Abstract
The purpose of this study was to evaluate whether CC-AAbs levels could predict prognosis in CHF patients. A total of 2096 patients with CHF (841 DCM patients and 1255 ICM patients) and 834 control subjects were recruited. CC-AAbs were detected and the relationship between CC-AAbs and patient prognosis was analyzed. During a median follow-up time of 52 months, there were 578 deaths. Of these, sudden cardiac death (SCD) occurred in 102 cases of DCM and 121 cases of ICM. The presence of CC-AAbs in patients was significantly higher than that of controls (both P < 0.001). Multivariate analysis revealed that positive CC-AAbs could predict SCD (HR 3.191, 95% CI 1.598–6.369 for DCM; HR 2.805, 95% CI 1.488–5.288 for ICM) and all-cause mortality (HR 1.733, 95% CI 1.042–2.883 for DCM; HR 2.219, 95% CI 1.461–3.371 for ICM) in CHF patients. A significant association between CC-AAbs and non-SCD (NSCD) was found in ICM patients (HR = 1.887, 95% CI 1.081–3.293). Our results demonstrated that the presence of CC-AAbs was higher in CHF patients versus controls and corresponds to a higher incidence of all-cause death and SCD. Positive CC-AAbs may serve as an independent predictor for SCD and all-cause death in these patients.
1. Introduction
Chronic heart failure (CHF) develops in the setting of left ventricular systolic and/or diastolic dysfunction and is a serious public health problem worldwide with increasing prevalence [1]. Long-term prognosis of CHF is poor and over 50% of CHF patients die within 5 years after diagnosis [2]. A major cause of mortality is sudden cardiac death (SCD) from ventricular arrhythmias [3, 4]. Thus, prediction and prevention of SCD are crucial to management of these patients.
Recently, evidence has been accumulating suggesting that autoimmunity plays a role in the occurrence and progression of CHF [5–7]. For example, β1-adrenergic receptor autoantibodies (functioning as receptor agonists) were detected in the serum of CHF patients [8–10] and removal of these autoantibodies has been shown to improve hemodynamic parameters [11]. In addition, antibodies against Na-K-ATPase exerted arrhythmogenic effects and correlated with SCD in certain DCM patients [12]. Immunization of rabbits with sarcolemmal Na-K-ATPase resulted in myocardial hypertrophy due to left ventricular pressure overload and myocardial fibrosis [13]. Therefore, characterizing these antibodies may be helpful in the understanding of CHF pathogenesis.
The L-type calcium channel plays an important role in cardiac excitation-contraction coupling [14]. Dysfunction of the channel often correlates with ventricular arrhythmias (VAs) [15]. Current studies suggest that autoantibodies are directly linked to SCD in DCM patients and calcium channel dysfunction contributes to pathogenesis [12, 16, 17]. Xiao et al. [18] detected the presence of calcium channel autoantibodies (CC-AAbs) in patients with DCM and found that CC-AAbs could prolong action potential duration (APD) and ultimately lead to VT in animal models. They believe that CC-AAbs could serve as a new biomarker for autoimmunity. Therefore, we set out to evaluate whether CC-AAbs could predict prognosis and SCD in CHF patients.
2. Materials and Methods
2.1. Patients Enrollment
From July 2005 to March 2010, 2096 CHF patients were recruited from 10 hospitals in mainland China. The inclusion criteria were CHF caused by DCM or ICM with NYHA (New York Heart Association) functional class II–IV despite optimized medical therapy and left ventricular ejection fraction (LVEF) ≤45% in DCM and ≤50% in ICM. DCM was diagnosed according to the guidelines for the study of familial DCM [19]. ICM was defined as ≥70% luminal stenosis of at least one major coronary artery diagnosed by coronary angiography and a history of myocardial infarction at least 3 months before enrollment. All cases were excluded if they had malignant tumors, severe liver and kidney dysfunctions or other uncontrollable diseases, pregnancy, and unwillingness to participate in the study.
2.2. Control Subjects
834 cases were selected as controls. Of them, 401 cases came from community-based inhabitants who underwent annual health examination and were free of structural heart disease, and 433 cases were hospitalized patients who underwent radiofrequency ablation for supraventricular tachycardia without structural heart disease. The exclusion criteria were the same for controls and CHF patients.
The investigation conformed to the principles outlined in the Declaration of Helsinki and was approved by the Ethics Committee of Fu Wai Cardiovascular Hospital (Beijing, China). All subjects who participated in the study provided written informed consent and reported themselves as Chinese Han nationality.
2.3. Serum Sampling and Peptide Synthesis
Blood sample was obtained from the antecubital vein and separated by centrifugation (3000 rpm, Sigma Centrifuge) for 10 min. Serum samples were stored at −80°C until needed for assay. A peptide corresponding to the sequence (residues 2~16) of α1c/CaV1.2 of the human L-type calcium channel (V-N-E-N-T-R-M-Y-I-P-E-E-N-H-Q) was synthesized by a commercial source (CL BIO-SCIENTIFIC CO.LTD). The purity of the peptides was determined by high performance liquid chromatography (HPLC) and direct sequence analysis with an automated amino acid analyzer.
