Abstract
Tektins (TEKTs) are composed of a family of filament-forming proteins localized in cilia and flagella. Five types of mammalian TEKTs have been reported, all of which have been verified to be present in sperm flagella. TEKT2, which is indispensable for sperm structure, mobility, and fertilization, was present at the periphery of the outer dense fiber (ODF) in the sperm flagella. By yeast two-hybrid screening, we intended to isolate flagellar proteins that could interact with TEKT2, which resulted in the isolation of novel two genes from the mouse testis library, referred as a TEKT2-binding protein 1 (TEKT2BP1) and -protein 2 (TEKT2BP2). In this study, we characterized TEKT2BP1, which is registered as a coiled-coil domain-containing protein 172 (Ccdc172) in the latest database. RT-PCR analysis indicated that TEKT2BP1 was predominantly expressed in rat testis and that its expression was increased after 3 weeks of postnatal development. Immunocytochemical studies discovered that TEKT2BP1 localized in the middle piece of rat spermatozoa, predominantly concentrated at the mitochondria sheath of the flagella. We hypothesize that the TEKT2-TEKT2BP1 complex might be involved in the structural linkage between the ODF and mitochondria in the middle piece of the sperm flagella.
Keywords: Tektin2, TEKT2BP1 (Ccdc172), spermatozoon, flagellum, peri-axoneme structure
Introduction
The flagella of spermatozoa are essential for sperm movement, and properly formed flagella are required for sperm movement to reach and fertilize eggs. Defects in flagellum molecules in spermatozoa result in an abnormality of the sperm flagella structure, which leads to motility disorders (Escalier 2006). Thus, it is important to understand the subcellular distribution and physiological functions of sperm tail components as well as the structural complexity of sperm flagella.
The core component of sperm flagella is the axoneme, which is composed of the central pair of microtubules, structural elements such as radial spokes, and nine outer doublet-microtubules. Each outer doublet-microtubule is made up of protofilaments consisting of tubulin heterodimers, dyneins, and other microtubule-associated proteins (Gibbons 1981; Olmsted 1986; Mohri 1993; Nojima et al. 1995; Sullivan 1998). Dynein motor proteins on the outer doublet microtubules generate the force for flagella propulsion.
Flagella of mammalian spermatozoa are divided into four parts: connecting piece, middle piece, principal piece, and end piece. Structural components are different between the compartments, because flagellum of mammalian spermatozoon have, in addition to the axoneme, three additional peri-axoneme components: the mitochondrial sheath in the middle piece, the outer dense fibers (ODFs) in the middle and the principal pieces, and the fibrous sheath (FS) in the principal piece; thus, mammalian sperm flagella are more complex. In the middle piece of the flagellum, nine ODFs surround the axoneme and are paired with nine peripheral doublets of microtubules, forming a “9+9+2” pattern. The mitochondrial sheath wraps around the ODFs in the middle piece. In the principal piece, ODF#3 and ODF#8 are replaced by the longitudinal columns of FS, which are held together by transverse ribs of this structure. The end piece lacking the ODF and FS contains only the axoneme. Mitochondria, ODFs, and the FS together form the peri-axoneme structure in the mammalian sperm flagella.
Tektins (TEKTs)—constitutive proteins of microtubules in cilia, flagella, basal bodies, and centrioles (Norrander et al. 1998; Steffen and Linck 1988; Larsson et al. 2000)—have been identified in various animals, including silkworms (Ota et al. 2002), mice (Norrander et al. 1998; Iguchi et al. 1999) and humans (Xu et al. 2001; Wolkowicz et al. 2002). They were originally isolated from sea urchins as a set of proteins: TEKT-A, -B and -C (Linck et al. 1982; Norrander et al. 1996). TEKTs of the sea urchin were shown to be highly coiled-coil molecules that form filaments in the wall of ciliary and flagellar axoneme microtubules (Norrander et al. 1996).
On the other hand, five types of TEKTs have been reported in mammals. TEKT1, which has been reported to localize in the centrosome in round spermatids as well as in the caudal ends of elongating spermatids (Larsson et al. 2000), has been recently verified to be a constituent of sperm flagella as well as the acrosome (Oiki et al. In press). TEKT2, which is identical to Tektin-t, has been reported to localize in the principal piece of human spermatozoa without detectable immunosignals in the middle piece or the end piece (Iguchi et al. 1999, 2002; Wolkowicz et al. 2002). TEKT3, which has been cloned as a testis-specific gene, is expressed in spermatocytes and spermatids (Roy et al. 2004). TEKT4, which was isolated as a molecule interacting with Spetex-1, localizes on the rat sperm flagella (Matsuyama et al. 2005). We have recently reported that TEKT5 is also a constituent of rat sperm flagella (Murayama et al. 2008). There is increasing evidence to show that, in contrast to TEKTs of sea urchin, mammalian TEKTs are associated with the peri-axoneme components of flagella; i.e., mitochondria, ODF, and FS.
Of mammalian TEKTs, TEKT2 has been found to be required for normal flagellar structure and function. Tekt2-null spermatozoa display flagellum bending and reduced motility (Tanaka et al. 2004) probably due to a disruption in the dynein inner arm. We previously demonstrated that TEKT2 might be present at the periphery of ODF as well as between ODFs and doublet microtubules in flagella (Shimasaki et al. 2010). In this study, we aimed to isolate novel TEKT2-interacting molecules to elucidate the molecular architecture of mammalian sperm flagella. We identified a 36-kDa TEKT2-bindng protein 1 (TEKT2BP1) as a flagella component predominantly associated with mitochondria in the middle piece of flagella.
Materials & Methods
Animals
Animal experiments were approved by the animal experimental committee of Kyushu University. Investigations were conducted in accordance with the National Research Council Guide for Care and Use of Laboratory Animals. Animals (ddY mouse and Wistar rats) for experiments were obtained from Kyudo Company (Fukuoka, Japan).
RNA Isolation
Total RNAs were isolated by QuickPrep Total RNA Extraction Kit (GE Healthcare, Piscataway, NJ) from testis, epididymis, lung, intestine, stomach, brain, heart, liver, skeletal muscle, and spleen of 12-week-old isoflurane-anesthetized Wistar rats as well as the testis of adult ddY mice. Total RNA was also isolated from testis of 1- to 8-week-old rats. cDNA strands were synthesized from 2 µg of total RNA using a PrimeScript 1st strand cDNA synthesis Kit (Takara Biotech. Co., Tokyo, Japan) with oligo-(dT) primers. The reverse-transcribed cDNA was used as a PCR template to synthesize genes. PCR was performed using Ex Taq DNA polymerase (Takara Biotech. Co.).
Yeast Two-hybrid Screening
Yeast two-hybrid screening with a mouse cDNA library was performed as previously reported (Doiguchi et al. 2007). Full-length mouse TEKT2 (accession number NM_011902) was obtained by reverse transcriptional PCR (RT-PCR) using two oligonucleotides: 5’- GAT TGT CGA CCC ATG GTG ACA CTA AGT TTC AAG C-3’ (forward) and 5’- ATA GTC GAC CTA GGT TAG CTC CAG CTG GCA G -3’ (reverse). PCR-amplified TEKT2 was sequenced using a DNA sequencer (Applied Biosystems, Foster City, CA). To construct the bait plasmid, TEKT2 cDNA was digested with SalI and subcloned into the multiple-cloning site of PAS404 to obtain PAS404-TEKT2. Saccharomyces cerevisiae strain Y190 was transformed with PAS404-TEKT2. The transformants were then re-transformed with mouse testis MATCHMAKER cDNA library (Clontech Lab. Palo Alto, CA) that was constructed to fuse to the GAL-4 activation domain in pACT2 containing the LEU2 marker. Transformants were selected on synthetic minimal medium with dextrose lacking tryptophan, leucine, and histidine in the presence of 25 mM 3-aminotriazole. Growing colonies were re-streaked and tested for galactosidase activity using the filter-binding assay. Plasmids were recovered from the positive colonies, and the inserts were sequenced using a DNA sequencer (Applied Biosystems).
