Abstract
Sudden cardiac death from ventricular fibrillation during acute myocardial infarction is a leading cause of total and cardiovascular mortality. To our knowledge, we here report the first genome-wide association study for this trait, conducted in a set of 972 individuals with a first acute myocardial infarction, 515 of whom had ventricular fibrillation and 457 of whom did not, from the Arrhythmia Genetics in The Netherlands (AGNES) study. The most significant association to ventricular fibrillation was found at 21q21 (rs2824292, odds ratio = 1.78, 95% CI 1.47–2.13, P = 3.3 × 10−10). The association of rs2824292 with ventricular fibrillation was replicated in an independent case-control set consisting of 146 out-of-hospital cardiac arrest individuals with myocardial infarction complicated by ventricular fibrillation and 391 individuals who survived a myocardial infarction (controls) (odds ratio = 1.49, 95% CI 1.14–1.95, P = 0.004). The closest gene to this SNP is CXADR, which encodes a viral receptor previously implicated in myocarditis and dilated cardiomyopathy and which has recently been identified as a modulator of cardiac conduction. This locus has not previously been implicated in arrhythmia susceptibility.
Sudden cardiac death (SCD) accounts for 15–20% of all natural deaths in adults in the United States and Western Europe and also for up to 50% of all cardiovascular deaths1. Ventricular fibrillation (uncoordinated contraction of the ventricles) is the most common underlying cardiac arrhythmia2. It arises through multiple mechanisms, depending on the affected individual’s underlying cardiac pathology3. In the last decade, considerable progress has been made in understanding the genetic, molecular and electrophysiological basis of SCD in the uncommon (monogenic) familial arrhythmia syndromes affecting structurally normal hearts and the rare inherited structural disorders associated with increased SCD risk4,5. However, the overwhelming majority (~80%) of SCDs in adults are caused by the sequelae of coronary artery disease, namely myocardial ischemia or acute myocardial infarction6,7, and SCD is the first clinically identified expression of heart disease in up to one-half of the instances of SCD8. Progress in understanding the genetic and molecular mechanisms that determine susceptibility to these common arrhythmias, affecting a much greater proportion of the population, has been limited3,9,10. The identification of genetic variants associated with these phenotypes is challenging due to difficulties in ascertaining individuals with these diseases.
Epidemiological studies have previously indicated that sudden death of a family member is a risk factor for SCD, suggesting a genetic component in susceptibility11,12. Recently, we also identified family history of sudden death as a strong risk factor for cardiac arrest during myocardial infarction in the AGNES study, in which we compared individuals with and without ventricular fibrillation during the early phase of a first acute myocardial infarction13.
Accordingly, in the present study, we sought to identify common genetic factors underlying susceptibility to ventricular fibrillation during myocardial infarction by conducting a genome-wide association study (GWAS) in the AGNES case-control set. The strategy of selecting this highly specific arrhythmia phenotype allowed us to avoid the problem of loss of statistical power inherent in analyzing cases or controls with multiple pathophysiologies. In particular, confining the case group to those with a first myocardial infarction minimizes the inclusion of individuals with extensive preexisting myocardial scarring from previous myocardial infarctions, in whom ventricular fibrillation may also arise from other mechanisms such as scar-related reentry.
A GWAS was performed in 515 AGNES cases (individuals having myocardial infarction with ventricular fibrillation) and 457 AGNES controls (individuals having myocardial infarction without ventricular fibrillation); all cases and controls were self-declared Dutch and of European ancestry. The clinical characteristics of AGNES cases and controls are presented in Table 1. Genotyping was carried out using the Illumina Human610-Quad v1 array. All but three DNA samples passed quality control criteria (Online Methods). Excluding the sex chromosome and mitochondrial DNA, this left 507,436 SNPs available for analysis. We next imputed nongenotyped SNPs from the HapMap European CEU reference panel (release 22). The association of each SNP with ventricular fibrillation was evaluated using a logistic regression model assuming an additive genetic model with adjustment for age and sex. The genomic control factor was small (λ = 1.026), indicating that overall inflation of genome-wide statistical results due to population stratification was minimal. There was an excess of SNPs associated with ventricular fibrillation compared to the number expected under the null hypothesis of no association (Supplementary Fig. 1).
Table 1.