2.4. Enzyme-Linked Immunoabsorbent Assay (ELISA)
ELISA was used to quantify the CC-AAbs. Briefly, microtiter plates were coated with 100 μL/well calcium channel peptides (5 μg/mL). After incubation at 37°C for 1 hour, the plates were washed with PBS-T 4 times. Nonspecific binding sites were blocked with fat-free milk solution for 1 h and then washed 4 times. The first antibody was added and incubated for 1 h at 37°C. The plates were then washed 4 times, incubated with horseradish peroxidase-streptavidin (Go a Hu IgG-HRP) solution (1 : 500) for 1 h at 37°C, washed 4 times, and developed for 5 min with substrate solution (3,3′,5,5′-tetramethylbenzidine, TMB) in absence of light. The reaction was terminated by stop solution. The optical density (OD) was read using ELISA plate reader (BIO-RAD model 550 USA) at 490 nm wavelength. Autoantibody positive was defined as a ratio (patient OD-blank OD/control OD-blank OD) ≥2.1. The intramicrotiter plate coefficient of variance (CV) level was evaluated using the variation of the negative control from well to well (n = 6), whereas the intermicrotiter plate CV level was obtained from different plates. The CVs of intraplates were less than 5%, and CVs of the interplates were less than 10%.
2.5. End Point Assessment
The patients were followed up to the end of March 2013 during regular outpatient clinic or through telephone contact. Median follow-up period was 52 months (0.40~92 months). End points included all-cause death, SCD (ICD appropriate discharge counter as SCD), and NSCD (heart transplantation regarded as NSCD). SCD was defined as unexpected death within 1 hour of onset of acute symptoms or unwitnessed death such as during sleep or unexpected death of someone last seen in stable medical condition <24 h with no evidence of a noncardiac cause [20].
2.6. Statistical Analysis
Statistical analyses were performed using SPSS 21.0 software (SPSS Inc, Chicago). Continuous values were expressed as mean ± SD, and categorical variables were shown as numbers (%). Student's t-test or Chi-square test was used to compare between groups; P < 0.05 was considered significant. Person-months of follow-up period started from the date of enrollment to the end of March 2013. Survival analysis in CHF patients was performed. 265 (12.64%) patients were lost to follow up and excluded in survival analysis. Kaplan-Meier curves using log rank test were performed based on presence or absence of CC-AAbs. By using Cox regression, the hazard ratios for time to all-cause death, SCD, and non-SCD from baseline were evaluated.
3. Results
3.1. Clinical Characteristics
A total of 1831 CHF patients (732 cases of DCM and 1099 cases of ICM) were successfully followed. As shown in Table 1, age and body mass index (BMI) distribution did not differ between CHF patients and controls (P > 0.05). Other possible CHF risk factors such as hypertension, hyperlipidemia, diabetes mellitus, premature ventricular contractions (PVCs), atrial fibrillation (AF), mean heart rate (MHR), LVEF, and left ventricular end-diastolic diameter (LVEDD) were more prevalent in CHF patients than in controls (P < 0.05). Hemodynamic parameters tested by echocardiography were similar between patients with DCM and with ICM (P > 0.05) with a trend towards higher NYHA classification in DCM versus in ICM patients (NYHA II: 21.45% versus 52.96%; NYHA III: 41.80% versus 30.76%; NYHA IV: 36.75% versus 16.28%, all P < 0.05). More DCM patients received diuretics and β-blockers, while ICM patients took more angiotensin-converting enzyme inhibitors (ACEIs) and calcium channel blockers (CCBs) for hypertension and prevention of coronary artery spasm.
Table 1.
Clinical data of control subjects and patients with CHF.
| Clinical characteristic | Control (834) | CHF | |||
|---|---|---|---|---|---|
| DCM (n = 732) | P | ICM (n = 1099) | P | ||
| Male, n (%) | 449 (53.84%) | 558 (76.23%) | <0.001 | 885 (80.53%) | <0.001 |
| Age (y) | 57.35 ± 12.68 | 58.86 ± 14.42 | =0.052 | 67.87 ± 10.48 | <0.001 |
| BMI | 24.63 ± 10.20 | 24.94 ± 17.05 | =0.587 | 25.02 ± 3.81 | =0.453 |
| NYHA class, n (%) | |||||
| I | 834 (100%) | 0 | — | 0 | — |
| II | 0 | 157 (21.45%) | — | 582 (52.96%) | — |
| III | 0 | 306 (41.80%) | — | 338 (30.76%) | — |
| IV | 0 | 269 (36.75%) | — | 179 (16.28%) | — |
| Hypertension, n (%) | 199 (23.86%) | 242 (33.06%) | =0.003 | 672 (61.15%) | <0.001 |
| Hyperlipidemia, n (%) | 53 (6.35%) | 76 (10.38%) | <0.001 | 329 (29.94%) | <0.001 |
| Diabetes mellitus, n (%) | 60 (7.19%) | 120 (16.39%) | <0.001 | 317 (28.84%) | <0.001 |
| ECG and arrhythmias | |||||
| MHR (beats/min) | 69.95 ± 10.60 | 79.69 ± 18.89 | <0.001 | 72.57 ± 14.37 | <0.001 |
| AF (n) | 0 | 177 (24.18%) | — | 130 (11.83%) | — |
| PVC (n) | 0 | 192 (26.23%) | — | 217 (19.75%) | — |
| QTc (ms) | 412.31 ± 81.21 | 446.59 ± 102.57 | <0.001 | 444.49 ± 88.67 | <0.001 |
| QRS duration (ms) | 94.26 ± 57.77 | 113.64 ± 38.23 | <0.001 | 104.88 ± 42.03 | <0.001 |
| Hemodynamic parameters | |||||
| LVEF (%) | 60.63 ± 9.09 | 32.91 ± 9.80 | <0.001 | 41.32 ± 8.67 | <0.001 |
| LVEDD (mm) | 45.34 ± 8.76 | 66.10 ± 11.96 | <0.001 | 57.59 ± 9.23 | <0.001 |
| Medications for CHF | |||||
| ACEI, n (%) | 0 | 437 (59.69%) | — | 710 (64.60%) | — |
| Diuretic, n (%) | 0 | 565 (77.18%) | — | 741 (67.42%) | — |
| Digoxin, n (%) | 0 | 483 (65.98%) | — | 697 (63.42%) | — |
| β-blocker, n (%) | 0 | 570 (77.87%) | — | 776 (70.61%) | — |
| CCBs, n (%) | 0 | 24 (3.28%) | — | 248 (22.57%) | — |
| ICD, n (%) | 0 | 10 (1.37%) | — | 28 (2.55%) | — |
Values are mean ± SD or number (%). P < 0.05 was considered significant compared with the control group. Premature vascular contraction (PVC) indicated >3000 beats/24 h.