Mouse genes encoding full-length TEKT2-binding protein 1 (TEKT2BP1, accession number XP_129272) and TEKT2-binding protein 2 (TEKT2BP2, accession number XP_922601) were obtained by RT-PCR. PCR-amplified genes were sequenced by DNA sequencing, digested by EcoRI and XhoI, and subcloned into pACT2 vector. SPERGEN-1 (Doiguchi et al. 2002b) (negative control) was also subcloned into pACT2 vector digested by EcoRI and XhoI. Y190 transformed with PAS404-TEKT2 was re-transformed by pACT2 containing full-length TEKT2BP1, TEKT2BP2, and SPERGEN-1, followed by selection on synthetic medium, and glowing colonies were processed for galactosidase assay.
Transfection of TEKT2BP1 and TEKT2 into COS7 cells
Expression vectors were constructed for transfection of TEKT2BP1 and TEKT2 into COS7 cells cultivated in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum.
Full-length mouse TEKT2 gene, of a molecular weight of approximately 50 kDa, was amplified by PCR using Phusion DNA polymerase (Finnzymes Oy, Espoo, Finland) and was subcloned in-frame into the EGFP expression vector (Promega Corp., Madison,WI). The full-length open reading frame of mouse TEKT2BP1 was similarly PCR-amplified (using 5’- GAT GAA TTC ACC ATG AGT CTG GAG TCC TTG TTTC -3’ (forward) and 5’- GAT GAA TTC CAA GTG TGG GGT GGC TCA TG -3’ (reverse) primers), and then amplified PCR products were digested by EcoRI and XhoI and cloned in-frame into pFLAG-CMV-5.1 expression vector (Sigma-Aldrich, St. Louis, MO). The PCR product sequence was confirmed using a DNA sequencer (Applied Biosystems).
Plasmid DNAs were transfected into COS7 cells using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA), following the manufacturer’s instructions. After 24 hr of culture, transfected cells were either fixed and examined under a fluorescence microscope (Leica DMRXA, Wetzlar, Germany) or lysed directly in SDS-PAGE buffer for electrophoresis and immunoblot analysis. Non-transfected COS7 cells were used as a control for immunoblotting. To evaluate the effects of microtubules on the distribution of expressed proteins in the transfected cells, microtubules were depolymerized by exposure to 20 μM Nocodazole (Sigma-Aldrich) for 3 hr before fixation.
For immunofluorescence microscopy, control and transfected COS7 cells were fixed using 4% paraformaldehyde in PBS at 4C, washed in PBS, and treated with 0.1% Triton X-100 for 5 min. Cells were then subjected to immunolabeling using either the anti-tubulin antibody (Sigma-Aldrich) diluted 1:200 or a polyclonal anti-FLAG antibody (Medical and Biological Laboratories, Nagoya, Japan) diluted 1:500, followed by incubation with Cy3-conjugated anti-rabbit IgG (GE Healthcare). Nuclear DNA was stained by Hoechst 33342.
Expression Analysis by RT-PCR
The reverse-transcribed cDNA described above was used as a template for PCR. Full-length rat TEKT2BP1 was PCR-amplified by Ex Taq DNA polymerase (Takara Biotech. Co.) using the following primers: 5’- ATG AGT CTG GAG TCG TTG TTT CAGC -3’ (forward) and 5’- GAC TCA TCG GCT CAA GGA GTT ATTG -3’ (reverse). Full-length TEKT2BP1 was cloned into pGEM-T easy vector and sequenced. Primers for glyceraldehyde-3-phosphate dehydrogenase (G3PDH) were: 5’- TGA AGG TCG GTG TCA ACG GAT TTG GC -3’ (forward) and 5’- CAT GTA GGC CAT GAG GTC CAC CAC -3’ (reverse).
Antibody Production
The peptide used for raising the antibody was derived from the hydrophilic region of TEKT2BP1 (RGKIKKATED) (see Fig. 1). The peptide was coupled to keyhole limpet hemocyanin (KLH, Sigma-Aldrich). The peptide coupled to KLH (1 mg total dose) was dissolved in 1 ml of saline, emulsified with 1 ml of Freund’s complete adjuvant, and injected at multiple sites on the back of a rabbit, as described previously (Katafuchi et al. 2000; Doiguchi et al. 2002a, 2002b). The antiserum was collected within two weeks after the final injection. Affinity purification of the antibody was carried out over a matrix of the peptide coupled to 2-fluoro-1-methylpyridinium toluene-4-sulfonate-activated Sephadex (Seikagaku Kougyo, Japan), as described previously (Iida et al. 2006).
Figure 1.
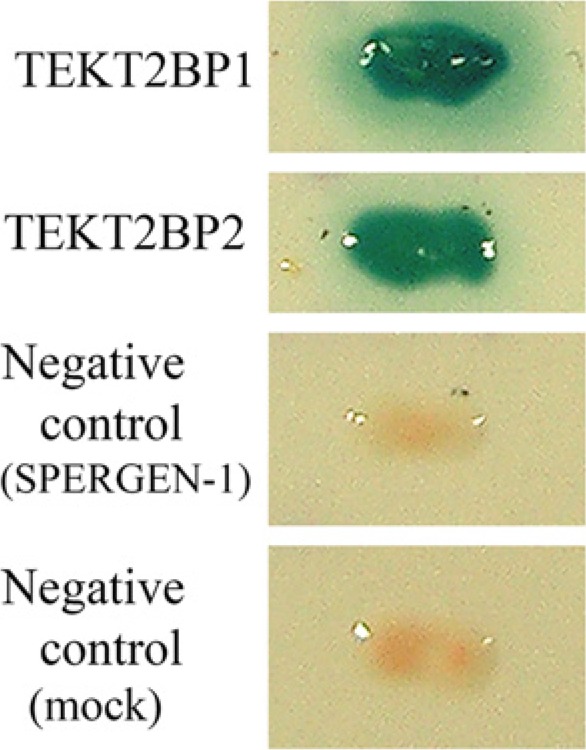
Yeast two-hybrid screening. PAS404-TEKT2-transformed Y190 yeast were re-transformed by pACT2 plasmid (Mock; negative control), pACT2-SPERGEN-1 (negative control), pACT2- TEKT2BP1 or pACT2-TEKT2BP2. Each transformant was cultivated on synthetic minimal medium with dextrose lacking tryptophan, leucine, and histidine in the presence of 25 mM 3-aminotriazole. Growing colonies were tested for galactosidase activity using the filter-binding assay. The co-transformed PAS404-TEKT2 with pACT2- TEKT2BP1 or TEKT2BP2 cells exhibited a clear galactosidase-positive phenotype.
Preparation of GST-fusion Proteins
Rat TEKT2BP1 has an open reading frame of 789 nucleotides encoding 262 amino acid residues (Fig. 2). The molecular size of TEKT2BP1 is 31 kDa. A 100-amino acid residue length (aa 1-100) of TEKT2BP1 was PCR-amplified using two primers; 5’- GAT GAA TTC ATG AGT CTG GAG TCG TTG TTT CAG -3’ (forward) and 5’- GAT CTC GAG TCA TTT CTT TAT AGC CTC GCA GGC TTG -3’ (reverse). After digestion with EcoR1 and Xho1, the PCR product was cloned in-frame at the COOH-terminus of GST (glutathione S-transferase) using pGEX4T1 system (GE Healthcare). Recombinant proteins were expressed in E. coli and purified onto glutathione-Sepharose (GE Healthcare), as described previously (Iida et al. 2006). The molecular size of the GST-fused TEKT2BP1 is 35 kDa. The GST-fused recombinant proteins, Rab3A, Rab3D, Rab6, Spergen3, Spetex-1, Syntaxin2, Hsp40, and Iba1, were similarly produced and purified. These recombinant proteins were used for immunoblot analysis.
Figure 2.