Baseline characteristics of the AGNES case-control set
| Characteristic | N a | Totalb | Casesc | Controlsd | P e |
|---|---|---|---|---|---|
| Sex (male)f | 783 (80.6) | 412 (80.0) | 371 (81.2) | 0.6850 | |
| Mean age at myocardial infarctiong | 56.4 ± 11 | 55.9 ± 11.2 | 56.9 ± 10.8 | 0.1740 | |
| Median ST segment deviation-mm (IQR) |
735, 379, 356 | 17 (19) | 22 (22) | 14 (14) | <0.0001 |
| Myocardial infarction location f | 962, 505, 457 | ||||
| Inferior myocardial infarction | 408 (42.4) | 203 (40.2) | 205 (44.9) | 0.1442h | |
| Anterior myocardial infarction | 554 (57.6) | 302 (59.8) | 252 (55.1) | ||
| Median CK-MB-μg/l (IQR) | 786, 359, 427 | 215 (299.2) | 225.6 (365.2) | 211.4 (257.7) | 0.0210 |
| Family history of sudden deathf | 909, 459, 450 | 291 (32.0) | 174 (37.9) | 117 (26.0) | <0.0001 |
| Beta blocker usagef | 936, 482, 454 | 85 (9.1) | 50 (10.4) | 35 (7.7) | 0.1726 |
| Cardiovascular risk factors f | |||||
| Current smoking | 914, 473, 441 | 565 (61.8) | 303 (64.1) | 262 (59.4) | 0.1531 |
| Diabetes mellitus | 893, 462, 431 | 62 (6.94) | 21 (4.6) | 41 (9.5) | 0.0040 |
| Hypertension | 294 (30.2) | 149 (28.9) | 145 (31.7) | 0.3633 | |
| Hypercholesterolemia | 297 (30.6) | 127 (24.7) | 170 (37.2) | <0.0001 | |
| BMIi | 879, 439, 440 | 26.5 ± 3.9 | 26.1 ± 3.8 | 26.9 ± 4.0 | 0.0042 |
IQR, interquartile range; CK-MB, creatine kinase-MB.
In case of missing values, the sample sizes of the total, case and control sets (total, case, control) for whom information was available are given.
n = 972.
n = 515.
n = 457.
P value for comparison of cases and controls for each item.
Number (%).
Age (years) ± s.d.
P value for comparison of inferior and anterior myocardial infarction between cases and controls.
Kg/m2 ± s.d.
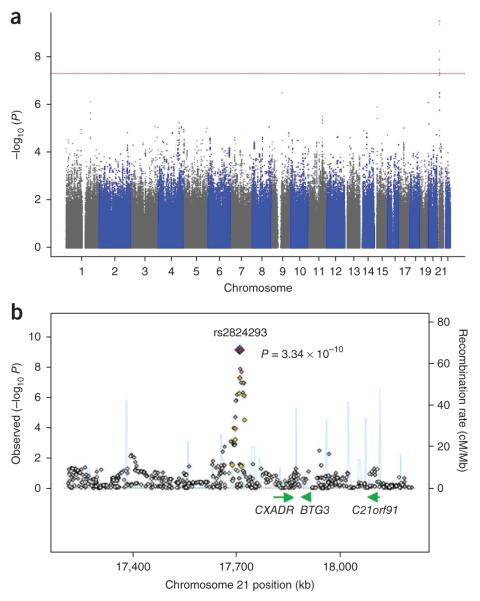
The distribution of P values for the associations of SNPs with ventricular fibrillation is shown in Figure 1, and SNPs displaying the strongest associations (chromosome (chr.) 21q21, chr. 9q21 and chr. 1q25) are presented in Table 2 and Supplementary Table 1. Eight SNPs on chr. 21q21, all in moderate to complete linkage disequilibrium with each other (r 2 = 0.4–1.0), surpassed our preset threshold for genome-wide significance of P < 5 × 10−8. Of these, the most significant associations were found for rs2824292 and rs2824293, which are 338 base pairs apart (r 2 = 1.0) and are situated 98 kb from CXADR (odds ratio = 1.78 per G allele, 95% CI, 1.47–2.13, P = 3.3 × 10−10). After adjustment for the main association signal (rs2824292), we saw a marked reduction in the association between ventricular fibrillation and the other seven SNPs on chromosome 21, indicating that these SNPs do not represent independent secondary association signals. The genotype frequencies for rs2824292 in AGNES cases and controls, as well as for rs1353342 (9q21) and rs12090554 (1q25), are presented in Supplementary Table 2, together with the genotype frequencies in a sample of the general Dutch population of European descent. As expected, the frequencies of the risk and protective alleles for each SNP in the general population were between those of AGNES cases and AGNES controls.
