Abstract
Background
Whether timeliness of follow-up after abnormal mammography differs at facilities serving vulnerable populations such as women with limited education or income, in rural areas, and racial/ethnic minorities is unknown.
Methods
We examined receipt of diagnostic evaluation following abnormal mammography using 1998-2006 Breast Cancer Surveillance Consortium-linked Medicare claims. We compared whether time to recommended breast imaging or biopsy depended on whether women attended facilities serving vulnerable populations. We characterized a facility by the proportion of mammograms performed on women with limited education or income, in rural areas, or racial/ethnic minorities.
Results
We analyzed 30,874 abnormal screening examinations recommended for follow-up imaging across 142 facilities and 10,049 abnormal diagnostic examinations recommended for biopsy across 114 facilities. Women at facilities serving populations with less education or more racial/ethnic minorities had lower rates of follow-up imaging (4-5% difference, p<0.05), and women at facilities serving more rural and low income populations had lower rates of biopsy (4-5% difference, p<0.05). Women undergoing biopsy at facilities serving vulnerable populations had longer times until biopsy than those at facilities serving non-vulnerable populations (21.6 days vs. 15.6 days; 95% CI for mean difference 4.1-7.7). The proportion of women receiving recommended imaging within 11 months and biopsy within 3 months varied across facilities (interquartile range 85.5%-96.5% for imaging and 79.4%-87.3% for biopsy).
Conclusions
Among Medicare recipients, follow-up rates were slightly lower at facilities serving vulnerable populations, and among those women who returned for diagnostic evaluation, time to follow-up was slightly longer at facilities that served vulnerable population. Interventions should target variability in follow-up rates across facilities, and evaluate effectiveness particularly at facilities serving vulnerable populations.
Keywords: mammography, timeliness, disparities, facility
Background
In breast cancer screening, up to 10% of screening mammography is abnormal, leading to women being recalled for diagnostic mammography or breast biopsy (1). Prompt diagnostic evaluation minimizes a woman's anxiety generated from having abnormal mammography (2). Furthermore, delays among symptomatic breast cancer patients of 12 or more weeks prior to presentation have been associated with higher breast cancer mortality (3), and the time to breast surgery among women with invasive early stage breast cancer impacts its treatability (4). Vulnerable populations such as women with low income, limited education, racial or ethnic minorities, and those who live in rural areas are more likely to face delays in breast cancer diagnosis and have worse breast cancer outcomes (5-12). It is important to understand the extent to which timeliness of mammography evaluation and loss to follow-up at facilities serving vulnerable populations differs from other facilities.
While there is no consensus as to the appropriate timeline for follow-up after abnormal mammography, from the woman's perspective, the sooner the better (2). The Mammography Quality Assurance Act requires that mammography facilities send or give directly to all women a written summary of the results of the mammography in lay terms no later than 30 days from the date of the examination. The American College of Radiology recommends contacting women regarding abnormal or concerning mammography within 5 days (13), while stricter European guidelines recommend that 95% of women receive examination results within 15 working days and 90% are offered follow-up within 5 working days (14). In the United States, most abnormal mammography are fully evaluated within 3 weeks, though there is significant variability by facility (15, 16). Neither the annual volume of mammography interpreted, the facility's recall rate, whether the facility is a specialty center, or the percentage of women who live in rural areas has been associated with the timeliness of evaluation (15). Loss to follow-up may differ for certain at-risk populations such as women with limited English proficiency (17). Limited workforce and capacity at non-profit facilities and in some rural areas (18, 19) may contribute to delays in follow-up care. It is unknown whether there are any differences in the timeliness of abnormal mammography evaluation at facilities on the basis of the proportion of women served who are racial or ethnic minorities, or women with limited income or education (20, 21). Prior studies evaluating the timeliness of follow-up care have been limited by the ability to ascertain imaging and biopsy procedures, and therefore have not evaluated differences in follow-up rates across facilities (15, 16).
Using a sample of Medicare recipients, this study estimates differences in receipt and timeliness of follow-up care after abnormal mammography at facilities based on the proportion of vulnerable women served. This study overcomes limitations of prior studies by using data from the Breast Cancer Surveillance Consortium (BCSC) – a collaborative network of mammography registries that includes clinical risk information, radiology interpretations, and cancer and pathology registry data – linked to Medicare claims to facilitate ascertainment of follow-up care. Prior work focused only on women observed to receive imaging and biopsy during follow-up due to the incomplete capture of breast ultrasound and biopsies (15). We hypothesized that facilities serving vulnerable women would have lower rates of women completing recommended follow-up, longer average times to follow-up among those who do, and more variable follow-up than facilities serving non-vulnerable women.
Methods
Data sources
The BCSC is a National Cancer Institute-funded consortium of pooled mammography registry data shown to be representative of US women undergoing mammography (1, 22). The registries prospectively collect women's self-reported demographic and clinical data at each mammography examination, together with radiologists’ mammography interpretations. Registries ascertain cancer outcomes through linkage with state tumor registries, regional Surveillance, Epidemiology, and End Results programs, and pathology databases, and pool data using a central Statistical Coordinating Center (SCC). Each registry and the SCC receive institutional review board approval for either active or passive consenting processes or a waiver of consent to enroll participants, link data, and perform analytic studies. All procedures are Health Insurance Portability and Accountability Act (HIPAA) compliant. All registries and the SCC have received a Federal Certificate of Confidentiality and protection for the identities of women, physicians, and facilities who are subjects of this research.