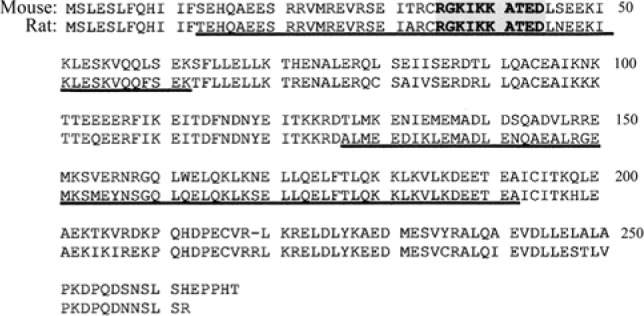
Predicted amino acid sequences of mouse (Mus musculus) TEKT2BP1 (accession no. XP_129272) and rat (Rattus norvegicus) TEKT2BP1 (accession no. XP_342071). Mouse TEKT2BP1 showed 84.4% identity with rat TEKT2BP1. Both TEKT2BP1 contain coiled-coil motifs at amino acid residues (aa) 13-62 and aa 127-192 (underlined). A polyclonal antibody was raised against the synthetic peptide (RGKIKKATED) corresponding to aa 35-44 of TEKT2BP1 (boldface type), which is shared by mouse and rat TEKT2BP1.
Purification of Spermatozoa
Spermatozoa that were released from caput and cauda epididymides of isoflurane-anesthetized adult Wistar rats were purified by Percoll-density gradient centrifugation, as reported previously (Iida et al., 1999). Highly purified spermatozoa were extracted using RIPA buffer [50 mM Tris (pH 7.2), 1 mM EDTA, 0.1% SDS, 0.1% Na deoxycholate, 1% Nonidet P-40, protease inhibitors cocktail (Roche diagnostics)] for 1 hr on ice, and then centrifuged for 15 min at 10,000 rpm to separate extracts from undissolved pellets. Both the extracts and the pellets were processed for electrophoresis and following immunoblot analysis.
Immunoblot Analysis
Protein samples dissolved in SDS-PAGE sample buffer were separated on SDS-PAGE using 12% acrylamide gels. Separated proteins were either stained with Coomassie brilliant blue or transferred to PVDF membranes (Hybond-P, GE Health). The membranes were incubated for 2 hr with either affinity-purified anti-TEKT2BP1 antibody diluted 1:10,000 in blocking buffer (PBS containing 5% nonfat milk and 0.05% Tween-20) or polyclonal anti-FLAG antibody (Medical and Biological Laboratories, Nagoya, Japan) diluted 1:5000. Membranes were then incubated with HRP-conjugated anti-rabbit IgG (BioRad, Richmond, CA) diluted 1:2000 in the same buffer. Antigen-antibody complexes were visualized using an ECL-Plus detection kit (GE Healthcare).
Immunofluorescence Microscopy of Spermatozoa
Rat spermatozoa that were purified from caput and cauda epididymis were fixed in 4% paraformaldehyde in PBS at 4C for 1 hr, washed in PBS, and then attached to poly-L-lysine-coated glass slides for immunolabeling. After treatment with 0.1% Triton X-100 for 5 min, the samples were incubated for 2 hr with anti-TEKT2BP1 antibody diluted 1:200 with blocking buffer (PBS containing 5% nonfat milk) followed by incubation with Cy3-conjugated goat anti-rabbit IgG (GE Healthcare). For DNA staining, immunostained samples were incubated for 30 min with PBS containing SYTOX Green (1:10,000 dilution; Molecular Probes, Eugene, OR). Some of the cauda spermatozoa were exposed for 10 min to PBS containing 1% Triton X-100, 0.1% SDS, and 1% 2-mercaptoethanol before immunolabeling. The samples were then washed with PBS and examined under a fluorescence microscope. For controls, the primary antibody was replaced by pre-immune serum.
Post-embedding Immunoelectron Microscopy
Adult rat epididymes were fixed in PBS containing 3% paraformaldehyde and 0.1% glutaraldehyde for 6 hr, washed overnight in PBS, dehydrated in a graded ethanol series, then embedded in LR White (London Resin Company, Berkshire, England), according to the protocol of the manufacturer. Ultra-thin sections were immunostained for 2 hr with the anti- TEKT2BP1 antibody, followed by incubation for 1 hr with 10 nm gold-conjugated goat anti-rabbit IgG (GE Healthcare). For controls, the primary antibody was replaced with pre-immune serum. The antibody was diluted in PBS containing 0.5% bovine serum albumin and 0.1% gelatin. Immunostained sections were examined using a Hitachi H-600 electron microscope (Tokyo, Japan) after staining with 2% uranyl acetate and lead citrate. On the electron micrographs, the number of gold particles present on cell organelles of immunostained spermatozoa was counted.
Results
Yeast Two-hybrid Screening
We performed yeast two-hybrid screening of a mouse testis cDNA library using full-length mouse TEKT2 as bait. Out of 1.3×109 colonies, 16 growing clones were selected based on their nutritional requirement for histidine, tryptophan, leucine as well as on galactosidase activity. Sequence data of these clones were subjected to homology search in the NCBI database employing FASTA and BLAST programs, and we found that they revealed high homology with two mouse genes encoding two hypothetical proteins: XP_129272 and XP_355274. These proteins have not been characterized yet, and were therefore designated as TEKT2-binding protein 1 (TEKT2BP1) and TEKT2-binding protein 2 (TEKT2BP2) in this study. Rat ortholog genes, XP_342071 (rat TEKT2BP1) and XP_573499 (rat TEKT2BP2), were also found in the database. In the latest databases, TEKT2BP1 and TEKT2BP2 are defined as coiled-coil domain-containing protein 172 (Ccdc172, NCBI Reference Sequence: NM_029372) and uncharacterized protein C1orf111 homolog (NCBI Reference Sequence: NP_001034682), respectively. In this study, the terms TEKT2BP1 and TEKT2BP2, rather than Ccdc172 and C1orf111 homolog, were used.
To confirm the interactive ability of TEKT2BP1 and TEKT2BP2 with TEKT2, the yeast Y190 strain, transformed by PAS404-TEKT2, was re-transformed by the rescue plasmid containing the full-length TEKT2BP1 (pACT2- TEKT2BP1) or TEKT2BP2 (pACT2- TEKT2BP2). The co-transformants of PAS404-TEKT2 with pACT2-TEKT2BP1 or pACT2- TEKT2BP2 exhibited a clear galactosidase-positive phenotype (Fig. 1). The negative controls (Mock and pACT2-SPERGEN-1) revealed no galactosidase activity. These results demonstrated that TEKT2 interacts strongly with TEKT2BP1 and TEKT2BP2 in the yeast two-hybrid system. Our preliminary immunocytochemical trial succeeded in detecting TEKT2BP1, but not TEKT2BP2 (data not shown), in rat spermatozoa; thus, we focused our attention on TEKT2BP1 hereafter.
Sequence of TEKT2BP1
The cDNA of mouse TEKT2BP1 contains an open reading frame (ORF) of 804 nucleotides encoding a protein of 267 amino acid residues. Rat ortholog TEKT2BP1 encodes a protein of 262 amino acid residues. The deduced amino acid sequences of rat and mouse TEKT2BP1 are shown in Figure 2. The predicted molecular mass of rat and mouse TEKT2BP1 is 31,074 Da and 31,482Da, respectively. At the amino acid level, mouse TEKT2BP1 showed 84.4% identity with rat TEKT2BP1. Full-length rat TEKT2BP1 was PCR-amplified, and its amino acid sequence was confirmed by DNA sequencing. NCBI (National Center of Biotechnology Information) Conserved Domain Search (http://ncbi.nem.nihgov/Structre/ccd/wrpsb.cgi) and SMART program (http://smart.embl-heidelberg.de/) predicted the residues aa 13-62 and aa 127-192 of rat TEKT2BP1 to be part of the coiled-coil domain (Fig. 2).