AF: atrial fibrillation; ACEI: angiotensin-converting enzyme inhibitor; BMI: body mass index; CHF: chronic heart failure; NYHA: New York Heart Association; DCM: dilated cardiomyopathy; ICM: ischaemic cardiomyopathy; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; MHR: mean heart rate; ICD: implantable cardioverter defibrillator; SCD: sudden cardiac death.
Table 2 lists relevant clinical characteristics between SCD and NSCD subgroups and shows that risk factors such as hypertension, hyperlipidemia, NYHA classification, LVEF, PVCs, and QTc as well as the CC-AAbs did not differ significantly between the two groups (all P > 0.05).
Table 2.
Clinical characteristics of CHF patients with SCD and NSCD subgroups.
| Characteristics | DCM (n = 732) | P | ICM (n = 1099) | P | ||
|---|---|---|---|---|---|---|
| NSCD (n = 146) | SCD (n = 102) | NSCD (n = 209) | SCD (n = 121) | |||
| Age (years) | 57.94 ± 14.67 | 57.98 ± 14.76 | 0.982 | 69.62 ± 10.84 | 68.78 ± 10.35 | 0.487 |
| Male gender, n (%) | 107 (73.29%) | 74 (72.55%) | 0.959 | 165 (78.95%) | 97 (80.17%) | 0.929 |
| MHR (beats/min) | 78.66 ± 18.39 | 79.43 ± 15.74 | 0.751 | 75.60 ± 16.39 | 75.48 ± 16.02 | 0.942 |
| Hypertension, n (%) | 48 (32.88%) | 31 (30.39%) | 0.766 | 130 (62.20%) | 69 (57.02%) | 0.643 |
| Hyperlipidemia, n (%) | 12 (8.22%) | 10 (9.80%) | 0.693 | 66 (31.58%) | 34 (28.10%) | 0.627 |
| Diabetes mellitus, n (%) | 18 (12.33%) | 15 (14.71%) | 0.636 | 76 (36.36%) | 29 (23.97%) | 0.089 |
| PVC, n (%) | 39 (26.71%) | 30 (29.41%) | 0.726 | 39 (18.66%) | 28 (23.14%) | 0.430 |
| AF, n (%) | 47 (32.19%) | 21 (20.59%) | 0.125 | 28 (13.39%) | 15 (12.40) | 0.819 |
| QRS duration (ms) | 119.49 ± 40.50 | 109.79 ± 32.71 | 0.156 | 102.38.10 ± 22.29 | 105.11 ± 24.00 | 0.921 |
| QTc (ms) | 453.81 ± 80.99 | 447.73 ± 106.04 | 0.651 | 443.74 ± 104.56 | 445.52 ± 62.58 | 0.862 |
| NYHA | ||||||
| II | 27 (18.49%) | 33 (32.35%) | 0.052 | 93 (44.50%) | 50 (41.32%) | 0.724 |
| III | 60 (41.10%) | 44 (43.14%) | 0.838 | 69 (33.01%) | 48 (39.67%) | 0.403 |
| IV | 59 (40.41%) | 25 (24.51%) | 0.064 | 47 (22.49%) | 23 (19.01%) | 0.546 |
| LVEF (%) | 31.26 ± 9.38 | 32.62 ± 9.15 | 0.880 | 40.09 ± 9.29 | 38.39 ± 9.33 | 0.261 |
| LVEDD (mm) | 68.15 ± 12.92 | 65.51 ± 9.85 | 0.292 | 58.08 ± 9.45 | 60.19 ± 9.29 | 0.151 |
| CC-AAbs | 10 (6.85%) | 13 (12.75%) | 0.153 | 17 (8.13%) | 12 (9.92%) | 0.614 |
| BNP (pg/mL) | 2127.51 ± 355.79 | 2123.03 ± 366.57 | 0.924 | 2002.27 ± 385.21 | 1995.56 ± 387.05 | 0.877 |
| ACEI, n (%) | 89 (60.96%) | 52 (50.98%) | 0.410 | 148 (70.81%) | 77 (63.64%) | 0.555 |
| Diuretic, n (%) | 101 (69.18%) | 76 (74.51%) | 0.709 | 146 (69.86%) | 82 (67.77%) | 0.865 |
| Digoxin, n (%) | 110 (75.34%) | 61 (59.80%) | 0.260 | 142 (67.94%) | 79 (65.29%) | 0.826 |
| β-blockers, n (%) | 108 (73.97%) | 78 (76.47%) | 0.866 | 151 (72.25%) | 85 (70.25%) | 0.874 |
| CCBs, n (%) | 2 (1.37%) | 0 | — | 34 (16.27) | 23 (19.01%) | 0.595 |
| ICD, n (%) | 1 (0.68%) | 1 (0.98%) | 0.801 | 4 (1.91%) | 2 (1.65%) | 0.867 |
Values are mean ± SD or number (%). P < 0.05 was considered significant comparing with NSCD group. PVC indicated frequent premature ventricular beats (more than 3000 beats/24 h). AF: atrial fibrillation; ACEI: angiotensin-converting enzyme inhibitor; BMI: body mass index; CHF: chronic heart failure; NYHA: New York Heart Association; CC-AAbs: calcium channel autoantibodies; DCM: dilated cardiomyopathy; ICM: ischaemic cardiomyopathy; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; MHR: mean heart rate; ICD: implantable cardioverter defibrillator; SCD: sudden cardiac death; NSCD: nonsudden cardiac death.