Figure 1.
Results of genome-wide association analysis in the AGNES case-control set. (a) Overview of the genome-wide association scan in the AGNES case-control population. P values corrected for age, sex and inflation factor are shown for each SNP tested. Within each chromosome, the results are plotted left to right from the p-terminal end. The horizontal red line indicates the preset threshold for genome-wide significance, P < 5 × 10−8. (b) Locus-specific association map, generated from genotyped and imputed SNPs, centered at rs2824293, located at 338 base pairs from rs2824292 and in complete linkage disequilibrium with it. Imputed SNPs are in gray; rs2824293 is depicted in blue; SNPs in red have r 2 ≥ 0.8 with rs2824293; SNPs in orange have r 2 = 0.5–0.8; SNPs in yellow have r 2 = 0.2–0.5; and SNPs in white have r 2 < 0.2 with the leading SNP. Superimposed on the plot are gene locations (green) and recombination rates (blue). Chromosome positions are based on HapMap release 22 build 36.2 and b was prepared using SNAP31.
Table 2.
Leading SNPs at the three loci most strongly associated with ventricular fibrillation in the GWAS in the AGNES case-control set
| Frequency of minor allele |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SNPa | Chromosome position | Positionb | Minor allele | Major allele | Total | Cases | Controls | Odds ratio (95% CI)c | P d | Closest genese |
| rs2824293 | 21q21 | 17,709,385 | G | A | 0.47 | 0.53 | 0.39 | 1.78 (1.47–2.13) | 3.34 × 10−10 | CXADR, BTG3 |
| rs2824292 | 21q21 | 17,709,047 | G | A | 0.47 | 0.53 | 0.39 | 1.78 (1.47–2.13) | 3.36 × 10−10 | CXADR, BTG3 |
| rs1353342 | 9q21 | 78,064,589 | A | C | 0.23 | 0.07 | 0.14 | 0.46 (0.34–0.63) | 3.34 × 10−7 | PCSK5, RFK, GCNT1 |
| rs12090554 | 1q25 | 183,818,971 | A | G | 0.23 | 0.19 | 0.28 | 0.58 (0.47–0.72) | 7.89 × 10−7 | HMCN1, IVNS1ABP |
Only rs2824293 was imputed; the rest of the SNPs were genotyped directly.
Based on Build 36.2.
Odds ratios are per copy of minor allele and are adjusted for age and sex.
The P values are corrected for genomic control factor.
Genes within an area of 1 Mb centered at the SNP are listed.
Because AGNES cases and controls differed in some characteristics, including risk factors for coronary artery disease and peak creatine kinase muscle-brain isoenzyme (CK-MB) levels, we carried out multifactor analysis to assess whether the rs2824292 was an independent predictor of ventricular fibrillation. Multifactor analysis for the association of rs2824292 with ventricular fibrillation adjusted for those baseline characteristics that were significantly (P < 0.05) different between AGNES cases and controls (Table 1) resulted in an odds ratio (OR) of 1.51 per G allele (95% CI 1.13–2.01, P = 0.005), indicating that this SNP is an independent risk factor for ventricular fibrillation. This was also the case for rs1353342 and rs12090554 (respectively, OR = 0.48, 95% CI 0.30–0.76, P = 0.002 and OR = 0.58, 95% CI 0.41–0.82, P = 0.002). Nevertheless, future research will be required in order to provide more evidence of the independent effects of these SNPs.
