Charles River Laboratories (St Constant, QC). Vendor surveillance records indicated that the rats were free of known bacterial, viral, and parasitic pathogens, including pneumonia virus of mice, sialodacryoadenitis virus, Kilham rat virus, Toolan H1 virus, rat parvovirus, reovirus 3, Theiler murine encephalomyelitis virus, lymphocytic choriomeningitis virus, Bordetella bronchiseptica, Corynebacterium kutscheri, Helicobacter hepaticus, Helicobacter bilis, Clostridium piliforme, Salmonella spp., Streptobacillus moniliformis, Streptococcus pneumoniae, Pseudomonas aeruginosa, Streptococcus β hemolytica, Klebsiella pneumoniae, Pasturella spp., Mycoplasma pulmonis, and common ecto- and endoparasites.
Rats initially were housed individually in standard shoebox-type cages (800 cm2; 26.7 cm × 43.2 cm; 18.5 cm high) on hardwood chip bedding supplied with a nylon chew toy at 21 ± 2 °C for 1 wk to acclimate to a 12:12-h reverse-lighting cycle (lights on at 1900). After acclimation to reversed-lighting conditions, rats were randomized into 2 groups of equal size and housed either singly (n = 24) or as pairs (n = 24) and allowed an additional week of acclimation prior to study start. One of the paired male rats was excluded from the study because of premature renal disease and anemia. This rat's partner was removed also, leaving 22 male rats in the pair-housed group at study initiation. Food (Global 2014 Chow, Harlan, Indianapolis, IN) and water were provided ad libitum throughout the study. The facilities and procedures are in compliance with the Animals for Research Act of Ontario29 and the Guidelines of the Canadian Council on Animal Care.7 The University of Guelph Animal Care Committee approved the study protocol.
Apparatus.
Six, custom-made (University of Guelph, Canada) place-conditioning boxes were used for this study. Each box was made of dark-gray PVC and was composed of 2 smaller boxes (30 × 40 × 26 cm3) connected by a transition box (23 × 30 × 26 cm3). Removable inserts, with or without small archway openings (10 × 10 cm2), defined the transition box enclosures. The 2 compartments differed primarily in visual cues: one compartment was dark gray whereas the other had a white wall and a 10-cm white stripe painted along the top of the other walls. In addition, there were cues that provided spatial information external to the compartments, such as posters on walls and the locations of benches, the room door, and lights. In this apparatus, rats do not display a significant spontaneous preference for any of the compartments. The entire apparatus was covered by black wire mesh to permit video tracking (EthoVision, version 3, Noldus Information Technology, Wageningen, Netherlands) of the rats during testing. This system was used to automatically record 2 dependent variables: time (s) spent in each compartment during tests for place preference and locomotor activity (total cm) during conditioning and reconditioning.
Drug.
Diacetylmorphine hydrochloride (heroin; MacFarlane Smith, Edinburgh, United Kingdom) was dissolved in 0.9% saline and injected subcutaneously at a volume of 1.0 mL/kg and dose of 0.3 mg/kg. Vehicle (0.9% normal saline; Baxter, Mississauga, Ontario, Canada) was injected subcutaneously at a volume of 1.0 mL/kg.
Clinical observation, body weight, and food consumption.
Rats were observed daily, and any abnormalities were noted. Body weight and food consumption were collected weekly. Food consumption was determined by weighing the food given for each week and subtracting the weight of the food remaining at the end of each week. Food consumption for pair-housed rats was halved to estimate the intake for each animal.
CPP procedure.
The CPP procedure involved 7 phases: habitation, conditioning, test I, extinction, tests of spontaneous recovery (tests II through IV), reconditioning, and test V.
Habituation (1 d).
Rats were handled twice for 5 min prior to being habituated to the apparatus. During this session, the inserts with openings were used, and rats had free access to all compartments for 20 min. Time spent in each of the 2 compartments was recorded to measure spontaneous preference or aversion.
Conditioning (4 d).
The day after habituation, the inserts with openings were replaced with solid inserts to fully separate the compartments. On each of the 4 d of conditioning, rats participated in one session in the morning (between 0800 and 1200) and one in the afternoon (between 1400 and 1800). For each rat, the minimal interval between the 2 sessions was 4 h. Each session included an injection of either vehicle or drug and subsequent confinement to one of the compartments for 30 min. The specific compartment chosen to be associated with drug was counterbalanced across rats. In addition, the time (morning or afternoon) of drug sessions was counterbalanced across rats and across days of conditioning for each rat. Locomotor activity was measured during each conditioning session.
Test I (1 d).
At 24 h after the last day of conditioning, the inserts with openings were used, and time spent in each compartment was measured over 20 min. No injections were given prior to this test.
Extinction (7 d).
This phase was similar to conditioning in that it included two 30-min sessions every day. However, during extinction, rats received vehicle injections before confinement to either compartment. Rats underwent a total of 7 extinction sessions.
Tests of spontaneous recovery (3 d).
These were carried out as described for Test I. Rats underwent 3 tests, at 1 wk (test II), 2 wk (test III), and 6 wk (test IV) after the last extinction session.
Reconditioning (1 d).
During this drug session, the solid inserts were used to fully separate the compartments, and rats underwent one session with heroin and the other with vehicle, in the compartments that were previously paired with heroin and vehicle, respectively. The occurrence of the heroin session (morning or afternoon) was counterbalanced across rats and, as done for conditioning, reconditioning sessions lasted 30 min. Injections were administered immediately before confinement in one of the 2 compartments.
Test V (1 d).
At 24 h after reconditioning, the inserts with openings were used, and time spent in each compartment was measured over 20 min. No injections were given prior to this test.
Total CORT analyses.