Four BCSC registries (North Carolina, Vermont, San Francisco, and New Hampshire) participated in a Medicare linkage. Women aged 65 and older who were enrolled in Medicare and had received mammography at one of these sites prior to 2006 were linked to the Center for Medicare and Medicaid Services’ (CMS) Medicare Program Master Enrollment file. Using this linked file, we identified Medicare enrollment information and Medicare claims data indicating when imaging, biopsies, and pathologic examinations occurred.
Study participants
Using BCSC mammograms from 1998-2006, we identified two samples of women: those with abnormal screening mammography requiring diagnostic imaging follow-up and those with abnormal diagnostic mammography requiring biopsy follow-up. We included women who had a corresponding mammography Medicare claim with the same date of service as the abnormal BCSC exam, and who had the potential to be linked to Medicare usage over the entire designated analytic follow-up periods (11 months for the screening analysis and 3 months for the diagnostic analysis, described in Outcomes below). We required enrollment in both Medicare Parts A and B and no enrollment in a Medicare HMO in the month of the abnormal mammogram and the months following. Women who died were excluded from the analysis as they did not have complete Medicare enrollment through the follow-up period.
An abnormal screening mammogram was defined according to the standard BCSC definition for screening mammography (bilateral views, indicated for routine screening, with no prior imaging within 9 months, and no prior breast cancer) and classified as abnormal if the Breast Imaging-Reporting and Data System (BI-RADS®) assessment was 0 (needs additional evaluation), 4 (suspicious abnormality), 5 (highly suggestive of malignancy), or 3 with a recommendation for immediate follow-up (probably benign) (17-19). An abnormal diagnostic mammogram was defined as a mammogram indicated by the radiologist as being recalled for additional work-up of an abnormal screening mammogram, short interval follow-up, or evaluation of a breast concern and interpreted by the radiologist as abnormal, having a BI-RADS® assessment of 4, 5, or 0 or 3 with a recommendation for biopsy, fine-needle aspiration, or surgical consult. Women with abnormal diagnostic mammography could have had prior breast cancer.
Outcomes
Claims for follow-up breast imaging and breast biopsies were identified in Medicare files (Carrier Claims, Inpatient, and Outpatient files) using International Classification of Disease (ICD)-9 procedure codes, Health Care Common Procedure Coding System (HCPCS) codes, and diagnosis-related groups (DRG) (Appendix A). We performed separate analyses for imaging follow-up among women with abnormal screening mammography, and for biopsy follow-up among women with abnormal diagnostic mammography. For the former, we determined the time to follow-up breast imaging within an 11 month period (e.g. diagnostic mammography, breast ultrasound, or breast magnetic resonance imaging). For the latter, we determined time to follow-up breast biopsy within a 3 month period. As there is no uniform definition of timeliness of follow-up for abnormal mammography, we selected these time periods as they encompass most abnormal mammography evaluations (15) and reflect a time frame by which all screening and diagnostic mammography should be resolved.
Facilities serving vulnerable women
Facilities were characterized as serving a vulnerable population based on the population of women served by the facility according to four socio-demographic characteristics: educational attainment, race/ethnicity, living in rural/urban areas, and household income (20, 21). A woman's educational attainment and race/ethnicity were self-reported in a survey at the time of mammography. Linkages between 2000 Census data and women's self-reported residential zip code were used to assign each woman an income measure corresponding to the median household income in the zip code and a rural/urban score corresponding to the percentage of rural residences in the zip code.
To describe the vulnerability of the population served by each mammography facility, we calculated a continuous facility-level vulnerability index using the entire population of women who receive mammography at the facility (regardless of insurance). First, we aggregated individual woman-level characteristics for the four vulnerability measures across all mammography examinations (both screening and diagnostic) conducted by a given facility during the 1998 to 2006 study period, not only those mammograms included in the analysis of time to follow-up. The continuous index measures were: (1) the percentage of the population with a high school education or less, (2) the percentage of the population composed of minorities (African-American race, or Hispanic/ Pacific-Islander/Hawaiian/Native American ethnicity), (3) the average median household income, and (4) the average percentage of rural residents. These measures were dichotomized providing a binary facility-level vulnerable/non-vulnerable classification for the population served by each facility: we classified facilities as serving a vulnerable population if: < 83% of women undergoing mammography at the facility had completed high school, > 30% minorities, average median household income < $45,000, percentage of rural residence > 52% (20). These thresholds identified facilities serving a higher proportion of women (1 standard deviation from the mean proportion) from a given vulnerability category based on data from all 7 BCSC sites (20), a population reflective of women receiving mammography in the U.S. (1).
We then developed a composite score adding one for each of the four vulnerability criteria met; each component was given equal weighting, resulting in a score ranging from 0 to 4. The vulnerability score is a characteristic of the mammography facility where the woman sought care, rather than a characteristic of the woman herself.