We next validated the association of the leading SNPs at the 21q21, 9q21 and 1q25 loci in an independent set of cases and controls (Online Methods and Table 3). Our P value threshold for this analysis was set to P < 0.017 (calculated as 0.05/3). Cases for the replication study (using the Amsterdam Resuscitation Study-Myocardial Infarction (ARREST-MI) cohort, n = 146, of which 80.1% were male with an average age ± standard deviation of 63.12 ± 13.10 years) were drawn from ARREST, a study aimed at establishing the genetic, clinical and environmental determinants of SCD in the general population. Dutch out-of-hospital cardiac arrest individuals of European ancestry in the ARREST dataset presenting with myocardial infarction at hospital admission and with electrocardiogram (ECG)-documented ventricular fibrillation were included as cases in the replication analysis. Controls for the replication analysis (from the Genetic Determinants of Restenosis-Myocardial Infarction study (GENDER-MI), n = 391, of which 79.5% were male with an average age of 61.7 ± 10.1 years) were myocardial infarction survivors drawn from the GENDER study14, which comprises individuals who underwent percutaneous transluminal coronary angioplasty (PTCA) at a time remote from a myocardial infarction. As observed in the AGNES case-control set, the risk G allele of SNP rs2824292 at chr. 21q21 was more abundant and the protective A allele was less abundant in the ARREST-MI cases compared to the GENDER-MI controls (Supplementary Table 2). The odds ratio per G allele in this case-control set was 1.49 (95% CI 1.14–1.95, P = 0.004), which is comparable to that found in the AGNES discovery set. rs12090554 at chr. 1q25 showed a trend for association with a direction of effect similar to that found in the AGNES discovery set (OR = 0.70 per copy of the A allele, 95% CI 0.49–1.01, P = 0.057). In contrast, rs1353342 on chr. 9q21 was not associated with ventricular fibrillation in the ARREST-MI compared to the GENDER-MI analysis (OR = 0.84, 95% CI 0.53–1.34, P = 0.46).
Table 3.
Replication analyses within the ARREST-MI and GENDER-MI case-control replication set
| SNP | Odds ratio (95% CI)a | P |
|---|---|---|
| rs2824292 | 1.49 (1.14–1.95) | 0.004 |
| rs1353342 | 0.84 (0.53–1.34) | 0.460 |
| rs12090554 | 0.70 (0.49–1.01) | 0.057 |
ARREST-MI cases, n = 146; GENDER-MI controls, n = 391.
Odds ratios are per copy of minor allele and are adjusted for age and sex.
The AGNES study did not include individuals who died before hospital arrival, and it is likely that some of these individuals died from ventricular fibrillation arising immediately or very soon after the initiation of chest pain and before the arrival of medical help. Thus, we cannot assert that the genetic association identified in this study also holds for the group of individuals who die immediately after the onset of symptoms. Nevertheless, an initial comparison of individuals presenting with ventricular fibrillation occurring within 5 min of onset of chest pain (n = 119) compared with those presenting with ventricular fibrillation occurring after more than 30 min of onset of chest pain (n = 204) showed no significant difference in the distribution of genotypes for rs2824292 (Supplementary Table 3).
The SNPs most strongly associated with ventricular fibrillation in this study appear to be located in an intergenic area that lacks linkage disequilibrium to SNPs within genes (Supplementary Fig. 2). These SNPs are not necessarily the functional variants for the observed effect, but they may serve as proxies for the actual functional variant(s). Thus, these SNPs, or functional SNPs in linkage disequilibrium with them, are likely to modulate ventricular fibrillation risk by exerting long-range effects on gene expression15. Inspection of a 1-Mb region spanning rs2824292 identified three genes all located within the area 0.5 Mb downstream of this SNP.
The gene closest to rs2824292 (located 98 kb away) is CXADR, encoding the coxsackievirus and adenovirus receptor (CAR) protein, a type I transmembrane protein of the tight junction16,17. CAR has a long-recognized role as viral receptor in the pathogenesis of viral myocarditis and its sequela of dilated cardiomyopathy18,19. Notably, active coxsackie B virus infection has been reported at a high frequency in a group of individuals with myocardial infarction who died suddenly20. More recently, two studies have reported a physiological role for CAR in localization of connexin 45 at the intercalated disks of the atrioventricular node cardiomyocytes as well as its role in conduction of the cardiac impulse within this cardiac compartment21,22. Thus, CXADR is a candidate gene for the association reported here.
Two other genes are located within 0.5 Mb of rs2824292. BTG3, located 179 kb from rs2824292, encodes B cell translocation gene 3, a member of the PC3, BTG and TOB family of growth inhibitory genes23. Its mRNA expression level is relatively high in the testis, lung and ovary but is low in heart23. C21orf91, located 374 kb from rs2824292, encodes the human ortholog of the chicken C21orf91 (also known as EURL, encoding the early undifferentiated retina and lens protein), which is known to be expressed in the developing chick retina and lens and has been suggested to play a role in the development of these structures24,25.