Also, we compared characteristics between DCM and ICM patients who were CC-AAbs positive and negative and found no significant differences in age, gender, medications, hemodynamic parameters, and other cardiovascular risk factors attributable to DCM or ICM status (Table 3, all P > 0.05).
Table 3.
Characteristics of CHF patients with CC-AAbs positive and negative.
| Characteristics | DCM (n = 732) | P | ICM (n = 1099) | P | ||
|---|---|---|---|---|---|---|
| CC-AAb(+) (n = 43) | CC-AAb(−) (n = 689) | CC-AAb(+) (n = 51) | CC-AAb (−) (n = 1048) | |||
| Age (years) | 59.14 ± 13.61 | 58.84 ± 14.48 | 0.895 | 67.69 ± 12.26 | 67.88 ± 10.39 | 0.899 |
| Male gender, n (%) | 34 (79.07%) | 524 (76.05%) | 0.869 | 40 (78.43%) | 845 (80.63%) | 0.898 |
| MHR (beats/min) | 79.29 ± 17.98 | 79.71 ± 18.95 | 0.883 | 74.73 ± 18.21 | 72.46 ± 14.16 | 0.273 |
| Hypertension, n (%) | 11 (25.58%) | 231 (33.53%) | 0.434 | 30 (58.82%) | 642 (61.26%) | 0.863 |
| Hyperlipidemia, n (%) | 3 (6.98%) | 73 (10.60%) | 0.793 | 18 (35.29%) | 256 (24.43%) | 0.191 |
| Diabetes mellitus, n (%) | 5 (11.63%) | 115 (16.69%) | 0.452 | 15 (29.41%) | 302 (28.82%) | 0.946 |
| PVC, n (%) | 14 (32.56%) | 178 (25.83%) | 0.467 | 7 (13.73%) | 210 (20.04%) | 0.353 |
| AF, n (%) | 11 (25.58%) | 166 (24.09%) | 0.864 | 4 (7.84%) | 126 (12.02%) | 0.415 |
| QRS duration (ms) | 110.71 ± 35.66 | 113.82 ± 38.41 | 0.610 | 103.25 ± 22.58 | 104.96 ± 42.76 | 0.777 |
| QTc (ms) | 463.61 ± 67.32 | 445.50 ± 104.37 | 0.268 | 452.41 ± 95.50 | 444.10 ± 88.34 | 0.514 |
| NYHA | ||||||
| II | 11 (25.58%) | 146 (21.19%) | 0.590 | 25 (49.02%) | 557 (53.15%) | 0.746 |
| III | 18 (41.86%) | 288 (41.80%) | 0.996 | 17 (33.33%) | 321 (30.63%) | 0.768 |
| IV | 14 (32.56%) | 255 (37.01%) | 0.685 | 9 (17.65%) | 170 (16.22%) | 0.820 |
| LVEF (%) | 33.46 ± 7.63 | 32.88 ± 9.92 | 0.718 | 41.38 ± 8.40 | 41.31 ± 8.69 | 0.958 |
| LVEDD (mm) | 66.87 ± 8.99 | 66.06 ± 12.13 | 0.686 | 56.10 ± 7.57 | 57.66 ± 9.31 | 0.254 |
| BNP (pg/mL) | 2139.17 ± 336.68 | 2089.10 ± 367.98 | 0.385 | 1984.44 ± 308.64 | 1982.79 ± 396.77 | 0.971 |
| Treatments | ||||||
| ACEI, n (%) | 26 (60.47%) | 411 (59.65%) | 0.958 | 34 (66.67%) | 676 (64.50%) | 0.884 |
| Diuretic, n (%) | 36 (83.72%) | 529 (76.77%) | 0.710 | 37 (72.55%) | 704 (67.18%) | 0.728 |
| Digoxin, n (%) | 35 (81.40%) | 448 (65.02%) | 0.340 | 34 (67.94%) | 663 (65.29%) | 0.826 |
| β-blockers, n (%) | 37 (86.05%) | 533 (77.36%) | 0.646 | 41 (80.39%) | 735 (70.13%) | 0.817 |
| CCBs, n (%) | 3 (6.98%) | 21 (3.05%) | 0.182 | 11 (21.57%) | 237 (22.61%) | 0.889 |
| ICD, n (%) | 2 (4.65%) | 8 (1.16%) | 0.119 | 3 (5.88%) | 25 (2.39%) | 0.147 |
Values are mean ± SD or number (%). P < 0.05 was considered significant comparing with NSCD group. PVC indicated frequent premature ventricular beats (more than 3000 beats/24 h). AF: atrial fibrillation; ACEI: angiotensin-converting enzyme inhibitor; BMI: body mass index; CHF: chronic heart failure; NYHA: New York Heart Association; CC-AAbs: calcium channel autoantibodies; DCM: dilated cardiomyopathy; ICM: ischaemic cardiomyopathy; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; MHR: mean heart rate; ICD: implantable cardioverter defibrillator; SCD: sudden cardiac death; NSCD: nonsudden cardiac death.