For CORT determination, cages were changed, and all fecal pellets produced during a light or dark period over 24 h were collected (that is, at 12-h intervals) and weighed at pretest, week 5, and week 10 of the study. Rats were not removed from cages during pellet collection, which took between 20 s to 45 s to perform, and light-phase samples were always collected first. Feces collection always occurred prior to any heroin treatments or CPP testing. Samples were frozen at −20 °C until extracted.14 Briefly, samples were dried for 2 h at 30 °C, weighed, and pulverized, and a 0.2-g sample was removed for extraction. To the fecal sample, 0.8 mL water and 5 mL dichloromethane were added, and samples were vortexed for 30 s in 5-s pulses. Samples then were centrifuged for 15 min at 1690 × g. The bottom (dichloromethane) fraction was transferred and washed with 1 mL 0.1 M NaOH by vortexing for 10 s, followed by centrifugation for 10 min at 1690 × g. The dichloromethane fraction was transferred and washed twice with water, centrifuged, and again transferred to a fresh tube. Of the final dichloromethane fraction, 1 mL was transferred and evaporated to dryness under N2 for approximately 15 min then stored at −20 °C until analyzed. Samples were resuspended in 1 mL 95% ethanol, vortexed, and diluted 1:25 with kit assay buffer. CORT concentration was determined by using the Correlate EIA Kit (Assay Designs, Ann Arbor, MI) according to manufacturer's instructions. ELISA plates were read on a BioTek PowerWave XS (Winooski, VT) ELISA plate reader at 405 nm. Concentration was determined as percentage bound by using a standard curve ranging from 32 pg/mL to 20,000 pg/mL (kit sensitivity, 27 pg/mL). Values were expressed according to the total feces collected over a time period and as nanograms of corticosterone per gram of feces. The assay kit has 28.6% crossreactivity with deoxycorticosterone and desoxycorticosterone, metabolites of corticosterone. Therefore, the values measured and reported largely represent corticosterone and these metabolites.
All samples were run in duplicate, and samples from different test periods were randomized to ELISA plates. The intraassay coefficient of variation was 3%, and the interassay coefficient of variation was 11%. Linear regression performed on the standard concentration to percentage corticoid bound curve demonstrated excellent correlation (R2 ≥ 0.98).
Organ weights.
At study end, rats were euthanized by CO2 inhalation, and liver, brain, and paired adrenal gland weights were collected. Organ:body weight ratios were calculated for each animal and expressed as a percentage (that is, relative organ weight).
Data analyses.
All data are reported as mean ± SEM. Between-group differences in body weights, food consumption, and organ:body weight ratios were evaluated by using Student t tests, with significance set at a P value less than 0.05. Group differences in diurnal and nocturnal levels of CORT over the course of the study were analyzed by using a 3-factor (cycle, week, housing) mixed-design ANOVA. When significant interactions or main effects were detected, posthoc multiple comparisons were conducted by using the Student–Newman–Keuls method (α level, 0.05).
In the testing apparatus described (that is, 2 larger compartments connected by a smaller transition box), CPP typically results from opposite shifts in time spent in the vehicle- and drug-paired compartments. Therefore, statistical analysis of CPP or locomotion during conditioning, extinction, or reconditioning across housing groups (between factors) involved 3-factor (treatment, week, housing) mixed-design ANOVA to compare time spent (seconds) and distance moved (centimeters) in each large compartment (repeated factor) at various tests (repeated factor). When significant interactions or main effects were detected, posthoc multiple comparisons were conducted by using the Student–Newman–Keuls method (α level, 0.05). We did not report the details of nonsignificant statistical analyses. All analyses were performed by using SigmaStat for Windows (version 3.5, Systat Software, Erkrath, Germany).
Results
Body weight, food consumption, and clinical observations.
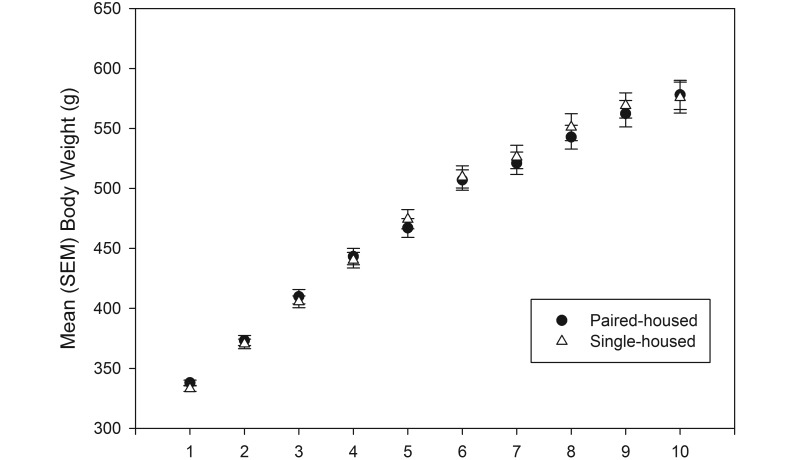
There were no significant (P = 0.9) differences in body weight (Figure 1) or food consumption (data not shown) between rats housed singly or in pairs. Rats demonstrated the expected body weight gain consistent with age-appropriate growth and maturation over the duration of the study.
Figure 1.
Weekly body weight (mean ± SEM) for single and pair-housed male rats over the course of the study. There was no significant difference in body weight between groups over time.
Scuffling and vocalization in pair-housed rats were common daily observations, especially during the early portion of the nocturnal phase, and 3 rats from different pairings had minor lacerations, interpreted to have arisen from agonistic interactions, by study end.
CPP.