Further studies, such as exploration of expression of these genes in human heart as a function of genotype at rs2824292 (or other linked SNPs), and functional studies are next steps.
SNPs in the NOS1AP locus, associated with variability in the heart rate–corrected QT (QTc) duration in the general population26–28, have recently been implicated as predictors of SCD risk in candidate SNP analyses29,30. In our GWAS, however, there was no association between SCD risk and SNPs at this locus.
Predicting and preventing SCD remains a major challenge for which new strategies are needed, and understanding the underlying molecular mechanisms of SCD represents a crucial step in this process. Although much of the focus on genetic influences on SCD risk in common diseases has been on variants that directly or indirectly influence ion channel function, the results of the current GWAS suggest new and possibly complementary biological pathways that may be implicated in ventricular fibrillation.
ONLINE METHODS
Study samples
All individuals studied were Dutch and of self-declared European descent. The medical ethics committees of the hospitals participating in the study approved the study protocols, and all participants gave written informed consent.
The AGNES sample
The AGNES case-control set consisted of individuals with a first acute ST-elevation myocardial infarction13. AGNES cases had ECG-registered ventricular fibrillation occurring before reperfusion therapy for an acute and first ST-elevation myocardial infarction. AGNES controls were individuals with a first acute ST-elevation myocardial infarction but without ventricular fibrillation. All cases and controls were recruited at seven heart centers in The Netherlands from 2001–2008. We excluded individuals with an actual non–ST-elevation myocardial infarction, prior myocardial infarction, congenital heart defects, known structural heart disease, severe comorbidity, electrolyte disturbances, trauma at presentation, recent surgery, previous coronary artery bypass graft or use of class I and III antiarrhythmic drugs. Individuals who developed ventricular fibrillation during or after percutaneous coronary intervention were not eligible. Furthermore, because early reperfusion limits the opportunity of developing ventricular fibrillation, potential control subjects undergoing percutaneous coronary intervention within 2 h after onset of myocardial ischemia symptoms were not included. This time interval was based on the observation that >90% of cases developed ventricular fibrillation within 2 h after onset of the complaint of symptoms.
The ARREST-MI sample
The ARREST-MI sample was drawn from the ARREST study. ARREST is an ongoing prospective population-based study aimed at establishing the genetic, clinical and environmental determinants of SCD in the general population. Dutch out-of-hospital cardiac arrest patients of European ancestry in the ARREST dataset presenting with myocardial infarction at hospital admission and with ECG-documented ventricular fibrillation were included in the replication analysis. The diagnosis of myocardial infarction was established at hospital admission by a cardiologist based on ECG abnormalities and cardiac enzymes and these data were retrieved retrospectively from hospital records. At the time of this study (August 2009), 146 such individuals were retrieved and all were included in the replication study.
The GENDER-MI sample
The GENDER-MI sample included individuals with a history of myocardial infarction drawn from the GENDER study14, a study aimed at investigating the association between genetic polymorphisms and clinical restenosis. The GENDER study comprises consecutive individuals who underwent successful PTCA for treatment of stable angina, non–ST-elevation acute coronary syndromes or silent ischemia at four referral centers for interventional cardiology in The Netherlands. The inclusion period lasted from March 1999 until June 2001 and in total, 3,104 consecutive individuals were included in this prospective, multicenter, follow-up study. Approximately 40% of subjects included in the GENDER study had a history of myocardial infarction.
A sample of 941 GENDER subjects was genotyped on the Illumina Human 610-Quad Beadchips32. The GENDER-MI sample used in the current study consisted of 391 of these genotyped cases, and all of these 391 individuals had a history of myocardial infarction.