Co-localization of TEKT2 and TEKT2BP1 in COS-7 Cells
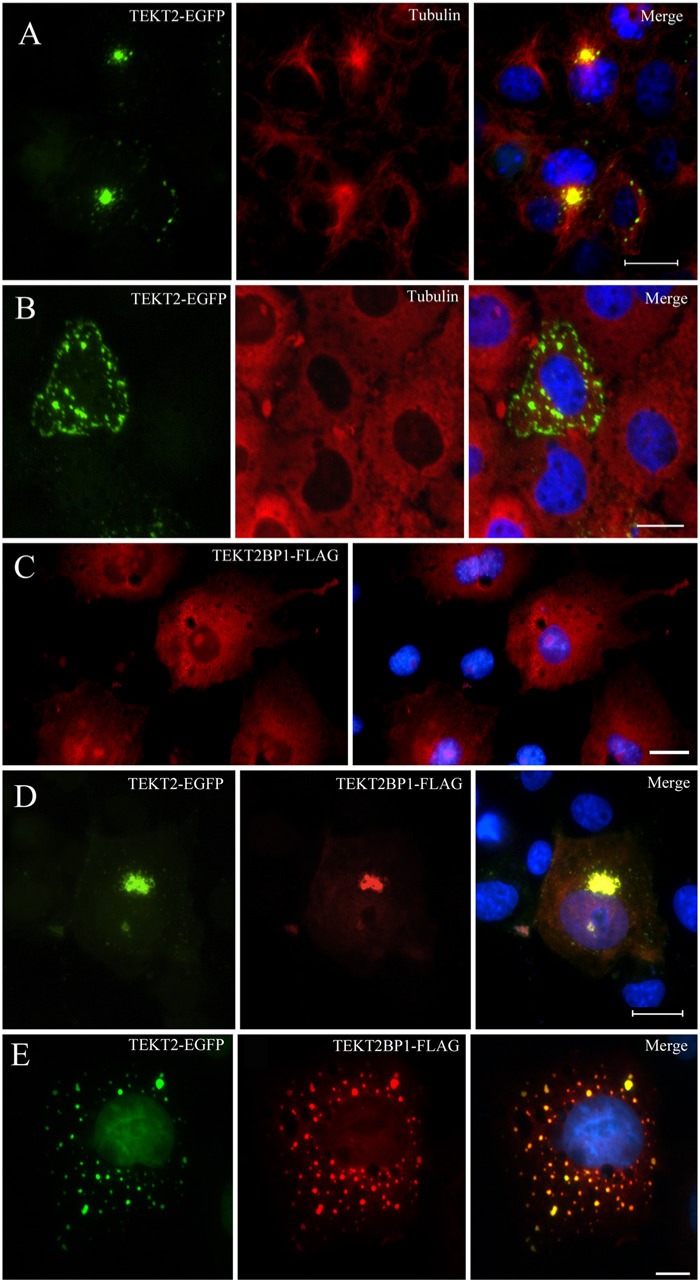
Physical interaction between TEKT2 and TEKT2BP1 was examined by transfection in COS7 cells (Fig. 3). When expressed in COS7 cells, TEKT2-EGFP appeared as small dots or aggregates at the microtubule organizing center (Fig. 3A), as reported in the previous study (Durcan et al. 2008). Depolymerization of microtubules by Nocodazole treatment resulted in dispersion of TEKT2-EGFP throughout the cytoplasm (Fig. 3B). TEKT2BP1-FLAG, on the other hand, appeared as a cytosolic protein distributed throughout the cytoplasm (Fig. 3C). When TEKT2-EGFP and TEKT2BP1-FLAG were co-expressed in the cells, the fluorescence of TEKT2-EGFP (green color) overlapped with that of TEKT2BP1-FLAG (red color) at the perinuclear region (Fig. 3D). After treatment with Nocodazole, the dispersed TEKT2-EGFP was accompanied by TEKT2BP1-FLAG (Fig. 3E). These observations suggested the existence of a physical interaction between the two proteins in COS7 cells.
Figure 3.
Physical interaction between TEKT2-EGFP and TEKT2BP1-FLAG expressed in COS7 cells. (A and B) COS-7 cells transfected with TEKT2-EGFP were cultivated in the absence (A) or presence (B) of 20 μM Nocodazole for 3 hr, fixed, and subjected to immunolabeling for tubulin. The green signal for TEKT2 (left) and red signal for tubulin (center) are merged in the right panels of the photographs. Nuclear DNA was stained using Hoechst 33342 (blue color). Note that expressed TEKT2-EGFP was found to be localized at the microtubule organizing centers (A), and Nocodazole treatment brought about the dispersal of TEKT2-EGFP throughout the cytoplasm (B). (C) COS-7 cells transfected with TEKT2BP1-FLAG (red). Nuclear DNA was stained by Hoechst 33342 (blue color). TEKT2BP1-FLAG appeared as cytosolic proteins distributed throughout the cytoplasm of the transfected COS7 cells. (D and E) Cells transfected with both TEKT2-EGFP (green) and TEKT2BP1-FLAG (red) were cultivated in the absence (D) or presence (E) of 20 μM Nocodazole for 3 hr. Cells were then fixed and subjected to immunolabeling for FLAG. The green signal for TEKT2 (left) and red signal for TEKT2BP1 (center) are merged (right panels). Nuclear DNA was stained by Hoechst 33342 (blue). TEKT2BP1-FLAG (red) co-localized with TEKT2-GFP (green) at the perinuclear region (D), and this co-localization was still observed after Nocodazole treatment (E). Scale bars, 20 μm in A, B, C and D; 10 μm in E.
Expression Profiles of TEKT2BP1
We next examined the expression of TEKT2BP1 in various organs of adult rats and the developmental expression of the gene in rat testes using RT-PCR. A 540-bp RT-PCR product of TEKT2BP1 was observed in testis, detected faintly in the epididymis, and detected at a much lower level in liver, stomach, intestine, spleen and skeletal muscle (Fig. 4A). PCR amplification of the gene was detectable faintly in the testes of 2-week-old rats and highly in 3-week-old rats that was sustained during the remaining postnatal developmental stages examined (Fig. 4B).
Figure 4.
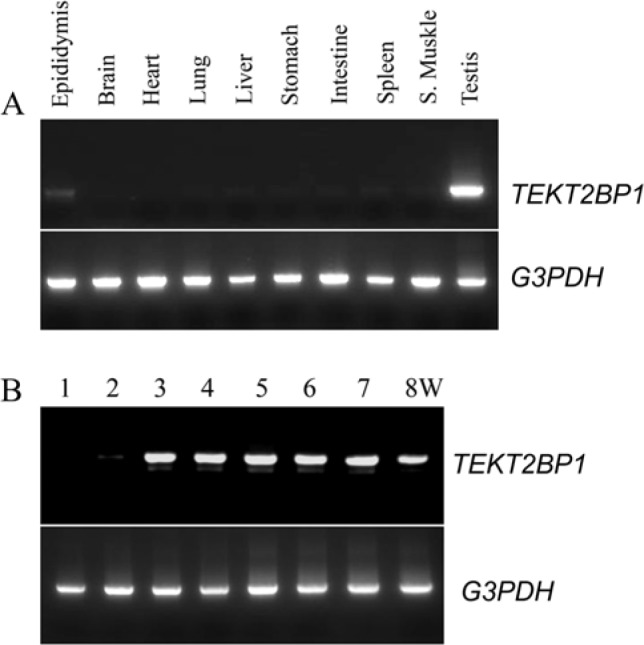
Expression analysis of rat TEKT2BP1 gene. (A) Expression analysis of TEKT2BP1 in various rat organs. RT-PCR analysis showed that TEKT2BP1 was highly expressed in testis, expressed at a low level in the epididymis, and at a much lower levels in the liver, stomach, intestine, spleen and skeletal muscle. (B) RT-PCR analysis was carried out to examine the expression levels of TEKT2BP1 in the testes of 1- to 8-week-old (W) rats. The expression of TEKT2BP1 was faintly detected at 2 weeks in the postnatal development and its expression level increased after 3 weeks. The expression of G3PDH was employed as a control for RT-PCR analysis.