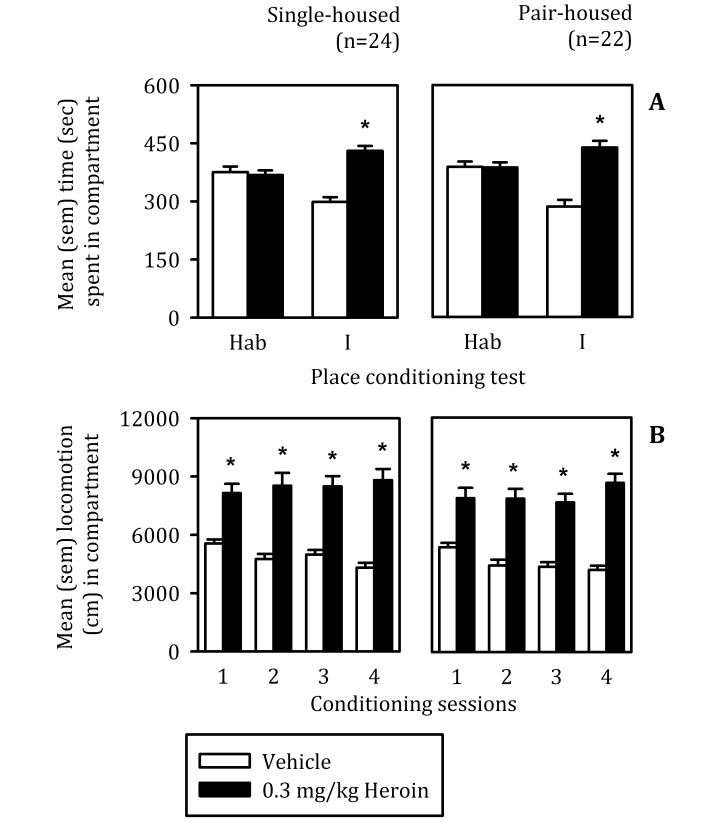
The conditioning protocol used in this study induced a significant preference for the heroin-paired compartment during test I, and the size of the CPP was virtually identical in singly and pair-housed groups (Figure 2 A; test by compartment interaction: F[1, 44] = 54.05, P < 0.001; main effect of test: F[1, 44] = 4.83, P < 0.05; main effect of compartment: F[1, 44] = 44.51, P < 0.001). During conditioning, the effect of 0.3 mg/kg heroin on locomotion was primarily stimulatory (Figure 2 B; conditioning session by conditioning day: F[3, 132] = 12.52; P < 0.001; main effect of conditioning session: F[1, 44] = 154.48, P < 0.001); no significant differences were found between housing groups. Therefore, singly and pair-housed rats did not differ in motor reactions to acute injections of 0.3 mg/kg heroin given during conditioning and displayed similar CPP for the compartment paired with the drug.
Figure 2.
(A) Time (mean ± SEM) spent in compartments paired with vehicle and 0.3 mg/kg heroin before (that is, habituation; Hab) and after (Test I) place conditioning in single- and pair-housed rats. *, Difference (α = 0.05) between compartments on test. (B) Locomotion (mean ± SEM) during conditioning after injections of vehicle and heroin in single- and pair-housed rats. *, Difference (α = 0.05) in activity between injections or compartments on each session of conditioning.
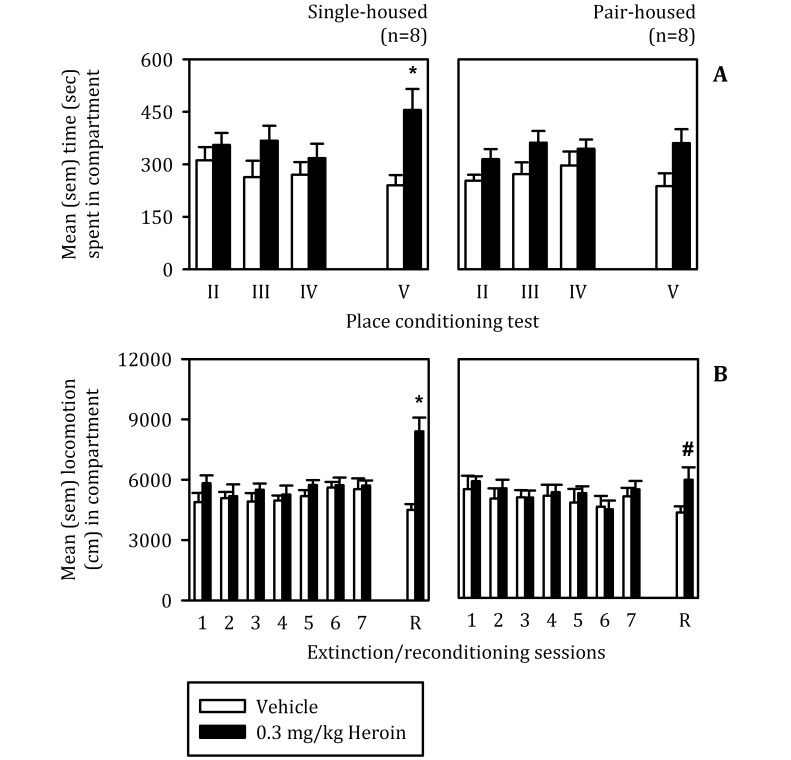
Because of lack of significant differences, 8 rats from each group were selected to continue CPP experiments and were tested for spontaneous recovery and reacquisition of CPP. The selection was based on the size of the CPP displayed on test I: a difference score was calculated, a median split was applied, and the top 8 rats were selected. Figure 3 A represents performance on preference tests (tests II through IV) given at different time intervals after extinction and the final test (test V), which was given 24 h after reconditioning. ANOVA identified a significant test by compartment interaction (F[3,42] = 3.78, P < 0.05) and a significant main effect of compartment (F[1,14] = 9.94, P < 0.05). Posthoc comparisons revealed a significant (P < 0.01) difference between time spent in vehicle and heroin compartments during test V in single-housed but not pair-housed rats. In other words, only single-housed rats displayed significant CPP reacquisition.