3.2. The Prognosis of Patients with CHF Correlates with CC-AAbs Expression
By the median of 52 months (0.40~92 months) of follow-up, 578 patients (248 cases of DCM and 330 cases of ICM) had died. Of these, 223 patients (102 cases of DCM and 121 cases of ICM) had SCD, while the rest had NSCD. As shown in Table 4, rates of CC-AAbs in DCM and ICM patients were significantly higher than those in controls (5.87% and 4.64% versus 1.20%, both P < 0.001). CC-AAbs rates were also significantly higher in all-cause mortality group (9.27% in DCM and 8.79% in ICM) compared to living patients (both P < 0.001). Further analysis indicated no difference in CC-AAbs status between SCD and NSCD groups in DCM (12.75% versus 6.85%, P > 0.05) and ICM (9.92% versus 8.13%, P > 0.05) patients.
Table 4.
The association between CC-AAbs and the prognosis of CHF patients.
| Control (n = 834) | DCM (n = 732) | ICM (n = 1099) | ||||||
|---|---|---|---|---|---|---|---|---|
| CC-AAb(+) | HR | CC-AAb(+) | HR (95% CI) | P | CC-AAb(+) | HR (95% CI) | P | |
| Total n (%) | 10 (1.20%) | 1 | 43 (5.87%) | 1 | <0.001 | 51 (4.64%) | 1 | <0.001 |
| No death n (%) | 10 (1.20%) | 1 | 20 (4.13%) | 1 | 0.030 | 22 (3.17%) | 1 | 0.178 |
| All-cause death, n (%) | 0 | 1 | 23 (9.27%) | 1.733 (1.042–2.883) | 0.034 | 29 (7.88%) | 2.219 (1.461–3.371) | <0.001 |
| Non-SCD n (%) | 0 | 1 | 10 (6.85%) | 1.049 (0.483–2.278) | 0.903 | 17 (5.74%) | 1.887 (1.081–3.293) | 0.025 |
| SCD n (%) | 0 | 1 | 13 (12.75%) | 3.191 (1.598–6.369) | 0.001 | 12 (11.57%) | 2.805 (1.488–5.288) | 0.001 |
The positive of calcium channel autoantibodies (CC-AAbs) increased hazard ratio. 95% confidence interval (95% CI) after adjustment for age, gender, BMI, MHR, hypertension, hyperlipidemia, diabetes mellitus, QTc, PVC, AF, NYHA class, LVEF, causes of HF, and medications. P < 0.05 was considered significant.
3.3. Positive CC-AAbs Indicated Higher Risk of SCD and All-Cause Death in CHF Patients
Kaplan-Meier curves showed that patients carrying CC-AAbs were susceptible to SCD and all-cause death in DCM (P = 0.002 and P < 0.001, resp.) and ICM patients (P < 0.001 and P = 0.001, resp.). However, a significant association between CC-AAbs and NSCD was only found in ICM patients (P = 0.002) (Figure 1).
Figure 1.
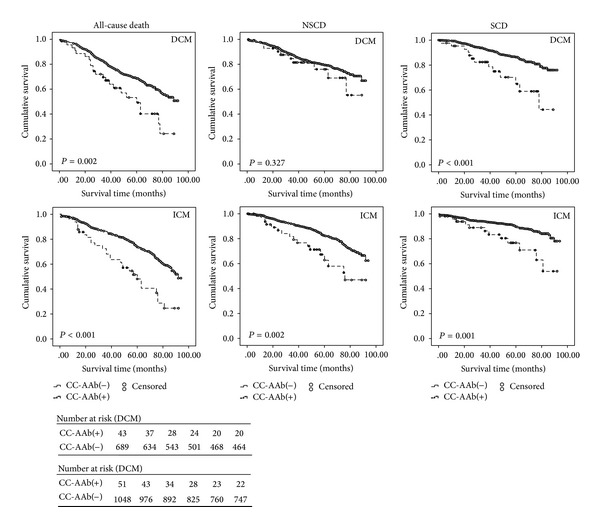
Kaplan-Meier curves for the probability of SCD and all-cause death in patients with DCM (upper panel) and ICM (lower panel) according to the presence or absence of CC-AAbs. Each censored case was marked with a circle dot. Patients with CC-AAbs positive were more susceptible to SCD and all-cause death than patients without carrying CC-AAbs both in DCM and ICM. The CC-AAbs were related with NSCD only in patients with ICM.
After adjusting for risk factors such as age, gender, BMI, HR, hypertension, diabetes mellitus, hyperlipidemia, NYHA classification, LVED, LVEF, QTc, PVCs, AF, and medications, Cox regression analysis revealed that the presence of CC-AAbs still remained an independent predictor for SCD (HR 3.191, 95% CI 1.598–6.369 for DCM; HR 2.805, 95% CI 1.488–5.288 for ICM) and all-cause mortality (HR 1.733, 95% CI 1.042–2.883 for DCM; HR 2.219, 95% CI 1.461–3.371 for ICM). Consistent with the results of univariate analysis, positive CC-AAbs correlated with NSCD only in ICM patients (HR = 1.887, 95% CI 1.081–3.293) (Table 4).
4. Discussion
Our large-scale prospective study demonstrated that more CHF patients had positive CC-AAbs levels than controls, and those positive patients had a high incidence of all-cause mortality as well as SCD. Thus, presence of CC-AAbs could serve as an independent predictor for SCD and mortality. This result is in line with a previous study that showed higher SCD in CC-AAbs positive DCM patients [18].