Analysis
We described the characteristics of the study samples overall, and by whether the facility performing the initial abnormal mammography met any of the vulnerability criteria. Characteristics included age, personal and family history of breast cancer, prior breast procedures, prior BCSC screening mammography, and BI-RADS® breast density.
We analyzed 1) the probability of receiving recommended follow-up within the designated follow-up period, and 2) among those receiving recommended follow-up procedures, the timeliness of that care. For analysis (1), we used a modified Poisson regression model estimated via generalized estimating equations (GEE) with clustering at the facility level to estimate adjusted relative risks (RR) for undergoing recommended follow-up procedures (imaging within 11 months for the screening population and biopsy within 3 months for the diagnostic population) on the basis of facility vulnerability status (23). We chose this model because it directly estimates relative risks, rather than odds ratios, which allow for ease of interpretation. We examined each of the four vulnerability indices and the composite score. For the screening population, the model was adjusted for mammography registry, age, and history of prior screening mammography. For the diagnostic population, the model was additionally adjusted for mammography indication and personal history of breast cancer. To provide estimates of proportions of women receiving recommended care, we used marginal standardization to standardize predicted probabilities based on our modified Poisson regression models to a common distribution of woman- and mammogram-characteristics (24).
For analysis (2), we estimated differences in average timeliness of follow-up among those receiving recommended follow-up procedures on the basis of facility population vulnerability using multiple linear regression models estimated via GEE with clustering at the facility level. These models were adjusted for the same covariates as included in the models for analysis (1), and adjusted estimates of the average days until follow-up were generated from the linear regression models using marginal standardization.
We described variability in follow-up rates and timeliness of follow-up across facilities by computing estimates for individual facilities and summarizing this information using boxplots. To ensure robust estimates at the facility level, we limited this analysis to facilities which contributed at least 50 mammograms. Tests of linear trend for the composite vulnerability scores in analyses (1) and (2) were based on generalized score statistics. All analyses were conducted using SAS software, version 9.2 (SAS Institute Inc., Cary, NC) and R, version 2.15.1 (R Foundation for Statistical Computing, Vienna, Austria).
We conducted several sensitivity analyses. We extended the time period for the biopsy analysis from 3 to 11 months. We refit models for the biopsy analysis after excluding women with a history of breast cancer. To our knowledge, there has not been a large scale validation of Medicare claims data for breast biopsies compared to chart review. To address the limitation that the Medicare claims data may have undercounted biopsies, we refit models assuming that women without a biopsy detected in the Medicare claims data but who had a new cancer diagnosis within 90 days detected in the Surveillance Epidemiology and End Results (SEER) registry or other pathology registries were compliant with follow-up and tested to see if there were differences in reporting compared to the SEER registry by population served.
Results
After excluding exams not meeting the Medicare follow-up enrollment criteria (n=1,421 total), the screening mammography sample included 30,874 mammograms recalled for subsequent imaging among 27,423 women across 142 facilities (Table 1). The diagnostic mammography sample included 10,049 mammograms among 9,592 women at 114 facilities. Distributions of these demographic characteristics did not differ greatly among included mammograms from the facilities serving vulnerable vs. non-vulnerable populations.
Table 1.
Demographics of Women Included in the Screening and Diagnostic Study Samples.
| Positive screening mammograms (N = 30,874) | Positive diagnostic mammograms (N = 10,049) | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-vulnerable facilities (composite=0) | Vulnerable facilities (composite >0) | Non-vulnerable facilities (composite=0) | Vulnerable facilities (composite >0) | |||||
| N | %a | N | %a | N | %a | N | %a | |
| Total | 12,888 | 17,986 | 4,614 | 5,435 | ||||
| N women | 11,579 | 15,879 | 4,372 | 5,227 | ||||
| Age, years | ||||||||
| 65-69 | 4,385 | 34.0 | 6,364 | 35.4 | 1,351 | 29.3 | 1,686 | 31.0 |
| 70-74 | 3,712 | 28.8 | 5,265 | 29.3 | 1,212 | 26.3 | 1,548 | 28.5 |
| 75-79 | 2,793 | 21.7 | 3,741 | 20.8 | 1,056 | 22.9 | 1,149 | 21.1 |
| 80+ | 1,998 | 15.5 | 2,616 | 14.5 | 995 | 21.6 | 1,052 | 19.4 |
| Prior BCSC screening mammogram (>9 months prior) | ||||||||
| No | 2,773 | 21.5 | 3,710 | 20.6 | 1,608 | 34.9 | 1,793 | 33.0 |
| Yes | 10,115 | 78.5 | 14,276 | 79.4 | 3,006 | 65.1 | 3,642 | 67.0 |
| BI-RADS breast density | ||||||||
| 1: Almost entirely fat | 685 | 6.4 | 1,015 | 6.1 | 312 | 9.0 | 295 | 6.4 |
| 2: Scattered fibroglandular densities | 5,535 | 52.1 | 9,188 | 55.4 | 1,787 | 51.6 | 2,628 | 57.2 |
| 3: Heterogeneously dense | 4,148 | 39.0 | 5,760 | 34.7 | 1,299 | 37.5 | 1,527 | 33.2 |
| 4: Extremely dense | 266 | 2.5 | 634 | 3.8 | 63 | 1.8 | 146 | 3.2 |
| Missing | 2,254 | 17.5 | 1,389 | 7.7 | 1,153 | 25.0 | 839 | 15.4 |
percent is among non-missing
Of facilities in which we analyzed screening mammography, 38 (26.8%) were designated as serving women with limited education, 19 (13.4%) served a high proportion of racial/ethnic minorities, 59 (41.5%) served a high proportion of rural residents, and 47 (33.1%) served low-income women (Table 2). The percentages of facilities designated as serving vulnerable women in the diagnostic mammography analysis were similar.