Figure 3.
(A) Time (mean ± SEM) spent in compartments paired with vehicle and 0.3 mg/kg heroin during various tests of place conditioning in single- and pair-housed rats. Tests II, III, and IV were given 1, 2 and 6 wk after the last extinction session, respectively. Test V was given 24 h after reconditioning. *, Difference (α = 0.05) between compartments on a given test. (B) Locomotion (mean ± SEM) during extinction (that is, 1 to 7 sessions) and reconditioning (R). During extinction, rats received vehicle injections prior to confinement to both CPP compartments. During reconditioning, rats received vehicle prior to confinement to the vehicle-paired compartment but 0.3 mg/kg heroin prior to confinement to the drug-paired compartment. *, Difference (α = 0.05) in activity between injections or compartments during extinction and reconditioning sessions; #, difference (α = 0.05) in activity between groups after the heroin injection given for the reconditioning session.
Figure 3 B represents locomotion during the 7 extinction sessions and the reconditioning session during the time that rats were confined to the appropriate compartments after injection of vehicle or 0.3 mg/kg heroin. ANOVA identified significant interactions between group and extinction or reconditioning session (F[7, 98] = 4.94, P < 0.001) and between compartment and extinction or reconditioning session (F[7,98] = 7.07, P < 0.001) as well as significant main effects of compartment (F[1,14] = 13.69, P < 0.05) and of extinction or reconditioning session (F[7,98] = 3.13, P < 0.01). Multiple comparisons revealed a heightened sensitivity to the stimulatory effects of 0.3 mg/kg heroin in singly housed rats. That is, on reconditioning, singly housed rats moved significantly more after an injection of heroin than after a vehicle injection and significantly more than did pair-housed rats injected with the same heroin dose. Therefore, although there was no effect of housing condition on spontaneous recovery of CPP, these data indicate that singly housed rats were more sensitive to the stimulatory properties of 0.3 mg/kg heroin during reconditioning and, unlike pair-housed rats, they displayed significant reacquisition.
CORT analyses.
Normal diurnal variation in fecal output was noted for both single- and pair-housed rats for the duration of the study, with markedly more feces produced during the dark phase than the light phase. At the beginning of the study, the total CORT levels were significantly different between the dark and light periods for both singly housed rats (3699 ± 555 ng and 1146 ± 195 ng, respectively) and pair-housed rats (3892 ± 513 ng and 1453 ± 129 ng, respectively), with a 3-fold difference noted in total CORT levels during the dark compared with light phases (Figure 4 A). The 3-way ANOVA revealed a significant main effect of cycle (F[1,14] = 38.2, P < 0.001), a significant main effect of week (F[2, 28] = 7.0, P < 0.005), and a significant cycle by week interaction (F[2, 28] = 18.0, P < 0.001). Similar results were obtained by evaluating CORT output relative to fecal output over the 12-h collection period (Figure 4 B).
Figure 4.
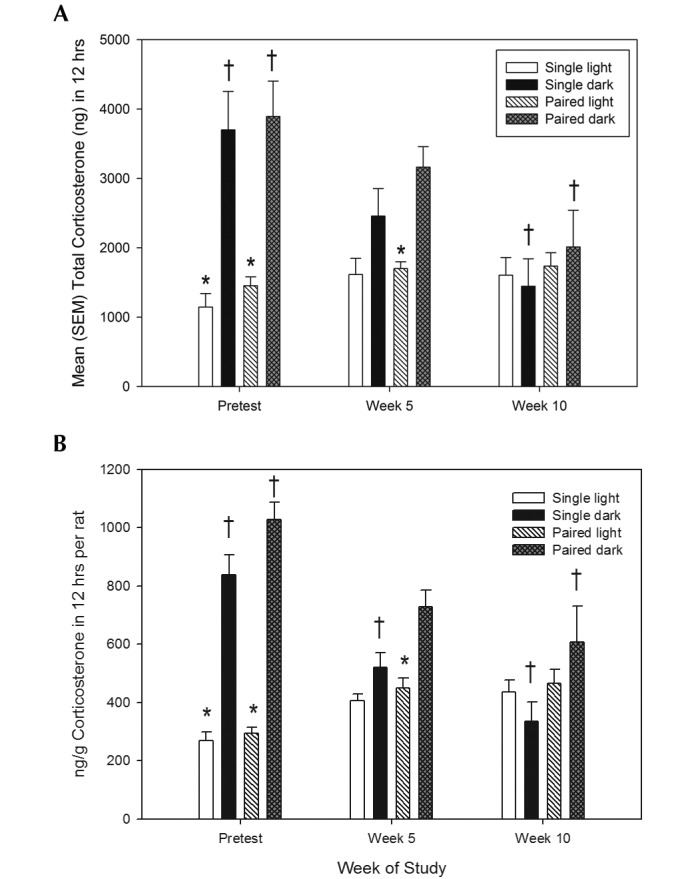
(A) Total CORT levels during 12-h light or dark period in single and pair-housed male Sprague–Dawley rats. (B) CORT levels relative to fecal output 12 h in single and pair-housed male Sprague–Dawley rats. *, Within-group differences (α = 0.05) between light-and dark-phase total fecal corticosterone levels; †, within-group differences (α = 0.05) in dark-phase CORT levels between test (week 5 or 10) compared with pretest levels.