In recent years, numerous prognostic indicators have been reported to be useful in predicting long-term prognosis of CHF patients: meta-analysis reviews have prompted a few candidates such as brain natriuretic peptide (BNP), ventricular tachycardia (VT), and late gadolinium enhancement (LGE) as predictors of SCD in DCM patients [21–23]. Previous research from us and other groups had also suggested β 1-receptor autoantibodies to be related to SCD in CHF patients [8, 16, 24]. Our present study expanded upon Xiao et al.'s [18] finding that CC-AAbs may be a risk factor for VT and SCD in a small DCM patient group and established that DCM and ICM patients positive for CC-AAbs sustained a 2- to 3-fold risk for SCD.
It is well known that L-type calcium channels play a critical role in signal transduction and electrophysiological activity of the heart [25, 26]. They help to maintain the shape and duration of the action potential plateau phase in ventricular myocytes. When activated by PKA or other factors, more calcium ions flow through the channels, which could trigger activation of the sodium/calcium exchanger and result in intercellular calcium overload, myocyte destruction, and cardiac electric instability [17, 27, 28]. Antibodies against adenine nucleotide translocator (ANT) have been reported to cross-react with a cell surface calcium channel protein to impair cardiac energy metabolism and function through increased intracellular calcium loading [29]. Similarly, autoantibodies against β 1-adrenoceptors have been seen to prolong APD by increasing the L-type calcium currents [16].
Animal experiments have shown that CC-AAbs can produce an agonist effect to calcium-channel function and verapamil, a calcium-channel antagonist, could attenuate immune-mediated myocyte damage [30, 31]. Researchers have suggested a few possible mechanisms by which CC-AAbs could bind to calcium channels: (1) the cell membrane might turn over and result in exposure of intracellular peptide after cardiomyocyte injury happens [32] and (2) autoantibodies could pass through the plasma membrane and penetrate adherent cells through gap junction under certain conditions [33, 34]. Once ventricular myocytes were exposed to affinity-purified CC-AAbs, the APD became prolonged significantly, and this was thought to promote the development of EAD induced ventricular arrhythmias and eventually SCD [18, 35]. These mechanisms may explain why more CC-AAbs were found in CHF patients with SCD.
Our study suggested that CC-AAbs could predict SCD and all-cause death in CHF patients. However, the rate of CC-AAbs observed in our study was lower than that of published data, which reported 48.8% [30]. One possible reason may be our much longer follow-up time, during which the autoantibody activity might have diminished. Nevertheless, we demonstrated CC-AAbs as a positive predictor for SCD and NSCD in ICM patients. Although unreported in the current literature, the prolonged Ca2+ current caused by CC-AAbs could delay cardiomyocyte repolarization and increase a risk of developing fatal arrhythmias independent of DCM or ICM etiology of CHF [14]. Multicenter clinical trials have shown that calcium channel antagonist diltiazem had protective effects on the myocardium in DCM patients, particularly during early stage disease [36–38].
5. Limitations
It is important to acknowledge some limitations in our current study. First, the ELISA method might have lower sensitivity and specificity compared to other methods such as a complex three-step screening strategy. The effect of CC-AAbs on the prediction of SCD in our study might be underestimated, which would limit its clinical application. Further studies are required to evaluate its diagnostic potential. Second, the CC-AAbs were only tested at baseline and the values immediately before the end point were unknown. Third, a relative high percentage (nearly 13%) of patients was lost during follow-up due to China's recent rapid urbanization. It is important to note that baseline data of these patients were not significantly different. Fourth, we did not follow up the control group, which we should do in the future studies. Finally, our study did not look at the underlying mechanisms behind our findings.
6. Conclusions
In conclusion, the presence of CC-AAbs was significantly higher in CHF patients than that in controls. Positive CC-AAbs could serve as an independent predictor for SCD and all-cause death in these patients and may lead to new preventive and therapeutic targets for CHF.
Acknowledgments
The authors thank Dr. Ying Yang from Qingdao Fu Wai Hospital, Dr. Daowen Wang from Wuhan Tongji Hospital, Dr. Chuanshi Xiao from the Second Hospital of Shanxi Medical University, Dr. JiangHong from Shanghai First People's Hospital, Dr. Weibin Huang from Xiamen University Zhongshan Hospital, Dr. Jianzhong Zhou from the First Affiliated Hospital of Chongqing Medical College, Dr. Xueqi Li from the Fourth Affiliated Hospital of Harbin Medical College, and Dr. He Jia from Hospital of Jilin Oilfield for assistance in collecting data and sera. This work was funded by the National Basic Research Program of China (973 program projects, Project no. 2013CB531105 to Jielin Pu).