Test of Specificity of the Anti-TEKT2BP1 Antibody in the Immunoblot Analyses
To examine the localization of TEKT2BP1 protein in rat spermatozoa, a polyclonal antibody was raised against the synthetic peptide (RGKIKKATED) corresponding to aa 35-44 of TEKT2BP1, the sequence of which is shared by the mouse and rat (Fig. 1). The anti-TEKT2BP1 antibody was used after affinity purification. The specificity of the anti-TEKT2BP1 antibody was first examined using western blotting of lysates from cells transfected with GST-fused TEKT2BP1 or one of several other GST-fused proteins. As shown in Figure 5A, the anti-TEKT2BP1 antibody recognized GST-TEKT2BP1 migrating at 35 kDa, but did not react with other GST-fused proteins. Next, COS7 cells expressing FLAG-tagged TEKT2BP1 were lysed, and the resultant proteins subjected to immunoblot analysis using either the anti-FLAG antibody or the anti-TEKT2BP1 antibody. As shown in Figure 5B, both the anti-FLAG antibody and the anti-TEKT2BP1 antibody recognized a protein migrating at approximately 35 kDa, which was not detected in the control samples (non- transfected cells); this indicated that the anti-TEKT2BP1 antibody recognizes TEKT2BP1 expressed in COS7 cells.
Figure 5.
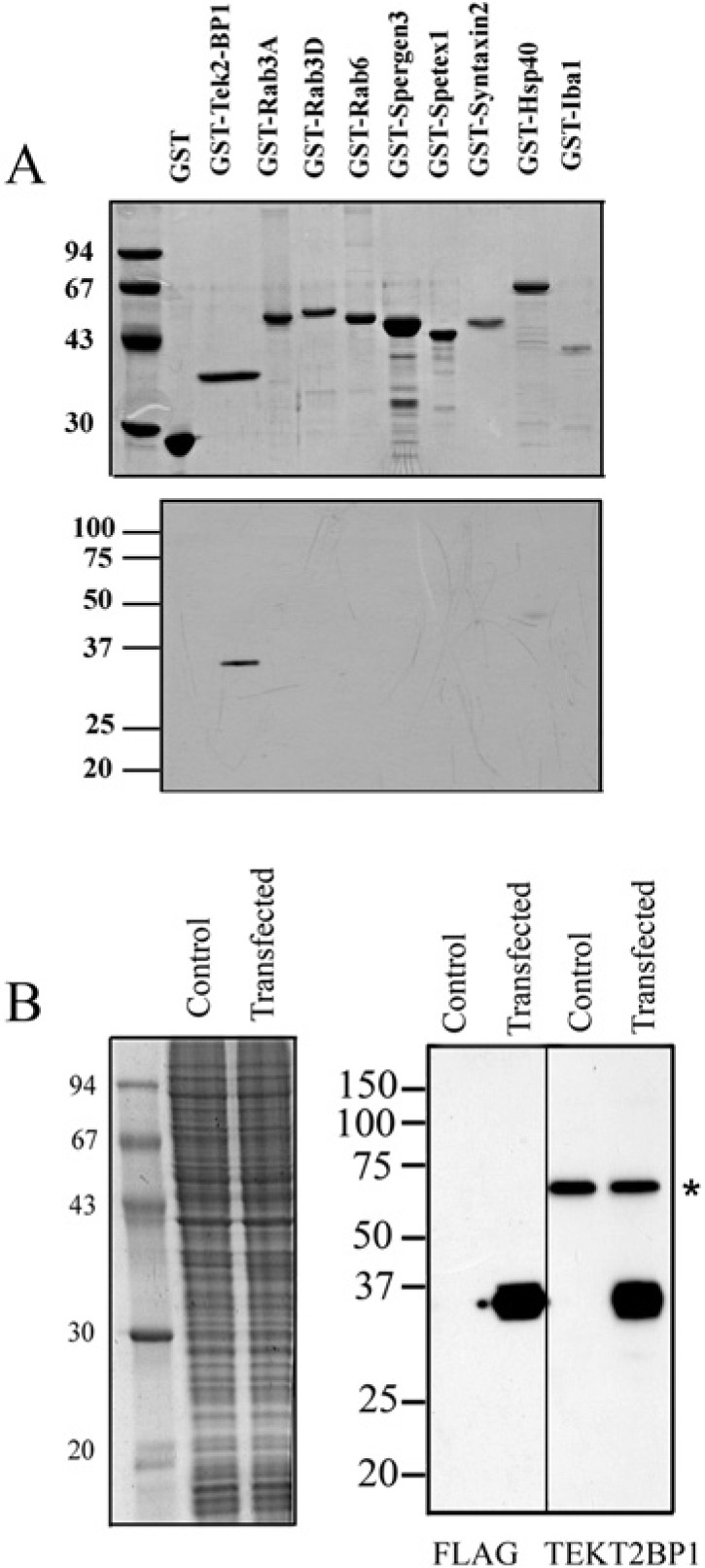
Specificity of the anti-TEKT2BP1 antibody. (A) Recombinant GST-fused proteins were produced in E. coli and separated by SDS-PAGE. Separated proteins were stained with Coomassie brilliant blue (upper panel) or transferred to a PVDF membrane for immunoblotting with the purified antibody against TEKT2BP1 (lower panel). The antibody recognized a 35-kDa GST-TEKT2BP1 protein without any prominent cross-reactivity to other GST-fused proteins. Molecular mass standards are shown in kilo-Daltons (left). (B) COS7 cells expressing FLAG-tagged TEKT2BP1 were lysed, and the resultant proteins separated on SDS-PAGE. Separated proteins were either stained with Coomassie brilliant blue (left panel) or transferred to PVDF membranes (right panel) for immunoblot analysis using either the anti-FLAG antibody (FLAG) or the anti-TEKT2BP1 antibody (TEKT2BP1). Both antibodies recognized a protein migrating at 35 kDa, which was not detected in the control samples (non-transfected cells). The asterisk indicates a nonspecific protein band of COS7 cells recognized by the anti-TEKT2BP1 antibody. Molecular mass standards are shown in kilo-Daltons (left).
Detection of TEKT2BP1 in Rat Spermatozoa
Because the expression and localization of TEKT2 have been previously examined in rat spermatozoa (Shimasaki et al. 2010), we also chose to investigate TEKT2BP1 in rat spermatozoa. Using the anti-TEKT2BP1 antibody, we examined by immunoblot analysis whether TEKT2BP1 is expressed in rat spermatozoa. Spermatozoa derived from the caput and cauda of rat epididymes were purified by Percoll-density gradient centrifugation, and purified spermatozoa were extracted using RIPA buffer, followed by centrifugation to separate the supernatant and the undissolved pellets. Both RIPA-extracted and undissolved proteins were subjected to SDS-PAGE and immunoblotting. We found that the anti-TEKT2BP1 antibody recognized a single protein migrating at 36 kDa in the extracted samples of both caput and cauda spermatozoa without detection in the undissolved pellets (Fig. 6). These data indicate the presence of TEKT2BP1 in spermatozoa. The size of 36 kDa is larger than the calculated molecular mass of full-length of TEKT2BP1 (31 kDa) deduced from the cDNA sequence; this slight increase may be due to protein modification, such as phosphorylation.
Figure 6.