Multiple comparisons indicated that the circadian difference in CORT levels was reduced after 10 wk of housing in the animal facility, and this decreased was primarily due to lower levels of CORT produced during the dark cycle. Although there was no overall significant main effect of group, a significant reduction of CORT in the dark period occurred after 5 wk in the socially isolated rats (F[1, 14] = 17.2, P < 0.01) but not until 10 wk in the pair-housed rats (F[1, 14] = 21.3, P < 0.05). Therefore, it appears that housing conditions had a minor effect on how rapidly circadian variations in CORT levels were altered. There were no between-group differences detected in total CORT levels during either phase of the light cycle (F[2, 28] = 0.44, P > 0.65).
Organ weights.
There were no significant differences in relative liver:body-weight ratios for rats between the 2 housing paradigms (0.038 for singly housed rats; 0.040 for pair-housed rats), confirming that food wastage and feed conversion efficiencies were similar for rats housed in either paradigm. As compared with pair-housed rats, singly-housed rats showed a small but significant increase in relative adrenal gland:body weight (5.9 × 10−5 and 4.5 × 10−5 for single-housed and with pair-housed rats, respectively; P < 0.043) and a decrease in relative brain:body weight (3.7 × 10−3 and 4.2 × 10−3 for singly and pair-housed rats, respectively; P < 0.005).
Discussion
In the current study, neither singly housed nor paired adult male rats showed initial differences in CPP induced by a single low dose of heroin, but differences emerged at later stages of testing after extinction and on reexposure to the drug. In fact, singly housed rats displayed a heightened sensitivity to the stimulatory effects of heroin assessed in the conditioning compartment, and only singly housed rats showed significant reacquisition of CPP. Why group differences were not observed during initial CPP conditioning and testing is unclear, but perhaps rats did not spend enough time in each of these housing conditions prior to behavioral testing. This reason could also explain why differences emerged on reconditioning, which occurred about 9 wk after initial conditioning and testing. Group differences might have emerged during initial CPP testing had different doses of heroin been used, and the use of a single dose level is one limitation of the current study. Finally, group differences might have emerged if the initial preference was not extinguished by explicitly pairing the drug-paired compartment with vehicle.
The CPP results suggest that duration and type of housing of adult laboratory rats can affect the animals’ sensitivity to the stimulatory and motivational effects of μ-opioid receptor agonists. More specifically, the current data suggest that social isolation may have sensitized rats to the effects of a low heroin dose, enhancing its ability to stimulate activity and promote reacquisition of conditioned place after its extinction.33 Given the postulated role of sensitization in addiction,34 the current CPP data indicate that social isolation may facilitate the development of addictive behaviors. The implications of these findings for humans or for other drugs of abuse remain unclear, and our study did not assess whether singly and pair-housed rats differed in the ability to learn or perform other tasks. The literature is not consistent with regard to the effects of social isolation and environmental enrichment on sensitivity to some effects of µ-opioid receptors agonists. For example, heroin CPP was reduced in group-housed mice reared from weaning to adulthood in enriched environments.13 However, others reported that group-housed Long–Evans male rats demonstrated greater CPP to low doses of heroin than did socially isolated rats,40 and, when isolated at weaning, rats were less sensitive to the drug than they were when they were isolated at maturity.39 Furthermore, other studies found no morphine CPP in isolated mice,10 no morphine CPP in isolation-reared Lister hooded rats,51 and reduced morphine CPP in rats reared in impoverished social conditions.3 Therefore, we suspect that rodent species, strain, test schedule, time of day testing, type of opiate, and opiate dose all may be critical determinants of the effect of housing conditions on CPP.
To our knowledge, this study is the first to report that chronic housing of male Sprague–Dawley rats, either singly or in pairs, in a standard laboratory animal environment leads to a blunting of circadian variation in CORT excretion. The effect is slightly more rapid in singly housed than pair-housed rats, although the overall difference by 12 wk was not significant. The experimental design in the present study was fundamentally different from most studies evaluating corticosterone levels in rats: most previous studies were short-term in nature (typically less than 2 wk in duration) and often introduced a stressor just prior to measuring CORT levels.14,17,24,36,45 In contrast, the current study lasted 12 wk, with most study activities (testing) occurring within the first 16 d and with little subsequent handling or testing of animals. Other than for planned periodic locomotion and CPP testing, rats were observed daily but were only handled once weekly for cage change or body-weight measurement. The reduction in nocturnal CORT levels occurred despite overall maintenance of the circadian variation in volume of feces produced by both groups, a finding that is consistent with a lack of correlation of biliary excretion of CORT with fecal mass.7 Stressed animals often produce less feces than do control animals, suggesting that the rats in the current study were not chronically stressed.17
Growth rates for rats in this study followed expected patterns for this stock,21 with steady increases in body weight throughout the experimental period and approximated by the steady increases in food consumption. This finding suggests that reduction of circadian corticoid rhythms in these rats did not adversely affect growth or appetite. These results also demonstrate that feed conversion efficiency and overall growth rates are unaffected by social housing paradigm in male Sprague–Dawley rats. Similarly, the relative liver and brain weights did not differ significantly from those published for male Sprague–Dawley rats, although the relative adrenal-gland weights obtained in this study were smaller than those reported for 12-wk-old male Sprague–Dawley rats (0.012 ± 0.002 g).21 This result is consistent with the decreased CORT found in these rats at week 10 and suggests a chronic reduction in adrenocortical activity, perhaps related to the lack of physical activity or to stimulation on a day-to-day basis. Others have determined that voluntary daily physical activity obtained via wheel running increased HPA system activity and total CORT production in male C57BL/6 mice and increased hippocampal neurogenesis.16 Although we noted significant differences in the relative brain and adrenal-gland weights between the paired and singly housed animals in the current study, the differences are small, are within expected variation for male Sprague–Dawley rats,23 and may not have biological significance. If meaningful, they would provide definitive evidence of adverse physiologic effects (such as decreased brain weight, leading to decreased plasticity and ability to cope with the environment) of single housing of rats. Other investigators have found evidence of small but significantly lower brain volumes in singly housed male Lister hooded rats after 15 wk; however, these investigators did not evaluate relative changes in brain weight, so it is difficult to compare their findings with those of the current study.15 In the cited study,15 lower brain volumes were found only in socially isolated male rats and not in group-housed male or female rats in either housing condition. Other stereologic findings from the previous study supporting the relevance of lower brain volumes included increased volume of the lateral ventricles and decreased hippocampal volume in singly housed male rats.15 Changes in hippocampal volume are not seen in socially isolated young male rhesus macaques;37 however, the isolated macaques were more physically active than were group-doused animals, which may help to maintain hippocampal neurogenesis.16,46
Shifts in the circadian rhythm of peak level expression of hypothalamic serotonin, hydroxytryptophan, and its metabolite, 5-hydroxy-indoleacetic acid, have been reported in Wistar rats after 5 wk of individual housing in soundproof cages,19 although absolute levels of these proteins remained the same. The cited study18 also evaluated plasma corticosterone levels in group- and individually housed rats housed in soundproof cages and reported higher peak corticosterone values in singly housed rats at the end of a 5-wk period. Social isolation combined with auditory isolation caused marked alterations in rat behavior, including increased reactivity to sound and alterations in vocalization patterns,28 which could contribute to higher serum corticosterone levels in singly housed rats held under conditions of sensory deprivation for 5 wk. In the present study, singly housed rats had visual, auditory, and olfactory contact with other rats in the room, which may account for the different trends in CORT levels noted for the 2 studies.