Table 2.
Vulnerability Characteristics of Facilities in Sample
| Screening analysis | Diagnostic analysis | |||
|---|---|---|---|---|
| N | % | N | % | |
| Number of facilities | 142 | 114 | ||
| Education | ||||
| Non-vulnerable | 104 | 73.2 | 86 | 75.4 |
| Vulnerable | 38 | 26.8 | 28 | 24.6 |
| Race/ethnicity | ||||
| Non-vulnerable | 123 | 86.6 | 101 | 88.6 |
| Vulnerable | 19 | 13.4 | 13 | 11.4 |
| Rural/urban residence | ||||
| Non-vulnerable | 83 | 58.5 | 61 | 53.5 |
| Vulnerable | 59 | 41.5 | 53 | 46.5 |
| Income | ||||
| Non-vulnerable | 95 | 66.9 | 76 | 66.7 |
| Vulnerable | 47 | 33.1 | 38 | 33.3 |
| Composite vulnerability score | ||||
| 0 | 54 | 38.0 | 41 | 36.0 |
| 1 | 44 | 31.0 | 38 | 33.3 |
| 2 | 21 | 14.8 | 17 | 14.9 |
| 3 | 15 | 10.6 | 12 | 10.5 |
| 4 | 8 | 5.6 | 6 | 5.3 |
Completeness of follow-up after abnormal screening mammography
Overall, 90.3% (95% CI 87.4, 93.2) of women returned within 11 months for imaging after being recalled. The supplemental digital content demonstrates cumulative incidence curves of follow-up for abnormal screening mammography by vulnerability of the population served (Figure, Supplemental Digital Content 1). After adjustment, we estimated significant differences for the race/ethnicity vulnerability index and limited education, with women at facilities serving vulnerable populations having lower rates of return (race/ethnicity-- 85.6% vs. 90.7%; RR: 0.94, 95% CI: 0.90 – 0.99; education- 87.1% vs. 90.8%; RR: 0.96, 95%: 0.92 - 1.00 (Table 3).
Table 3.
Percent of Women Receiving Follow-up Imaging by 11 Months and Time to Follow-up Imaging, Adjusted Analysis
| ADJUSTED ESTIMATES§ | % Receiving Follow-up Imaging | Relative Risk (RR) for Follow-up Imaging | Mean Days to Imaging, among Those Who Return | Difference in Mean Days to Imaging | ||
|---|---|---|---|---|---|---|
| Vulnerability Index * | Non-vulnerable | Vulnerable | RR (95% CI) | Non-vulnerable | Vulnerable | Mean diff |
| Education | 90.8 | 87.1 | 0.96 (0.92, 1.00) | 15.4 | 24.5 | 9.1 (3.7, 14.5) |
| Race/ethnicity | 90.7 | 85.6 | 0.94 (0.90, 0.99) | 16.1 | 23.0 | 6.9 (−1.1, 14.9) |
| Rural/urban residence | 91.5 | 88.6 | 0.97 (0.91, 1.03) | 15.4 | 18.2 | 2.8 (−0.3, 5.9) |
| Income | 89.8 | 91.3 | 1.02 (0.97, 1.06) | 16.2 | 17.7 | 1.5 (−2.2, 5.1) |
| Any (Composite>0) | 91.9 | 89.1 | 0.97 (0.92, 1.02) | 15.0 | 17.8 | 2.8 (−0.5, 6.1) |
| Composite vulnerability score | ||||||
| 0 | 91.9 | Reference | 15.0 | Reference | ||
| 1 | 88.4 | 0.96 (0.89, 1.04) | 15.8 | 0.7 (−2.7, 4.1) | ||
| 2 | 92.9 | 1.01 (0.97, 1.05) | 18.1 | 3.0 (−2.5, 8.5) | ||
| 3 | 87.8 | 0.95 (0.90, 1.02) | 24.8 | 9.7 (3.1, 16.3) | ||
| 4 | 83.6 | 0.91 (0.81, 1.01) | 21.5 | 6.5 (−0.1, 13.0) | ||
| p for trend = 0.10 | p for trend = 0.03 | |||||
Separate models were fit for each of the vulnerability indices (and the composite score). For reported RRs, non-vulnerable is the reference category for all binary vulnerability indices. Thus RR<1 suggests those at vulnerable facilities have lower rates of imaging follow-up than those at non-vulnerable facilities. For reported differences in mean days to imaging, we used vulnerable minus non-vulnerable.