Abbreviations
- ACEI:
Angiotensin-converting enzyme inhibitors
- AF:
Atrial fibrillation
- BMI:
Body mass index
- CC-AAbs:
Calcium channel autoantibody
- CCBs:
Calcium channel blockers
- CHF:
Chronic heart failure
- DCM:
Dilated cardiomyopathy
- ELISA:
Enzyme-linked immunoabsorbent assay
- HPLC:
High performance liquid chromatography
- ICM:
Ischemic cardiomyopathy
- LVEDD:
Left ventricular end-diastolic diameter
- LVEF:
Left ventricular ejection fraction
- NSCD:
Nonsudden cardiac death
- NYHA:
New York Heart Association
- SCD:
Sudden cardiac death
- VAs:
Ventricular arrhythmias.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Remme WJ, Swedberg K. Guidelines for the diagnosis and treatment of chronic heart failure. European Heart Journal. 2001;22(17):1527–1560. doi: 10.1053/euhj.2001.2783. [DOI] [PubMed] [Google Scholar]
- 2.Levy D, Kenchaiah S, Glarson M, et al. Long-term trends in the incidence of and survival with heart failure. New England Journal of Medicine. 2002;347(18):1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 3.Linde C, Daubert C. Cardiac resynchronization therapy in patients with New York heart association class i and II heart failure: an approach to 2010. Circulation. 2010;122(10):1037–1043. doi: 10.1161/CIRCULATIONAHA.109.923094. [DOI] [PubMed] [Google Scholar]
- 4.Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. New England Journal of Medicine. 2000;342(15):1077–1084. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 5.Kaya Z, Leib C, Katus HA. Autoantibodies in heart failure and cardiac dysfunction. Circulation Research. 2012;110(1):145–158. doi: 10.1161/CIRCRESAHA.111.243360. [DOI] [PubMed] [Google Scholar]
- 6.Caforio AL, Mahon NG, Baig MK, et al. Prospective familial assessment in dilated cardiomyopathy: cardiac autoantibodies predict disease development in asymptomatic relatives. Circulation. 2007;115(1):76–83. doi: 10.1161/CIRCULATIONAHA.106.641472. [DOI] [PubMed] [Google Scholar]
- 7.Muller J, Wallukat G, Dandel M, et al. Immunoglobulin adsorption in patients with idiopathic dilated cardiomyopathy. Circulation. 2000;101(4):385–391. doi: 10.1161/01.cir.101.4.385. [DOI] [PubMed] [Google Scholar]
- 8.Pei J, Li N, Chen J, et al. The predictive values of beta1-adrenergic and M2 muscarinic receptor autoantibodies for sudden cardiac death in patients with chronic heart failure. European Journal of Heart Failure. 2012;14(8):887–894. doi: 10.1093/eurjhf/hfs082. [DOI] [PubMed] [Google Scholar]
- 9.Miao GB, Liu JC, Liu MB, et al. Autoantibody against β1-adrenergic receptor and left ventricular remodeling changes in response to metoprolol treatment. European Journal of Clinical Investigation. 2006;36(9):614–620. doi: 10.1111/j.1365-2362.2006.01705.x. [DOI] [PubMed] [Google Scholar]
- 10.Jahns R, Boivin V, Siegmund C, et al. Autoantibodies activating human β1-adrenergic receptors are associated with reduced cardiac function in chronic heart failure. Circulation. 1999;99(5):649–654. doi: 10.1161/01.cir.99.5.649. [DOI] [PubMed] [Google Scholar]
- 11.Dorffel WV, Felix SB, Wallukat G, et al. Short-term hemodynamic effects of immunoadsorption in dilated cardiomyopathy. Circulation. 1997;95(8):1994–1997. doi: 10.1161/01.cir.95.8.1994. [DOI] [PubMed] [Google Scholar]
- 12.Baba A, Yoshikawa T, Ogawa S. Autoantibodies produced against sarcolemmal Na-K-ATPase: possible upstream targets of arrhythmias and sudden death in patients with dilated cardiomyopathy. Journal of the American College of Cardiology. 2002;40(6):1153–1159. doi: 10.1016/s0735-1097(02)02075-2. [DOI] [PubMed] [Google Scholar]
- 13.Baba A, Yoshikawa T, Iwata M, et al. Antigen-specific effects of autoantibodies against sarcolemmal Na-K-ATPase pump in immunized cardiomyopathic rabbits. International Journal of Cardiology. 2006;112(1):15–20. doi: 10.1016/j.ijcard.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 14.Splawski I, Timothy KW, Sharpe LM, et al. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119(1):19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Zimetbaum PJ, Buxton AE, Batsford W, et al. Electrocardiographic predictors of arrhythmic death and total mortality in the multicenter unsustained tachycardia trial. Circulation. 2004;110(7):766–769. doi: 10.1161/01.CIR.0000139311.32278.32. [DOI] [PubMed] [Google Scholar]
- 16.Christ T, Wettwer E, Dobrev D, et al. Autoantibodies against the β1-adrenoceptor from patients with dilated cardiomyopathy prolong action potential duration and enhance contractility in isolated cardiomyocytes. Journal of Molecular and Cellular Cardiology. 2001;33(8):1515–1525. doi: 10.1006/jmcc.2001.1414. [DOI] [PubMed] [Google Scholar]
- 17.Iwata M, Yoshikawa T, Baba A, Anzai T, Mitamura H, Ogawa S. Autoantibodies against the second extracellular loop of beta1-adrenergic receptors predict ventricular tachycardia and sudden death in patients with idiopathic dilated cardiomyopathy. Journal of the American College of Cardiology. 2001;37(2):418–424. doi: 10.1016/s0735-1097(00)01109-8. [DOI] [PubMed] [Google Scholar]
- 18.Xiao H, Wang M, Du Y, et al. Arrhythmogenic autoantibodies against calcium channel lead to sudden death in idiopathic dilated cardiomyopathy. European Journal of Heart Failure. 2011;13(3):264–270. doi: 10.1093/eurjhf/hfq198. [DOI] [PubMed] [Google Scholar]