A recent study evaluated CORT levels in 84-wk-old male and female F344 rats.9 The study demonstrated that minor alterations in routine husbandry resulted in higher CORT levels9 but did not assess levels in young rats, so no time course differential was obtained. Furthermore, the authors of that study9 evaluated CORT levels only at specific times in response to a potential stressor, rather than collecting all feces produced during each time period, making it impossible to compare total CORT levels between studies.
In their natural state, rats are highly intelligent and exploratory animals, with a remarkable ability to adapt to new environments.4,11,20 Therefore, long-term housing of rats under standard laboratory conditions (standard-sized caging with solid bottom and substrate) without regular physical activity might eventually reduce HPA CORT output. Some studies have indirectly demonstrated that social housing alone may not affect physiologic responses of male rats to their environments. One study found that group and singly housed Sprague–Dawley rats under standard laboratory conditions for 13 wk did not demonstrate differences in hippocampal glucocorticoid receptor mRNA expression.50 Others similarly found no effect of housing condition on the response to a chronic stressor in paired or singly housed adult male Sprague–Dawley rats.17 Recent studies have indicated that neuronal connections, particularly within the hippocampus, and cortical complexity are constantly remodelled in mammals according to their experiences.21,27,38 Environmental enrichment may protect synaptic formation and complexity and brain plasticity in rats as they age, possibly through NO-mediated pathways.21,27,38 A reduction of the nocturnal HPA axis activity in rats held in a constant environment may represent an adaptive mechanism. However, in a study that investigated a shorter duration of single and pair housing, CORT levels did not reveal reduced HPA nocturnal activity in male rats that were handled daily for oral gavage.47
The results of the current study have potential implications for defining optimal housing conditions for rats in laboratory environments. Although researchers generally strive to standardize housing conditions that may contribute to experimental variation, the regular and unvarying environment of the rat in a contemporary vivarium, with little opportunity for regular physical activity, may undermine normal physiologic processes and HPA axis function.
Acknowledgments
This work was funded by the Natural Science and Engineering Research Council of Canada (Leri). Amanda Healy was supported by an Ontario Veterinary College Summer Research Student Scholarship.
References
- 1.Andersen SL, Teicher MH. 2004. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology 29:1988–1993 [DOI] [PubMed] [Google Scholar]
- 2.Bamberg E, Palme R, Meingassner JG. 2001. Excretion of corticosteroid metabolites in urine and faeces of rats. Lab Anim 35:307–314 [DOI] [PubMed] [Google Scholar]
- 3.Bardo MT, Robinet PM, Hammer RF., Jr 1997. Effect of differential rearing environments on morphine-induced behaviors, opioid receptors, and dopamine synthesis. Neuropharmacology 36:251–259 [DOI] [PubMed] [Google Scholar]
- 4.Barnett SA. 1958. Exploratory behaviour. Br J Psychol 49:289–310 [DOI] [PubMed] [Google Scholar]
- 5.Bowling SL, Bardo MT. 1994. Locomotor and rewarding effects of amphetamine in enriched, social, and isolate reared rats. Pharmacol Biochem Behav 48:459–464 [DOI] [PubMed] [Google Scholar]
- 6.Brunson KL, Chen Y, Avishai-Eliner S, Baram TZ. 2003. Stress and the developing hippocampus: a double-edged sword? Mol Neurobiol 27:121–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canadian Council on Animal Care. [Internet] 1993. CCAC guide to care and use of experimental animals, vol1. [Cited 2 February 2014]. Available at: http://www.ccac.ca/Documents/Standards/Guidelines/Experimental_Animals_Vol1.pdf
- 8.Cavigelli SA, Guhad FA, Ceballos RM, Whetzel CA, Nevalainen T, Lang CM, Klein LC. 2006. Fecal corticoid metabolites in aged male and female rats after husbandry-related disturbances in the colony room. J Am Assoc Lab Anim Sci 45:17–21 [PubMed] [Google Scholar]
- 9.Cavigelli SA, Monfort SL, Whitney TK, Mechref YS, Novotny M, McClintock MK. 2005. Frequent serial fecal corticoid measures from rats reflect circadian and ovarian corticosterone rhythms. J Endocrinol 184:153–163 [DOI] [PubMed] [Google Scholar]
- 10.Coudereau JP, Debray M, Monier C, Bourre JM, Frances H. 1997. Isolation impairs place preference conditioning to morphine but not aversive learning in mice. Psychopharmacology (Berl) 130:117–123 [DOI] [PubMed] [Google Scholar]