Random sample of the Dutch general population
The random sample of the Dutch general population constituted the European descent component of the Surinamese in The Netherlands: Study on Ethnicity and Health study, a cross-sectional study that aimed to assess the cardiovascular risk profile of three ethnic groups in The Netherlands: Creole, Hindustani and individuals of European ancestry33. It was based on a sample of 35–60-year-old, noninstitutionalized people from southeast Amsterdam. A random sample of 2,000 Surinamese individuals and approximately 1,000 Dutch individuals of European ancestry were drawn from the Amsterdam population register between 2001 and 2003. People were classified as Dutch if they and both of their parents were born in The Netherlands. The overall response rate was 61% among these Dutch individuals. Those who responded to the oral interview were invited for a medical examination, at which time blood for DNA extraction was drawn. The response rate at this stage among the Dutch individuals was 90%, resulting in a total of 508 subjects with DNA samples available. DNA samples from these 508 subjects were used for genotyping in the current study.
Genotyping
Genome-wide genotyping of SNPs on the 515 AGNES cases and 457 AGNES controls was done using the Illumina Human610-Quad v1 array. The analysis was performed with the BeadStudio software and the Genotyping module version 3.2.33 using the cluster file provided by Illumina (Human610-Quadv1_B.egt). The GenCall score cutoff was 0.15 and the average call rate was 99.45%.
Multiple quality control measures were implemented. The estimated sex for each individual determined by genotyping was compared with their phenotypic sex. Exclusion criteria included deviation from Hardy-Weinberg equilibrium at P < 10−4 (in controls), sample call rate <0.95 and SNP call rate <0.98.
For GENDER-MI, genotypes at rs2824292, rs1353342 and rs12090554 were extracted from previously obtained genotypes using the Illumina Human 610-Quad Beadchip32. Quality control criteria for the genotypic data for the GENDER study were the same as those applied in the AGNES study.
For ARREST-MI and for the sample of the Dutch European descent population, genotypes at rs2824292, rs1353342 and rs12090554 were determined by means of a Taqman assay (Applied Biosystems).
Statistical analysis and imputation
Differences in continuous phenotypic variables between cases and controls were tested using an independent t test when data were normally distributed or a Mann-Whitney U test otherwise. Values are presented as means ± s.d. or median and interquartile range, respectively. Differences in categorical variables were compared using a Fisher exact test, and values are presented as number and percentages.
Genotype imputation was done using the Markov-chain Monte Carlo method implemented in MACH1.034. An r 2 threshold of 0.3 was implemented in the program to identify and discard low-quality imputations.
Each SNP was analyzed for association with ventricular fibrillation using logistic regression assuming an additive genetic model with adjustment for age and sex, using the ProbABEL analysis package35. SNPs with a minor allele frequency >0.05 were analyzed. The P value for genome-wide significance was set at P < 5 × 10−8, corresponding to a target α (or P value) of 0.05 with a Bonferroni correction for 1 million independent tests36. The P values were corrected for the genomic control factor. A two-sided P value of P < 0.017 (Bonferroni-corrected P value for the three independent SNPs) was considered as significant in the replication study.
In the multifactor analysis, we adjusted for age, sex, hypercholesterolemia, diabetes, ST segment deviation, maximal CK-MB, body mass index and family history of sudden cardiac death.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by research grants from The Netherlands Heart Foundation (grants 2001D019, 2003T302 and 2007B202), the Leducq Foundation (grant 05-CVD), the Center for Translational Molecular Medicine (CTMM COHFAR) and the Interuniversity Cardiology Institute of The Netherlands (project 27). C.R.B. is an Established Investigator of The Netherlands Heart Foundation (grant 2005T024). H.L.T. is supported by The Netherlands Organization for Scientific Research (NWO, grant ZonMW Vici 918.86.616) and the Medicines Evaluation Board Netherlands (CBG). A.B. was supported by The Netherlands Organization for Scientific Research (NWO, grant Mozaiek 017.003.084). A.H. Zwinderman and E.P.A. van Iperen provided the genotype data from the GENDER study. We thank L. Beekman for technical support and N. Bruinsma for assistance in collection of subject data.
Footnotes
Note: Supplementary information is available on the Nature Genetics website.