- 19.Fatkin D. Guidelines for the diagnosis and management of familial dilated cardiomyopathy. Heart Lung and Circulation. 2007;16(1):19–21. doi: 10.1016/j.hlc.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) Journal of the American College of Cardiology. 2006;48(5):e247–e346. doi: 10.1016/j.jacc.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Scott PA, Barry J, Roberts PR, Morgan JM. Brain natriuretic peptide for the prediction of sudden cardiac death and ventricular arrhythmias: a meta-analysis. European Journal of Heart Failure. 2009;11(10):958–966. doi: 10.1093/eurjhf/hfp123. [DOI] [PubMed] [Google Scholar]
- 22.de Sousa MR, Morillo CA, Rabelo FT, Filho AMN, Ribeiro ALP. Non-sustained ventricular tachycardia as a predictor of sudden cardiac death in patients with left ventricular dysfunction: a meta-analysis. European Journal of Heart Failure. 2008;10(10):1007–1014. doi: 10.1016/j.ejheart.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Shi HW, Pu P, Deng W, et al. Prognostic value of late gadolinium enhancement in dilated cardiomyopathy patients. A meta-analysis. Saudi Medical Journal. 2013;34(7):719–726. [PubMed] [Google Scholar]
- 24.Yoshikawa T, Baba A, Nagatomo Y. Autoimmune mechanisms underlying dilated cardiomyopathy. Circulation Journal. 2009;73(4):602–607. doi: 10.1253/circj.cj-08-1151. [DOI] [PubMed] [Google Scholar]
- 25.Bourinet E, Mangoni ME, Nargeot J. Dissecting the functional role of different isoforms of the L-type Ca2+ channel. The Journal of Clinical Investigation. 2004;113(10):1382–1384. doi: 10.1172/JCI21815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun F, Hayama E, Katsube Y, Matsuoka R, Nakanishi T. The role of the large-conductance voltage-dependent and calcium-activated potassium (BKca) channels in the regulation of rat ductus arteriosus tone. Heart and Vessels. 2010;25(6):556–564. doi: 10.1007/s00380-010-0008-1. [DOI] [PubMed] [Google Scholar]
- 27.Chiale PA, Ferrari I, Mahler E, et al. Differential profile and biochemical effects of antiautonomic membrane receptor antibodies in ventricular arrhythmias and sinus node dysfunction. Circulation. 2001;103(13):1765–1771. doi: 10.1161/01.cir.103.13.1765. [DOI] [PubMed] [Google Scholar]
- 28.Nagy ZA, Virag L, Toth A, et al. Selective inhibition of sodium-calcium exchanger by SEA-0400 decreases early and delayed afterdepolarization in canine heart. British Journal of Pharmacology. 2004;143(7):827–831. doi: 10.1038/sj.bjp.0706026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schulze K, Heineman FW, Schultheiss HP, Balaban RS. Impairment of myocardial calcium homeostasis by antibodies against the adenine nucleotide translocator. Cell Calcium. 1999;25(5):361–370. doi: 10.1054/ceca.1999.0039. [DOI] [PubMed] [Google Scholar]
- 30.Xiao H, Wang M, Du Y, et al. Agonist-like autoantibodies against calcium channel in patients with dilated cardiomyopathy. Heart and Vessels. 2012;27(5):486–492. doi: 10.1007/s00380-011-0176-7. [DOI] [PubMed] [Google Scholar]
- 31.Dong R, Liu P, Wee L, Butany J, Sole MJ. Verapamil ameliorates the clinical and pathological course of murine myocarditis. The Journal of Clinical Investigation. 1992;90(5):2022–2030. doi: 10.1172/JCI116082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okazaki T, Honjo T. Pathogenic roles of cardiac autoantibodies in dilated cardiomyopathy. Trends in Molecular Medicine. 2005;11(7):322–326. doi: 10.1016/j.molmed.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Reichlin M. Cellular dysfunction induced by penetration of autoantibodies into living cells: cellular damage and dysfunction mediated by antibodies to dsDNA and ribosomal P proteins. Journal of Autoimmunity. 1998;11(5):557–561. doi: 10.1006/jaut.1998.0219. [DOI] [PubMed] [Google Scholar]
- 34.Koscec M, Koren E, Wolfson-Reichlin M, et al. Autoantibodies to ribosomal P proteins penetrate into live hepatocytes and cause cellular dysfunction in culture. Journal of Immunology. 1997;159(4):2033–2041. [PubMed] [Google Scholar]
- 35.Shah M, Akar FG, Tomaselli GF. Molecular basis of arrhythmias. Circulation. 2005;112(16):2517–2529. doi: 10.1161/CIRCULATIONAHA.104.494476. [DOI] [PubMed] [Google Scholar]
- 36.Figulla HR, Gietzen F, Zeymer U, et al. Diltiazem improves cardiac function and exercise capacity in patients with idiopathic dilated cardiomyopathy: results of the diltiazem in dilated cardiomyopathy trial. Circulation. 1996;94(3):346–352. doi: 10.1161/01.cir.94.3.346. [DOI] [PubMed] [Google Scholar]
- 37.Neglia D, Sambuceti G, Giorgetti A, et al. Effects of long-term treatment with verapamil on left ventricular function and myocardial blood flow in patients with dilated cardiomyopathy without overt heart failure. Journal of Cardiovascular Pharmacology. 2000;36(6):744–750. doi: 10.1097/00005344-200012000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Liao Y-H. Interventional study of diltiazem in dilated cardiomyopathy: a report of multiple centre clinical trial in China. Chinese cooperative group of diltiazem intervention trial in dilated cardiomyopathy. International Journal of Cardiology. 1998;64(1):25–30. doi: 10.1016/s0167-5273(97)00310-0. [DOI] [PubMed] [Google Scholar]