- 11.Davis H. 1996. Underestimating the rat's intelligence. Brain Res Cogn Brain Res 3:291–298 [DOI] [PubMed] [Google Scholar]
- 12.Deroche V, Piazza PV, Le Moal M, Simon H. 1994. Social isolation-induced enhancement of the psychomotor effects of morphine depends on corticosterone secretion. Brain Res 640:136–139 [DOI] [PubMed] [Google Scholar]
- 13.El Rawas R, Thiriet N, Lardeux V, Jaber M, Solinas M. 2009. Environmental enrichment decreases the rewarding but not the activating effects of heroin. Psychopharmacolology (Berl) 203:561–570 [DOI] [PubMed] [Google Scholar]
- 14.Eriksson E, Royo F, Lyberg K, Carlsson H-E, Hau J. 2004. Effect of metabolic cage housing on immunoglobulin A and corticosterone excretion in faeces and urine of young male rats. Exp Physiol 89:427–433 [DOI] [PubMed] [Google Scholar]
- 15.Fabricius K, Helboe L, Steiniger-Brach B, Fink-Jensen A, Pakkenberg B. 2010. Stereological brain volume changes in postweaned socially isolated rats. Brain Res 1345:233–239 [DOI] [PubMed] [Google Scholar]
- 16.Fuss J, Ben Abdallah NM, Vogt MA, Touma C, Pacifici PG, Palme R, Witzemann V, Hellweg R, Gass P. 2010. Voluntary exercise induces anxiety-like behavior in adult C57BL/6J mice correlating with hippocampal neurogenesis. Hippocampus 20:364–376 [DOI] [PubMed] [Google Scholar]
- 17.Giralt M, Armario A. 1989. Individual housing does not influence the adaptation of the pituitary–adrenal axis and other physiological variables to chronic stress in adult male rats. Physiol Behav 45:477–481 [DOI] [PubMed] [Google Scholar]
- 18.Greco AM, Gambardella P, Sticchi R, D'Aponte D, Di Renzo G, De Franciscis P. 1989. Effects of individual housing on circadian rhythms of adult rats. Physiol Behav 45:363–366 [DOI] [PubMed] [Google Scholar]
- 19.Hall FS, Huang S, Fong GW, Pert A, Linnoila M. 1998. Effects of isolation-rearing on locomotion, anxiety, and responses to ethanol in fawn hooded and Wistar rats. Psychopharmacology (Berl) 139:203–209 [DOI] [PubMed] [Google Scholar]
- 20.Hofer MA. 1994. Early relationships as regulators of infant physiology and behavior. Acta Paediatr Suppl 397 Suppl:9–18 [DOI] [PubMed] [Google Scholar]
- 21.Johansson BB. 2004. Brain plasticity in health and disease. Keio J Med 53:231–246 [DOI] [PubMed] [Google Scholar]
- 22.Konkle ATM, Kentner AC, Baker SL, Stewart A, Bielajew C. 2010. Environmental-enrichment–related variations in behavioral, biochemical, and physiologic responses of Sprague–Dawley and Long Evans rats. J Am Assoc Lab Anim Sci 49:427–436 [PMC free article] [PubMed] [Google Scholar]
- 23.Lang PL, White WJ. 1994. Growth, development, and survival of the Crl:CD(SD)BR stock and CDF(F344)/CrlBR strain, p 588–608. In: Dungworth DL, Mohr U, Capen CC, editors. Pathobiology of the aging rat, vol 1. Washington (DC): International Life Sciences Institute. [Google Scholar]
- 24.Lepschy M, Touma C, Hruby R, Palme R. 2007. Noninvasive measurement of adrenocortical activity in male and female rats. Lab Anim 41:372–387 [DOI] [PubMed] [Google Scholar]
- 25.Leri F, Rizos Z. 2005. Reconditioning of drug-related cues: a potential contributor to relapse after drug reexposure. Pharmacol Biochem Behav 80:621–630 [DOI] [PubMed] [Google Scholar]
- 26.Lightman SL, Wiles CC, Atkinson HC, Henley DE, Russell GM, Leendertz JA, McKenna MA, Spiga F, Wood SA, Conway-Campbell BL. 2008. The significance of glucocorticoid pulsatility. Eur J Pharmacol 583:255–262 [DOI] [PubMed] [Google Scholar]
- 27.Lores-Arnaiz S, Bustamante J, Arismendi M, Vilas S, Paglia N, Basso N, Capani F, Coirini H, L'opez Costa JJ, Lores Arnaiz MR. 2006. Extensive enriched environments protect old rats from the aging dependent impairment of spatial cognition, synaptic plasticity, and nitric oxide production. Behav Brain Res 169:294–302 [DOI] [PubMed] [Google Scholar]
- 28.Nunes Mamede Rosa ML, Nobre MJ, Oliveira AR, Brandão ML. 2005. Isolation-induced changes in ultrasonic vocalization, fear-potentiated startle, and prepulse inhibition in rats. Neuropsychobiology 51:248–255 [DOI] [PubMed] [Google Scholar]
- 29.Ontario Ministry of Agriculture and Food. Animals for Research Act. [Internet] R.R.O. 1990, Regulation 24 Research Facilities and Supply Facilities. [Cited 2 February 2014]. Available at: http://www.e-laws.gov.on.ca/html/regs/english/elaws_regs_900024_e.htm
- 30.Pryce CR, Feldon J. 2003. Long-term neurobehavioural impact of the postnatal environment in rats: manipulations, effects, and mediating mechanisms. Neurosci Biobehav Rev 27:57–71 [DOI] [PubMed] [Google Scholar]
- 31.Raz S, Berger BD. 2010. Social isolation increases morphine intake: behavioral and psychopharmacological aspects. Behav Pharmacol 21:39–46 [DOI] [PubMed] [Google Scholar]
- 32.Rescorla RA. 2001. Retraining of extinguished Pavlovian stimuli. J Exp Psychol Anim Behav Process 27:115–124 [PubMed] [Google Scholar]