Adjusted for mammography registry, age, and history of prior screening mammography.
Timeliness of follow-up after abnormal screening mammography among those completing follow-up imaging
Among women who underwent recommended follow-up imaging, the mean number of days to imaging was 16.6 (95% CI 15.1, 18.2). Comparing adjusted estimates of average days until imaging among these women on the basis of facility population vulnerability yielded statistically significant differences for the education index; it took longer for women at facilities serving women with less education to undergo follow-up imaging (24.5 days vs. 15.4 days; 95% CI for mean difference 3.7-14.5) than at facilities serving women with more education. There was also a statistically significant trend (p = 0.03) toward longer follow-up times with greater composite vulnerability scores.
Completeness of follow-up after abnormal diagnostic mammography
Overall, 83.2% (95% CI 81.5, 85.0) of women who were recommended for biopsy returned for biopsy within 3 months. The supplemental digital content provides the cumulative incidence curves for biopsy after abnormal diagnostic mammography by vulnerability of the population served (Figure, Supplemental Digital Content 2). After adjustment, we estimated significant differences for the rural/urban vulnerability index, the income vulnerability index, and the composite index. Women at the facilities serving more vulnerable populations tended to have lower rates of return for biopsy (residence 80.6% vs. 84.9; RR: 0.95, 95% CI: 0.92 – 0.98; income 80.0% vs. 84.6%; RR: 0.95, 95% CI 0.91 – 0.98; any vulnerability 80.8% vs. 86.2%; RR: 0.94, 95% CI: 0.91- 0.97 (Table 4).
Table 4.
Percent of Women Receiving Biopsy by 3 Months and Time to Follow-up Biopsy, Adjusted Analysis
| ADJUSTED ESTIMATES§ | % Receiving Follow-up Biopsy | Relative Risk (RR) for Follow-up Biopsy | Mean Days to Biopsy, among Those Who Return | Difference in Mean Days to Biopsy | ||
|---|---|---|---|---|---|---|
| Vulnerability Index * | Non-vulnerable | Vulnerable | RR (95% CI) | Non-vulnerable | Vulnerable | Mean diff |
| Education | 83.6 | 80.7 | 0.96 (0.91, 1.02) | 18.6 | 20.2 | 1.6 (−1.7, 5.0) |
| Race/ethnicity | 83.4 | 80.7 | 0.97 (0.90, 1.04) | 18.5 | 21.5 | 3.0 (−0.7, 6.6) |
| Rural/urban residence | 84.9 | 80.6 | 0.95 (0.92, 0.98) | 17.3 | 21.2 | 3.9 (1.4, 6.5) |
| Income | 84.6 | 80.0 | 0.95 (0.91, 0.98) | 17.2 | 22.6 | 5.4 (3.0, 7.8) |
| Any (Composite>0) | 86.2 | 80.8 | 0.94 (0.91, 0.97) | 15.6 | 21.6 | 5.9 (4.1, 7.7) |
| Composite vulnerability score | ||||||
| 0 | 86.1 | Reference | 15.7 | Reference | ||
| 1 | 81.9 | 0.95 (0.91, 0.99) | 21.7 | 6.0 (4.1, 7.9) | ||
| 2 | 77.0 | 0.89 (0.84, 0.95) | 23.4 | 7.7 (4.5, 10.9) | ||
| 3 | 81.8 | 0.95 (0.90, 1.00) | 18.6 | 3.0 (0.1, 5.8) | ||
| 4 | 82.7 | 0.96 (0.91, 1.01) | 22.9 | 7.2 (3.3, 11.2) | ||
| p for trend = 1.0 | p for trend = 0.03 | |||||
Separate models were fit for each of the vulnerability indices (and the composite score). For reported RRs, non-vulnerable is the reference category for all binary vulnerability indices. Thus RR<1 suggests those at vulnerable facilities have lower rates of biopsy follow-up than those at non-vulnerable facilities. For reported differences in mean days to biopsy, we used vulnerable minus non-vulnerable.
Adjusted for mammography registry, mammography indication, age, history of prior screening mammography, and personal history of breast cancer.
Timeliness of follow-up after abnormal diagnostic mammography among those completing follow-up tests
Among the women receiving biopsy, the average time to biopsy was 18.8 days (95% CI 17.5-20.0) and was longer for women at facilities serving vulnerable populations on the basis of residence, income, and the composite index. Adjusted estimates of the average difference in timeliness of biopsy were: residence (21.2 days vs. 17.3 days; 95% CI for mean difference 1.4 – 6.5); income (22.6 days vs. 17.2 days; 95% CI for mean difference 3.0-7.8); and any vulnerability (21.6 days vs. 15.6 days; 95% CI for mean difference 4.1-7.7).