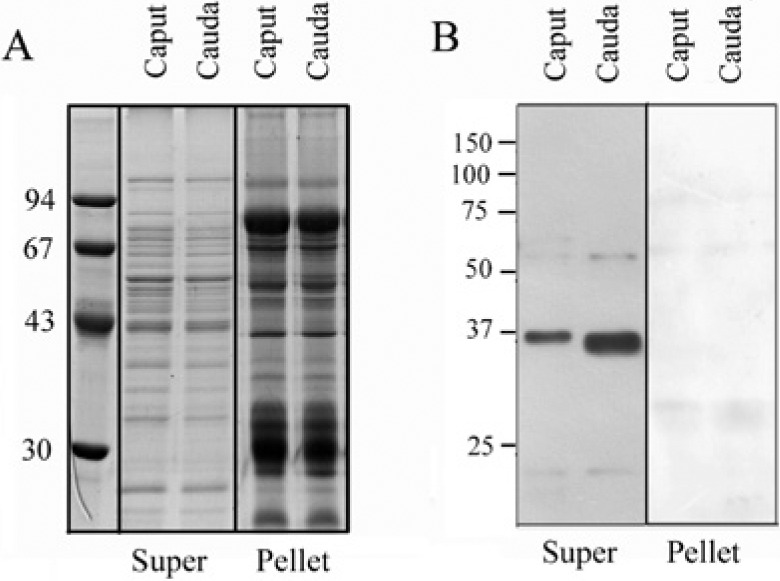
Immunoblot analysis of TEKT2BP1 in spermatozoa. Rat spermatozoa from caput and cauda epididymis were purified by Percoll-density gradient centrifugation, and purified spermatozoa were lysed by RIPA buffer. Both extracted (Super) and undissolved proteins (Pellet) were processed for SDS-PAGE. Separated proteins were either stained with Coomassie brilliant blue (A) or transferred to PVDF membrane for immunoblot analysis using the antibody against TEKT2BP1 (B). A protein migrating at approximately 36 kDa was detected in the supernatants of both caput and cauda spermatozoa. No band was detected in the undissolved samples (pellet). Molecular masses of the standard proteins are shown in the left in kilo-Daltons.
Using the anti-TEKT2BP1 antibody, we examined by fluorescence microscopy the distribution of TEKT2BP1 protein in rat epididymal spermatozoa. Spermatozoa derived from the caput and cauda were separately immunostained for TEKT2BP1. TEKT2BP1was visualized by the anti-TEKT2BP1 antibody and Cy3-conjugated anti-rabbit IgG. The immunovisualized samples were counterstained by SYTOX Green to visualize nuclear DNA.
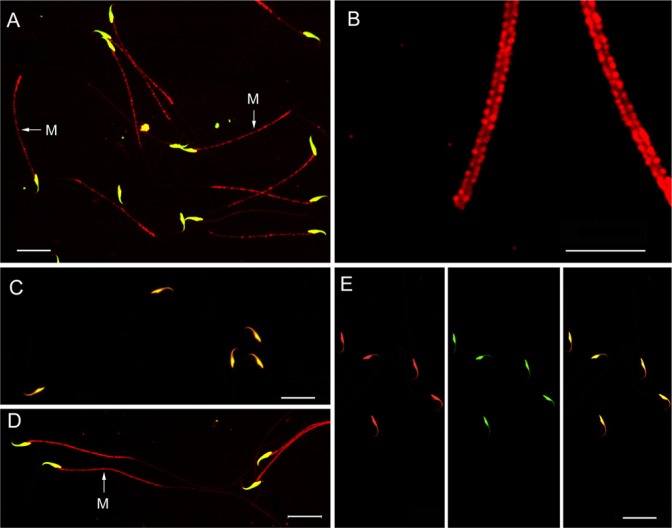
In caput spermatozoa, TEKT2BP1 was detected in the middle piece, but not in the principal piece of the flagella in most spermatozoa (Fig. 7A). The nuclear immunofluorescence by anti-TEKT2BP1 and Cy3-conjugated anti-rabbit IgG in spermatozoa might be non-specific. At high magnification of Fig. 7A, TEKT2BP1 immunostaining appeared as two dotted lines along the sides, but not in the central axis region of flagella (Fig. 7B), showing the localization of TEKT2BP1 on the mitochondrial sheath in the middle piece. On the other hand, cauda spermatozoa showed faint or very weak immunolabeling for TEKT2BP1 (Fig. 7C). However, when cauda spermatozoa were exposed to PBS containing 1% Triton, 0.1% SDS, and 1% 2-mercaptoethanol for 10 min before immunostaining, immunolabeling for TEKT2BP1 at the middle piece of flagella was completely recovered (Fig. 7D). At high magnification, we confirmed that the mitochondrial sheath in the middle piece was immunostained (data not shown). Replacement of the anti-TEKT2BP1 antibody with pre-immune serum (Fig. 7E) produced little immunolabeling on spermatozoa, with the exception of the signal in the sperm nuclei.
Figure 7.
Immunocytochemical localization of TEKT2BP1 in purified rat spermatozoa derived from the caput (A, B and E) and cauda (C and D). Fixed spermatozoa were treated either with 0.1% Triton X-100 for 5 min (A-C, E) or with 1% Triton X-100, 0.1% SDS, and 1% 2-mercaptoethanol (D) before immunostaining with the anti-TEKT2BP1 antibody followed by incubation with Cy3-conjugated goat anti-rabbit IgG (red). Immunostained samples were counter-stained by SYTOX Green to visualize nuclear DNA (green). Merged immunofluorescence images for TEKT2BP1 and SYTOX Green are shown in A-D. TEKT2BP1 immunosignal was found to be present in the middle piece of caput spermatozoa The middle pieces of immunolabeled spermatozoa in (A) are enlarged in (B). The TEKT2BP1 immunosignal was faint in cauda spermatozoa treated with 0.1% Triton X-100 (C), whereas pre-treatment of spermatozoa with 1% Triton X-100, 0.1% SDS, and 1% 2-mercaptoethanol produced distinct TEKT2BP1 immunostaining at the middle piece of the flagella (D). Replacement of the antibody with pre-immune serum caused immunostaining on the nuclei of the sperm heads (E), indicating that the nuclear signal was not caused by TEKT2BP1. M: middle piece. Scale bars, 20 μm in A, C, D and E; 10 μm in B.
Post-embedding Immunoelectron Microscopy
To determine the subcellular localization of TEKT2BP1 in sperm flagella, ultra-thin sections of caput spermatozoa were processed for post-embedding immunoelectron microscopy using the anti-TEKT2BP1 antibody and 10 nm gold-conjugated secondary antibody. The gold particles complexed with the anti-TEKT2BP1 antibody were found to be associated mostly with mitochondria, and modestly with ODF in the middle piece of the flagella (Fig. 8A and 8B). Axoneme and FS were devoid of labeling (Fig. 8A and B). Little or no gold particles were noticed in the control samples for which the primary antibody was replaced by pre-immune serum (Fig. 8C). We counted the number of immunogold particles found on cellular organelles or other cellular spaces on printed electron micrographs. Of 2,484 particles, 1,556 gold particles (62.6%) were found on mitochondria, 539 particles (21.7%) on ODF, and 389 particles (15.7%) between the mitochondria and ODF.
Figure 8.
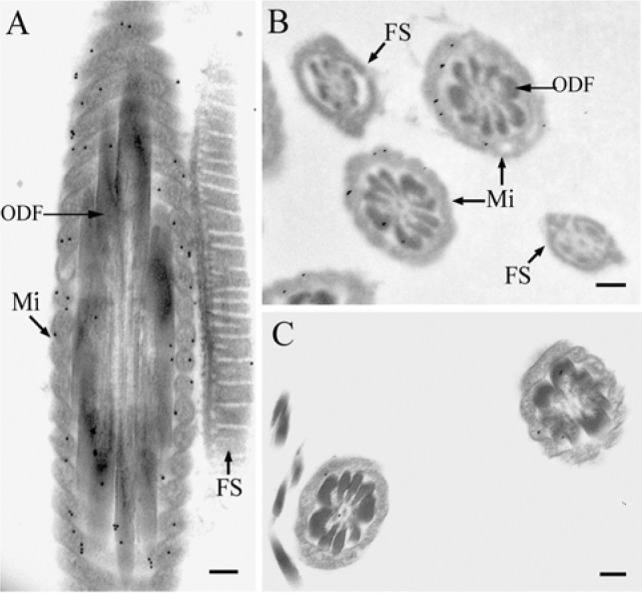
Post-embedding immunoelectron microscopy. Spermatozoa from caput epididymes were fixed and embedded in LR White. Ultrathin sections were immunostained for 2 hr with the anti-TEKT2BP1 antibody, followed by incubation for 1 hr with 10 nm gold-conjugated goat anti-rabbit IgG (A and B). For controls, the primary antibody was replaced with pre-immune serum (C). Gold particles complexed with the anti-TEKT2BP1 antibody were found to be associated with mitochondria (Mi) and, to a lesser extent, with ODF in the middle piece of the flagella, whereas the axoneme and the fibrous sheath (FS) were devoid of immunolabeling. Scale bars, 200 nm.