AUTHOR CONTRIBUTIONS C.R.B., A.A.M.W. and L.R.C.D. conceived the study. C.R.B. and A.A.M.W. supervised the study. L.R.C.D., J.S.S.G.d.J. and R.F.M. collected clinical data and DNA from the AGNES samples. A.B., M.T.B. and H.L.T. collected clinical data and DNA from the ARREST-MI samples. J.W.J. collected clinical data and DNA from the GENDER-MI samples. N.R.B. collected DNA from the Dutch European descent population sample. B.P.S., A.P., P.L. and T.M. performed the genome-wide genotyping. R.F.M. performed the genotyping in the replication case-control set and in the sample of the Dutch European descent population. R.P., A.P. and M.W.T.T. performed statistical analysis of the data. R.P. and M.W.T.T. carried out SNP imputation. C.R.B., R.P., S.S.C., S.K., X.J., R.J.M., D.M.R. and A.A.M.W. wrote the manuscript. C.R.B., A.A.M.W., D.M.R., N.H.B. and R.J.M. obtained funding for the study.
COMPETING FINANCIAL INTERESTS The authors declare competing financial interests: details accompany the full-text HTML version of the paper at http://www.nature.com/naturegenetics/.
References
- 1.Myerburg RJ, Castellanos A. Cardiac arrest and sudden cardiac death. In: Libby P, Bonow RO, Mann DL, Zipes DP, editors. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. Elsevier; Oxford, UK: 2007. pp. 933–974. [Google Scholar]
- 2.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N. Engl. J. Med. 2001;345:1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 3.Arking DE, Chugh SS, Chakravarti A, Spooner PM. Genomics in sudden cardiac death. Circ. Res. 2004;94:712–723. doi: 10.1161/01.RES.0000123861.16082.95. [DOI] [PubMed] [Google Scholar]
- 4.Wilde AA, Bezzina CR. Genetics of cardiac arrhythmias. Heart. 2005;91:1352–1358. doi: 10.1136/hrt.2004.046334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watkins H, Ashrafian H, McKenna WJ. The genetics of hypertrophic cardiomyopathy: teare redux. Heart. 2008;94:1264–1268. doi: 10.1136/hrt.2008.154104. [DOI] [PubMed] [Google Scholar]
- 6.Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334–2351. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 7.Zipes DP, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e385–e484. doi: 10.1161/CIRCULATIONAHA.106.178233. [DOI] [PubMed] [Google Scholar]
- 8.Myerburg RJ. Sudden cardiac death: exploring the limits of our knowledge. J. Cardiovasc. Electrophysiol. 2001;12:369–381. doi: 10.1046/j.1540-8167.2001.00369.x. [DOI] [PubMed] [Google Scholar]
- 9.Noseworthy PA, Newton-Cheh C. Genetic determinants of sudden cardiac death. Circulation. 2008;118:1854–1863. doi: 10.1161/CIRCULATIONAHA.108.783654. [DOI] [PubMed] [Google Scholar]
- 10.Myerburg RJ, Castellanos A. Emerging paradigms of the epidemiology and demographics of sudden cardiac arrest. Heart Rhythm. 2006;3:235–239. doi: 10.1016/j.hrthm.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 11.Friedlander Y, et al. Family history as a risk factor for primary cardiac arrest. Circulation. 1998;97:155–160. doi: 10.1161/01.cir.97.2.155. [DOI] [PubMed] [Google Scholar]
- 12.Jouven X, Desnos M, Guerot C, Ducimetiere P. Predicting sudden death in the population: the Paris Prospective Study I. Circulation. 1999;99:1978–1983. doi: 10.1161/01.cir.99.15.1978. [DOI] [PubMed] [Google Scholar]
- 13.Dekker LR, et al. Familial sudden death is an important risk factor for primary ventricular fibrillation: a case-control study in acute myocardial infarction patients. Circulation. 2006;114:1140–1145. doi: 10.1161/CIRCULATIONAHA.105.606145. [DOI] [PubMed] [Google Scholar]
- 14.Agema WR, et al. Current PTCA practice and clinical outcomes in The Netherlands: the real world in the pre-drug-eluting stent era. Eur. Heart J. 2004;25:1163–1170. doi: 10.1016/j.ehj.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Kleinjan DA, van Heyningen V. Long-range control of gene expression: emerging mechanisms and disruption in disease. Am. J. Hum. Genet. 2005;76:8–32. doi: 10.1086/426833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergelson JM, et al. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 17.Tomko RP, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowles NE, Richardson PJ, Olsen EG, Archard LC. Detection of coxsackie-B-virus-specific RNA sequences in myocardial biopsy samples from patients with myocarditis and dilated cardiomyopathy. Lancet. 1986;1:1120–1123. doi: 10.1016/s0140-6736(86)91837-4. [DOI] [PubMed] [Google Scholar]