Variation in follow-up across facilities
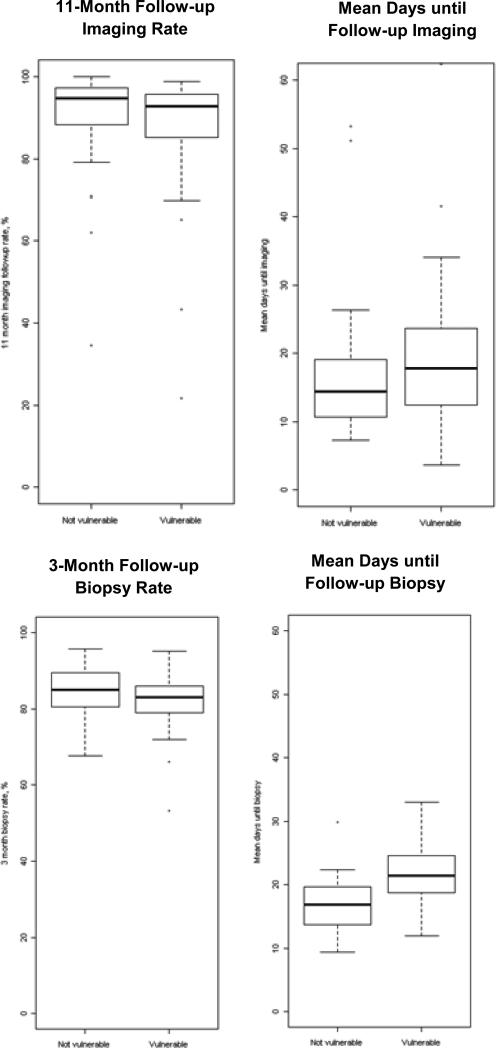
Limiting the imaging analysis to facilities with at least 50 mammograms there were a total of 93 facilities (of which 55 served vulnerable women, i.e. composite>0). Limiting the biopsy analysis to facilities with at least 50 mammograms there were a total of 51 facilities (of which 35 served vulnerable women, i.e. composite>0). Raw follow-up imaging and biopsy rates were highly variable across facilities, both for those that serve vulnerable vs. non-vulnerable populations (Figure 1). The interquartile ranges (IQRs, i.e. range of rates among middle 50% of facility rates) of follow-up imaging rates were: vulnerable 85.3%-95.7% and non-vulnerable 88.3%-97.3%; the IQRs of follow-up biopsy rates were: vulnerable 79.0%-86.0% and non-vulnerable 80.5%-89.5%. The within-facility average time to follow-up imaging among women receiving recommended follow-up (IQR: vulnerable 12.4-23.6 days; non-vulnerable 10.6-19.1 days) and biopsy (IQR: vulnerable 18.7-24.6 days; non-vulnerable 13.7-19.7 days) was also variable.
Figure 1.
Follow-up Imaging and Follow-up Biopsy Boxplots
Sensitivity analyses
The biopsy analysis did not substantially differ when we extended the follow-up period to 11 months, when we excluded women with prior history of breast cancer, or when we included new cancer diagnoses in the SEER registry as a surrogate for a biopsy not captured in the Medicare claims data (N=224) (results not shown).
Discussion
On average, women insured by Medicare fee-for-service who underwent recommended follow-up care for abnormal mammography did so within a few weeks. However, 17% of women did not return for biopsy within 3 months, and a statistically significantly higher percentage did not return at facilities serving low income women and those living in rural areas. For both completeness and timeliness of follow-up, facilities that serve vulnerable populations tended to have higher loss to follow-up and slightly (arguably clinically insignificant) longer time to follow-up for both imaging after abnormal screening and biopsies after abnormal diagnostic mammography. Importantly, there was variability in time to follow-up imaging and biopsy across both facilities serving vulnerable and non-vulnerable women, providing evidence of gaps in timeliness of care.
Our findings that up to 17% of women at facilities serving vulnerable and non-vulnerable women did not receive biopsy within three months of an abnormal mammography is concerning. Perhaps even more concerning is the evidence of the variability in follow-up completeness across facilities. Our study includes women insured by Medicare, which suggests that insurance alone does not ensure timely follow-up. Notably, in neither analysis can we determine whether women were offered biopsy, but declined. Our use of Medicare claims allowed for a more robust capture of follow-up imaging and biopsies than in prior work. How this degree of loss to follow-up contributes to cancer outcomes is unknown.
Our study suggests that populations in rural and low income settings may be at particular risk for loss to follow-up after abnormal diagnostic mammography. Prior research has identified social context and life factors such as competing health issues, economic hardship, distrust of health care providers, and inflexible work policies that contribute to loss to follow-up among vulnerable women (25). Understanding what interventions facilities could put in place to improve follow-up, particularly for high risk individuals, is critical to ensuring women are receiving the care they would like in a timely fashion.
Patient navigators have gained significant attention as a potential strategy to improve the timeliness of evaluation (26-30). Patient navigation is a barrier-focused intervention provided to women with abnormal mammography to help ensure access to recommended follow-up tests and cancer care in a timely manner (29). These programs may decrease wait times and improve follow-up rates particularly at facilities serving vulnerable women. The extent to which patient navigators or other types of patient or health system-level interventions account for the variability across mammography facilities is unknown.