Discussion
The cellular organelles of the peri-axoneme structure in the middle piece of mammalian spermatozoa are mitochondria and ODF. Sperm mitochondria are assembled into an organized sheath surrounding the ODF and the axoneme in the middle piece. Each mitochondrion is arranged so that its surface faces three different cellular organelles; i.e., the plasma membrane, neighboring mitochondria, and the ODF. Mitochondria of sperm flagella are unique in that the surface of the mitochondrial outer membrane is decorated by highly ordered particulate arrays. The surface of the mitochondrion that faces the plasma membrane is decorated by closely packed rods that are haphazardly dispersed and composed of two to four 70- to 80-Å particles, while the concave surface of the organelle facing ODF displays rods assembled in stepladder pattern (Friend and Heuser 1981). ODFs, which differ from one another in terms of their size and cross-sectional profile at the ultrastructural level, are not uniform structures. They have a thin, striated cortical layer (cortex) with an electron-dense inner medulla (Fawcett 1975). The cortex is continuous over the abaxial surface of the ODFs but it is usually absent on the side nearer to the axoneme. Surface replica of naked ODFs exhibits an oblique striation at the cortex, and the striation appears to be composed of globular subunits (Fawcett 1975; Woolley 1971). Between ODF and mitochondrial sheath, there is a narrow space in which an electron-dense matrix, called the submitochondrial reticulum (SMR), exists (Olson and Winfrey 1986, 1990, 1992). The SMR is attached to the outer mitochondrial membrane and extends from the connecting piece to the annulus in spermatozoa. The molecules coating the surface of mitochondria and ODF as well as the SMR might be involved in the highly ordered organization of the peri-axoneme structure in mammalian sperm flagella. The peri-axoneme structure is attached to outer doublet microtubules of the axoneme through axoneme-ODF linkage.
We have recently identified several TEKTs as the components associated with the peri-axoneme structures of mammalian sperm flagella: TEKT2 at the periphery of ODF (Shimasaki et al. 2010), TEKT3 at the ODF and mitochondria (Takiguchi et al. 2011), TEKT4 at the cortex of ODF (Iida et al. 2006), and TEKT5 at the concave surface of the mitochondria (Murayama et al. 2008). It is probable that, after filament formation by homo- or hetero dimerization (Setter et al. 2006; Norrander et al. 1996), TEKTs might play roles as adhesive molecules to integrate ODF, mitochondria and SMR into the peri-axoneme structure in the middle piece of flagella.
In this study, we isolated and identified TEKT2BP1 (Ccdc172) as a partner of TEKT2 by yeast two-hybrid screening. TEKT2BP1 was predominantly expressed in the testis and its expression level increased after 3 weeks of postnatal development. Co-localization of TEKT2 and TEKT2BP1 in both intact and microtubule-disrupted COS7 cells supports the physical interaction between these two proteins. Immunofluorescence microscopy and immunoelectron microscopy suggest that TEKT2-BP1 might be associated with mitochondria, and, to a lesser extent, with ODF and the space between the two structures. In view of the finding that TEKT2 is present on the surface of ODF in flagella (Shimasaki et al. 2010), TEKT2 and TEKT2BP1 might form a protein complex either on the surface of ODF or between the ODF and the mitochondria. There is a possibility that the TEKT2-TEKT2BP1 complex might be involved in the structural linkage between the ODF and the mitochondria in the middle piece of the flagella.
We sometimes encountered difficulties in immunostaining TEKTs in spermatozoa. Pre-treatment of spermatozoa by autoclave is essential for immunocytochemical detection of TEKT5 in rat sperm flagella (Murayama et al. 2008). Immunosignals for TEKT2 in the principal piece was much stronger than for that in the middle piece in the cauda rat spermatozoa despite the presence of TEKT2 throughout the flagella (Shimasaki et al. 2010). In this study we showed that pre-treatment of cauda spermatozoa with PBS containing Triton X-100, SDS, and 2-mercaptoethanol was essential for immunocytochemical detection of TEKT2BP1. One possible explanation for such difficulties is that disulfide bond formation between molecules in flagella, which occurs during spermiogenesis in the testis and during maturation in the epididymis (Miranda-Vizuete et al. 2001), prohibits the antibodies from accessing the antigen in the flagellum.
Although the peri-axoneme structures of the flagella, i.e., ODF and FS, were once considered as passive supportive structures that provided mechanical reinforcement for the axoneme (Baltz et al. 1990), there is now accumulating evidence to suggest that the structures might be involved in a variety of critical physiological processes, such as energy production, signal transduction, protection against oxidative damage (Carrera et al. 1994; Godeas et al. 1997; Miki and Eddy 1999; Miki et al. 2004). More recently, Cao et al. (2006) identified additional signaling and metabolic proteins in the peri-axoneme structures. More studies of the peri-axoneme structure should cast new light on the structural and functional aspect of mammalian spermatozoa.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Grant-in-Aid for Scientific Research of Japan Society for the Promotion of Science.
References
- Baltz JM, Williams PO, Cone RA. (1990). Dense fibers protect mammalian sperm against damage. Biol Reprod 43:485-491 [DOI] [PubMed] [Google Scholar]
- Cao W, Gerton GL, Moss SB. (2006). Proteomic profiling of accessory structures from the mouse sperm flagellum. Mol Cell Proteomics 5:801-810 [DOI] [PubMed] [Google Scholar]
- Carrera A, Gerton GL, Moss SB. (1994). The major fibrous sheath polypeptide of mouse sperm: structural and functional similarities to the A-kinase anchoring proteins. Dev Biol 165:272-284 [DOI] [PubMed] [Google Scholar]
- Doiguchi M, Yamashita H, Ichinose J, Mori T, Shibata Y, Iida H. (2002a). Complementary DNA cloning and characterization of rat spergen-1, a spermatogenic cell-specific gene-1, containing a mitochondria-targeting signal. Biol Reprod 66:1462-1470 [DOI] [PubMed] [Google Scholar]
- Doiguchi M, Mori T, Toshimori K, Shibata Y, Iida H. (2002b). Spergen-1 might be an adhesive molecule associated with mitochondria in the middle piece of spermatozoa. Dev Biol 252:127-137 [DOI] [PubMed] [Google Scholar]
- Doiguchi M, Kaneko T, Urasoko A, Nishitani H, Iida H. (2007). Identification of heat shock protein Hsp40/DnaJ-1 as an acrosome- and a tail-associated component in rodent spermatozoa. Mol Reprod Dev 74:223-232 [DOI] [PubMed] [Google Scholar]
- Durcan TM, Halpin ES, Rao T, Collins NS, Tribble EK, Hornick JE, Hinchcliffe EH. (2008). Tektin 2 is required for central spindle microtubule organization and the completion of cytokinesis. J Cell Biol 181: 595–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalier D. (2006). Knockout mouse models of sperm flagellum anomalies. Human Reprod Update 12:449-461 [DOI] [PubMed] [Google Scholar]