- 19.Pauschinger M, et al. Detection of adenoviral genome in the myocardium of adult patients with idiopathic left ventricular dysfunction. Circulation. 1999;99:1348–1354. doi: 10.1161/01.cir.99.10.1348. [DOI] [PubMed] [Google Scholar]
- 20.Andréoletti L, et al. Active coxsackieviral B infection is associated with disruption of dystrophin in endomyocardial tissue of patients who died suddenly of acute myocardial infarction. J. Am. Coll. Cardiol. 2007;50:2207–2214. doi: 10.1016/j.jacc.2007.07.080. [DOI] [PubMed] [Google Scholar]
- 21.Lisewski U, et al. The tight junction protein CAR regulates cardiac conduction and cell-cell communication. J. Exp. Med. 2008;205:2369–2379. doi: 10.1084/jem.20080897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim BK, et al. Coxsackievirus and adenovirus receptor (CAR) mediates atrioventricular-node function and connexin 45 localization in the murine heart. J. Clin. Invest. 2008;118:2758–2770. doi: 10.1172/JCI34777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshida Y, et al. ANA, a novel member of Tob/BTG1 family, is expressed in the ventricular zone of the developing central nervous system. Oncogene. 1998;16:2687–2693. doi: 10.1038/sj.onc.1201805. [DOI] [PubMed] [Google Scholar]
- 24.Strausberg RL, et al. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc. Natl. Acad. Sci. USA. 2002;99:16899–16903. doi: 10.1073/pnas.242603899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Godbout R, Andison R, Katyal S, Bisgrove DA. Isolation of a novel cDNA enriched in the undifferentiated chick retina and lens. Dev. Dyn. 2003;227:409–415. doi: 10.1002/dvdy.10310. [DOI] [PubMed] [Google Scholar]
- 26.Arking DE, et al. A common genetic variant in the NOS1 regulator NOS1AP modulates cardiac repolarization. Nat. Genet. 2006;38:644–651. doi: 10.1038/ng1790. [DOI] [PubMed] [Google Scholar]
- 27.Newton-Cheh C, et al. Common variants at ten loci influence QT interval duration in the QTGEN Study. Nat. Genet. 2009;41:399–406. doi: 10.1038/ng.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfeufer A, et al. Common variants at ten loci modulate the QT interval duration in the QTSCD Study. Nat. Genet. 2009;41:407–414. doi: 10.1038/ng.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kao WH, et al. Genetic variations in nitric oxide synthase 1 adaptor protein are associated with sudden cardiac death in US white community-based populations. Circulation. 2009;119:940–951. doi: 10.1161/CIRCULATIONAHA.108.791723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eijgelsheim M, et al. Genetic variation in NOS1AP is associated with sudden cardiac death: evidence from the Rotterdam Study. Hum. Mol. Genet. 2009;18:4213–4218. doi: 10.1093/hmg/ddp356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson AD, et al. SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics. 2008;24:2938–2939. doi: 10.1093/bioinformatics/btn564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sampietro ML, et al. A genome wide association analysis in the GENDER study. Neth. Heart J. 2009;17:262–264. doi: 10.1007/BF03086261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agyemang C, et al. Prevalence, awareness, treatment, and control of hypertension among Black Surinamese, South Asian Surinamese and White Dutch in Amsterdam, The Netherlands: the SUNSET study. J. Hypertens. 2005;23:1971–1977. doi: 10.1097/01.hjh.0000186835.63996.d4. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Mach Abecasis GR. 1.0: Rapid haplotype reconstruction and missing genotype inference. Am. J. Hum. Genet. 2006;s79:2290. [Google Scholar]
- 35.Aulchenko YS, Ripke S, Isaacs A, van Duijn CM. GenABEL: an R library for genome-wide association analysis. Bioinformatics. 2007;23:1294–1296. doi: 10.1093/bioinformatics/btm108. [DOI] [PubMed] [Google Scholar]
- 36.Hoggart CJ, Clark TG, De IM, Whittaker JC, Balding DJ. Genome-wide significance for dense SNP and resequencing data. Genet. Epidemiol. 2008;32:179–185. doi: 10.1002/gepi.20292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.