- 33.Rescorla RA. 2004. Spontaneous recovery. Learn Mem 11:501–509 [DOI] [PubMed] [Google Scholar]
- 34.Robinson TE, Berridge KC. 2003. Addiction. Annu Rev Psychol 54:25–53 [DOI] [PubMed] [Google Scholar]
- 35.Rosenbrock H, Koros E, Bloching A, Podhorna J, Borsini F. 2005. Effect of chronic intermittent restraint stress on hippocampal expression of marker proteins for synaptic plasticity and progenitor cell proliferation in rats. Brain Res 1040:55–63 [DOI] [PubMed] [Google Scholar]
- 36.Royo F, Bjork N, Carlsson HE, Mayo S, Hau J. 2004. Impact of chronic catheterization and automated blood sampling (Accusampler) on serum corticosterone and fecal immunoreactive corticosterone metaqbolites and immunoglobulin A in male rats. J Endocrinol 180:145–153 [DOI] [PubMed] [Google Scholar]
- 37.Sánchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. 1998. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Res 812:38–49 [DOI] [PubMed] [Google Scholar]
- 38.Saito S, Kobayashi S, Ohashi Y, Igarashi M, Komiya Y, Ando S. 1994. Decreased synaptic density in aged brains and its prevention by rearing under enriched environment as revealed by synaptophysin contents. J Neurosci Res 39:57–62 [DOI] [PubMed] [Google Scholar]
- 39.Schenk S, Ellison F, Hunt T, Amit Z. 1985. An examination of heroin conditioning in preferred and nonpreferred environments and in differentially housed mature and immature rats. Pharmacol Biochem Behav 22:215–220 [DOI] [PubMed] [Google Scholar]
- 40.Schenk S, Hunt T, Colle L, Amit Z. 1983. Isolation versus grouped housing in rats: differential effects of low doses of heroin in the place-preference paradigm. Life Sci 32:1129–1134 [DOI] [PubMed] [Google Scholar]
- 41.Schrijver NCA, Bahr NI, Weiss IC, Wurbel H. 2002. Dissociable effects of isolation rearing and environmental enrichment on exploration, spatial learning, and HPA activity in adult rats. Pharmacol Biochem Behav 73:209–224 [DOI] [PubMed] [Google Scholar]
- 42.Smith MA, Chisholm KA, Bryant PA, Greene JL, McClean JM, Stoops WW, Yancey DL. 2005. Social and environmental influences on opioid sensitivity in rats: importance of an opioid's relative efficacy at the µ receptor. Psychopharmacology (Berl) 181:27–37 [DOI] [PubMed] [Google Scholar]
- 43.Thanos PK, Cavigelli SA, Michaelides M, Olvet DM, Patel U, Diep MN, Volkow ND. 2009. A noninvasive method for detecting the metabolic stress response in rodents: characterization and disruption of the circadian corticosterone rhythm. Physiol Res 58:219–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thorsell A, Slawecki CJ, El Khoury A, Mathe AA, Ehlers CL. 2006. The effects of social isolation on neuropeptide Y levels, exploratory, and anxiety-related behaviors in rats. Pharmacol Biochem Behav 83:28–34 [DOI] [PubMed] [Google Scholar]
- 45.Touma C, Palme R, Sachser N. 2004. Analyzing corticosterone metabolites in fecal samples of mice: a noninvasive technique to monitor stress hormones. Horm Behav 45:10–22 [DOI] [PubMed] [Google Scholar]
- 46.Trejo JL, Carro E, Torres-Aleman I. 2001. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci 21:1628–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turner PV, Vaughn E, Sunohara-Neilson E, Ovari J, Leri F. 2012. Oral gavage in rats: Animal welfare evaluation. J Am Assoc Lab Anim Sci 51:25–30 [PMC free article] [PubMed] [Google Scholar]
- 48.Valles R, Rocha A, Nation JR. 2006. The effects of acquisition training schedule on extinction and reinstatement of cocaine self-administration in male rats. Exp Clin Psychopharmacol 14:245–253 [DOI] [PubMed] [Google Scholar]
- 49.Weiss IC, Di Iorio L, Feldon J, Domeney AM. 2000. Strain differences in the isolation-induced effects on prepulse inhibition of the acoustic startle response and on locomotor activity. Behav Neurosci 114:364–373 [PubMed] [Google Scholar]
- 50.Weiss IC, Pryce CR, Jongen-Relo AL, Nanz-Bahr NI, Feldon J. 2004. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res 152:279–295 [DOI] [PubMed] [Google Scholar]
- 51.Wongwitdecha N, Marsden CA. 1996. Effect of social isolation on the reinforcing properties of morphine in the conditioned place-preference test. Pharmacol Biochem Behav 53:531–534 [DOI] [PubMed] [Google Scholar]
- 52.Zakharova E, Miller J, Unterwald E, Wade D, Izenwasser S. 2009. Social and physical environment alter cocaine-conditioned place preference and dopaminergic markers in adolescent male rats. Neuroscience 163:890–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zimmermann E, Critchlow V. 1967. Effects of diurnal variation in plasma corticosterone levels on adrenocortical response to stress. Proc Soc Exp Biol Med 125:658–663 [DOI] [PubMed] [Google Scholar]