- Fawcett DW. (1975). The mammalian spermatozoon. Dev Biol 44:394-436 [DOI] [PubMed] [Google Scholar]
- Friend DS, Heuser JE. (1981). Orderly particle arrays on the mitochondrial outer membrane in rapidly-frozen sperm. Anat Rec 199:159-75 [DOI] [PubMed] [Google Scholar]
- Gibbons IR. (1981). Cilia and flagella of eukaryotes. J Cell Biol 91:107-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godeas C, Tramer F, Micali F, Soranzo M, Sandri G, Panfili E. (1997). Distribution and possible novel role of phospholipid hydroperoxide glutathione peroxidase in rat epididymal spermatozoa. Biol Reprod 57:1502-1508 [DOI] [PubMed] [Google Scholar]
- Iida H, Yoshinaga Y, Tanaka S, Toshimori K, Mori T. (1999). Identification of rab3A GTPase as an acrosome-associated small GTP binding protein in rat sperm. Dev Biol 211:144-155 [DOI] [PubMed] [Google Scholar]
- Iida H, Honda Y, Matsuyama T, Shibata Y, Inai T. (2006). Tektin 4 is located on outer dense fibers, not associated with axonemal tubulins of flagella in rodent spermatozoa. Mol Reprod Dev 73:929-936 [DOI] [PubMed] [Google Scholar]
- Iguchi N, Tanaka H, Fujii T, Tamura K, Kaneko Y, Nojima H, Nishimune Y. (1999). Molecular cloning of haploid germ cell-specific tektin cDNA and analysis of the protein in mouse testis. FEBS Lett 456:315-321 [DOI] [PubMed] [Google Scholar]
- Iguchi N, Tanaka H, Nakamura Y, Nozaki M, Fujiwara T, Nishimune Y. (2002). Cloning and characterization of the human tektin-t gene. Mol Hum Reprod 8:525-530 [DOI] [PubMed] [Google Scholar]
- Katafuchi K, Mori T, Toshimori K, Iida H. (2000). Localization of a syntaxin isoform, syntaxin 2, to the acrosomal region of rodent spermatozoa. Mol Reprod Dev 57: 375-383 [DOI] [PubMed] [Google Scholar]
- Larsson M, Norrander J, Graslund S, Brundell E, Linck R, Stahl S, Hoog C. (2000). The spatial and temporal expression of Tekt1, a mouse tektin C homologue, during spermatogenesis suggests that it is involved in the development of the sperm tail basal body and axoneme. Eur J Cell Biol 79:718-725 [DOI] [PubMed] [Google Scholar]
- Linck RW, Albertini DF, Kenney DM, Langevin GL. (1982). Tektin filaments: chemically unique filaments of sperm flagellar microtubules. Prog Clin Biol Res 80:127–132 [DOI] [PubMed] [Google Scholar]
- Matsuyama T, Honda Y, Doiguchi M, Iida H. (2005). Molecular cloning of a new member of TEKTIN family, tektin4, located to the flagella of rat spermatozoa. Mol Reprod Dev 72:120-128 [DOI] [PubMed] [Google Scholar]
- Miki K, Eddy EM. (1999). Single amino acids determine specificity of binding of protein kinase A regulatory subunits by protein kinase A anchoring proteins. J Biol Chem 274:29057-29062 [DOI] [PubMed] [Google Scholar]
- Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, Strader LF, Perreault SD, Eddy EM, O’Brien DA. (2004). Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci U S A 101:16501-16506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Vizuete A, Ljung J, Damdimopoulos AE, Gustafsson JA, Oko R, Pelto-Huikko M, Spyrou G. (2001). Characterization of Sptrx, a novel member of the thioredoxin family specifically expressed in human spermatozoa. J Biol Chem 276:31567-74 [DOI] [PubMed] [Google Scholar]
- Mohri H. (1993). Role of tubulin and dynein in spermatozoan motility. Mol Reprod Dev 36:221-22 [DOI] [PubMed] [Google Scholar]
- Murayama E, Yamamoto E, Kaneko T, Shibata Y, Inai T, Iida H. (2008). Tektin5, a new TEKTIN family member, is a component of the middle piece of flagella in rat spermatozoa. Mol Reprod Dev 75:650-658 [DOI] [PubMed] [Google Scholar]
- Nojima D, Linck RW, Egelman EH. (1995). At least one of the protofilaments in flagellar microtubules is not composed of tubulin. Curr Biol 5:158-167 [DOI] [PubMed] [Google Scholar]
- Norrander JM, Perrone CA, Amos LA, Linck RW. (1996). Structural comparison of tektins and evidence for their determination of complex spacings in flagellar microtubules. J Mol Biol 257:385-397 [DOI] [PubMed] [Google Scholar]
- Norrander J, Larsson M, Stahl S, Hoog C, Linck R. (1998). Expression of ciliary tektins in brain and sensory development. J Neurosci 18:8912-8918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oiki S, Hiyama E, Gotoh T, Iida H. Localization of Tektin 1 at both acrosome and flagella of mouse and bull spermatozoa. Zoolog Sci. In press. [DOI] [PubMed] [Google Scholar]
- Olmsted JB. (1986). Microtubule-associated protein. Annu Rev Cell Biol 2:421-457 [DOI] [PubMed] [Google Scholar]
- Olson GE, Winfrey VP. (1986). Identification of a cytoskeletal network adherent to the mitochondria of mammalian spermatozoa. J Ultrastruct Mol Struct Res 94:131-139 [DOI] [PubMed] [Google Scholar]
- Olson GE, Winfrey VP. (1990). Mitochondria-cytoskeleton interactions in the sperm midpiece. J Struct Biol 103:13-22 [DOI] [PubMed] [Google Scholar]
- Olson GE, Winfrey VP. (1992). Structural organization of surface domains of sperm mitochondria. Mol Reprod Dev 33:89-98 [DOI] [PubMed] [Google Scholar]
- Ota A, Kusakabe T, Sugimoto Y, Takahashi M, Nakajima Y, Kawaguchi Y, Koga K. (2002). Cloning and characterization of testis-specific tektin in Bombyx mori. Comp Biochem Physiol B 133:371-382 [DOI] [PubMed] [Google Scholar]
- Roy A, Yan W, Burns KH, Matzuk MM. (2004). Tektin3 encodes an evolutionarily conserved putative testicular microtubules-related protein expressed preferentially in male germ cells. Mol Reprod Dev 67:295-302 [DOI] [PubMed] [Google Scholar]
- Setter PW, Malvey-Dornb E, Steffenc W, Stephensd RE, Lincka RW. (2006). Tektin interactions and a model for molecular functions. Exp Cell Res 312:2880-2896 [DOI] [PubMed] [Google Scholar]
- Shimasaki S, Yamamoto E, Murayama E, Kurio H, Kaneko T, Shibata Y, Inai T, Iida H. (2010). Subcellular localization of Tektin2 in rat sperm flagellum. Zoolog Sci 27: 755-761 [DOI] [PubMed] [Google Scholar]
- Steffen W, Linck RW. (1988). Evidence for tektins in centrioles and axonemal doublet microtubules. Proc Natl Acad Sci U S A 85:2643-2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KF. (1998). Structure and utilization of tubulin isotype. Annu Rev Cell Biol 4:687-716 [DOI] [PubMed] [Google Scholar]
- Takiguchi H, Murayama E, Kaneko T, Kurio H, Toshimori K, Iida H. (2011). Characterization and subcellular localization of Tektin 3 in rat spermatozoa. Mol Reprod Dev 78:611- 620 [DOI] [PubMed] [Google Scholar]
- Tanaka H, Iguchi N, Toyama Y, Kitamura K, Takahashi T, Kaseda K, Maekawa M, Nishimune Y. (2004). Mice deficient in the axonemal protein Tektin-t exhibit male infertility and immotile-cilium syndrome due to impaired inner arm dynein function. Mol Cell Biol 24:7958-7964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkowicz MJ, Naaby-Hansen S, Gamble AR, Reddi PP, Flickinger CL, Herr JC. (2002). Tektin B1 demonstrates flagellar localization in human sperm. Biol Reprod 66:241-250 [DOI] [PubMed] [Google Scholar]
- Woolley DM. (1971). Striations in the periperal fibers of rat and mouse spermatozoa. J. Cell Biol 49: 936-939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Zhou Z, Cheng C, Zhao W, Tang R, Huang Y, Wang W, Xu J, Zeng L, Xie Y, Mao Y. (2001). Cloning and characterization of a novel human TEKTIN1 gene. Int J Biochem Cell Biol 33:1172-1182 [DOI] [PubMed] [Google Scholar]