Other strategies mammography facilities can use to help ensure timely follow-up for women with abnormal mammography such as electronic tracking systems or approaches to communicating abnormal mammography results to women are discussed in the literature (31, 32), but there are no clear recommendations yet as to best practices for facilities to adopt. Our study demonstrates the variability in timeliness that exists across facilities and some degree of disparities in follow-up highlighting that there is still important work to do to clarify what strategies are successful and in what settings.
The strengths of our study include the use of clinically rich data in a large cohort of women across four states, and the linkage to Medicare claims for capture of biopsy and follow-up imaging. However, our study has several limitations. Our study was limited to women with Medicare, and therefore, may not be generalizable to younger populations or women without Medicare. We evaluated several measures of whether facilities served vulnerable women. However, two of these metrics are based on zip code based averages, and our approach has not been externally validated. Our definition for facilities that serve vulnerable populations may not be generalizable to areas of the country with more extreme poverty or greater predominance of racial/ethnic minorities than those reflected in the BCSC. We chose to link to Medicare claims data to improve the identification of imaging and biopsy procedures. However, we found that some women were identified as having cancer without having a claim in the Medicare data, suggesting that some biopsies may still be missed. In addition, data were not available that may explain differences between facilities that serve vulnerable populations and those that do not including the effect of patient characteristics such as patient knowledge and attitudes towards mammography screening, the geographic accessibility of facilities to patients, or facility/physician characteristics such as facility capacity, radiologist communication with women, or reminder systems.
In conclusion, among women with fee-for-service Medicare who had an abnormal screening or diagnostic mammography, the time to follow-up imaging and biopsy among women who completed follow-up tests was not on average clinically meaningfully different between facilities that served vulnerable and non-vulnerable women. However, there was variability in loss to follow-up across facilities, and the overall rates of loss to follow-up, particularly for biopsy, were relatively high, and were worse at facilities that served rural and low income women. Efforts to improve the timeliness of evaluation and decrease cancer disparities should focus on identifying successful strategies to decrease loss to follow-up across all facilities, and specifically evaluate effectiveness particularly at facilities serving rural and low income women.
Supplementary Material
Acknowledgments
This work was supported by the Agency for Health Care Research and Quality, Grant #1 K08 HS018090-01, NIH/NCRR/OD UCSF-CTSI Grant Number KL2 RR024130, California Breast Cancer Research Project, Grant #14IB-0062, and the National Cancer Institute-funded Breast Cancer Surveillance Consortium (U01CA63740, U01CA86076, U01CA86082, U01CA63736, U01CA70013, U01CA69976, U01CA63731, U01CA70040, HHSN261201100031C). The collection of cancer and vital status data used in this study was supported in part by several state public health departments and cancer registries throughout the U.S. For a full description of these sources, please see: http://www.breastscreening.cancer.gov/work/acknowledgement.html. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. We thank the participating women, mammography facilities, and radiologists for the data they have provided for this study. A list of the BCSC investigators and procedures for requesting BCSC data for research purposes are provided at: http://breastscreening.cancer.gov/.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosenberg RD, Yankaskas BC, Abraham LA, et al. Performance benchmarks for screening mammography. Radiology. 2006;241:55–66. doi: 10.1148/radiol.2411051504. [DOI] [PubMed] [Google Scholar]
- 2.Brewer NT, Salz T, Lillie SE. Systematic review: the long-term effects of false-positive mammograms. Ann Intern Med. 2007;146:502–510. doi: 10.7326/0003-4819-146-7-200704030-00006. [DOI] [PubMed] [Google Scholar]
- 3.Richards MA, Smith P, Ramirez AJ, et al. The influence on survival of delay in the presentation and treatment of symptomatic breast cancer. Br J Cancer. 1999;79:858–864. doi: 10.1038/sj.bjc.6690137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vujovic O, Yu E, Cherian A, et al. Effect of interval to definitive breast surgery on clinical presentation and survival in early-stage invasive breast cancer. Int J Radiat Oncol Biol Phys. 2009;75:771–774. doi: 10.1016/j.ijrobp.2008.11.049. [DOI] [PubMed] [Google Scholar]
- 5.Sabatino SA, Thompson TD, Richardson LC, et al. Health insurance and other factors associated with mammography surveillance among breast cancer survivors: results from a national survey. Med Care. 2012;50:270–276. doi: 10.1097/MLR.0b013e318244d294. [DOI] [PubMed] [Google Scholar]
- 6.Williams DL, Tortu S, Thomson J. Factors associated with delays to diagnosis and treatment of breast cancer in women in a Louisiana urban safety net hospital. Women Health. 2010;50:705–718. doi: 10.1080/03630242.2010.530928. [DOI] [PubMed] [Google Scholar]
- 7.Tian N, Goovaerts P, Zhan FB, et al. Identifying Risk Factors for Disparities in Breast Cancer Mortality among African-American and Hispanic Women. Womens Health Issues. 2012 doi: 10.1016/j.whi.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu XC, Lund MJ, Kimmick GG, et al. Influence of race, insurance, socioeconomic status, and hospital type on receipt of guideline-concordant adjuvant systemic therapy for locoregional breast cancers. J Clin Oncol. 2012;30:142–150. doi: 10.1200/JCO.2011.36.8399. [DOI] [PubMed] [Google Scholar]
- 9.Elmore JG, Nakano CY, Linden HM, et al. Racial inequities in the timing of breast cancer detection, diagnosis, and initiation of treatment. Med Care. 2005;43:141–148. doi: 10.1097/00005650-200502000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Chlebowski RT, Chen Z, Anderson GL, et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst. 2005;97:439–448. doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 11.Dalton SO, During M, Ross L, et al. The relation between socioeconomic and demographic factors and tumour stage in women diagnosed with breast cancer in Denmark, 1983-1999. Br J Cancer. 2006;95:653–659. doi: 10.1038/sj.bjc.6603294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayanian JZ, Kohler BA, Abe T, et al. The relation between health insurance coverage and clinical outcomes among women with breast cancer. N Engl J Med. 1993;329:326–331. doi: 10.1056/NEJM199307293290507. [DOI] [PubMed] [Google Scholar]
- 13.ACR Practice Guideline for the Performance of Screening and Diagnostic Mammography. American College of Radiology; 2008. [Google Scholar]
- 14.Perry N. BM, de Wolf C, Tornberg S, Holland R, von Karsa L. European guidelines for quality assurance in breast cancer screening and diagnosis-Fourth edition. Office for Official Publications of the European Communities; Luxembourg: 2006. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg RD, Haneuse SJ, Geller BM, et al. Timeliness of follow-up after abnormal screening mammogram: variability of facilities. Radiology. 2011;261:404–413. doi: 10.1148/radiol.11102472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yabroff KR, Ashbeck E, Rosenberg R. Trends in time to completion of mammographic screening and follow-up services. AJR Am J Roentgenol. 2007;188:242–245. doi: 10.2214/AJR.04.1730. [DOI] [PubMed] [Google Scholar]
- 17.Karliner LS, Ma L, Hofmann M, et al. Language barriers, location of care, and delays in follow-up of abnormal mammograms. Med Care. 2012;50:171–178. doi: 10.1097/MLR.0b013e31822dcf2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Orsi C, Tu SP, Nakano C, et al. Current realities of delivering mammography services in the community: do challenges with staffing and scheduling exist? Radiology. 2005;235:391–395. doi: 10.1148/radiol.2352040132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Artz DR, Brown ML, Barrett MJ. The supply of mammography resources in West Virginia. W V Med J. 1992;88:142–146. [PubMed] [Google Scholar]
- 20.Goldman LE, Walker R, Miglioretti DL, et al. Accuracy of diagnostic mammography at facilities serving vulnerable women. Med Care. 2011;49:67–75. doi: 10.1097/MLR.0b013e3181f380e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldman LE, Haneuse SJ, Miglioretti DL, et al. An Assessment of the Quality of Mammography Care at Facilities Treating Medically Vulnerable Populations. Med Care. 2008;46:701–708. doi: 10.1097/MLR.0b013e3181789329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sickles EA, Miglioretti DL, Ballard-Barbash R, et al. Performance benchmarks for diagnostic mammography. Radiology. 2005;235:775–790. doi: 10.1148/radiol.2353040738. [DOI] [PubMed] [Google Scholar]
- 23.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 24.Lane PW, Nelder JA. Analysis of covariance and standardization as instances of prediction. Biometrics. 1982;38:613–621. [PubMed] [Google Scholar]
- 25.Shelton RC, Goldman RE, Emmons KM, et al. An investigation into the social context of low-income, urban Black and Latina women: implications for adherence to recommended health behaviors. Health Educ Behav. 2011;38:471–481. doi: 10.1177/1090198110382502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donelan K, Mailhot JR, Dutwin D, et al. Patient perspectives of clinical care and patient navigation in follow-up of abnormal mammography. J Gen Intern Med. 2011;26:116–122. doi: 10.1007/s11606-010-1436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ell K, Padgett D, Vourlekis B, et al. Abnormal mammogram follow-up: a pilot study women with low income. Cancer Pract. 2002;10:130–138. doi: 10.1046/j.1523-5394.2002.103009.x. [DOI] [PubMed] [Google Scholar]
- 28.Ell K, Vourlekis B, Lee PJ, et al. Patient navigation and case management following an abnormal mammogram: a randomized clinical trial. Prev Med. 2007;44:26–33. doi: 10.1016/j.ypmed.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Paskett ED, Harrop JP, Wells KJ. Patient navigation: an update on the state of the science. CA Cancer J Clin. 2011;61:237–249. doi: 10.3322/caac.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Institute NC [June 28, 2012];2012 Available at: http://crchd.cancer.gov/attachments/brochures/pnrp_brochure.pdf.
- 31.Carney PA, Kettler M, Cook AJ, et al. An assessment of the likelihood, frequency, and content of verbal communication between radiologists and women receiving screening and diagnostic mammography. Acad Radiol. 2009;16:1056–1063. doi: 10.1016/j.acra.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maly RC, Leake B, Mojica CM, et al. What influences diagnostic delay in low-income women with breast cancer? J Womens Health (Larchmt) 2011;20:1017–1023. doi: 10.1089/jwh.2010.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.